Background:
Inflamed endothelial cells (ECs) trigger atherogenesis, especially at arterial regions experiencing disturbed blood flow. UCP2 (Uncoupling protein 2), a key mitochondrial antioxidant protein, improves endothelium-dependent relaxation in obese mice. However, whether UCP2 can be regulated by shear flow is unknown, and the role of endothelial UCP2 in regulating inflammation and atherosclerosis remains unclear. This study aims to investigate the mechanoregulation of UCP2 expression in ECs and the effect of UCP2 on endothelial inflammation and atherogenesis.
Methods:
In vitro shear stress simulation system was used to investigate the regulation of UCP2 expression by shear flow. EC-specific Ucp2 knockout mice were used to investigate the role of UCP2 in flow-associated atherosclerosis.
Results:
Shear stress experiments showed that KLF2 (Krüppel-like factor 2) mediates fluid shear stress-dependent regulation of UCP2 expression in human aortic and human umbilical vein ECs. Unidirectional shear stress, statins, and resveratrol upregulate whereas oscillatory shear stress and proinflammatory stimuli inhibit UCP2 expression through altered KLF2 expression. KLF2 directly binds to UCP2 promoter to upregulate its transcription in human umbilical vein ECs. UCP2 knockdown induced expression of genes involved in proinflammatory and profibrotic signaling, resulting in a proatherogenic endothelial phenotype. EC-specific Ucp2 deletion promotes atherogenesis and collagen production. Additionally, we found endothelial Ucp2 deficiency aggravates whereas adeno-associated virus-mediated EC-Ucp2 overexpression inhibits carotid atherosclerotic plaque formation in disturbed flow-enhanced atherosclerosis mouse model. RNA-sequencing analysis revealed FoxO1 (forkhead box protein O1) as the major proinflammatory transcriptional regulator activated by UCP2 knockdown, and FoxO1 inhibition reduced vascular inflammation and disturbed flow-enhanced atherosclerosis. We showed further that UCP2 level is critical for phosphorylation of AMPK (AMP-activated protein kinase), which is required for UCP2-induced inhibition of FoxO1.
Conclusions:
Altogether, our studies uncover that UCP2 is novel mechanosensitive gene under the control of fluid shear stress and KLF2 in ECs. UCP2 expression is critical for endothelial proinflammatory response and atherogenesis. Therapeutic strategies enhancing UCP2 level may have therapeutic potential against atherosclerosis.
Keywords: antioxidants, atherosclerosis, endothelial cell, inflammation, resveratrol
Novelty and Significance.
What Is Known?
Endothelial dysfunction, characterized by inflammation, oxidative stress, and fibrosis, is an early pathophysiological change prone to atherogenesis.
UCP2 (Uncoupling protein 2) protects against endothelial dysfunction in diet-induced obese mice through suppression of oxidative stress.
In mice, global knockout of Ucp2 aggravates atherosclerosis and endothelial deficiency of Ucp2 increases vascular dysfunction in pulmonary hypertension.
What New Information Does This Article Contribute?
Oscillatory shear stress and unidirectional shear stress oppositely regulate UCP2 expression by altering KLF2 (Krüppel-like factor 2) in endothelial cells (ECs). Vasoprotective agents statins and resveratrol upregulate whereas proinflammatory stimuli suppress UCP2 mRNA expression. UCP2 is a novel KLF2 target gene in ECs.
UCP2 expression is critical for endothelial proinflammatory phenotype and UCP2 knockdown inactivates AMPK (AMP-activated protein kinase), which leads to activation of FoxO1 (forkhead box protein O1) transcription factor that critically regulates the expression of proinflammatory genes.
Endothelial Ucp2 deficiency exaggerates atherogenesis while Ucp2 overexpression in ECs attenuates disturbed flow-enhanced atherosclerosis. Inhibition of FoxO1 by AS1842856 in endothelial Ucp2 knockout mice reduces disturbed flow-enhanced atherosclerotic plaque formation.
Blood flow patterns play a critical role in the site-specific distribution of atherosclerotic plaques along the arterial tree. UCP2 protects against endothelial dysfunction by reducing oxidative stress in obese mice. Here, we demonstrate that endothelial UCP2 is a novel transcriptional target of KLF2. UCP2 expression is differentially regulated by distinct flow patterns and upregulated by statins and resveratrol through KLF2-dependent mechanism. UCP2 is a mechanosensitive suppressor of inflammation and UCP2 insufficiency inactivates AMPK to induce FoxO1 activation, which drives endothelial inflammation. We show further that Ucp2 knockout in ECs aggravates western diet/disturbed flow-induced atherosclerosis, while EC-specific Ucp2 overexpression attenuates plaque formation in disturbed flow-enhanced atherosclerosis. Furthermore, FoxO1 inhibitor AS1842856 reduces disturbed flow-enhanced atherosclerotic plaque formation in EC-specific Ucp2 knockout mice. Our findings suggest that enhancing UCP2 expression/activity is a potential therapeutic strategy to treat atherosclerosis.
Meet the First Author, see p 369
Endothelial cells (ECs) are professional mechanosensing cells that constantly perceive and respond to the hemodynamic forces generated by blood flow.1 The hemodynamic forces play a critical role in the development and geometric distribution of atherosclerosis,2 a leading cause of death in patients with stroke and myocardial infarction.3 Both the preclinical and clinical data showed that atherosclerotic plaque occurs preferentially in the branched or curved areas of blood vessels, where blood flow is oscillatory or disturbed.4,5 However, much less plaque is detected in the straight part of blood vessel, where blood flow is unidirectional.5 Investigating the underpinning mechanisms of such site-specific distribution of atherosclerotic plaques will critically contribute to the development of better therapeutic options for treating atherosclerosis.
In recent years, targeting inflammation was proposed as a rational measure to combat against atherosclerosis and ongoing clinical trials using monoclonal antibodies against IL (interleukin)-1β and IL-6 in patients showed promising anti-atherosclerotic efficacy.6,7 Multiple types of cells participate in atherogenesis and dysfunction of ECs is essential in the initiation of atherosclerosis.8–10 In the early phase of atherogenesis, elevated circulating levels of ox-LDL (oxidized low-density lipoprotein) and proinflammatory mediators induce inflammation and oxidative stress on the vascular wall and ECs become damaged and express elevated levels of proinflammatory genes including vascular cell adhesion molecule 1. The inflamed ECs orchestrate the recruitment of immune cells to the injury sites and modulate the differentiation of proinflammatory cells during the buildup of plaques.11,12 One promising anti-inflammatory target in ECs is KLF2 (Krüppel-like factor 2), a transcription factor inducible by unidirectional shear stress (USS) and lipid-lowering drugs statins.13,14 Importantly, KLF2 exerts anti-inflammatory and antioxidant effects in ECs.15,16 Although hemizygous deficiency of KLF2 in mice was reported to promote atherosclerosis,17 the mechanism of its anti-atherosclerotic effect in ECs remains to be further revealed.
UCP2 (Uncoupling protein 2) is a mitochondrial proton anion that controls production of mitochondrial superoxide anion.18 UCP2 inhibits oxidative stress to preserve nitric oxide bioavailability and endothelial function in diet-induced obese mice.19 Endothelial UCP2 regulates mitophagy and prevents excessive EC apoptosis but UCP2 deficiency exaggerates intermittent hypoxia-induced pulmonary hypertension.20 Although UCP2 in bone marrow-derived cells was reported to be protective against atherosclerosis,21 it remains untested whether endothelial UCP2 is an critical player in atherogenesis. In addition, except for a role of AMPK (AMP-activated protein kinase) in induction of UCP2,22 little is known about other regulatory mechanisms of UCP2 expression in ECs. Therefore, it is necessary to examine the endothelium-specific role of UCP2 in atherogenesis and the molecular regulatory mechanism of UCP2 expression in ECs for the determination of the pathophysiological function of endothelial UCP2 in atherosclerosis.
In the present study, we demonstrate that UCP2 expression is inhibited by disturbed flow (DF) and inflammatory cytokines. By contrast, USS and vasoprotective agents (statins and resveratrol) upregulate UCP2 expression through a KLF2-dependent mechanism. We further reveal that UCP2 is a direct transcriptional target of KLF2 in ECs and endothelial UCP2 deficiency induces proinflammatory response. UCP2 deficiency in ECs aggravates both western diet-induced atherosclerosis and DF-associated atherosclerosis, while EC-specific UCP2 overexpression attenuates plaque formation in DF-induced atherosclerosis in the carotid artery. We used RNA-sequencing analysis and identified FoxO1, a transcription factor inactivated by PI3K (phosphoinositide 3-kinases)-Akt (protein kinase B) signaling and an critical regulator of endothelial metabolic activity,23 is a critical proinflammatory mediator downstream of UCP2-AMPK axis.
Methods
Data Availability
All supporting data are available within the article and its Supplemental Material. For details on the experimental procedures and materials used, please refer to the Major Resources Table and the materials and methods section in the Supplemental Material.
Results
Differential Regulation of UCP2 Expression by Distinct Flow Patterns
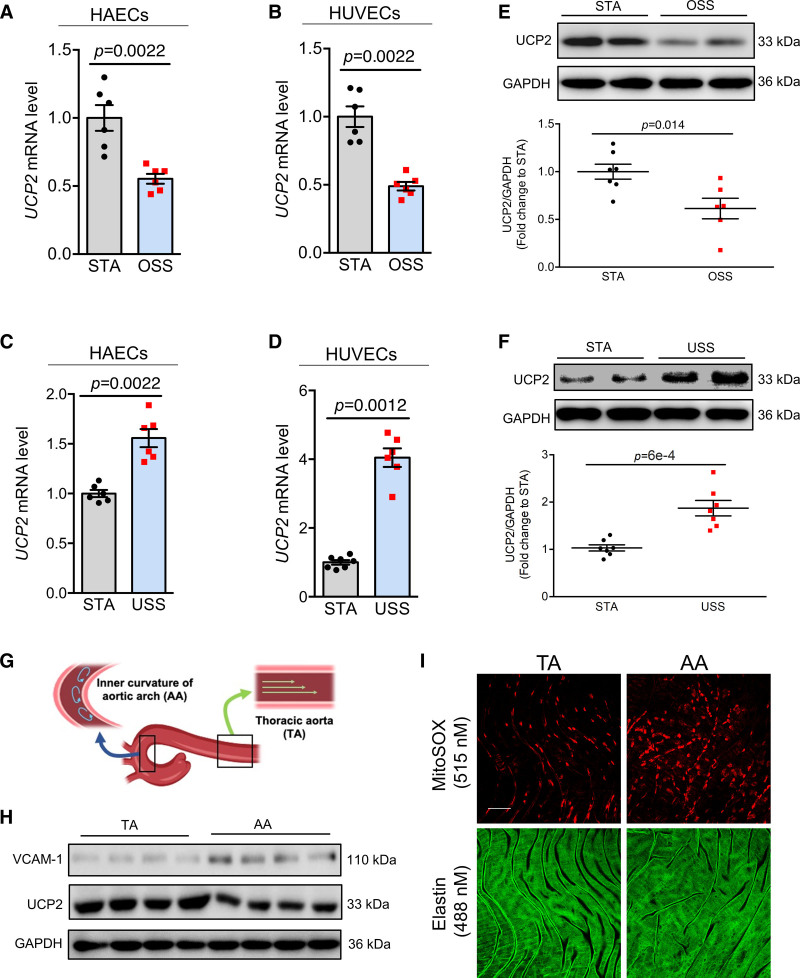
To determine the expression of UCP2 in ECs in response to different types of shear stress generated by blood flow, both human aortic ECs (HAECs) and human umbilical vein ECs (HUVECs) were exposed for different durations to oscillatory shear stress (OSS) or USS generated by the Ibidi pump system. Gene expression results showed that, compared with cells kept static (STA), UCP2 mRNA expression was inhibited by OSS (Figure 1A and 1B and Figure S1A and S1B). By contrast, USS upregulated UCP2 mRNA (Figure 1C and 1D and Figure S1C). Consistent with the mRNA expression data, Western blotting results showed 24-hour OSS decreased (Figure 1E) while 24-hour USS increased (Figure 1F) the UCP2 protein level in HUVECs. To detect the relative UCP2 expression levels in different aortic regions, we isolated aortic arch and thoracic aorta (TA) from mouse aortas for Western blotting analysis and found UCP2 expression in aortic arch, where blood flow is disturbed, is lower than that in TA, where blood flow is unidirectional (Figure 1G and 1H and Figure S1G), thus revealing that UCP2 expression is also inhibited by OSS in vivo. UCP2 is known to suppress mitochondrial reactive oxygen species (ROS) in ECs.19 Indeed, knockdown of UCP2 using shRNA in HAECs promoted generation of mitochondrial ROS which was inhibited by ROS scavenger tempol (Figure S1D through S1F). En face endothelium staining of mitochondrial ROS showed that the ECs in aortic arch accumulated a higher level of mitochondrial ROS than those in TA (Figure 1I and Figure S1H), suggesting that the OSS-suppressed UCP2 expression likely contributes to increased oxidative stress in aortic arch.
