Abstract
Objective
The safety and feasibility of preoperative pembrolizumab combined with chemoradiotherapy (PPCT) for resectable esophageal squamous cell carcinoma have been confirmed by the prior Preoperative Anti-PD-1 Antibody combined with Chemoradiotherapy for Locally Advanced Squmous Cell Carcinoma of Esophageus (PALACE)-1 trial. Potential therapeutic benefit was also observed with a pathologic complete response rate of 55.6% after PPCT. We will conduct the multicenter single-arm PALACE-2 study to investigate the efficacy and to further confirm the safety of PPCT (ClinicalTrials.gov ID: NCT04435197).
Methods
A total of 143 patients with previously untreated, locally advanced, and surgically resectable esophageal squamous cell carcinoma (T2 through T4a, N0 through N+, M0) will be enrolled in PALACE-2. Main exclusion criteria are autoimmune disease, interstitial lung disease, ongoing immunosuppressive therapy, and having received chemotherapy, radiotherapy, target therapy, or immune therapy for this or any other malignancies. Positive programmed cell death ligand 1 expression is not mandatory for enrollment. Patients will receive PPCT, which includes concurrent pembrolizumab (200 mg on day 1 and day 22), carboplatin (area under the curve = 2, once a week for 5 weeks), nab-paclitaxel (50 mg/m2, once a week for 5 weeks), and radiotherapy (23 fractions of 1.8 Gy, 5 fractions a week). Esophagectomy will be performed within 4 to 6 weeks after the completion of PPCT.
Results
The primary end point is the rate of pathologic complete response. Secondary outcome measures are 3-year disease-free survival rate, 3-year overall survival rate, R0 resection rate, and adverse events during neoadjuvant and perioperative periods.
Conclusions
PPCT was preliminarily demonstrated to be safe, feasible, and to provide potential therapeutic benefits by the PALACE-1 trial. The subsequent multicenter PALACE-2 study will investigate the efficacy and further confirm the safety of PPCT for locally advanced, resectable esophageal squamous cell carcinoma.
Key Words: esophageal squamous cell carcinoma, neoadjuvant therapy, chemoradiotherapy, immunotherapy
