Abstract
A K+/H+ antiporter regulates cytoplasmic pH in Enterococcus hirae growing at alkaline pH. Mutants defective in this antiport activity were alkaline pH sensitive. One mutant, Pop1, lacked both K+/methylamine exchange at pH 9.5 and concomitant acidification of cytoplasmic pH. Pop1 grew well at pHs below 8 but did not at pHs above 9, conditions under which cytoplasmic pH was not fully acidified.
Potassium is a major cytoplasmic cation in growing bacterial cells and plays important roles in cell physiology (1, 8). K+ movement across the cell membrane is involved in maintenance of turgor and in the regulation of cytoplasmic pH (pHin) (1, 9, 19). For these purposes, bacteria have evolved diverse potassium transport systems (1, 3). In a gram-positive bacterium, Enterococcus hirae, two K+ uptake systems, KtrI (2) and KtrII (12, 17), were reported. In addition to these K+ uptake systems, a unique K+ extrusion system has been discovered in E. hirae (13). In contrast to the secondary antiport of K+ for protons or for ammonium ion (1, 19) found in other organisms, E. hirae apparently uses a primary transport system, which expels K+ even against a K+ concentration gradient by exchange of H+ and functions at alkaline pHin. Concomitant with K+ extrusion, the pHin falls from 9 to 8, at which point K+ extrusion stops (13). This system is probably constitutive, since the activity was observed in cells cultured not only at pH 9.0 but also at pH 7.5 or 6.0 in high-K+ medium (13). This system seems important for homeostasis of K+ and H+, especially for pHin regulation, in E. hirae in an alkaline environment (11, 14). In this study we report the isolation of mutants defective in the function of this antiporter.
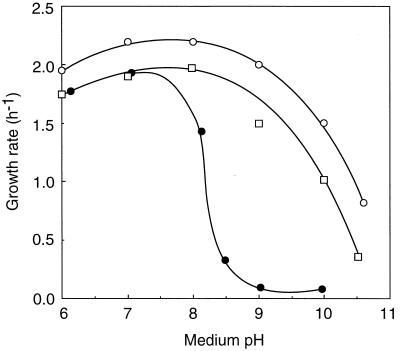
All of the experiments were done with E. hirae ATCC 9790 and the mutants derived from this strain. We expected that a mutant deficient in this antiporter would be alkaline pH sensitive for two reasons. Firstly, this antiport system is active at alkaline pHin in resting cells (13). Secondly, we recently found that wild-type cells cultured in a defined synthetic medium lack this activity, presumably owing to lack of trace metal ion(s) (11a), and that in this medium this bacterium grew normally at acidic to neutral pHs but not at an alkaline pH beyond 9 (15). This system is thus indispensable for the growth of E. hirae at alkaline pH. Thirty-two mutants, which did not grow at pH 9.5 but grew at pH 7.5 in KTY medium (10 g of Difco tryptone, 5 g of Difco yeast extract, 10 g of K2HPO4, and 10 g of glucose per liter) (12), were isolated after mutagenesis with N-methyl-N′-nitro-N-nitrosoguanidine (200 μg/ml) at 37°C for 30 min and enrichment by two cycles of penicillin (200 U/ml) treatment in KTY medium at pH 9.5. The medium pH was controlled by addition of HCl, KHCO3, or K2CO3, and in all cases the K+ concentration was adjusted to 200 mM by addition of KCl. Glucose was omitted from the selective plates to minimize the pH drop during growth. All mutants grew well on SF medium (the selective medium for enterococci, including E. hirae; Difco Laboratories). The spontaneous reversion frequency of Pop1, one of the alkaline pH-sensitive mutants, was 10−6, indicating a point mutation. Figure 1 shows the effect of medium pH on the growth rates of strain 9790 (parent strain), Pop1, and Pop1R, a spontaneous revertant of Pop1, in KTY medium. Pop1 did not grow at pHs above 9.0, but it grew well at an acidic pH range, similarly to the parent strain, indicating that Pop1 is specifically sensitive to alkaline pH under these culture conditions. Other alkaline pH-sensitive mutants showed nearly the same property of growth when tested at various external pHs. Pop1R recovered the ability to grow at alkaline external pH. Importantly, Pop1 did not grow at pH 9.0, even when the medium pH was adjusted by addition of Na2CO3 instead of K2CO3. Thus, the sensitivity of Pop1 to alkaline pH did not depend upon the concentration ratio of K+ versus Na+ in the medium.
