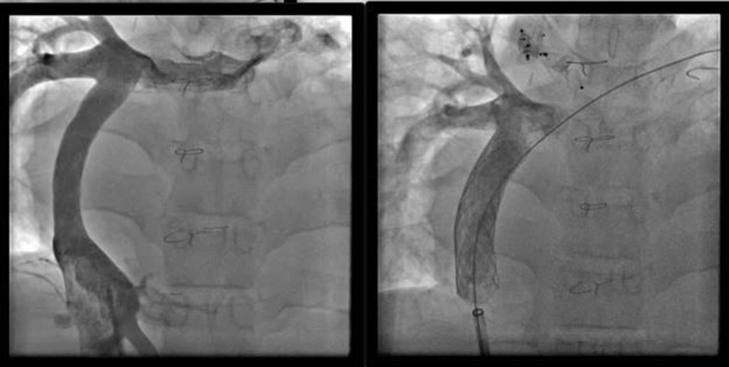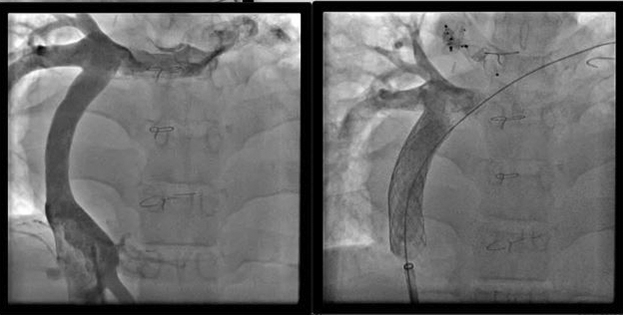
Upsizing of a 14-mm extracardiac conduit with two 18-mm stents.
Central Message.
Intervention on the pulmonary arteries and atrioventricular valve enhances candidacy for Fontan. Optimization of the Fontan circuit, pulmonary arteries, and valves might improve late outcomes after Fontan surgery.
See Commentaries on pages 233 and 235.
Feature Editor's Introduction—Experts in congenital heart disease have unified efforts to increase survival and reduce the morbidity of single ventricle patients for decades. Tremendous technical and medical refinements have been instrumental in making it possible for the majority of our single ventricle patients survive postnatal heart surgery and become candidates for the completion of single ventricle palliation. We have long understood that the ultimate success of third-stage single ventricle palliation depends on the early and optimal management of these patients undergoing this not the most technically demanding operation with significant chronic suboptimal physiological state of the circulation.
In this issue, Dr d’Udekem and expert colleagues graciously offer a comprehensive review of optimizing Fontan candidacy and the Fontan circulation, including possible interventions beyond Fontan completion. The authors focus on critical circulatory elements from myocardial to atrioventricular valve function and pulmonary arteries that impact the Fontan circulation. They emphasize the proper strategies and proper timing of interventions and provide a further window into innovative approaches proposed to improve the quality of life of patients with a Fontan circulation. Their perspective on this Fontan conundrum is the result of years of research and clinical work. We thank the authors for their outstanding contribution.
Can Yerebakan, MD, PhD
We now accept that the vast majority of patients born with single ventricle physiology who survive the Fontan procedure will live into adulthood.1 Accordingly, our goal has shifted from achieving mere survival to a much larger scope: we now want more patients with a Fontan circulation to live longer and to have a better quality of life. There might be several interventions that may help us to achieve these goals before Fontan completion, in the early years that follow Fontan completion, and maybe even several decades after completion of the Fontan circulation.
Candidacy for Fontan Completion
A long time has passed since the publication of the “Ten Commandments” by Drs Choussat and Fontan.2 Several of these initial recommendations have since been disproven.3,4 We now understand that there are limitations to the Fontan circulation, and that we should perform this procedure only in ideal candidates. As an example, we have learned our lesson in terms of preserving the pulmonary vasculature before Fontan completion by avoiding pulmonary overcirculation and the associated high pulmonary vascular resistance. In countries with extensive primary healthcare, we no longer see patients with extremely high pulmonary blood flow and increased pulmonary vascular resistance. None of us would offer a Fontan to patients who have been identified to have elevated pulmonary artery (PA) pressure during catheterization. Pulmonary venous obstruction remains an issue, and only few of us would be brave enough to proceed with Fontan circulation in patients significantly affected by this restriction.