Abbreviations and Acronyms: AEs, adverse events; CROSS, Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study; CT, computed tomography; ESCC, esophageal squamous cell carcinoma; ICI, immune checkpoint inhibitor; nCRT, neoadjuvant chemoradiotherapy; OS, overall survival; PALACE, Preoperative Anti-PD-1 Antibody combined with Chemoradiotherapy for Locally Advanced Squmous Cell Carcinoma of Esophageus; pCR, pathologic complete response; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; PET-CT, positron emission tomography-computed tomography; PPCT, preoperative pembrolizumab combined with chemoradiotherapy
Graphical abstract
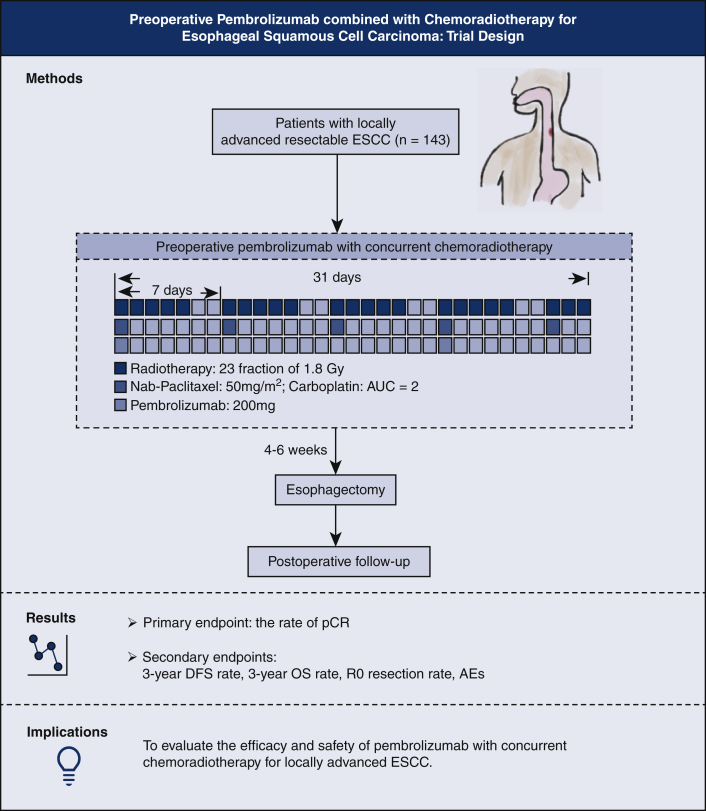
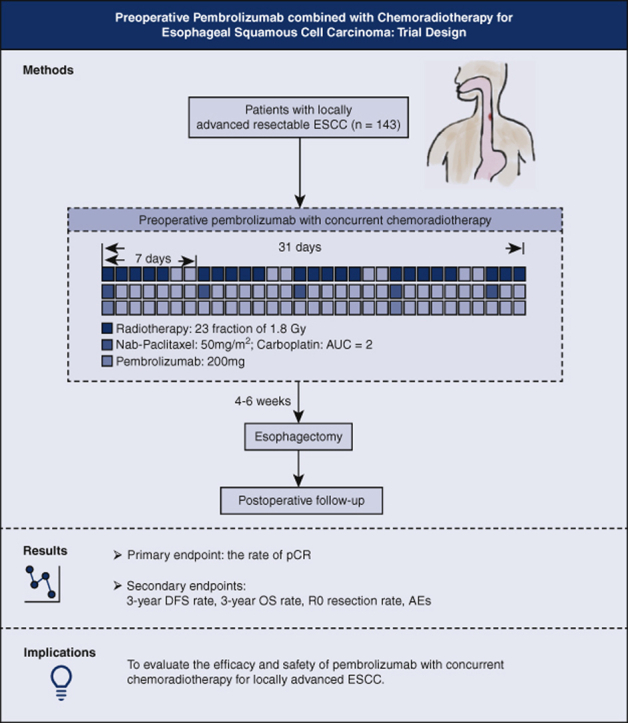
Trial design and flowchart of the Preoperative Anti-PD-1 Antibody combined with Chemoradiotherapy for Locally Advanced Squmous Cell Carcinoma of Esophageus-2 trial. ESCC, Esophageal squamous cell carcinoma; AUC, area under the curve; pCR, pathologic complete response; DFS, disease-free survival; OS, overall survival; AEs, adverse events.
Flowchart of the PALACE-2 trial.
Central Message.
This multicenter single-arm PALACE-2 trial will evaluate the efficacy and safety of preoperative pembrolizumab with chemoradiotherapy for locally advanced esophageal squamous cell carcinoma.
Perspective.
Preoperative immunotherapy with concurrent chemoradiotherapy was preliminarily demonstrated to be safe, feasible, and provide potential therapeutic benefits for locally advanced, resectable esophageal squamous cell carcinoma by the PALACE-1 trial. This subsequent multicenter single-arm PALACE-2 trial will evaluate the efficacy and further confirm the safety of this newly developed neoadjuvant regimen.
See Commentaries on pages 300 and 302.
Esophageal cancer is the seventh most common malignancy around the world.1 In the Asian population, more than 90% of the diagnosed esophageal cancers are esophageal squamous cell carcinoma (ESCC).2 Given the difficulties of early screening, nearly half of patients are diagnosed as having locally advanced disease, and neoadjuvant chemoradiotherapy (nCRT) followed by surgery has been introduced as the recommended treatment. According to the Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study (CROSS) trial, 29% of patients achieved pathologic complete response (pCR) after nCRT, with a substantially improved median overall survival (OS) of 48.6 months.3,4 However, the 5-year OS rate was about 47%, and 49% of patients developed either local-regional recurrence or distant metastasis even after nCRT.3 For those patients who respond poorly to nCRT, a more effective treatment strategy is required for further improvement of survival.
