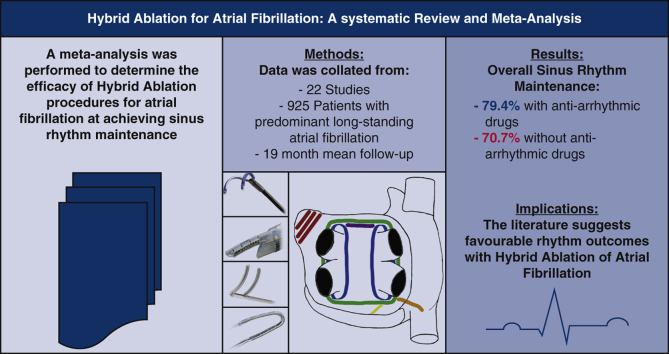
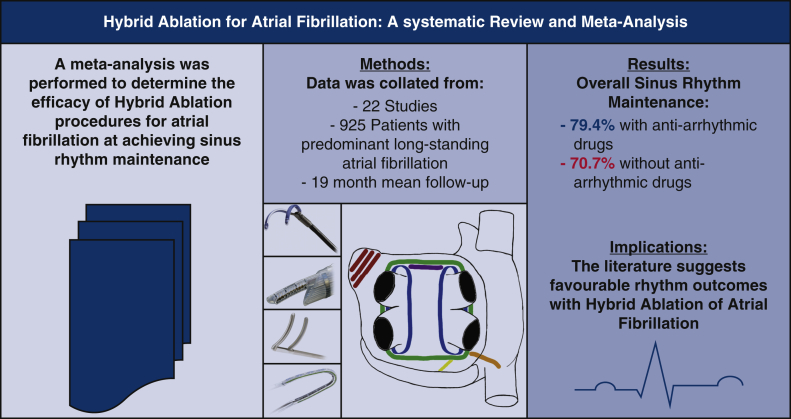
Abstract
Background
Both catheter and surgical ablation strategies offer effective treatments of atrial fibrillation (AF). The hybrid (joint surgical and catheter) ablation for AF is an emerging rhythm control strategy. We sought to determine the efficacy and safety of hybrid ablation of AF.
Methods
Systematic review and meta-analysis interrogating PubMed, EMBASE, and Cochrane databases from January 1, 1991, to November 30, 2017, using the following search terms: “Cox-maze,” “mini-maze,” “ablation methods (including radiofrequency, cryoablation, cryomaze),” and “surgery.” Included studies required ablation procedures to be hybrid and report rhythm follow-up.
Results
We included 925 patients with AF (38% persistent, 51% longstanding persistent) from 22 single-center studies (mean follow-up of 19 months). The surgical lesion set consisted of pulmonary vein isolation (n = 11) or box lesion (n = 11) with variable additional linear ablation. This was followed by sequential (n = 9), staged (n = 9), or combination (n = 4) catheter-based ablation to ensure isolation of pulmonary veins and to facilitate additional ablation or consolidation of surgically ablated lines. Overall, sinus rhythm maintenance was 79.4% (95% confidence interval [CI], 72.4-85.7] and 70.7% (95% CI, 62.2-78.7) with and without antiarrhythmic drugs, respectively at 19 ± 25 (range, 6-128) months. The use of the bipolar AtriCure Synergy system and left atrial appendage exclusion conferred superior rhythm outcome without antiarrhythmic drugs (P ≤ .01). The overall complication rate was 6.5% (95% CI, 3.4-10.2): mortality 0.2% (95% CI, 0-0.9); stroke 0.3% (95% CI, 0-1.1); reoperation for bleeding 1.6% (95% CI, 0.6-3.0); permanent pacing ~0% (95% CI, 0-0.5); conversion to sternotomy 0.3% (95% CI, 0-1.1); atrioesophageal fistula ~0% (95% CI, 0-0.5); and phrenic nerve injury 0.3% (95% CI, 0-1.1).
Conclusions
Hybrid ablation therapy for AF demonstrates favorable rhythm outcome with acceptable complication rates.
Key Words: atrial fibrillation, catheter ablation, surgical ablation, hybrid ablation, radiofrequency
Abbreviations and Acronyms: AAD, antiarrhythmic drug; AF, atrial fibrillation; CI, confidence interval; LAA, left atrial appendage; PVI, pulmonary vein isolation; RF, radiofrequency; SRM, sinus rhythm maintenance; VATS, video-assisted thoracoscopic surgery
Graphical abstract
Common lesion sets across studies.
Central Message.
The hybrid ablation procedure for atrial fibrillation (AF) shows favorable outcomes with low complication rates and is worth increasing consideration in the management of symptomatic AF.
Perspective.
This analysis provides a current understanding of the efficacy and safety of the hybrid ablation of atrial fibrillation. Through this understanding, the patients most suited for this type of therapy may better be identified and the focus of future studies to enhance our understanding of this treatment will be brought to light.
Atrial fibrillation (AF) remains a significant public health burden.1 Catheter ablation has been demonstrated to be superior to medical therapy for symptoms, with reduced mortality and hospitalizations also shown in those with concomitant heart failure.2 However, the success rates of catheter ablation in persistent and longstanding persistent AF have been limited despite greater amount of ablation.3 In addition, reports suggest a gradual attrition following initial successful catheter ablation. In comparison, the rates of freedom from AF with the surgical Cox-maze III and Cox-maze IV procedures were reported at 96% and 91% with antiarrhythmic drugs (AAD), or 83% and 78% without AAD, at up to 5.4 years of follow-up.4,5 These apparent superior results may be due to the ability to obtain direct or videoscopic visualization, better stabilization, and confirmation of lesion transmurality, particularly when the lesions are cut and sewn. However, the invasiveness of the surgical approach is also accompanied by greater perioperative morbidity and potential major complications including death, bleeding, and stroke.5
The hybrid approach comprising initial surgical epicardial ablation with concurrent or sequential endocardial catheter-based ablation has emerged, with initial studies showing promising rates of freedom from AF.6 This strategy seeks to combine the strengths and minimize the drawbacks of the individual approaches by balancing invasiveness and duration of ablation procedure with improved delivery of ablative lesions sets followed by endocardial electrophysiologic evaluation and additional consolidative ablation if required. Therefore, we undertook a systematic literature review and meta-analysis to evaluate the efficacy and safety of this hybrid approach at sinus rhythm maintenance (SRM) in patients with AF.
Methods
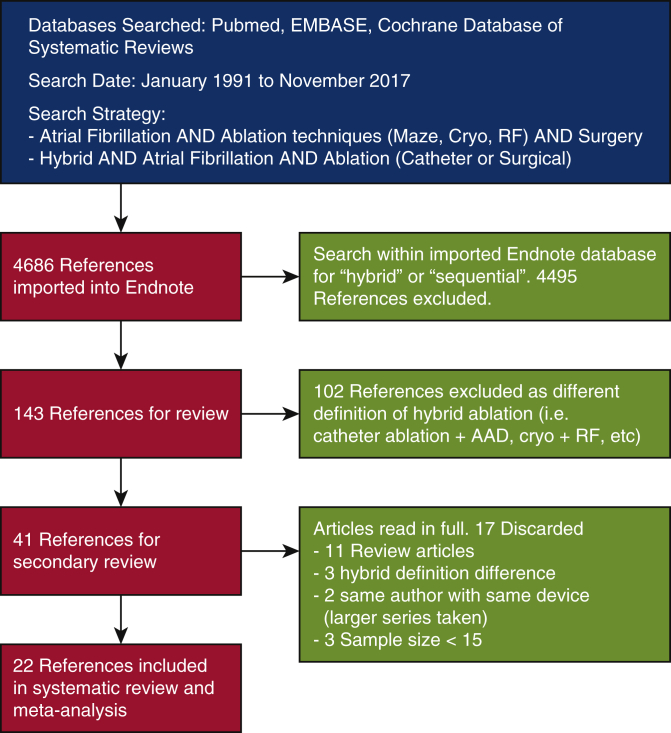
This study was registered with the PROSPERO International prospective register of systematic reviews website (Registration Number CRD42017059106). We followed the MOOSE (Meta-analysis of Observational Studies in Epidemiology.) guidelines for meta-analyses and systematic reviews of observational studies. A search of electronic databases including PubMed, Embase, and Cochrane database of systematic reviews was carried out using the following search terms: “AF AND ablation techniques (Maze, Cryo, radiofrequency [RF]) AND Surgery” and “hybrid AND AF AND ablation (Catheter or Surgical)” (Figure E1). The search was conducted from January 1, 1991, to November 30, 2017. No written consent from patients was obtained due to the study being a meta-analysis with no direct patient involvement.
Figure E1.
Search strategy utilised for article inclusion.