Figure 1.
Differential regulation of UCP2 (uncoupling protein 2) by distinct flow patterns. Oscillatory shear stress (OSS) for 12 h downregulated UCP2 mRNA level in human aortic endothelial cells (HAECs; A) and human umbilical vein ECs (HUVECs; B), n=6, nonparametric Mann-Whitney test. Unidirectional shear stress (USS) for 24 h upregulates UCP2 mRNA level in HAECs (C) and HUVECs (D), n=6, nonparametric Mann-Whitney test. E, Representative and summarized Western blotting data showing inhibited UCP2 protein expression in HUVECs exposed to OSS for 24 h. n=6, nonparametric Mann-Whitney test. F, Representative and summarized Western blotting data showing increased expression of UCP2 protein level in HUVECs exposed to USS for 24 h. n=6, nonparametric Mann-Whitney test. G, Diagram showing the location of thoracic aorta (TA) and aortic arch (AA) in the mouse aortas. H, Western blotting data showing reduced UCP2 protein level in AA compared with TA, VCAM-1 level was known to be increased in AA and used as a positive control here, n=4. I, En face MitoSOX staining indicative of mitochondrial ROS in the en face endothelium of aortas shows that AA has higher level of mitochondrial ROS compared with TA. Scale bar: 50 μm. Representative images were selected as they best represent images obtained as well as the mean values of each condition. STA indicates static.
KLF2 Transcriptionally Regulates UCP2 Expression
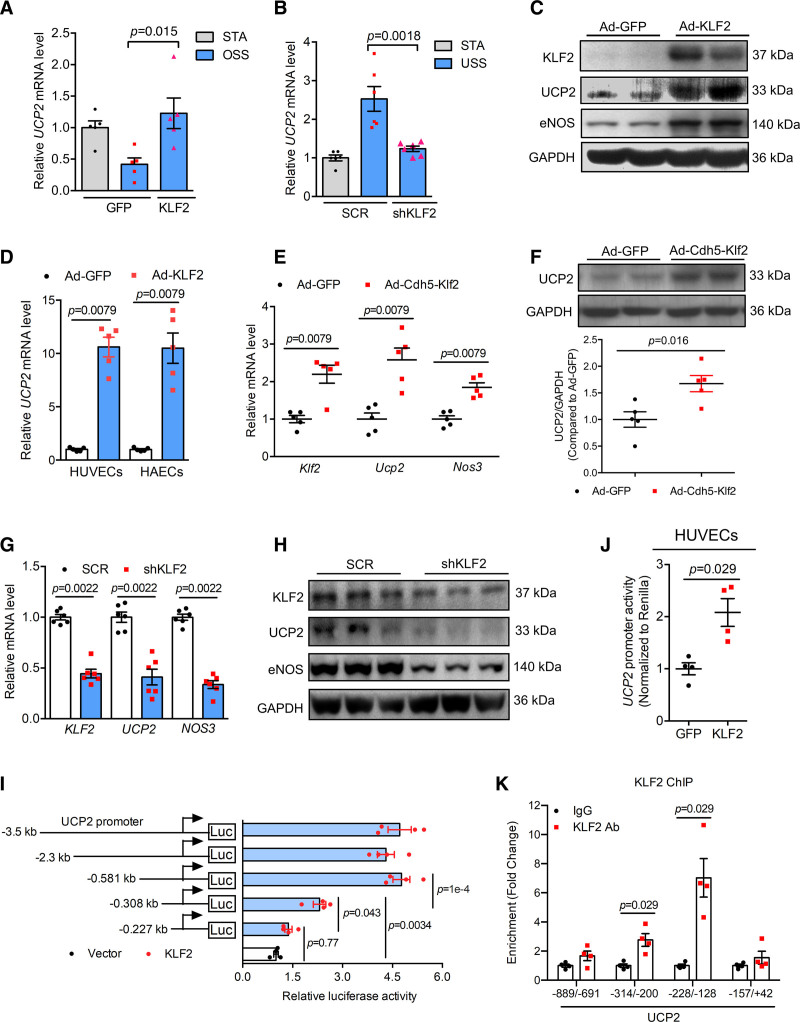
The transcription factor KLF2 controls the expression of a large number of genes responsive to shear stress and KLF2 expression is also differentially regulated by different flow patterns. We next asked whether KLF2 plays a role in controlling UCP2 expression in ECs in response to hemodynamic forces. Overexpression of KLF2 by adenovirus KLF2 (Ad-KLF2) in HUVECs reversed the inhibitory effect of OSS on UCP2 mRNA expression (Figure 2A), suggesting that KLF2 can increase UCP2 expression. However, knockdown of KLF2 in HUVECs by adenovirus KLF2-shRNA (Ad-shKLF2) abolished USS-induced UCP2 mRNA upregulation (Figure 2B). Likewise, overexpression of a DNA-binding defective form of KLF2 (Ad-KLF2ΔZnF) in HUVECs reduced USS-induced UCP2 mRNA expression (Figure S1I). These results clearly indicate that KLF2 expression is required for UCP2 transcription in ECs in response to USS. To test whether KLF2 can directly induce UCP2 expression, we determined basal UCP2 expression and found that Ad-KLF2 significantly elevated both UCP2 protein expression in HUVECs (Figure 2C and Figure S2A) and UCP2 mRNA expression in HAECs, HUVECs and mouse aortic ECs (Figure 2D and Figure S2B). To determine whether KLF2 induces UCP2 expression in vivo, we generated EC-specific overexpression adenoviral Klf2 (Figure S3A) whose expression is under the control of mouse VE-Cadherin promoter (Ad-Cdh5-Klf2) and injected it to mice via tail vein for 7 days. Compared with Ad-GFP (green fluorescent protein)-injected control, the mRNA level of mouse Klf2, Ucp2, and Nos3 (a known target gene of KLF2) in mouse aortas, hearts and livers was all upregulated by Ad-Cdh5-Klf2 (Figure 2E and Figure S3B and S3C). Consistently, UCP2 protein level in mouse aortas was also increased by Ad-Cdh5-Klf2 (Figure 2F). In contrast to the effect of KLF2 overexpression, knockdown of KLF2 by shRNA downregulated the expression of UCP2 mRNA and protein in HUVECs (Figure 2G and 2H). Moreover, overexpression of Ad-KLF2ΔZnF decreased basal UCP2 mRNA and protein expression (Figure S2C and S2D), suggesting that KLF2 likely regulates UCP2 expression through transcriptional mechanisms.
Figure 2.
UCP2 (Uncoupling protein 2) is a target of KLF2 (Krüppel-like factor 2). A, Real-time quantitative polymerase chain reaction (qPCR) data showing KLF2 overexpression by adenovirus KLF2 (Ad-KLF2) reversed oscillatory shear stress (OSS)-induced downregulation of UCP2 mRNA in human umbilical vein ECs (HUVECs), OSS time is 12 h, n=5, 2-way ANOVA, Tukey post hoc. B, Real-time qPCR data showing KLF2 knockdown by KLF2-shRNA abolished unidirectional shear stress (USS)-induced upregulation of UCP2 mRNA in HUVECs, USS time is 24 h, n=5, 2-way ANOVA, Tukey post hoc. C, Western blotting data showing Ad-KLF2-mediated KLF2 overexpression for 24 h induces UCP2 protein expression in HUVECs. D, Ad-KLF2 overexpression for 24 h induces UCP2 mRNA expression in HUVECs and human aortic ECs (HAECs). n=5, nonparametric Mann-Whitney test. E, Endothelium-specific overexpression of Klf2 mediated by Ad-Cdh5-Klf2 in C57 mice for 7 d upregulated mRNA levels of Klf2, Ucp2, and Nos3 in mouse aortas. n=5, nonparametric Mann-Whitney test. Representative western blots and summarized data (F) showing that endothelium-specific overexpression of Klf2 mediated by Ad-Cdh5-Klf2 in C57 mice for 7 d upregulated UCP2 protein expression in mouse aortas. n=5, nonparametric Mann-Whitney test. KLF2 knockdown by KLF2-shRNA downregulated mRNA (G) and protein (H) levels of KLF2, UCP2, and eNOS (endothelial nitric oxide synthase) in HUVECs. n=6, nonparametric Mann-Whitney test. I, Dual luciferase activity assay results showing KLF2 regulates UCP2 promoter activity in HEK293A cells transfected with different lengths of human UCP2 promoters. n=4, 2-way ANOVA, Tukey post hoc. J, KLF2 increases UCP2 promoter activity in HUVECs electroporated a −508 bp UCP2 promoter. n=4, nonparametric Mann-Whitney test. K, Chromatin immunoprecipitation (ChIP)-qPCR assay in HUVECs transduced with Ad-KLF2 showing enriched KLF2 binding to UCP2 promoter. n=4, nonparametric Mann-Whitney test. Representative images were selected as they best represent images obtained. SCR indicates scramble.
