Abstract
Research on aquatic plant viruses is lagging behind that of their terrestrial counterparts. To address this knowledge gap, here we identified viruses associated with freshwater macrophytes, a taxonomically diverse group of aquatic phototrophs that are visible with the naked eye. We surveyed pooled macrophyte samples collected at four spring sites in Florida, USA through next generation sequencing of RNA extracted from purified viral particles. Sequencing efforts resulted in the detection of 156 freshwater macrophyte associated (FMA) viral contigs, 37 of which approximate complete genomes or segments. FMA viral contigs represent putative members from all five major phyla of the RNA viral kingdom Orthornavirae. Similar to viral types found in land plants, viral sequences identified in macrophytes were dominated by positive-sense RNA viruses. Over half of the FMA viral contigs were most similar to viruses reported from diverse hosts in aquatic environments, including phototrophs, invertebrates, and fungi. The detection of FMA viruses from orders dominated by plant viruses, namely Patatavirales and Tymovirales, indicate that members of these orders may thrive in aquatic hosts. PCR assays confirmed the presence of putative FMA plant viruses in asymptomatic vascular plants, indicating that viruses with persistent lifestyles are widespread in macrophytes. The detection of potato virus Y and oat blue dwarf virus in submerged macrophytes suggests that terrestrial plant viruses infect underwater plants and highlights a potential terrestrial-freshwater plant virus continuum. Defining the virome of unexplored macrophytes will improve our understanding of virus evolution in terrestrial and aquatic primary producers and reveal the potential ecological impacts of viral infection in macrophytes.
Keywords: Macrophyte, RNA virus, Submerged aquatic vegetation, Virome, Metagenomics, Potato virus Y, Oat blue dwarf virus, Freshwater springs, Vallisneria, Aquatic plant
Introduction
Over a decade of research has confirmed the critical roles of viruses in the evolution and ecology of wild terrestrial vegetation (Malmstrom, Melcher & Bosque-Pérez, 2011; Roossinck, 2015; Shates et al., 2019). Yet, little is known about plant virus ecology in aquatic systems. The sparse information regarding viruses infecting aquatic vegetation was noted over 50 years ago when researchers considered viruses as an alternative strategy to control harmful freshwater plants (Zettler & Freeman, 1972). This biocontrol idea was based upon evidence from the 1950’s demonstrating viral infection in freshwater vascular plants (MacClement & Richards, 1956). During the past 70 years there has been little progress in investigating viral infection in aquatic vegetation, with the exception of single-celled phototrophs (Brussaard, 2004; Coy et al., 2018; Nagasaki, 2008; Van Etten, Agarkova & Dunigan, 2019), a knowledge gap addressed herein.
In contrast to terrestrial systems where vascular plants dominate, macrophytes, together with phytoplankton, are the principal primary producers in aquatic ecosystems (Jänes et al., 2017; Nõges, Luup & Feldmann, 2010). Macrophytes are a group of taxonomically diverse photosynthetic organisms that are visible to the naked eye and actively grow, permanently or periodically, in aquatic environments (Chambers et al., 2008; Lesiv, Polishchuk & Antonyak, 2020). This diverse group includes phototrophs spanning from cyanobacterial mats (Cyanophyta) to seven divisions within Archaeplastida, including macroalgae (Charophyceae, Chlorophyta, Rhodophyta, and Xanthophyta), non-vascular plants (Bryophyta) and vascular plants (Pteridophyta and Spermatophyta) (Chambers et al., 2008). Macrophytes play essential roles in aquatic systems by influencing habitat structure and function (e.g., modifying water current and sediment conditions), serving as food sources for a wide range of herbivores, providing habitat and a structurally complex environment, cycling carbon and nutrients, and improving water quality (Dibble, Thomaz & Padial, 2006; Duarte et al., 2013; Heck et al., 2008; Lamb et al., 2017; Lesiv, Polishchuk & Antonyak, 2020; Reitsema, Meire & Schoelynck, 2018; Rennie & Jackson, 2005; Srivastava, Gupta & Chandra, 2008; Waycott et al., 2009). Although macrophytes are important for maintaining aquatic ecosystem health and function, many species require management as they can become harmful when overgrown due to nutrient loading and/or new habitat invasion (Anderson, 2003; Smetacek & Zingone, 2013). A better understanding of factors shaping macrophyte ecology will lead to more effective conservation and management strategies for aquatic ecosystems (Chambers et al., 1999).
Viruses are expected to impact macrophytes given their effects on terrestrial plant ecology and evolution. Studies of plant viral infection in wild populations and those at the interface between agricultural and unmanaged vegetation indicate that viruses play a significant evolutionary role in plants by affecting plant fitness, population dynamics, and diversity (Kelley et al., 1994; Montes, Alonso-Blanco & García-Arenal, 2019; Remold, 2002; van Mölken & Stuefer, 2011). Although viruses are often viewed as disease agents, viral infections are prevalent in natural terrestrial systems where viruses often coexist with their plant hosts without causing negative effects, displaying mutualistic or even beneficial interactions (Boccardo et al., 1987; Roossinck, 2011; Roossinck, 2015; Roossinck & Schultz-Cherry, 2015). Limited sampling of kelp and seagrass in marine environments indicates that viral infection is also prevalent in marine macrophytes, with over 60% of sampled individuals testing positive for viruses (McKeown et al., 2018; McKeown et al., 2017; Van Bogaert et al., 2019). Similar to what has been observed in terrestrial wild vegetation (Kamitani et al., 2016; Roossinck, Martin & Roumagnac, 2015; Susi et al., 2019), these prevalent macrophyte viral infections are mainly asymptomatic. Research in freshwater systems has mainly focused on viruses infecting charophytic algae (Chara spp.), which are interesting hosts from an evolutionary standpoint due to their ancestral position relative to land plants (Zhong, Sun & Penny, 2015). Indeed, Chara-infecting viruses have unique features resembling various groups of terrestrial plant viruses and therefore may hold clues regarding plant virus evolution (Gibbs et al., 2011; Vlok, Gibbs & Suttle, 2019). More virological research on macrophytes will lead to a deeper understanding of their ecology and potential evolutionary links among freshwater, marine, and terrestrial plant viruses.
To broadly investigate freshwater macrophyte associated (FMA) viruses, we surveyed pooled samples of macrophyte species collected at four spring sites in Florida, USA. The springs have significant cultural, ecological, and economic value for the state of Florida and this study joins efforts to investigate understudied viral dynamics in these freshwater systems (Malki et al., 2020; Malki et al., 2021). The captured diversity includes viruses from all five major phyla of the RNA viral kingdom Orthornavirae that are currently recognized by the International Committee on Virus Taxonomy (ICTV). We explored taxonomic affiliations of detected viral sequences to evaluate how FMA viruses fit within the known RNA virosphere (Koonin et al., 2020). PCR assays for putative FMA plant viruses demonstrated widespread infections in sampled vascular plants. The detection of two known crop-infecting viral pathogens in submerged macrophytes suggests that terrestrial plant viruses infect underwater plants and highlights a potential terrestrial-freshwater plant virus continuum.
Materials and Methods
Study site and sample collection
Macrophyte samples were collected during July 2017 from freshwater springs located within four Florida State Parks, namely Ichetucknee, Rainbow, Manatee, and Blue Springs State Parks, in accordance with permit 06011710 from the Florida Department of Environmental Protection. Three sampling points were selected within each spring site (Table 1), starting at the spring head where underground water emerges (Location ID 1) and moving ~0.2 miles downstream in a stepwise manner (Location IDs 2 and 3, respectively). Two samples from each visually distinct macrophyte species observed within each spring location were hand-picked through wading and snorkeling. Each macrophyte sample was rinsed on site with spring water and placed into individual Ziplock© bags. Samples were kept on ice during transport to the lab where collected species were identified based on morphological features whenever possible and stored at −80 °C until further processing.
Table 1. Sampling locations and macrophytes collected within four freshwater springs.
| Spring (ID) | Latitude/Longitude (Location ID) | Collected species (Common name) | Specimen description |
|---|---|---|---|
| Blue | 28.947483/−81.339574 (1) | Lyngbya wollei * | Mat-forming cyanobacteria |
| Typha spp. (cattail) | Require perpetually moist soil | ||
| Unidentified | n/a | ||
| Hydrocotyle umbellate L. (Pennywort) | Rooted, grows in water or on land | ||
| 28.947163/−81.33964 (2) | Lyngbya wollei | Mat-forming cyanobacteria | |
| Sagittaria kurziana Glück (Springtape) | Rooted submerged plant | ||
| Tillandsia usneoides L. (Spanish moss)** | Epiphytic and rootless ‘air-plant’ | ||
| 28.944765/−81.339414 (3) | Ludwigia repens J.R. Forst. (Red ludwigia) | Rooted, grows partially or fully submerged | |
| Sagittaria lancifolia L. (Lanceleaf arrowhead) | Rooted, grows in shallow-water habitats | ||
| Hydrocotyle umbellata L. (Pennywort) | Rooted, grows in water or on land | ||
| Unidentifed | n/a | ||
| Lyngbya wollei * | Mat-forming cyanobacteria | ||
| Ichetucknee | 29.984065/−82.761744 (1) | Unidentified | n/a |
| Vallisneria americana Michx. (Tapegrass) | Rooted submerged plant | ||
| Ludwigia repens J.R. Forst. (Red ludwigia) | Rooted, grows partially or fully submerged | ||
| Hydrocotyle umbellata L. (Pennywort) | Rooted, grows in water or on land | ||
| Chara sp. (Muskgrass) | Branched macroalgae | ||
| 29.982173/−82.760423 (2) | Vallisneria americana Michx. (Tapegrass) | Rooted submerged plant | |
| Hydrocotyle umbellata L. (Pennywort) | Rooted, grows in water or on land | ||
| 29.981734/−82.760234 (3) | Ludwigia repens J.R. Forst. (Red ludwigia) | Rooted, grows partially or fully submerged | |
| Ceratophyllum demersum L. (Hornwort) | Grows free-floating and submerged | ||
| Hydrocotyle umbellata L. (Pennywort) | Rooted, grows in water or on land | ||
| Vallisneria americana Michx. (Tapegrass) | Rooted submerged plant | ||
| Manatee | 29.489562/−82.977069 (1) | Lyngbya wollei * | Mat-forming cyanobacteria |
| 29.489403/−82.977678 (2) | Lyngbya wollei * | Mat-forming cyanobacteria | |
| 29.489216/−82.978378 (3) | Lyngbya wollei * | Mat-forming cyanobacteria | |
| Unidentified | n/a | ||
| Unidentified | n/a | ||
| Unidentified | n/a | ||
| Rainbow | 29.1023/−82.437633 (1) | Hydrilla verticillata (L. f.) Royle (Waterthyme) | Rooted submerged plant |
|
Potamogeton pectinatus L. (Fennel pondweed) |
Rooted submerged plant | ||
| Utricularia sp. (Bladderwort) | Submerged or free-floating carnivorous plant | ||
|
Myriophyllum heterophyllum Michx. (Broadleaf watermilfoil) |
Rooted submerged plant | ||
|
Potamogeton illinoensis Morong (Illinois pondweed) |
Rooted submerged plant | ||
| Sagittaria kurziana Glück (Springtape) | Rooted submerged plant | ||
| 29.101762/−82.437174 (2) |
Myriophyllum heterophyllum Michx. (Broadleaf watermilfoil) |
Rooted submerged plant | |
|
Potamogeton illinoensis Morong (Illinois pondweed) |
Rooted submerged plant | ||
| Sagittaria kurziana Glück (Springtape) | Rooted submerged plant | ||
| Utricularia sp. (Bladderwort) | Submerged or free-floating carnivorous plant | ||
| Ceratophyllum demersum L. (Hornwort) | Grows free-floating and submerged | ||
|
Najas guadalupensis (Spreng.) Magnus (Southern waternymph) |
Rooted submerged plant | ||
| 29.101305/−82.436856 (3) | Sagittaria kurziana Glück (Springtape) | Rooted submerged plant | |
| Cladium jamaicense L. (Saw-grass) | Rooted, water-loving (grows in wet or dry soil) | ||
| Ludwigia repens J.R. Forst. (Red ludwigia) | Rooted, grows partially or fully submerged | ||
| Hydrocotyle umbellata L. (Pennywort) | Rooted, grows in water or on land |
Notes:
Lyngbya wollei is currently regarded as a synonym of Microseira wollei.
Spanish moss is not considered a macrophyte.
n/a, not available.
Virus particle purification from macrophyte tissues and nucleic acid extraction
Virus particles were purified from macrophyte tissues prior to nucleic acid extractions following previously described methods for submerged aquatic vegetation (Van Bogaert et al., 2019). Samples were thawed, rinsed with MilliQ water, and visible epiphytes were carefully removed using sterile scalpel blades. Approximately 200 mg of tissue from each macrophyte species (i.e., 100 mg per each individual plant from a given species from each location) were placed in a 1.5 ml Zymo© bead beating tube containing 2 mm ceramic beads (Zymo Research, Irvine, CA, USA) with 800 µl of Suspension Medium (SM) buffer (0.1 M NaCl, 50 mM Tris-HCl (pH 7.5), 10 mM MgSO4). Tissues were homogenized through bead-beating using a Fisherbrand™ Bead Mill 4 Homogenizer (Fisher Scientific, Waltham, MA, USA) at maximum speed for 90 s. Homogenates were then centrifuged at 4 °C for 10 min at 10,000×g and supernatants were filtered through a 0.22 µm Sterivex (Millipore, Burlington, MA, USA). Filtrates containing partially purified virus particles were treated with chloroform (20% final concentration), vortexed vigorously, and incubated at room temperature for 10 min to disrupt any remaining lipid-containing entities such as cell membranes. After centrifuging the chloroform mixture for 30 s at room temperature, the aqueous fraction was collected and nuclease-treated to remove non-encapsidated nucleic acids. Nuclease treatment was performed by incubating the aqueous fraction for 1.5 h at 37 °C with a nuclease cocktail consisting of 1X Turbo DNase Buffer (Invitrogen, Waltham, MA, USA), 21 U of Turbo DNase (Invitrogen, Waltham, MA, USA), 4.5 U of Baseline-ZERO DNase (Epicentre, Paris, France), 112.5 U Benzonase (EMD Millipore, Burlington, MA, USA), and 400 U RNase I (Ambion, Austin, TX, USA) (Ng et al., 2012; Victoria et al., 2009). Nucleases were inactivated with 20 mM EDTA (pH = 8.0) prior to nucleic acid extractions. Nucleic acids were extracted from 200 µl of purified viral fraction in one of two ways (Table 2). Samples that were used for an initial round of next-generation sequencing (NGS) were extracted using the QIAamp MinElute Virus Spin kit (Qiagen, Hilden, Germany). Samples used for a second NGS run were extracted using the RNeasy kit (Qiagen, Hilden, Germany) with the on-column DNase I digestion. Both types of extractions were performed following manufacturer’s protocols.
