Abstract
Objective
We aimed to clarify the clinical features and surgical outcomes of descending necrotizing mediastinitis (DNM) to provide a guide for its surgical treatment, focusing on the type of extension and the deployed procedures.
Methods
As a joint study of the Japan Broncho-esophagological Society and the Japanese Association for Chest Surgery (JBES1703/JACS1806 study), the clinical data of consecutive patients with DNM who underwent surgical drainage between 2012 and 2016 were collected from 131 participating institutions. The infection limited to the area superior to the carina level was defined as type I; while spreading to the lower mediastinum (LM) as type II. The LM infection limited to the anterior LM, that spread to both the anterior and posterior LM and that limited the posterior LM (type IIC) were further categorized as type IIA, IIB, and IIC, respectively.
Results
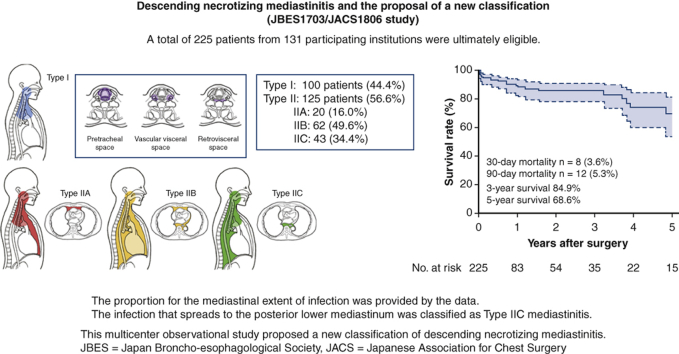
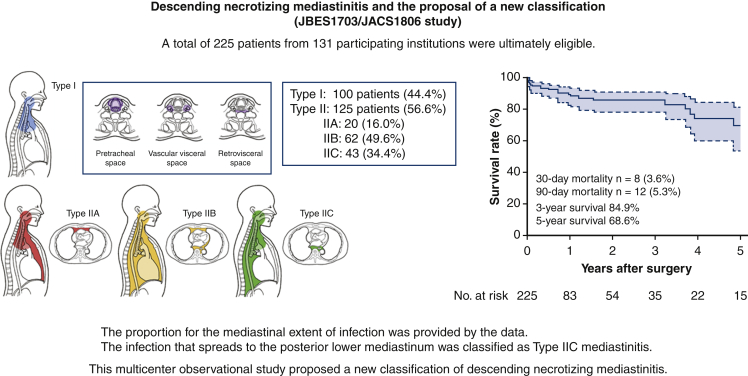
A total of 225 patients were ultimately eligible. One hundred patients (44.4%) were categorized as type I, whereas 125 patients were type II (56.6%); The number of type IIA, IIB, and IIC cases was 20 (16%), 62 (49.6%) and 43 (34.4%), respectively. Patients with type I and IIC infections more commonly underwent cervical drainage than patients with type IIA and IIB infections (34.3% and 13.4%, respectively). A total of 8 patients died within 30 days (3.6%, type I/II: 1/7). The 5-year overall survival rate was 68.6%. Type II infection was associated with the 90-day mortality (odds ratio, 5.18; P = .045).
Conclusions
This study demonstrated a previously unclassified group of lower mediastinal extent that is localized within the posterior mediastinum (type IIC). We proposed a new DNM classification including type IIC mediastinitis.
Key Words: descending necrotizing mediastinitis, multicenter observation study, classification, infection
Abbreviation and Acronyms: CRF, case report form; DM, diabetes mellitus; DNM, descending necrotizing mediastinitis; JACS, Japanese Association for Chest Surgery; JATS, Japanese Association for Thoracic Surgery; JBES, Japan Broncho-esophagological Society; LM, lower mediastinum; VATS, video-assisted thoracic surgery
Graphical abstract
New classification of extension type for descending necrotizing mediastinitis.
Central Message.
A new classification of descending necrotizing mediastinitis was proposed on the basis of a multicenter observational study.
Perspective.
A multicenter observational study was conducted on surgically treated patients with descending necrotizing mediastinitis (DNM) in Japan. The study found a new type of disease extension and proposed a new DNM classification. The 30-day mortality and 90-day mortality were 3.6% and 5.3%, respectively.
Descending necrotizing mediastinitis (DNM) is an acute, life-threating infection of both the neck and mediastinum. It originates from an oropharyngeal or cervical infection and advances into the mediastinal spaces.1 The mediastinal abscess often spreads aggressively into the mediastinal connective tissues, and patients easily develop a severe general condition with sepsis. Emergent surgical treatment including wide drainage is necessary for patients to prevent a critical state; however, the reported surgical outcomes in the past have been dismal, with a mortality rate of 15.5% to 35%.2, 3, 4, 5 The Japanese Association for Thoracic Surgery (JATS) reported that 100 cases underwent surgery in 2016, and hospital mortality was seen in 14 cases (14%).6
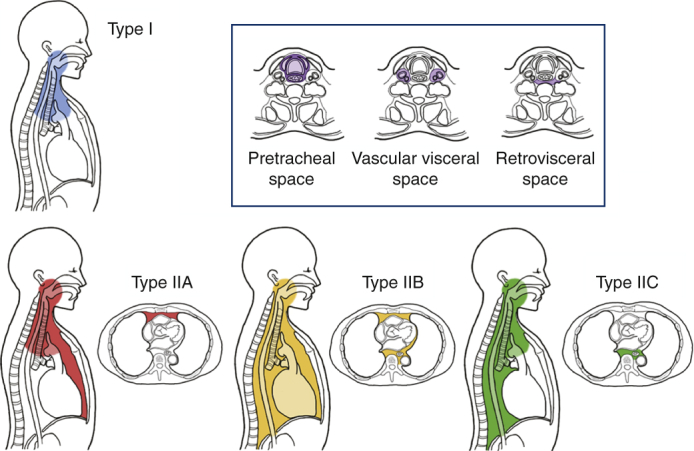
Estrera and colleagues7 proposed the following diagnostic criteria for DNM in 1983: (1) clinical manifestations of severe infection; (2) demonstration of characteristic radiographic findings; (3) documentation of necrotizing mediastinal infection at operation; and (4) establishment of the relationship of oropharyngeal or cervical infection with the development of the necrotizing mediastinal process. Generally, odontogenic, pharyngeal, or cervical infections cause cervical necrotizing fasciitis, extending to loose cervical spaces that are bordered by deep cervical fascia. Three potential spaces—pretracheal space, vascular visceral space, and retrovisceral/prevertebral space—are considered to serve as descending infection routes to the thorax.8,9 Regarding the mediastinal infection route, Endo and colleagues10 proposed a disease classification according to the degree of mediastinal extent: type I, infection localized to the upper mediastinum above the carina; type IIA, infection extending to the lower anterior mediastinum; and type IIB, infection extending to both the anterior and posterior mediastinum. Thus far, this classification has been practically used.
DNM is a relatively rare disease in practice, so individual medical institutions are typically inexperienced with treating DNM.11 Therefore, no large case series has been presented thus far.12 Some studies consisting of several dozen cases have reported different surgical approaches, and the extent of surgery and treatment outcomes have varied.13, 14, 15, 16 Furthermore, the relationship between the cervical infection and mediastinal infection remains unclear due to the limited literature.