We next sought to investigate whether KLF2 can bind to UCP2 promoter. We first checked the putative KLF2 binding core sequences (CA/GCCC) in UCP2 promoter region and found that there are a number of CA/GCCC sequences within 1000 bp DNA sequences before transcription start site (Figure S2F). We cloned a UCP2 promoter 3500 bp in length to pGL3 vector and cotransfected the promoter with KLF2 wild type and mutant plasmids to HEK293A cells. Dual luciferase assay showed that wild type-KLF2 increased luciferase signal while KLF2 mutants KLF2ΔZnF and ZnF decreased basal UCP2 promoter activity in HEK293A cells transfected with a 3500 bp UCP2 promoter (Figure S2E). To identify the putative KLF2-binding region(s) in the UCP2 promoter, we performed the UCP2 promoter truncation analysis and showed that, compared with the luciferase signal produced by −581 bp fragment of UCP2 promoter plasmid, the plasmid with −308 bp fragment of UCP2 promoter showed a significant reduction in luciferase activity, indicating that KLF2 is most likely to bind to the DNA regions from −581 to −308 bp (Figure 2I). In addition, compared with the luciferase signal produced by −308 bp fragment of UCP2 promoter plasmid, the plasmid with −227 bp fragment of UCP2 promoter also showed a significant decrease in producing luciferase signal (Figure 2I), suggesting that the DNA region from −308 bp to −227 bp in UCP2 promoter can also be occupied by KLF2. Furthermore, electroporation of the −581 bp UCP2 promoter to HUVECs also showed that KLF2 increased UCP2 promoter activity (Figure 2J).
To determine whether KLF2 directly binds to UCP2 promoter, we performed chromatin immunoprecipitation assay in HUVECs. Compared with IgG controls, KLF2 chromatin immunoprecipitation has an enrichment in UCP2 promoter region (Figure 2K and Figure S2G), demonstrating that KLF2 is capable of a direct binding to UCP2 promoter. Previous studies revealed that UCP2 expression is positively regulated by PGC-1α (peroxisome proliferator-activated receptor gamma 1α, coactivator) in muscle cells.24 Indeed, PGC-1α knockdown decreased UCP2 mRNA level (Figure S2H), indicating that PGC-1α maintains the basal UCP2 transcription in ECs. However, KLF2-induced UCP2 upregulation appears independent of PGC-1α as knockdown of PGC-1α did not affect KLF2 overexpression-induced UCP2 mRNA expression (Figure S2I). In cancer cells, UCP2 is negatively regulated by SMAD4.25 In congruence, SMAD4 knockdown by shRNA upregulated UCP2 mRNA level in HUVECs (Figure S2I), but KLF2-induced UCP2 expression was not affected by SMAD4 knockdown (Figure S2J through S2L), thus confirming that SMAD4 as a negative regulator of UCP2 is not involved in KLF2-regulated UCP2 expression in ECs. Taken together, the present results demonstrate that UCP2 is a new direct target of KLF2 in ECs.
Regulation of UCP2 by Proinflammatory Stimuli and Vasoprotective Agents
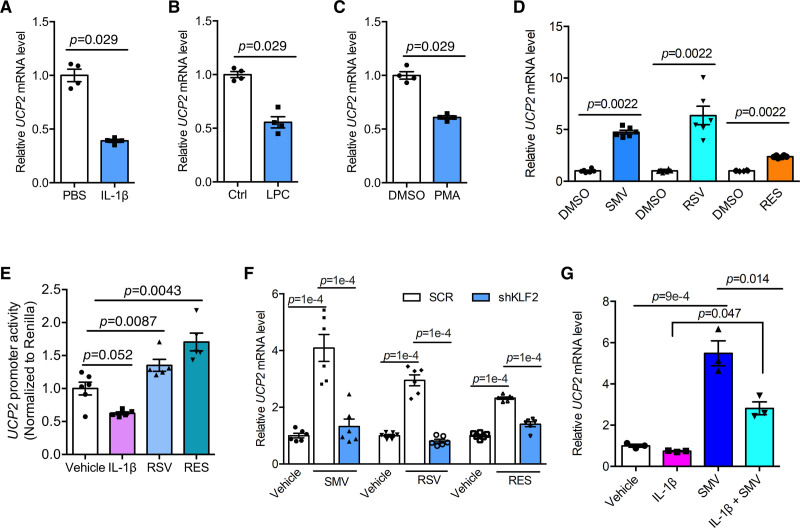
KLF2 expression is suppressed by proinflammatory mediators including cytokines and ox-LDL.26,27 We hypothesized that KLF2-UCP2 signaling axis participates in endothelial proinflammatory response. In contrast to other antioxidant proteins which were upregulated by IL-1β (Figure S3D), UCP2 mRNA expression was inhibited by IL-1β (Figure 3A). In addition, treatment of HUVECs with lysophosphatidylcholine, an important component of ox-LDL28 and phorbol 12-myristate 13-acetate downregulated UCP2 mRNA expression (Figure 3B and 3C). These results indicate that UCP2 is an endothelial antioxidant protein susceptible to be inhibited by inflammatory stimuli.
Figure 3.
Regulation of UCP2 (uncoupling protein 2) by inflammation and vasoprotective agents. A–C, Real-time quantitative polymerase chain reaction (qPCR) data showing UCP2 mRNA expression is inhibited by proinflammatory mediators IL (interleukin)-1β (10 ng/mL), lysophosphatidylcholine (LPC; 100 μmol/L), and phorbol 12-myristate 13-acetate (PMA; 1 μmol/L), nonparametric Mann-Whitney test. D, Human umbilical vein ECs (HUVECs) treated with simvastatin (SMV, 1 μmol/L), rosuvastatin (RSV, 10 μmol/L), and resveratrol (RES, 50 μmol/L) for 24 h showed upregulated expression of UCP2 mRNA, nonparametric Mann-Whitney test. E, Opposite regulation of UCP2 promoter activity in HUVECs transfected with UCP2 promoter plasmid (581 bp in length) by IL-1β and vasoprotective agents RSV and RES. Two-way ANOVA, Tukey post hoc. F, KLF2 (Krüppel-like factor 2) knockdown in HUVECs by KLF2-shRNA abolished induction of UCP2 mRNA by treatment with SMV, RSV, and RES for 24 h. Two-way ANOVA, Tukey post hoc. G, SMV treatment antagonized the inhibitory effect of IL-1β on UCP2 mRNA expression. Two-way ANOVA, Tukey post hoc. PBS indicates phosphate-buffered saline.
Vasoprotective agents, such as statins and resveratrol, were reported to induce KLF2 expression in ECs.13,29 To test whether KLF2-UCP2 axis can be targeted by these therapeutic agents, we treated HUVECs with rosuvastatin and resveratrol and found that UCP2 mRNA expression was time-dependently upregulated by both drugs (Figure S3E and S3F). Likewise, simvastatin, rosuvastatin, and resveratrol all upregulated UCP2 mRNA in HAECs (Figure 3D). UCP2 promoter activity was inhibited by IL-1β but increased by rosuvastatin and resveratrol (Figure 3E), suggesting the involvement of transcriptional regulatory mechanism of UCP2 expression by these treatments. To determine whether KLF2 is required for UCP2 induction by these agents, KLF2 was silenced by KLF2-shRNA before treatment. KLF2 knockdown abolished UCP2 induction by 24-hour treatment with simvastatin, rosuvastatin, and resveratrol (Figure 3F and Figure S3G). Moreover, simvastatin treatment was antagonistic against IL-1β-induced inhibition of UCP2 expression in HUVECs (Figure 3G). Altogether, the present results demonstrate that targeting the KLF2-UCP2 signaling axis holds much therapeutic potential against vascular inflammation.
UCP2 Knockdown Induces a Proinflammatory and Profibrotic EC Phenotype
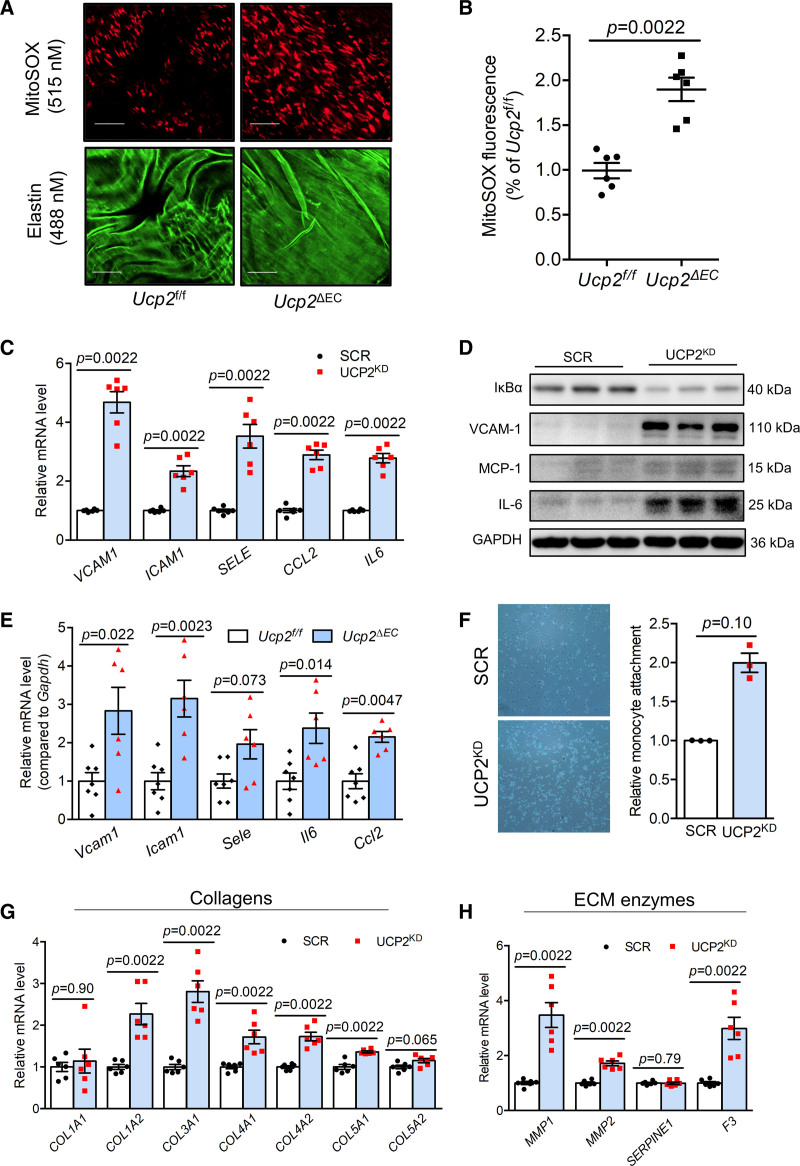
The negative regulation of UCP2 by proinflammatory mediators prompted us to wonder whether UCP2 is critical in regulating proinflammatory responses in ECs. We generated EC-specific UCP2 knockout mice (Ucp2ΔEC, Figure S4A through S4D) which exhibited increased oxidative stress in the aortic endothelium as indicated by elevated mitochondrial ROS level (Figure 4A and 4B). Knockdown of UCP2 in HAECs induced the expression of antioxidant genes like HMOX1, SOD2, and GPX3 (Figure S4E) and HO-1 (HMOXQ1 [heme oxygenase 1]) protein (Figure S4F). The mRNA expression analysis showed that Hmox1 but not Nqo1 was upregulated in aortas from Ucp2ΔEC mice (Figure S4G). The upregulation of cytoprotective HO-1 in UCP2-silenced ECs is likely attributed to the increased oxidative stress which normally triggers activation of antioxidant pathways.