By enhancing the antitumor activity of T cells, immunotherapy is currently considered a promising treatment for various types of malignancies, including esophageal cancer. Given the overall high level of tumor mutation burden and the high rate of positive programmed cell death ligand 1 (PD-L1) expression,5, 6, 7, 8, 9 ESCC patients are expected to experience an inspiring clinical benefit after immunotherapy targeting programmed cell death protein 1 (PD-1)/PD-L1 checkpoints.10, 11, 12
A widely used immune checkpoint inhibitor (ICI) targeting PD-1, pembrolizumab has been confirmed to be effective in the treatment of advanced esophageal cancer. According to the Phase 2 trial KEYNOTE-180, pembrolizumab monotherapy was proven to be safe and effective for heavily pretreated ESCC, with an objective response rate of 14.3%.13 The further Phase 3 randomized controlled trial KEYNOTE-181 compared pembrolizumab with chemotherapy as second-line treatment for advanced/metastatic esophageal cancer. For patients with combined positive score (the ratio of the number of all PD-L1 expressing cells to the number of all tumor cells) ≥10 in esophageal tumor, OS was significantly prolonged after pembrolizumab treatment (9.3 vs 6.7 months; P = .0074). The response rate was also improved together with a lower frequency of adverse events (AEs).14
Immunotherapy is a promising treatment for advanced ESCC. Meanwhile, preclinical studies have also proven the synergy between ICI and chemoradiotherapy.15,16 Therefore, it is reasonable to evaluate the addition of immunotherapy to the neoadjuvant regimen for locally advanced disease. There are several ongoing prospective clinical trials that focus mainly on the combination of immunotherapy and neoadjuvant chemotherapy/nCRT for esophageal cancer. In the recently completed Preoperative Anti-PD-1 Antibody combined with Chemoradiotherapy for Locally Advanced Squmous Cell Carcinoma of Esophageus (PALACE)-1 trial (ClinicalTrials.gov ID: NCT03792347), we investigated the safety and feasibility of preoperative pembrolizumab combined with chemoradiotherapy (PPCT) followed by surgery in treating locally advanced ESCC. Twenty patients were enrolled, and 18 underwent surgery. PPCT was shown to be safe and feasible and induced a pCR in 55.6% of resected specimens.17
The results of PALACE-1 study justify a further clinical trial. Therefore, we designed and conducted a subsequent multicenter single-arm PALACE-2 trial to investigate the efficacy and to further confirm the safety of PPCT (ClinicalTrials.gov ID: NCT04435197).
Materials and Methods
Study Design
The PALACE-2 study is a prospective, multicenter, single-arm clinical trial. Five high-volume medical centers in China are participating in this study (Ruijin hospital, Shanghai Jiao Tong University School of Medicine; the First Affiliated Hospital, Zhejiang University School of Medicine; the First Affiliated Hospital of Xiamen University; the First Affiliated Hospital of Fujian Medical University; and the First Affiliated Hospital of Nanchang University). This study was initiated during August 2020. With an estimated inclusion period of about 3 years, the primary end point is anticipated to be achieved in June 2023. The general study design was presented in Video 1.
Eligibility Criteria
Patients with histologically conformed, locally advanced, and surgically resectable ESCC will be enrolled in this study. Positive PD-L1 expression is not mandatory for enrollment. The detailed inclusion criteria are:
-
•
Histologically confirmed ESCC, with a clinical stage of cT2 though T4a, N0 through N3, M0;
-
•
Age ranging from 18 to 75 years;
-
•
Eastern Cooperative Oncology Group performance status score of 0 to 1; and
-
•
Patients approve and sign the informed consent.
The exclusion criteria are as follows:
-
•
Patients with active autoimmune diseases or history of autoimmune diseases;
-
•
Patients who need systemic treatment with either corticosteroids or other immunosuppressive drugs;
-
•
Patients with symptomatic interstitial pulmonary disease;
-
•
Patients who are allergic to drugs used in the trial;
-
•
Pregnant or lactating women;
-
•
Patients of childbearing age who are not willing to use contraceptive measures;
-
•
Patients who have previously received targeted therapy, immunotherapy, chemotherapy, or radiotherapy for this or any other prior malignancies; and
-
•
Underlying medical conditions that, in the investigator's opinion, will increase the risk of medication use or obscure the interpretation of toxicity and AEs.
Baseline Evaluation
Before enrollment and initiation of neoadjuvant treatment, baseline evaluation will be arranged for each patient to stage the disease and to exclude distant metastasis and any other excluding factors. The evaluation includes physical examination, upper gastrointestinal endoscopy with tumor biopsy (if not previously examined), endoscopic ultrasonography, contrast-enhanced chest computed tomography (CT), positron emission tomography-computed tomography (PET-CT), routine blood test, echocardiography, pulmonary function, and electrocardiogram. Ultrasonography with fine-needle aspiration will be performed for any suspected cervical lymph node metastasis.