Given this, we conducted a multicenter study including institutions for head and neck surgery and thoracic surgery as a joint study of the Japan Broncho-esophagological Society (JBES) and the Japanese Association for Chest Surgery (JACS). Based on this database, we explored the type of extension from the neck to the mediastinum and suggested a new classification of the disease (Video 1).
Methods
Eligibility Criteria of the Patients
This study was designed as a multicenter retrospective study of the JBES and JACS in which 131 institutions participated (JBES1703/JACS1806 study). The participating institutions are listed at the Appendix 1. Consecutive patients who underwent surgical drainage for DNM at the participating hospitals between January 2012 and December 2016 were collected. The data collection was opened on May 1, 2018, and closed on October 31, 2018.
DNM was defined by the criteria of Estrera and colleagues,7 where computed tomography was used to identify the spread of infection; (1) clinical evidence of severe oropharyngeal infection, (2) characteristic roentgenographic features of mediastinitis, (3) documentation of necrotizing mediastinal infection at operation, and (4) establishment of the relationship of oropharyngeal or cervical infection with the development of the necrotizing mediastinal process. Treatment for DNM was selected and performed according to the physicians' preference in each institute. Patients who opted out of providing their clinical data were excluded from the study. The institutional review board of each institution approved the study protocol with a waiver of informed patient consent. This study is registered in the UMIN Clinical Trial Database (Study ID: UMIN000035328; October 13, 2017 [#1310]).
Data Collection
Participating institutions registered each eligible patient using their case report form (CRF) as an electronic file. The items collected through the CRF were as follows: (1) patient's physical status (age, sex, height, weight, comorbidities, performance status); (2) clinical data at the time of the diagnosis (cause and initial site of the infection, date of the initial assessment for the disease, date of the DNM diagnosis, type of the radiological examination at the diagnosis, initial patient's symptoms, cervical and mediastinal route of the infection, extent of the infection spread in the mediastinum [Endo type], existence of pleural effusion, existence of empyema). The infection limited to the area superior to the carina level was defined as type I, whereas spreading to the lower mediastinum (LM) was defined as type II according to the Endo's classification. The LM infection limited to the anterior LM, that spread to both the anterior and posterior LM and that limited the posterior LM were further categorized as type IIA, IIB, and IIC, respectively (Figure E1); (3) clinical information on the operation (date of the drainage, operation time, estimated blood loss, approach for the drainage, the number of drainage tube, presence of combined tracheostomy, detected microbes); (4) clinical course after the surgery (duration of mechanical ventilation, dwelling time of the drainage tube, presence and date of repeat surgeries, used antibiotics during the course); and (5) treatment outcomes (morbidity, hospitalization period, mortality, date of death, cause of death, latest presence of the patients, residual disabilities).
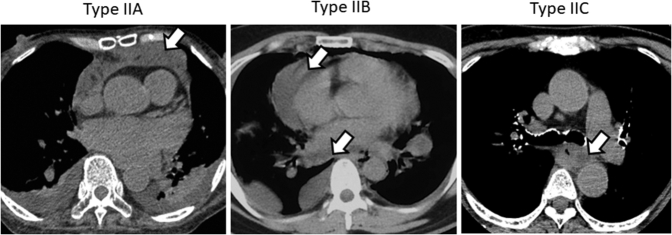
Figure E1.
Computed tomography imaging shows type II mediastinitis. Open arrows indicate abscess in the mediastinum.
Statistical Analyses
The primary end point of the study was 30-day mortality. Secondary end points of the study were 90-day mortality and overall survival. Survival curves were estimated using the Kaplan–Meier method. The overall survival was defined as the time between the operation and death from any cause.
The statistical significance of the factors was evaluated using the Pearson χ2 test or Fisher exact tests. A multivariate analysis by a Cox proportional hazards model was used to test the significance of the prognostic factors. All statistical analyses were performed using JMP Statistical Discovery software program (version 11.0; SAS Institute, Cary, NC).
Results
Patients' Physical Status and Symptoms on Admission
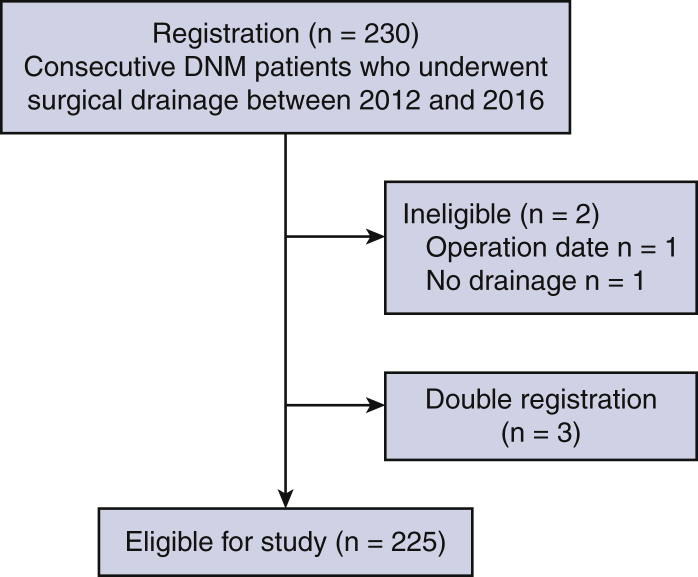
Two hundred thirty patients were registered from the participating institutions. One patient who underwent surgery after the period and another who received no surgical drainage were excluded. After we excluded 3 double-registered patients, the data of a total of 225 patients were finally eligible for the study (Figure 1).
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram for the study. A total of 230 patients were registered from the 131 participating institutions. The data of a total of 225 patients were finally eligible for the study. DNM, Descending necrotizing mediastinitis.
The clinical background characteristics of these 225 patients on admission are described in Table 1. There were more male patients than female patients (59.1% vs 40.9%). Diabetes mellitus (DM) was the most frequent comorbidity of the patients (28.4%), followed by hypertension (17.8%), malignant neoplasm (6.7%), and renal dysfunction (6.7%). One half of the patients showed a good performance status (PS0), whereas 9% had PS3 or PS4.
Table 1.