Figure 4.
UCP2 (Uncoupling protein 2) knockdown induces a proinflammatory and profibrotic endothelial cell (EC) phenotype. A, En face endothelium MitoSox staining showing elevated MitoSox fluorescence signal in endothelium of mouse aortas from Ucp2ΔEC mice. Scale bar: 50 μm. B, Statistical summary of MitoSOX fluorescence intensity in en face endothelium of mouse aortas from Ucp2f/f and Ucp2ΔEC mice. Nonparametric Mann-Whitney test. C, Elevated expression of vascular proinflammatory genes VCAM1, ICAM1, SELE, CCL2, and IL6, nonparametric Mann-Whitney test, and upregulated protein expression of VCAM-1, MCP-1, and IL-6 but inhibited expression of IκBα in in UCP2 silenced human aortic ECs (HAECs; D). E, Compared with aortas from Ucp2f/f mice, the aortas from Ucp2ΔEC mice express higher mRNA level of Vcam1, Icam1, Sele, Ccl2, and Il6, nonparametric Mann-Whitney test. F, Monocyte attachment assay showed that more monocyte attached to UCP2 silenced HAECs, figure magnification ×20; nonparametric Mann-Whitney test. UCP2 knockdown in HAECs induces expression of (G) collagen genes COL1A2, COL3A1, COL4A1, COL4A2, and COL5A1 and (H) genes involved in extracellular matrix (ECM) degradation MMP1, MMP2, and F3, nonparametric Mann-Whitney test.
Sustained oxidative stress can result in chronic inflammation in many cell types. We detected the expression of proinflammatory genes in UCP2-silenced ECs. The mRNA level of vascular adhesion molecules VCAM1, ICAM1, and SELE, chemokine CCL2 and cytokine IL6 was all upregulated in UCP2-silenced HAECs and HUVECs (Figure 4C and Figure S5A). We observed a reduced expression of IκBα, which negatively regulates NF-κB (nuclear factor kappa B) activity, together with increased protein expression of VCAM-1 (vascular cell adhesion molecule 1), MCP-1 (monocyte chemoattractant protein 1), and IL-6 in UCP2KD HAECs (Figure 4D). UCP2-silenced HUVECs also expressed much greater protein levels of VCAM-1 and MCP-1 (Figure S5B through S5D). Consistent with in vitro observations, the mRNA level of Vcam1, Icam1, Sele, Ccl2, and Il6 was elevated in the aortas from Ucp2ΔEC mice (Figure 4E). Moreover, Ccl2 and Sele levels were increased in isolated lung ECs from Ucp2ΔEC mice (Figure S5E). Furthermore, monocyte attachment assay showed that UCP2 knockdown promoted adhesion of monocytes to HAECs (Figure 4F). These results demonstrate that UCP2 insufficiency in ECs is proinflammatory.
As ECs is a critical cell type in initiating vascular diseases which are often characterized with fibrosis,30 we next interrogated whether UCP2 silencing plays a role in expression of fibrotic genes. Analysis of the expression of a number of collagen genes revealed that UCP2 regulates expression of fibrillar collagens COL1A2, COL3A1, and COL5A1 (Figure 4G). The expression of type IV collagen which forms basement membrane was also upregulated in UCP2-silenced HAECs (Figure 4G). Accordingly, the expression of collagenases MMP1 and MMP2 and tissue factor F3 were also increased in these UCP2-silenced HAECs (Figure 4H), indicating remodeled extracellular matrix when UCP2 expression is inhibited. In summary, UCP2 expression is critical for EC homeostasis and suppressed UCP2 expression results in proinflammatory and profibrotic responses.
EC-Specific UCP2 Deletion Aggravates Whereas UCP2 Overexpression Attenuates Atherosclerosis
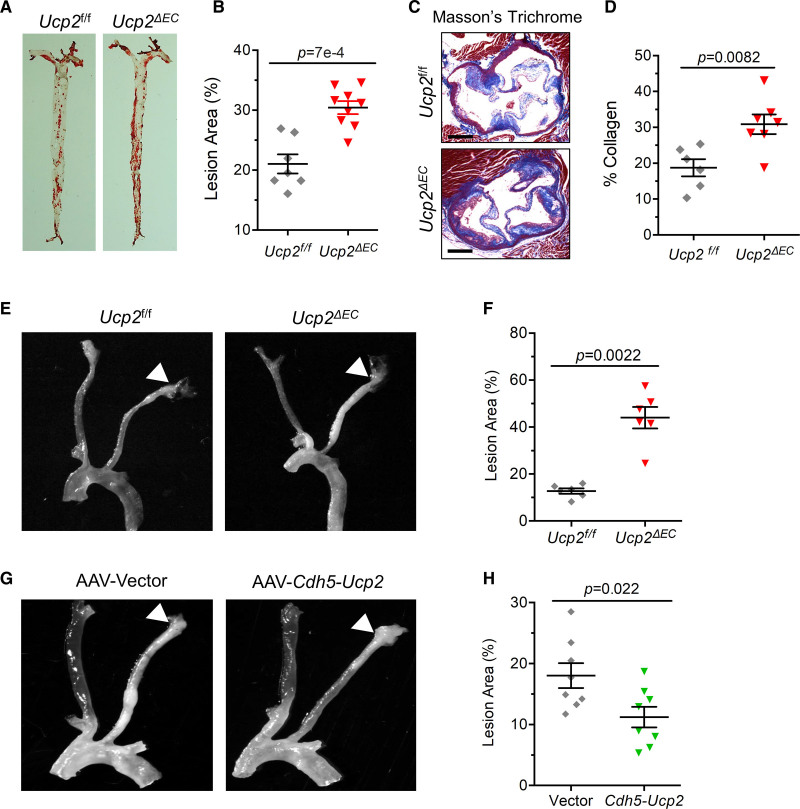
The critical role of UCP2 in regulating expression of proinflammatory and profibrotic genes led us to hypothesize that endothelial UCP2 is important for the pathogenesis of atherosclerosis, a vascular disorder of chronic inflammation. We endeavored to use a method for induction of atherosclerosis in mice by injecting the adeno-associated virus proprotein convertase subtilisin/kexin type 9 (AAV-Pcsk9), which degrades LDL receptor in mouse livers (Figure S6A and S6B), leading to increased serum lipids. Indeed, serum lipid level was rapidly elevated in AAV-Pcsk9-injected mice on a western diet for 1 week (Figure S6C through S6F). To investigate whether EC-specific deletion of Ucp2 affects atherogenesis, the AAV-Pcsk9-injected Ucp2f/f and Ucp2ΔEC mice were fed a western diet for 15 weeks. Oil Red O staining of mouse aortas showed that UCP2 deficiency in ECs aggravated atherosclerosis (Figure 5A and 5B), without affecting the serum lipid profile (Figure S6G through S6J). In agreement with the in vitro data showing increased expression of collagen genes in UCP2-silenced ECs (Figure 4G), the Masson trichrome staining of collagens in aortic roots showed that, compared with Ucp2f/f mice, Ucp2ΔEC mouse aortic roots expressed higher levels of collagens (Figure 5C and 5D).
Figure 5.
Endothelial UCP2 (uncoupling protein 2) is critical for atherogenesis. Oil Red O staining (A) and summarized data (B) showing endothelial cell (EC)-specific UCP2 knockout promotes atherosclerotic plaque formation, nonparametric Mann-Whitney test. Increased collagen level visualized by Masson trichrome staining (C) and summarized data (D) for elevated collagen level in aortic roots from Ucp2ΔEC mice, nonparametric Mann-Whitney test. E and F, Increased plaque formation in Ucp2ΔEC mice receiving left carotid partial ligation surgery, nonparametric Mann-Whitney test. G and H, AAV-Cdh5-Ucp2-mediated overexpression of UCP2 specifically in ECs reduced plaque formation in ApoE−/− mice receiving left carotid partial ligation surgery, unpaired t test. Scale bar: 200 μm.
Given UCP2 expression is inhibited by OSS, we reasoned that UCP2 is a critical regulator of DF-associated atherosclerosis. To test this hypothesis in vivo, mice were received left carotid artery partial ligation surgery to develop DF-enhanced atherosclerosis. The AAV-Pcsk9-injected Ucp2f/f and Ucp2ΔEC mice were fed a western diet immediately after recovery from surgery. Fifteen days postsurgery, more plaques developed in left carotid arteries from Ucp2ΔEC mice than those from Ucp2f/f mice (Figure 5E and 5F and Figure S7A). By contrast, ApoE−/− mice with EC-specific Ucp2 overexpression using EC-enhanced AAV9 vector that is driven by Ve-Cadherin promoter exhibited a reduced plaque formation in left carotid arteries 3 weeks postsurgery (Figure 5G and 5H and Figure S7B through S7D). Moreover, the in vitro UCP2 knockdown and overexpression experiments in HUVECs showed that UCP2 level is critical for KLF2 expression; UCP2 knockdown reduced whereas UCP2 overexpression increased KLF2 mRNA level (Figure S7E and S7F), indicating a reciprocal regulation between UCP2 and KLF2 in ECs. Furthermore, UCP2 downregulation was observed in the plaque area of human renal arteries (Figure S11C). Taken together, both gain- and loss-of-function experiments in vivo demonstrate the importance of endothelial UCP2 in DF-associated atherogenesis.