FIG. 1.
Growth rates of E. hirae at various pHs. Cells were cultured at 37°C in KTY complex medium at the indicated pHs, and the growth rates were determined by measuring the optical density at 600 nm with a Perkin-Elmer spectrophotometer (model 35). The growth rates were determined between the optical densities of 0.1 and 0.2. There was no significant change in the medium pH during this period. The medium pH indicated represents the initial pH. Symbols: ○, strain 9790; ●, Pop1; □, Pop1R.
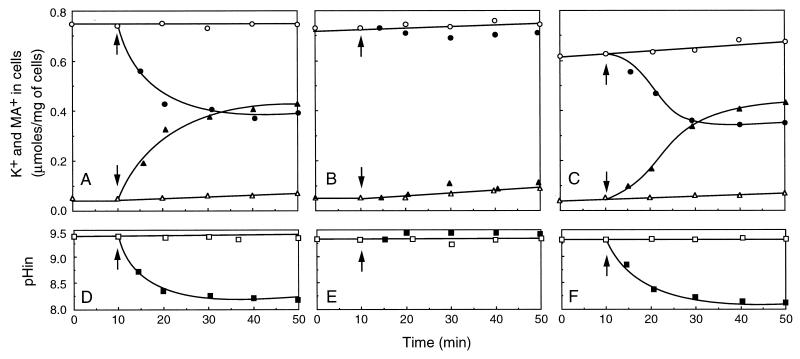
Uphill K+ extrusion activity was measured in all these alkaline pH-sensitive mutants cultured in KTY medium at pH 7.5. Cells were harvested at late logarithmic phase, washed twice with 2 mM MgSO4, and incubated in 2 mM MgSO4 for 30 min on ice. By this treatment, energy depletion was accomplished (4); the ATP level decreased to less than 10% in growing cells. For measurement of uphill K+ extrusion, cells were resuspended in 50 mM 2-[N-cyclohexylamino]ethanesulfonic acid (CHES)–KOH (pH 9.5) containing 0.2 M KCl and 50 mM methylamine-HCl, at a cell density of 1 mg/ml, and incubated at 25°C. Glucose (10 mM) was added. At intervals, the cell samples (0.1 ml) were collected by filtration through a Millipore filter (0.45-μm pore size), washed twice with 2 mM MgSO4, and extracted with hot 5% trichloroacetic acid. Aliquots were then analyzed for K+ by flame photometry. Background due to the binding of buffer K+ to the filters was subtracted from the data. The glucose-induced K+ efflux from the K+-loaded cells measured in this way was normal or somewhat decreased in most of the mutants. However, we found three mutants (Pop1, Pop2, and Pop3) that were totally defective in uphill K+ extrusion. Figure 2 shows the K+/H+ antiport activity of strain 9790, Pop1, and Pop1R. The K+ concentration of the buffer used for the experiment was about 250 mM. Since the internal K+ concentrations of these cells were from 300 to 365 mM, the K+ gradients ([K+]in/[K+]out) of these cells were insignificant under the experimental conditions. Nevertheless, upon addition of glucose, K+ was extruded by the parent strain 9790 (Fig. 2A). In these experiments, the uptake of [14C]methylamine (0.37 MBq/mmol) was measured at the same time. Cell samples collected on filters were counted in a liquid scintillation counter. Cytoplasmic water space was taken to be 2 μl/mg of cells (10). This showed that an equivalent amount of methylamine was also accumulated in 9790 cells (Fig. 2A). Judging from the pKa of methylamine, 10.6 at 25°C, we presume that most of the internal base was protonated. We infer that the cells exchange K+ for an equivalent amount of the protonated amine. In the same experiment, the pHin (interior acid) of resting cells was determined by the use of [14C]benzylamine-HCl (10 μM; 0.148 MBq/mmol) as described previously (13). This showed that, concurrent with K+ extrusion and amine accumulation, the pHin of strain 9790 was acidified from 9.3 to 8.1, at which point the exchange of K+ for methylamine leveled off (Fig. 2D). These transport reactions were not observed in Pop1 (Fig. 2B and E), but they were recovered in the revertant Pop1R (Fig. 2C and F).
FIG. 2.
