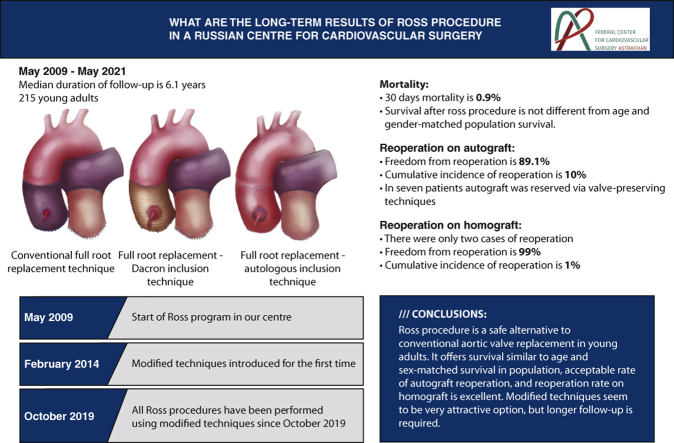
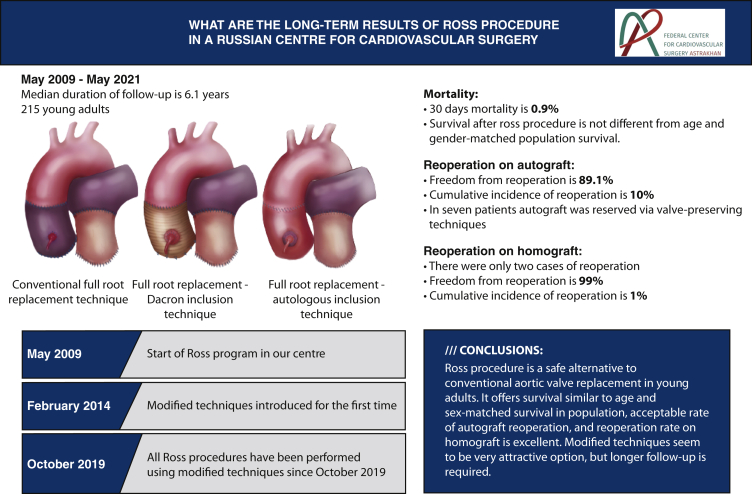
Abstract
Objectives
To evaluate our 12-year experience with the Ross procedure in adults.
Methods
A retrospective analysis of 215 cases of the Ross procedure was performed. The mean age of the patients was 36 ± 11.1 years, and the male to female ratio was 75% to 25%, respectively. The pulmonary autograft was placed into the aortic position using the full-root replacement technique and its modified versions. The right ventricular outflow tract was reconstructed using a pulmonary homograft in all cases.
Results
The 30-day mortality after the operation was 0.9% (2 patients). The median duration of follow-up was 6.1 years (interquartile range, 6.5 years) and was complete in 86% of cases. The survival at 12 years was 94.7% and was comparable with the survival rate of the general population matched for age and sex. At the end of the follow-up, freedom from reoperation due to pulmonary autograft and homograft dysfunction was 89.1% and 99%, respectively.
Conclusions
In our series, the Ross procedure resulted in low early mortality and excellent survival in adults. The long-term survival was not statistically different from the survival of the general population. The pulmonary homograft offered an excellent durability and freedom from reoperation.
Key Words: Ross procedure, aortic valve replacement, pulmonary autograft
Abbreviations and Acronyms: AV, aortic valve; AVR, aortic valve replacement; CI, confidence interval; IQR, interquartile range; RVOT, right ventricular outflow tract
Graphical abstract
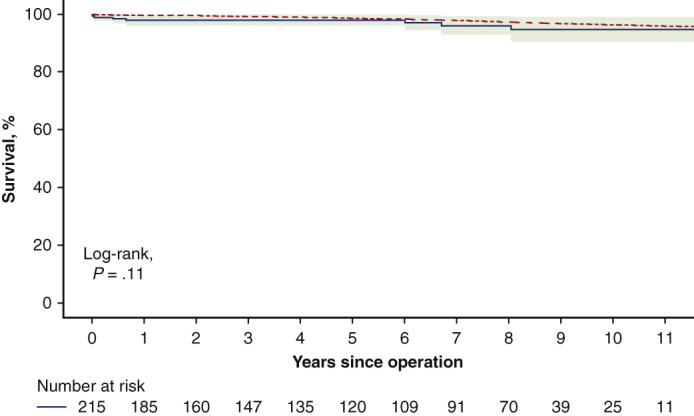
Survival of patients after the Ross procedure compared with the general population.
Central Message.
The Ross procedure is a safe operation in young adults with survival similar to general population and an acceptable rate of reoperations.
Perspective.