In the current era, the 3 key elements to obtain a successful Fontan circulation are appropriate myocardial systolic and diastolic function, absence of atrioventricular (AV) valve regurgitation, and well-developed PAs.3,5,6 It now has been clearly identified that individuals without preserved ventricular function, large PAs, and nonregurgitant AV valves will be at greater risk of Fontan failure. Studies have shown, for example, that when AV valve intervention is needed at or before Fontan, 18% of patients will experience Fontan failure within 3.5 years.6 After 5 decades of experience with the Fontan palliation, the question going forward is whether we can intervene to improve these 3 parameters before Fontan completion.
Myocardial Function
In single ventricle circulation, the majority of patients who will reach the time of Fontan completion with poor ventricular function will be patients with hypoplastic left heart syndrome or unbalanced AV septal defect with a common AV valve. It is still unclear whether myocardial dysfunction is preventable in those patients. It is possible that excessive early loading of the ventricular cavities will lead to irreversible damage in ventricular function. If that were the case, then better management of ventricular loading early in life might improve the long-term outcomes of these patients. Policies of restrictive shunting may be beneficial. Some teams are now trying to preserve or improve ventricular function by injection of stem cells.7,8 It is far too early to know whether stem cell therapy will be a viable option for these patients, although limited data in patients with Ebstein anomaly suggest possible benefits of intramyocardial injection of cell-based therapy.9
AV Valve Regurgitation
The burden of AV valve regurgitation in the lifetime of patients with single ventricle conditions seems enormous. We have demonstrated that valves in single ventricles fail at much faster rates than valves of normal hearts with 2 ventricles.6 We have not yet identified why these valves are failing at such higher rates, and it seems as though we are not yet mastering the techniques of repair that might improve these patients' outcomes. We have identified that the presence of more than moderate AV valve regurgitation in these patients more than doubles their risk of death and transplantation after Fontan surgery.6 Most of us can agree that the presence of more than moderate AV valve regurgitation necessitates a separate intervention before Fontan completion. A separate procedure should be favored, for the following reasons: (1) prolonging the cross-clamp time at the time of Fontan increases the risk of early Fontan failure; (2) these repairs are difficult and sometimes necessitate a second run; the Fontan circuit is then always obstructing the vision and access to the repaired valve, making immediate redo surgery difficult; (3) the quality of this repair is unpredictable, and performing the repair before Fontan completion allows either repeat surgery or even replacement of the valve at the same time or as another separate procedure before Fontan; and (4) for reasons not yet elucidated, this surgery seems far better tolerated before Fontan completion than after.10 Because our rate of failure of our current AV valve repairs are suboptimal, we should explore other techniques. For example, it has been suggested that patients with single ventricles may have larger annuli than leaflet surfaces, and thus it might be necessary to proceed with leaflet augmentation to achieve more successful repairs. We have had some success with patch augmentation of the anterior leaflet of the tricuspid valve.
PAs
We know that the functional outcome and longevity of patients after Fontan completion will be directly dependent on the quality of their pulmonary vasculature. Performing a Fontan circulation on 1 lung has demonstrated extremely poor results, with one-quarter of the patients dying within 5 years of Fontan completion.11 Enlarging the PAs by stenting or by surgery at the time of the bidirectional Glenn procedure has become routine in most institutions. Data have shown that augmentation of 1 or both small PAs does not adversely affect long-term outcomes in patients when performed at or before the Fontan procedure.12 Some teams have adopted a strategy of performing an additional intervention to develop the distal pulmonary vasculature of patients with a small PA. Sughimoto and colleagues13 described an alternative solution to full reconstruction of the central PAs by excising the stenosed PA and anastomosing a Gore-Tex conduit to both ends of the central PA. More recently, Seaman and colleagues14 followed patients after interim shunt placement into the smaller PA and showed that the induced enlargement of the PAs was maintained after 5 years. Many of us would patch any small PA using various techniques at the time of Fontan. An Australian study showed that patients who underwent pulmonary arterioplasty actually had very similar outcomes as their counterparts who did not need PA patching.15 Optimization of the size of the PAs before, at the time of, or after Fontan completion should be considered mandatory.
The size of the extracardiac conduit is another important consideration. Simply put, the pressure in the inferior vena cava needs to be minimized to mitigate the long-term issues with liver disease in the years after Fontan. Implanting small conduits during childhood will commit these patients to either conduit replacement or transcatheter stent augmentation in the future.