Neoadjuvant Treatment
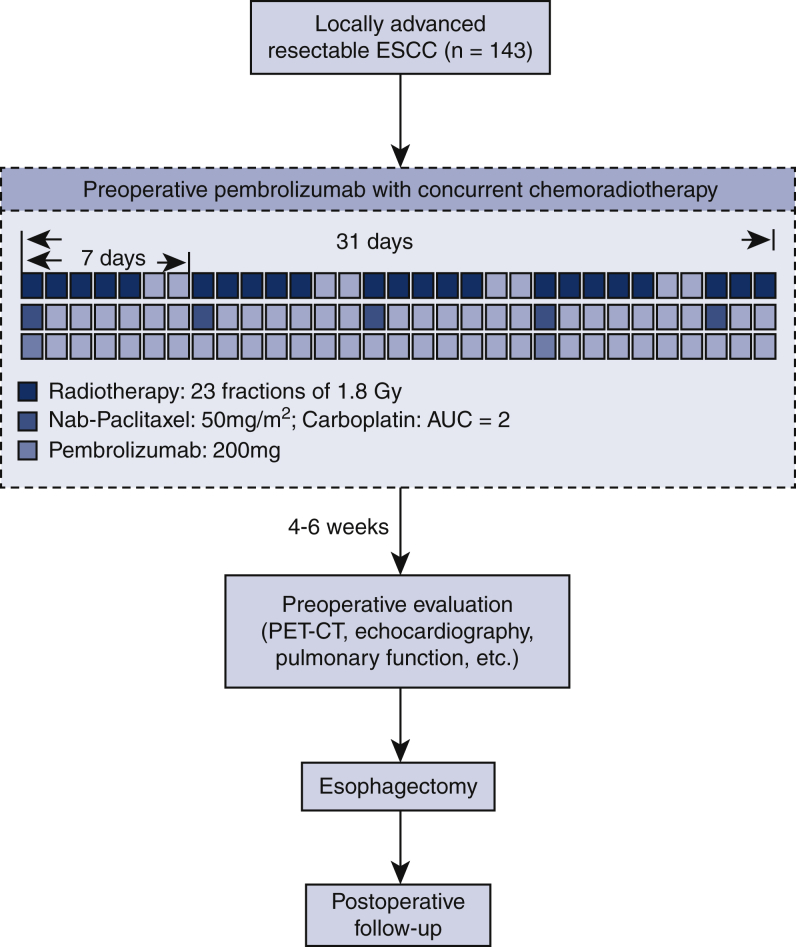
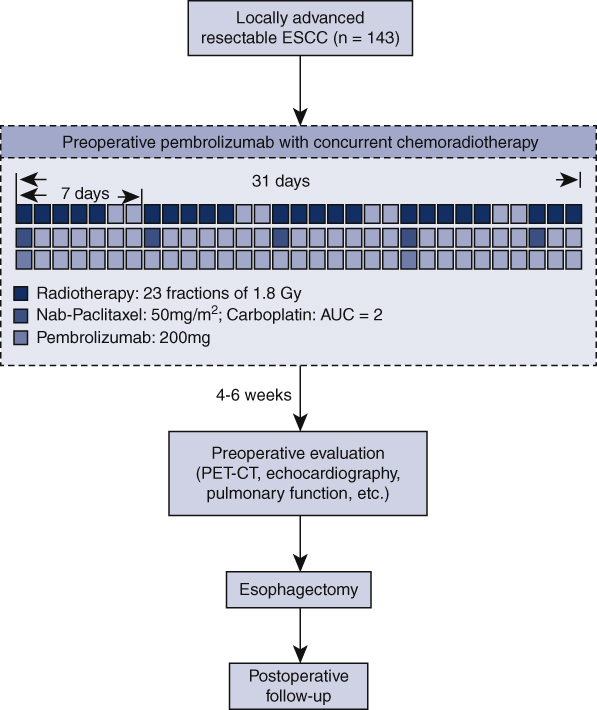
Preoperative treatment is composed of chemotherapy, radiotherapy, and immunotherapy (Figure 1). Neoadjuvant chemoradiotherapy will be given according to the CROSS regimen.3 Chemotherapy includes carboplatin (area under the curve of 2 mg/mL/min) and nab-paclitaxel (50 mg/m2) intravenously, once a week for 5 weeks (on day 1, 8, 15, 22, and 29 of the neoadjuvant treatment period). Concurrent radiotherapy will be given by means of external-beam radiation. The total radiation dose is 41.4 Gy, which will be given by 23 fractions, 5 fractions per week, with 1.8 Gy for each fraction. Pembrolizumab (ICI targeting PD-1) will be given concurrently on day 1 and 22 of the neoadjuvant therapy at a dose of 200 mg. For patients weighing <50 kg, pembrolizumab will be given at a dose of 100 mg.
Figure 1.