The Ross procedure resulted in low early mortality and excellent survival in adults that was not statistically different from the survival of the general population. The pulmonary homograft offered an excellent durability and freedom from reoperation.
Over the last 60 years, the pursuit for an ideal aortic valve (AV) substitute has led to various options, including bioprosthetic and mechanical valves, homografts, and the pulmonary autograft (Ross procedure).1 Although the latter has become an attractive option in the pediatric cardiac surgical armamentarium due to its favorable hemodynamics, the issue of durability of the pulmonary autograft in the long term remained unresolved. The complexity of the Ross procedure along with pulmonary homograft calcification often outweighs the intrinsic drawbacks of mechanical valves such as the need for lifelong anticoagulation and reoperation in the young adults, in favor of mechanical substitutes. As a result, the most recent European Society of Cardiology and the European Association for Cardio-Thoracic Surgery guidelines on valvular heart disease 2021 do not include the procedure for treatment of the AV disease,2 whereas the recent American College of Cardiology and the American Heart Association guidelines mention the Ross procedure as an option in the highly selected patients.3 In this study, we aimed to analyze our 12-year experience in the Ross procedure in view of identifying the key factors influencing survival and late pulmonary autograft dysfunction after this operation.
Methods
Patients
Between May 2009 and May 2021, in our center for cardiac surgery, 215 consecutive adult patients (mean age 36 ± 11.1 years, range, 18-63 years) underwent the Ross procedure. A retrospective analysis of the prospectively collected data was performed. The ethics committee approval was waived, as the data were reviewed retrospectively.
The indications for the operations were as follows: aortic stenosis in 69 cases (32.1%), aortic regurgitation in 90 cases (41.9%), and mixed lesions in 56 cases (26%). The Ross procedure was offered as an alternative treatment option to young adult patients with an active lifestyle and thoroughly discussed with them. Also, the Ross procedure was performed in those who wanted to avoid lifelong anticoagulation or the latter was contraindicated. Table 1 shows the clinical profile of all patients before the operation.
Table 1.
Baseline patient characteristics
| Ross procedure overall | Conventional Ross procedure | Dacron graft inclusion technique | Autologous inclusion technique | |
|---|---|---|---|---|
| Number of patients | 215 | 159 | 26 | 30 |
| Age, mean ± SD | 36 ± 11.1 | 35.1 ± 10.9 | 36.5 ± 10 | 39.9 ± 12.3 |
| Sex, n (%) | ||||
| Male | 162 (75.3) | 119 (55.3) | 19 (8.8) | 24 (11.2) |
| Female | 53 (24.7) | 40 (18.6) | 7 (3.3) | 6 (2.8) |
| AV pathology, n (%) | ||||
| BAV | 147 (50.1) | 103 (35.1) | 19 (6.5) | 25 (8.5) |
| Other congenital | 5 (2.3) | 5 (2.3) | 0 | 0 |
| IE | 56 (26.1) | 44 (20.5) | 9 (4.2) | 3 (1.4) |
| Rheumatic | 32 (14) | 28 (12.3) | 1 (0.4) | 3 (1.3) |
| Degenerative | 13 (6.1) | 8 (3.8) | 2 (0.9) | 3 (1.4) |
| PVD | 3 (1.4) | 3 (1.4) | 0 | 0 |
| Previous interventions, n (%) | ||||
| AVR | 3 (1.4) | 3 (1.4) | 0 | 0 |
| AV commissurotomy | 3 (1.4) | 3 (1.4) | 0 | 0 |
| Coarctation repair | 3 (1.4) | 3 (1.4) | 0 | 0 |
| NYHA class, n (%) | ||||
| I | 95 (44.2) | 67 (31.2) | 13 (6) | 15 (7) |
| II | 109 (50.7) | 81 (37.7) | 13 (6) | 15 (7) |
| III | 10 (4.6) | 10 (4.6) | 0 | 0 |
| IV | 1 (0.5) | 1 (0.5) | 0 | 0 |
| Preoperative echocardiographic data | ||||
| LVEF, median (IQR) | 60 (10) | 60 (10) | 58 (10.3) | 58 (8) |
| LVEF ≥55%, n (%) | 155 (72) | 114 (52.9) | 18 (8.4) | 23 (10.7) |
| LVEF 40%-55%, n (%) | 50 (23.3) | 36 (16.8) | 8 (3.7) | 6 (2.8) |
| LVEF ≤39%, n (%) | 6 (2.8) | 5 (2.3) | 0 | 1 (0.5) |
| Missing values, n (%) | 4 (1.9) | 4 (1.9) | 0 | 0 |
| Aortic annulus, mm, median (IQR) | 24 (3) | 24 (3.75) | 24 (2.5) | 24.5 (3.5) |
| Aortic annulus ≥27 mm, n (%) | 42 (19.5) | 34 (15.8) | 2 (0.9) | 6 (2.8) |
| Sinuses of Valsalva, mm, median (IQR) | 36 (7) | 35 (9) | 34 (4.75) | 38 (3.75) |
| Ascending aorta (proximal), mm, median (IQR) | 36 (9) | 36 (9.75) | 34.5 (10.5) | 40 (9.75) |
| Ascending aorta ≥45 mm, n (%) | 27 (12.6) | 12 (5.6) | 4 (1.9) | 11 (5.1) |
Variables with missing values were simply excluded from analysis. SD, Standard deviation; AV, aortic valve; BAV, bicuspid aortic valve; IE, infective endocarditis; PVD, prosthetic valve dysfunction; AVR, aortic valve replacement; NYHA, New York Heart Association functional class; LVEF, left ventricle ejection fraction; IQR, interquartile range.