Additional Considerations
It is becoming clear that the best outcomes after Fontan completion are achieved in patients who are physically active and lean.16 There is a direct relationship between body mass index in patients and risk of Fontan failure, with Cao and colleagues16 reporting that with every 1% increase in body fat percentage, there was a 10% increased risk of developing Fontan failure. From this perspective, individuals who are incapable of exercising (eg, after brain injury or because of a neuromuscular disorder) will be poor candidates for Fontan completion.
Optimization
We have often told our patients that the Fontan procedure was the last in a series of operations for single ventricle palliation. We now realize that many of these patients need reoperation.17,18 Approximately one-half need a reintervention within the 3 decades following their Fontan.17 Today, a limited proportion of those patients need intervention on the Fontan circuit (2-4%), PAs (3-11%), AV valve (11%), or semilunar valve (<1%),6,17,18 but as the mortality and morbidity of these procedures decrease and our population of patients with a Fontan circulation ages, a large number of patients likely will require optimization procedures.
Fontan Circuit
There has been much debate regarding the ideal size of the conduit used for extracardiac Fontan. Many of us have concerns about the size of the Gore-Tex tube for extracardiac Fontan when 16 mm or smaller. There is no indication that using a tube larger than 18 mm would be beneficial or deleterious. It is amazing to note that the diameter of the conduit decreases with time. Patel and associates19,20 showed a significant 25% reduction in the minimal cross-sectional area of the Fontan conduit within a year of Fontan completion. They found no benefit of oversizing the Fontan circuitry. Is it possible that the reduction in the inner diameter of these conduits is dependent and inversely proportional to the flow inside the conduit? Is it possible that a circuit that had been placed at age 3 to 4 years needs to be increased in size as the patient enters adolescence and adulthood? Because the Gore-Tex conduit has some elasticity, we may consider dilating the conduit that appears to be too small as the patient reaches adulthood.
Another issue is the presence of narrowing and kinking on the anastomosis of the circuit itself. This narrowing is not always hemodynamically significant because it does not generate a pressure gradient. Lee and colleagues21 reported that patients with smaller conduits had better exercise capacity. Interestingly, Itatani and associates22 observed a backward flow of fluid during exercise in children with larger conduits. There is a growing body of evidence generated with 4-dimensional computational flow dynamics magnetic resonance imaging studies showing that oversizing the Fontan conduits leads to worse exercise capacity and even impaired liver function in the long term. Even though there are few reports of dilatation and stenting of Fontan circuitry,23,24 at this stage, it appears to be a very innocuous procedure that may provide long-term sustained benefits (Figure 1).
Figure 1.
Left, Angiogram from an 18-year-old with pulmonary atresia with intact ventricular septum. There was diffuse narrowing of a 14-mm Gore-Tex extracardiac conduit that had been placed during a Fontan operation at age 2.5 years. Right, Improved Fontan extracardiac conduit diameter after placement of two 18-mm stents.
PAs
Do we not suspect that the majority of patients with a Fontan circulation have PA sizes inferior to normal? We do not yet have evidence that these PAs, especially the left PA, are smaller. The central vessels are easily accessible to interventional catheterization, and again it seems that post-Fontan intervention on these PAs does not lead to higher rates of morbidity. It has not yet been demonstrated that the stenting of small PAs increases the longevity or exercise capacity of these patients, but some of these benefits could be expected.
AV Valve
We have now identified that developing AV valve regurgitation after Fontan completion more than doubles the risk of death and transplantation. While there seems to be a rationale for operating on these patients, this has not come into current practice at this early stage of our failure. A review of the Australia and New Zealand Fontan Registry (ANZFR) found that only 17 of 158 candidates (11%) underwent valve intervention after their Fontan completion.6 The likely reason for the lack of intervention is because at the early stage of failure, the patients remain asymptomatic, and the majority of them are active adolescents or young adults. The success rate of surgical intervention on the AV valve is not equivalent to that of valve repair in 2-ventricle circulation, with the majority of studies reporting 50 percent of failure within 5 to 7 years following valve repair.6,25,26 This procedure has a reported risk of mortality ranging from 3% to 19%.10,17,27
Unfortunately, we are not yet able to provide a risk–benefit estimation of these procedures. Failure of the AV valve after Fontan completion might be an indication for implantation of stented bioprostheses by catheter intervention. In the worst of these patients, a bioprosthetic valve rather than a mechanical valve is often indicated because their long-term outcomes are compromised. The currently reported results of AV valve repair are suboptimal. The annulus of these patients is invariably dilated, and they clearly would benefit from less invasive procedures.
