ABSTRACT
Introduction
Plant-derived proteins have received considerable attention as an alternative to animal-based proteins and are now frequently used in both plant-based diets and sports nutrition products. However, little information is available on the anabolic properties of potato-derived protein. This study compares muscle protein synthesis rates after the ingestion of 30 g potato protein versus 30 g milk protein at rest and during recovery from a single bout of resistance exercise in healthy, young males.
Methods
In a randomized, double-blind, parallel-group design, 24 healthy young males (24 ± 4 yr) received primed continuous l-[ring-13C6]-phenylalanine infusions while ingesting 30 g potato-derived protein or 30 g milk protein after a single bout of unilateral resistance exercise. Blood and muscle biopsies were collected for 5 h after protein ingestion to assess postprandial plasma amino acid profiles and mixed muscle protein synthesis rates at rest and during recovery from exercise.
Results
Ingestion of both potato and milk protein increased mixed muscle protein synthesis rates when compared with basal postabsorptive values (from 0.020% ± 0.011% to 0.053% ± 0.017%·h−1 and from 0.021% ± 0.014% to 0.050% ± 0.012%·h−1, respectively; P < 0.001), with no differences between treatments (P = 0.54). In the exercised leg, mixed muscle protein synthesis rates increased to 0.069% ± 0.019% and 0.064% ± 0.015%·h−1 after ingesting potato and milk protein, respectively (P < 0.001), with no differences between treatments (P = 0.52). The muscle protein synthetic response was greater in the exercised compared with the resting leg (P < 0.05).
Conclusions
Ingestion of 30 g potato protein concentrate increases muscle protein synthesis rates at rest and during recovery from exercise in healthy, young males. Muscle protein synthesis rates after the ingestion of 30 g potato protein do not differ from rates observed after ingesting an equivalent amount of milk protein.
Key Words: MUSCLE PROTEIN SYNTHESIS, MILK PROTEIN, RESISTANCE EXERCISE, SPORTS NUTRITION
Protein ingestion (1–3) and physical activity (4) stimulate muscle protein synthesis and are essential for the maintenance and accretion of skeletal muscle mass. Protein ingested during recovery from exercise further augments muscle protein synthesis rates (5–7) and supports the skeletal muscle adaptive response to more prolonged exercise training (8). The muscle protein synthetic response to protein ingestion is driven by the postprandial increase in circulating essential amino acids (EAA) concentrations (9), with plasma leucine being of particular relevance (10–12). Postprandial muscle protein synthesis rates at rest and during recovery from exercise have been reported to differ substantially after the ingestion of different protein sources (13–15). The anabolic properties of a protein source are largely determined by its protein digestion and amino acid (AA) absorption kinetics, as well as the AA composition of the protein (9,16–18).
Our habitual protein intake originates from both animal- and plant-based sources (19,20). In general, plant-based proteins are considered to provide a lesser anabolic stimulus after ingestion when compared with animal-based proteins. This is mainly attributed to their lower digestibility and incomplete AA profile, characterized by low leucine, lysine, and/or methionine contents (19,20). Plant-based proteins already comprise a large part of our daily protein intake, but their contribution will become much greater because of the growing interest in consuming more plant-based diets and plant-based proteins (21). The trend of consuming a more plant-based diet has also reached the athletic community, where sports supplements containing whey or egg protein are now frequently traded in for supplements providing plant-derived protein isolates or concentrates. Despite their popularity, only few studies have actually compared the anabolic properties of animal- versus plant-derived proteins (15,22–24). Lesser anabolic properties have been reported after soy (15,23,24) and wheat (25) protein ingestion when compared with dairy protein both at rest and/or during recovery from exercise. However, these differences are not always apparent (15,26,27). As there is a large variety in plant-derived protein characteristics (28), more plant-derived proteins should be evaluated for their anabolic properties both at rest and during recovery from exercise.
Potatoes are the third most consumed crop worldwide (29,30). Potatoes contain a mere ~1.5% protein based on their fresh weight (30). However, when potatoes are used for starch extraction, a residue remains (potato fruit juice), which is generally used for feed production or discarded as a waste product. From this residue, a potato protein concentrate can be extracted (31). We previously identified the AA profile of potato-derived protein along with various other plant-based protein sources (28). The analysis revealed an AA composition of potato protein that closely resembles milk protein. In contrast to most other plant-derived proteins, potato protein provides sufficient amounts of all individual EAA according to the WHO/FAO/UNU AA requirements, with no apparent deficiencies (28). However, whether this favorable AA profile of potato-derived protein also translates to strong anabolic properties upon ingestion remains to be established.
We hypothesize that the ingestion of 30 g potato protein concentrate increases muscle protein synthesis rates both at rest and during recovery from exercise in healthy, young men. Furthermore, we hypothesize that the muscle protein synthetic response after the ingestion of 30 g potato protein does not differ from the ingestion of 30 g milk protein. To test our hypotheses, we assessed postabsorptive and postprandial muscle protein synthesis rates after the ingestion of either 30 g potato- or milk-derived protein concentrate after a single bout of unilateral resistance exercise in 24 healthy, young males.
METHODS
Participants
Twenty-four healthy, recreationally active males (24 ± 4 yr, 1.79 ± 0.07 m, 72.4 ± 7.1 kg) volunteered to participate in this parallel-group, double-blind, randomized controlled trial (subjects’ characteristics are presented in Table 1). The trial was registered at the Netherlands Trial Register (NTR7152) and was conducted between April 2018 and February 2020 at Maastricht University in Maastricht, The Netherlands (see Supplemental Fig. 1, Supplemental Digital Content, for the CONSORT (Consolidated Standards of Reporting Trials) flow diagram, http://links.lww.com/MSS/C572). All participants were informed on the purpose of the study, the experimental procedures, and the possible risks before providing informed written consent to participate. The procedures followed were in accordance with the ethical standards of the medical ethics committee of the Maastricht University Medical Centre+ (METC 173053) on research involving human participants and in accordance with the Helsinki Declaration of 1975 as revised in October 2013. The study was independently monitored by the Clinical Trial Centre Maastricht (CTCM).
TABLE 1.
Participants’ characteristics.
| POTATO | MILK | |
|---|---|---|
| Age (yr) | 23 ± 3 | 25 ± 4 |
| Height (m) | 1.81 ± 0.04 | 1.77 ± 0.8 |
| Body mass (kg) | 73.7 ± 6.4 | 71.2 ± 7.9 |
| BMI (kg·m−2) | 22.7 ± 1.4 | 22.7 ± 1.7 |
| Systolic blood pressure (mm Hg) | 119 ± 11 | 119 ± 12 |
| Diastolic blood Pressure (mm Hg) | 63 ± 8 | 68 ± 11 |
| Resting heart rate (bpm) | 63 ± 10 | 62 ± 8 |
| Lean body mass (kg) | 57.7 ± 6.1 | 52.6 ± 5.7 |
| Body fat (%) | 19.7 ± 3.1 | 22.8 ± 4.3 |
| Leg press 1-RM (kg) | 115 ± 26 | 98 ± 22 |
| Knee extension 1-RM (kg) | 61 ± 10 | 54 ± 9 |
Values represent mean ± SD. n = 12 per nutritional intervention group. POTATO: 30 g of potato-derived protein; MILK: 30 g milk protein. Independent-samples t-test for POTATO vs MILK all P ≥ 0.05.