Simultaneous measurements of K+/amine exchange and pHin in E. hirae. Strain 9790 (A and D), Pop1 (B and E), and Pop1R (C and F) were grown in KTY medium at pH 7.5, washed, and suspended in 50 mM CHES-KOH buffer (pH 9.5) containing 200 mM KCl and 50 mM methylamine-HCl. At intervals, aliquots were collected and cellular K+ (circles) contents and the pHin (interior acid) (squares) were determined as described in the text. Accumulation of methylamine (MA+) (triangles) was measured by use of [14C]methylamine (0.37 MBq/mmol). At the times indicated by the arrows, 10 mM glucose was added. Open and closed symbols represent absence and presence of glucose, respectively. The internal water space was taken as 2 μl/mg (dry weight) (10).
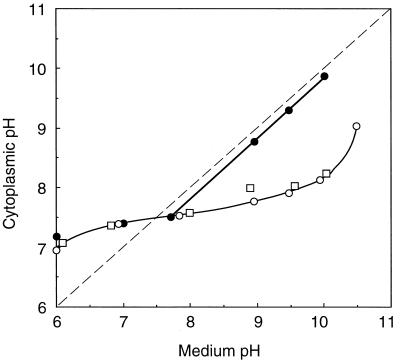
The internal pHs of cells growing in KTY medium at different pHs were estimated (Fig. 3). The internal pH of strain 9790 was maintained within a relatively narrow range between 7.0 and 8.3 when the medium pH was between 6 and 10. The mutant Pop1 also showed alkalinization of the internal pH at acidic external pH values; for example, the pHin was estimated as 7.2 when the medium pH was 6.0. However, the pHin of Pop1 varied proportionally to medium pH when the medium pH was above 8 (Fig. 3), suggesting a defect in acidification of the internal pH at an alkaline medium pH. In the revertant Pop1R, acidification of the internal pH at alkaline external pH was restored. These results suggested that the K+/H+ antiport system participates in pH acidification of this bacterium growing at alkaline external pH.
FIG. 3.
pHin values of growing E. hirae at various external pHs. Cells were cultured in KTY medium at the indicated medium pHs, and the pHin of cells was measured as follows. When the turbidity of the culture reached an optical density of 0.2, [carboxyl-14C]acetylsalicylic acid (10 μM; 0.078 MBq/mol) or [14C]benzylamine (10 μM; 0.148 MBq/mmol) was added to 10 ml of culture to measure the interior alkaline or acid pH, respectively (10, 16). The accumulation of the radioactive probe was determined by filtering culture fluid through a Whatman GF/C glass filter and measuring the counts per minute on the filters. Nonspecific binding of the probe to cells was determined by using boiled cells or cells treated with n-butanol (10). Before filtration of the culture, turbidity was measured at 540 nm and the dry weight of cells was calculated. Symbols: ○, strain 9790; ●, Pop1; □, Pop1R.
There are other possibilities to explain the lack of the K+/H+ antiport activity in Pop1: alteration of the permeability of the cell membrane for K+ and H+ or a defect in energy production by glucose metabolism. The passive K+ efflux rate was examined in these strains suspended in K+-free buffer containing 200 mM NaCl at pH 9.5. Proton permeability was also measured by monitoring the change of the medium pH after a proton pulse as described elsewhere (18). There was no significant difference in flux rates of K+ and H+ in these strains (data not shown). No remarkable difference between these strains in glucose-induced acid production was observed. These results suggest that a mutation in Pop1 does not affect membrane permeabilities for K+ and H+. Instead, active exchange of K+ for H+ at alkaline pH was absent in Pop1. Pop2 and Pop3 were also defective in the antiport activity.
Most of the other alkaline pH-sensitive mutants were not wholly or partially defective in the antiport activity. In the mutants having this antiport activity, acidification of the pHin at alkaline external pH was normal. We know that the metabolism of E. hirae growing at alkaline pH is not identical with that at neutral or acidic pH. For instance, carbonate (CO2) is essential for the growth of E. hirae at alkaline pH (7, 10); the glycolytic metabolism may be switched to a more effective system for ATP production in the presence of CO2 (5, 6). It is possible that some key steps in metabolic pathways essential for growth at alkaline pH are damaged in these mutants. A few alkaline pH-sensitive mutants were leaky to K+.