Semilunar Valve
Few reports of reoperation on the semilunar valves in patients with Fontan circulation have been published. This may change rapidly in the coming years, however. Concerns have been raised regarding the capacity of the pulmonary root needed to sustain pulmonary circulation over a lifetime.28 There are anecdotal reports of dissection and rupture of these roots.29 Regardless, procedures on these dilated pulmonary roots likely are becoming more frequent.
Arrhythmia
The most frequent intervention after Fontan is pacemaker implantation. The ANZFR found that of 435 patients who required at least 1 reintervention following Fontan, 63 (14%) had at least 1 pacemaker insertion surgery.17 We have demonstrated that implanting a pacemaker in a patient with Fontan circulation increases the risk of death by 14-fold and is the most potent predictor of death and transplantation in this population.30 Some small studies have shown that multisite pacing may reduce the adverse outcomes observed after pacing in these patients.31, 32, 33
Beyond Fontan
At this time of the progressive decline of the Fontan circulation, we seem to have only one option available: heart transplantation. Some of these patients may be candidates for mechanical circulatory support as a bridge to heart transplantation. Several groups are attempting to develop right cavopulmonary assist devices that would act as the missing right ventricle in these patients.34,35 The majority of these patients who are facing failure of their Fontan circulation have preserved systolic ventricular function.36 A question that needs to be answered within the next decade is whether the decline of Fontan circulation is reversible. In an otherwise healthy patient with preserved systolic function, a good circuit, good-sized PAs, and no AV valve regurgitation, it is possible that the decline of the circulation could be related to a progressive increase in pulmonary vascular resistance through a lack of pulsatility and progressive diastolic dysfunction of the systemic ventricle.
Could these 2 phenomena be reversible? It is likely that exercise, known to be an essential measure to prevent the decline of the Fontan circulation, is acting by intermittently providing some degree of pulsatility in the PA37 along with intermittent loading of the left ventricle preventing progressive diastolic dysfunction. Will it be possible to revalidate the Fontan circulation by stretching the PAs and systemic ventricle by volume and pressure loading with a right cavopulmonary assist device? There is no limit to the potential options available for optimizing Fontan circulation. A summary of our recommended potential avenues is provided in Table 1.
Table 1.
Potential avenues for improving candidacy and optimized Fontan circulation
| Areas considered for candidacy and/or optimization | Current areas of concern | Potential improvements in technology and/or clinical practice |
|---|---|---|
| Myocardial function | Excessive early loading of ventricles leads to irreversible damage | Reduced volume loading in early life (restrictive shunting policies?); cell-based therapies |
| AV valve | Repair technique for AV valve regurgitation | Investigate new repair techniques (ie, patching of anterior leaflet of tricuspid valve) |
| Timing of repair for AV valve regurgitation | Identify best timing of repair by building a risk/benefits analysis in a large dataset | |
| AV valve failure | Implantation of stented bioprostheses by catheter intervention | |
| PA | Small pulmonary vasculature | Intermediate procedure to grow the PAs (interim shunt placement); more systematic PA patch augmentation |
| Smaller left PA | Stenting/dilatation; revalidation of PA via cavopulmonary assist device | |
| Increase in pulmonary vascular resistance | Exercise to increase pulsatility and increase intermittent loading of ventricle | |
| PA stenosis | Stenting of small PAs | |
| Fontan circuit | Size decreases with time; relative conduit obstruction | Stent Gore-Tex conduit as a teenager; use 4-dimensional magnetic resonance imaging flow studies to evaluate optimal sizing |
| Semilunar valve | Pulmonary root potential to dilate | Early surgical intervention |
| Arrhythmia | Pacemaker increases mortality | Multisite pacing |
| Body mass index | Higher body mass index and adverse body composition (increased fat and decreased lean muscle mass) linked to greater likelihood of failure | Stress physical activity and proper nutrition prior to Fontan; Initiate systematic exercise programs from an early age. |
AV, Atrioventricular; PA, pulmonary artery.