1-RM, one-repetition maximum of the exercised leg.
Preliminary screening
Participants 18–35 yr old, with BMI >18.5 and <27.5 kg·m−2, underwent an initial screening session to assess eligibility. For this purpose, height, weight, blood pressure, and body composition (by dual-energy x-ray absorptiometry; Discovery A, Hologic) were determined. Participants were deemed healthy based on their responses to a medical questionnaire and were excluded from participation if smoking, performing progressive resistance exercise training, using medication that affected protein metabolism, or intolerant to the investigated protein products. After initial screening, the participants were familiarized with the exercise testing protocol and the exercise equipment. Unilateral one-repetition maximum (1-RM) strength was assessed for both legs separately, on the supine leg press (Technogym BV, Capelle aan den Ijssel, the Netherlands) and seated knee extension (Technogym BV) exercise using the multiple repetition testing procedure (32). Before testing, participants performed a unilateral warm-up at low resistance for 20 repetitions to become familiarized with the equipment and to have exercise technique assessed and adjusted. Working sets of 8 repetitions were then performed with progressively increased loads until failure, to perform a valid estimation within 1–8 repetitions of the set. A repetition was considered valid if the subject was able to complete it in a controlled manner. A 2-min rest period was allowed between successive sets. In between the screening session and the experimental trial, subjects reported to the laboratory for an additional visit to perform a true 1-RM strength test.
1-RM strength test
During this visit, the participant’s unilateral 1-RM strength was determined for each leg separately, starting with the supine leg press, followed by the seated knee extension. The estimated 1-RM obtained during the screening visit was used to determine the initial load for the actual 1-RM test (33). Before testing each exercise, participants performed 2 sets of unilateral warm-up at low weight: first 20 repetitions at 25% of the estimated 1-RM followed by 8 repetitions at 50% of the estimated 1-RM. During these sets, the exercise technique was again closely assessed and adjusted when necessary. After warm-up, the 1-RM was determined based on the protocol described by Kraemer and Fry (34). In short, for the first attempt, the load was set at 90% of the estimated 1-RM and was increased by 2.5%–5% after each successful lift until failure. A 2-min rest period was allowed between successive attempts. A lift was deemed successful when performed in a controlled manner, without assistance, and for the full range of motion. The range of motion for the supine leg press started at a knee angle of 70° until full extension (without locking the knee); for the seated knee extension, the knee angle was set from 70° to 160°. The 1-RM testing and experimental trials were separated by at least 3 d.
Study design
Participants were randomly assigned to ingest a 400-mL beverage containing 30 g potato protein (POTATO) or 30 g milk protein (MILK). After beverage ingestion, the bottle was rinsed with 150 mL of water. Potato protein concentrate (Solanic 100) was supplied by AVEBE (Veendam, the Netherlands), and milk protein concentrate (MPC80) was obtained from FrieslandCampina (Wageningen, the Netherlands). Participants were allocated to a treatment according to a block randomization list performed using a computerized randomizer (http://www.randomization.com/). An independent researcher was responsible for random assignment (n = 12 per group) and preparation of the study treatment beverages, which were sequentially numbered according to subject number. The beverages were prepared in nontransparent protein-shakers.
Diet and physical activity
Participants refrained from sports and strenuous physical activities (such as heavy lifting) and alcohol consumption for 3 d before the experimental trial. In addition, all participants filled out a food and activity diary for 3 d before the experimental trial. For the evening before the trial, all participants consumed the same standardized meal containing 2.3 MJ, with 20% energy provided as carbohydrate, 65% as fat, and 15% as protein, before 2200 h after which they remained fasted.
Experimental protocol
At ~7:30 am, participants arrived at the laboratory in the overnight fasted state. A catheter was inserted into an antecubital vein for stable isotope AA infusion, while a second catheter was inserted retrogradely into a dorsal hand vein of the contralateral arm for arterialized blood sampling. To obtain arterialized blood samples, the hand was placed in a hot box (60°C) for 10 min before each blood sample collection (35).
After taking a baseline blood sample (t = −180 min), the plasma phenylalanine pool was primed with a single dose of l-[ring-13C6]-phenylalanine (2.25 μmol·kg−1). Thereafter, a continuous intravenous infusion of l-[ring-13C6]-phenylalanine (0.05 μmol·kg−1⋅min−1) was initiated (t = −180 min) using a calibrated IVAC 598 pump (San Diego, CA). While resting in a supine position, arterialized blood samples were collected in EDTA-containing tubes 60 and 120 min (t = −120 and t = −60, respectively) after the initiation of the tracer infusion. At 130 min (t = −50), the unilateral exercise session commenced. After the exercise session (t = −10 min), the participants returned to the resting position. At t = 0 min, an arterialized blood sample was obtained as well as bilateral muscle biopsy samples from the m. vastus lateralis of the rested and exercised leg. Immediately after the muscle biopsy, participants ingested the beverage corresponding to their randomized treatment allocation, i.e., POTATO (n = 12) or MILK (n = 12). To minimize dilution of the steady-state plasma l-[ring-13C6]-phenylalanine precursor pool, 3.85% l-[ring-13C6]-phenylalanine was added to the drinks. Arterialized blood samples were then collected at t = 15, 30, 45, 60, 90, 120, 150, 180, 210, 240, and 300 min into the postprandial period. Second and third muscle biopsies were collected at t = 120 and 300 min, respectively, to determine postprandial muscle protein synthesis rates from 0 to 120, from 120 to 300, and from 0 to 300 min. Muscle biopsy collection was performed from both the rested and the exercised leg during each time point, starting with the exercised leg. Blood samples were collected into EDTA-containing tubes and centrifuged at 1200g for 10 min at 4°C. Aliquots of plasma were frozen in liquid nitrogen and stored at −80°C. Biopsy samples were collected using a 5-mm Bergström needle (36) custom adapted for manual suction. Samples were obtained from separate incisions from the middle region of the m. vastus lateralis, ~15 cm above the patella and ~3 cm below entry through the fascia. Local anesthetic (1% Xylocaine with adrenaline 1:100,000) was applied to numb the skin and fascia. Muscle samples were freed from any visible nonmuscle material, immediately frozen in liquid nitrogen, and stored at −80°C until further processing. When the experimental protocol was complete, cannulae were removed and participants were fed and assessed for ~30 min before leaving the laboratory. For a schematic representation of the infusion protocol, see Figure 1.
FIGURE 1.
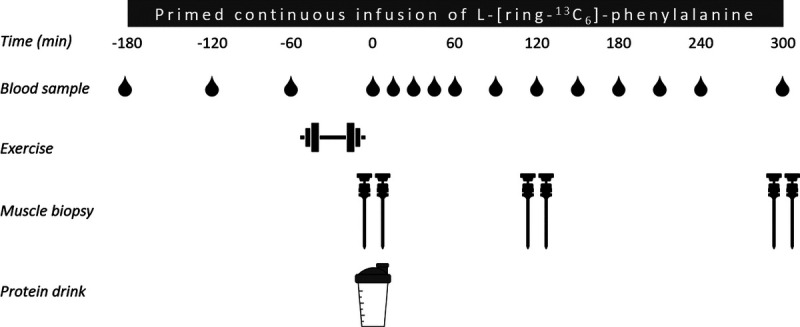
Schematic representation of the experimental design.