Table 2. Methods used for preparing and sequencing samples through two independent NGS runs.
| Site | NGS round | Extraction kit (Qiagen) | Reverse transcription approach* | Product clean-up** |
Library type | # of indexing PCR cycles | Illumina platform |
|---|---|---|---|---|---|---|---|
| Blue | 1 | QIAamp MinElute Virus Spin kit | Random hexamers | AMpure XP beads | cDNA | 20 | NextSeq |
| 2 | RNeasy kit | Random hexamers | Spin Column | cDNA | 18 | HiSeq | |
| 2 | RNeasy kit | SISPA | Spin Column | SISPA | 5 | HiSeq | |
| Ichetucknee | 1 | QIAamp MinElute Virus Spin kit | Random hexamers | AMpure XP beads | cDNA | 20 | NextSeq |
| 2 | RNeasy kit | Random hexamers | Spin Column | cDNA | 18 | HiSeq | |
| 2 | RNeasy kit | SISPA | Spin Column | SISPA | 5 | HiSeq | |
| Manatee | 1 | QIAamp MinElute Virus Spin kit | Random hexamers | AMpure XP beads | cDNA | 20 | NextSeq |
| 2 | RNeasy kit | Random hexamers | Spin Column | cDNA | 18 | HiSeq | |
| 2 | RNeasy kit | SISPA | Spin Column | SISPA | 5 | HiSeq | |
| Rainbow | 1 | QIAamp MinElute Virus Spin kit | Random hexamers | AMpure XP beads | cDNA | 20 | NextSeq |
Notes:
Distinguishes if cDNA was obtained using random hexamers included with the Superscript First-Strand Synthesis System (Invitrogen) or primers used for sequence-independent, single-primer amplification (SISPA).
Procedure used to clean-up Klenow reaction products (cDNA libraries) and SISPA products prior to NGS library preparation. The spin column procedure was performed with the DNA Clean & Concentrator®-25 kit (Zymo Research).
Reverse-transcription for NGS library preparation
Extracted nucleic acids from individual macrophyte species were pooled by spring site, resulting in the following four pools: Blue (12 species), Ichetucknee (11 species), Manatee (six species), and Rainbow (16 species) (Table 1). Pooled nucleic acids were reversed-transcribed for two independent NGS efforts (Table 2). Reverse transcription was performed with the Superscript IV First-Strand Synthesis System for RT-PCR (Invitrogen, Waltham, MA, USA) using random hexamers provided by the manufacturer or a random primer tagged with a known linker sequence following manufacturer’s protocols. Products from the former were used without pre-amplification for NGS library preparation (cDNA libraries), whereas products from the latter were used for sequence-independent, single-primer amplification (SISPA libraries, see below) (Table 2). Reverse-transcribed products obtained with random hexamers for cDNA libraries were subjected to second-strand synthesis with the Klenow Fragment DNA Polymerase (New England Biolabs, Ipswich, MA, USA). For cDNA libraries, 80 µl of double-stranded reverse-transcribed product were prepared from each spring site pool and purified with either Agencourt AMpure XP beads (Beckman-Coulter, Brea, CA, USA) or the DNA Clean & Concentrator®-25 (Zymo Research, Irvine, CA, USA) for fragmentation and NGS library preparation (Table 2).
SISPA for NGS library preparation
Randomly generated cDNA products used for SISPA were obtained using Primer_A (5′-GTTTCCCAGTCACGATANNNNNNNNN-3′) (Gaynor et al., 2007) and Primer_N1-8 (5′-CCTTGAAGGCGGACTGTGAGNNNNNNNN-3′) (Ng et al., 2015) in separate reactions. Complementary strands for these reverse-transcribed products were synthesized with the Klenow Fragment DNA Polymerase (New England Biolabs, Ipswich, MA, USA). Double-stranded cDNA products were then amplified using 2 µM of the appropriate primer containing the linker sequence alone (underlined primer sequence above) (Table S1). The PCR reaction contained 5 µl of template, 3.7 U AmpliTaq Gold polymerase (Thermo Fisher Scientific, Waltham, MA, USA), 4 mM MgCl2, 0.25 mM dNTPs, and 1X PCR Gold buffer in a 50 µl reaction volume. For reactions using Primer_N1, thermocycling conditions were performed with an initial denaturation at 95 °C for 5 min, followed by 5 cycles of [95 °C for 1 min, 59 °C for 1 min, 72 °C for 90 s], 40 cycles of [95 °C for 30 s, 59 °C for 30 s, 72 °C for 90 s with an increased extension time of 2 s per cycle], and a final extension at 72 °C for 10 min. Reactions with Primer_B used the same thermocycling settings with the exception of no incremental extension time as the 40 cycles progressed. SISPA products were confirmed through gel electrophoresis using a 1.5% agarose gel stained with ethidium bromide. After visualization, all SISPA products were cleaned with the Zymo DNA & Concentrator Kit-25 (Zymo Research, Irvine, CA, USA). SISPA products from Primer A/B and N1-8/N reactions were pooled by spring site by combining 40 µl of cleaned product from each reaction prior to NGS library preparation.
NGS library preparation
Samples were sequenced through two independent NGS runs of opportunity (Table 2). Non-amplified double-stranded cDNA samples were fragmented to 300 bp using a Covaris M220 instrument and used as templates for NGS library preparation (cDNA libraries), whereas SISPA products were not further fragmented prior to library preparation (SISPA libraries). All libraries were prepared for multiplexing using the Accel-NGS 1S Plus DNA Library kit for Illumina Platforms (Swift Biosciences, Ann Arbor, MI, USA) following the manufacturer’s instructions for DNA inputs <1 ng/µl for the cDNA libraries and >1 ng/µl for SISPA libraries. For the first NGS round, four cDNA libraries representing pooled samples from each spring site were paired-end sequenced (2 × 150 bp) using a mid-output v2.5 (300 cycles) kit on a NextSeq 500 platform (Illumina, San Diego, CA, USA) at the University of Colorado BioFrontiers Next-Gen Sequencing core facility. The second NGS round included cDNA (n = 3) and SISPA (n = 3) libraries from the Blue, Ichetucknee, and Manatee spring sites. Libraries from the second round were commercially paired-end sequenced (2 × 150 bp) on a HiSeq system (Illumina, San Diego, CA, USA) at GENEWIZ. Raw NGS data can be found in the Sequence Read Archive (SRA) database under BioProject accession number PRJNA826216.
Sequence analysis
Sequences from both NGS rounds were analyzed using the University of South Florida high performance computing cluster. Raw sequences were trimmed for quality and to remove indexing adapters and SISPA primers (if applicable) using Trimmomatic version 0.36.0 (Bolger, Lohse & Usadel, 2014) with default parameters except for a read head crop of 10 bp instead of zero. Sequence quality after trimming was verified with FastQC version 0.11.5 (Andrews, 2010). Various assembly strategies using the SPAdes assembly toolkit (Nurk et al., 2013), including command line flags for rna-, single-cell, and meta-SPAdes, were evaluated and final strategies were selected based on the approach producing the longest contigs as determined by QUAST (Gurevich et al., 2013). Due to the high number of indexing PCR cycles (Table 2), quality-filtered sequences from cDNA libraries were assembled following a pipeline for PCR amplified libraries (Roux et al., 2019). To do this, identical reads (no mismatches) were deduplicated using the Clumpify tool (parameters: “dedupe subs=0 passes=2”) from the BBtools package (sourceforge.net/projects/bbmap/). Deduplicated sequences were then assembled using single-cell SPAdes (Bankevich et al., 2012). Quality-filtered sequences from SISPA libraries were assembled using metaSPAdes v 3.11.1 with default parameters (Nurk et al., 2017). For all libraries, assembled contigs larger than 1,000 bp were selected using the Galaxy public server (usegalaxy.org) (Afgan et al., 2018) and compared against the NCBI Reference Sequence viral protein database (RefSeq Release number 93, https://www.ncbi.nlm.nih.gov/refseq/) using BLASTx (e-value < 10−10). BLAST results were explored using MEGAN6 Community Edition (Huson et al., 2016) to identify contigs with matches to RNA viral sequences. These contigs were then compared against the GenBank non-redundant (nr) database (BLASTx, e-value 0.001) to remove contig sequences that had higher identities with non-viral sequences (i.e., false positives). Libraries from NGS Round 2 were accidentally contaminated with turtlegrass virus X (TVX; accession number MH077559). Therefore, contigs representing TVX were also removed from further analyses.
Once viral contigs were identified from each library, a non-redundant file containing contigs from all libraries was created for read mapping analyses. To do this, open reading frames (ORFs) >450 nt were identified using the getorf application from the EMBOSS suite (Rice, Longden & Bleasby, 2000) as implemented in the Galaxy public server. These ORFs were then used to identify viral contigs containing ORFs sharing >90% identity using CD-HIT (Fu et al., 2012). Trimmed and deduplicated forward reads from each library were mapped to the non-redundant viral contig file using BowtieBatch v 1.0.1 and Read2RefMapper v 1.0.1 applications in the CyVerse Cyberinfrastructure (Goff et al., 2011) as part of the iVirus pipeline (Bolduc et al., 2017). Reads were mapped if they shared >90% identity with a given contig and contigs were considered present in a given sample pool if reads mapped to >75% of the contig length. The number of reads mapping to a given contig was normalized by contig length. Read mapping was used to evaluate the relative distribution of contigs within a given sample pool and whether contigs were present in more than one pool (i.e., spring site). The relative distribution of viral contigs in each pool was summarized in a heatmap created using the superheat package in R (https://rlbarter.github.io/superheat/).
Near-complete genomes
Viral contigs approximating complete genomes or genome segments based on known lengths for their predicted taxonomic group were annotated using Geneious v R8. Potential assembly errors in some contigs, based on known genome organizations, were verified by reassembling reads and contigs using the default Geneious overlap layout consensus assembler and/or mapping against the original contig using default parameters to evaluate coverage across the genome. ORFs were compared against GenBank nr and Conserved Domain (CDD) databases for annotation purposes. If no significant matches were found in these databases, ORFs were compared against potential remote homologs using HHpred (Hildebrand et al., 2009) as implemented in the MPI Bioinformatics Toolkit public server (https://toolkit.tuebingen.mpg.de/) (Zimmermann et al., 2018). Near-complete RNA viral genomes or segments are available through GenBank under accession numbers ON125107 to ON125143.
Phylogenetic analyses
Novel RNA viral sequences identified in this study were compared to previously reported RNA viruses by constructing RNA-directed RNA polymerase (RdRp) phylogenetic trees to evaluate taxonomic affiliations. Only FMA RdRp amino acid sequences that did not contain early stop codons and were >60% the expected length based on reported sequences for a given taxon were included in the analysis. To construct phylogenetic trees, the most similar RdRp amino acid sequences to a given sequence of interest were retrieved through BLAST. Additionally, representative curated RdRp amino acid sequences previously used for a comprehensive analysis of the five major branches of the global RNA virome were retrieved from the supplemental materials provided by Wolf et al. (2018). For each group of interest, sequences were aligned using the MAFFT alignment server (Katoh, Rozewicki & Yamada, 2019). Homologous protein sequences were automatically added based on structural alignments from the Database of Aligned Structural Homologs (DASH) to guide alignments, but these sequences were removed from the output alignment file. Poorly aligned regions were removed from alignments using TrimAl (Capella-Gutierrez, Silla-Martinez & Gabaldon, 2009) with the gappyout method as implemented in the Phylemon2 server (Sanchez et al., 2011). The trimmed alignments were used to construct maximum likelihood (ML) phylogenetic trees using PhyML (Guindon et al., 2010) with default parameters and automatic selection of best substitution model based on Akaike information criterion (Lefort, Longueville & Gascuel, 2017). Support for specific nodes on the trees were assessed using the approximate likelihood ratio test (aLRT) with the nonparametric Shimodaira-Hasegawa-like procedure (Guindon et al., 2010). Output tree files were visualized and edited using the ggtree R package (Yu, 2020; Yu et al., 2017).
PCR assays for detecting putative plant viruses
Since NGS was performed on pooled macrophytes from each site, PCR assays were designed to determine which macrophyte species contained putative plant viruses. Specific PCR primers for each putative FMA plant virus were designed using Primer3 (Untergasser et al., 2012) (Table S1) and applied to nucleic acid extracts from purified virus particles from individual macrophyte species collected at each spring site. Single-stranded cDNA was synthesized from extracts using the Superscript IV First-Strand Synthesis System for RT-PCR (Invitrogen, Waltham, MA, USA) with random hexamers provided by the manufacturer. All PCRs were performed using the AmpliTaq Gold™ 360 Master Mix with GC enhancer (Thermo Fisher Scientific, Waltham, MA, USA). Each 25 µl PCR reaction contained 2 µl of cDNA, 1X AmpliTaq Gold™ 360 Master Mix, 0.96 µM of each primer, and 1 µl 360 GC Enhancer. Thermocycling conditions were performed with an initial denaturation at 95 °C for 10 min, followed by 40 cycles of [95 °C for 30 s, 50 °C for 30 s, 72 °C for 60 s], and a final extension at 72 °C for 10 min. A sample was considered positive for a given virus if a single band of the expected length (Table S1) was observed through gel electrophoresis using a 1.5% agarose gel stained with ethidium bromide.
Results and discussion
FMA viruses are diverse and dominated by positive-stranded RNA viruses
This study surveyed the diversity of RNA viruses associated with macrophytes in four freshwater springs located in Florida, USA. Viromic sequencing efforts resulted in the detection of 156 distinct RNA viral contigs >1 kilobase (kb) in length, 37 of which represent near-complete genomes or genomic segments (Data S2, Table S2). Two distinct approaches using two independent NGS runs of opportunity were used to sequence viral nucleic acids purified from macrophyte tissues with the goal of capturing a diversity of RNA viruses (Table 2). One approach targeted viral cDNA without preamplification for NGS (cDNA libraries). The second approach exploited SISPA, a random amplification technique prior to library preparation that has been previously used for viral discovery in macrophytes (Van Bogaert et al., 2019). The 156 FMA viral contigs presented here describe the combined results, although it is noteworthy that each NGS round and approach identified unique viral contigs (Fig. S1). When comparing results from the same sequencing round (NGS Round 2), it is clear that few viral contigs were recovered from the SISPA libraries that were not also identified in the cDNA libraries. Therefore, cDNA libraries were a more fruitful approach for viral discovery in freshwater macrophytes (Fig. S1).
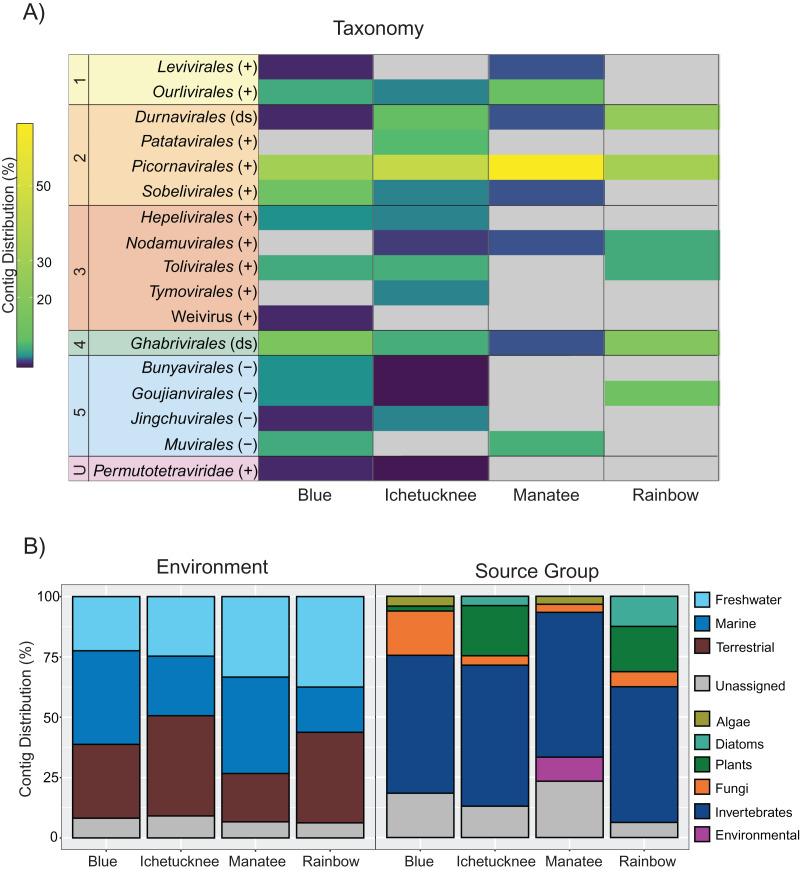
Viral contigs were detected in pooled macrophyte samples from each of the four surveyed spring sites, including Blue (n = 49), Ichetucknee (n = 76), Manatee (n = 30), and Rainbow (n = 16) (Fig. S2). Identified contigs in each spring site were dominated by those representing positive-sense, single-stranded (+) RNA viruses (Fig. 1A). This is consistent with the dominance of +RNA viral types identified in land plant (Dolja, Krupovic & Koonin, 2020) and global RNA viromes (Wolf et al., 2018), including those from aquatic environments (Vlok, Lang & Suttle, 2019b; Wolf et al., 2020). Notably, over 50% of the contigs from macrophyte pools from all the investigated springs were most similar to viral sequences retrieved from aquatic organisms or environments (Fig. 1B).