Conclusions
In his seminal work explaining the principle on which the Fontan circulation works, Marc de Leval claimed that we should only perform “good” Fontan operations. We should still follow the same principle, but we now realize that we can improve the candidacy for Fontan by repetitive intervention on the PAs and AV valves. Hopefully, optimizing the Fontan circulation by stenting the Fontan circuit or PAs, along with more aggressive intervention on the AV valves of these patients, will be beneficial. At this stage, we might not yet have explored all possibilities available to provide the maximum lifespan for these patients.
Conflict of Interest Statement
Dr d'Udekem serves as a consultant for Actelion. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
References
- 1.d'Udekem Y., Iyengar A.J., Galati J.C., Forsdick V., Weintraub R.G., Wheaton G.R., et al. Redefining expectations of long-term survival after the Fontan procedure: twenty-five years of follow-up from the entire population of Australia and New Zealand. Circulation. 2014;130(11 Suppl 1):S32–S38. doi: 10.1161/CIRCULATIONAHA.113.007764. [DOI] [PubMed] [Google Scholar]
- 2.Choussat A., Fontan F., Besse P., Vallot F., Chauve A. In: Pediatric Cardiology. Anderson R., Shinebourne E.A., editors. Churchill Livingstone; Edinburgh, UK: 1978. Selection criteria for Fontan's procedure; pp. 559–566. [Google Scholar]
- 3.Hosein R.B.M., Clarke A.J.B., McGuirk S.P., Griselli M., Stumper O., De Giovanni J.V., et al. Factors influencing early and late outcome following the Fontan procedure in the current era. The ‘Two Commandments'? Eur J Cardiothorac Surg. 2007;31:344–352. doi: 10.1016/j.ejcts.2006.11.043. discussion 353. [DOI] [PubMed] [Google Scholar]
- 4.Stern H.J. Fontan “Ten Commandments” revisited and revised. Pediatr Cardiol. 2010;31:1131–1134. doi: 10.1007/s00246-010-9811-9. [DOI] [PubMed] [Google Scholar]
- 5.Gewillig M. The Fontan circulation. Heart. 2005;91:839–846. doi: 10.1136/hrt.2004.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King G., Ayer J., Celermajer D., Zentner D., Justo R., Disney P., et al. Atrioventricular valve failure in fontan palliation. J Am Coll Cardiol. 2019;73:810–822. doi: 10.1016/j.jacc.2018.12.025. [DOI] [PubMed] [Google Scholar]
- 7.Ishigami S., Ohtsuki S., Eitoku T., Ousaka D., Kondo M., Kurita Y., et al. Intracoronary cardiac progenitor cells in single ventricle physiology: the PERSEUS (Cardiac Progenitor Cell Infusion to Treat Univentricular Heart Disease) randomized phase 2 trial. Circ Res. 2017;120:1162–1173. doi: 10.1161/CIRCRESAHA.116.310253. [DOI] [PubMed] [Google Scholar]
- 8.Burkhart H.M., Qureshi M.Y., Rossano J.W., Cantero Peral S., O'Leary P.W., Hathcock M., et al. Autologous stem cell therapy for hypoplastic left heart syndrome: safety and feasibility of intraoperative intramyocardial injections. J Thorac Cardiovasc Surg. 2019;158:1614–1623. doi: 10.1016/j.jtcvs.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 9.O'Leary P.W., Qureshi M.Y., Cetta F., Nelson T.J., Holst K.A., Dearani J.A. Cone reconstruction for Ebstein anomaly: ventricular remodeling and preliminary impact of stem cell therapy. Mayo Clin Proc. 2021;96:3053–3061. doi: 10.1016/j.mayocp.2021.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Stephens E.H., Dearani J.A., Niaz T., Arghami A., Phillips S.D., Cetta F. Effect of earlier atrioventricular valve intervention on survival after the Fontan operation. Am J Cardiol. 2020;137:103–110. doi: 10.1016/j.amjcard.2020.09.028. [DOI] [PubMed] [Google Scholar]
- 11.Fujii Y., Sano S., Asou T., Imoto Y., Oshima Y., Kawasaki S., et al. Outcomes of one-lung Fontan operation: a retrospective multicenter study in Japan. Ann Thorac Surg. 2012;94:1275–1280. doi: 10.1016/j.athoracsur.2012.04.080. [DOI] [PubMed] [Google Scholar]