Exercise protocol
Participants began with a standardized warm-up on the supine leg press (20 repetitions at 25% 1-RM followed by 8 repetitions at 50% 1-RM) followed by 3 sets of 8 repetitions at ~80% 1-RM. For the fourth set, participants were instructed to perform as many repetitions as possible. Participants then carried out the same exercise protocol (i.e., same warm-up, number of sets and repetitions at percentage of estimated 1-RM) on the seated knee extension machine. Each set was separated by 2 min of passive recovery during which the participant remained seated. Strong verbal encouragement was provided by one of the study investigators during each set. Participants were randomly allocated to perform the exercise session with their dominant or nondominant leg. The randomization scheme ensured an equal amount of participants performed the exercise with the dominant (n = 6) as well as nondominant (n = 6) leg within each interventional group (n = 12).
Dietary protein analysis
Batch-specific nitrogen contents of both potato and milk were provided by the manufacturer. Milk protein content was determined as nitrogen content ×6.38 (37,38) and potato protein content as nitrogen content ×6.25 (39). AA contents in protein were determined by acid hydrolysis in triplicate. In particular, the AA contents were liberated from the protein powders (~4 mg) by adding 2 mL of 6 M HCl and heating to 110°C for 12 h. The hydrolyzed proteins were subsequently dried under a nitrogen stream while heated to 120°C. Before analysis, the hydrolysate was dissolved in 5 mL of 0.1 M HCl and 20 μL of AccQ/Tag derivatizing reagent solution (Waters, Saint/Quentin, France) was added as described here below for the plasma AA concentration analysis. AA composition of the proteins is presented in Table 2.
TABLE 2.
AA composition of the protein concentrates.
| POTATO | MILK | |
|---|---|---|
| Alanine | 1.4 | 1.0 |
| Arginine | 1.3 | 0.9 |
| Aspartic acid | 2.6 | 1.8 |
| Cystine | 0.2 | 0.1 |
| Glutamic acid | 2.5 | 5.5 |
| Glycine | 1.3 | 0.5 |
| Histidine | 0.5 | 0.7 |
| Isoleucine | 0.9 | 1.0 |
| Leucine | 2.6 | 2.6 |
| Lysine | 1.8 | 2.1 |
| Methionine | 0.6 | 0.6 |
| Phenylalanine | 1.5 | 1.3 |
| Proline | 1.4 | 2.9 |
| Serine | 1.4 | 1.3 |
| Threonine | 1.4 | 1.1 |
| Tyrosine | 0.7 | 0.7 |
| Valine | 1.1 | 1.2 |
| TAA | 23.2 | 25.3 |
| EAA | 10.5 | 10.7 |
| BCAA | 4.7 | 4.9 |
| Nitrogen content (%) | 13.1 | 12.8 |
| Protein content (%) | 81.9a | 81.5b |
Values for AA contents are in grams per 30 g protein. POTATO: 30 g potato-derived protein; MILK: 30 g of milk protein.
aProtein as nitrogen × 6.25.
bProtein as nitrogen content × 6.38.
BCAA, branched chain amino acids; TAA, total amino acids.
Plasma analysis
Plasma glucose and insulin concentrations were analyzed using commercially available kits (ref. no. A11A01667, Glucose HK CP, ABX Diagnostics, Montpellier, France; and ref. no. HI-14 K, Millipore, St. Louis, MO, respectively). Plasma l-[ring-13C6]-phenylalanine, enrichments were determined by gas chromatography–mass spectrometry (GC-MS; Agilent 7890A GC/5975C MSD; Agilent Technologies). In particular, the plasma was deproteinized on ice with dry 5-sulfosalicyclic acid. Free AA were purified using cation exchange AG 50 W-X8 resin (mesh size, 100–200; ionic form, hydrogen; Bio-Rad Laboratories, Hercules, CA) columns. The free AA were converted to their tert-butyl dimethylsilyl (TBDMS) derivative before analysis by GC-MS using selected ion monitoring of masses 336 and 342 for unlabeled and labeled l-[ring-13C6]-phenylalanine, respectively. Standard regression curves were applied from a series of known standard enrichment values against the measured values to assess the linearity of the mass spectrometer and to account for any isotope fraction which may have occurred during the analysis.
To determine basal mixed muscle fractional synthetic rate (FSR), the single biopsy approach was applied as described by Burd et al. 2012 (40). In short, plasma protein obtained before tracer infusion (t = −180 min) was used to determine baseline l-[ring-13C6]-phenylalanine enrichments. For this purpose, the plasma sample was precipitated by adding perchloric acid. Subsequently, similarly as for the mixed muscle protein fraction, the denaturized plasma protein pellet was hydrolyzed, passed over a Dowex exchange resin, and the resulting AA samples were derivatized to their N(O,S)-ethoxycarbonyl-ethylesters before being measured by GC-IRMS, as explained below.
Plasma AA concentrations were determined by ultraperformance liquid chromatography–mass spectrometry (UPLC-MS; ACQUITY UPLC H-Class with QDa, Waters). In particular, 50 μL blood plasma was deproteinized using 100 μL of 10% SSA with 50 μM of MSK-A2 internal standard (Cambridge Isotope Laboratories, Tewksbury, MA). Subsequently, 50 μL of ultrapure demineralized water was added, and samples were centrifuged. After centrifugation, 10 μL of supernatant was added to 70 μL of Borate reaction buffer (Waters). In addition, 20 μL of AccQ/Tag derivatizing reagent solution (Waters) was added after which the solution was heated to 55°C for 10 min. Of this, 100 μL derivative 1 μL was injected and measured using UPLC-MS.
Muscle analysis
A piece of wet muscle (~50–70 mg) was freeze dried for 48 h. Collagen, excessive blood, and other nonmuscle materials were subsequently removed from the muscle fibers under a light microscope. The isolated muscle fiber mass was weighed, and 35 volumes (7× wet weight of isolated muscle fibers × wet-to-dry ratio 5:1) of ice-cold 2% perchloric acid was added. Thereafter, the tissue was homogenized by sonification and centrifuged to separate the supernatant from the protein pellet. The supernatants containing the muscle tissue free AA were purified and derivatized before analysis by GC-MS, similarly as for the plasma l-[ring13C6]-phenylalanine enrichments. The protein pellet was washed 3 times with 1 mL 2% perchloric acid. The AA were liberated from the mixed muscle enriched protein fraction by adding 2 mL of 6 M HCl and heating to 110°C for 15.5 h. The hydrolyzed mixed muscle protein fractions were dried under a nitrogen stream while heated to 110°C. The dried mixed muscle protein fraction was dissolved in a 50% acetic acid solution. The AA concentrations from the mixed muscle protein fraction were passed over a Dowex exchange resin (AG 50 W-X8, 100–200 mesh hydrogen form, Bio-Rad Laboratories) using 2 M NH4OH. Subsequently, the purified AA solution was dried under a nitrogen stream at room temperature, followed by derivatization to their N(O,S)-ethoxycarbonyl-ethylesters. The ratio of 13C/12C of mixed muscle protein-bound phenylalanine was determined using gas chromatography–combustion–isotope ratio mass spectrometry (GC-IRMS; Delta V; Thermo Scientific, Bremen, Germany) by monitoring ion masses 44, 45, and 46. Standard regression curves were applied from a series of known standard enrichment values against the measured values to assess the linearity of the mass spectrometer and to account for any isotope fractionation, which may have occurred during the analysis.