UCP2 Deficiency Activates FoxO1 to Induce Endothelial Inflammation
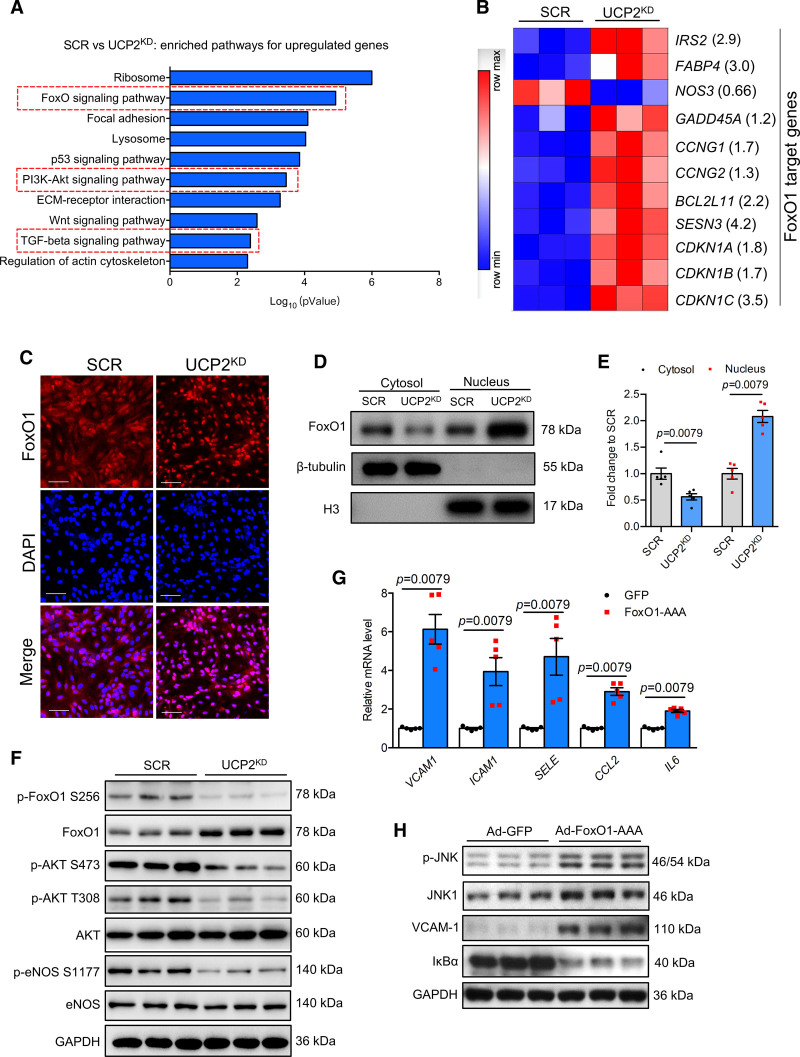
Given the importance of UCP2 in endothelial proinflammatory responses and atherogenesis, we next investigated the underlying mechanisms for UCP2 deficiency-induced inflammation in ECs by performing whole transcriptome analysis using RNA sequencing in UCP2KD HAECs. Database for Annotation, Visualization, and Integrated Discovery (DAVID) Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis revealed several enriched pathways that are important in cell metabolism and proliferation (Figure 6A and Figure S8A), including Ribosome, FoxO signaling pathway, focal adhesion, cell cycle, DNA replication, and pyrimidine metabolism. We further analyzed the differentially expressed genes and found that a panel of differentially expressed genes are FoxO1 target genes (Figure 6B). FoxO1 is a transcription factor critical for the metabolic activity and growth of vascular endothelium, and its activity can be inhibited by the PI3K-Akt pathway, which is also a top ranked enriched pathway in UCP2KD HAECs (Figure 6A and Figure S8A). FoxO1 nuclear level was increased in UCP2KD HAECs (Figure 6C through 6E). Western blotting analysis showed that total FoxO1 expression was increased while the levels of phosphorylated FoxO1 at serine 256 and phosphorylated Akt and eNOS (endothelial nitric oxide synthase) were reduced in UCP2KD HAECs (Figure 6F), indicating diminished Akt activity and increased FoxO1 nuclear translocation. These results demonstrate that FoxO1 is a transcriptional regulator that is activated by UCP2 deficiency.
Figure 6.
UCP2 (Uncoupling protein 2) regulates PI3K (phosphoinositide 3-kinases)-Akt (protein kinase B)-FoxO1 (forkhead box protein O1) pathway in endothelial cells (ECs). A, Enriched KEGG pathways for upregulated genes in UCP2KD human aortic endothelial cells (HAECs). B, Heatmap showing differentially expressed FoxO1 target genes in scramble (SCR) and UCP2KD HAECs, fold change value of each gene was shown in the bracket. FoxO1 target genes were selected according to published data showing FoxO1 directly controls the transcription of these genes. C, FoxO1 nuclear translocation visualized by immunofluorescence staining was increased in UCP2KD HAECs, Scale bar: 100 μm. D and E, Nuclear/cytosolic fractionation of HAECs protein lysates followed by Western blotting analysis showing FoxO1 nuclear level is increased by UCP2 knockdown. n=4, nonparametric Mann-Whitney test. F, Western blotting results showing increased expression of total FoxO1 but reduced level of p-FoxO1 S256, p-Akt S473, p-Akt T308, p-eNOS (endothelial nitric oxide synthase) S1177 in UCP2KD HAECs. Ad-FoxO1-AAA induced mRNA expression (G) of VCAM1, ICAM1, SELE, CCL2, and IL6 and inhibited IκBα expression but increased protein level (H) of p-JNK, JNK1, and VCAM-1, nonparametric Mann-Whitney test. TGF indicates transforming growth factor.
Endothelial FoxO1 ablation was reported to be anti-inflammatory and protective from atherosclerosis in mice.31 To further reveal the role of FoxO1 in mediating UCP2 deficiency-mediated endothelial inflammation, we first overexpressed a mutant form of FoxO1 with 3 Akt phosphorylation sites mutated to alanine (FoxO1-AAA) in HAECs and found that FoxO1-AAA significantly induced the expression of proinflammatory genes that are also inducible by UCP2 knockdown (Figure 6G). FoxO1-AAA also increased JNK phosphorylation, JNK1, and VCAM-1 expression but inhibited IκBα expression (Figure 6H), suggesting that FoxO1 can activate JNK and NF-κB pathways to induce inflammation in ECs. Luciferase reporter assays further confirmed that FoxO1 promoted the transcriptional activity of NF-κB and AP-1 (Figure S8B and S8C).
FoxO1 Inhibition Reduces Endothelial Inflammation and Atherogenesis Induced by UCP2 Deficiency
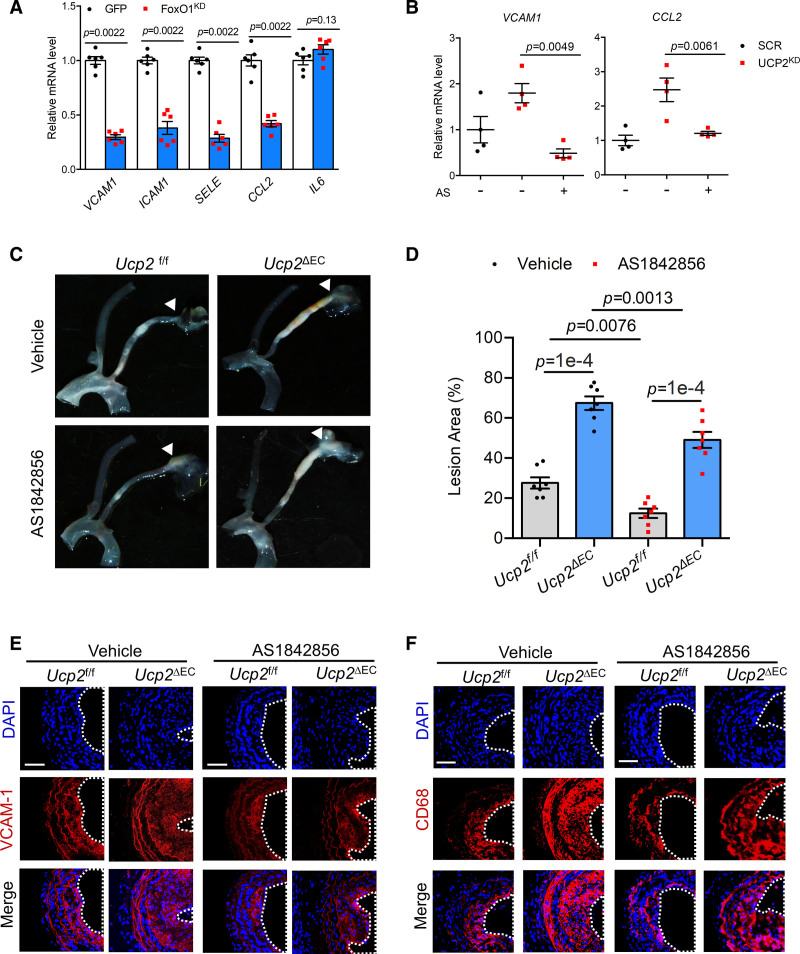
In contrast to the proinflammatory effect of FoxO1 activation, knockdown of FoxO1 reduces, except for IL6, basal expression of proinflammatory genes VCAM1, ICAM1, SELE, and CCL2 in HAECs (Figure 7A and Figure S8D and S8E). FoxO1 knockdown also reduces the basal transcriptional activity of NF-κB (Figure S6F). To determine the effect of FoxO1 activation in UCP2KD-induced endothelial inflammation, we used FoxO1 inhibitor AS1842856 that blocks FoxO1 transcription activity. AS1842856 treatment abolished UCP2KD-induced FoxO1 expression and reversed the induction of CDKN1B, a well-known FoxO1 transcription target (Figure S8G through S8I). AS1842856 also reversed UCP2KD-induced expression of VCAM1 and CCL2 (Figure 7B), thus confirming that FoxO1 is a proinflammatory mediator that is activated by UCP2 knockdown.
Figure 7.
FoxO1 (Forkhead box protein O1) inactivation reduces inflammation and attenuates UCP2 (uncoupling protein 2) deficiency-induced atherosclerotic plaque formation. A, FoxO1 knockdown by shRNA reduced basal mRNA level of VCAM1, ICAM1, SELE, and CCL2, nonparametric Mann-Whitney test. B, FoxO1 inhibitor AS1842856 (1 μmol/L) abolished mRNA expression of VCAM1 and CCL2 induced by UCP2 knockdown in human aortic endothelial cells (HAECs). Two-way ANOVA, Tukey post hoc. C and D, FoxO1 inhibitor AS1842856 reduced atherosclerotic plaque formation in Ucp2f/f and Ucp2ΔEC mice receiving left carotid partial ligation surgery, 2-way ANOVA, Tukey post hoc. Immunofluorescence staining showing AS1842856 suppressed (E) VCAM-1 level and (F) macrophage infiltration in atherosclerotic carotid arteries exposed to disturbed flow generated by partial ligation.
To determine whether increased FoxO1 signaling contributes to the endothelial UCP2 knockout phenotype, we used FoxO1 inhibitor AS1842856 to treat the Ucp2f/f and Ucp2ΔEC mice subjected to partial ligation of carotid arteries. The results showed that the formation of atherosclerotic plaques in Ucp2f/f mice was attenuated by FoxO1 inhibition, and the aggravated plaque formation in Ucp2ΔEC mice was also reduced by AS1842856 (Figure 7C and 7D and Figure S9A), suggesting FoxO1 signaling not only contributes to normal but also UCP2 knockout-accelerated atherogenesis. The reduced formation of atherosclerotic plaques in AS1842856-treated mice was accompanied by a decreased expression of VCAM-1 (Figure 7E) in carotid arteries and a panel of proinflammatory genes (Vcam1, Sele, Ccl2, and Icam1) in aortas (Figure S9B and S9C). Moreover, the infiltration of macrophage to vascular tissues was reduced by AS1842856 (Figure 7F) in Ucp2f/f and Ucp2ΔEC mice. Furthermore, histological analysis of the carotid arteries showed that AS1842856 decreased DF-enhanced vascular remodeling (Figure S10A).