- 12.Shearer L., Justo R.N., Marathe S.P., Betts K., Venugopal P., Winlaw D.S., et al. Augmentation of the pulmonary arteries at or prior to the Fontan procedure is not associated with worse long-term outcomes: a propensity-matched analysis from the Australia-New Zealand Fontan Registry†. Eur J Cardiothorac Surg. 2019;55:829–836. doi: 10.1093/ejcts/ezy376. [DOI] [PubMed] [Google Scholar]
- 13.Sughimoto K., Konstantinov I.E., Brizard C.P., d'Udekem Y. Hilum-to-hilum Gore-Tex tube replacement of central pulmonary arteries. Ann Thorac Surg. 2015;99:340–342. doi: 10.1016/j.athoracsur.2014.06.085. [DOI] [PubMed] [Google Scholar]
- 14.Seaman C.S., d'udekem Y., Jones B.O., Brizard C.P.R., Cheung M.M.H. Augmentation of pulmonary arterial growth in single ventricle patients by interim selective shunts. Semin Thorac Cardiovasc Surg. 2021;33:483–489. doi: 10.1053/j.semtcvs.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Brink J., MacIver R., Lee M.G.Y., Konstantinov I.E., Cheung M., Brizard C.P., et al. Neonatal pulmonary artery reconstruction during shunting to treat and prevent juxtaductal coarctation. Ann Thorac Surg. 2015;99:641–647. doi: 10.1016/j.athoracsur.2014.09.034. [DOI] [PubMed] [Google Scholar]
- 16.Cao J.Y., Tran D., Briody J., Attard C., Hassan E.B., Simm P., et al. Impact of adiposity on clinical outcomes in people living with a Fontan circulation. Int J Cardiol. 2021;329:82–88. doi: 10.1016/j.ijcard.2020.12.066. [DOI] [PubMed] [Google Scholar]
- 17.Daley M., du Plessis K., Zannino D., Hornung T., Disney P., Cordina R., et al. Reintervention and survival in 1428 patients in the Australian and New Zealand Fontan Registry. Heart. 2020;106:751–757. doi: 10.1136/heartjnl-2019-315430. [DOI] [PubMed] [Google Scholar]
- 18.Downing T.E., Allen K.Y., Goldberg D.J., Rogers L.S., Ravishankar C., Rychik J., et al. Surgical and catheter-based reinterventions are common in long-term survivors of the Fontan operation. Circ Cardiovasc Interv. 2017;10:e004924. doi: 10.1161/CIRCINTERVENTIONS.116.004924. [DOI] [PubMed] [Google Scholar]
- 19.Patel N.D., Friedman C., Herrington C., Wood J.C., Cheng A.L. Progression in Fontan conduit stenosis and hemodynamic impact during childhood and adolescence. J Thorac Cardiovasc Surg. 2021;162:372–380.e2. doi: 10.1016/j.jtcvs.2020.09.140. [DOI] [PubMed] [Google Scholar]
- 20.Tongut A., d'Udekem Y. Commentary: you should occasionally look at the results!! J Thorac Cardiovasc Surg. 2021;162:381–382. doi: 10.1016/j.jtcvs.2020.11.055. [DOI] [PubMed] [Google Scholar]
- 21.Lee S.Y., Song M.K., Kim G.B., Bae E.J., Kim S.H., Jang S.I., et al. Relation between exercise capacity and extracardiac conduit size in patients with Fontan circulation. Pediatr Cardiol. 2019;40:1584–1590. doi: 10.1007/s00246-019-02190-4. [DOI] [PubMed] [Google Scholar]
- 22.Itatani K., Miyaji K., Tomoyasu T., Nakahata Y., Ohara K., Takamoto S., et al. Optimal conduit size of the extracardiac Fontan operation based on energy loss and flow stagnation. Ann Thorac Surg. 2009;88:565–572. doi: 10.1016/j.athoracsur.2009.04.109. discussion 572-573. [DOI] [PubMed] [Google Scholar]
- 23.Hagler D.J., Miranda W.R., Haggerty B.J., Anderson J.H., Johnson J.N., Cetta F., et al. Fate of the Fontan connection: mechanisms of stenosis and management. Congenit Heart Dis. 2019;14:571–581. doi: 10.1111/chd.12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ten Cate F.E., Trieschmann U., Germund I., Hannes T., Emmel M., Bennink G., et al. Stenting the Fontan pathway in paediatric patients with obstructed extracardiac conduits. Heart. 2017;103:1111–1116. doi: 10.1136/heartjnl-2016-310511. [DOI] [PubMed] [Google Scholar]