Figure 1. Distribution of freshwater macrophyte associated (FMA) viral contigs.
(A) Heatmap showing contig distribution based on taxonomic groups. (B) Graphs showing contig distribution based on information about the closest BLASTx match, including type of environment (left) and isolation source organism (right). The color scale on the heatmap represents low (dark purple) to high (yellow) proportion of contigs in a given taxonomic group based on the total number of viral contigs identified in a given spring dataset. Gray color on the heatmap indicates taxonomic groups that were not detected in a spring dataset. Numbers on the left-hand side of the heatmap highlight groups representing each of the five major phyla of the Orthornavirae kingdom, including Lenarviricota (1), Pisuviricota (2), Kitrinaviricota (3), Duplornaviricota (4), and Negarnaviricota (5), whereas the letter “U” specifies an unclassified group. Genome types for each group are indicated within parentheses, including positive (+) and negative (−) single-stranded and double-stranded (ds) RNA viruses. ‘Unassigned’ in bottom panels refers to contigs that could not be assigned to a given category due to BLASTx matches to viruses from multiple categories.
FMA viral contigs spanned five major phyla within the Orthornavirae kingdom (RdRp-encoding viruses) as well as unclassified viruses that have not been accommodated within the current taxonomic framework accepted by the ICTV (Koonin et al., 2020) (Fig. 1A). Due to low amino acid sequence identities to known viruses (as low as 20% in some cases, Table S2), most genomic sequences representing novel viruses were labelled here at the order rank to evaluate trends at broader levels of resolution. Datasets from all spring sites were dominated by contigs most similar to members of the order Picornavirales and contained contigs representing members of the orders Durnavirales and Ghabrivirales (Fig. 1A). However, few contigs were shared across macrophyte pools (Fig. S2). This was expected considering that pools were composed of different macrophytes with little species overlap and no single macrophyte species was collected from all four spring sites (Table 1). The highest number of shared contigs (n = 6) was observed between Manatee and Blue datasets (Table S2). Manatee had the lowest diversity of collected macrophytes, but half of the macrophytes represented the cyanobacterium Lyngbya wollei. Blue was the only other spring where L. wollei was found, and it was collected from each of the three collection locations within the spring. However, it remains to be determined if shared contigs from Blue and Manatee datasets represent viruses associated with L. wollei.
FMA viruses are most similar to viruses infecting a diversity of hosts
Although the survey presented here was conducted in an attempt to identify potential macrophyte-infecting viruses, identified FMA contigs shared similarities with viruses infecting diverse hosts. More than half of the identified contigs in each pool were most similar to viruses associated with invertebrates (Fig. 1B). All of the datasets contained contigs most similar to mycoviruses (i.e., fungi-infecting viruses) and viruses infecting photosynthetic organisms. Putative plant viruses were identified in macrophyte pools from three spring sites, including Blue, Ichetucknee, and Rainbow. Viruses similar to diatom-infecting viruses were identified in Ichetucknee and Rainbow, whereas viruses most similar to those infecting algae were identified in Blue and Manatee macrophyte pools.
Given the low identities to known viruses it is not possible to predict the hosts of most FMA viruses identified here based on sequence information alone. This is further complicated by shared evolutionary histories among plant-, invertebrate- (i.e., arthropods), and fungi-infecting viruses that result from complex symbiotic relationships among their hosts (Dolja & Koonin, 2018; Dolja, Krupovic & Koonin, 2020; Lefeuvre et al., 2019; Roossinck, 2019). Symbiotic associations may allow extensive horizontal virus transfers among disparate hosts. For example, the closest relatives of plant viruses include fungal and arthropod viruses, reflecting strong ties among their hosts (Dolja, Krupovic & Koonin, 2020; Lefeuvre et al., 2019; Roossinck, 2019). Moreover, some plant viruses may replicate within fungi (Andika et al., 2017; Mascia et al., 2019) and arthropod vectors (Hogenhout et al., 2008) further highlighting cross-kingdom viral infections and blurring the lines of what is called a ‘plant virus’ (Lefeuvre et al., 2019).
Despite our limited ability to predict hosts, the viral diversity captured here indicates that macrophytes harbor novel viruses infecting these essential primary producers and members of their holobionts. Macrophytes may also serve as reservoirs for viruses infecting organisms that interact with these autotrophs (e.g., invertebrates). Below we outline taxonomic relationships for each phylum representing the five major branches of the Orthornavirae (Koonin et al., 2020) to highlight how the viral diversity recovered from freshwater macrophytes fits within the known RNA virosphere. Phylogenetic relationships did not tease apart potential hosts in many cases due to overlapping viromes among fungi, plants, and metazoans (Dolja & Koonin, 2018; Dolja, Krupovic & Koonin, 2020) and similarities to viruses for which definitive hosts have not been identified. Nevertheless, the genetic data presented here provides a critical starting point to design targeted assays to further investigate FMA viruses and their hosts.
Branch 1: Lenarviricota FMA viruses include putative bacterial and invertebrate viruses
The phylum Lenarviricota occupies the most basal position of the Orthornavirae RdRp tree and includes viruses that infect prokaryotes and eukaryotes (Callanan et al., 2021; Koonin et al., 2020; Wolf et al., 2018). Lenarviricota FMA viruses include putative members of the class Leviviricetes and order Ourlivirales, designated here FMA levi-like viruses and FMA ourli-like viruses, respectively.
The majority of Lenarviricota FMA viral contigs (8 out of 10) are most similar to members of the Ourlivirales, including one near-complete genome (Fig. 2, Table S2). Currently classified members of this order fall within the family Botourmiaviridae and include plant viruses and mycoviruses (Ayllón et al., 2020). However, a diversity of invertebrate-associated viruses originally labelled as ‘narna-like’ viruses also belong to this family (Shi et al., 2016; Wolf et al., 2018). Although invertebrate-associated botourmiavirus hosts remain unverified, intermediate to high (>0.1% to >1% of non-ribosomal RNA reads) abundance levels for several of these viruses in sampled specimens suggest that they are transcriptionally active in invertebrates (Shi et al., 2016). All FMA ourli-like viral sequences are most similar to invertebrate-associated botourmiaviruses from aquatic environments, with the exception of FMA ourli-like virus 4 which is most similar to a virus discovered from the root of an apple tree (apple narna-like virus 2) (Table S2). Phylogenetic analyses confirmed that FMA ourli-like viral sequences cluster within the Botourmiaviridae clade (Fig. S3).
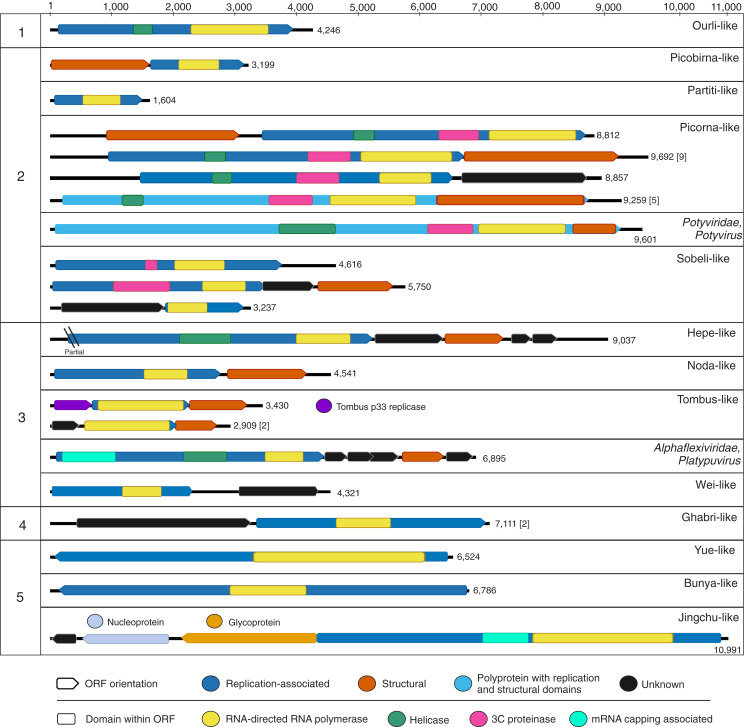
Figure 2. Genome schematics of near-complete freshwater macrophyte associated (FMA) viral genomes or segments.
Numbers across the top depict a genome size ruler, whereas numbers on the left-hand side group sequences based on the five major branches of the Orthornavirae kingdom representing the Lenarviricota (1), Pisuviricota (2), Kitrinaviricota (3), Duplornaviricota (4), and Negarnaviricota (5) phyla. Numbers on the right-hand side of each genome schematic specify genome size. Genomes with a similar organization are represented by a single schematic and the total number of represented genomes is summarized within brackets. The legend at the bottom of the figure highlights open reading frames (ORF) and predicted protein domains observed in more than one sequence. ORFs observed in a single genome or segment are indicated within their respective panels.
Botourmiaviruses infecting different host groups have distinguishing genome features. Plant-infecting botourmiviruses (genus Ourmiavirus) are segmented, with each segment encoding a single protein including RdRp, movement and capsid proteins. On the other hand, mycovirus genomes belonging to the Botoulivirus, Magoulivirus and Scleroulivirus genera are monocistronic and non-segmented, only encoding for the RdRp. Reported invertebrate-associated botourmiaviruses are non-segmented and encode RdRp alone, similar to mycoviruses from this group, or exhibit a novel organization encoding RdRp and capsid proteins in a putative dicistronic genome (Shi et al., 2016). Moreover, several of the invertebrate-associated monocistronic botourmiaviruses contain a picorna-like helicase domain (pfam 00910) within the RdRp ORF that has not been observed in other members of the Ourlivirales. One such virus is Wenzhou shrimp virus 10, which was presumed to be transcriptionally active in sampled shrimp based on a high proportion of viral RNA transcripts (Shi et al., 2016). The FMA ourli-like virus 1 near-complete genome length (~4.2 kb) and organization matches that of Wenzhou shrimp virus 10 and other monocistronic invertebrate-associated botourmiaviruses (Fig. 2). The remaining FMA viral sequences were most similar to monocistronic and bicistronic invertebrate-associated botourmiaviruses and the monocistronic Apple narna-like virus 2 (Table S2). We were not able to distinguish or predict genome organizations based on the phylogenetic position of individual invertebrate-associated botourmiaviruses. Nevertheless, similarities to viruses containing genomic features that have only been observed in invertebrate-associated viruses indicate that FMA ourli-like viruses may infect invertebrates rather than fungi or macrophytes.
The remaining two Lenarviricota FMA viral contigs (FMA levi-like virus 1 and 2) are most similar to a virus identified from a freshwater mollusk, namely Wenzhou levi-like virus 1 (Table S2). Note that levi-like viruses are distinct from members of the family Fiersviridae (formerly named Leviviridae), which infect gram-negative bacteria (Bollback & Huelsenbeck, 2001). Although levi-like viruses are expected to infect prokaryotes, these viruses have only been discovered through metaviromics and metatranscriptomic studies investigating viruses associated with invertebrates (Shi et al., 2016) and environmental samples (Callanan et al., 2020; Krishnamurthy et al., 2016; Starr et al., 2019). Phylogenetic analyses confirmed that FMA levi-like RdRp sequences cluster with novel levi-like viruses (Fig. S3). Specifically, FMA levi-like sequences group in a small clade with levi-like viruses associated with freshwater mollusks and crustaceans. Interestingly, FMA levi-like viruses were detected in the Manatee and Blue datasets, which were the only macrophyte pools that included extracts from L. wollei cyanobacterial mats. PCR data confirmed the association of FMA levi-like viral sequences with L. wollei (Table 3). Future work is needed to definitively determine if FMA levi-like viruses infect L. wollei. This is a topic of substantial interest since the presence of cyanobacterial mats in freshwater systems signals ecosystem degradation (Hudon, Sève & Cattaneo, 2014) and L. wollei’s widespread distribution and high coverage in Florida’s springs has become a management concern (Stevenson et al., 2007). Therefore, a phage that infects L. wollei might serve as a potential biocontrol agent.
Table 3. Putative plant viruses identified in individual macrophyte species through PCR.
| Spring (Site)* | Species | FMA virus** | Top BLASTx match*** | Identity (%) |
|---|---|---|---|---|
| Blue (1) | Unidentified | levi-like virus 1 | Wenzhou levi-like virus 1 | 42 |
| Blue (2) | Lyngbya wollei | levi-like virus 2A | Wenzhou levi-like virus 1 | 72 |
| Tillandsia usneoides L. | tombus-like virus 6 | Soybean yellow mottle mosaic virus | 37 | |
| Iche (1) | Vallisneria americana Michx. | partiti-like virus 3B | Rose cryptic virus 1 | 42 |
| Iche (2) | Vallisneria americana Michx. | alphaflexi-like virus 1C | Donkey orchid symptomless virus | 38 |
| poty-like virus 2 | Potato virus A | 57 | ||
| poty-like virus 3 | Malva vein clearing virus | 64 | ||
| poty-like virus 5 | Pokeweed mosaic virus | 72 | ||
| poty-like virus 6D | Turnip mosaic virus | 54 | ||
| poty-like virus 4 | Potato virus B | 33 | ||
| tymo-like virus 2 | Oat blue dwarf virus | 92 | ||
| Hydrocotyle umbellate L. | partiti-like virus 3B | Rose cryptic virus 1 | 42 | |
| picorna-like virus 55E | Cherry virus Trakiya | 26 | ||
| Iche (3) |
Ludwigia repens J.R. Forst. |
partiti-like virus 11 | Melon partitivirus | 65 |
| potato virus Y | Potato virus Y | 95 | ||
| poty-like virus 6D | Turnip mosaic virus | 54 | ||
| Hydrocotyle umbellate L. | partiti-like virus 3B | Rose cryptic virus 1 | 42 | |
| picorna-like virus 55E | Cherry virus Trakiya | 26 | ||
| Vallisneria americana Michx. | alphaflexi-like virus 1C | Donkey orchid symptomless virus | 38 | |
| picorna-like virus 55E | Cherry virus Trakiya | 26 | ||
| sobeli-like virus 1 | Kummerowia striatad enamovirus | 34 | ||
| Man (1) | Lyngbya wollei | levi-like virus 2A | Wenzhou levi-like virus 1 | 72 |
| Man (2) | Lyngbya wollei | levi-like virus 2A | Wenzhou levi-like virus 1 | 72 |
| Man (3) | Lyngbya wollei | levi-like virus 2A | Wenzhou levi-like virus 1 | 72 |
| Rain (2) | Potamogeton illinoensis Morong | partiti-like virus 4F | Rose cryptic virus 1 | 43 |
| partiti-like virus 10G | Pepper cryptic virus 1 | 43 | ||
| Sagittaria kurziana Glück | partiti-like virus 4F | Rose cryptic virus 1 | 43 | |
| Utricularia sp | partiti-like virus 10G | Pepper cryptic virus 1 | 43 | |
| Ceratophyllum demersum L. | partiti-like virus 10G | Pepper cryptic virus 1 | 43 | |
| Najas guadalupensis (Spreng.) Magnus | partiti-like virus 4F | Rose cryptic virus 1 | 43 | |
| partiti-like virus 10G | Pepper cryptic virus 1 | 43 |
Branch 2: Pisuviricota viruses dominate FMA viral diversity
Pisuviricota is the most diverse phylum of the Orthornavirae kingdom in terms of genome architectures and number of species (Koonin et al., 2020). Pisuviricota members infect four out of five eukaryotic supergroups, suggesting that viruses from this group evolved prior to the radiation of eukaryotes (Koonin et al., 2008). Half of the identified FMA viruses fall within this phylum, with putative members from the orders Durnavirales, Picornavirales, Sobelivirales and Patatavirales. These groups are composed of +RNA viruses, with the exception of Durnavirales which includes the only double-stranded (ds) RNA viruses within the phylum.