Clinical characteristics of patients on admission and etiology of descending necrotizing mediastinitis (DNM)
| Category | n | % |
|---|---|---|
| Age | ||
| Median, y (range) | 64 | (19-93) |
| <20 y/o | 2 | |
| 20-29 | 4 | |
| 30-39 | 7 | |
| 40-49 | 26 | |
| 50-59 | 41 | |
| 60-69 | 63 | |
| 70-79 | 57 | |
| 80-90 | 23 | |
| ≥90 | 2 | |
| Sex | ||
| Male | 133 | 59.1 |
| Female | 92 | 40.9 |
| Height average, cm (range) | 160.7 | (137-182) |
| Weight average, kg (range) | 57.9 | (30-106) |
| Comorbidity | ||
| Diabetes mellitus | 64 | 28.4 |
| Malignant neoplasm | 15 | 6.7 |
| Autoimmune disease | 7 | 3.1 |
| Steroid use | 6 | 2.7 |
| Ischemic heart disease | 8 | 3.6 |
| Cerebrovascular disease | 9 | 4.0 |
| Renal dysfunction | 15 | 6.7 |
| Hypertension | 40 | 17.8 |
| Others | 58 | 25.8 |
| Performance status | ||
| 0 | 118 | 52.4 |
| 1 | 36 | 16.0 |
| 2 | 19 | 8.4 |
| 3 | 20 | 8.9 |
| 4 | 21 | 9.3 |
| Not evaluated | 11 | 4.9 |
| Symptoms | ||
| Pain | 171 | 76.0 |
| Fever | 164 | 72.9 |
| Neck swelling | 134 | 59.6 |
| Neck redness | 81 | 36.0 |
| Dyspnea | 79 | 35.1 |
| Dysphagia | 76 | 33.8 |
| Septic status | 40 | 17.8 |
| Subcutaneous emphysema | 13 | 5.8 |
| Impaired consciousness | 11 | 4.9 |
| Palpitation | 10 | 4.4 |
| Others | 27 | 12.0 |
| Type of origin | ||
| Traumatic | 8 | 3.6 |
| Medical procedure related | 14 | 6.2 |
| Infection | 203 | 90.2 |
| Source of infection | ||
| Odontogenic | 38 | 16.9 |
| Pharyngeal | 114 | 50.7 |
| Cervical | 55 | 24.4 |
| Esophageal | 6 | 2.7 |
| Unclear or others | 12 | 5.3 |
The most frequently observed symptoms were pain (n = 171, 76%; neck, n = 140; chest, n = 14; neck + chest, n = 14; others, n = 3), followed by a fever (n = 164, 72%), neck swelling (n = 134, 59.6%), neck redness (n = 81, 36.0%), dyspnea (n = 79, 35.1%), and dysphagia (n = 76, 33.8%). A septic condition was noted in 18% (n = 40) of patients.
Etiology of DNM
A total of 90% (n = 203) of DNM cases were caused by some type of primary infection, while 6% (n = 14) were iatrogenic, and 3.6% (n = 8) were traumatic (Table 1). Abscess had mainly developed from a pharyngeal infection (n = 114, 50.7%), while other sources of abscess included other cervical infection (24.4%) and odontogenic infection (16.9%). An esophageal origin was seen in only 2.7% of patients (n = 6). The average duration between the first diagnosis of infection source and the diagnosis of DNM was 4 days (range, 0-69 days).
Microbiological examinations revealed bacterial or fungal infections in 208 patients (92.4%), listed in Table E1. Among them, 93 (41.3%) had a single-microbial infection, whereas 115 (51.1%) had polymicrobial infection. Streptococcus species were the most common species in single-microbial infections, being found in up to 41.9%. In polymicrobial infections, 78.3% of patients showed infection with Streptococcus species, an aerobic bacterium, followed by Prevotella (49.6%), an anaerobic bacterium. This indicates that a majority of polymicrobial infections were mixed aerobic and anaerobic infections.
Classification of Mediastinal Extent of Infection
As recommended previously,10 we also defined DNM limited to the area above the carina as type I and that extending into the LM as type II. Regarding extension of mediastinal infection, the infection was limited to the area superior to the carina level in 100 patients (type I, 44.4%), whereas in 125 patients, the infection had spread below the carina level into the LM (type II, 56.6%) (Table 2). Among the 125 type II cases, 20 (16%) extended to the anterior LM (type IIA), and 62 (49.6%) extended to both the anterior and posterior LM (type IIB). A total of 43 cases (34.4%) extended to the posterior LM only; this type was not mentioned in the previous classification.10 We therefore newly classified this type of spreading as type IIC (Table 2, Figure 2).
Table 2.
Regions of neck infection and extension to mediastinitis
| n | % | |
|---|---|---|
| Cervical route of infection | ||
| Pretracheal space | 111 | 49.3 |
| Vascular visceral space | 117 | 52.0 |
| Retrovisceral space | 137 | 60.9 |
| NA | 3 | 1.3 |
| Route of cervical infection: single or multiple | ||
| Single | 117 | 52.0 |
| Multiple | 105 | 46.7 |
| NA | 3 | 1.3 |
| Mediastinal extent of infection | ||
| Anterior mediastinum | 120 | 53.3 |
| Middle mediastinum | 108 | 48.0 |
| Posterior mediastinum | 112 | 49.8 |
| NA | 7 | 3.1 |
| Regions of mediastinal infection: single or multiple | ||
| Single | 126 | 56.0 |
| Multiple | 92 | 40.9 |
| NA | 7 | 3.1 |
| Level of mediastinal extent | ||
| Above the carina (type I) | 100 | 44.4 |
| Anterior lower mediastinum (type IIA) | 20 | 8.9 |
| Both the anterior and posterior lower mediastinum (type IIB) | 62 | 27.6 |
| Posterior lower mediastinum (type IIC) | 43 | 19.1 |
NA, Not available.
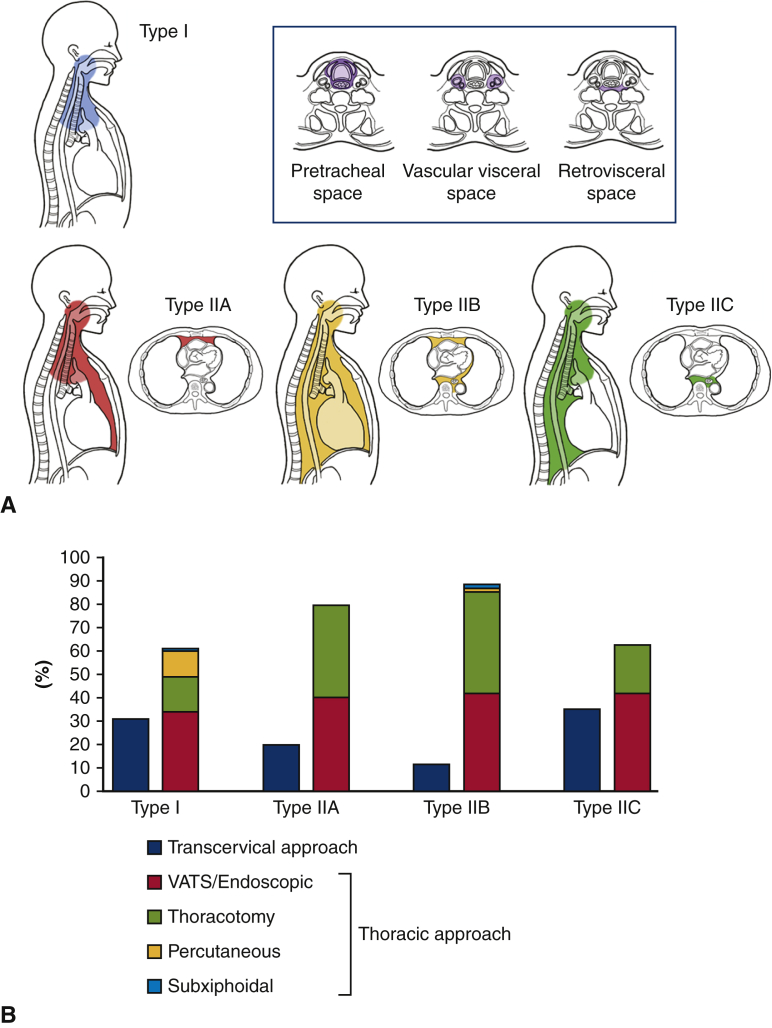
Figure 2.