Definitions
We defined hybrid AF ablation as a combined surgical and endocardial catheter-based approach, regardless of surgical access, to encompass any combined ablation approach. This is consistent with the 2017 Heart Rhythm Society/European Heart Rhythm Association/European Cardiac Arrhythmia Society expert consensus statement on catheter and surgical ablation for AF that has broadly defined “hybrid” procedures for the ablation of AF as those combine surgical and catheter ablation.7 Typically, the catheter-based procedure either follows the surgical ablation. The aim is to confirm the integrity of the surgically placed ablation lines with endocardial mapping, consolidate these ablation lesion sets as required, and perform additional substrate-based ablation. The surgical ablation is usually performed via sternotomy, thoracotomy, video-assisted thoracoscopic surgery (VATS), or subxiphoid/laparoscopic transdiaphragmatic approach. Surgical lesions were defined as any series of lesions placed on the heart (cut and sew/RF/cryothermy/microwave) for the management of AF. The surgical lesions were categorized into box or pulmonary vein isolation, whereas all other ablations were also specified. We also analyzed the role of surgical exclusion of the left atrial appendage (LAA). Efficacy outcomes assessed were SRM with and without AAD. Safety outcomes assessed were the reporting of mortality, cerebrovascular accident, reoperation for bleeding, phrenic nerve injury, atrioesophageal fistula, conversion to open procedure, postoperative pacemaker implantation, and length of stay. The classification of AF was according to 2017 Heart Rhythm Society/European Heart Rhythm Association/European Cardiac Arrhythmia expert consensus statement on catheter and surgical ablation for AF.7
Statistical Analysis
Meta-analyses were conducted using the meta packages of the R statistical software (version 3.6; The R Foundation for Statistical Computing, Vienna, Austria). Specifically, calculation of a pooled proportion from studies reporting single proportions was carried out using the ‘metaprop’ function using the Freeman–Turkey Double arcsine transformation. Mean and standard deviation of continuous variables and exact binomial lower and upper confidence limits of categorical variables were pooled across studies and analyzed using a random effects meta-analysis model. Statistical heterogeneity was assessed using the I2 statistic. Metaregression was performed using the ‘Metafor’ package. All metaregression analyses were performed using univariate random-effects models with heterogeneity assessed using the restricted maximum likelihood estimator. Meta-regression analysis was done to evaluate associations between outcomes of SRM and complications in each paper versus ablation method, lesions set used, exclusion of LAA, and use of randomized control trial, weighting for number of patients who completed follow-up. Assumptions of a linear model were upheld throughout.
Results
Our literature search (Figure E1) yielded 4636 references. Review of the abstracts resulted in 141 potentially relevant articles. In total, 102 references were excluded, as the “hybrid” ablation approach did not meet our definition of combined surgical and catheter-based procedures. A further 17 were excluded following secondary review of full-text articles (n = 9 review articles, n = 2 duplicates, n = 3 sample size <15 and n = 3 failed to meet definition of hybrid ablation). A total of 22 studies were included (Table 1).6,8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28
Table 1.
Baseline study characteristics
| Authors | Year | Cohort size | Mean age, y | Male, n (%) | PAF, n (%) | PersAF, n (%) | Long-standing PersAF, n (%) | Mean AF duration, y | HT, % | DM, % | BMI, kg/m2 | Mean LA size, mm | Mean LVEF, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bisleri et al9 | 2013 | 45 | 62 ± 10 | 33 (73) | 0 | 0 | 45 (100) | 7 ± 5.8 | 76 | 31 | 17∗ | 51 ± 10 | 56 ± 7 |
| Bulava et al10 | 2015 | 50 | 62 ± 7 | 32 (64) | 0 | 0 | 50 (100) | 3.5 ± 2.9 | 64 | 0 | 31 ± 5 | 48 ± 5 | 63 ± 8 |
| de Asmundis et al11 | 2017 | 64 | 60 ± 9 | 56 (88) | 0 | 21 (33) | 43 (67) | 5.2 ± 3.7 | 52 | 9 | 28 ± 4 | 50 ± 7 | 53 ± 11 |
| Edgerton et al21 | 2016 | 24 | 64 ± 9 | 22 (92) | 0 | 0 | 24 (100) | 6.8 ± 3.5 | 63 | 4 | 31 ± 5 | – | 53 ± 9 |
| Eisenberger et al27 | 2015 | 35 | 71 ± 6 | 23 (66) | 0 | 9 (26) | 26 (74) | 4.3 ± 0.9 | 83 | 23 | 29 ± 5 | 50 ± 5 | 63 ± 7 |
| Gaita et al26 | 2013 | 33 | 58 ± 13 | 15 (45) | 0 | 0 | 33 (100) | 2.4 ± 2.6 | – | – | – | 51 ± 6 | 53 ± 11 |
| Gehi et al22 | 2013 | 101 | 63 ± 10 | 79 (78) | 17 (17) | 47 (47) | 37 (37) | 5.9 ± 5.5 | 61 | 19 | 59∗ | 51 ± 1 | 50 ± 11 |
| Gersak et al8 | 2012 | 50 | 56 ± 11 | 42 (84) | 3 (6) | 8 (16) | 39 (78) | 5 ± 4.7 | 74 | 4 | 29 ± 4 | 48 ± 5 | 59 ± 11 |
| Kiser et al23 | 2010 | 28 | – | – | 0 | 5 (18) | 23 (82) | 8 | – | – | – | 53 | - |
| Krul et al19 | 2011 | 31 | 57 ± 7 | 25 (81) | 16 (52) | 13 (42) | 2 (6) | 8† | 32 | 3 | 29 ± 4 | 47 ± 7 | - |
| Kumar et al12 | 2015 | 38 | 62 ± 7 | 34 (89) | 14 (37) | 19 (50) | 15 (13) | 6.8 ± 4.4 | 42 | 13 | 28 ± 3 | – | 52 ± 14 |
| Kurfirst et al13 | 2014 | 30 | 61 ± 8 | 20 (67) | 0 | 4 (13) | 26 (87) | 2.8 ± 2.3 | 67 | – | 30 ± 5 | 48 ± 5 | 62 ± 8 |
| La Meir et al14 | 2012 | 19 | 61 ± 9 | 16 (84) | 5 (26) | 4 (21) | 10 (53) | 5 | 37 | – | 28 ± 5 | 50 ± 1 | - |
| La Meir et al15 | 2013 | 35 | 57 ± 10 | 24 (69) | 16 (46) | 8 (23) | 11 (31) | 5 | 43 | 3 | 27 ± 4 | 52 ± 1 | - |
| Lee et al28 | 2011 | 25 | 61 ± 11 | 19 (76) | 16 (64) | 5 (20) | 4 (16) | 3† | 52 | 12 | 29 ± 6 | – | 57 ± 9 |
| Mahapatra et al16 | 2011 | 15 | 60 ± 2 | 8 (53) | 0 | 9 (60) | 6 (40) | 5.4 ± 0.6 | 47 | 20 | – | 52 ± 10 | 47 ± 3 |
| Muneretto et al6 | 2012 | 36 | 62 ± 10 | 17 (47) | 0 | 8 (22) | 28 (78) | 6.1 | 42 | 19 | – | 50 ± 6 | 53 ± 3 |
| Osmancik et al20 | 2016 | 33 | 60 ± 11.6 | 24 (73) | 0 | 22 (73) | 8 (27) | 2.5 ± 2.7 | – | – | 33 ± 5 | 47 ± 9 | 54 ± 12 |
| Pison et al17 | 2012 | 26 | 57 ± 9 | 18 (69) | 15 (58) | 11 (42) | 0 | 5.6 ± 3.6 | 46 | – | 27 ± 4 | 43 ± 6 | 59 ± 7 |
| Richardson et al18 | 2016 | 83 | 63† | 68 (82) | 1 (1) | 82 (99) | 0 | – | – | – | 49† | 55† | |
| Toplisek et al24 | 2016 | 37 | 54 ± 11 | 32 (86) | 0 | 37 (100) | 0 | 4† | 59 | 5 | 29 ± 4 | 47 ± 6 | 59 ± 11 |
| Zembala et al25 | 2017 | 90 | 55 ± 10 | 70 (78) | 0 | 39 (43) | 51 (57) | 4.5 ± 3.7 | 69 | 13 | 29 ± 4 | 45 ± 6 | 49 ± 10 |
| Mean | 42.1 | 60.5 | 74% | 12% | 38% | 51% | 5.1 | 59.3 | 12.1 | 29 | 59.1 | 54.8% |
Underline indicates the mean value has included a study that reported a median value.
PAF, Paroxysmal, PersAF, persistent; AF, atrial fibrillation; HT, hypertension; DM, diabetes mellitus; BMI, body mass index; LA, left atrium; LVEF, left ventricular ejection fraction.
Number with obesity.
Median.