Durnavirales FMA viruses include plant partiti-like viruses and an aquatic picobirna-like virus
Durnavirales FMA viral sequences were most similar to members of two distinct families, the Partitiviridae and Picobirnaviridae. These viruses were preliminarily designated FMA partiti-like viruses 1 through 11 and FMA picorbirna-like virus 1, respectively. Both families are characterized by viruses with segmented dsRNA genomes. Partitiviruses can have more than two segments with two essential genome segments, dsRNA1 and dsRNA2, encoding RdRp and capsid proteins, respectively, whereas picobirnaviruses are bisegmented and each segment encodes a single (RdRp) or two (capsid and hypothetical) proteins. Based on known segment lengths, we identified the near-complete sequence of the RdRp-encoding segment of FMA partiti-like viruses 1 through 3 and FMA picobirna-like virus 1 (Fig. 2).
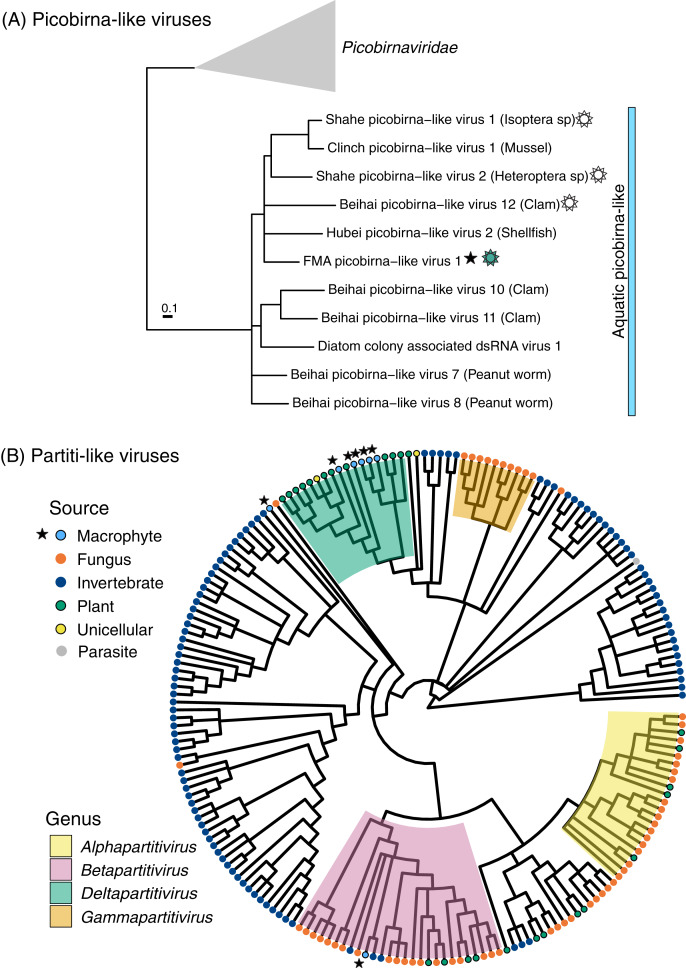
The majority of FMA viruses representing Durnavirales (11 out of 12) were most similar to members of the family Partitiviridae with matches to partiti-like RdRps or capsids associated with invertebrates (n = 3), fungi (n = 1), and plants (n = 7) (Table S2). A phylogenetic analysis including seven FMA viral RdRps revealed that the majority (five) of these sequences clustered within the genus Deltapartitivirus, which is composed of plant viruses (Fig. 3) (Vainio et al., 2018). One of the remaining sequences clustered with the genus Betapartitivirus, which includes plant viruses and mycoviruses (Vainio et al., 2018), as well as invertebrate-associated partiti-like viruses for which a host has not been determined. Therefore, the majority of FMA partiti-like sequences detected here likely represent novel plant deltapartitiviruses. Remaining sequences may represent invertebrate viruses or mycoviruses, a determination that cannot be made based on sequence similarities and phylogeny alone.
Figure 3. Midpoint-rooted maximum likelihood phylogenetic trees for members of the order Durnavirales, including picobirna-like (A) and partiti-like (B) viruses based on predicted RdRp amino acid sequences.
Freshwater macrophyte associated (FMA) sequences are highlighted with a star. The blue bar on the picobirna-like virus tree highlights sequences that were retrieved from aquatic organisms, which are specified within parenthesis after virus names. The sun symbol points to putative non-segmented genomes in this ‘aquatic’ picobirna-like clade. Branches with <90% and <70% aLRT support values in picobirna- and partiti-like virus trees, respectively, were collapsed.
FMA picobirna-like virus 1 is most similar to an arthropod-associated virus, Shahe picobirna-like virus 2 (Table S2). In addition to encoding an RdRp, this putative genome segment encodes a second hypothetical protein with matches to picobirnavirus capsids based on HHpred searches (Fig. 2). This is a novel organization for picobirnaviruses, but some genomes reported from invertebrates, including Shahe picobirna-like virus 2, contain a similar genome organization (Shi et al., 2016). Picobirnaviruses were discovered from fecal samples of various vertebrates, mainly mammals, but the picobirnavirus host range remains unclear (Ghosh & Malik, 2021). The presence of conserved ribosomal binding sites (RBS) in the genomes of picobirnaviruses reported from vertebrates suggested that these viruses infect bacteria (Boros et al., 2018; Krishnamurthy & Wang, 2018). The potential host range for picobirnaviruses has been extended to include invertebrates due to their identification in transcriptomes; however, none of the detected viruses were considered transcriptionally active within sampled invertebrates (Shi et al., 2016). Additionally, exploration of alternative codons has prompted the possibility that some picobirnaviruses may infect fungi with a lifestyle reminiscent of mitoviruses (Ghosh & Malik, 2021) and a scenario where picobirna-like viruses infect unicellular eukaryotes has been noted (Green et al., 1999).
A phylogenetic analysis of RdRps found using the standard code confirmed that FMA picobirna-like virus 1 clusters with invertebrate-associated picobirna-like viruses as opposed to clustering with members of the family Picobirnaviridae, which includes viruses isolated from vertebrates (Fig. 3). RBS were not detected in FMA picobirna-like virus 1 or any other members of the invertebrate-associated picobirna-like virus clade, which were all retrieved from aquatic environments. This clade also includes a virus discovered from diatoms using a technique that targets intracellular dsRNA, suggesting that the virus infects diatoms (Urayama, Takaki & Nunoura, 2016). Although the lack of RBS is not predictive of an eukaryote-infecting virus (Krishnamurthy & Wang, 2018), the distinct clade of invertebrate- and diatom-associated picobirna-like viruses with unique genomic features (i.e., lack of RBS and dicistronic segments in some cases) raises the possibility of a picobirnavirus lineage that infects eukaryotes in aquatic environments. More sampling and targeted studies are needed to evaluate this possibility.
Picornavirales FMA viruses are dominated by putative aquatic invertebrate viruses
The order Picornavirales encompasses an expansive group of +RNA viruses infecting unicellular organisms, plants, and metazoans and represents the largest order of the phylum Pisuviricota (Koonin et al., 2020; Wolf et al., 2018). FMA viruses from all spring sites were dominated by members of this order (Fig. 1A). There are 66 viral contigs matching picorna-like viruses (Table S2), including 16 near-complete genomes that exhibit the typical Helicase-Proteinase-Polymerase domain organization within polyproteins or replication-associated ORFs (Fig. 2) (Koonin et al., 2008). These near-complete genomes contained two main genome organizations, monocistronic genomes encoding a polyprotein (n = 5) and dicistronic genomes encoding non-structural and structural proteins in separate ORFs (n = 11). All five monocistronic genomes have the Helicase-Proteinase-Polymerase-Capsid domain organization reminiscent of some members of the family Marnaviridae (Vlok, Lang & Suttle, 2019a). Nine of the dicistronic genomes have an organization similar to that of members of the family Dicistroviridae with a non-structural ORF followed by a second ORF encoding a dicistro-like capsid. One of the remaining dicistronic genomes does not have a recognizable structural ORF, while the other had a unique organization, with a structural ORF upstream from the non-structural ORF. This novel organization has also been observed in an invertebrate-associated picorna-like virus, Beihai picorna-like virus 105.
The overwhelming majority of FMA picorna-like viral contigs are most closely related to unclassified invertebrate-associated viruses found within this supergroup, with the exception of three contigs that are most similar to picorna-like viruses discovered in seawater (Table S2). Moreover, 61% of FMA picorna-like viral contigs were most similar to invertebrate-associated viruses found within a previously described ‘aquatic picorna-like’ cluster (Shi et al., 2016). This aquatic cluster is most closely related to members of the Marnaviridae and includes viruses infecting unicellular photosynthetic organisms (algae and diatoms) and viruses predicted to infect invertebrates given that they appeared to be transcriptionally active within sampled hosts. Phylogenetic analysis including 20 RdRps from FMA picorna-like viral sequences confirmed a high proportion (65%) of contigs clustering within the aquatic picorna-like clade (Fig. S4). Most of the remaining FMA picorna-like viral contigs were most similar to invertebrate-associated members of the Dicistroviridae. Therefore, most of the FMA picorna-like viral contigs likely represent novel viruses infecting invertebrates and/or aquatic unicellular eukaryotes.
Sobelivirales FMA viruses potentially represent novel invertebrate viruses
FMA viruses within the order Sobelivirales, labelled here FMA sobeli-like viruses, are most similar to ‘sobemo-like’ viruses (Table S2). Sobemo-like viruses represent a diverse assemblage of invertebrate-associated viruses that are not bona fide members of the family Solemoviridae, which infect plants (Shi et al., 2016; Wolf et al., 2018). We identified ten sobeli-like FMA viral contigs, three of which appear to be near-complete genomes based on known genome lengths (Fig. 2). Although these three putative near-complete genomes were most similar to sobemo-like viruses, we did not detect certain features that have been reported from invertebrate sobemo-like viruses (Shi et al., 2016). Specifically, we did not detect trypsin-like peptidase domains within any of the RdRp ORFs and only one of the near-complete genomes contained an identifiable capsid-encoding ORF. FMA sobeli-like virus 1 only contained a single ORF encoding RdRp with no recognizable structural domains, which is not typical of sobemo-like viruses or members of the Solemoviridae. FMA sobeli-like virus 2 and 3 have similar genome organization to viruses identified from arthropods including ticks and shrimp, respectively. Given that sobemo-like viruses have been identified in invertebrates, FMA sobemo-like viruses may represent viruses associated with invertebrates that interact with the sampled macrophytes.
Patatavirales FMA viruses include potato virus Y
The order Patatavirales is composed of the largest family of RNA plant viruses, namely Potyviridae (Wylie et al., 2017). We identified six FMA viral contigs with similarities to potyviruses, including a near-complete genome (Fig. 2). Based on limited similarities to known viruses, the viral contigs represent at least three novel potyviruses. However, the FMA potato virus Y (PVY) genome shares 91% genome-wide pairwise identity with a PVY isolate retrieved from potatoes (Dullemans et al., 2011). Phylogenetic analysis indicates that the FMA PVY belongs to phylogroup C, which has been identified in tomatoes, peppers, and potatoes and is thought to have diverged in Europe (Gibbs et al., 2017) (Fig. 4). In addition to infecting solanaceous crops, PVY is known to infect wild plants, including solanaceous and non-solanaceous weeds, and even ornamentals (e.g., Kaliciak & Syller, 2009; Turina et al., 2006). Therefore, it is currently unknown if FMA PVY infects freshwater macrophytes and/or reflects agricultural runoff or other terrestrial inputs into the springs.
Figure 4. Midpoint-rooted maximum likelihood phylogenetic tree showing potato virus Y phylogroups (Gibbs et al., 2017) based on polyprotein nucleotide sequences.
The freshwater macrophyte associated (FMA) sequence is highlighted with a star. Branches with <70% aLRT support values were collapsed.
Branch 3: Kitrinoviricota FMA viruses include putative invertebrate and plant viruses
The phylum Kitrinoviricota represents a diverse group of viruses but, in contrast to Pisuviricota, only includes +RNA eukaryotic viruses (Koonin et al., 2020). Kitrinoviricota FMA viruses represent four out of the six orders that currently make up the phylum, including Hepelivirales, Nodamuvirales, Tolivirales, and Tymovirales as well as “weiviruses”, which have not been classified within an order. Identified viruses within these groups are most similar to plant and invertebrate viruses.
Hepelivirales and “weivirus” FMA viruses are most similar to aquatic viruses
FMA viruses representing members of the Hepelivirales and “weiviruses” are most similar to viruses associated with aquatic organisms, mainly invertebrates. The order Hepelivirales contains vertebrate, invertebrate, and plant viruses. We identified five FMA hepe-like viruses most similar to viruses associated with aquatic invertebrates (Table S2). One of the contigs, FMA hepe-like virus 1, represents a near-complete genome that was most similar to a putative crab-infecting virus (Shi et al., 2016). “Weiviruses” have only been identified from aquatic invertebrates (Shi et al., 2016) and seawater (Wolf et al., 2020). BLAST searches also revealed a wei-like virus associated with a coral dinoflagellate symbiont, Symbiodinium +RNA virus TR74740 (Levin et al., 2017). We detected a single contig similar to “weiviruses”, FMA wei-like virus 1, which represents a near-complete genome (Fig. 2). FMA wei-like virus 1 is most similar to Beihai weivirus-like virus 7, a virus detected from an octopus transcriptome (Table S2). FMA wei-like virus 1 contains genomic features similar to those of “weiviruses”, including two major ORFs encoding the RdRp and a hypothetical protein.