Flowchart of the Preoperative Anti-PD-1 Antibody combined with Chemoradiotherapy for Locally Advanced Squmous Cell Carcinoma of Esophageus-2 trial. ESCC, Esophageal squamous cell carcinoma; AUC, area under the curve; PET-CT, positron emission tomography-computed tomography.
For chemoradiotherapy-related AEs occurred during the neoadjuvant period, clinical observation, dose modification, suspension of the chemotherapy or radiotherapy, and symptomatic treatment can be applied according to the type and severity of the AEs and based on multidisciplinary discussion. When facing immune-related AEs, dose modification of pembrolizumab is not recommended.
After the completion of PPCT, physical examination, routine blood test, contrast-enhanced chest CT, PET-CT, echocardiography, pulmonary function, and electrocardiogram will be undertaken to reevaluate the disease and exclude cases with any surgical contraindications.
Surgery
Each patient will undergo an open or minimally invasive esophagectomy (video-assisted or robotic-assisted esophagectomy), with 2-field or 3-field lymphadenectomy. Surgery should be arranged 4 to 6 weeks after completion of PPCT. For tumors located in the upper third of the esophagus, McKeown esophagectomy will be performed, whereas for middle and lower third located tumors, Ivor Lewis esophagectomy will be performed.
Pathological and Radiological Evaluation
Each resected specimen will be evaluated by 2 pathologists independently. Pathological reports should describe tumor size, extent of tumor invasion, overall and positive lymph nodes dissected, resection margin, grade of differentiation, and grade of tumor regression. Pathological stage will be determined according to the American Joint Committee on Cancer criteria for esophageal carcinoma (eighth edition).18 R0 resection is predefined as a tumor-free resection margin. To evaluate and grade the response of esophageal tumor to PPCT, the extent of residual disease will be divided into 4 categories: grade I, no sign of residual carcinoma; grade II, <10% vital residual tumor cell; grade III, 11% to 50%; grade IV, >50%.3,19 Major pathologic response is predefined as no more than 10% of residual viable tumor cells in primary lesions.20,21
During radiological evaluation of esophageal tumor, thickening of the esophageal wall observed in chest CT examination will be recorded as T3, and direct involvement of adjacent organs will be recorded as T4.22 The status of complete metabolic response will be used for radiological evaluation, which is predefined as maximum standardized uptake value <4 for esophageal tumor with no nodal uptake according to PET-CT after PPCT.17,23 In case of doubt regarding clinical staging of esophageal cancer, multidisciplinary discussion will be recommended.
Follow-up
The postoperative follow-up should be arranged in accordance with the National Comprehensive Cancer Network guideline for esophageal cancer.24 Follow-up visits will be scheduled at 1, 6, 12, 18, 24, 30, 36, 48, and 60 months after surgery. When patients are facing suspected local recurrence or distance metastasis, additional visit will be scheduled, and contrast-enhanced chest CT, PET-CT, and/or upper gastrointestinal endoscopy will be performed.
Study End Point
The primary study parameter of PALACE-2 trial is the pCR rate. pCR is defined as the absence of any signs of cancer in resected tissue samples examined by pathologists. The secondary study parameters include:
-
•
Three-year disease-free survival rate;
-
•
Three-year OS rate;
-
•
R0 resection rate, defined as the percentage of patients who undergo surgery and achieve a tumor-free resection margin; and
-
•
Rate of AEs during neoadjuvant therapy and perioperative period. AEs will be evaluated and recorded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).
Statistical Analysis
According to a review of current literature, the pCR rate after neoadjuvant chemoradiotherapy for locally advanced ESCC in Asian population was expected to be 43.2%.25 Meanwhile, the pCR rate after PPCT was assumed to be 56%, based on the short-term results of our prior PALACE-1 trial.17 With a power of 80%, a sample size of 130 will be required to detect a difference in pCR rate between PPCT and chemoradiotherapy at a significance level of 5%. To allow for a 10% of dropout cases, the sample size was increased to 143 in this PALACE-2 trial.
Data analysis will comply with the intention-to-treat principle. Subgroups will be defined according to pathological and radiological responses, baseline characteristics, long-term survival, and PD-L1 expression status. Data will be recorded and collected via standardized case report form and will be analyzed centrally.
Discussion
This PALACE-2 study is a prospective, multicenter, single-arm clinical trial investigating the combination of neoadjuvant immunotherapy and concurrent chemoradiotherapy followed by esophagectomy for locally advanced ESCC, which has been studied preliminarily by the PALACE-1 trial. A graphical representation that summarizes the method and the study end points is shown in Figure 2. By conducting this trial, we aim to provide evidence concerning the efficacy and safety of this novel therapeutic strategy. If the results are promising, a further multicenter, randomized controlled trial (PALACE-3 trial) will be conducted, comparing PPCT with conventional nCRT.