A, Extension type and surgical procedures of mediastinal infection. Classification of descending necrotizing mediastinitis (DNM) extension type; we defined DNM l limited to the area above the carina as type I and DNM that had extended into the lower mediastinum as type IIA (anterior lower mediastinum), type IIB (both the anterior and posterior lower mediastinum), and type IIC (posterior lower mediastinum). B, Relationship between the type of mediastinal spread and surgical procedures for mediastinal drainage. All approaches were first divided into 2 categories, “transcervical approach” and “thoracic approach.” Then the “thoracic approach” was subdivided into 4 categories “VATS,” “thoracotomy,” “percutaneous,” and “subxiphoidal.” The distribution of the 2 major category showed significant difference between each mediastinitis type (P = .0070). VATS, Video-assisted thoracic surgery.
Pleural effusion was found in 145 patients (64.4%). One-fourth of the patients were accompanied by empyema (n = 58, 25.8%); among them, 34.5% of empyema cases were found in bilateral pleural spaces, whereas 43.1% were right only and 22.4% left only.
Relationship Between the Cervical Spread Route and the Type of Mediastinal Extent
Regarding cervical infection, the retrovisceral space was the most frequent site of development (n = 137, 60.9%), and the frequencies of development through the pretracheal space and the vascular visceral space were similar (n = 111, 49.3% and n = 117, 52.0%). Fifty-two percent of cases developed through a single route in the neck, while 46.7% developed through multiple routes. Mediastinal infection developed more frequently through a single route than through multiple routes (56.0% vs 40.9%) (Table 2). Pearson χ2 test revealed a strong connection between each cervical route and mediastinal extent of infection; Pretracheal space infection was significantly associated with anterior mediastinal infection (P < .0001); a similar association was also seen between the vascular visceral space and middle mediastinum and the retrovisceral space and posterior mediastinum (P < .0001 and P < .0001, respectively). The relationship between the type of cervical infection and the type of mediastinitis was described in Tables E2 and E3; the distribution of each cervical infection to the mediastinum is described in Table E2, whereas the contribution of the cervical route to each mediastinal spreading type is shown in Table E3. Type IIA mediastinal infections more frequently spread through the pretracheal space (70%) than the retrovisceral space (20%). In contrast, type IIC infections more frequently spread through the retrovisceral space (88%) than the pretracheal space (26%). The contribution to each Endo type was significantly different among the pretracheal space infection (P = .0002) and among the retrovisceral space infection (P < .0001).
Differences in the mediastinal extent were significant in the pretracheal and retrovisceral infections (P = .0002 and P < .0001, retrospectively). Type IIA mediastinitis more frequently occurred through a single region than type IIB and IIC.
Treatment of DNM
The median duration time from the initial medical assessment to surgical drainage was 2 days (0-69 days, 4.0 days in average) (Table 3). A total of 162 patients (72%) underwent surgery on the day of the DNM diagnosis. Fifty patients (22.2%) underwent surgery 1 to 3 days after the diagnosis, whereas the remaining 13 (5.8%) received surgery 4 or more days later.
Table 3.
Treatments and outcomes of descending necrotizing mediastinitis (DNM)
| Category | n | % |
|---|---|---|
| Duration from the initial medical assessment to surgical drainage | ||
| Median, d (range) | 2 (0-69) | |
| Average, d | 4.0 | |
| Duration from the DNM diagnosis to surgical drainage | ||
| Median, d (range) | 0 (0-10) | |
| Average, d | 0.64 | |
| Operation time | ||
| Median, min (range) | 171 (22-614) | |
| Average, min | 186.3 | |
| Estimated blood loss | ||
| Median, mL (range) | 79 (0-2560) | |
| Average, mL | 185.6 | |
| Approach for cervical drainage | ||
| Percutaneous | 19 | 8.4 |
| Endoscopic | 4 | 1.8 |
| Cervicotomy | 189 | 84.0 |
| NA | 11 | 4.9 |
| Approach for mediastinal drainage | ||
| Transcervical | 57 | 25.3 |
| Percutaneous | 12 | 5.3 |
| Subxiphoidal | 2 | 0.8 |
| VATS/endoscopic | 86 | 38.2 |
| Thoracotomy | 59 | 26.2 |
| NA | 9 | 4.0 |
| Dwelling time of cervical drainage | ||
| Median, d | 14 | |
| Average, d | 16.6 | |
| Dwelling time of mediastinal drainage | ||
| Median, d | 17 | |
| Average, d | 20.5 | |
| Repeat operation for cervical drainage | ||
| No | 158 | 70.2 |
| Yes | 67 | 29.8 |
| Repeat operation for mediastinal drainage | ||
| No | 179 | 79.6 |
| Yes | 46 | 20.4 |
| Tracheostomy | ||
| No | 64 | 28.4 |
| Yes | 161 | 71.6 |
| Mechanical ventilation | ||
| No | 70 | 31.1 |
| Yes | 155 | 68.9 |
| Duration of mechanical ventilation | ||
| Median, d (range) | 10 (1-218) | |
| Average, d | 20.4 | |
| Hospital stay | ||
| Median, d (range) | 47 (3-1378) | |
| Average, d | 66.4 | |
| Operation-related mortality | ||
| 30-d mortality | 8 | 3.6 |
| 90-d mortality | 12 | 5.3 |
NA, Not available; VATS, video-assisted thoracoscopic surgery.
Drainage of the neck was performed via cervicotomy in 84% of cases, whereas a percutaneous approach was selected in 8.4% and an endoscopic approach in 1.8%. The approach to mediastinal drainage was divided into transcervical (25.3%), percutaneous (5.3%), subxiphoidal (0.8%), video-assisted thoracic surgery (VATS)/endoscopic (38.2%), or thoracotomy (26.2%). Tracheostomy was performed for 161 patients (71.6%), and 155 patients (68.9%) received mechanical ventilation after the operation.
The relationship between the type of mediastinal spread and the surgical approach for mediastinal drainage is described in Table E4 and summarized in Figure 2, B. Patients with type I and IIC infections more commonly underwent cervical drainage than patients with type IIA and IIB infections (34.3% and 13.4%, respectively). Thoracotomy was used more frequently for type IIA and IIB infections compared with type I and IIC (42.6% and 16.7%, respectively).
Clinical Course after the Operation
The median dwelling time of the cervical drainage tube was 14 days after the operation, whereas that of the mediastinal/thoracic drainage tube was 17 days (Table 3). Thirty percent (n = 67) of patients received repeat operations for a cervical drainage for recurrent/remnant abscess, whereas 20% (n = 46) received repeat operations for a mediastinal drainage. Repeat operation is defined here as surgical drainage that follows any type of initial operation, whether cervical or mediastinal.
The median duration of mechanical ventilation was 10 days after the operation. The median hospital stay was 47 days. To assess the link between the surgical modality and treatment outcomes, the influence of each surgical modality to the incidence of repeat thoracic surgery was analyzed (Table E5). It revealed that the patients with type IIB who underwent thoracotomy experienced significantly less repeat surgery than patients with type IIB who did undergo no VATS/thoracotomy approach did (P = .0022).