Nodamuvirales and Tolivirales are dominated by putative invertebrate viruses
The majority of FMA viruses representing the orders Nodamuvirales and Tolivirales are most similar to invertebrate noda-like and tombus-like associated viruses, respectively. We identified four noda-like viral contigs, one of which represented a near complete genome (Fig. 2). Members of the family Nodaviridae, which include fish and invertebrate viruses, have bisegmented genomes with the RNA1 segment encoding for the RdRp and RNA2 encoding a capsid protein (Sahul Hameed et al., 2019). However, the FMA noda-like virus 1 genome has a dicistronic organization with ORFs encoding for the RdRp and capsid proteins. This organization has also been described from aquatic invertebrate noda-like viruses (Shi et al., 2016) suggesting that aquatic noda-like viruses may represent a novel group. Tombusviruses represent a diverse group of plant- and invertebrate-associated viruses (Wolf et al., 2018). We identified eight FMA tombus-like viral contigs, including three near-complete genomes (Fig. 2). The three genomes had similar organization to invertebrate tombus-like viruses. Notably, these genomes did not contain ORFs with similarities to movement proteins seen in plant tombusviruses. Therefore, it is likely that the majority of FMA tombus-like viruses are associated with invertebrates.
Tymovirales FMA viruses include viruses most similar to aquatic and terrestrial plant viruses
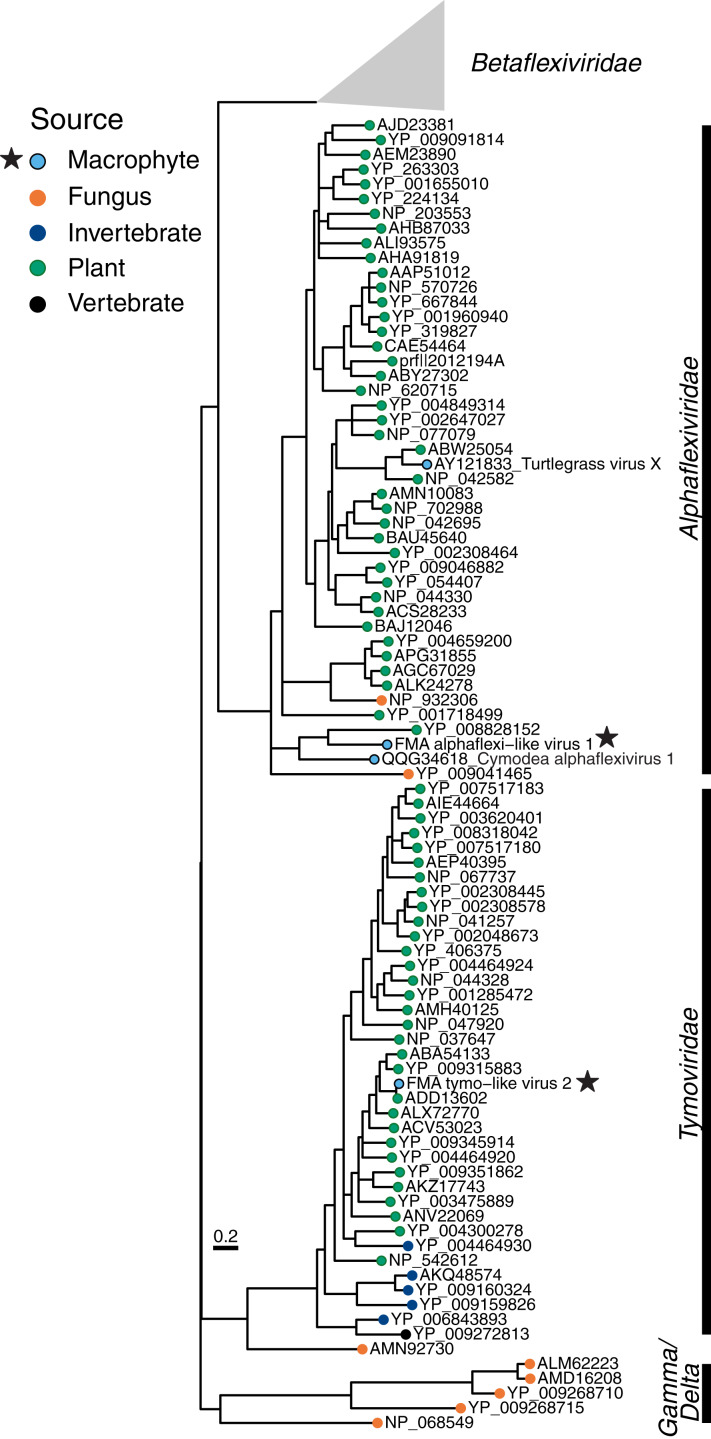
The Tymovirales is the only order within the phylum Kitrinoviricota that is dominated by plant viruses. We detected three FMA tymo-like viral contigs most similar to members from two out of five families within this order, namely Tymoviridae and Alphaflexiviridae, including a near-complete genome (Fig. 2) (Table S2). The FMA alphaflexi-like virus 1 genome is most similar to donkey orchid symptomless virus, the sole member of the genus Platypuvirus within the family Alphaflexiviridae. The genus was named after the platypus because the donkey orchid symptomless virus genome encodes proteins from disparate origins, including RdRp and capsid proteins that are related to viruses of the family Alphaflexiviridae but a movement protein (MP) that is most similar to that of dianthoviruses in the family Tombusviridae (Wylie, Li & Jones, 2013). Phylogenetic analysis and genome organization support that FMA alphaflexi-like virus 1 represents a novel member of the Platypuvirus genus (Fig. 5). BLAST searches resulted in the detection of a potential third member of this genus based on similarities to the RdRp alone, namely the seagrass-associated virus Cymodea alphaflexivirus 1 (Bejerman & Debat, 2021). Therefore, two out of three putative members of the genus Platypuvirus are associated with aquatic macrophytes. The remaining two FMA tymo-like viral contigs were most similar to members of the Tymoviridae, including one contig, FMA tymo-like virus 2, with high amino acid identity (92%) to oat blue dwarf virus (OBDV). Interestingly, there are other macrophyte viruses in the families Alphaflexiviridae, and Betaflexiviridae, including turtlegrass virus X (Van Bogaert et al., 2019) and Cymodocea nodosa foveavirus 1 (Bejerman & Debat, 2021), respectively. The presence of macrophyte viruses in three out of the five families within the Tymovirales indicates that viruses from this order might thrive in both terrestrial and aquatic plants.
Figure 5. Midpoint-rooted maximum likelihood phylogenetic trees for members of the order Tymovirales based on predicted RdRp amino acid sequences.
Freshwater macrophyte associated (FMA) sequences are highlighted with a star. Branches with aLRT support values <70% were collapsed. Gammaflexiviridae and Deltaflexiviridae families are abbreviated as ‘Gamma’ and ‘Delta’, respectively.
Branch 4: Duplornaviricota FMA viruses include putative novel mycoviruses- and diatom-infecting viruses
The phylum Duplornaviricota includes the majority of known eukaryotic dsRNA viruses, which are distributed among three orders, namely Reovirales, Ghabrivirales, and Mindivirales. We identified 16 FMA viral contigs most similar to members of the order Ghabrivirales (Table S2), including two near-complete genomes or segments (Fig. 2). Members of the Ghabrivirales include viruses with a diverse array of genome lengths and arrangements, which infect protists, fungi, plants, and metazoans (Wolf et al., 2018). Nine of the FMA ghabri-like viral contigs were most similar to mycoviruses from the family Megabirnaviridae. The remaining ghabri-like viral contigs were most similar to unclassified viruses associated with diatoms (n = 6) and mosquitoes (n = 1). FMA ghabri-like viral contigs most similar to diatom-associated viruses had matches to diatom colony associated dsRNA virus 16, which had high coverage in RNA libraries targeting diatom intracellular dsRNA and likely infects diatoms (Urayama, Takaki & Nunoura, 2016). The two FMA ghabri-like near complete genomes represent either a single segment encoding RdRp and capsid proteins, similar to segment 1 of Megabirnaviridae (Sato et al., 2019), or ‘minimal’ dsRNA genomes, similar to members of the Totiviridae (Wolf et al., 2018). Phylogenetic analysis indicates that the four FMA ghabri-like viral RdRps cluster closely with segmented viruses from the Megabirnaviridae and invertebrate-associated toti-like viruses (Fig. S5). Altogether, these analyses suggest that the majority of FMA ghabri-like viral contigs likely represent novel mycoviruses and/or diatom-associated viruses.
Branch 5: Negarnaviricota FMA viruses potentially represent protist and invertebrate viruses
The phylum Negarnaviricota includes the vast majority of negative-sense RNA viruses currently classified by the ICTV. We identified FMA viruses most similar to viruses from four out of seven orders within this phylum, including Muvirales, Goujianvirales, Bunyavirales, and Jingchuvirales. Members of the Muvirales and Goujianvirales were discovered in invertebrates and nematodes (Shi et al., 2016) and are classified within the families Qinviridae and Yueviridae, respectively. Sequences similar to qinviruses and yueviruses have also been reported from protists (Chiapello et al., 2020) and soil samples (Starr et al., 2019). Additionally, BLAST searches revealed qin- and yue-like viral proteins from protozoan (Brachionus plicatilis, accession no. RNA03874), algal (Chara braunii, accession no. GBG68844) and plant (Vigna unguiculata, accession no. QCE01079) genome projects, suggesting that these viruses may be associated with a diversity of organisms including autotrophs. We identified four FMA yue-like viral contigs, including a near-complete genome (Fig. 2), and three FMA qin-like viral contigs (Table S2). The FMA yue-like viral contigs were most similar to yueviruses associated with freshwater invertebrates, whereas FMA qin-like viruses had matches to nematode and soil viruses, as well as proteins associated with algae and plants. The FMA yue-like virus 1 contig may represent a single monocistronic segment encoding the RdRp, given that yueviruses are bisegmented (Shi et al., 2016). Phylogenetic analysis indicates that detected FMA yue- and qin-like viruses cluster with viruses associated with protists, including oomycetes and rotifers (Fig. S6).
Bunyavirales and Jingchuvirales are two large orders within the Negarnaviricota that contain viruses with segmented and mainly unsegmented genomes, respectively. We identified two FMA bunya-like viral contigs. FMA bunya-like virus 1 represents a near-complete segment encoding the RdRp (L segment) (Fig. 2), which is most closely related to a mycovirus, Rhizoctonia solani bunya/phlebo-like virus 1 (Table S2). Phylogenetic analysis indicates that FMA bunya-like viral sequences cluster closest to oomycete viruses (Chiapello et al., 2020) and Rhizoctonia solani bunya/phlebo-like virus 1 (Picarelli et al., 2019), in a clade composed of viral genomes for which structural proteins have not been reported (Fig. S7). This clade also includes viruses associated with cestodes (Schistocephalus solidus bunya-like virus 1) (Hahn et al., 2020) and invertebrates (Beihai barnacle virus 5 and Barns Ness serrated wrack bunya/phlebo-like virus 1) (Shi et al., 2016; Waldron, Stone & Obbard, 2018). Whereas FMA bunya-like viruses are most closely related to mycoviruses and oomycete viruses, FMA jingchu-like viruses are most similar to invertebrate-associated viruses. We identified four FMA jingchu-like viral contigs, including a near-complete genome (Fig. 2). The FMA jingchu-like virus 1 is most similar to viruses identified in snakes and freshwater shrimp based on BLAST searches using ORFs encoding the RdRp and glycoprotein (Table S2). Phylogenetic analysis did not provide further insight since the FMA jingchu-like virus 1 RdRp sequence clustered just outside a clade including snake and shrimp-associated viruses (Fig. S8). However, the nucleoprotein encoding ORF is most similar to insect-associated viruses. BLAST searches using the FMA jingchu-like virus 1 genome as a query resulted in top matches to a glycoprotein detected in the transcriptome from a freshwater amphipod (accession number XP_018024392). Therefore, we suspect that FMA jingchu-like viruses represent invertebrate-associated viruses rather than vertebrate-infecting viruses.
Putative FMA plant viruses are widespread in vascular aquatic vegetation
Given the limited information regarding viral infection in freshwater aquatic vegetation, we used PCR to identify which macrophyte species contained putative FMA plant viruses. Based on BLAST matches and/or phylogenetic analysis we identified 15 distinct FMA viral contigs potentially representing plant viruses (Table 3). All of the putative FMA plant viruses were detected in perennial, mainly vascular, plants and the majority (10 out 15) were limited to a single macrophyte species, Vallisneria americana Michx. Poty-like (40%) and partiti-like (27%) viruses most similar to members of the Potyviridae and Partitiviridae families, respectively, dominated the diversity of FMA viruses representing plant viruses. However, poty-like viruses were only identified in two macrophyte species sampled in Ichetucknee, namely V. americana and Ludwigia repens J.R. Forst., whereas cryptic partiti-like viruses were detected in eight species collected throughout Ichetucknee and Rainbow spring sites. This observation extends to individual FMA viruses given that the most prevalent FMA virus was FMA partiti-like virus 10, which was detected in four macrophyte species. Therefore, partiti-like viruses most similar to persistent cryptic viruses that cause asymptomatic infections (Vainio et al., 2018) may be more widespread in spring macrophytes than poty-like viruses.
The putative plant viruses we identified in freshwater macrophytes reflect what has been observed in natural terrestrial ecosystems, where there are an abundance of viral groups with persistent lifestyles (Prendeville et al., 2012; Roossinck, 2015). At the time of macrophyte sample collection there were no evident signs of symptomatic viral infection, and all macrophytes where putative plant viruses were detected are considered perennials. Persistent viruses, which establish long-term infections, are expected to spread in their host plant without causing critical damage and such a lifestyle may be more easily maintained in perennial hosts (Shates et al., 2019; Takahashi et al., 2019). Although persistent lifestyles are typically associated with viruses with dsRNA genomes (Roossinck, 2010), a diversity of +RNA viruses also establish persistent infections (Takahashi et al., 2019). Future research should address the role of persistent viral infection in the ecology of aquatic vegetation given that these interactions may have important implications for the host plant, including potential beneficial outcomes (Roossinck, 2011; Takahashi et al., 2019).
The majority of putative FMA plant viruses had low amino acid level identities to known viruses, indicating their genetic novelty (Table 3). However, FMA tymo-like virus 2 and FMA PVY had high similarities (>85% nucleotide identity) to oat blue dwarf virus (OBDV) and potato virus Y (PVY), respectively. OBDV infects grasses of economic interest, such as oat and maize (Edwards & Weiland, 2009; Edwards & Weiland, 2010), and PVY is a major agricultural pathogen (Torrance & Talianksy, 2020). Although OBDV and PVY have broad host ranges and infect weeds that may serve as viral reservoirs (Cervantes & Alvarez, 2011; Westdal, 2011), to our knowledge, this is the first study to detect these viruses in macrophytes. Some PVY strains are transmissible in water suggesting that water-mediated infection is plausible (Mehle et al., 2014). However, OBDV is a phloem-limited virus that is transmitted by insect vectors and the mechanism of its introduction to submerged aquatic vegetation is less clear. Regardless, our findings highlight that macrophytes may harbor terrestrial plant viruses, a possibility that was noted over 70 years ago when researchers observed lesions in terrestrial indicator plants inoculated with homogenates from aquatic plants (MacClement & Richards, 1956). However, the identities of the viruses causing symptoms in terrestrial indicator plants were not determined in that study. Future work will examine the distribution of PVY and OBDV in freshwater springs, if their presence in macrophytes is related to agricultural runoff, and if these viruses can replicate and be transmitted among macrophytes.