Figure 2.
Trial design and flow chart of the Preoperative Anti-PD-1 Antibody combined with Chemoradiotherapy for Locally Advanced Squmous Cell Carcinoma of Esophageus-2 trial. ESCC, Esophageal squamous cell carcinoma; AUC, area under the curve; pCR, pathologic complete response; DFS, disease-free survival; OS, overall survival; AEs, adverse events.
Emerging preclinical studies have demonstrated the synergistic effects between immunotherapy and chemotherapy/radiotherapy.15,16,26 Conventional chemotherapy could induce DNA damage in cancer cells, which was reported to consequently enhance the presentation of tumor-associated antigen and recruitment of antigen presenting cells.27,28 Chemotherapy could also result in the destruction of original immune cells, and the subsequent homeostatic T cell reconstruction, which helps to reshape the immune response of T cells to malignancies.26 In addition, the restoration of immunosurveillance by chemotherapy has also been reported, and potential mechanisms have been revealed.29 Radiation has also been reported to modulate antitumor immune response, by upregulating major histocompatibility complex class I expression,30 enhancing tumor infiltration of lymphocytes,31,32 modulating the expression of immune checkpoints.33 Based on these findings, we anticipate an improved short-term efficacy and long-term survival after the combination therapy of pembrolizumab and chemoradiotherapy in the PALACE-2 trial.
In this PALACE-2 trial, only patients with ESCC will be included. According to the CROSS trial, ESCC was associated with a significantly higher pCR rate after nCRT (49% for squamous cell carcinoma vs 23% for adenocarcinoma; P = .008) and a far better long-term survival than adenocarcinoma.3,4 ESCC patients also seemed to benefit more from immunotherapy than adenocarcinoma patients. In the Phase 3 KEYNOTE-181 study, the median OS after pembrolizumab was 8.2 months for patients with ESCC and 7.1 months for all patients.14 These results were consistent with those from the KEYNOTE-180 study. Taken together, ESCC is expected to have a better response after PPCT and therefore was set as the study population. However, the lack of an adenocarcinoma group still makes application of the results limited.
PD-L1 expression status is among the most promising immune biomarkers and has been used as a selection criterion for immunotherapy in the clinical management of advanced/metastatic esophageal cancer.24 However, we do not employ PD-L1 expression among the inclusion criteria in this PALACE-2 trial. Previous studies have demonstrated that chemotherapy and radiotherapy potentially upregulate immune checkpoints including PD-L1.33, 34, 35 Hence, the PD-L1 status could be modulated during concurrent chemoradiotherapy and immunotherapy, which makes it a less persuasive biomarker than ICI monotherapy. Although a high PD-L1 expression was demonstrated to be correlated with a better prognosis after immunotherapy, PD-L1 negative ESCC could also benefit from ICI.13 Furthermore, the prior PALACE-1 trial also demonstrated the random distribution of positive and negative PD-L1 expression in either pCR or non-pCR patients (30% of patients with pCR and 63% without pCR had positive PD-L1 expression).17
We recently completed a prospective, single-arm clinical trial (ie, PALACE-1) to preliminarily investigate the safety and feasibility of neoadjuvant pembrolizumab with concurrent chemoradiotherapy.17 Twenty patients with locally advanced ESCC were enrolled and given PPCT followed by esophagectomy. Of these patients, 19 received complete neoadjuvant treatment and 18 underwent surgery. PPCT was shown to be safe and feasible, with potential improvement for patient survival. Grade III and higher-grade AEs during neoadjuvant treatment were observed in 65% of patients. No treatment-related delay of surgery was observed. The pCR rate after PPCT reached 55.6%. Based on these results, we designed and initiated this multicenter PALACE-2 trial, to investigate the efficacy and to further confirm the safety of PPCT. Currently, several prospective clinical trials are also being conducted to investigate the application of neoadjuvant immunotherapy for locally advanced ESCC (eg, ClinicalTrials.gov IDs: NCT04644250, NCT04006041, NCT04225364, NCT04437212, NCT04625543, NCT03985670, NCT04229459, and NCT04460066). Most of these studies are Phase 1 or Phase 2 trials with limited sample size, which mainly aim to explore novel neoadjuvant combination regimens with ICI and chemotherapy or chemoradiotherapy. Notably, a Phase 3, multicenter, randomized clinical trial (ie, MA-EC-II-004 study) is being conducted in approximately 20 Chinese hospitals to investigate the neoadjuvant immunotherapy (camrelizumab) combined with chemotherapy for locally advanced ESCC. The future combination of results from PALACE-2 and other ongoing clinical trials is important to better reveal the significance of preoperative immunotherapy for ESCC.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://aats.blob.core.windows.net/media/21%20AM/AM21_TH06/AM21_TH06_8.mp4.

Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
The authors thank every member of the Preoperative Anti-PD-1 Antibody combined with Chemoradiotherapy for Locally Advanced Squmous Cell Carcinoma of Esophageus study group for their participation and important contributions to this ongoing trial.
Footnotes
Supported by the Chinese Scoiety of Clinical Oncology-Merck Sharp & Dohme Cancer Research Fund (Y-MSD2020-0279).
Drs Zheng and Li contributed equally to this article.
Supplementary Data
The presentation of professor Hecheng Li for the American Association for Thoracic Surgery 101st Annual Meeting. Video available at: https://www.jtcvs.org/article/S2666-2736(21)00422-8/fulltext.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Abnet C.C., Arnold M., Wei W.Q. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154:360–373. doi: 10.1053/j.gastro.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Hagen P., Hulshof M.C., van Lanschot J.J., Steyerberg E.W., van Berge Henegouwen M.I., Wijnhoven B.P., et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro J., van Lanschot J.J.B., Hulshof M., van Hagen P., van Berge Henegouwen M.I., Wijnhoven B.P.L., et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 5.Lin D.C., Hao J.J., Nagata Y., Xu L., Shang L., Meng X., et al. Genomic and molecular characterization of esophageal squamous cell carcinoma. Nat Genet. 2014;46:467–473. doi: 10.1038/ng.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song Y., Li L., Ou Y., Gao Z., Li E., Li X., et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature. 2014;509:91–95. doi: 10.1038/nature13176. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L., Zhou Y., Cheng C., Cui H., Cheng L., Kong P., et al. Genomic analyses reveal mutational signatures and frequently altered genes in esophageal squamous cell carcinoma. Am J Hum Genet. 2015;96:597–611. doi: 10.1016/j.ajhg.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas Research Network, Analysis Working Group: Asan University, BC Cancer Agency, Brigham and Women's Hospital, Broad Institute, Brown University, et al. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541:169–175. doi: 10.1038/nature20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohigashi Y., Sho M., Yamada Y., Tsurui Y., Hamada K., Ikeda N., et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 10.Sholl L.M., Hirsch F.R., Hwang D., Botling J., Lopez-Rios F., Bubendorf L., et al. The promises and challenges of tumor mutation burden as an immunotherapy biomarker: a perspective from the International Association for the Study of Lung Cancer Pathology Committee. J Thorac Oncol. 2020;15:1409–1424. doi: 10.1016/j.jtho.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yarchoan M., Johnson B.A., III, Lutz E.R., Laheru D.A., Jaffee E.M. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer. 2017;17:209–222. doi: 10.1038/nrc.2016.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jardim D.L., Goodman A., de Melo Gagliato D., Kurzrock R. The challenges of tumor mutational burden as an immunotherapy biomarker. Cancer Cell. 2021;39:154–173. doi: 10.1016/j.ccell.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah M.A., Kojima T., Hochhauser D., Enzinger P., Raimbourg J., Hollebecque A., et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: the phase 2 KEYNOTE-180 Study. JAMA Oncol. 2019;5:546–550. doi: 10.1001/jamaoncol.2018.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kojima T., Shah M.A., Muro K., Francois E., Adenis A., Hsu C.H., et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38:4138–4148. doi: 10.1200/JCO.20.01888. [DOI] [PubMed] [Google Scholar]
- 15.Sharma P., Allison J.P. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 16.Sharabi A.B., Lim M., DeWeese T.L., Drake C.G. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16:e498–e509. doi: 10.1016/S1470-2045(15)00007-8. [DOI] [PubMed] [Google Scholar]
- 17.Li C., Zhao S., Zheng Y., Han Y., Chen X., Cheng Z., et al. Preoperative Pembrolizumab Combined with Chemoradiotherapy for Oesophageal Squamous Cell Carcinoma (PALACE-1) Eur J Cancer. 2021;144:232–241. doi: 10.1016/j.ejca.2020.11.039. [DOI] [PubMed] [Google Scholar]