- 25.Menon S.C., Dearani J.A., Cetta F. Long-term outcome after atrioventricular valve surgery following modified Fontan operation. Cardiol Young. 2011;21:83–88. doi: 10.1017/S1047951110001538. [DOI] [PubMed] [Google Scholar]
- 26.Muntaner C.D., King G., Zannino D., Alphonso N., Finucance K., Winlaw D., et al. Poor late outcomes after tricuspid valve repair in a single ventricle: experience of 103 patients. Ann Thorac Surg. 2021;111:987–994. doi: 10.1016/j.athoracsur.2020.05.070. [DOI] [PubMed] [Google Scholar]
- 27.Sughimoto K., Konstantinov I.E., d'Udekem Y., Brink J., Zannino D., Brizard C.P. Mid-term outcomes of congenital mitral valve surgery: Shone's syndrome is a risk factor for death and reintervention. Interact Cardiovasc Thorac Surg. 2017;25:734–739. doi: 10.1093/icvts/ivx211. [DOI] [PubMed] [Google Scholar]
- 28.Cohen M.S., Marino B.S., McElhinney D.B., Robbers-Visser D., van der Woerd W., Gaynor J.W., et al. Neo-aortic root dilation and valve regurgitation up to 21 years after staged reconstruction for hypoplastic left heart syndrome. J Am Coll Cardiol. 2003;42:533–540. doi: 10.1016/s0735-1097(03)00715-0. [DOI] [PubMed] [Google Scholar]
- 29.Umezu K., Harada Y., Sakamoto T., Okamura T., Shintomi S., Takigiku K., et al. Neo-aortic insufficiency late after staged reconstruction for hypoplastic left heart syndrome: impact of differences in initial palliative procedures. Heart Vessels. 2019;34:1456–1463. doi: 10.1007/s00380-019-01376-3. [DOI] [PubMed] [Google Scholar]
- 30.Poh C.L., d'Udekem Y. Life after surviving Fontan surgery: a meta-analysis of the incidence and predictors of late death. Heart Lung Circ. 2018;27:552–559. doi: 10.1016/j.hlc.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Bacha E.A., Zimmerman F.J., Mor-Avi V., Weinert L., Starr J.P., Sugeng L., et al. Ventricular resynchronization by multisite pacing improves myocardial performance in the postoperative single-ventricle patient. Ann Thorac Surg. 2004;78:1678–1683. doi: 10.1016/j.athoracsur.2004.04.065. [DOI] [PubMed] [Google Scholar]
- 32.Sojak V., Mazic U., Cesen M., Schrader J., Danojevic N. Cardiac resynchronization therapy for the failing Fontan patient. Ann Thorac Surg. 2008;85:2136–2138. doi: 10.1016/j.athoracsur.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Joyce J., O'Leary E.T., Mah D.Y., Harrild D.M., Rhodes J. Cardiac resynchronization therapy improves the ventricular function of patients with Fontan physiology. Am Heart J. 2020;230:82–92. doi: 10.1016/j.ahj.2020.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Rodefeld M.D., Marsden A., Figliola R., Jonas T., Neary M., Giridharan G.A. Cavopulmonary assist: long-term reversal of the Fontan paradox. J Thorac Cardiovasc Surg. 2019;158:1627–1636. doi: 10.1016/j.jtcvs.2019.06.112. [DOI] [PubMed] [Google Scholar]
- 35.Sinha P., Deutsch N., Ratnayaka K., Lederman R., He D., Nuszkowski M., et al. Effect of mechanical assistance of the systemic ventricle in single ventricle circulation with cavopulmonary connection. J Thorac Cardiovasc Surg. 2014;147:1271–1275. doi: 10.1016/j.jtcvs.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kotani Y., Chetan D., Zhu J., Saedi A., Zhao L., Mertens L., et al. Fontan failure and death in contemporary Fontan circulation: analysis from the last two decades. Ann Thorac Surg. 2018;105:1240–1247. doi: 10.1016/j.athoracsur.2017.10.047. [DOI] [PubMed] [Google Scholar]
- 37.Cordina R., Celermajer D.S., d'Udekem Y. Lower limb exercise generates pulsatile flow into the pulmonary vascular bed in the setting of the Fontan circulation. Cardiol Young. 2018;28:732–733. doi: 10.1017/S104795111800015X. [DOI] [PubMed] [Google Scholar]