UCP2 Modulates AMPK Activity to Impact on Akt-FoxO1 Pathway
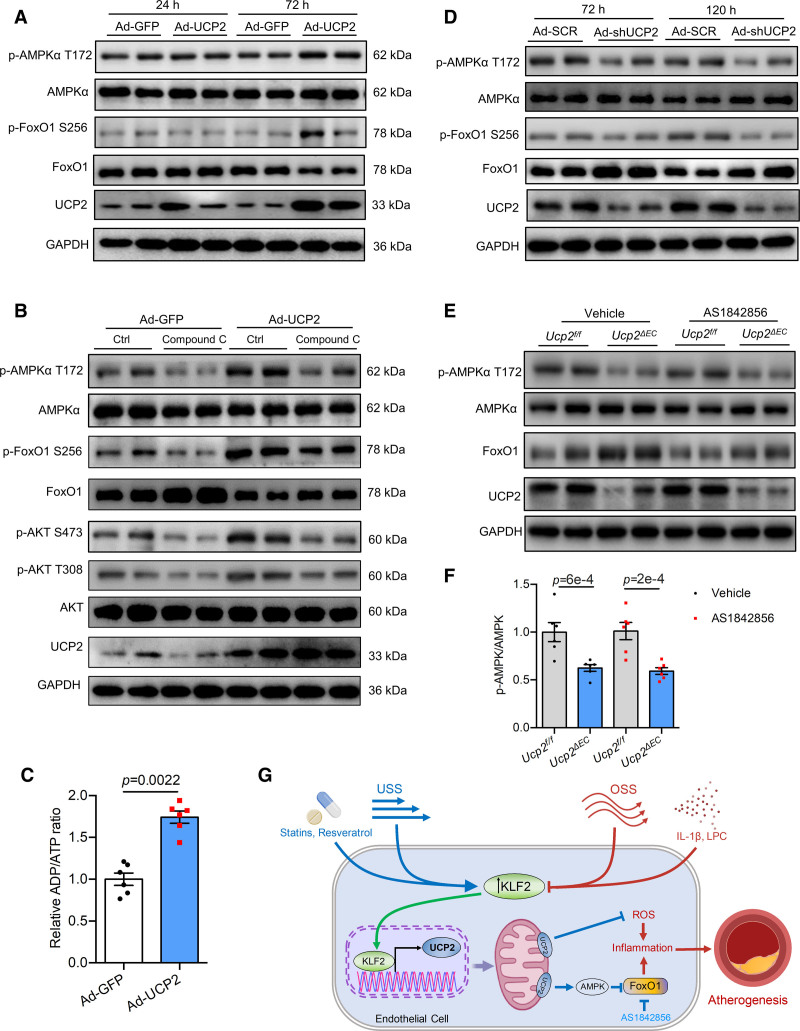
UCP2 is a mild uncoupler of ATP synthesis in the electron transport chain, and increased UCP2 expression may inhibit cellular ATP production and induce energy stress in ECs. AMPK is a master regulator of cellular homeostasis under energy stress and critically regulates atherosclerosis.32,33 Therefore, we hypothesize that UCP2 modulates AMPK activity to impact on Akt-FoxO1 pathway. We found that UCP2 overexpression increased phosphorylated level of AMPK at threonine 172 (p-AMPK T172) in HUVECs (Figure 8A and Figure S10B), suggesting increased AMPK activity by UCP2. To determine whether elevated AMPK activity by UCP2 overexpression plays a role in regulation of Akt-FoxO1 pathway, we used Compound C to inhibit AMPK. Basal levels of p-FoxO1 S256, p-Akt S473, and p-Akt T308 were suppressed, while the total FoxO1 level was increased by Compound C in control HUVECs (Figure 8B and Figure S10C). AMPK inhibition abolished UCP2-induced p-AMPK T172, p-FoxO1 S256, and p-Akt S473/T308 (Figure 8B and Figure S10C). To support the role of UCP2 overexpression in AMPK activation, we measured cellular ADP/ATP level and mitochondrial membrane potential. The result showed that UCP2 overexpression decreased mitochondrial membrane potential indicated by the dye MitoTracker Red CMXRos (Figure S11A). ADP/ATP assay showed that there was a significant increase of ADP/ATP ratio in Ad-UCP2-treated group (Figure 8C), indicating increased cellular energy stress by UCP2 overexpression. By contrast to the effect of UCP2 overexpression on AMPK activation, knockdown of UCP2 reduced level of p-AMPK T172 (Figure 8D and Figure S11B). In consistent with in vitro data, endothelial UCP2 deficiency leads to a reduced p-AMPK T172 in mouse aortas (Figure 8E and 8F and Figure S11C). These results demonstrate that AMPK is critical for UCP2-induced activation of Akt, which subsequently phosphorylates FoxO1 to render it inactive.
Figure 8.
AMPK (AMP-activated protein kinase) is a key mediator of the inhibitory effect of UCP2 (uncoupling protein 2) on FoxO1 (forkhead box protein O1). A, UCP2 knockdown inhibits AMPK activity but increases FoxO1 activity and expression in human umbilical vein ECs (HUVECs). B, UCP2 overexpression for 72 h increases p-AMPK T172 level and inhibits FoxO1 activity and expression in HUVECs. C, ADP/ATP assay showing UCP2 overexpression for 72 h increased ADP/ATP ratio in HUVECs. n=6, nonparametric Mann-Whitney test. D, AMPK inhibition by Compound C abolished the inhibitory effect of UCP2 overexpression on FoxO1 activity and expression in HUVECs. E, EC-specific Ucp2 knockout inhibits AMPK activity but induces FoxO1 expression in mouse aortas. F, Summarized data showing EC-specific Ucp2 knockout inhibits AMPK activity in mouse aortas, 2-way ANOVA, Tukey post hoc. G. Schematic overview of endothelial UCP2 regulation and signaling in blood flow-associated atherosclerosis.
Discussion
Here, we demonstrate that UCP2 expression is negatively regulated by atheroprone OSS and proinflammatory mediators in ECs. The atheroprotective USS and vasoprotective agents including simvastatin, rosuvastatin, and resveratrol upregulate UCP2 expression through the mechanosensitive transcription factor KLF2. We further uncover that UCP2 is a novel mechanosensitive gene under a direct control of KLF2. Mechanistic studies of the UCP2-regulated inflammation reveal that FoxO1, whose activity is regulated by UCP2-induced AMPK, as a proinflammatory mediator in ECs when UCP2 is silenced. The in vivo investigation using gain- and loss-of-function approaches support that UCP2-FoxO1 signaling is a critical for endothelial proinflammatory response and DF-associated atherosclerosis. These novel findings suggest that activation of the KLF2-UCP2 signaling axis may represent an effective anti-inflammatory strategy for treating atherosclerosis.
KLF2 is a shear stress-responsive transcription factor inducible by USS.34 The differential regulation of UCP2 by OSS and USS prompted us to investigate whether KLF2 regulates UCP2 in ECs in response to hemodynamic forces. The identification of UCP2 as a novel target of KLF2 helps gain deeper mechanistic insights into previously unexplained effects of USS and KLF2. For example, earlier studies suggested that the antioxidant effects of USS and KLF2 are independent of SOD2 and catalase, 2 important cellular oxidative defense proteins.35 It is of great interest to further investigate whether UCP2 mediates the antioxidant effect of USS against oxidant stress challenge.
While the present study shows that KLF2 directly regulates UCP2 expression through transcriptional regulatory mechanism, we cannot exclude other minor mechanisms that may indirectly contribute to flow dependent UCP2 expression. We found that UCP2 expression can be negatively regulated by SMAD4 in ECs (Figure S2I). We went further to evaluate the relative contribution of SMAD4 inhibition by KLF2 to UCP2 upregulation and found that SMAD4 plays a minimal role in KLF2-mediated upregulation of UCP2 (Figure S2J through S2L). SMAD4 is a common SMAD protein that integrates the extracellular signals from bone morphogenetic proteins and TGF (transforming growth factor)-β.36 TGF-β–induced SMAD3/4 transcriptional activity is suppressed by KLF2 and prolonged atheroprotective flow in ECs.16,37 These reports indicate that the induction of UCP2 mRNA by USS is likely to be partly contributed by SMAD4 inhibition. Further study is needed to understand the degree of the effect of TGF-β-SMAD3/4 pathway on UCP2 expression induced by USS.
Most KLF2 activators were discovered based on their ability to increase KLF2 expression in ECs. For example, suberanilohydroxamic acid, a pan-inhibitor of histone deacetylase inhibitor, was found to induce KLF2 expression and demonstrated to be atheroprotective in mouse models of atherosclerosis.38 In addition, based on luciferase-based assay, tannic acid was found as a novel KLF2 activator that inhibits endothelial inflammation and atherosclerosis.39 These findings provide the proof of concept that targeting KLF2 as a therapeutic strategy has promise for treating atherosclerosis. However, desirable as KLF2 activation might be to treat atherosclerotic diseases, KLF2 as a transcription factor is difficult to be directly targeted using pharmacological approaches. In the present study, we sought to establish KLF2-UCP2 signaling axis as a targetable pathway with better therapeutic prospects. UCP2 meets several criteria for pharmacological targeting. First, we show that UCP2 is differentially regulated by distinct flow patterns, negatively regulated by proinflammatory mediators, and a direct target of KLF2. Second, the vasoprotective statins upregulate UCP2 expression through KLF2 activation, suggesting that targeting UCP2 holds high therapeutic potential. Third, UCP2 knockout in bone marrow-derived cells promoted atherosclerosis,21 a similar effect is achieved by EC-specific UCP2 knockout in the present study (Figure 5A, 5B, 5E, and 5F). This implies that pharmacological targeting at UCP2 in vivo will produce more desired effects as both macrophages and ECs are important cell types participate in atherogenesis. More importantly, direct overexpression of UCP2 in ECs attenuated DF-induced plaque formation (Figure 5G and 5H), suggesting that increasing endothelial UCP2 level alone is sufficient to achieve beneficial effect against atherosclerosis.
Endothelial inflammation is induced by OSS but suppressed by USS. Our finding of the differential regulation of UCP2 by OSS and USS as well as inhibition of UCP2 by proinflammatory mediators led us to ask whether UCP2 as a direct target of KLF2 also plays a regulatory role in inflammation. We first detected the expression of antioxidant genes which are often upregulated in response to oxidative stress and proinflammatory insult.40,41 We showed that the cytoprotective protein HO-1 was strongly induced when UCP2 is silenced. This result further confirms that UCP2 is an antioxidant protein whose deficiency rapidly activates cellular antioxidant defense system. Next, we checked the expression of vascular adhesion molecules and chemokines that are important in endothelial inflammation and atherogenesis and found that lack of UCP2 inhibited expression of IκBα which suppresses NF-κB nuclear translocation. This result helps explain the effect of UCP2 knockdown on induction of a wide range of proinflammatory genes.
UCP2 downregulation was observed in the plaque region of human renal arteries. Further in vivo evaluation of the functional effect of UCP2 against atherogenesis revealed that endothelial UCP2 is a critical atheroprotective mitochondrial protein. The accelerated western diet-induced atherosclerosis and DF-associated atherosclerosis observed in EC-specific Ucp2 knockout mice are likely attributed to the proinflammatory and profibrotic effects of UCP2 deficiency in ECs. UCP2 silencing in HAECs results in greater adhesion of monocytes to ECs, indicating that the vascular wall of EC-specific Ucp2 knockout mice attracts more infiltrated macrophages, which promotes atherosclerotic progression. In contrast to the detrimental effect of UCP2 deficiency, EC-specific overexpression of UCP2 exerts a protective effect against DF-enhanced atherosclerosis. These in vitro and in vivo results further support the notion that UCP2 can be targeted for therapeutic purposes against vascular inflammatory disorders.