Calculations
The FSR (%·h−1) of mixed muscle protein enriched fractions was calculated by the standard precursor–product equation (41):
where Eb is the increment in mixed muscle protein-bound l-[ring-13C6]-phenylalanine enrichment (mole % excess) during the tracer incorporation period, and t is the tracer incorporation time in hours. Weighted mean plasma enrichments were calculated by taking the measured enrichment between consecutive time points and correcting for the time between these sampling time points (Eprecursor). For calculation for postprandial FSR, biopsy samples at t = 0, 120 and 300 min were used. For the calculation of basal FSR, Eb2 represented the protein-bound l-[ring-13C6]-phenylalanine enrichments in the muscle of the rested leg at t = 0 min, and Eb1 represented the protein-bound l-[ring-13C6]-phenylalanine enrichments in plasma albumin at t = −180 min.
Net incremental area under the curve (iAUC) was determined for plasma AA concentrations during the 5-h postprandial period after protein ingestion. The iAUC was calculated using the trapezoid rule, with plasma concentrations before beverage ingestion (t = 0 min) serving as baseline.
Outcome measures
The primary outcome measure is mixed muscle FSR over the aggregate (i.e., 0–300 min) postprandial period, comparing POTATO versus MILK in the rested and exercised leg. Secondary outcome measures were mixed muscle FSR changes from basal (i.e., −180 to 0 min and 0 to 300 min) and changes from basal to the early and late postprandial period (i.e., −180 to 0 min, 0 to 120 min, and 120 to 300 min), comparing POTATO versus MILK in the rested and exercised leg. Additional secondary outcome measures were plasma glucose, insulin and AA concentrations, and plasma AA iAUC. Plasma glucose, insulin, and AA peak concentrations and time to peak were tertiary outcomes.
Statistical analysis
A power calculation was performed with differences in postprandial muscle FSR between the two interventional groups as primary outcome measure. A sample size of 12 participants per treatment, including a 10% dropout rate, was calculated using a power of 80%, a significance level of 0.05, an SD of 0.0065%·h−1, and a difference in FSR of 0.008%·h−1 between treatments (or ~20% when expressed as a relative difference). Participants’ characteristics were analyzed by an independent-samples t-test. Plasma glucose, insulin, and AA concentrations and AA enrichments were analyzed by a two-factor (treatment–time) repeated-measures ANOVA. Plasma AA iAUC as well as plasma glucose, insulin, and AA peak concentrations and time to peak were analyzed by an independent-samples t-test. Basal postabsorptive mixed muscle protein synthesis rates for the rested leg were analyzed by an independent-samples t-test. Similarly, postprandial mixed muscle protein synthesis rates during the early (0–120 min) and the entire (0–300 min) postprandial period were analyzed by independent-samples t-test for MILK versus POTATO in the rested leg as well as exercised leg. Changes in muscle protein synthesis rates over time (−180 to 0, 0 to 120, and 120 to 300) were analyzed by a two-factor repeated-measures ANOVA. For the repeated-measures ANOVA tests, Bonferroni post hoc analysis were performed whenever a significant F-ratio was found to isolate specific differences. Statistical analyses were performed with a software package (IBM SPSS statistics for Windows, version 26.0, IBM Corp., Armonk, NY, USA). Mean values were considered to be significantly different for P values <0.05.
RESULTS
Plasma glucose and insulin concentrations
No significant changes in plasma glucose concentrations were observed after protein ingestion, with no differences between interventions (time–treatment, P = 0.12; Fig. 2A). Plasma insulin concentrations increased after MILK but not after POTATO ingestion (time–treatment, P < 0.001), with a modest peak value of 26 ± 12 mU·L−1 achieved 30 min after MILK ingestion (Fig. 2B).
FIGURE 2.
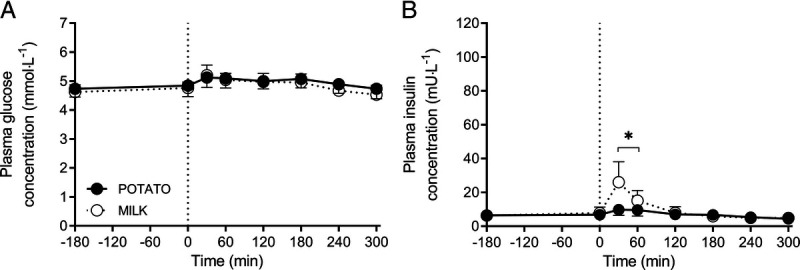
Postprandial plasma glucose (A) and insulin (B) concentrations during the 300-min period after the ingestion of POTATO vs MILK in 24 healthy young males (n = 12 per group). Time 0 min represents time of beverage intake. POTATO: 30 g potato-derived protein; MILK: 30 g milk protein. Values represent mean ± SD; repeated-measures ANOVA with time as within-subject variable and interventional drink (treatment) as between-subject variable. Time–treatment, P = 0.12 (A); P < 0.001 (B).
Plasma AA concentrations
Plasma EAA concentrations increased after protein ingestion (Fig. 3A), with a delayed and smaller postprandial rise after POTATO compared with MILK ingestion (time–treatment, P < 0.001). Overall, plasma EAA concentrations were 16% lower after POTATO versus MILK protein ingestion (iAUC: 108 ± 20 vs 129 ± 29 mmol per 300 min·L−1, respectively, P = 0.04; Fig. 3B). The lower postprandial EAA availability was also accompanied by 22% lower peak EAA concentrations (1402 ± 118 vs 1788 ± 250 μmol·L−1, respectively, P < 0.001) that were reached 143 ± 54 and 48 ± 27 min after POTATO versus MILK ingestion (P < 0.001).
FIGURE 3.