Surgical Ablation Procedures
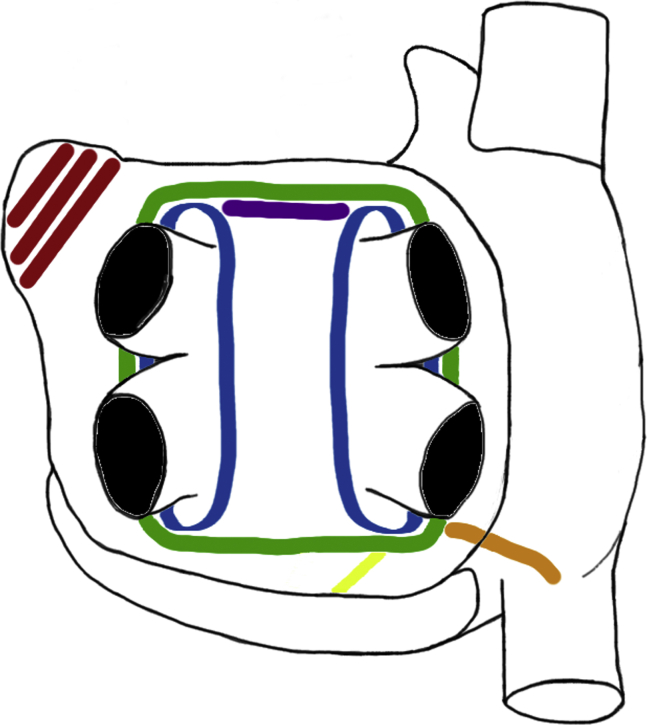
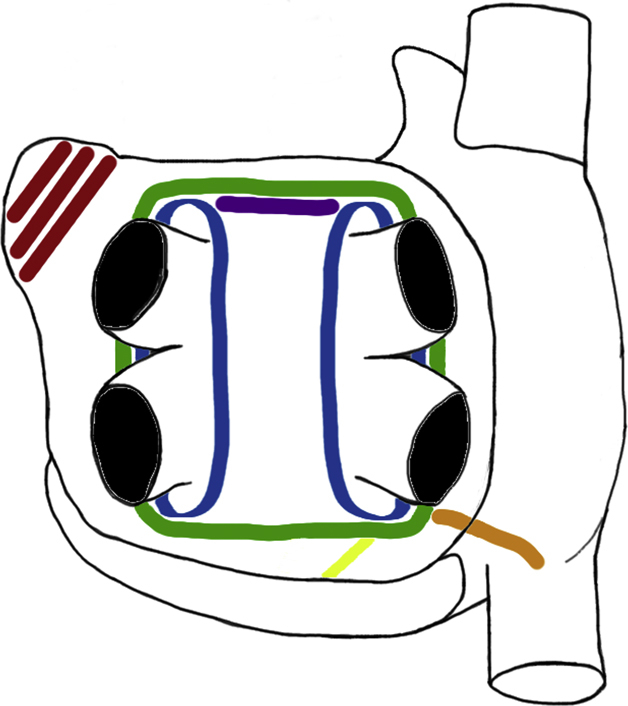
Both surgical access and ablation lesion sets varied among the studies, with interpretation of the lesion set from published figures required in one study (Table 2).8 Surgical access was via unilateral or bilateral VATS (n = 13),6,9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 subxiphoid/transdiaphragmatic laparoscopy (n = 6),8,21, 22, 23, 24, 25 sternotomy (n = 2),26,27 and mini-thoracotomy (n = 1).28 Ablation lesion sets included pulmonary vein isolation (PVI) in 11,8,13,15, 16, 17,19,22, 23, 24,26,28 and box isolation in 116,9, 10, 11, 12,14,18,20,21,25,27; additional lesions are detailed in Table 2: roof line, floor line, mitral isthmus line, ganglionated plexi ablation, ligament of Marshall ablation, between the right inferior pulmonary vein and inferior vena cava, superior vena cava, inferior vena cava, bicaval line, cavotricuspid isthmus, between the right inferior pulmonary vein and Thebesian valve, oblique sinus, Waterson's groove line, and complete posterior wall ablation. The LAA was surgically managed in 11 (n = 296 patients, 30.7%); stapled (n = 5),12,16,17,19,28 clipped (n = 3),10,11,13 combination of stapled or clipped (n = 2),15,18 and unspecified (n = 1).27 Additional concomitant procedures included removal of the fat along Waterson's groove to facilitate disruption of the ganglionic plexi6; valve replacement in 19 patients, coronary artery bypass grafts in 7 patients, and combination of these procedures in 8 patients27; and minimally invasive mitral valve repair in 1 patient.23 In the study by Pison and colleagues,17 the lesions were placed in a stepwise fashion with additional lines only added when sustained AF remained inducible with isoproterenol. The commonly employed surgical lesions are depicted in Figure 1, Figure 2, Figure 3.
Table 2.
Surgical procedural characteristics
| Study | Access | Device | Energy source | Basic lesion | Additional linear ablation | GP | LAA closure | Procedural duration, min |
|
|---|---|---|---|---|---|---|---|---|---|
| Surgical | Total | ||||||||
| Bisleri et al9 | VATS (unilateral R) | Cobra | Unipolar RF | Box | N | N | N | 85 ± 9 | – |
| Bulava et al10 | VATS (bilateral) | AtriCure | Bipolar RF | Box | LOM, Trigone | Y | Y (42/50) | 190 ± 30 | |
| de Asmundis et al11 | VATS (bilateral) | AtriCure | Bipolar RF | Box | SVC (34 [53%]) + caval line (if RA dilated) +ML (4 [6%]) | N | Y (47/64) | – | 268 ± 71 |
| Edgerton et al21 | Laparoscopic | nContact | Unipolar RF | Box | LOM, RA line | N | N | – | 277 ± 64 |
| Eisenberger et al27 | Sternotomy | Medtronic | Cryoablation | Box | LOM, mitral isthmus, cavotricuspid isthmus | N | Y | – | – |
| Gaita et al26 | Sternotomy | Frigitronics | Cryoablation | PVI | Roof line, mitral isthmus | N | Y | – | – |
| Gehi et al22 | Laparoscopic | nContact | Unipolar RF | PVI | LOM, roof line (90/101), Floor line, mitral isthmus (84/101), RIPV-IVC, WG | N | N | – | – |
| Gersak et al8 | Laparoscopic | nContact | Unipolar RF | PVI | LOM, roof line, RIPV-IVC | Y | N | – | 313 ± 94 |
| Kiser et al23 | Laparoscopic | nContact | Unipolar RF | PVI | LOM, roof line, mitral isthmus, RIPV-IVC, RIPV-Thebesian valve, oblique sinus, WG | N | N | 102 | – |
| Krul et al19 | VATS (bilateral) | AtriCure | Bipolar RF | PVI | LOM +/– (RL + FL + Trigone in LSP and P patients) | Y | Y | – | 205∗ |
| Kumar et al12 | VATS (bilateral) | AtriCure | Bipolar RF | Box | N | N | – | 223 ± 57 | |
| Kurfirst et al13 | VATS (bilateral) | AtriCure | Bi/Unipolar RF | PVI | LOM (29/30), roof + floor lines (29/30), mitral isthmus (26/30) | Y | Y (19/30) | – | 201 ± 30 |
| La Meir et al14 | VATS (bilateral) | Cobra | Unipolar RF | Box | N | Y | N | – | 216∗ |
| La Meir et al15 | VATS (bilateral) | AtriCure | Bi/Unipolar RF | PVI | Roof line (32/35), floor line (31/35), mitral isthmus (7/35) | Y | Y (15/35) | – | 268∗ |
| Lee et al28 | Mini thoracotomy | Gemini-X | Bipolar RF | PVI | N | Y | Y | – | – |
| Mahapatra et al16 | VATS (unilateral R) | AtriCure | Bipolar RF | PVI | SVC, roof line, mitral isthmus, LOM | Y | Y | – | 450 ± 20 |
| Muneretto et al6 | VATS (unilateral R) | Cobra | Unipolar RF | Box | N | N | N | 80 ± 7 | – |
| Osmancik et al20 | VATS (unilateral R) | Cobra | Bi/Unipolar RF | Box | N | N | Y (8/30) | 115 ± 30 | – |
| Pison et al17 | VATS (bi/unilateral R) | AtriCure | Bipolar RF | PVI/Box | Mitral isthmus (3/26), bicaval line (9/26), SVC (7/26), IVC (3/26), roof line (1/26) | N | Y | – | 280 ± 84 |
| Richardson et al18 | VATS (bilateral) | AtriCure | Bipolar RF | Box | SVC + IVC + LOM | Y | Y | – | – |
| Toplisek et al24 | Laparoscopic | nContact | Unipolar RF | PVI | LOM, RL, inferior RPV → IVC | Y | N | – | – |
| Zembala et al25 | Laparoscopic | nContact | Unipolar RF | PVI | LOM, RA line +/– complete posterior wall | N | N | 141 ± 25 | – |
GP, Ganglionated plexi; LAA, left atrial appendage; VATS, video-assisted thoracoscopic surgery; R, right; RF, radiofrequency; N, no; LOM, ligament of Marshall; Y, yes; SVC, superior vena cava; RA, right atrium; ML, mitral line; PVI, pulmonary vein isolation; RIPV, right inferior pulmonary vein; IVC, inferior vena cava; WG, Waterson's grove; FL, floor line; LSP, long standing persistent; P, persistent.
Denotes median value.
Figure 1.