Treatment Outcomes
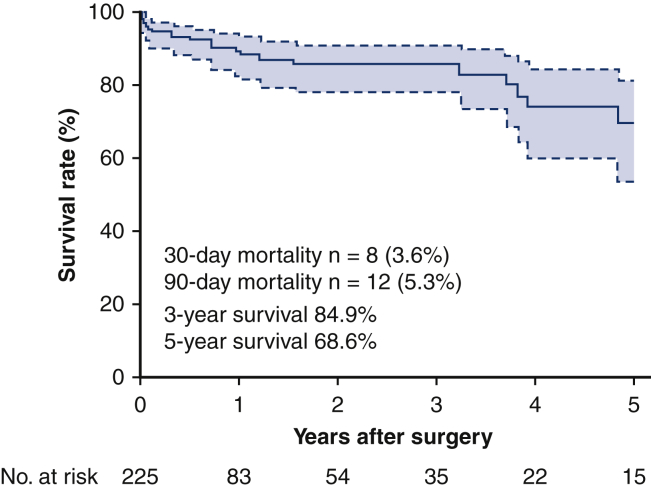
As the primary end point, 8 patients (3.6%) died within 30 days, including 1 patient with type I and 7 patients with type II. As a secondary end point, 12 patients (5.3%) died within 90 days, including 2 patients with type I and 10 patients with type II (Table 3). Overall death within the follow-up period was seen in 28 patients (12.4%). The cause of death was DNM in 7 patients (25%), any type of cancer in 5 (17.9%), pneumonia in 5 (17.9%), multiple organ failure in 4 (14.3%), and others in 5 (17.9%). No cancer death was observed either in the 30-day mortality or in the 90-day mortality. Residual disabilities after treatment were dysphagia in 30 patients (13.3%), chronic respiratory failure in 6 (2.7%), dysarthria in 3 (1.3%) and gait disturbance in 3 (1.3%) (Table E6). Sixty percent of patients (n = 136) reported no obvious residual disabilities after the discharge. The 3-year overall survival rate was 84.9%, and the 5-year overall survival rate was 68.6% with the median follow-up time of 6.5 months (Figure 3).
Figure 3.
Kaplan–Meier curves of the overall survival in 225 cases with descending necrotizing mediastinitis. Survival curves were estimated using the Kaplan–Meier method. The overall survival was defined as the time between the operation and death from any cause.
In the analyses searching for factors potentially related to prognosis, the extent of mediastinal infection (type II vs type I) was associated with the 90-day mortality (logistic regression model; odds ratio, 4.63; P = .034) and age was associated with overall survival (Cox proportional hazard model; hazard ratio 1.04 per 1-year increase, P = .0027; Table 4).
Table 4.
Analyses of factors associated with the 90-day mortality (logistic regression models) and overall survival (Cox proportional hazard models)
| Category | n | Univariate analyses |
Multivariate analyses |
||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | ||
| 90-d mortality | |||||
| Age | |||||
| Per 1-y increase | 1.02 (0.97-1.06) | .42 | 1.02 (0.98-1.07) | .40 | |
| Sex | |||||
| F/M | 92/133 | 2.11 (0.65-6.86) | .21 | 2.08 (0.61-7.65) | .25 |
| Diabetes mellitus | |||||
| Yes/no | 64/161 | 0.49 (0.07-1.93) | .36 | 0.60 (0.09-2.51) | .52 |
| Performance status∗ | |||||
| 2-4/0-1 | 60/154 | 1.91 (0.70-8.97) | .29 | 2.42 (0.66-8.64) | .18 |
| Extent of mediastinitis | |||||
| Lower mediastinum/above carina | 125/100 | 4.26 (1.09-28.2) | .036 | 4.63 (1.12-31.9) | .034 |
| Duration to drainage | |||||
| Per 1-d increase | 1.02 (0.94-1.08) | .43 | 1.01 (0.93-1.07) | .68 | |
| n | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
|---|---|---|---|---|---|
| Overall survival | |||||
| Age | |||||
| Per 1-y increase | – | 1.04 (1.01-1.08) | .022 | 1.04 (1.01-1.08) | .027 |
| Sex | |||||
| F/M | 92/133 | 1.07 (0.51-2.27) | .86 | 0.87 (0.38-1.98) | .74 |
| Diabetes mellitus | |||||
| Yes/no | 64/161 | 0.75 (0.32-1.78) | .52 | 1.01 (0.41-2.48) | .98 |
| Performance status∗ | |||||
| 2-4/0-1 | 60/154 | 0.95 (0.40-2.26) | .92 | 1.00 (0.41-2.48) | .99 |
| Extent of mediastinitis | |||||
| Lower mediastinum/above carina | 125/100 | 1.43 (0.66-3.10) | .36 | 1.57 (0.69-3.56) | .28 |
| Duration to drainage | |||||
| Per 1-d increase | – | 1.04 (1.00-1.06) | .032 | 1.03 (0.99-1.05) | .10 |
CI, Confidence interval; F, female; M, male.
11 patients were not evaluable.
Discussion
We ultimately analyzed the data of 225 patients who received surgical drainage for DNM from nationwide institutions that specialize in cervical or thoracic diseases. To our knowledge, there have been no such comprehensive studies on the clinical and therapeutic status of DNM thus far. Most previous studies were case series studies of fewer than 50 cases.12 Over the past decade, the JATS has been collecting data on the incidence of surgical treatment for DNM from institutions that specialize in general thoracic surgery.6 These efforts have revealed 491 cases of surgical treatment of DNM over the 5 years that the present study covers. The JATS studies did not include ENT institutions, so patients who were treated by only ENT staff were likely excluded, whereas the present study involved both ENT institutions and thoracic surgery institutions. Since almost all patients with type I DNMs are treated with cervical drainage only, real data concerning the clinical practice of treating DNM should be collected from both clinical fields. Therefore, we believe that the cases collected in the present study represent the demographics of patients with DNM.
The age group with the greatest number of patients with DNM was the patients in their 60s. Although a majority of the patients were elderly people, as expected, there were a certain number of younger patients; indeed, 29.8% of patients were in their 40s and 50s, and 5.8% were younger than 40 years old. With regard to the comorbidities of the patients, DM was the most frequently observed (n = 64, 28.4%). The inclusion of other comorbidities on the list suggested that hosts compromised by any means were the most vulnerable to this disease. Since DM is a major comorbidity in the adult population in Japan, the proportion of DM among patients with DNM was greater than in some previous studies (11%-18%).3,17,18
With regard to cases of cervical infection, the present data showed that the retrovisceral space was the most frequent route of infection, occurring approximately 10% more frequently than the other routes. One half of the patients present a single route of infection among the 3 major routes. Since there were no detailed data concerning the cervical route of DNM available in the literature, we were unable to evaluate the proportions. As previous studies suggested, there was a significant association between the cervical infection spaces and the mediastinal infection routes.7
We further analyzed in detail the extent of mediastinal infection. We found that nearly one third of type II mediastinitis cases belonged to a previously unreported type, where the infection had spread into the LM but was still limited to the posterior mediastinum. Therefore, we named this type IIC DNM. We also showed that this type IIC was strongly associated with retrovisceral space infection in the neck, whereas type IIA was associated with pretracheal space infection. Furthermore, many of the type IIA cases had spread through a single cervical route, which suggested that type IIA mediastinitis was actually milder than the other types.