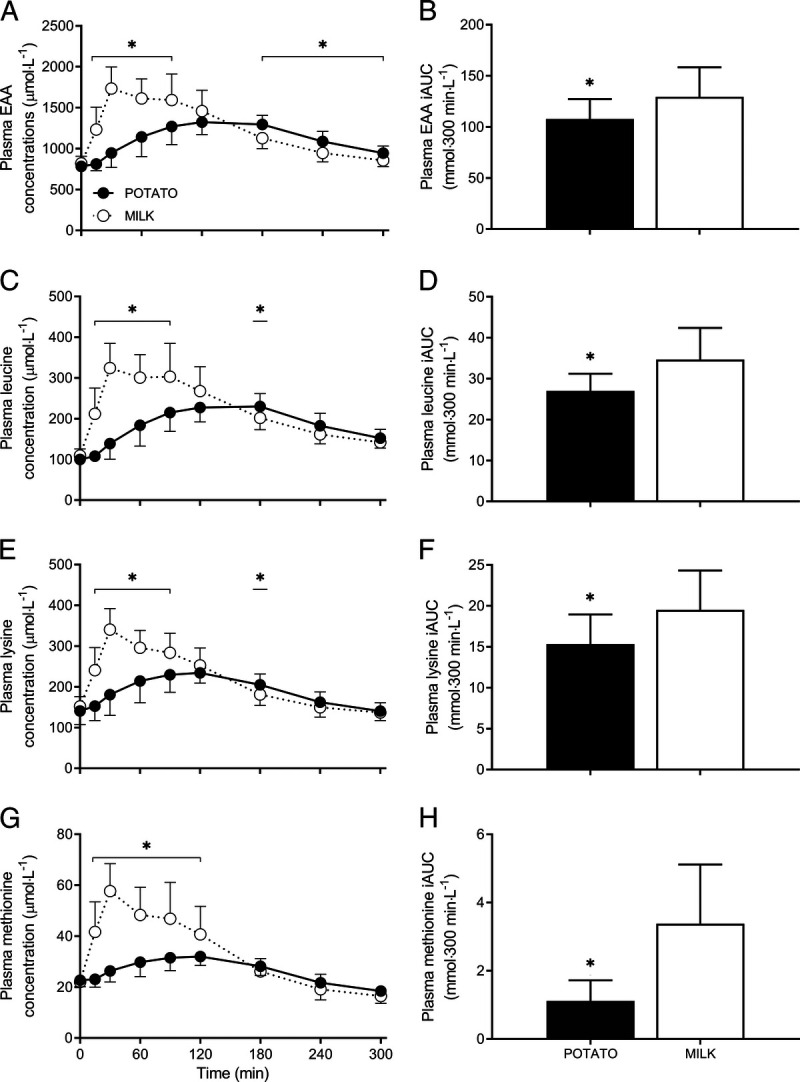
Postprandial plasma EAA (EAA, A), leucine (C), lysine (E), and methionine (G) concentrations during the 300-min postprandial period after the ingestion of POTATO vs MILK. Time 0 min represents time of beverage intake. Panels B, D, F, and H represent the 0- to 5-h iAUC after protein ingestion. POTATO: 30 g potato-derived protein; MILK: 30 g milk protein. Values represent mean ± SD; *Significantly different for POTATO vs MILK (P < 0.05). Repeated-measures ANOVA with time as within-subject variable and interventional drink (treatment) as between-subject variable. Time–treatment, P < 0.001 (A, C, E, and G).
The postprandial rise in circulating plasma leucine (Fig. 3C), lysine (Fig. 3E), and methionine (Fig. 3G) concentrations was delayed and smaller after POTATO when compared with MILK ingestion (time–treatment, all P < 0.001). Postprandial plasma leucine (Fig. 3D), lysine (Fig. 3F), and methionine (Fig. 3H) availability were respectively 23%, 21%, and 67% lower for POTATO when compared with MILK (iAUC: 27 ± 4 vs 35 ± 8, 15 ± 4 vs 19 ± 5, and 1 ± 1 vs 3 ± 2 mmol per 300 min·L−1, respectively; all P < 0.05). Peak values were also respectively 26%, 29%, and 41% lower for POTATO versus MILK (252 ± 23 vs 341 ± 65, 247 ± 34 vs 347 ± 43, and 34 ± 3 vs 58 ± 11 μmol·L−1, respectively; all P < 0.001). Time to reach peak values was significantly longer for POTATO when compared with MILK ingestion (153 ± 50 vs 48 ± 27, 100 ± 35 vs 40 ± 20, and 103 ± 41 vs 40 ± 23 min, respectively; all P < 0.001).
In general, all postprandial plasma AA concentrations revealed similar differences between treatments (see Supplemental Fig. 2, Supplemental Digital Content, Post-prandial plasma amino concentrations, http://links.lww.com/MSS/C572; time–treatment, all P < 0.05). The overall proline and valine concentrations (iAUC) were lower for POTATO versus MILK, whereas the overall glycine concentrations were higher for POTATO versus MILK (Supplemental Fig. 2, Supplemental Digital Content, http://links.lww.com/MSS/C572). Collectively, when evaluating the total sum of all AA (TAA), the postprandial increase over time differed significantly between protein sources (time–treatment, P < 0.001), with a trend toward overall lower plasma AA availability after POTATO versus MILK ingestion (iAUC: 115 ± 43 vs 147 ± 47 mmol per 300 min·L−1, respectively; P = 0.095). In line, peak TAA concentrations were 37% lower for POTATO versus MILK (2884 ± 230 vs 3626 ± 440 μmol·L−1, respectively; P < 0.001) and were reached 118 ± 56 and 43 ± 24 min after protein ingestion, respectively (P < 0.001).
Plasma and muscle l-[ring-13C6]-phenylalanine enrichments
Plasma phenylalanine concentrations and l-[ring-13C6]-phenylalanine enrichments over time are presented in Figures 4A and 4B, respectively. Plasma l-[ring-13C6]-phenylalanine enrichments over time were higher during the first 60 min and lower during the last 150 min after POTATO versus MILK ingestion (time–treatment, P < 0.001). Weighted mean plasma l-[ring-13C6]-phenylalanine enrichments averaged 6.63 ± 0.46 and 6.75 ± 0.55 MPE during the basal postabsorptive period (P = 0.55) and 6.26 ± 0.41 and 6.59 ± 0.48 MPE during the postprandial period (P = 0.09) for POTATO and MILK, respectively.
FIGURE 4.
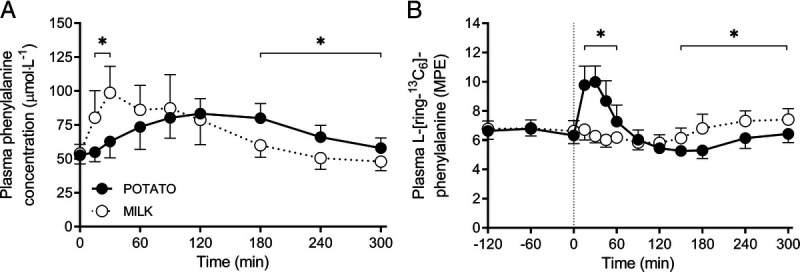
Postprandial plasma phenylalanine concentrations (A) and plasma l-[ring-13C6]-phenylalanine enrichments (B) during the 300-min period after the ingestion of POTATO vs MILK in healthy, young males (n = 12 per group). Time 0 min represents time of protein ingestion. POTATO: 30 g potato protein; MILK: 30 g milk protein. Values represent mean ± SD. *Significantly different for MILK vs WHEAT (P < 0.05). Repeated-measures ANOVA with time as within-subject variable and interventional drink (treatment) as between-subject variable. Time–treatment, P < 0.001 (A), P < 0.001 (B).
In the rested leg, mixed muscle protein-bound l-[ring-13C6]-phenylalanine enrichments increased after the ingestion of POTATO and MILK from 0.0046 ± 0.0028 and 0.0046 ± 0.0029 MPE (at t = 0 min, P = 0.99) to 0.0135 ± 0.0058 and 0.0124 ± 0.0041 MPE (at t = 120 min, P = 0.61), reaching 0.0230 ± 0.0076 and 0.0220 ± 0.0052 MPE, respectively, at 300 min after protein ingestion (at t = 300 min, P = 0.72; time–treatment, P = 0.70).
In the exercised leg, mixed muscle protein-bound l-[ring-13C6]-phenylalanine enrichments increased after POTATO and MILK ingestion from 0.0050 ± 0.0036 and 0.0044 ± 0.0026 MPE (at t = 0 min, P = 0.68) to 0.0156 ± 0.0063 and 0.0128 ± 0.0035 MPE (at t = 120 min, P = 0.20), reaching 0.0280 ± 0.0096 and 0.0262 ± 0.0049 MPE, respectively, at 300 min after protein ingestion (at t = 300 min, P = 0.56; time–treatment, P = 0.50). Collectively, 300 min after protein ingestion, the mixed muscle protein-bound l-[ring-13C6]-phenylalanine enrichments were higher in the exercised compared with the rested leg (both treatments; P < 0.05).