To elucidate the molecular mechanisms for UCP2-deficiency-induced inflammation, we checked global mRNA expression in UCP2KD HAECs using RNA sequencing. Our bioinformatic analysis together with experimental approaches identified transcription factor FoxO1 as a critical mediator of the proinflammatory effect seen in UCP2KD HAECs. FoxO1 is an important factor involved in a variety of cellular processes including proliferation, differentiation, metabolism, and stress response.23,42,43 PI3K-Akt signaling is a critical upstream regulatory pathway of FoxO1 activity via direct phosphorylation to inactivate FoxO1.44 We observed an increased FoxO1 expression and nuclear level but reduced Akt activity in UCP2KD HAECs. Moreover, overexpression of FoxO1-AAA mimics the proinflammatory effect observed in UCP2 knockdown conditions. Furthermore, knockdown or inhibition of FoxO1 in HAECs greatly reduced the expression of proinflammatory genes. These findings are consistent with previous reports showing that endothelial knockout of Akt1 is proinflammatory and atheroprone while FoxO1 deletion in ECs is anti-inflammatory and atheroprotective.31,45 In addition to the contribution of inflammation to atherogenesis, dysregulated cellular metabolism in both immune and vascular cells was suggested to play a pivotal role in providing energy and building materials for the remodeled blood vessel.46–48 It is plausible that the inflammation observed in UCP2-silenced ECs is secondary to the impact of UCP2 on cellular metabolism. Following this possibility, we focused on the role of AMPK, a critical regulator of cellular energy metabolism, in regulation of FoxO1. We found endothelial UCP2 level is critical for the phosphorylated level of AMPK both in vitro and in vivo. UCP2 was previously found as a downstream effector of metformin-activated AMPK.49,50 Our new data showing reduced AMPK activity in aortas from endothelial Ucp2 knockout mice and in UCP2KD HUVECs suggest that a reciprocal regulation between AMPK and UCP2 in ECs.
Although we obtain substantial evidence showing the regulatory mechanism of UCP2 expression and the proinflammatory and atheroprone effect of UCP2 deficiency in ECs, the present study has several limitations. First, the more detailed mechanisms for FoxO1 activation under the condition of UCP2 deficiency need to be explored in the future. It will be interesting to uncover how AMPK regulates Akt-FoxO1 signaling in ECs. Under stress, AMPK was shown to drive Akt activation through phosphorylation of Skp2 serine 256 in cancer cells.51 It is likely that endothelial UCP2 level is an indicator of cellular stress state and therefore it signals to FoxO1 via AMPK-driven Akt activity. Second, our mechanistic studies focus on the proinflammatory effect of FoxO1 through NF-κB and AP-1 transcription factors, but it remains to be demonstrated whether FoxO1 directly works together with NF-κB or AP-1 to control the expression of proinflammatory genes. Third, the present study did not evaluate the role of endothelial UCP2 signaling in plaque rupture, a critical clinical event in the pathogenesis of human atherosclerosis. Further study employing an Apoe−/− Fbn1 C1039G+/− mouse line, a good genetic plaque rupture mouse model, to study the effect of UCP2 deficiency on plaque vulnerability might be interesting. Last, the present study used a Paigen diet, which contains cholate, to induce atherosclerosis in mice. Cholate can influence transcription factors controlling genes involved with regulating hepatic lipoprotein metabolism, which may confound interpretation of the results obtained.52 Although the cholate-containing diet is widely used in atherosclerotic research, even up to the present time,53 we think that the use of cholate in induction of atherosclerosis should be carefully evaluated based on the nature of the studies to be carried out.
In summary, we provide novel evidence demonstrating that UCP2 is a novel KLF2 target in ECs and UCP2 expression is differentially regulated by OSS and USS. UCP2 deficiency in ECs induces inflammation and aggravates western diet-induced atherosclerosis and DF-associated atherosclerosis, while EC-specific overexpression of UCP2 attenuates plaque formation in DF-induced carotid atherosclerosis. UCP2 is an inflammatory regulator whose deficiency inactivates AMPK, which is required for UCP2-induced inhibition of FoxO1 in ECs. Pharmacological targeting the KLF2-UCP2 pathway may represent a promising therapeutic strategy for the treatment of atherosclerotic diseases.
Article Information
Acknowledgments
We thank members of the Y.H. group for the helpful discussions and Dr Lingshan Gou for critical comments.
Author Contributions
The conceptualization was performed by J.-Y. Luo and C.K. Cheng. The funding acquisition was performed by Y. Huang, J.-Y. Luo, and A. Xu. The investigation was performed by J.-Y. Luo, C.K. Cheng, L. He, Y. Pu, C.W. Lau, and X.Y. Tian. The methodology was performed by J.-Y. Luo, C.K. Cheng, C.W. Lau, and X.Y. Tian. The resources were given by Y. Huang, H. Jo, A. Xu, R.C.W. Ma and X.Y. Tian. The supervision was done by Y. Huang. The validation was performed by C.K. Cheng. The manuscript was written by J.-Y. Luo and Y. Huang. The manuscript was reviewed and edited by J.-Y. Luo, C.K. Cheng, and Y. Huang.
Sources of Funding
This study was supported by grants from Hong Kong RGC-Senior Research Fellow Scheme (SRFS2021-4S04), Hong Kong RGC-General Research Fund (14112919), RGC Postdoctoral Fellowship Scheme (PDFS 2022/23), National Natural Science Foundation of China (91939302, 82000251), Hong Kong RGC-Area of Excellence (AoE/M/707-18), Hong Kong RGC-Research Impact Fund (R4012-18) and CityU Start-up Fund.
Disclosures
None.
Supplemental Material
Expanded Materials & Methods
Figures S1–S11
Tables S1–S3
Data Set 1–3
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AKT
- protein kinase B
- AMPK
- AMP-activated protein kinase
- DF
- disturbed flow
- ECs
- endothelial cells
- eNOS
- endothelial nitric oxide synthase
- FoxO1
- forkhead box protein O1
- HAECs
- human aortic endothelial cells
- HO-1
- HMOX1
- HUVECs
- human umbilical vein endothelial cells
- IL
- interleukin
- KLF2
- Krüppel-like factor 2
- OSS
- oscillatory shear stress
- ox-LDL
- oxidized low-density lipoprotein
- PI3K
- phosphoinositide 3-kinases
- ROS
- reactive oxygen species
- TGF
- transforming growth factor
- UCP2
- uncoupling protein 2
- USS
- unidirectional shear stress
This manuscript was sent to Francisco Violi, Senior Guest Editor, for review by expert referees, editorial decision, and final disposition.
J.-Y. Luo and C.K. Cheng contributed equally.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCRESAHA.122.321187.
For Sources of Funding and Disclosures, see page 439.
References
- 1.Ku KH, Subramaniam N, Marsden PA. Epigenetic determinants of flow-mediated vascular endothelial gene expression. Hypertension. 2019;74:467–476. doi: 10.1161/HYPERTENSIONAHA.119.13342 [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Luo JY, Li B, Tian XY, Chen LJ, Huang Y, Liu J, Deng D, Lau CW, Wan S, et al. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature. 2016;540:579–582. doi: 10.1038/nature20602 [DOI] [PubMed] [Google Scholar]
- 3.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538 [DOI] [PubMed] [Google Scholar]
- 4.VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004;24:12–22. doi: 10.1161/01.ATV.0000105054.43931.f0 [DOI] [PubMed] [Google Scholar]
- 5.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327–387. doi: 10.1152/physrev.00047.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridker PM, Devalaraja M, Baeres FMM, Engelmann MDM, Hovingh GK, Ivkovic M, Lo L, Kling D, Pergola P, Raj D, et al. ; RESCUE Investigators. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2021;397:2060–2069. doi: 10.1016/S0140-6736(21)00520-1 [DOI] [PubMed] [Google Scholar]
- 7.Soehnlein O, Libby P. Targeting inflammation in atherosclerosis - from experimental insights to the clinic. Nat Rev Drug Discov. 2021;20:589–610. doi: 10.1038/s41573-021-00198-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeo KP, Lim HY, Thiam CH, Azhar SH, Tan C, Tang Y, See WQ, Koh XH, Zhao MH, Phua ML, et al. Efficient aortic lymphatic drainage is necessary for atherosclerosis regression induced by ezetimibe. Sci Adv. 2020;6:eabc2697. doi: 10.1126/sciadv.abc2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cahill PA, Redmond EM. Vascular endothelium - Gatekeeper of vessel health. Atherosclerosis. 2016;248:97–109. doi: 10.1016/j.atherosclerosis.2016.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc [DOI] [PubMed] [Google Scholar]
- 11.Gimbrone MA, Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–636. doi: 10.1161/CIRCRESAHA.115.306301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergheanu SC, Bodde MC, Jukema JW. Pathophysiology and treatment of atherosclerosis: Current view and future perspective on lipoprotein modification treatment. Neth Heart J. 2017;25:231–242. doi: 10.1007/s12471-017-0959-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sen-Banerjee S, Mir S, Lin Z, Hamik A, Atkins GB, Das H, Banerjee P, Kumar A, Jain MK. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation. 2005;112:720–726. doi: 10.1161/CIRCULATIONAHA.104.525774 [DOI] [PubMed] [Google Scholar]
- 14.Hergenreider E, Heydt S, Tréguer K, Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–256. doi: 10.1038/ncb2441 [DOI] [PubMed] [Google Scholar]
- 15.Fledderus JO, van Thienen JV, Boon RA, Dekker RJ, Rohlena J, Volger OL, Bijnens AP, Daemen MJ, Kuiper J, van Berkel TJ, et al. Prolonged shear stress and KLF2 suppress constitutive proinflammatory transcription through inhibition of ATF2. Blood. 2007;109:4249–4257. doi: 10.1182/blood-2006-07-036020 [DOI] [PubMed] [Google Scholar]
- 16.Fledderus JO, Boon RA, Volger OL, Hurttila H, Ylä-Herttuala S, Pannekoek H, Levonen AL, Horrevoets AJ. KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:1339–1346. doi: 10.1161/ATVBAHA.108.165811 [DOI] [PubMed] [Google Scholar]