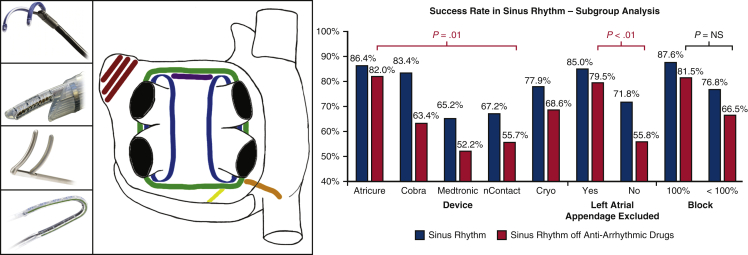
Ablation devices, ablations lines and success rate in SRM. Common surgical devices and ablation lines placed are shown. The subgroup analysis of these different devices, the exclusion of the left atrial appendage, and the achievement of conduction block on the SRM at final follow-up is displayed. The only statistically significant comparators were AtriCure versus nContact and left atrial appendage exclusion on SRM without AAD. Left (from top to bottom), The RF devices used in the studies: Medtronic Gemini, nContact system, AtriCure Synergy and Estech Cobra. Middle, Commonly placed surgical lines of PVI (blue), box lesion (green), roof line (purple), mitral line (yellow), RIPV to right atrium line (orange), LAA exclusion (red). Right, Procedural success in obtaining SRM at final follow-up related to device used and left atrial appendage exclusion. Image sources: (top to bottom). Medtronic: http://www.medtronic.com/us-en/healthcare-professionals/products/cardiovascular/ablation-surgical/cardioblate-gemini-irrigated-rf-surgical-ablation-system.html. nContact: Provided by nContact for reproduction in publication. AtriCure: Provided by nStenning sales representative. Estech: http://www.axle-international.com/manufacturers/right_estech/left_surgical-ablation.html. NS, Not significant.
Figure 2.
Common ablation lines placed.
Figure 3.
Core findings of our meta-analysis.
Twenty-studies used RF for surgical ablation,6,8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25,28 whereas 2 used cryothermy.26,27 RF devices for surgical ablation included the monopolar Cobra Adhere XL device (Estech, San Ramon, Calif; n = 3),6,9,14 bipolar Cobra Fusion 150 (Estech; n = 1),20 bipolar AtriCure Synergy clamp with or without rail device (Coolrail; AtriCure, West Chester, Ohio; n = 9),10, 11, 12, 13,15, 16, 17, 18, 19 nContact (nContact Surgical, Morrisville, NC; n = 6),8,21, 22, 23, 24, 25,29 and Gemini-X (10%; Medtronic, Minneapolis, Minn; n = 1; 10% of the cases with the AtriCure).28 Of the 2 studies using cryo-energy; 1 used the Frigitronics cryothermy (Frigitronics, Cooper Surgical, Shelton, CT),26 and 1 the CryoFlex (Medtronic).27
Only 10 reported on amount of RF energy delivered per lesion, which ranged from at least 1 minute18 to variable number of applications (at least 2,6 3,15,19,28 4-6,11,17 3-7,16 and at least 5 applications10,13). One of the 2 cryoablation studies reported 2-minutes of ablation time per lesion.27 The end point of the surgical procedure was conduction block in 12,6,8, 9, 10,13,15,16,18, 19, 20,22,28 with 5 of those being confirmed endocardially by the electrophysiologist,6,9,16,20,22 completion of the planned lesion set in 9,11,12,14,17,21,24, 25, 26, 27 and visual confirmation of contiguity of ablation lines in 1.23 Ten studies reported total procedural time (218 ± 88 minutes),8,9,11,12,14, 15, 16, 17,19,21 whereas 6 reported surgical time (119 ± 45 minutes).6,10,13,20,23,25
Catheter-Based Ablation Procedures
Details of catheter-based procedures are listed in Table 3. Nine-studies undertook immediate follow on catheter-based ablation in a hybrid theater,11,12,14,15,17,19,21, 22, 23 whereas 9 used a staged approach with a variable interval period ranging from 4 to 167 days before the endocardial procedure,6,9,10,13,16,20,26, 27, 28 and 4 used a combination of both sequential or staged approaches.8,18,24,25 Of note, Lee and colleagues28 only performed catheter-based procedures in patients with recurrent AF more than 3 months after the initial surgical procedure. In this study, only 23 of 25 underwent further electrophysiologic study and ablation, with 2 patients declining further intervention given symptomatic improvement.28 Further, the study of Gersak and colleagues8 had 5 patients who declined the endocardial ablation stage of the procedure.
Table 3.
Electrophysiologic procedural characteristics
| Study | Interval, d | Additional ablation (n) | Ablation source | 3D system | Procedure end point | Block check |
Procedural duration, min |
||
|---|---|---|---|---|---|---|---|---|---|
| Preablation | Postablation | Fluoro time | Total | ||||||
| Bisleri et al9 | 30- 45 | PV (3), CFAE (20), CTI (11) | RF | Carto | Block, AF stimulation test | Y (Bi: 91%) | Y (Bi: 100%) | – | – |
| Bulava et al10 | 42-56 | CTI + ML | RF | Carto | Block, AF stimulation test (pacing induced) | Y (37 [74%]) | Y (100) | 8 ± 4 | 137 ± 41 |
| de Asmundis et al11 | Sequential | CTI + ML + CFAE | RF | Carto | Block/isolation | Y (47 [73%]) | Y (100%) | 22 ± 9 | – |
| Edgerton et al21 | Sequential | CS +/– LAA +/– CFAE | RF | NS | SR, Block, Isopren challenge | Y (#NS) | Y (#NS) | 36 ± 15 | – |
| Eisenberger et al27 | 90 | – | NS | Carto | Block, AF stimulation test (pacing induced) | Y (0% gap free lesions) | Y (31 [89%]) | 7∗ | 130∗ |
| Gaita et al26 | 90 | – | RF | Carto | Block/isolation | Y (58%) | Y (79%) | – | – |
| Gehi et al22 | Sequential | CS + CTI (99), CS (73), +CFAE (29) | RF | NavX/Carto | Block/isolation | – | Y (97) | – | – |
| Gersak et al8 | Combined cohort | - | RF | NS | Block/isolation | – | Y (100%†) | – | – |
| Kiser et al23 | Sequential | CS + CTI | NS | Carto | SR, Isopren challenge, Block (line confirmed) | – | Y (#NS) | – | 85 |
| Krul et al19 | Sequential | - | NS | NS | Block | – | Y (#NS) | – | – |
| Kumar et al12 | Sequential | CTI + CFAE | RF | Carto | Block, AF stimulation test with pacing | Y (26 [65%]) | Y (#NS) | 19 ± 9 | – |
| Kurfirst et al13 | 90 | CTI (if in SR) + ML | RF | Carto | Block. Test with atrial tachy pacing (300 bpm) | Y (33%) | Y (100%) | – | – |
| La Meir et al14 | Sequential | ML +/– CTI | RF | NS | Block | Y (Bi:0) | Y (Bi:0) | – | – |
| La Meir et al15 | Sequential | SVC-IVC (10), SVC (8), IVC (3), CTI (3) | RF | NS | Block + widely separated double potentials. Stim test (pacing + iso) | Y (#NS) | Y (Bi:100%) | – | – |
| Lee et al28 | 167 ± 89 | RL + ML | NS | NS | Block, AF stimulation test | Y (0%) | Y (#NS) | – | – |
| Mahapatra et al16 | 4 ± 1 | CS+CTI, CFAE (2) | RF | NavX/Carto | SR, Block, Iso challenge | Y (46.7%) | Y (#NS) | – | – |
| Muneretto et al6 | 33 ± 2 | CTI (21), CFAE (6) | NS | Carto | Block, AF stimulation test | Y (Bi: 83.3) | Y (#NS) | 18 ± 3 | – |
| Osmancik et al20 | 96 ± 73 | GP + CTI | RF | Carto | SR, Block | Y (40%) | Y (88.8%) | 19 ± 7 | 216 ± 64 |
| Pison et al17 | Sequential | CTI | RF/Cryo | NS | SR, Block, Iso challenge | Y (#NS) | Y (#NS) | – | – |
| Richardson et al18 | Combined | CTI + ML + CFAE | RF | Carto | Block/isolation | Y (38 [45.8%]) | Y (#NS) | – | – |
| Toplisek et al24 | Combined | RL + SVC + IVC | RF | NS | Block/isolation | – | Y (#NS) | – | – |
| Zembala et al25 | Combined | CS + CTI | RF | Carto | Block | – | Y (#NS) | – | – |
3D, 3-Dimensional; PV, pulmonary vein; CFAE, complex fractionated atrial electrogram; CTI, cavotricuspid isthmus line; RF, radiofrequency; AF, atrial fibrillation; Y, yes; ML, mitral line; CS, coronary sinus line; LAA, left atrial appendage; NS, not stated; SR, sinus rhythm; SVC-IVC, superior vena cava/inferior vena cava; GP, ganglionated plexi.
Denotes median value.
Only tested in if still in AF.