Muscle protein synthesis rates
In the rested leg, postabsorptive fractional mixed muscle protein synthesis rates averaged 0.020% ± 0.011% and 0.021% ± 0.014%·h−1 in the POTATO and MILK trial, respectively, with no differences between groups (P = 0.88; Fig. 5). POTATO and MILK ingestion both strongly increased mixed muscle protein synthesis rates (main effect of time P < 0.001), with no time–treatment interaction (P = 0.52) from the basal postabsorptive to 5 h postprandial period. No differences in postprandial mixed muscle protein synthesis rates were observed between POTATO and MILK ingestion during the early (e.g., 0–120 min; 0.051% ± 0.019% and 0.055% ± 0.017%·h−1, respectively; P = 0.55), late (e.g., 120–300 min; 0.055% ± 0.023% and 0.046% ± 0.017%·h−1, respectively; P = 0.33), or entire postprandial period (e.g., 0–300 min; 0.053% ± 0.017% and 0.050% ± 0.012%·h−1, respectively; P = 0.54).
FIGURE 5.
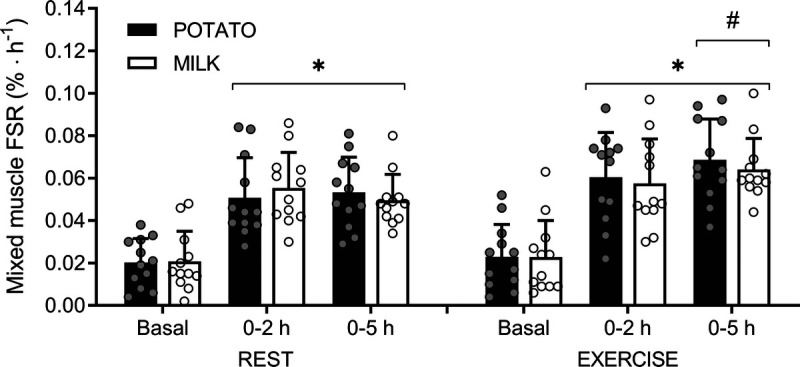
Mixed muscle FSR in the basal postabsorptive and postprandial period after the ingestion of POTATO vs MILK in the rested and exercised leg. POTATO: 30 g potato-derived protein; MILK: 30 g milk protein; REST: rested leg; EXERCISE: exercised leg. Values represent mean ± SD. *Significantly different from basal, P < 0.05. #Significantly different from rested leg, P < 0.05. Independent-samples t-test POTATO vs MILK: REST: P = 0.88, P = 0.55, and P = 0.54 for basal, 0–120, and 0–300, respectively. EXERCISE: P = 0.97, P = 0.73, and P = 0.52 for basal, 0–120, and 0–300, respectively.
In the exercised leg, postabsorptive mixed muscle protein synthesis rates averaged 0.023% ± 0.015% and 0.023% ± 0.017%·h−1 for POTATO and MILK, respectively, with no differences between groups (P = 0.97; Fig. 5). POTATO and MILK ingestion both strongly increased mixed muscle protein synthesis rates after exercise (main effect of time P < 0.001), with no time–treatment interaction (P = 0.58) from the basal postabsorptive to 5 h postprandial period. No differences in postprandial muscle protein synthesis rates were observed after POTATO and MILK ingestion during the early (0.060% ± 0.021% and 0.058% ± 0.021%·h−1, respectively; P = 0.74), late (0.071% ± 0.031% and 0.065% ± 0.021%·h−1, respectively; P = 0.25), and entire postprandial period (0.069% ± 0.019% and 0.064% ± 0.015%·h−1, respectively; P = 0.52). Postprandial muscle protein synthesis rates over the 5-h period after exercise were significantly higher in the exercised versus rested leg, for both treatments (P < 0.05).
Mixed muscle protein synthesis rates determined with the intracellular l-[ring-13C6]-phenylalanine enrichments used as precursor pool (see Supplemental Fig. 3, Supplemental Digital Content, Muscle intracellular l-[ring-13C6] phenylalanine enrichments, http://links.lww.com/MSS/C572) resulted in similar findings with no differences between treatments (see Supplemental Fig. 4, Supplemental Digital Content, Mixed muscle fractional synthetic rate, http://links.lww.com/MSS/C572).
DISCUSSION
The present study shows that the ingestion of 30 g potato-derived protein strongly increases muscle protein synthesis rates at rest and during recovery from exercise in healthy, young males. Despite the observation of a lesser and more delayed postprandial rise in plasma EAA availability after potato when compared with milk protein ingestion, postprandial mixed muscle protein synthesis rates did not differ between protein sources at rest or during recovery from exercise.
The anabolic properties of plant-derived proteins are generally considered to be lower when compared with animal-derived proteins (19,20). This has been, at least partly, attributed to plant-derived proteins providing overall less EAA and the prevalence of one or more specific AA deficiencies in these proteins (19,20). In contrast to many plant-derived proteins (28), we observed that potato-derived protein provides sufficient amounts of all EAA according to the WHO/FAO guidelines for protein requirements. In fact, 30 g of the applied potato-derived protein was shown to provide similar amounts of EAA (10.5 vs 10.7 g), leucine (2.6 vs 2.6 g), lysine (1.8 vs 2.1 g), and methionine (0.6 vs 0.6 g) when compared with the equivalent amount of milk protein (Table 2). Despite similar AA composition, the postprandial rise in circulating (essential) AA was attenuated after the ingestion of potato compared with milk protein (Fig. 3), resulting in lower peak EAA, leucine, lysine, and methionine concentrations (−22%, −26%, −29%, and −41%) that were reached at a much later point in time (+200, +221, +150, and +156 min, respectively). Consequently, postprandial plasma AA availability was substantially lower throughout the 5-h postprandial period after potato ingestion when compared with milk protein ingestion (Fig. 3). Based on the phenylalanine tracer kinetics (Fig. 4), we attribute this to a more delayed protein digestion and AA absorption, an increased AA retention in splanchnic tissues, and/or a less efficient digestion of potato compared with milk protein. As the intrinsically labeled protein approach (42) simply cannot be applied in the case of plant-derived proteins, it is impossible to directly quantify the exact amount of potato protein-derived AA that were released in the circulation, as we have done previously for milk- (2) and mealworm-derived protein (43).
Despite the attenuated postprandial rise in circulating AA after the ingestion of potato-derived protein, we observed a strong increase in muscle protein synthesis rates (Fig. 5). A response that did not differ from the response observed after ingesting an equivalent amount of milk protein (Fig. 5). Clearly, the provided potato-derived protein is capable of strongly stimulating muscle protein synthesis in vivo in humans. Whether the absence of any differences in the anabolic response to potato versus milk protein ingestion can be attributed to the favorable AA profile of potato protein when compared with other plant-derived proteins remains unclear, as previous work (26,27) but certainly not all studies (15,23–25) have reported no differences in the anabolic response to the ingestion of similar amounts of plant- versus animal-derived protein. Obviously, the observed postprandial rise in circulating AA after the ingestion of 30 g potato protein concentrate was sufficient to elevate muscle protein synthesis rates. The more sustained release of AA throughout the latter stages of the postprandial period may have compensated for a potential lesser initial increase in postprandial plasma AA availability, allowing a postprandial muscle protein synthetic response that did not differ from the ingestion of 30 g of milk protein. However, our data did not show an early attenuated postprandial increase in muscle protein synthesis rates after potato protein when compared with milk protein ingestion, with FSR values calculated using plasma (Fig. 5) and tissue free enrichments (Supplemental Fig. 3, Supplemental Digital Content, http://links.lww.com/MSS/C572) as precursor pools. As there was some initial disbalance between l-[ring-13C6]-phenylalanine release and overall phenylalanine kinetics (Fig. 4B), we cannot exclude that this may have caused a minor overestimation of the early postprandial FSR in the potato group.