Operative Technique
In most cases, the surgical approach to the heart was through median sternotomy, and there were only 2 cases of right upper partial sternotomy. Cardiopulmonary bypass was established with aortic and bicaval cannulation without hypothermia. Myocardial protection was accomplished using antegrade cold crystalloid cardioplegia in all cases. The pulmonary root was harvested in a scalloped fashion with 3 to 4 mm below the attachments of the pulmonary valve cusps and secured in the aortic position using interrupted 4-0 braided polyester sutures for the proximal anastomosis. The conventional full-root replacement technique was used in most of the patients (74%). However, since 2014, we have gradually implemented modified techniques, and all operations performed in the last 2 years were performed using either a Dacron graft or autologous inclusion techniques. In the case of dilated aortic annulus, we used the Dacron graft for the inclusion technique with annulus reduction, whereas the autologous inclusion technique was used if the ascending aorta was dilated up to 45 mm with normal aortic annulus size. The autologous inclusion technique we used is similar to the one described by Skillington and colleagues,4 with the exception of the aortic annulus support. We did not reduce the aortic annulus, as we implemented this technique if the aortic annulus size was normal. In case of the ascending aorta sizes 45 mm or more, the ascending aorta replacement was used depending on the presence of aortopathy, at the discretion of the operating surgeon. To note, no aortoplasty techniques were implemented. Key steps of autologous technique are demonstrated in Video 1.
Until 2014, if the diameter of the aortic annulus was greater than of the pulmonary one by ≥2 mm, the annulus was reinforced with a subannular purse-string suture or an external Dacron strip. The distal anastomosis was completed 2 mm above the level of the autograft commissures. No foreign material was used to support the distal anastomosis.
Fresh and cryopreserved pulmonary homografts were used to reconstruct the right ventricular outflow tract (RVOT) in all cases. Unfortunately, we cannot present accurate data regarding the proportion of homograft types used, as it was not properly recorded initially in electronic history of almost one half of patients. However, vast majority of homografts used were fresh ones. The intraoperative data are presented in Table 2.
Table 2.
Operative patient characteristics
| Operative characteristics, median (IQR) | |
| Cardiopulmonary bypass time, min | 139 (32) |
| Crossclamp time, min | 116 (24) |
| Procedure time, min | 225 (65) |
| Operative technique, n (%) | |
| Conventional full-root replacement technique | 159 (74) |
| Autologous inclusion technique | 30 (13.9) |
| Dacron graft inclusion technique | 26 (12.1) |
| Annulus reduction | 40 (18.6) |
| Isolated Ross procedure | 164 (76.3) |
| Concomitant procedures, n (%) | |
| Elective CABG | 7 (3.3) |
| Mitral valve surgery | 15 (7) |
| Tricuspid valve repair | 4 (1.9) |
| Ascending aorta replacement | 14 (6.5) |
| Pulmonary homograft size, median (IQR) | 27 (3) |
IQR, Interquartile range; CABG, coronary artery bypass grafting.
Follow-up
Transthoracic echocardiography was performed before the hospital discharge in all patients. After discharge, the first outpatient appointment was scheduled in 6 months, then annually. When a patient visit to the center was not possible, the follow-up data were obtained by contacting patients or their relatives via a phone call and/or an e-mail. Echocardiogram examination was done at the time of the follow-up visit, or it was sent by an e-mail, if performed elsewhere. In July 2021, the completeness of the follow-up was 86%, and the median duration was 6.1 years (interquartile range [IQR], 6.5 years).
Early or hospital mortality was defined as death from any cause occurring in the hospital or 30 days after the operation. Death from any cause beyond that time limit was defined as late mortality. Reoperation was defined as any surgical intervention for pulmonary autograft or pulmonary homograft. More than mild insufficiency of the pulmonary autograft was described as an autograft dysfunction or failure.