Vallisneria americana Michx was the macrophyte species with the highest diversity of putative plant viruses resulting in the detection of ten viruses, including OBDV. Half of the viruses detected in this macrophyte represent poty-like viruses. The global distribution, wide host range (including cultivated and wild vegetation), and fixed hypervariable genomic regions of members of the Potyviridae point to their adaptability to new hosts and environments (Nigam et al., 2019; Wylie et al., 2017). The detection of a diversity of poty-like viruses in V. americana indicate that potyviruses may also thrive in freshwater vegetation. Notably, V. americana is a submerged monocotyledonous plant and freshwater member of the Alismatales, the only order also encompassing marine flowering plants (i.e., angiosperms), namely seagrasses. Poty-like viruses have not been reported yet from marine angiosperms; however, there are reports of brown macroalgae containing flexuous virus particles reminiscent of potyviruses that reacted to potyvirus monoclonal antibodies (Easton, Lewis & Pearson, 1997).
The findings presented here, together with reports from seagrasses (Bejerman & Debat, 2021; Van Bogaert et al., 2019), suggest that some viruses identified in freshwater and marine angiosperms fall within the known diversity of terrestrial plant viruses. This was not expected considering that the core Alismatales, an order mainly composed of plants that have a completely submerged seedling phase, evolved over 120 million years ago (Du & Wang, 2016; Givnish et al., 2018). Aquatic angiosperms are generally thought to have evolved from terrestrial plants (Les, Cleland & Waycott, 1997; Papenbrock, 2012). However, more recent analyses suggest an alternative scenario where some angiosperm lineages, including the Alismatales, originated and dispersed in aquatic environments (Du & Wang, 2016; Givnish et al., 2018; Gomez et al., 2015). Our findings suggest that despite the divergence between aquatic and terrestrial angiosperms, there may be close evolutionary relationships among their viruses. Moreover, the discovery of a diversity of RNA viruses in microalgae (Chlorophyta and Chlorarachniophyceae) suggest that there may be more complex and closer evolutionary relationships among viruses infecting aquatic phototrophs and land plants than previously thought (Charon et al., 2020). More sampling of unexplored aquatic phototrophs, including macrophytes, is needed to evaluate how virome composition in aquatic primary producers relates to changes in plant evolution (Mushegian, Shipunov & Elena, 2016) and the potential ecological impacts of viral infection in macrophytes.
Conclusions
Here we described viral diversity associated with macrophytes from freshwater springs, further expanding the known RNA viral diversity associated with aquatic phototrophs. FMA viruses include viruses associated with a diversity of organisms that are presumed to be part of macrophyte holobionts as well as organisms that directly interact with macrophytes (e.g., invertebrate herbivores). Although macrophytes play a vital role in aquatic ecosystems, viral infection may have escaped detection due to persistent and asymptomatic infections that go unnoticed. Despite the lack of obvious symptomatic disease, persistent viral infections play important roles in terrestrial plant ecology (Lefeuvre et al., 2019; Roossinck, 2015; Takahashi et al., 2019) and are likely to affect macrophytes. Known terrestrial plant pathogens identified in submerged aquatic vegetation highlight a potential terrestrial-aquatic continuum for plant viruses. This is important since agricultural runoff is considered one of the major nonpoint pollution sources impacting freshwater systems (Xia et al., 2020) and it is likely that abiotic and biotic farmland components, such as viruses, reach aquatic habitats. Although there have been concerns about plant viral pathogens in freshwater sources used for crop irrigation (Hong, 2017; Mehle & Ravnikar, 2012; Rosario et al., 2009), the potential effects of terrestrial plant viruses in aquatic vegetation have not been explored. The springs act as ‘natural flowing water chemostats’ with unparalleled temporal stability in physicochemical parameters that render them suitable for studying autotroph homeostasis (Nifong, Cohen & Cropper, 2014). The genetic data gathered here can be used to design molecular assays to investigate virus-macrophyte interactions in these natural freshwater laboratories and further investigate plant virus evolution by confirming virus-host associations.
Supplemental Information
Table S1 provides a list of primers used for PCR and sequence-independent single-primer amplification (SISPA) assays. Table S2 provides detailed information for the 156 viral contigs identified in macrophyte datasets.
Acknowledgments
The authors would like to thank the Florida Department of Environmental Protection Division of Recreation and Parks and the Florida Park Service for their assistance in obtaining sampling permits and access to the springs. The authors also acknowledge Kema Malki, Kaitlin Mettel, Robin Jung and William E. Landry for all their help during field sample collection and sample processing.
Funding Statement
This project was funded by grant DEB-1555854 from the National Science Foundation to Mya Breitbart and a grant to Noémi Van Bogaert from the Belgian American Educational Foundation (BAEF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Karyna Rosario, Email: krosari2@usf.edu.
Mya Breitbart, Email: mya@usf.edu.
Additional Information and Declarations
Competing Interests
Mya Breitbart is an Academic Editor for PeerJ.
Author Contributions
Karyna Rosario conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Noémi Van Bogaert conceived and designed the experiments, performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Natalia B. López-Figueroa performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Haris Paliogiannis performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Mason Kerr performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Mya Breitbart conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
Field samples were collected from freshwater springs in Florida, USA in accordance with a permit from the Florida Department of Environmental Protection (permit # 06011710).
DNA Deposition
Data Availability
The following information was supplied regarding data availability:
Next generation sequencing data are available at the Sequence Read Archive (SRA) database under BioProject PRJNA826216 (BioSample accession numbers SAMN27553344 through SAMN27553353).
References
- Afgan et al. (2018).Afgan E, Baker D, Batut B, van den Beek M, Bouvier D, Cech M, Chilton J, Clements D, Coraor N, Gruning BA, Guerler A, Hillman-Jackson J, Hiltemann S, Jalili V, Rasche H, Soranzo N, Goecks J, Taylor J, Nekrutenko A, Blankenberg D. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Research. 2018;46(W1):W537–W544. doi: 10.1093/nar/gky379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson (2003).Anderson LWJ. A review of aquatic weed biology and management research conducted by the United States Department of Agriculture—Agricultural Research Service. Pest Management Science. 2003;59(6–7):801–813. doi: 10.1002/ps.725. [DOI] [PubMed] [Google Scholar]
- Andika et al. (2017).Andika IB, Wei S, Cao C, Salaipeth L, Kondo H, Sun L. Phytopathogenic fungus hosts a plant virus: a naturally occurring cross-kingdom viral infection. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(46):12267–12272. doi: 10.1073/pnas.1714916114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews (2010).Andrews S. FastQC: a quality control tool for high throughput sequence data. 2010. http://www.bioinformatics.babraham.ac.uk/projects/fastqc http://www.bioinformatics.babraham.ac.uk/projects/fastqc
- Ayllón et al. (2020).Ayllón Mía A, Turina M, Xie J, Nerva L, Marzano S-YL, Donaire L, Jiang D, Consortium ICTVR. ICTV virus taxonomy profile: Botourmiaviridae. Journal of General Virology. 2020;101(5):454–455. doi: 10.1099/jgv.0.001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankevich et al. (2012).Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. Journal of Computational Biology. 2012;19(5):455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejerman & Debat (2021).Bejerman N, Debat H. Exploring the tymovirids landscape through metatranscriptomics data. BioRxiv. 2021 doi: 10.1101/2021.1107.1115.452586. [DOI] [Google Scholar]
- Boccardo et al. (1987).Boccardo G, Lisa V, Luisoni E, Milne RG. Cryptic plant viruses. In: Maramorosch K, Murphy FA, Shatkin AJ, editors. Advances in Virus Research. Cambridge: Academic Press; 1987. pp. 171–214. [DOI] [PubMed] [Google Scholar]
- Bolduc et al. (2017).Bolduc B, Youens-Clark K, Roux S, Hurwitz BL, Sullivan MB. iVirus: facilitating new insights in viral ecology with software and community data sets imbedded in a cyberinfrastructure. ISME Journal. 2017;11(1):7–14. doi: 10.1038/ismej.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger, Lohse & Usadel (2014).Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollback & Huelsenbeck (2001).Bollback JP, Huelsenbeck JP. Phylogeny, genome evolution, and host specificity of single-stranded RNA bacteriophage (family Leviviridae) Journal of Molecular Evolution. 2001;52(2):117–128. doi: 10.1007/s002390010140. [DOI] [PubMed] [Google Scholar]
- Boros et al. (2018).Boros Á, Polgár B, Pankovics P, Fenyvesi H, Engelmann P, Phan TG, Delwart E, Reuter G. Multiple divergent picobirnaviruses with functional prokaryotic Shine-Dalgarno ribosome binding sites present in cloacal sample of a diarrheic chicken. Virology. 2018;525(10):62–72. doi: 10.1016/j.virol.2018.09.008. [DOI] [PubMed] [Google Scholar]
- Brussaard (2004).Brussaard CPD. Viral control of phytoplankton populations—a review. Journal of Eukaryotic Microbiology. 2004;51(2):125–138. doi: 10.1111/j.1550-7408.2004.tb00537.x. [DOI] [PubMed] [Google Scholar]
- Callanan et al. (2021).Callanan J, Stockdale SR, Adriaenssens EM, Kuhn JH, Rumnieks J, Pallen MJ, Shkoporov AN, Draper LA, Ross RP, Hill C. Leviviricetes: expanding and restructuring the taxonomy of bacteria-infecting single-stranded RNA viruses. Microbial Genomics. 2021;7(11):000686. doi: 10.1099/mgen.0.000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callanan et al. (2020).Callanan J, Stockdale SR, Shkoporov A, Draper LA, Ross RP, Hill C. Expansion of known ssRNA phage genomes: from tens to over a thousand. Science Advances. 2020;6(6):eaay5981. doi: 10.1126/sciadv.aay5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capella-Gutierrez, Silla-Martinez & Gabaldon (2009).Capella-Gutierrez S, Silla-Martinez JM, Gabaldon T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25(15):1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes & Alvarez (2011).Cervantes FA, Alvarez JM. Within plant distribution of Potato Virus Y in hairy nightshade (Solanum sarrachoides): an inoculum source affecting PVY aphid transmission. Virus Research. 2011;159(2):194–200. doi: 10.1016/j.virusres.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Chambers et al. (1999).Chambers PA, DeWreede RE, Irlandi EA, Vandermeulen H. Management issues in aquatic macrophyte ecology: a Canadian perspective. Canadian Journal of Botany. 1999;77(4):471–487. doi: 10.1139/b99-092. [DOI] [Google Scholar]
- Chambers et al. (2008).Chambers PA, Lacoul P, Murphy KJ, Thomaz SM. Global diversity of aquatic macrophytes in freshwater. Hydrobiologia. 2008;595(1):9–26. doi: 10.1007/s10750-007-9154-6. [DOI] [Google Scholar]
- Charon et al. (2020).Charon J, Marcelino VR, Wetherbee R, Verbruggen H, Holmes EC. Metatranscriptomic identification of diverse and divergent RNA viruses in green and chlorarachniophyte algae cultures. Viruses. 2020;12(10):1180. doi: 10.3390/v12101180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiapello et al. (2020).Chiapello M, Rodríguez-Romero J, Ayllón MA, Turina M. Analysis of the virome associated to grapevine downy mildew lesions reveals new mycovirus lineages. Virus Evolution. 2020;6(2):e00711. doi: 10.1093/ve/veaa058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coy et al. (2018).Coy SR, Gann ER, Pound HL, Short SM, Wilhelm SW. Viruses of eukaryotic algae: diversity, methods for detection, and future directions. Viruses. 2018;10(9):487. doi: 10.3390/v10090487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibble, Thomaz & Padial (2006).Dibble ED, Thomaz SM, Padial AA. Spatial complexity measured at a multi-scale in three aquatic plant species. Journal of Freshwater Ecology. 2006;21(2):239–247. doi: 10.1080/02705060.2006.9664992. [DOI] [Google Scholar]
- Dolja & Koonin (2018).Dolja VV, Koonin EV. Metagenomics reshapes the concepts of RNA virus evolution by revealing extensive horizontal virus transfer. Virus Research. 2018;244:36–52. doi: 10.1016/j.virusres.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolja, Krupovic & Koonin (2020).Dolja VV, Krupovic M, Koonin EV. Deep roots and splendid boughs of the global plant virome. Annual Review of Phytopathology. 2020;58(1):23–53. doi: 10.1146/annurev-phyto-030320-041346. [DOI] [PubMed] [Google Scholar]
- Du & Wang (2016).Du Z-Y, Wang Q-F, China Phylogeny Consortium Phylogenetic tree of vascular plants reveals the origins of aquatic angiosperms. Journal of Systematics and Evolution. 2016;54(4):342–348. doi: 10.1111/jse.12182. [DOI] [Google Scholar]
- Duarte et al. (2013).Duarte CM, Kennedy H, Marbà N, Hendriks I. Assessing the capacity of seagrass meadows for carbon burial: current limitations and future strategies. Ocean & Coastal Management. 2013;83:32–38. doi: 10.1016/j.ocecoaman.2011.09.001. [DOI] [Google Scholar]
- Dullemans et al. (2011).Dullemans AM, Cuperus C, Verbeek M, van der Vlugt RAA. Complete nucleotide sequence of a potato isolate of strain group C of Potato virus Y from 1938. Archives of Virology. 2011;156(3):473–477. doi: 10.1007/s00705-010-0853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton, Lewis & Pearson (1997).Easton LM, Lewis GD, Pearson MN. Virus-like particles associated with dieback symptoms in the brown alga Ecklonia radiata. Diseases of Aquatic Organisms. 1997;30:217–222. doi: 10.3354/dao030217. [DOI] [Google Scholar]
- Edwards & Weiland (2009).Edwards MC, Weiland JJ. Maize as a new host for Oat blue dwarf virus. Plant Disease. 2009;93(9):972. doi: 10.1094/pdis-93-9-0972a. [DOI] [PubMed] [Google Scholar]
- Edwards & Weiland (2010).Edwards MC, Weiland JJ. First infectious clone of the propagatively transmitted Oat blue dwarf virus. Archives of Virology. 2010;155(4):463–470. doi: 10.1007/s00705-010-0603-6. [DOI] [PubMed] [Google Scholar]