Exercise has previously been shown to sensitize skeletal muscle tissue to the anabolic properties of protein ingestion (44). In the current study, we applied a unilateral exercise design to allow assessment of postprandial muscle protein synthesis rates both at rest as well as during recovery from exercise. We observed a strong increase in muscle protein synthesis rates in the exercised leg after both potato ingestion as well as milk protein ingestion (Fig. 5), with responses that were greater when compared with the rested leg. Again, no differences were observed in postexercise muscle protein synthesis rates after the ingestion of 30 g potato versus 30 g milk protein. These data imply that plant-derived protein concentrates can be applied effectively to support postexercise muscle conditioning in athletes. These findings are in contrast to some (15,23,24) but certainly not all (15,26) studies comparing postexercise muscle protein synthesis rates after soy compared with dairy protein ingestion. The apparent discrepancy may be, at least partly, explained by the amount of protein provided. In the present study, we provided 30 g potato or milk protein, which is more than the amount of egg or milk protein (20 g) that has previously been suggested to be required to maximize postexercise muscle protein synthesis rates (45). Although we can only speculate on the impact of ingesting smaller amounts of potato-derived protein on postprandial muscle protein synthesis rates, our data imply that a maximal postexercise muscle protein synthetic response can be obtained by ingesting up to 30 g of a high-quality plant-derived protein concentrate.
There is an increasing interest in the consumption of food products and sports supplements containing alternative, more sustainable, sources of protein (20). The present study extends on prior work evaluating the postprandial and/or postexercise muscle protein synthetic responses after soy- and wheat-derived protein ingestion (15,23–27), showing that potato protein ingestion can strongly increase muscle protein synthesis rates at rest and during recovery from exercise. In support, Oikawa and colleagues (46) observed increases in daily muscle protein synthesis rates after more prolonged potato protein supplementation in females during a period of exercise training. Furthermore, increases in muscle mass and strength gains have been reported after both plant- as well as animal-derived protein supplementation during prolonged resistance-type exercise training (47–50). The present data clearly show that there are ample opportunities for the use of plant-derived proteins in sports nutrition, but more research will be needed to evaluate the anabolic properties of the various plant-derived proteins that are currently available and their potential blends (27).
CONCLUSIONS
In conclusion, the ingestion of 30 g potato-derived protein concentrate strongly increases muscle protein synthesis rates at rest and during recovery from exercise in vivo in healthy, young males. The postprandial muscle protein synthetic response after the ingestion of 30 g potato protein does not differ from the response after the ingestion of an equivalent amount of milk protein. Plant-derived proteins may be applied effectively in vegan protein products and sports nutrition supplements to support skeletal muscle conditioning during recovery from exercise.
Supplementary Material
Acknowledgments
The authors thank Lisanne H.P. Houben for her medical assistance, Francesco Recchia for this practical assistance, and Annemarie P. Gijssen, Wendy E. Sluijsmans, and Hasibe Aydeniz for their analytical work. They also extend their gratitude to all study participants for their time and commitment.
This study was partially funded by the Alliance for Potato Research & Education (APRE), Chicago, IL, and AVEBE, Veendam, The Netherlands.
L. J. C. vL. has received research grants, consulting fees, speaking honoraria, or a combination of these from Friesland Campina, Tereos Syral, and Pepsico. P. J. M. P., F. K. H., W. J. H. H., J. P. B. G., J. M. S., J. M. X. vK., W. K. H. W., and T. S. report no conflicts of interest. The results of the present study do not constitute endorsement by the American College of Sports Medicine. The authors declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Clinical Trial Registry number: Nederlands Trial Register: NTR7152. https://www.trialregister.nl/
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
Contributor Information
PHILIPPE J. M. PINCKAERS, Email: philippe.pinckaers@maastrichtuniversity.nl.
FLORIS K. HENDRIKS, Email: f.hendriks@maastrichtuniversity.nl.
WESLEY J.H. HERMANS, Email: w.hermans@maastrichtuniversity.nl.
JOY P.B. GOESSENS, Email: joy.goessens@maastrichtuniversity.nl.
JOAN M. SENDEN, Email: j.senden@maastrichtuniversity.nl.
JANNEAU M. X. VAN KRANENBURG, Email: j.vankranenburg@maastrichtuniversity.nl.
WILL K. H. W. WODZIG, Email: will.wodzig@mumc.nl.
TIM SNIJDERS, Email: tim.snijders@maastrichtuniversity.nl.
REFERENCES
- 1.Gorissen SH, Remond D, van Loon LJ. The muscle protein synthetic response to food ingestion. Meat Sci. 2015;109:96–100. [DOI] [PubMed] [Google Scholar]
- 2.Groen BB Horstman AM Hamer HM, et al. Post-prandial protein handling: you are what you just ate. PLoS One. 2015;10(11):e0141582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rennie MJ Edwards RH Halliday D, et al. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin Sci (Lond). 1982;63(6):519–23. [DOI] [PubMed] [Google Scholar]
- 4.Chesley A MacDougall JD Tarnopolsky MA, et al. Changes in human muscle protein synthesis after resistance exercise. J Appl Physiol (1985). 1992;73(4):1383–8. [DOI] [PubMed] [Google Scholar]
- 5.Biolo G Tipton KD Klein S, et al. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol. 1997;273(1 Pt 1):E122–9. [DOI] [PubMed] [Google Scholar]
- 6.Moore DR Tang JE Burd NA, et al. Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol. 2009;587(Pt 4):897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tipton KD Ferrando AA Phillips SM, et al. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol. 1999;276(4):E628–34. [DOI] [PubMed] [Google Scholar]
- 8.Cermak NM Res PT de Groot LC, et al. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr. 2012;96(6):1454–64. [DOI] [PubMed] [Google Scholar]
- 9.Volpi E Kobayashi H Sheffield-Moore M, et al. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78(2):250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rieu I Balage M Sornet C, et al. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiol. 2006;575(1):305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wall BT Hamer HM de Lange A, et al. Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clin Nutr. 2013;32(3):412–9. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson DJ Hossain T Hill DS, et al. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J Physiol. 2013;591(11):2911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burd NA Yang Y Moore DR, et al. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. Micellar casein at rest and after resistance exercise in elderly men. Br J Nutr. 2012;108(6):958–62. [DOI] [PubMed] [Google Scholar]
- 14.Pennings B Boirie Y Senden JM, et al. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr. 2011;93(5):997–1005. [DOI] [PubMed] [Google Scholar]