- 18.Rice T.W., Ishwaran H., Ferguson M.K., Blackstone E.H., Goldstraw P. Cancer of the esophagus and esophagogastric junction: an eighth edition staging primer. J Thorac Oncol. 2017;12:36–42. doi: 10.1016/j.jtho.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chirieac L.R., Swisher S.G., Ajani J.A., Komaki R.R., Correa A.M., Morris J.S., et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. 2005;103:1347–1355. doi: 10.1002/cncr.20916. [DOI] [PubMed] [Google Scholar]
- 20.Li C.Y., Huang P.M., Chu P.Y., Chen P.M., Lin M.W., Kuo S.W., et al. Predictors of survival in esophageal squamous cell carcinoma with pathologic major response after neoadjuvant chemoradiation therapy and surgery: the impact of chemotherapy protocols. Biomed Res Int. 2016;2016:6423297. doi: 10.1155/2016/6423297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Travis W.D., Dacic S., Wistuba I., Sholl L., Adusumilli P., Bubendorf L., et al. IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy. J Thorac Oncol. 2020;15:709–740. doi: 10.1016/j.jtho.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishimaki T., Tanaka O., Ando N., Ide H., Watanabe H., Shinoda M., et al. Evaluation of the accuracy of preoperative staging in thoracic esophageal cancer. Ann Thorac Surg. 1999;68:2059–2064. doi: 10.1016/s0003-4975(99)01171-6. [DOI] [PubMed] [Google Scholar]
- 23.Elliott J.A., O'Farrell N.J., King S., Halpenny D., Malik V., Muldoon C., et al. Value of CT-PET after neoadjuvant chemoradiation in the prediction of histological tumour regression, nodal status and survival in oesophageal adenocarcinoma. Br J Surg. 2014;101:1702–1711. doi: 10.1002/bjs.9670. [DOI] [PubMed] [Google Scholar]
- 24.Ajani J.A., D'Amico T.A., Bentrem D.J., Chao J., Corvera C., Das P., et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:855–883. doi: 10.6004/jnccn.2019.0033. [DOI] [PubMed] [Google Scholar]
- 25.Yang H., Liu H., Chen Y., Zhu C., Fang W., Yu Z., et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase iii multicenter, randomized, open-label clinical trial. J Clin Oncol. 2018;36:2796–2803. doi: 10.1200/JCO.2018.79.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davern M., Lysaght J. Cooperation between chemotherapy and immunotherapy in gastroesophageal cancers. Cancer Lett. 2020;495:89–99. doi: 10.1016/j.canlet.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Shurin G.V., Tourkova I.L., Kaneno R., Shurin M.R. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J Immunol. 2009;183:137–144. doi: 10.4049/jimmunol.0900734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma Y., Adjemian S., Mattarollo S.R., Yamazaki T., Aymeric L., Yang H., et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38:729–741. doi: 10.1016/j.immuni.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y.J., Fletcher R., Yu J., Zhang L. Immunogenic effects of chemotherapy-induced tumor cell death. Genes Dis. 2018;5:194–203. doi: 10.1016/j.gendis.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reits E.A., Hodge J.W., Herberts C.A., Groothuis T.A., Chakraborty M., Wansley E.K., et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumura S., Wang B., Kawashima N., Braunstein S., Badura M., Cameron T.O., et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181:3099–3107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hallahan D., Kuchibhotla J., Wyble C. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer Res. 1996;56:5150–5155. [PubMed] [Google Scholar]
- 33.Dovedi S.J., Adlard A.L., Lipowska-Bhalla G., McKenna C., Jones S., Cheadle E.J., et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–5468. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 34.Tran L., Allen C.T., Xiao R., Moore E., Davis R., Park S.J., et al. Cisplatin alters antitumor immunity and synergizes with PD-1/PD-L1 inhibition in head and neck squamous cell carcinoma. Cancer Immunol Res. 2017;5:1141–1151. doi: 10.1158/2326-6066.CIR-17-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng H.Y., Li J., Tao L., Lam A.K., Chan K.W., Ko J.M.Y., et al. Chemotherapeutic treatments increase PD-L1 expression in esophageal squamous cell carcinoma through EGFR/ERK activation. Transl Oncol. 2018;11:1323–1333. doi: 10.1016/j.tranon.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The presentation of professor Hecheng Li for the American Association for Thoracic Surgery 101st Annual Meeting. Video available at: https://www.jtcvs.org/article/S2666-2736(21)00422-8/fulltext.