There has been no clear consensus concerning the ideal type for adequate surgical drainage in DNM. Misthos and colleagues19 adopted a less-aggressive approach via the transcervical and/or subxiphoid route in 66% of patients with type IIA, which led to undesirable outcomes and a 34% mortality rate. Endo and collleagues10 proposed in their 1999 paper that the type of approach for surgical drainage should be determined according to the extent of mediastinal infection; they suggested that type I may not require aggressive mediastinal drainage, and a transcervical approach would be sufficient to control the infection. For type IIA, subxiphoidal mediastinal drainage without sternotomy may achieve adequate drainage, whereas type IIB usually requires complete mediastinal drainage through thoracotomy. Palma and colleagues20 reported successful infection control using the same strategy for their 34-case series. The present study shows the recent practice of specialized physicians in Japan; For type I mediastinitis, 25% of patients underwent a minimal approach that included subcutaneous, transcervical and subxiphoid approaches, and only 15% received thoracotomy. Interestingly, patients with type IIC mainly underwent VATS (41.9%), followed by thoracotomy (20.9%) and transcervical approach (34.9%). We conducted additional prognostic analyses comparing each subtype of type II mediastinitis with regard to 90-day mortality and overall survival, which showed no significant difference (Table E7).
According to recent review articles that include cases reported after 1990, the mortality rate after surgical intervention for DNM lies between 15.5% and 23%.2, 3, 4 However, the summed data of JATS surveys for 10 years (from 2005 to 2014) showed a 30-day mortality rate of 4.7% (n = 40 per total 858 cases) and in-hospital mortality rate of 7.1% (n = 61). This gap in the mortality between the JATS survey and previous review articles may be due to the different periods of treatment; generally, the cases in the review article were treated during older period, whereas the JATS cases were treated more recently. Alternatively, differences in the patient ethnicity or medical systems may account for the discrepancy. In the present study, the 30-day mortality (3.6%) and 90-day mortality (5.3%) rates were even lower than in the JATS survey, although the in-hospital mortality rate was not included in the present study. The good prognosis in the present study may be attributed to the composition of the study participants. The present study included many ENT (ear, nose, and throat) departments as well as general thoracic departments in Japan, while the JATS study included only general thoracic departments. Therefore, patients with less-advanced disease who were able to be treated by head and neck specialists alone may have been included in the present study.
The present study provided long-term survival data on surgically treated DNM patients (3-year overall survival of 84.9% and 5-year overall survival of 68.6%). This is the first report to describe the long-term outcomes of surgery among dozens of studies on DNM. An older age was shown to be the only significant factor influencing a poor long-term survival of patients. Since many of the patients who suffer from DNM are elderly and have comorbidities or are physically unfit, pneumonia and neoplasm were among the major causalities of death. Remaining dysphagia, which is a major residual disability, may also have affected patients' daily lives after surgery. We did not find “duration to drainage” to be a significant risk factor for 90-day mortality or for overall survival in the multivariate analysis. We speculate one possible reason for this may be that the study was statistically underpowered since 80% of patients underwent surgery within 6 days after the initial presentation to the hospital. Another possibility would be that patients who underwent delayed surgery might have a more indolent bacterial infection, which would not be as harmful to the host.
Several limitations associated with the present study warrant mention. First, this study was conducted as a retrospective observational study in which physicians in multiple institutions participated through a registered CRF with multiple choices allowed for single categories. The physician's choice may have been influenced by his or her own interpretation, which may harm the quality of data. In addition, we did not do any central reviews for this classification; the lack of standardization for the classification may harm the quality of the study. However, Endo and colleagues' classification is generally well penetrated throughout the thoracic surgeons in Japan, which, we believe, help to keep the categorization qualified. There will also always be selection bias when patient accrual largely depends on the quality of the archived patient chart. A lead-time bias is also inevitable in this type of retrospective study. To decrease the influence of these biases, we restricted the eligible patients to those who underwent surgical drainage within a period of 5 years and asked the participants to make an effort to register consecutive patients who were eligible within the period. A large-scale, prospective registered study is warranted to resolve these issues.
Conclusions
The present multicenter retrospective study revealed better surgical outcomes than previous reports, with a 30-day mortality rate of 3.6% and a 3-year survival of 84.9%. This large-scale study produced a new DNM classification, including type IIC, which is localized within the posterior mediastinum (Figure 4). The extension of infection to the LM (type II vs type I) was significantly associated with the short-term outcome of severe infectious disease.
Figure 4.
Methods, results, and implications of the study. A multicenter observational study was conducted on surgically treated patients with descending necrotizing mediastinitis (DNM) in Japan. The study found a new type of disease extension and proposed a new DNM classification. JBES, Japan Broncho-esophagological Society; JACS, Japanese Association for Chest Surgery.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
We give special thanks to Ms Yukiko Nishihara for her support in data management and Dr Miyuki Abe for drawing Figure 2.
Footnotes
Drs Kenji Sugio and Tatsuro Okamoto contributed equally to this article.
Supplementary Data
A brief summary of a multicenter observational study (JBES1703/JACS1806). The study retrospectively investigated clinical features and treatment outcomes of patients with descending necrotizing mediastinitis who were surgically treated. Video available at: https://www.jtcvs.org/article/S2666-2736(21)00225-4/fulltext.
Appendix 1
Hospitals That Contributed to Registration of Six Patients or More
Kitasato University School of Medicine (Dr Yamashita); Kobe City Medical Center General Hospital (Dr Shinohara); Chiba University Graduate School of Medicine (Dr Yoshino); Kobe University Hospital (Dr Shinomiya); St Marianna University School of Medicine (Dr Koizuka); Tokai University Hospital (Dr Iwasaki); Jichi Medical University Hospital (Dr Endo).
Participating Hospitals
Asahikawa Medical University Hospital; Hokkaido University Hospital; Sapporo City General Hospital; Sapporo Medical University Hospital; Teine Keijinkai Hospital; Hirosaki University, School of Medicine; Iwate Medical University Hospital; Iwate Prefectural Isawa Hospital; Akita University Hospital; Yamagata University Hospital; Yamagata Prefectural Central Hospital; Fukushima Medical University Hospital; Ibaraki Prefectural Central Hospital, Ibaraki Cancer Center; Tsukuba Medical Center Hospital; Kiryu Kosei General Hospital; Gunma University Hospital; Isesaki Municipal Hospital; Dokkyo Medical University, Saitama Medical Center; University Hospital; Tokyo Medical University Ibaraki Medical Center; The University of Tokyo Hospital; Tokyo Medical and dental University; Tokyo Metropolitan Tama Medical Center; Tokyo Medical University Hospital; Tokyo Women's Medical University, Tokyo Medical University Hachioji Medical Center; The Jikei University Hospital; The Jikei University Katsushika Medical Center; Kyorin University Hospital; Japanese Red Cross Medical Center; Toho University Omori Medical Center; Teikyo University Hospital; Japanese Red Cross Maebashi Hospital; Tokyo Medical Center; Showa University Hospital; Mito Saiseikai General Hospital; Mito Medical Center; Yachiyo Medical Center; Funabashi Municipal Medical Center; Yokohama City Minato Red Cross Hospital; Yokohama Sakae Kyosai Hospital; Kanagawa Cancer Center; Showa University Northern Yokohama Hospital; Kanazawa University Hospital; Yokosuka Kyosai Hospital; Yokosuka General Hospital Uwamachi; Kanazawa Medical University; Fujisawa City Hospital; Hamamatsu University School of Medicine; Seirei Mikatahara General Hospital; University of Fukui Hospital; Toyama University Hospital; Shinshu University Hospital; Nagoya University Hospital; Nagoya City University Hospital; Fujita Health University Hospital, Ichinomiya Municipal Hospital; Gifu University Hospital; Ogaki Municipal Hospital; Mie University Hospital, Japanese Red Cross Ise Hospital; Higashi-ohmi General Medical Center; Kyoto University Hospital; Kyoto Okamoto Memorial Hospital; Ayabe City Hospital; The Tazuke Kofukai Medical Research Institute, Kitano Hospital; Osaka University Hospital; Kansai Medical University Hospital; Osaka City University Hospital; Osaka City General Hospital; Otemae Hospital; Kindai University Hospital; Yodogawa Christian Hospital; Kishiwada City Hospital; Japanese Red Cross Wakayama Medical Center; Kobe City Nishi-Kobe Medical Center; Hyogo Prefectural Amagasaki General Medical Center; Hyogo College of Medicine; Miyoshi Central Hospital; Shimane University Hospital; Shimane Prefectural Central Hospital; Tottori University Hospital; Okayama University Hospital; Kawasaki Medical School General Medical Center; Hiroshima City Asa Citizens Hospital; Hiroshima City Hiroshima Citizens Hospital; Fukuyama Medical Center; Saiseikai Yamaguchi hospital; Kagawa University Hospital; Kagawa Prefectural Central Hospital; Takamatsu Municipal Hospital; Matsuyama Shimin Hospital; Ehime University Hospital; Kochi Medical School Hospital; Kyushu University Hospital; Fukuoka-higashi Medical Center; Kurume University Hospital; St. Mary's Hospital; Japan Community Health care Organization Kyushu Hospital; Hospital of the University of Occupational and Environmental Health; Nagasaki University Hospital; Sasebo City General Hospital; Kumamoto University Hospital; Oita University Hospital; Oita Prefectural Hospital; Shinbeppu Hospital; Kagoshima University Hospital; Urasoe General Hospital.