- 15.Tang JE Moore DR Kujbida GW, et al. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol (1985). 2009;107(3):987–92. [DOI] [PubMed] [Google Scholar]
- 16.Boirie Y Dangin M Gachon P, et al. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci U S A. 1997;94(26):14930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koopman R Walrand S Beelen M, et al. Dietary protein digestion and absorption rates and the subsequent postprandial muscle protein synthetic response do not differ between young and elderly men. J Nutr. 2009;139(9):1707–13. [DOI] [PubMed] [Google Scholar]
- 18.Tang JE, Phillips SM. Maximizing muscle protein anabolism: the role of protein quality. Curr Opin Clin Nutr Metab Care. 2009;12(1):66–71. [DOI] [PubMed] [Google Scholar]
- 19.Gorissen SHM, Witard OC. Characterising the muscle anabolic potential of dairy, meat and plant-based protein sources in older adults. Proc Nutr Soc. 2018;77(1):20–31. [DOI] [PubMed] [Google Scholar]
- 20.van Vliet S, Burd NA, van Loon LJ. The skeletal muscle anabolic response to plant- versus animal-based protein consumption. J Nutr. 2015;145(9):1981–91. [DOI] [PubMed] [Google Scholar]
- 21.Medawar E Huhn S Villringer A, et al. The effects of plant-based diets on the body and the brain: a systematic review. Transl Psychiatry. 2019;9(1):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips SM, Tang JE, Moore DR. The role of milk- and soy-based protein in support of muscle protein synthesis and muscle protein accretion in young and elderly persons. J Am Coll Nutr. 2009;28(4):343–54. [DOI] [PubMed] [Google Scholar]
- 23.Wilkinson SB Tarnopolsky MA Macdonald MJ, et al. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr. 2007;85(4):1031–40. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y Churchward-Venne TA Burd NA, et al. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr Metab (Lond). 2012;9(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorissen SH Horstman AM Franssen R, et al. Ingestion of wheat protein increases in vivo muscle protein synthesis rates in healthy older men in a randomized trial. J Nutr. 2016;146(9):1651–9. [DOI] [PubMed] [Google Scholar]
- 26.Churchward-Venne TA Pinckaers PJM Smeets JSJ, et al. Myofibrillar and mitochondrial protein synthesis rates do not differ in young men following the ingestion of carbohydrate with whey, soy, or leucine-enriched soy protein after concurrent resistance- and endurance-type exercise. J Nutr. 2019;149(2):210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinckaers PJM Kouw IWK Hendriks FK, et al. No differences in muscle protein synthesis rates following ingestion of wheat protein, milk protein, and their protein blend in healthy, young males. Br J Nutr. 2021;126(12):1832–42. [DOI] [PubMed] [Google Scholar]
- 28.Gorissen SHM Crombag JJR Senden JMG, et al. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids. 2018;50(12):1685–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devaux A, Kromann P, Ortiz O. Potatoes for sustainable global food security. Potato Res. 2014;57(3–4):185–99. [Google Scholar]
- 30.Camire ME, Kubow S, Donnelly DJ. Potatoes and human health. Crit Rev Food Sci Nutr. 2009;49(10):823–40. [DOI] [PubMed] [Google Scholar]
- 31.Laus MC, Klip G, Giuseppin ML. Improved extraction and sample cleanup of tri-glycoalkaloids α-solanine and α-chaconine in non-denatured potato protein isolates. Food Anal Methods. 2017;10(4):845–53. [Google Scholar]
- 32.Mayhew JL Prinster J Ware J, et al. Muscular endurance repetitions to predict bench press strength in men of different training levels. J Sports Med Phys Fitness. 1995;35(2):108–13. [PubMed] [Google Scholar]
- 33.Verdijk LB van Loon L Meijer K, et al. One-repetition maximum strength test represents a valid means to assess leg strength in vivo in humans. J Sports Sci. 2009;27(1):59–68. [DOI] [PubMed] [Google Scholar]
- 34.Kraemer W Fry A Maud P, et al. In: Maud P, Foster C, editors. Physiological Assessment of Human Fitness. Strength Testing: Development and Evaluation Methodology. Champaign (IL): Human Kinetics; 1995. pp. 115–37. [Google Scholar]
- 35.Abumrad NN Rabin D Diamond MP, et al. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism. 1981;30(9):936–40. [DOI] [PubMed] [Google Scholar]
- 36.Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35(7):609–16. [PubMed] [Google Scholar]
- 37.Jones DB. Factors for Converting Percentages of Nitrogen in Foods and Feeds into Percentages of Protein. Washington (DC): US Department of Agriculture; 1941. [Google Scholar]
- 38.Mariotti F, Tome D, Mirand PP. Converting nitrogen into protein—beyond 6.25 and Jones’ factors. Crit Rev Food Sci Nutr. 2008;48(2):177–84. [DOI] [PubMed] [Google Scholar]
- 39.Van Gelder W. Conversion factor from nitrogen to protein for potato tuber protein. Potato Res. 1981;24(4):423. [Google Scholar]
- 40.Burd NA Pennings B Groen BB, et al. The single biopsy approach is reliable for the measurement of muscle protein synthesis rates in vivo in older men. J Appl Physiol (1985). 2012;113(6):896–902. [DOI] [PubMed] [Google Scholar]
- 41.Schierbeek H. Mass Spectrometry and Stable Isotopes in Nutritional and Pediatric Research. New Jersey: John Wiley & Sons, Inc; 2017. pp. 56–61. [Google Scholar]
- 42.Trommelen J Holwerda AM Pinckaers PJM, et al. Comprehensive assessment of post-prandial protein handling by the application of intrinsically labelled protein in vivo in human subjects. Proc Nutr Soc. 2021;80(2):221–9. [DOI] [PubMed] [Google Scholar]
- 43.Hermans WJH Senden JM Churchward-Venne TA, et al. Insects are a viable protein source for human consumption: from insect protein digestion to postprandial muscle protein synthesis in vivo in humans: a double-blind randomized trial. Am J Clin Nutr. 2021;114(3):934–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biolo G Maggi SP Williams BD, et al. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol. 1995;268(3 Pt 1):E514–20. [DOI] [PubMed] [Google Scholar]
- 45.Moore DR Robinson MJ Fry JL, et al. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr. 2009;89(1):161–8. [DOI] [PubMed] [Google Scholar]
- 46.Oikawa SY Bahniwal R Holloway TM, et al. Potato protein isolate stimulates muscle protein synthesis at rest and with resistance exercise in young women. Nutrients. 2020;12(5):1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Volek JS Volk BM Gómez AL, et al. Whey protein supplementation during resistance training augments lean body mass. J Am Coll Nutr. 2013;32(2):122–35. [DOI] [PubMed] [Google Scholar]
- 48.Babault N Païzis C Deley G, et al. Pea proteins oral supplementation promotes muscle thickness gains during resistance training: a double-blind, randomized, Placebo-controlled clinical trial vs. whey protein. J Int Soc Sports Nutr. 2015;12(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joy JM Lowery RP Wilson JM, et al. The effects of 8 weeks of whey or rice protein supplementation on body composition and exercise performance. Nutr J. 2013;12(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lynch HM Buman MP Dickinson JM, et al. No significant differences in muscle growth and strength development when consuming soy and whey protein supplements matched for leucine following a 12 week resistance training program in men and women: a randomized trial. Int J Environ Res Public Health. 2020;17(11):3871. [DOI] [PMC free article] [PubMed] [Google Scholar]