Seventeen-studies used RF energy for catheter ablation (n = 13, Thermocool; Biosense Webster, Irvine, Calif8, 9, 10, 11,13, 14, 15, 16,18,24, 25, 26, 27; n = 2 Thermocool SmartTouch, Biosense Webster12,20; n = 1 Thermocool or irrigated Blazer II [Boston Scientific, Boston, Mass],22 and n = 1 combination of Thermocool and cryo-balloon technology [Arctic Front; CryoCath, Montreal, Quebec, Canada]).17 Five studies did not state the device employed for the catheter procedure (Table 3).6,19,21,23,28 Fourteen studies reported the use of 3-dimensional electroanatomical mapping system (n = 12 CARTO)6,9, 10, 11, 12, 13,18,20,23,25, 26, 27 and n = 2 CARTO or NavX.16,22 Eight-studies did not specify the mapping system.8,14,15,17,19,21,24,28 Catheter ablation strategy differed among the studies beyond PVI (Table 3) with addition of cavo-tricuspid isthmus line (n = 11),10, 11, 12, 13, 14, 15,17,18,20,22,23 mitral isthmus line (n = 6),10,11,14,18,24,28 coronary sinus ablation (n = 4),16,21,22,25 roof-line (n = 3),8,24,28 superior/inferior venous cava ablation (n = 4),8,15,16,24 ganglionated plexi ablation (n = 1),20 and complex fractionated atrial electrogram ablation (n = 6).11,12,16,18,21,22 Five studies did not perform additional lines of ablation other than consolidating the surgically placed lines as required.6,9,19,26,27 Two studies did not provide a specific written description of catheter ablation lines, necessitating interpretation from diagrams in the respective studies.8,29
At the beginning of the procedure, 16 reported checking for conduction block from the surgical ablation with variable results in gap free lesions (range, 0%-91.1%).6,9, 10, 11, 12, 13, 14, 15, 16, 17, 18,20,21,26, 27, 28 Conduction block following catheter ablation was described in all studies although only 11 reported this in detail.8, 9, 10, 11,13, 14, 15,20,22,26,27 Further testing with AF induction was performed using pacing maneuvers in 46,9,13,28 and isoproterenol challenge in 4 studies.15, 16, 17,23 Only 5 studies reported rhythm status of their patients at the end of the procedure.9,17,20,22,23 The procedural time for the catheter-based component was not uniformly reported, with 13 not reporting any procedural time indices.8,9,13, 14, 15,17, 18, 19,22,24, 25, 26,28 Seven studies reported time of fluoroscopy of 18.3 minutes (range, 7.3-36.2)10, 11, 12,16,20,21,27 and 5 reported procedure time of 112.3 minutes (range, 18-216).6,10,20,23,27 The study by Gaita and colleagues26 reported that no fluoroscopy was used.
Postprocedural Follow-up
Postablation follow-up included the use primarily of implantable loop recorders in 5,6,8,9,18,24 Holter monitoring (1-7 day) in 16,10, 11, 12, 13, 14, 15, 16, 17,19, 20, 21, 22, 23,25, 26, 27 and 12-lead ECG in 1.28 However, the follow-up frequency was variable as detailed in Table 4. The overall follow-up duration was 19 months (range, 6-128.4) after initial surgical procedure with follow-up completed by 93.3%. Definition of recurrent arrhythmia varied across studies with 13 studies defining AF recurrence as any atrial tachyarrhythmia lasting >30 seconds10, 11, 12, 13,15, 16, 17, 18, 19,21,22,25,27 and 3 studies as monthly AF burden >0.5%.6,9,24 AF recurrence was not defined in the remaining 6 studies.8,14,20,23,26,28
Table 4.
Follow-up and results
| Study | Method |
Mean F/U, mo | Number complete F/U (%) | SR with AAD (%) | SR without AAD (%) | Need for further catheter ablation | |||
|---|---|---|---|---|---|---|---|---|---|
| ECG | 1 d | 7 d | ILR | ||||||
| Bisleri et al9 | – | – | – | Y | 28.4 ± 2 | 45/45 (100) | 40/45 (89) | – | – |
| Bulava et al10 | – | – | 3, 6, 9, 12 | – | 12 | 50/50 (100) | 47/50 (94) | 43/50 (86) | 2 |
| de Asmundis et al11 | – | 6, 12 | – | – | 23.1 ± 14 | 64/64 (100) | 43/64 (67) | 43/64 (67) | 14 |
| Edgerton et al21 | – | – | 3, 6, 9, 12 | – | 24∗ | 21/24 (88) | 4/21 (19) | 3/21 (14) | – |
| Eisenberger et al27 | – | – | 3, 6, 12 | – | 12 | 35/35 (100) | 32/35 (91) | 30/35 (86) | 1 |
| Gaita et al26 | 3, 6, 12 | 3, 6, 12 | – | – | 128.4 ± 37 | 33/33 (100) | 24/33 (73) | 16/33 (48) | 4 |
| Gehi et al22 | 3, 6, 12 | 3, 6, 12 | Y | 12∗ | 101/101 (100) | 66/101 (65) | – | 6 | |
| Gersak et al8 | 6, 12, 18, 24 | – | – | Y | 12∗ | 32/50 (64) | 28/32 (88) | 24/32 (75) | 1 |
| Kiser et al23 | – | 3 | 6 | – | 6∗ | 25/28 (89) | 21/25 (84) | 19/25 (76) | – |
| Krul et al19 | 3/12 | 3/12 | – | – | 12.5† | 22/31 (71) | 19/22 (86) | 19/22 (86) | – |
| Kumar et al12 | – | – | 3, 6, 9, 12 | – | 11.2 ± 2 | 38/38 (100) | 35/38 (92) | 31/38 (82) | 3 |
| Kurfirst et al13 | – | – | 3, 6, 12 | – | 6.93 | 30/30 (100) | 28/30 (93) | 27/30 (90) | 1 |
| La Meir et al14 | – | – | 3, 6, 12 | – | 12∗ | 19/19 (100) | 12/19 (63) | 7/19 (37) | – |
| La Meir et al15 | 3,6, 12 | – | 3, 6, 12 | – | 12 | 35/35 (100) | 32/35 (91) | 32/35 (91) | – |
| Lee et al28 | 3,6,24 | – | – | – | 14 | 23/25 (92) | 21/23 (91) | 12/23 (52) | 2 |
| Mahapatra et al16 | 1,3,6,9,12 | 9, 18, 24 | 3, 6, 12 | – | 20.7 | 15/15 (100) | 13/15 (86) | 14/15 (93) | – |
| Muneretto et al6 | – | – | – | Y | 30 | 36/36 (100) | 33/36 (92) | 28/36 (77) | – |
| Osmancik et al20 | – | 3, 6, 9, 12 | 6, 12 | – | 9.7 ± 3.6 | 30/30 (100) | 24/30 (80) | 21/30 (70) | 2 |
| Pison et al17 | – | if 7 d NA | 3, 6, 9, 12 | – | 15.6 ± 5 | 24/26 (92) | 22/24 (92) | 22/24 (92) | 2 |
| Richardson et al18 | – | – | – | Y | 12 | 79/83 (95) | 56/79 (71) | 45/79 (57) | – |
| Toplisek et al24 | 12 | – | – | Y | 12 | 37/37 (100) | 19/37 (51) | 18/37 (49) | – |
| Zembala et al25 | – | 3 | 6, 12 | Y | 12 | 69/90 (77) | 58/69 (84) | 43/69 (62) | 1 |
ECG, Electrocardiogram; 1d, 24-hour Holter monitor; 7d, 7-day Holter monitor; ILR, implantable loop recorder; F/U, follow-up; SR, sinus rhythm; AAD, antiarrhythmic drugs; Y, yes, NA, not applicable.
50% of cohort.
Median.
Outcomes
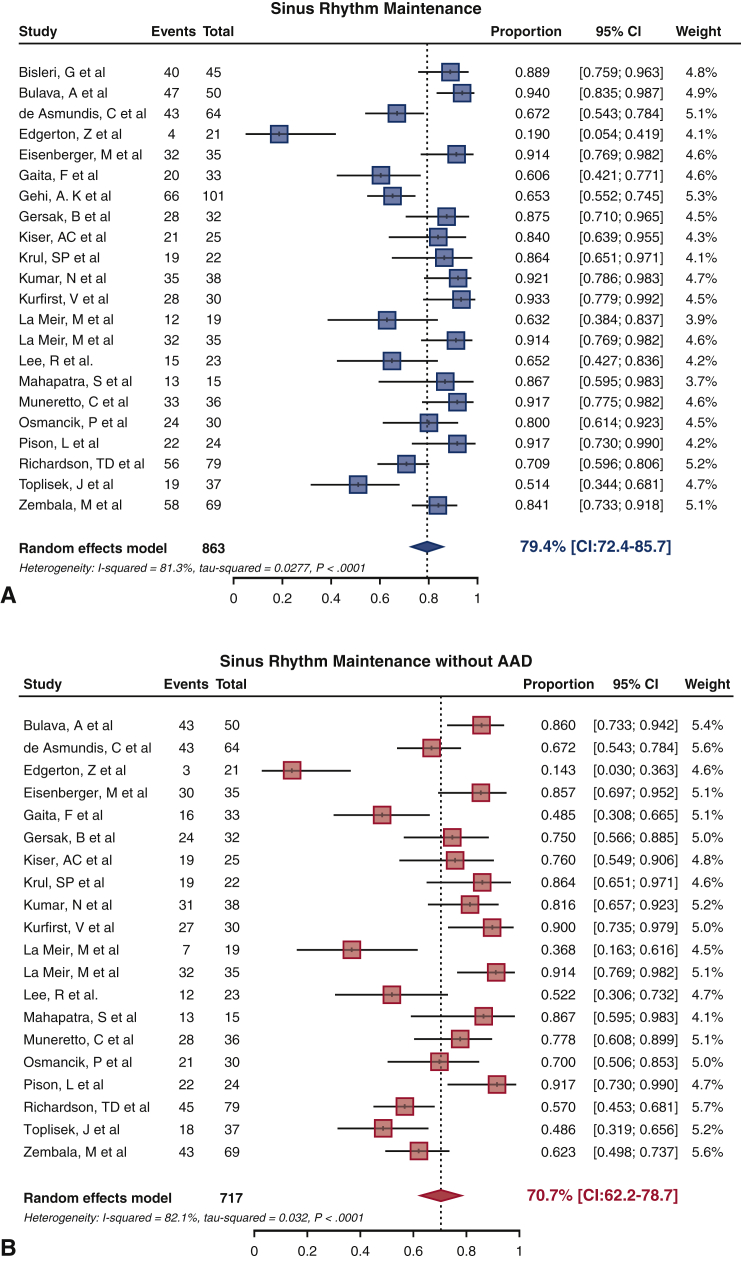
Overall SRM at final follow-up was 79.4% (95% confidence interval [CI], 72.4-85.7) with and 70.7% (95% CI, 62.2-78.7) without AAD (Figure 4). Of note SRM off AAD was not reported in 2 studies.9,22 There was evidence of statistical heterogeneity in the analysis of SRM both with (I2 = 81.3%, P < .001) and without AAD (I2 = 82.1%, P < .001). To explore the potential sources of heterogeneity, univariate analyses were conducted across the variables of age, AF type, left atrial diameter, AF duration, and follow-up length, with none shown to be statistically significant in predicting SRM. There were no significant differences in SRM outcome with or without AAD between the studies using the sequential or staged or a combination of both approaches (all P = NS). Similarly, no significant differences in SRM outcome with or without AAD were found between studies using fundamental surgical lesion sets of box or PVI (all P = NS). Although no significant difference in SRM with AAD existed between the groups who underwent LAA exclusion as opposed to those who did not, a superior result in SRM without AAD was seen in the LAA exclusion group (79.5% [95% CI, 71.2-86.8] vs 55.8% [95% CI, 41.4-69.8], P < .001). In addition, we did not find any statistically significant differences in SRM outcome according to surgical access or ablation energy source used (all P = NS).