Table E1.
Microbial study
| Single-microbial infections (n = 93) | n | % |
|---|---|---|
| Streptococcus spp. | 39 | 41.9 |
| Staphylococcus spp. | 14 | 15.1 |
| Prevotella spp. | 5 | 5.4 |
| Peptostreptococcus spp. | 4 | 4.3 |
| Parvimonas micra/Peptostreptocuccus micros | 4 | 4.3 |
| Klebsiella spp. | 3 | 3.2 |
| Stenotrophomonas spp. | 3 | 3.2 |
| Others | 21 | 22.6 |
| Polymicrobial infections (n = 115) | n (total 293) | % |
|---|---|---|
| Streptococcus spp. | 90 | 78.3 |
| Prevotella spp. | 57 | 49.6 |
| Peptostreptococcus spp. | 23 | 20.0 |
| Staphylococcus spp. | 16 | 13.9 |
| Parvimonas micra/Peptostreptocuccus micros | 16 | 13.9 |
| Fusobacterium spp. | 16 | 13.9 |
| Bacteroides spp. | 8 | 7.0 |
| Corynebacterium spp. | 7 | 6.1 |
| Others | 60 | 52.2 |
Table E2.
Relationship between the neck infection and the type of mediastinal extent
| Neck infection | n | Type of mediastinitis (%) |
|||
|---|---|---|---|---|---|
| Type I (n = 98∗) | Type IIA (n = 20) | Type IIB (n = 61∗) | Type IIC (N = 43) | ||
| Pretracheal space | 43 | 19 | 11 | 12 | 1 |
| Vascular visceral space | 24 | 15 | 3 | 3 | 3 |
| Retrovisceral space | 50 | 23 | 2 | 9 | 16 |
| Pretracheal + vascular | 18 | 8 | 2 | 7 | 1 |
| Vascular + retrovisceral | 37 | 14 | 1 | 9 | 13 |
| Retrovisceral + pretracheal | 12 | 7 | 0 | 3 | 2 |
| Pretracheal + vascular + retrovisceral | 38 | 12 | 1 | 18 | 7 |
The cervical routes of 2 patients with type I and 1 patient with type IIB were not available.
Table E3.
Relationship between the type of mediastinal extent and the cervical route of infection
| n | Pretracheal space∗ (n = 111) | Vascular visceral space (n = 117) | Retrovisceral space† (n = 137) | Single/Multiple routes (n = 117/105) | |
|---|---|---|---|---|---|
| Type I | 98 | 46 | 49 | 56 | 57/41 |
| Type IIA | 20 | 14 | 7 | 4 | 16/4 |
| Type IIB | 61 | 40 | 37 | 39 | 24/37 |
| Type IIC | 43 | 11 | 24 | 38 | 20/23 |
The contribution to each Endo type was significantly different.
among the pretracheal space infection (Pearson χ2 test; P = .0002)
among the retrovisceral space infection (P < .0001).
Table E4.
Approach for mediastinal drainage according to the DNM type
| Type I |
Type IIA |
Type IIB |
Type IIC |
|||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Transcervical | 31 | 31.0 | 4 | 20.0 | 7 | 11.3 | 15 | 34.9 |
| Percutaneous | 11 | 11.0 | 0 | 0 | 1 | 1.6 | 0 | 0 |
| Subxiphoid | 1 | 1.0 | 0 | 0 | 1 | 1.6 | 0 | 0 |
| VATS/endoscopic | 34 | 34.0 | 8 | 40.0 | 26 | 41.9 | 18 | 41.9 |
| Thoracotomy | 15 | 15.0 | 8 | 40.0 | 27 | 43.6 | 9 | 20.9 |
| NA | 8 | 8.0 | 0 | 0 | 0 | 0 | 1 | 2.3 |
VATS, Video-assisted thoracoscopic surgery; NA, not available.
Table E5.
Analyses of factors associated with the repeat thoracic surgery (logistic regression models)
| n | Odds ratio (95% CI) | P value | |
|---|---|---|---|
| Type I | |||
| Approach | |||
| No VATS/thoracotomy | 43 | 1 | |
| VATS | 34 | 4.10 (0.996-16.9) | .051 |
| Thoracotomy | 15 | 2.05 (0.31-13.7) | .46 |
| Type IIA | |||
| Approach | |||
| No VATS/thoracotomy | 4 | 1 (0.22-6.02) | .87 |
| VATS | 8 | 0.14 (0.01-2.51) | .18 |
| Thoracotomy | 8 | 0.14 (0.01-2.51) | .18 |
| Type IIB | |||
| Approach | |||
| No VATS/thoracotomy | 9 | 1 (0.22-6.02) | .87 |
| VATS | 26 | 0.24 (0.05-1.19) | .081 |
| Thoracotomy | 27 | 0.14 (0.03-0.75) | .022 |
| Type IIC | |||
| Approach | |||
| No VATS/thoracotomy | 15 | 1 (0.22-6.02) | .87 |
| VATS | 18 | 0.64 (0.14-2.87) | .56 |
| Thoracotomy | 9 | 0.51 (0.08-3.49) | .50 |
CI, Confidence interval; VATS, video-assisted thoracoscopic surgery.
Table E6.
Residual disabilities
| Category | n | % |
|---|---|---|
| Residual disabilities | ||
| None | 136 | 60.4 |
| Dysphagia | 3 | 1.3 |
| Dysarthria | 3 | 1.3 |
| Gait disturbance | 3 | 1.3 |
| Chronic respiratory failure | 6 | 2.7 |
| Impaired consciousness | 1 | 0.4 |
| Executive dysfunction | 1 | 0.4 |
| Others | 8 | 3.6 |
| NA | 46 | 20.4 |
NA, Not available.
Table E7.