- Fu et al. (2012).Fu L, Niu B, Zhu Z, Wu S, Li W. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics. 2012;28(23):3150–3152. doi: 10.1093/bioinformatics/bts565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor et al. (2007).Gaynor AM, Nissen MD, Whiley DM, Mackay IM, Lambert SB, Wu G, Brennan DC, Storch GA, Sloots TP, Wang D. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLOS Pathogens. 2007;3(5):e64. doi: 10.1371/journal.ppat.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh & Malik (2021).Ghosh S, Malik YS. The true host/s of picobirnaviruses. Frontiers in Veterinary Science. 2021;7:615293. doi: 10.3389/fvets.2020.615293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs et al. (2017).Gibbs AJ, Ohshima K, Yasaka R, Mohammadi M, Gibbs MJ, Jones RAC. The phylogenetics of the global population of potato virus Y and its necrogenic recombinants. Virus Evolution. 2017;3(1):vex002. doi: 10.1093/ve/vex002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs et al. (2011).Gibbs AJ, Torronen M, Mackenzie AM, Wood JT, Armstrong JS, Kondo H, Tamada T, Keese PL. The enigmatic genome of Chara australis virus. Journal of General Virology. 2011;92(11):2679–2690. doi: 10.1099/vir.0.033852-0. [DOI] [PubMed] [Google Scholar]
- Givnish et al. (2018).Givnish TJ, Zuluaga A, Spalink D, Soto Gomez M, Lam VKY, Saarela JM, Sass C, Iles WJD, de Sousa DJL, Leebens-Mack J, Chris Pires J, Zomlefer WB, Gandolfo MA, Davis JI, Stevenson DW, dePamphilis C, Specht CD, Graham SW, Barrett CF, Ané C. Monocot plastid phylogenomics, timeline, net rates of species diversification, the power of multi-gene analyses, and a functional model for the origin of monocots. American Journal of Botany. 2018;105(11):1888–1910. doi: 10.1002/ajb2.1178. [DOI] [PubMed] [Google Scholar]
- Goff et al. (2011).Goff S, Vaughn M, McKay S, Lyons E, Stapleton A, Gessler D, Matasci N, Wang L, Hanlon M, Lenards A, Muir A, Merchant N, Lowry S, Mock S, Helmke M, Kubach A, Narro M, Hopkins N, Micklos D, Hilgert U, Gonzales M, Jordan C, Skidmore E, Dooley R, Cazes J, McLay R, Lu Z, Pasternak S, Koesterke L, Piel W, Grene R, Noutsos C, Gendler K, Feng X, Tang C, Lent M, S-j Kim, Kvilekval K, Manjunath BS, Tannen V, Stamatakis A, Sanderson M, Welch S, Cranston K, Soltis P, Soltis D, O’Meara B, Ane C, Brutnell T, Kleibenstein D, White J, Leebens-Mack J, Donoghue M, Spalding E, Vision T, Myers C, Lowenthal D, Enquist B, Boyle B, Akoglu A, Andrews G, Ram S, Ware D, Stein L, Stanzione D. The iPlant collaborative: cyberinfrastructure for plant biology. Frontiers in Plant Science. 2011;2:34. doi: 10.3389/fpls.2011.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez et al. (2015).Gomez B, Daviero-Gomez V, Coiffard C, Martín-Closas C, Dilcher DL. Montsechia, an ancient aquatic angiosperm. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(35):10985–10988. doi: 10.1073/pnas.1509241112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green et al. (1999).Green J, Gallimore CI, Clewley JP, Brown DWG. Genomic characterisation of the large segment of a rabbit picobirnavirus and comparison with the atypical picobirnavirus of Cryptosporidium parvum. Archives of Virology. 1999;144(12):2457–2465. doi: 10.1007/s007050050658. [DOI] [PubMed] [Google Scholar]
- Guindon et al. (2010).Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology. 2010;59(3):307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- Gurevich et al. (2013).Gurevich A, Saveliev V, Vyahhi N, Tesler G. QUAST: quality assessment tool for genome assemblies. Bioinformatics. 2013;29(8):1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn et al. (2020).Hahn MA, Rosario K, Lucas P, Dheilly NM. Characterization of viruses in a tapeworm: phylogenetic position, vertical transmission, and transmission to the parasitized host. ISME Journal. 2020;14(7):1755–1767. doi: 10.1038/s41396-020-0642-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck et al. (2008).Heck KL, Carruthers TJB, Duarte CM, Hughes AR, Kendrick G, Orth RJ, Williams SW. Trophic transfers from seagrass meadows subsidize diverse marine and terrestrial consumers. Ecosystems. 2008;11(7):1198–1210. doi: 10.1007/s10021-008-9155-y. [DOI] [Google Scholar]
- Hildebrand et al. (2009).Hildebrand A, Remmert M, Biegert A, Soding J. Fast and accurate automatic structure prediction with HHpred. Proteins-structure Function and Bioinformatics. 2009;77(S9):128–132. doi: 10.1002/prot.22499. [DOI] [PubMed] [Google Scholar]
- Hogenhout et al. (2008).Hogenhout SA, Ammar E-D, Whitfield AE, Redinbaugh MG. Insect vector interactions with persistently transmitted viruses. Annual Review of Phytopathology. 2008;46(1):327–359. doi: 10.1146/annurev.phyto.022508.092135. [DOI] [PubMed] [Google Scholar]
- Hong (2017).Hong C. CHAPTER 11: component analyses of irrigation water in plant disease epidemiology. In: Hong C, Moorman GW, Wohanka W, Buttner C, editors. Biology, Detection, and Management of Plant Pathogens in Irrigation Water. St. Paul: The American Phytopathological Society (APS); 2017. pp. 111–121. [DOI] [Google Scholar]
- Hudon, Sève & Cattaneo (2014).Hudon C, Sève MD, Cattaneo A. Increasing occurrence of the benthic filamentous cyanobacterium Lyngbya wollei: a symptom of freshwater ecosystem degradation. Freshwater Science. 2014;33(2):606–618. doi: 10.1086/675932. [DOI] [Google Scholar]
- Huson et al. (2016).Huson DH, Beier S, Flade I, Górska A, El-Hadidi M, Mitra S, Ruscheweyh H-J, Tappu R. MEGAN Community Edition—Interactive exploration and analysis of large-scale microbiome sequencing sata. PLOS Computational Biology. 2016;12(6):e1004957. doi: 10.1371/journal.pcbi.1004957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänes et al. (2017).Jänes H, Kotta J, Pärnoja M, Crowe TP, Rindi F, Orav-Kotta H. Functional traits of marine macrophytes predict primary production. Functional Ecology. 2017;31(4):975–986. doi: 10.1111/1365-2435.12798. [DOI] [Google Scholar]
- Kaliciak & Syller (2009).Kaliciak A, Syller J. New hosts of Potato virus Y (PVY) among common wild plants in Europe. European Journal of Plant Pathology. 2009;124(4):707–713. doi: 10.1007/s10658-009-9452-0. [DOI] [Google Scholar]
- Kamitani et al. (2016).Kamitani M, Nagano AJ, Honjo MN, Kudoh H. RNA-Seq reveals virus–virus and virus–plant interactions in nature. FEMS Microbiology Ecology. 2016;92(11):fiw176. doi: 10.1093/femsec/fiw176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh, Rozewicki & Yamada (2019).Katoh K, Rozewicki J, Yamada KD. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics. 2019;20(4):1160–1166. doi: 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley et al. (1994).Kelley SE, Shykoff J, Hamilton WD, Howard JC. Viral pathogens and the advantage of sex in the perennial grass Anthoxanthum odoratum. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 1994;346(1317):295–302. doi: 10.1098/rstb.1994.0146. [DOI] [Google Scholar]
- Koonin et al. (2020).Koonin EV, Dolja VV, Krupovic M, Varsani A, Wolf YI, Yutin N, Zerbini FM, Kuhn JH. Global organization and proposed megataxonomy of the virus world. Microbiology and Molecular Biology Reviews. 2020;84(2):e00061. doi: 10.1128/MMBR.00061-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin et al. (2008).Koonin EV, Wolf YI, Nagasaki K, Dolja VV. The Big Bang of picorna-like virus evolution antedates the radiation of eukaryotic supergroups. Nature Reviews Microbiology. 2008;6(12):925–939. doi: 10.1038/nrmicro2030. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy et al. (2016).Krishnamurthy SR, Janowski AB, Zhao G, Barouch D, Wang D. Hyperexpansion of RNA bacteriophage diversity. PLOS Biology. 2016;14(3):e1002409. doi: 10.1371/journal.pbio.1002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy & Wang (2018).Krishnamurthy SR, Wang D. Extensive conservation of prokaryotic ribosomal binding sites in known and novel picobirnaviruses. Virology. 2018;516:108–114. doi: 10.1016/j.virol.2018.01.006. [DOI] [PubMed] [Google Scholar]
- Lamb et al. (2017).Lamb JB, van de Water JAJM, Bourne DG, Altier C, Hein MY, Fiorenza EA, Abu N, Jompa J, Harvell CD. Seagrass ecosystems reduce exposure to bacterial pathogens of humans, fishes, and invertebrates. Science. 2017;355(6326):731–733. doi: 10.1126/science.aal1956. [DOI] [PubMed] [Google Scholar]
- Lefeuvre et al. (2019).Lefeuvre P, Martin DP, Elena SF, Shepherd DN, Roumagnac P, Varsani A. Evolution and ecology of plant viruses. Nature Reviews Microbiology. 2019;17(10):632–644. doi: 10.1038/s41579-019-0232-3. [DOI] [PubMed] [Google Scholar]
- Lefort, Longueville & Gascuel (2017).Lefort V, Longueville J-E, Gascuel O. SMS: smart model selection in PhyML. Molecular Biology and Evolution. 2017;34(9):2422–2424. doi: 10.1093/molbev/msx149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Les, Cleland & Waycott (1997).Les DH, Cleland MA, Waycott M. Phylogenetic studies in Alismatidae, II: evolution of marine angiosperms (seagrasses) and hydrophily. Systematic Botany. 1997;22(3):443–463. doi: 10.2307/2419820. [DOI] [Google Scholar]
- Lesiv, Polishchuk & Antonyak (2020).Lesiv MS, Polishchuk AI, Antonyak HL. Aquatic macrophytes: ecological features and functions. Studia Biologica. 2020;14(2):79–94. doi: 10.30970/sbi.1402.619. [DOI] [Google Scholar]
- Levin et al. (2017).Levin RA, Voolstra CR, Weynberg KD, van Oppen MJH. Evidence for a role of viruses in the thermal sensitivity of coral photosymbionts. ISME Journal. 2017;11(3):808–812. doi: 10.1038/ismej.2016.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacClement & Richards (1956).MacClement WD, Richards MG. Virus in wild plants. Canadian Journal of Botany. 1956;34(5):793–799. doi: 10.1139/b56-060. [DOI] [Google Scholar]
- Malki et al. (2020).Malki K, Rosario K, Sawaya NA, Székely AJ, Tisza MJ, Breitbart M. Prokaryotic and viral community composition of freshwater springs in Florida, USA. mBio. 2020;11(2):e00436. doi: 10.1128/mBio.00436-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malki et al. (2021).Malki K, Sawaya NA, Tisza MJ, Coutinho FH, Rosario K, Székely AJ, Breitbart M. Spatial and temporal dynamics of prokaryotic and viral community assemblages in a lotic system (Manatee Springs, Florida) Applied and Environmental Microbiology. 2021;87(18):e00646. doi: 10.1128/AEM.00646-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmstrom, Melcher & Bosque-Pérez (2011).Malmstrom CM, Melcher U, Bosque-Pérez NA. The expanding field of plant virus ecology: historical foundations, knowledge gaps, and research directions. Virus Research. 2011;159(2):84–94. doi: 10.1016/j.virusres.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Mascia et al. (2019).Mascia T, Vučurović A, Minutillo SA, Nigro F, Labarile R, Savoia MA, Palukaitis P, Gallitelli D. Infection of Colletotrichum acutatum and Phytophthora infestans by taxonomically different plant viruses. European Journal of Plant Pathology. 2019;153(4):1001–1017. doi: 10.1007/s10658-018-01615-9. [DOI] [Google Scholar]
- McKeown et al. (2018).McKeown DA, Schroeder JL, Stevens K, Peters AF, Saez CA, Park J, Rothman MD, Bolton JJ, Brown MT, Schroeder DC. Phaeoviral infections are present in Macrocystis, Ecklonia and Undaria (Laminariales) and are influenced by wave exposure in Ectocarpales. Viruses. 2018;10(8):410. doi: 10.3390/v10080410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown et al. (2017).McKeown DA, Stevens K, Peters AF, Bond P, Harper GM, Brownlee C, Brown MT, Schroeder DC. Phaeoviruses discovered in kelp (Laminariales) ISME Journal. 2017;11(12):2869–2873. doi: 10.1038/ismej.2017.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehle et al. (2014).Mehle N, Gutiérrez-Aguirre I, Prezelj N, Delić D, Vidic U, Ravnikar M. Survival and transmission of potato virus Y, pepino mosaic virus, and potato spindle tuber viroid in water. Applied and Environmental Microbiology. 2014;80(4):1455–1462. doi: 10.1128/AEM.03349-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehle & Ravnikar (2012).Mehle N, Ravnikar M. Plant viruses in aqueous environment—survival, water mediated transmission and detection. Water Research. 2012;46(16):4902–4917. doi: 10.1016/j.watres.2012.07.027. [DOI] [PubMed] [Google Scholar]
- Montes, Alonso-Blanco & García-Arenal (2019).Montes N, Alonso-Blanco C, García-Arenal F. Cucumber mosaic virus infection as a potential selective pressure on Arabidopsis thaliana populations. PLOS Pathogens. 2019;15(5):e1007810. doi: 10.1371/journal.ppat.1007810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushegian, Shipunov & Elena (2016).Mushegian A, Shipunov A, Elena SF. Changes in the composition of the RNA virome mark evolutionary transitions in green plants. BMC Biology. 2016;14(1):68. doi: 10.1186/s12915-016-0288-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaki (2008).Nagasaki K. Dinoflagellates, diatoms, and their viruses. Journal of Microbiology. 2008;46(3):235–243. doi: 10.1007/s12275-008-0098-y. [DOI] [PubMed] [Google Scholar]
- Ng et al. (2015).Ng TF, Kondov NO, Deng X, Van Eenennaam A, Neibergs HL, Delwart E. A metagenomics and case-control study to identify viruses associated with bovine respiratory disease. Journal of Virology. 2015;89(10):5340–5349. doi: 10.1128/JVI.00064-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng et al. (2012).Ng TF, Marine R, Wang C, Simmonds P, Kapusinszky B, Bodhidatta L, Oderinde BS, Wommack KE, Delwart E. High variety of known and new RNA and DNA viruses of diverse origins in untreated sewage. Journal of Virology. 2012;86(22):12161–12175. doi: 10.1128/JVI.00869-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nifong, Cohen & Cropper (2014).Nifong RL, Cohen MJ, Cropper WP., Jr Homeostasis and nutrient limitation of benthic autotrophs in natural chemostats. Limnology and Oceanography. 2014;59(6):2101–2111. doi: 10.4319/lo.2014.59.6.2101. [DOI] [Google Scholar]
- Nigam et al. (2019).Nigam D, LaTourrette K, Souza PFN, Garcia-Ruiz H. Genome-wide variation in potyviruses. Frontiers in Plant Science. 2019;10:1439. doi: 10.3389/fpls.2019.01439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nõges, Luup & Feldmann (2010).Nõges T, Luup H, Feldmann T. Primary production of aquatic macrophytes and their epiphytes in two shallow lakes (Peipsi and Võrtsjärv) in Estonia. Aquatic Ecology. 2010;44(1):83–92. doi: 10.1007/s10452-009-9249-4. [DOI] [Google Scholar]