- 17.Atkins GB, Wang Y, Mahabeleshwar GH, Shi H, Gao H, Kawanami D, Natesan V, Lin Z, Simon DI, Jain MK. Hemizygous deficiency of Krüppel-like factor 2 augments experimental atherosclerosis. Circ Res. 2008;103:690–693. doi: 10.1161/CIRCRESAHA.108.184663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian XY, Ma S, Tse G, Wong WT, Huang Y. Uncoupling protein 2 in cardiovascular health and disease. Front Physiol. 2018;9:1060. doi: 10.3389/fphys.2018.01060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian XY, Wong WT, Xu A, Lu Y, Zhang Y, Wang L, Cheang WS, Wang Y, Yao X, Huang Y. Uncoupling protein-2 protects endothelial function in diet-induced obese mice. Circ Res. 2012;110:1211–1216. doi: 10.1161/CIRCRESAHA.111.262170 [DOI] [PubMed] [Google Scholar]
- 20.Haslip M, Dostanic I, Huang Y, Zhang Y, Russell KS, Jurczak MJ, Mannam P, Giordano F, Erzurum SC, Lee PJ. Endothelial uncoupling protein 2 regulates mitophagy and pulmonary hypertension during intermittent hypoxia. Arterioscler Thromb Vasc Biol. 2015;35:1166–1178. doi: 10.1161/ATVBAHA.114.304865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanc J, Alves-Guerra MC, Esposito B, Rousset S, Gourdy P, Ricquier D, Tedgui A, Miroux B, Mallat Z. Protective role of uncoupling protein 2 in atherosclerosis. Circulation. 2003;107:388–390. doi: 10.1161/01.cir.0000051722.66074.60 [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Liu J, Tian XY, Wong WT, Lau CW, Xu A, Xu G, Ng CF, Yao X, Gao Y, et al. Uncoupling protein-2 mediates DPP-4 inhibitor-induced restoration of endothelial function in hypertension through reducing oxidative stress. Antioxid Redox Signal. 2014;21:1571–1581. doi: 10.1089/ars.2013.5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilhelm K, Happel K, Eelen G, Schoors S, Oellerich MF, Lim R, Zimmermann B, Aspalter IM, Franco CA, Boettger T, et al. FOXO1 couples metabolic activity and growth state in the vascular endothelium. Nature. 2016;529:216–220. doi: 10.1038/nature16498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 25.Sayeed A, Meng Z, Luciani G, Chen LC, Bennington JL, Dairkee SH. Negative regulation of UCP2 by TGFβ signaling characterizes low and intermediate-grade primary breast cancer. Cell Death Dis. 2010;1:e53. doi: 10.1038/cddis.2010.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar A, Kumar S, Vikram A, Hoffman TA, Naqvi A, Lewarchik CM, Kim YR, Irani K. Histone and DNA methylation-mediated epigenetic downregulation of endothelial Kruppel-like factor 2 by low-density lipoprotein cholesterol. Arterioscler Thromb Vasc Biol. 2013;33:1936–1942. doi: 10.1161/ATVBAHA.113.301765 [DOI] [PubMed] [Google Scholar]
- 27.SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, Feinberg MW, Chen Z, Simon DI, Luscinskas FW, et al. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199:1305–1315. doi: 10.1084/jem.20031132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu R, Huang YH, Elinder LS, Frostegård J. Lysophosphatidylcholine is involved in the antigenicity of oxidized LDL. Arterioscler Thromb Vasc Biol. 1998;18:626–630. doi: 10.1161/01.atv.18.4.626 [DOI] [PubMed] [Google Scholar]
- 29.Gracia-Sancho J, Villarreal G, Jr, Zhang Y, García-Cardeña G. Activation of SIRT1 by resveratrol induces KLF2 expression conferring an endothelial vasoprotective phenotype. Cardiovasc Res. 2010;85:514–519. doi: 10.1093/cvr/cvp337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steffensen LB, Rasmussen LM. A role for collagen type IV in cardiovascular disease? Am J Physiol Heart Circ Physiol. 2018;315:H610–H625. doi: 10.1152/ajpheart.00070.2018 [DOI] [PubMed] [Google Scholar]
- 31.Tsuchiya K, Tanaka J, Shuiqing Y, Welch CL, DePinho RA, Tabas I, Tall AR, Goldberg IJ, Accili D. FoxOs integrate pleiotropic actions of insulin in vascular endothelium to protect mice from atherosclerosis. Cell Metab. 2012;15:372–381. doi: 10.1016/j.cmet.2012.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Q, Xu J, Ma Q, Liu Z, Sudhahar V, Cao Y, Wang L, Zeng X, Zhou Y, Zhang M, et al. PRKAA1/AMPKα1-driven glycolysis in endothelial cells exposed to disturbed flow protects against atherosclerosis. Nat Commun. 2018;9:4667. doi: 10.1038/s41467-018-07132-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seneviratne A, Cave L, Hyde G, Moestrup SK, Carling D, Mason JC, Haskard DO, Boyle JJ. Metformin directly suppresses atherosclerosis in normoglycaemic mice via haematopoietic adenosine monophosphate-activated protein kinase. Cardiovasc Res. 2021;117:1295–1308. doi: 10.1093/cvr/cvaa171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang J, Pu Y, Zhang H, Xie L, He L, Zhang CL, Cheng CK, Huo Y, Wan S, Chen S, et al. KLF2 Mediates the Suppressive Effect of Laminar Flow on Vascular Calcification by Inhibiting Endothelial BMP/SMAD1/5 Signaling. Circ Res. 2021;129:e87–e100. doi: 10.1161/CIRCRESAHA.120.318690 [DOI] [PubMed] [Google Scholar]
- 35.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA, Jr, et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo JY, Zhang Y, Wang L, Huang Y. Regulators and effectors of bone morphogenetic protein signalling in the cardiovascular system. J Physiol. 2015;593:2995–3011. doi: 10.1113/JP270207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boon RA, Fledderus JO, Volger OL, van Wanrooij EJ, Pardali E, Weesie F, Kuiper J, Pannekoek H, ten Dijke P, Horrevoets AJ. KLF2 suppresses TGF-beta signaling in endothelium through induction of Smad7 and inhibition of AP-1. Arterioscler Thromb Vasc Biol. 2007;27:532–539. doi: 10.1161/01.ATV.0000256466.65450.ce [DOI] [PubMed] [Google Scholar]
- 38.Xu Y, Xu S, Liu P, Koroleva M, Zhang S, Si S, Jin ZG. Suberanilohydroxamic acid as a pharmacological kruppel-like factor 2 activator that represses vascular inflammation and atherosclerosis. J Am Heart Assoc. 2017;6: e007134. doi: 10.1161/JAHA.117.007134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Y, Liu P, Xu S, Koroleva M, Zhang S, Si S, Jin ZG. Tannic acid as a plant-derived polyphenol exerts vasoprotection via enhancing KLF2 expression in endothelial cells. Sci Rep. 2017;7:6686. doi: 10.1038/s41598-017-06803-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishikawa K, Navab M, Leitinger N, Fogelman AM, Lusis AJ. Induction of heme oxygenase-1 inhibits the monocyte transmigration induced by mildly oxidized LDL. J Clin Invest. 1997;100:1209–1216. doi: 10.1172/JCI119634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cox CS, McKay SE, Holmbeck MA, Christian BE, Scortea AC, Tsay AJ, Newman LE, Shadel GS. Mitohormesis in mice via sustained basal activation of mitochondrial and antioxidant signaling. Cell Metab. 2018;28:776–786.e5. doi: 10.1016/j.cmet.2018.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oellerich MF, Potente M. FOXOs and sirtuins in vascular growth, maintenance, and aging. Circ Res. 2012;110:1238–1251. doi: 10.1161/CIRCRESAHA.111.246488 [DOI] [PubMed] [Google Scholar]
- 43.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086 [DOI] [PubMed] [Google Scholar]
- 44.Tang ED, Nuñez G, Barr FG, Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741 [DOI] [PubMed] [Google Scholar]
- 45.Fernández-Hernando C, Ackah E, Yu J, Suárez Y, Murata T, Iwakiri Y, Prendergast J, Miao RQ, Birnbaum MJ, Sessa WC. Loss of Akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell Metab. 2007;6:446–457. doi: 10.1016/j.cmet.2007.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Z, Zhu H, Dai X, Wang C, Ding Y, Song P, Zou MH. Macrophage liver kinase B1 inhibits foam cell formation and atherosclerosis. Circ Res. 2017;121:1047–1057. doi: 10.1161/CIRCRESAHA.117.311546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bories GFP, Leitinger N. Macrophage metabolism in atherosclerosis. FEBS Lett. 2017;591:3042–3060. doi: 10.1002/1873-3468.12786 [DOI] [PubMed] [Google Scholar]
- 48.Nitz K, Lacy M, Atzler D. Amino acids and their metabolism in atherosclerosis. Arterioscler Thromb Vasc Biol. 2019;39:319–330. doi: 10.1161/ATVBAHA.118.311572 [DOI] [PubMed] [Google Scholar]
- 49.Xie Z, Zhang J, Wu J, Viollet B, Zou MH. Upregulation of mitochondrial uncoupling protein-2 by the AMP-activated protein kinase in endothelial cells attenuates oxidative stress in diabetes. Diabetes. 2008;57:3222–3230. doi: 10.2337/db08-0610 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Xu MJ, Song P, Shirwany N, Liang B, Xing J, Viollet B, Wang X, Zhu Y, Zou MH. Impaired expression of uncoupling protein 2 causes defective postischemic angiogenesis in mice deficient in AMP-activated protein kinase α subunits. Arterioscler Thromb Vasc Biol. 2011;31:1757–1765. doi: 10.1161/ATVBAHA.111.227991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han F, Li CF, Cai Z, Zhang X, Jin G, Zhang WN, Xu C, Wang CY, Morrow J, Zhang S, et al. The critical role of AMPK in driving Akt activation under stress, tumorigenesis and drug resistance. Nat Commun. 2018;9:4728. doi: 10.1038/s41467-018-07188-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Getz GS, Reardon CA. Diet and murine atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:242–249. doi: 10.1161/01.ATV.0000201071.49029.17 [DOI] [PubMed] [Google Scholar]
- 53.Liu Q, Pan J, Bao L, Xu C, Qi Y, Jiang B, Wang D, Zhu X, Li X, Zhang H, et al. Major vault protein prevents atherosclerotic plaque destabilization by suppressing macrophage ASK1-JNK signaling. Arterioscler Thromb Vasc Biol. 2022;42:580–596. doi: 10.1161/ATVBAHA.121.316662 [DOI] [PubMed] [Google Scholar]
- 54.Gory S, Vernet M, Laurent M, Dejana E, Dalmon J, Huber P. The vascular endothelial-cadherin promoter directs endothelial-specific expression in transgenic mice. Blood. 1999;93:184–192. [PubMed] [Google Scholar]
- 55.Varadi K, Michelfelder S, Korff T, Hecker M, Trepel M, Katus HA, Kleinschmidt JA, Müller OJ. Novel random peptide libraries displayed on AAV serotype 9 for selection of endothelial cell-directed gene transfer vectors. Gene Ther. 2012;19:800–809. doi: 10.1038/gt.2011.143 [DOI] [PubMed] [Google Scholar]
- 56.Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol. 2009;297:H1535–H1543. doi: 10.1152/ajpheart.00510.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Korshunov VA, Berk BC. Flow-induced vascular remodeling in the mouse: a model for carotid intima-media thickening. Arterioscler Thromb Vasc Biol. 2003;23:2185–2191. doi: 10.1161/01.ATV.0000103120.06092.14 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All supporting data are available within the article and its Supplemental Material. For details on the experimental procedures and materials used, please refer to the Major Resources Table and the materials and methods section in the Supplemental Material.