Analyses of factors associated with the 90-day mortality (logistic regression models) and overall survival (Cox proportional hazard models)
| Extent of mediastinitis | n | Odds ratio (95% CI) | P value |
|---|---|---|---|
| 90-d mortality | |||
| Type IIB/type IIA | 62/20 | 1.14 (0.22-6.02) | .87 |
| Type IIC/type IIA | 43/20 | 0.21 (0.01-2.37) | .20 |
| Type IIC/type IIB | 43/62 | 0.19 (0.02-1.58) | .12 |
| n | Hazard ratio (95% CI) | P value | |
|---|---|---|---|
| Overall survival | |||
| Type IIB/type IIA | 62/20 | 1.83 (0.40-8.26) | .43 |
| Type IIC/type IIA | 43/20 | 1.02 (0.20-5.30) | .98 |
| Type IIC/type IIB | 43/62 | 0.54 (0.19-1.57) | .26 |
CI, Confidence interval.
References
- 1.Pearse H.E. Mediastinitis following cervical suppuration. Ann Surg. 1938;108:588–611. doi: 10.1097/00000658-193810000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiernan P.D., Hernandez A., Byrne W.D., Bloom R., Dicicco B., Hetrick V., et al. Descending cervical mediastinitis. Ann Thorac Surg. 1998;65:1483–1488. doi: 10.1016/s0003-4975(98)00142-8. [DOI] [PubMed] [Google Scholar]
- 3.Ridder G.J., Maier W., Kinzer S., Teszler C.B., Boedeker C.C., Pfeiffer J. Descending necrotizing mediastinitis: contemporary trends in etiology, diagnosis, management, and outcome. Ann Surg. 2010;251:528–534. doi: 10.1097/SLA.0b013e3181c1b0d1. [DOI] [PubMed] [Google Scholar]
- 4.Prado-Calleros H.M., Jimenez-Fuentes E., Jimenez-Escobar I. Descending necrotizing mediastinitis: systematic review on its treatment in the last 6 years, 75 years after its description. Head Neck. 2016;38(Suppl 1):E2275–E2283. doi: 10.1002/hed.24183. [DOI] [PubMed] [Google Scholar]
- 5.Wheatley M.J., Stirling M.C., Kirsh M.M., Gago O., Orringer M.B. Descending necrotizing mediastinitis: transcervical drainage is not enough. Ann Thorac Surg. 1990;49:780–784. doi: 10.1016/0003-4975(90)90022-x. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu H., Endo S., Natsugoe S., Doki Y., Hirata Y., Kobayashi J., et al. Thoracic and cardiovascular surgery in Japan in 2016: annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg. 2019;67:377–411. doi: 10.1007/s11748-019-01068-9. [DOI] [PubMed] [Google Scholar]
- 7.Estrera A.S., Landay M.J., Grisham J.M., Sinn D.P., Platt M.R. Descending necrotizing mediastinitis. Surg Gynecol Obstet. 1983;157:545–552. [PubMed] [Google Scholar]
- 8.Roccia F., Pecorari G.C., Oliaro A., Passet E., Rossi P., Nadalin J., et al. Ten years of descending necrotizing mediastinitis: management of 23 cases. J Oral Maxillofac Surg. 2007;65:1716–1724. doi: 10.1016/j.joms.2006.10.060. [DOI] [PubMed] [Google Scholar]
- 9.Pinto A., Scaglione M., Scuderi M.G., Tortora G., Daniele S., Romano L. Infections of the neck leading to descending necrotizing mediastinitis: role of multi-detector row computed tomography. Eur J Radiol. 2008;65:389–394. doi: 10.1016/j.ejrad.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 10.Endo S., Murayama F., Hasegawa T., Yamamoto S., Yamaguchi T., Sohara Y., et al. Guideline of surgical management based on diffusion of descending necrotizing mediastinitis. Jpn J Thorac Cardiovasc Surg. 1999;47:14–19. doi: 10.1007/BF03217934. [DOI] [PubMed] [Google Scholar]
- 11.Freeman R.K., Vallieres E., Verrier E.D., Karmy-Jones R., Wood D.E. Descending necrotizing mediastinitis: an analysis of the effects of serial surgical debridement on patient mortality. J Thorac Cardiovasc Surg. 2000;119:260–267. doi: 10.1016/S0022-5223(00)70181-4. [DOI] [PubMed] [Google Scholar]
- 12.Mazzella A., Santagata M., Cecere A., La Mart E., Fiorelli A., Tartaro G., et al. Descending necrotizing mediastinitis in the elderly patients. Open Med (Wars) 2016;11:449–460. doi: 10.1515/med-2016-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ris H.B., Banic A., Furrer M., Caversaccio M., Cerny A., Zbaren P. Descending necrotizing mediastinitis: surgical treatment via clamshell approach. Ann Thorac Surg. 1996;62:1650–1654. doi: 10.1016/s0003-4975(96)00683-2. [DOI] [PubMed] [Google Scholar]
- 14.Roberts J.R., Smythe W.R., Weber R.W., Lanutti M., Rosengard B.R., Kaiser L.R. Thoracoscopic management of descending necrotizing mediastinitis. Chest. 1997;112:850–854. doi: 10.1378/chest.112.3.850. [DOI] [PubMed] [Google Scholar]
- 15.Stella F., Petrella F. Transsternal transpericardial approach for acute descending necrotizing mediastinitis. J Thorac Cardiovasc Surg. 2005;129:212–214. doi: 10.1016/j.jtcvs.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 16.Iwata T., Sekine Y., Shibuya K., Yasufuku K., Iyoda A., Iizasa T., et al. Early open thoracotomy and mediastinopleural irrigation for severe descending necrotizing mediastinitis. Eur J Cardiothorac Surg. 2005;28:384–388. doi: 10.1016/j.ejcts.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Kocher G.J., Hoksch B., Caversaccio M., Wiegand J., Schmid R.A. Diffuse descending necrotizing mediastinitis: surgical therapy and outcome in a single-centre series. Eur J Cardiothorac Surg. 2012;42:e66–e72. doi: 10.1093/ejcts/ezs385. [DOI] [PubMed] [Google Scholar]
- 18.Neville S.E., Boye K.S., Montgomery W.S., Iwamoto K., Okamura M., Hayes R.P. Diabetes in Japan: a review of disease burden and approaches to treatment. Diabetes Metab Res Rev. 2009;25:705–716. doi: 10.1002/dmrr.1012. [DOI] [PubMed] [Google Scholar]
- 19.Misthos P., Katsaragakis S., Kakaris S., Theodorou D., Skottis I. Descending necrotizing anterior mediastinitis: analysis of survival and surgical treatment modalities. J Oral Maxillofac Surg. 2007;65:635–639. doi: 10.1016/j.joms.2006.06.287. [DOI] [PubMed] [Google Scholar]
- 20.Palma D.M., Giuliano S., Cracchiolo A.N., Falcone M., Ceccarelli G., Tetamo R., et al. Clinical features and outcome of patients with descending necrotizing mediastinitis: prospective analysis of 34 cases. Infection. 2016;44:77–84. doi: 10.1007/s15010-015-0838-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A brief summary of a multicenter observational study (JBES1703/JACS1806). The study retrospectively investigated clinical features and treatment outcomes of patients with descending necrotizing mediastinitis who were surgically treated. Video available at: https://www.jtcvs.org/article/S2666-2736(21)00225-4/fulltext.