- Nurk et al. (2013).Nurk S, Bankevich A, Antipov D, Gurevich AA, Korobeynikov A, Lapidus A, Prjibelski AD, Pyshkin A, Sirotkin A, Sirotkin Y, Stepanauskas R, Clingenpeel SR, Woyke T, McLean JS, Lasken R, Tesler G, Alekseyev MA, Pevzner PA. Assembling single-cell genomes and mini-metagenomes from chimeric MDA products. Journal of Computational Biology. 2013;20(10):714–737. doi: 10.1089/cmb.2013.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurk et al. (2017).Nurk S, Meleshko D, Korobeynikov A, Pevzner PA. metaSPAdes: a new versatile metagenomic assembler. Genome Research. 2017;27(5):824–834. doi: 10.1101/gr.213959.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papenbrock (2012).Papenbrock J. Highlights in seagrasses’ phylogeny, physiology, and metabolism: what makes them special? ISRN Botany. 2012;2012(7):103892. doi: 10.5402/2012/103892. [DOI] [Google Scholar]
- Picarelli et al. (2019).Picarelli MASC, Forgia M, Rivas EB, Nerva L, Chiapello M, Turina M, Colariccio A. Extreme diversity of mycoviruses present in isolates of Rhizoctonia solani AG2-2 LP from Zoysia japonica from Brazil. Frontiers in Cellular and Infection Microbiology. 2019;9:244. doi: 10.3389/fcimb.2019.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendeville et al. (2012).Prendeville HR, Ye X, Jack Morris T, Pilson D. Virus infections in wild plant populations are both frequent and often unapparent. American Journal of Botany. 2012;99(6):1033–1042. doi: 10.3732/ajb.1100509. [DOI] [PubMed] [Google Scholar]
- Reitsema, Meire & Schoelynck (2018).Reitsema RE, Meire P, Schoelynck J. The future of freshwater macrophytes in a changing world: dissolved organic carbon quantity and quality and its interactions with macrophytes. Frontiers in Plant Science. 2018;9:629. doi: 10.3389/fpls.2018.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remold (2002).Remold SK. Unapparent virus infection and host fitness in three weedy grass species. Journal of Ecology. 2002;90:967–977. doi: 10.1046/j.1365-2745.2002.00731.x. [DOI] [Google Scholar]
- Rennie & Jackson (2005).Rennie MD, Jackson LJ. The influence of habitat complexity on littoral invertebrate distributions: patterns differ in shallow prairie lakes with and without fish. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62(9):2088–2099. doi: 10.1139/f05-123. [DOI] [Google Scholar]
- Rice, Longden & Bleasby (2000).Rice P, Longden I, Bleasby A. EMBOSS: the European molecular biology open software suite. Trends in Genetics. 2000;16(6):276–277. doi: 10.1016/S0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- Roossinck (2010).Roossinck MJ. Lifestyles of plant viruses. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365(1548):1899–1905. doi: 10.1098/rstb.2010.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roossinck (2011).Roossinck MJ. The good viruses: viral mutualistic symbioses. Nature Reviews Microbiology. 2011;9(2):99–108. doi: 10.1038/nrmicro2491. [DOI] [PubMed] [Google Scholar]
- Roossinck (2015).Roossinck MJ. Plants, viruses and the environment: ecology and mutualism. Virology. 2015;479–480(e3):271–277. doi: 10.1016/j.virol.2015.03.041. [DOI] [PubMed] [Google Scholar]
- Roossinck (2019).Roossinck MJ. Evolutionary and ecological links between plant and fungal viruses. New Phytologist. 2019;221(1):86–92. doi: 10.1111/nph.15364. [DOI] [PubMed] [Google Scholar]
- Roossinck, Martin & Roumagnac (2015).Roossinck MJ, Martin DP, Roumagnac P. Plant virus metagenomics: advances in virus discovery. Phytopathology. 2015;105(6):716–727. doi: 10.1094/PHYTO-12-14-0356-RVW. [DOI] [PubMed] [Google Scholar]
- Roossinck & Schultz-Cherry (2015).Roossinck MJ, Schultz-Cherry S. Move over, bacteria! Viruses make their mark as mutualistic microbial symbionts. Journal of Virology. 2015;89(13):6532–6535. doi: 10.1128/JVI.02974-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario et al. (2009).Rosario K, Nilsson C, Lim YW, Ruan Y, Breitbart M. Metagenomic analysis of viruses in reclaimed water. Environmental Microbiology. 2009;11(11):2806–2820. doi: 10.1111/j.1462-2920.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- Roux et al. (2019).Roux S, Trubl G, Goudeau D, Nath N, Couradeau E, Ahlgren NA, Zhan Y, Marsan D, Chen F, Fuhrman JA, Northen TR, Sullivan MB, Rich VI, Malmstrom RR, Eloe-Fadrosh EA. Optimizing de novo genome assembly from PCR-amplified metagenomes. PeerJ. 2019;7(5879):e6902. doi: 10.7717/peerj.6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahul Hameed et al. (2019).Sahul Hameed AS, Ninawe AS, Nakai T, Chi SC, Johnson KL, ICTV Report Consortium ICTV virus taxonomy profile: nodaviridae. Journal of General Virology. 2019;100(1):3–4. doi: 10.1099/jgv.0.001170. [DOI] [PubMed] [Google Scholar]
- Sanchez et al. (2011).Sanchez R, Serra F, Tarraga J, Medina I, Carbonell J, Pulido L, de Maria A, Capella-Gutierrez S, Huerta-Cepas J, Gabaldon T, Dopazo J, Dopazo H. Phylemon 2.0: a suite of web-tools for molecular evolution, phylogenetics, phylogenomics and hypotheses testing. Nucleic Acids Research. 2011;39(suppl):W470–W474. doi: 10.1093/nar/gkr408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato et al. (2019).Sato Y, Miyazaki N, Kanematsu S, Xie J, Ghabrial SA, Hillman BI, Suzuki N, ICTV Report Consortium ICTV virus taxonomy profile: Megabirnaviridae. Journal of General Virology. 2019;100(9):1269–1270. doi: 10.1099/jgv.0.001297. [DOI] [PubMed] [Google Scholar]
- Shates et al. (2019).Shates TM, Sun P, Malmstrom CM, Dominguez C, Mauck KE. Addressing research needs in the field of plant virus ecology by defining knowledge gaps and developing wild dicot study systems. Frontiers in Microbiology. 2019;9:3305. doi: 10.3389/fmicb.2018.03305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi et al. (2016).Shi M, Lin XD, Tian JH, Chen LJ, Chen X, Li CX, Qin XC, Li J, Cao JP, Eden JS, Buchmann J, Wang W, Xu J, Holmes EC, Zhang YZ. Redefining the invertebrate RNA virosphere. Nature. 2016;540(7634):539–543. doi: 10.1038/nature20167. [DOI] [PubMed] [Google Scholar]
- Smetacek & Zingone (2013).Smetacek V, Zingone A. Green and golden seaweed tides on the rise. Nature. 2013;504(7478):84–88. doi: 10.1038/nature12860. [DOI] [PubMed] [Google Scholar]
- Srivastava, Gupta & Chandra (2008).Srivastava J, Gupta A, Chandra H. Managing water quality with aquatic macrophytes. Reviews in Environmental Science and Biotechnology. 2008;7(3):255–266. doi: 10.1007/s11157-008-9135-x. [DOI] [Google Scholar]
- Starr et al. (2019).Starr EP, Nuccio EE, Pett-Ridge J, Banfield JF, Firestone MK. Metatranscriptomic reconstruction reveals RNA viruses with the potential to shape carbon cycling in soil. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(51):25900–25908. doi: 10.1073/pnas.1908291116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson et al. (2007).Stevenson RJ, Pinowska A, Albertin A, Sickman J. Ecological condition of algae and nutrients in Florida Springs: the synthesis report. 2007. pp. 1–58. Environmental Assessment Section, Florida Department of Environmental Protection, Tallahassee, FL, USA.
- Susi et al. (2019).Susi H, Filloux D, Frilander MJ, Roumagnac P, Laine A-L. Diverse and variable virus communities in wild plant populations revealed by metagenomic tools. PeerJ. 2019;7(1844):e6140. doi: 10.7717/peerj.6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi et al. (2019).Takahashi H, Fukuhara T, Kitazawa H, Kormelink R. Virus latency and the impact on plants. Frontiers in Microbiology. 2019;10:2764. doi: 10.3389/fmicb.2019.02764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrance & Talianksy (2020).Torrance L, Talianksy ME. Potato virus Y emergence and evolution from the Andes of South America to become a major destructive pathogen of potato and other solanaceous crops worldwide. Viruses. 2020;12(12):1430. doi: 10.3390/v12121430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turina et al. (2006).Turina M, Ciuffo M, Lenzi R, Rostagno L, Mela L, Derin E, Palmano S. Characterization of four viral species belonging to the family Potyviridae isolated from Ranunculus asiaticus. Phytopathology. 2006;96(6):560–566. doi: 10.1094/phyto-96-0560. [DOI] [PubMed] [Google Scholar]
- Untergasser et al. (2012).Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. Primer3—new capabilities and interfaces. Nucleic Acids Research. 2012;40(15):e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urayama, Takaki & Nunoura (2016).Urayama S-I, Takaki Y, Nunoura T. FLDS: a comprehensive dsRNA sequencing method for intracellular RNA virus surveillance. Microbes and Environments. 2016;31(1):33–40. doi: 10.1264/jsme2.ME15171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio et al. (2018).Vainio EJ, Chiba S, Ghabrial SA, Maiss E, Roossinck M, Sabanadzovic S, Suzuki N, Xie J, Nibert M, ICTV Report Consortium ICTV virus taxonomy profile: Partitiviridae. Journal of General Virology. 2018;99(1):17–18. doi: 10.1099/jgv.0.000985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bogaert et al. (2019).Van Bogaert N, Rosario K, Furman BT, Hall MO, Greco AM, Breitbart M. Discovery of a novel potexvirus in the seagrass Thalassia testudinum from Tampa Bay, Florida. Limnology and Oceanography Letters. 2019;4(1):1–8. doi: 10.1002/lol2.10098. [DOI] [Google Scholar]
- Van Etten, Agarkova & Dunigan (2019).Van Etten JL, Agarkova IV, Dunigan DD. Chloroviruses. Viruses. 2019;12(1):20. doi: 10.3390/v12010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Mölken & Stuefer (2011).van Mölken T, Stuefer JF. The potential of plant viruses to promote genotypic diversity via genotype × environment interactions. Annals of Botany. 2011;107(8):1391–1397. doi: 10.1093/aob/mcr078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoria et al. (2009).Victoria JG, Kapoor A, Li L, Blinkova O, Slikas B, Wang C, Naeem A, Zaidi S, Delwart E. Metagenomic analyses of viruses in stool samples from children with acute flaccid paralysis. Journal of Virology. 2009;83(9):4642–4651. doi: 10.1128/JVI.02301-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlok, Gibbs & Suttle (2019).Vlok M, Gibbs AJ, Suttle CA. Metagenomes of a freshwater charavirus from British Columbia provide a window into ancient lineages of viruses. Viruses. 2019;11(3):299. doi: 10.3390/v11030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlok, Lang & Suttle (2019a).Vlok M, Lang AS, Suttle CA. Application of a sequence-based taxonomic classification method to uncultured and unclassified marine single-stranded RNA viruses in the order Picornavirales. Virus Evolution. 2019a;5:vez056. doi: 10.1093/ve/vez056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlok, Lang & Suttle (2019b).Vlok M, Lang AS, Suttle CA. Marine RNA virus quasispecies are distributed throughout the oceans. mSphere. 2019b;4(2):e00157. doi: 10.1128/mSphereDirect.00157-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron, Stone & Obbard (2018).Waldron FM, Stone GN, Obbard DJ. Metagenomic sequencing suggests a diversity of RNA interference-like responses to viruses across multicellular eukaryotes. PLOS Genetics. 2018;14(7):e1007533. doi: 10.1371/journal.pgen.1007533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waycott et al. (2009).Waycott M, Duarte CM, Carruthers TJB, Orth RJ, Dennison WC, Olyarnik S, Calladine A, Fourqurean JW, Heck KL, Hughes AR, Kendrick GA, Kenworthy WJ, Short FT, Williams SL. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(30):12377–12381. doi: 10.1073/pnas.0905620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westdal (2011).Westdal P. Host range studies of oat blue dwarf virus. Canadian Journal of Botany. 2011;46(11):1431–1435. doi: 10.1139/b68-194. [DOI] [Google Scholar]
- Wolf et al. (2018).Wolf YI, Kazlauskas D, Iranzo J, Lucia-Sanz A, Kuhn JH, Krupovic M, Dolja VV, Koonin EV. Origins and evolution of the global RNA virome. mBio. 2018;9(6):e02329. doi: 10.1128/mBio.02329-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf et al. (2020).Wolf YI, Silas S, Wang Y, Wu S, Bocek M, Kazlauskas D, Krupovic M, Fire A, Dolja VV, Koonin EV. Doubling of the known set of RNA viruses by metagenomic analysis of an aquatic virome. Nature Microbiology. 2020;5(10):1262–1270. doi: 10.1038/s41564-020-0755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie et al. (2017).Wylie SJ, Adams M, Chalam C, Kreuze J, López-Moya JJ, Ohshima K, Praveen S, Rabenstein F, Stenger D, Wang A, Zerbini FM, ICTV Report Consortium ICTV virus taxonomy profile: potyviridae. Journal of General Virology. 2017;98(3):352–354. doi: 10.1099/jgv.0.000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie, Li & Jones (2013).Wylie SJ, Li H, Jones MGK. Donkey orchid symptomless virus: a viral ‘platypus’ from Australian terrestrial orchids. PLOS ONE. 2013;8(11):e79587. doi: 10.1371/journal.pone.0079587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia et al. (2020).Xia Y, Zhang M, Tsang DCW, Geng N, Lu D, Zhu L, Igalavithana AD, Dissanayake PD, Rinklebe J, Yang X, Ok YS. Recent advances in control technologies for non-point source pollution with nitrogen and phosphorous from agricultural runoff: current practices and future prospects. Applied Biological Chemistry. 2020;63(1):8. doi: 10.1186/s13765-020-0493-6. [DOI] [Google Scholar]
- Yu (2020).Yu G. Using ggtree to visualize data on tree-like structures. Current Protocols in Bioinformatics. 2020;69(1):e96. doi: 10.1002/cpbi.96. [DOI] [PubMed] [Google Scholar]
- Yu et al. (2017).Yu G, Smith DK, Zhu H, Guan Y, Lam TT-Y. ggtree: an R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods in Ecology and Evolution. 2017;8(1):28–36. doi: 10.1111/2041-210X.12628. [DOI] [Google Scholar]
- Zettler & Freeman (1972).Zettler FW, Freeman TE. Plant pathogens as biocontrols of aquatic weeds. Annual Review of Phytopathology. 1972;10(1):455–470. doi: 10.1146/annurev.py.10.090172.002323. [DOI] [Google Scholar]
- Zhong, Sun & Penny (2015).Zhong B, Sun L, Penny D. The origin of land plants: a phylogenomic perspective. Evolutionary Bioinformatics. 2015;11:137–141. doi: 10.4137/EBO.S29089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann et al. (2018).Zimmermann L, Stephens A, Nam S-Z, Rau D, Kübler J, Lozajic M, Gabler F, Söding J, Lupas AN, Alva V. A completely reimplemented MPI Bioinformatics Toolkit with a new HHpred server at its core. Journal of Molecular Biology. 2018;430(15):2237–2243. doi: 10.1016/j.jmb.2017.12.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 provides a list of primers used for PCR and sequence-independent single-primer amplification (SISPA) assays. Table S2 provides detailed information for the 156 viral contigs identified in macrophyte datasets.
Data Availability Statement
The following information was supplied regarding data availability:
Next generation sequencing data are available at the Sequence Read Archive (SRA) database under BioProject PRJNA826216 (BioSample accession numbers SAMN27553344 through SAMN27553353).