Figure 4.
Sinus rhythm maintenance (SRM) forest plots. A, SRM achieved across studies with antiarrhythmic drugs (AAD) and B, without AAD. CI, Confidence interval.
Specific to the endocardial catheter-based component, there were no statistically significant differences in SRM outcome with or without AAD whether conduction block was achieved across all lesion sets (all P = NS). Likewise, ablation targeting ganglionated plexi did not afford additional benefits in SRM with or without AAD. No differences in SRM outcomes were seen between studies utilizing different catheter type (irrigated, nonirrigated, or contact force sensing). AAD usage was found to be highly variable between studies, with n = 6 initiating an unspecified AAD postoperatively,6,14,15,22,23,26 n = 6 using amiodarone postoperatively but with varying protocols,8,13,16,20,24,25 n = 4 initiating an AAD patients preoperatively,11,12,17,21 n = 2 discharging patients without any AAD postoperatively,10,27 and n = 1 continuing whichever AAD the patient was on throughout the admission.19 Three studies did not state their AAD usage protocol.9,18,28
Procedural Complications
Table 5 outlines the various procedural complications from the 22 studies. Ten studies reported postoperative length of stay of 5 days (range, 3.4-7.6).6,9, 10, 11,13,16,17,20,22,28 Of note, 5 studies reported a median length of stay,14,15,18,19,21 and 7 did not report this parameter.8,13,23, 24, 25, 26, 27 The total complication rate across the studies was 6.5% (95% CI, 3.4-10.2, I2 = 69.8%, P < .001) comprising: mortality rate of 0.2% (95% CI, 0-0.9, I2 = 0%, P = .9), stroke rate of 0.3% (95% CI, 0-1.1, I2 = 10.1%, P = .3), reoperation for bleeding rate of 1.6% (95% CI, 0.6-3.0, I2 = 19.9%, P = .2), conversion to sternotomy rate of 0.3% (95% CI, 0-1.1, I2 = 0.8%, P = .5), and phrenic nerve injury (temporary or permanent) of 0.3% (95% CI, 0-1.1, I2 = 0%, P = .7). Six patients (0.6%) required a permanent pacemaker implant, and there were 4 cases (0.4%) of atrioesophageal fistula. Of note, all atrioesophageal fistula occurred in studies that used mono-polar RF device. Mortality was considered in the first 30 days of either surgical or catheter procedure with 8 deaths in total: 3 atrioesophageal fistula, 1 bleeding and cardiac tamponade, 1 sudden cardiac death, 1 stroke, and 2 undetermined. Comparison across the studies did not reveal any statistically significant predictors of major complications.
Table 5.
Complications
| Study | Mean LOS, d | 30-d mortality, n (%) | Stroke, n (%) | Reoperation for bleeding/effusion, n (%) | Phrenic nerve injury, n (%) | AO fistula, n (%) | Conversion to open procedure, n (%) | Postoperative PPM, n (%) | Total, N |
|---|---|---|---|---|---|---|---|---|---|
| Bisleri et al9 | 3.9 ± 1.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bulava et al10 | 4.1 ± 1.1 | 0 | 0 | 1 (2) | 4 (8) | 0 | 2 (4) | 0 | 7 |
| de Asmundis et al11 | 6 ± 3 | 0 | 0 | 4 (6) | 0 | 0 | 2 (3) | 0 | 6 |
| Edgerton et al21 | 5∗ | 3 (13) | 2 (8) | 1 (4) | 1 (4) | 1 (4) | 0 | 0 | 8 |
| Eisenberger et al27 | – | 0 | 0 | 4 (11) | 2 (6) | 0 | – | 0 | 6 |
| Gaita et al26 | – | 0 | 4 | 0 | 0 | 0 | – | 5 (15) | 9 |
| Gehi et al22 | 4.4 | 2 (2) | 0 | 2 | 0 | 1 (1) | 0 | 0 | 7 |
| Gersak et al8 | – | 1 (2) | 1 | 0 | 0 | 2 (4) | 0 | 0 | 5 |
| Kiser et al23 | – | 0 | 0 | 0 | 0 | 0 | 0 | 2 | |
| Krul et al19 | 6∗ | 0 | 0 | 0 | 0 | 0 | 3 (10) | 0 | 3 |
| Kumar et al12 | – | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Kurfirst et al13 | 4.5 ± 3 | 0 | 0 | 0 | 0 | 0 | 2 (7) | 0 | 3 |
| La Meir et al14 | 3.6∗ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| La Meir et al15 | 3.4∗ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lee et al28 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mahapatra et al16 | 4.1 ± 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Muneretto et al6 | 4 ± 1.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Osmancik et al20 | 7.6 ± 5.3 | 0 | 1 (3) | 0 | 1 (3) | 0 | 0 | 0 | 2 |
| Pison et al17 | 7 ± 2 | 0 | 0 | 1 (4) | 0 | 0 | 0 | 0 | 1 |
| Richardson et al18 | 6∗ | 1 (1) | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) | 3 |
| Toplisek et al24 | – | 0 | 1 (3) | 2 (5) | 0 | 0 | 0 | 0 | 3 |
| Zembala et al25 | – | 1 (1) | 2 (2) | 3 (3) | 1 (1) | 0 | 1 | 0 | 8 |
| Median [CI] | 5.0 | 0.2 [0–0.9] | 0.3 [0–1.1] | 1.6 [0.6–3.0] | 0.3 [0–1.1] | ~0 [0–0.5] | 0.3 [0–1.1] | ~0 [0–0.5] | 6.5 [3.4–10.2] |
LOS, Length of stay; AO, atrioesophageal; PPM, permanent pacemaker; CI, confidence interval.
Median
Discussion
Hybrid (joint surgical and catheter-based) ablation for AF is an emerging technique purporting to improve rhythm control outcomes in patients with more persistent forms of AF. This systematic review and meta-analysis on the hybrid ablation approach in an overall cohort of 925 (89% nonparoxysmal AF; 38% persistent and 51% longstanding persistent) subjects demonstrates the following key findings:
-
1.
Favorable overall SRM of 79% and 71% with and without AAD at mean follow-up of 19 months;
-
2.
Complication rates of approximately 6.5%, which represent comparable figures to earlier catheter-based ablation series;
-
3.
There was no difference in rhythm outcome whether the catheter ablation was performed immediately following or staged for a later date; and
-
4.
Studies that reported exclusion of LAA were associated with greater SRM outcome off AAD.
Whilst in its infancy, the hybrid approach demonstrates adequate safety and efficacy when compared to catheter ablation alone. This approach has the potential to improve the longer-term durability of ablation lesions and reduce the need for multiple procedures. This would result in significant cost savings to the health care system, with a recent study showing that a 1% reduction in repeat ablation procedures could save 30 million US dollars to the US health care system.30
Outcomes and Complications of Hybrid Ablation
The overall SRM of 79% and 71% with or without AAD at a mean 19-month follow-up with the hybrid ablation approach in largely patients with nonparoxysmal AF (89% of overall cohort) appears to be favorable in comparison with other established catheter-based approaches, although the heterogeneity of the included studies must also be taken into consideration. Outcomes of catheter ablation approach have demonstrated SRM at 2-year follow-up to be 60% after single ablation procedure and 80% following multiple procedures. Further, the single procedural catheter ablation outcome for those with nonparoxysmal AF was only 42% at 2-year follow-up. However, it must be pointed out that the follow-up methods in these studies were variable. Importantly, the overall complication rate with the hybrid approach was similar to earlier catheter ablation series at 6.5%.31 With increasing experience, this figure is likely to improve akin to the catheter ablation data whereby complication rates were found to reduce by approximately one-third over a decade.32 Further, this study did not find any statistically significant differences in complications according to ablation energy source, device, lesion set, or procedure type from the overall low number of reported complications. However, further study is required to tease out why all incidences of atrioesophageal fistula occurred with the use of nContact unipolar RF device.