The K+/H+ antiport activity was also observed in arginine-adapted cells, in which ATP can be generated via the arginine deiminase pathway (13). Since the common product between glycolysis and arginine metabolism is ATP, it is plausible that ATP is linked with this system. Attempts to detect any difference in K+-activated ATPase activity of the membrane vesicles of strain 9790 and Pop1 were unsuccessful. We are now trying to clone the gene complementing alkaline pH sensitivity of Pop1.
Acknowledgments
We thank I. Yamato for critical reading of the manuscript.
This work is supported in part by a grant-in-aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan.
REFERENCES
- 1.Bakker E P. Alkali cation transport systems in prokaryotes. Boca Raton, Fla: CRC Press; 1993. [Google Scholar]
- 2.Bakker E P, Harold F M. Energy coupling to potassium transport in Streptococcus faecalis: interplay of ATP and the protonmotive force. J Biol Chem. 1980;255:433–440. [PubMed] [Google Scholar]
- 3.Epstein W, Burrman E, McLaggan D, Naprstek J. Multiple mechanisms, roles and controls of K+ transport in Escherichia coli. Biochem Soc Trans. 1993;21:1006–1010. doi: 10.1042/bst0211006. [DOI] [PubMed] [Google Scholar]
- 4.Forrest W W, Walker D J. Synthesis of reserve materials for endogenous metabolism in Streptococcus faecalis. J Bacteriol. 1965;89:1448–1452. doi: 10.1128/jb.89.6.1448-1452.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham A F, Lund B M. The effect of alkaline pH on growth and metabolic products of a motile, yellow-pigmented Streptococcus sp. J Gen Microbiol. 1983;129:2429–2435. [Google Scholar]
- 6.Gunsalus I C, Niven C F. The effect of pH on the lactic acid fermentation. J Biol Chem. 1942;145:131–136. [Google Scholar]
- 7.Hardie J M. Genus Streptococcus. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 2. Baltimore, Md: Williams & Wilkins; 1986. pp. 1043–1071. [Google Scholar]
- 8.Harold F M. The vital force—a study of bioenergetics. New York, N.Y: Freeman Co.; 1990. [Google Scholar]
- 9.Harold F M, Maloney P. Energy transduction by ion currents. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella. 2nd ed. Vol. 1. Washington, D.C: ASM Press; 1996. pp. 283–306. [Google Scholar]
- 10.Kakinuma Y. Lowering of cytoplasmic pH is essential for growth of Streptococcus faecalis at high pH. J Bacteriol. 1987;169:4403–4405. doi: 10.1128/jb.169.9.4403-4405.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kakinuma Y. K+ transport in Enterococcus hirae. In: Bakker E P, editor. Alkali cation transport systems in prokaryotes. Boca Raton, Fla: CRC Press; 1993. pp. 277–290. [Google Scholar]
- 11a.Kakinuma, Y., et al. Unpublished data.
- 12.Kakinuma Y, Harold F M. ATP-driven exchange of Na+ and K+ ions by Streptococcus faecalis. J Biol Chem. 1985;260:2086–2091. [PubMed] [Google Scholar]
- 13.Kakinuma Y, Igarashi K. Active potassium extrusion regulated by intracellular pH in Streptococcus faecalis. J Biol Chem. 1988;263:14166–14170. [PubMed] [Google Scholar]
- 14.Kakinuma Y, Igarashi K. Potassium/proton antiport system of Enterococcus hirae growing at high pH. J Bacteriol. 1995;177:2227–2229. doi: 10.1128/jb.177.8.2227-2229.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kakinuma Y, Yasumura K, Igarashi K. Potassium/proton antiport system is dispensable for growth of Enterococcus hirae at low pH. Biosci Biotechnol Biochem. 1999;63:875–878. doi: 10.1271/bbb.63.875. [DOI] [PubMed] [Google Scholar]
- 16.Kashket E R. The proton motive force in bacteria: a critical assessment of methods. Annu Rev Microbiol. 1985;39:219–242. doi: 10.1146/annurev.mi.39.100185.001251. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi H. Second system for potassium transport in Streptococcus faecalis. J Bacteriol. 1982;150:506–511. doi: 10.1128/jb.150.2.506-511.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi H, Unemoto T. Streptococcus faecalis mutants defective in regulation of cytoplasmic pH. J Bacteriol. 1980;143:1187–1193. doi: 10.1128/jb.143.3.1187-1193.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silver S. Transport of inorganic cations. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella. 2nd ed. Vol. 1. Washington, D.C: ASM Press; 1996. pp. 1091–1102. [Google Scholar]