The hybrid procedure offers the advantage of surgical epicardial ablation whereby broad, continuous lesions can be applied to isolate the pulmonary veins with additional linear lines and LAA exclusion in a relatively quick time frame of 80 to 200 minutes. The endocardial catheter-based component allows mapping to confirm conduction block and further targeted endocardial ablation to ensure lesion contiguity and electrical isolation. In the study by Muneretto and colleagues,6 this combined approach resulted in reduced procedure time with the surgical procedure taking 80 ± 7 minutes, the endocardial mapping taking 18 ± 2 minutes, and additional endocardial ablation taking 25 ± 4 minutes. However, the length of hospital stay of 5 days with the hybrid approach was longer than the typical 1 to 2 day stays with the catheter-based approach. Further refinement of the hybrid approach to optimize outcomes while minimizing invasiveness and complications would help to consolidate the role of this emerging technique.
Lessons From Early Experience
The move to a hybrid procedure from a surgical standpoint represents the potential for a less-invasive surgical procedure than that of the traditional Cox-maze procedure while potentially offering equivalent results without cardiopulmonary bypass and median sternotomy. Although advances in surgical techniques have allowed the Cox-maze procedure to be completed via mini-thoracotomy or VATS approach, the hybrid procedure allows an additional dimension through endocardial mapping to guide more targeted ablation to complete the lesion sets.33,34 This review identified several important technical considerations with this novel hybrid ablation technique. First, there was no statistically significant difference in outcome when the hybrid procedure was performed with the endocardial component performed sequentially in the same setting or staged with an interval of weeks to months. There are potential advantages offered in each procedure type. Sequential procedures offer the potential for immediate identification of lesion gaps that can be treated at the same time with greater probability of achieving sinus rhythm at the outset and greater chance for reverse remodeling. In contrast, the staged approach affords time for complete lesion maturation and regression of edema such that lesion gaps can be more definitely identified for endocardial ablation. Further, scheduling of the staged approach would be easier, as it does not require the hybrid theater and cross-discipline expertise to be available at the same time. Second, we were unable to identify a superior outcome related to lesion set application. In our consideration of this point, we sought to determine whether other variables in the procedure could have bearing on the procedural outcome in terms of SRM. While no significant difference was identified between devices in terms of SR with AAD, on further statistical analysis when comparing devices types, the use of the AtriCure Synergy system was found to be superior to the nContact system in SRM without AAD (AtriCure: 82% [95% CI, 72.5-90] vs nContact: 68.6% [95% CI, 29.4-96.9], P = .01) (Figure 1). Given that all atrioesophageal fistulae occurred with the use of the nContact unipolar RF devices, the use of bipolar RF devices may be protective against this serious complication. However, this statistical difference may become attenuated as further experience is added to the literature. Last, the potential merits of LAA exclusion seen in this review requires further investigation in light of recent randomized catheter ablation data showing improved long-term freedom from atrial arrhythmias following empirical electrical isolation of the LAA in patients with longstanding persistent AF.35
Clinical Implications
The hybrid ablation approach for AF appears promising, with this systemic review and meta-analysis demonstrating favorable outcomes without significant additional procedural-related complications. This novel technique may fill the gap in our current armamentarium of ablating patients with more persistent form of AF, given that the optimal catheter ablation approach for this subgroup of patients remains unclear.3 The invasiveness and complexity of the Cox-maze III procedure might have limited its uptake despite excellent SRM results.5 In contrast, the hybrid ablation procedure is less invasive, does not require cardiopulmonary bypass, and allows for shorter hospitalization. The hybrid ablation approach can potentially be extended to patients with AF undergoing concomitant valvular or coronary artery bypass graft surgery in place of a traditional Cox-maze procedure, to reduce cardiopulmonary bypass time and associated complications. As with all novel techniques, there remains room for improvement with further reduction in complication rates following greater experience, improvement in ablation devices, and ablation techniques. Concurrent aggressive targeting of AF risk factors may further improve SRM with the novel hybrid ablation approach.36
Study Limitations
The overall number of patients included is relatively small and is a function of the emerging nature of this procedure. We are unable to detail the specific hybrid ablation outcomes according to types of AF (paroxysmal vs nonparoxysmal), given the lack of available patient-level data provided and thus interpretation of results must be considered in light of this. We recognize the limitations of the individual included studies may affect the robustness of this review and meta-analysis. First, the average number of subjects in most of the included studies is low. Second, all the available studies on hybrid ablation were nonrandomized, single-center cohort series. Third, there is significant heterogeneity of the hybrid ablation approach with variable epicardial and endocardial lesion sets, different ablation devices, and surgical access employed. Last, there is a lack of reporting standard in the included studies with variable follow-up methodology and time points. Nevertheless, these limitations serve to highlight areas of focus for future studies. The upcoming CONVERGE trial (Convergence Of Epicardial And Endocardial Radiofrequency [RF] Ablation For The Treatment Of Symptomatic Persistent AF) is one such study that is randomizing patients between a hybrid ablation procedure and standalone endocardial catheter ablation that should provide greater insight into the relative efficacy of these approaches.
Conclusions
The hybrid ablation procedure for AF shows favorable outcome with complication rates that are comparable with the early catheter-based ablation approach. Early results suggest a potential role for this novel strategy in selected patients with AF, given the suboptimal results of catheter-based approach in those with a more persistent form of the arrhythmia. Further studies are needed to determine the optimal hybrid ablation technique and longer-term outcome results.
Conflict of Interest Statement
Dr Lau reports that the University of Adelaide receives on his behalf lecture and/or consulting fees from Biotronik, Bayer, Medtronic, Abbott Medical, Boehringer Ingelheim, and Pfizer. Mr Chapman is employed by CathRx. Dr Sanders reports having served on the advisory board of Medtronic, Abbott Medical, Boston-Scientific, Pacemate, and CathRx. Dr Sanders reports that the University of Adelaide receives on his behalf lecture and/or consulting fees from Medtronic, Abbott Medical, and Boston-Scientific. Dr Sanders reports that the University of Adelaide receives on his behalf research funding from Medtronic, Abbott Medical, Boston-Scientific, and Microport. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
We acknowledge our statistical consultant, Dr Gijo Thomas, University of Adelaide.
Footnotes
Dr Lau is supported by the Robert J. Craig Lectureship from the University of Adelaide. Dr Elliott is supported by an Early Career Fellowship from the National Heart Foundation of Australia. Dr Sanders is supported by a Practitioner Fellowship from the National Health and Medical Research Council of Australia and by the National Heart Foundation of Australia.
Appendix
References
- 1.Gallagher C., Hendriks J.M., Giles L., Karnon J., Pham C., Elliott A.D., et al. Increasing trends in hospitalisations due to atrial fibrillation in Australia from 1993 to 2013. Heart. 2019;105:1358–1363. doi: 10.1136/heartjnl-2018-314471. [DOI] [PubMed] [Google Scholar]
- 2.Marrouche N.F., Brachmann J., Andresen D., Siebels J., Boersma L., Jordaens L., et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. doi: 10.1056/NEJMoa1707855. [DOI] [PubMed] [Google Scholar]
- 3.Clarnette J.A., Brooks A.G., Mahajan R., Elliott A.D., Twomey D.J., Pathak R.K., et al. Outcomes of persistent and long-standing persistent atrial fibrillation ablation: a systematic review and meta-analysis. Europace. 2018;20:f366–f376. doi: 10.1093/europace/eux297. [DOI] [PubMed] [Google Scholar]
- 4.Damiano R.J., Jr., Schwartz F.H., Bailey M.S., Maniar H.S., Munfakh N.A., Moon M.R., et al. The Cox maze IV procedure: predictors of late recurrence. J Thorac Cardiovasc Surg. 2011;141:113–121. doi: 10.1016/j.jtcvs.2010.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prasad S.M., Maniar H.S., Camillo C.J., Schuessler R.B., Boineau J.P., Sundt T.M., III, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg. 2003;126:1822–1828. doi: 10.1016/s0022-5223(03)01287-x. [DOI] [PubMed] [Google Scholar]
- 6.Muneretto C., Bisleri G., Bontempi L., Curnis A. Durable staged hybrid ablation with thoracoscopic and percutaneous approach for treatment of long-standing atrial fibrillation: a 30-month assessment with continuous monitoring. J Thorac Cardiovasc Surg. 2012;144:1460–1465. doi: 10.1016/j.jtcvs.2012.08.069. discussion 1465. [DOI] [PubMed] [Google Scholar]
- 7.Calkins H., Hindricks G., Cappato R., Kim Y.H., Saad E.B., Aguinaga L., et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–e444. doi: 10.1016/j.hrthm.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gersak B., Pernat A., Robic B., Sinkovec M. Low rate of atrial fibrillation recurrence verified by implantable loop recorder monitoring following a convergent epicardial and endocardial ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2012;23:1059–1066. doi: 10.1111/j.1540-8167.2012.02355.x. [DOI] [PubMed] [Google Scholar]
- 9.Bisleri G., Rosati F., Bontempi L., Curnis A., Muneretto C. Hybrid approach for the treatment of long-standing persistent atrial fibrillation: electrophysiological findings and clinical results. Eur J Cardiothorac Surg. 2013;44:919–923. doi: 10.1093/ejcts/ezt115. [DOI] [PubMed] [Google Scholar]
- 10.Bulava A., Mokracek A., Hanis J., Kurfirst V., Eisenberger M., Pesl L. Sequential hybrid procedure for persistent atrial fibrillation. J Am Heart Assoc. 2015;4:e001754. doi: 10.1161/JAHA.114.001754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Asmundis C., Chierchia G.B., Mugnai G., Van Loo I., Nijs J., Czapla J., et al. Midterm clinical outcomes of concomitant thoracoscopic epicardial and transcatheter endocardial ablation for persistent and long-standing persistent atrial fibrillation: a single-centre experience. Europace. 2017;19:58–65. doi: 10.1093/europace/euw026. [DOI] [PubMed] [Google Scholar]
- 12.Kumar N., Pison L., Lozekoot P., Choudhury R., La Meir M., Gelsomino S., et al. The symbiosis of contact force catheter use for hybrid ablation for atrial fibrillation. Neth Heart J. 2015;23:438–446. doi: 10.1007/s12471-015-0729-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurfirst V., Mokracek A., Bulava A., Canadyova J., Hanis J., Pesl L. Two-staged hybrid treatment of persistent atrial fibrillation: short-term single-centre results. Interact Cardiovasc Thorac Surg. 2014;18:451–456. doi: 10.1093/icvts/ivt538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.La Meir M., Gelsomino S., Lorusso R., Luca F., Pison L., Parise O., et al. The hybrid approach for the surgical treatment of lone atrial fibrillation: one-year results employing a monopolar radiofrequency source. J Cardiothorac Surg. 2012;7:71. doi: 10.1186/1749-8090-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.La Meir M., Gelsomino S., Luca F., Pison L., Parise O., Colella A., et al. Minimally invasive surgical treatment of lone atrial fibrillation: early results of hybrid versus standard minimally invasive approach employing radiofrequency sources. Int J Cardiol. 2013;167:1469–1475. doi: 10.1016/j.ijcard.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 16.Mahapatra S., LaPar D.J., Kamath S., Payne J., Bilchick K.C., Mangrum J.M., et al. Initial experience of sequential surgical epicardial-catheter endocardial ablation for persistent and long-standing persistent atrial fibrillation with long-term follow-up. Ann Thorac Surg. 2011;91:1890–1898. doi: 10.1016/j.athoracsur.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pison L., La Meir M., van Opstal J., Blaauw Y., Maessen J., Crijns H.J. Hybrid thoracoscopic surgical and transvenous catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2012;60:54–61. doi: 10.1016/j.jacc.2011.12.055. [DOI] [PubMed] [Google Scholar]
- 18.Richardson T.D., Shoemaker M.B., Whalen S.P., Hoff S.J., Ellis C.R. Staged versus simultaneous thoracoscopic hybrid ablation for persistent atrial fibrillation does not affect time to recurrence of atrial arrhythmia. J Cardiovasc Electrophysiol. 2016;27:428–434. doi: 10.1111/jce.12906. [DOI] [PubMed] [Google Scholar]
- 19.Krul S.P., Driessen A.H., van Boven W.J., Linnenbank A.C., Geuzebroek G.S., Jackman W.M., et al. Thoracoscopic video-assisted pulmonary vein antrum isolation, ganglionated plexus ablation, and periprocedural confirmation of ablation lesions: first results of a hybrid surgical-electrophysiological approach for atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:262–270. doi: 10.1161/CIRCEP.111.961862. [DOI] [PubMed] [Google Scholar]
- 20.Osmancik P., Budera P., Zdarska J., Herman D., Petr R., Straka Z. Electrophysiological findings after surgical thoracoscopic atrial fibrillation ablation. Heart Rhythm. 2016;13:1246–1252. doi: 10.1016/j.hrthm.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Edgerton Z., Perini A.P., Horton R., Trivedi C., Santangeli P., Bai R., et al. Hybrid procedure (endo/epicardial) versus standard manual ablation in patients undergoing ablation of longstanding persistent atrial fibrillation: results from a single center. J Cardiovasc Electrophysiol. 2016;27:524–530. doi: 10.1111/jce.12926. [DOI] [PubMed] [Google Scholar]
- 22.Gehi A.K., Mounsey J.P., Pursell I., Landers M., Boyce K., Chung E.H., et al. Hybrid epicardial-endocardial ablation using a pericardioscopic technique for the treatment of atrial fibrillation. Heart Rhythm. 2013;10:22–28. doi: 10.1016/j.hrthm.2012.08.044. [DOI] [PubMed] [Google Scholar]
- 23.Kiser A.C., Landers M., Horton R., Hume A., Natale A., Gersak B. The convergent procedure: a multidisciplinary atrial fibrillation treatment. Heart Surg Forum. 2010;13:E317–E321. doi: 10.1532/HSF98.20091112. [DOI] [PubMed] [Google Scholar]
- 24.Toplisek J., Pernat A., Ruzic N., Robic B., Sinkovec M., Cvijic M., et al. Improvement of atrial and ventricular remodeling with low atrial fibrillation burden after hybrid ablation of persistent atrial fibrillation. Pacing Clin Electrophysiol. 2016;39:216–224. doi: 10.1111/pace.12791. [DOI] [PubMed] [Google Scholar]
- 25.Zembala M., Filipiak K., Kowalski O., Buchta P., Niklewski T., Nadziakiewicz P., et al. Staged hybrid ablation for persistent and longstanding persistent atrial fibrillation effectively restores sinus rhythm in long-term observation. Arch Med Sci. 2017;13:109–117. doi: 10.5114/aoms.2015.53960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaita F., Ebrille E., Scaglione M., Caponi D., Garberoglio L., Vivalda L., et al. Very long-term results of surgical and transcatheter ablation of long-standing persistent atrial fibrillation. Ann Thorac Surg. 2013;96:1273–1278. doi: 10.1016/j.athoracsur.2013.05.054. [DOI] [PubMed] [Google Scholar]
- 27.Eisenberger M., Bulava A., Mokracek A., Hanis J., Kurfirst V., Dusek L. Sequential hybrid surgical cryomaze and transvenous catheter ablation of atrial fibrillation. Pacing Clin Electrophysiol. 2015;38:1379–1385. doi: 10.1111/pace.12686. [DOI] [PubMed] [Google Scholar]
- 28.Lee R., McCarthy P.M., Passman R.S., Kruse J., Malaisrie S.C., McGee E.C., et al. Surgical treatment for isolated atrial fibrillation: minimally invasive vs. classic cut and sew maze. Innovations (Phila) 2011;6:373–377. doi: 10.1097/IMI.0b013e318248f3f4. [DOI] [PubMed] [Google Scholar]
- 29.Zembala M., Filipiak K., Kowalski O., Boidol J., Sokal A., Lenarczyk R., et al. Minimally invasive hybrid ablation procedure for the treatment of persistent atrial fibrillation: one year results. Kardiol Pol. 2012;70:819–828. [PubMed] [Google Scholar]
- 30.Mansour M., Karst E., Heist E.K., Dalal N., Wasfy J.H., Packer D.L., et al. The impact of first procedure success rate on the economics of atrial fibrillation ablation. JACC Clin Electrophysiol. 2017;3:129–138. doi: 10.1016/j.jacep.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Cappato R., Calkins H., Chen S.A., Davies W., Iesaka Y., Kalman J., et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100–1105. doi: 10.1161/01.CIR.0000157153.30978.67. [DOI] [PubMed] [Google Scholar]
- 32.Gupta A., Perera T., Ganesan A., Sullivan T., Lau D.H., Roberts-Thomson K.C., et al. Complications of catheter ablation of atrial fibrillation: a systematic review. Circ Arrhythm Electrophysiol. 2013;6:1082–1088. doi: 10.1161/CIRCEP.113.000768. [DOI] [PubMed] [Google Scholar]
- 33.Ad N., Henry L., Friehling T., Wish M., Holmes S.D. Minimally invasive stand-alone Cox-maze procedure for patients with nonparoxysmal atrial fibrillation. Ann Thorac Surg. 2013;96:792–798. doi: 10.1016/j.athoracsur.2013.05.007. discussion 798-9. [DOI] [PubMed] [Google Scholar]
- 34.Sirak J.H., Schwartzman D. Interim results of the 5-box thoracoscopic maze procedure. Ann Thorac Surg. 2012;94:1880–1884. doi: 10.1016/j.athoracsur.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Di Biase L., Burkhardt J.D., Mohanty P., Mohanty S., Sanchez J.E., Trivedi C., et al. Left Atrial appendage isolation in patients with longstanding persistent AF undergoing catheter ablation: BELIEF trial. J Am Coll Cardiol. 2016;68:1929–1940. doi: 10.1016/j.jacc.2016.07.770. [DOI] [PubMed] [Google Scholar]
- 36.Pathak R.K., Middeldorp M.E., Lau D.H., Mehta A.B., Mahajan R., Twomey D., et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. 2014;64:2222–2231. doi: 10.1016/j.jacc.2014.09.028. [DOI] [PubMed] [Google Scholar]