Statistical Analysis
Data were analyzed using R (R version 4.0.5, R Foundation for Statistical Computing). Continuous variables with normal distribution were expressed as the mean ± standard deviation (SD) or median with IQR if the distribution was skewed, whereas categorical data were presented as absolute numbers and relative frequencies. The Wilcoxon signed-rank test (non-normal distribution) or t test (normal distribution) was used to compare echocardiographic data obtained just before hospital discharge and the last available one during the follow-up. The Kaplan–Meier method was used to evaluate survival and freedom from reoperation. Freedom from reoperation was investigated considering death as a competing risk with cumulative incidence function. The results were truncated at 11 years because of the sample size. Comparison of expected and observed survival was made via a one-sample log-rank test. Age- and sex-matched Russian general population survival estimates were obtained from the World Health Organization (www.who.int, last available data, 2019).
Results
Mortality and Morbidity
There were 2 in-hospital deaths (0.9%). The causes of early mortality were massive bleeding soon after the operation and sepsis. A list of complications is presented in Table 3. The median stay in the intensive care unit was 21 hours (IQR, 24 hours). The median time for hospital stay was 12 days (IQR, 5 days).
Table 3.
Early postoperative complications after the Ross procedure
| Death early after surgery, n (%) | 2 (0.9) |
| Nonelective CABG, n (%) | 10 (4.6) |
| Perioperative myocardial infarction, n (%) | 10 (4.6) |
| Atrial fibrillation, n (%) | 10 (4.6) |
| Re-exploration for bleeding, n (%) | 8 (3.7) |
| Pacemaker implantation, n (%) | 5 (2.3) |
| Wound infection, n (%) | 4 (1.9) |
| Stroke, n (%) | 1 (0.5) |
The patients with myocardial infarction were the same who underwent unplanned coronary artery bypass grafting. No technical issues with pulling the sutures through the aortic root basement were experienced. CABG, Coronary artery bypass grafting.
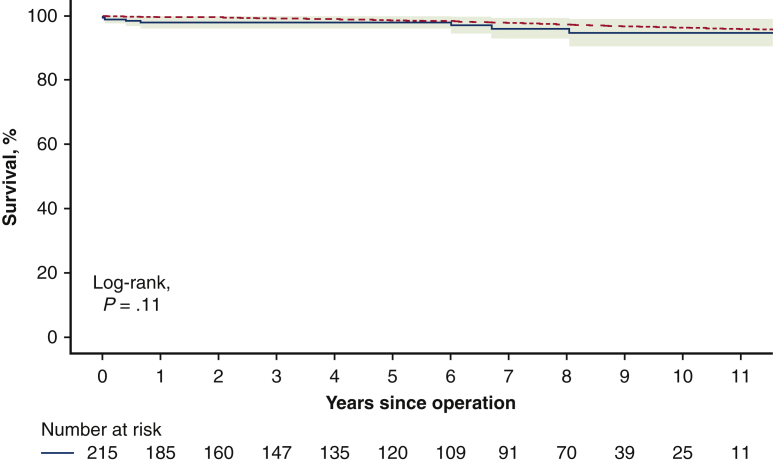
There were 5 late deaths during the follow-up (2.3%). The known causes of deaths were stroke and suicide, and the remaining 3 deaths were sudden and unexplained. The survival rate at 12 years was 94.7% (95% confidence interval [CI], 90.5%-99.0%) which was not significantly different from the survival of an age- and sex-matched general Russian population (P = .11; Figure 1).
Figure 1.
Survival of patients after the Ross procedure compared with the general population using a one-sample log-rank test. The red dotted line represents survival of general population, and the blue solid line with green shading represents survival of Ross sample with 95% confidence interval (CI).
Reoperations
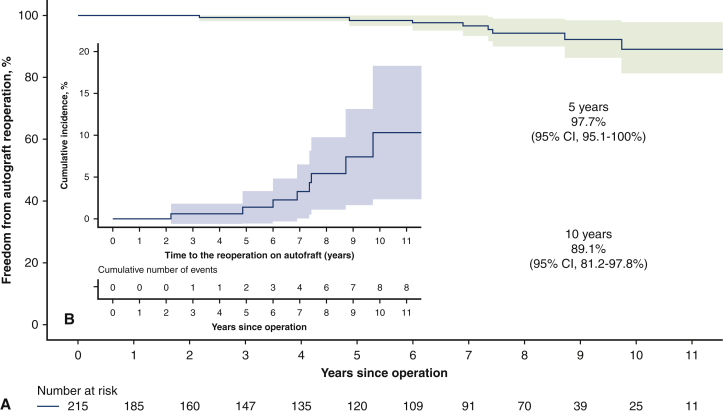
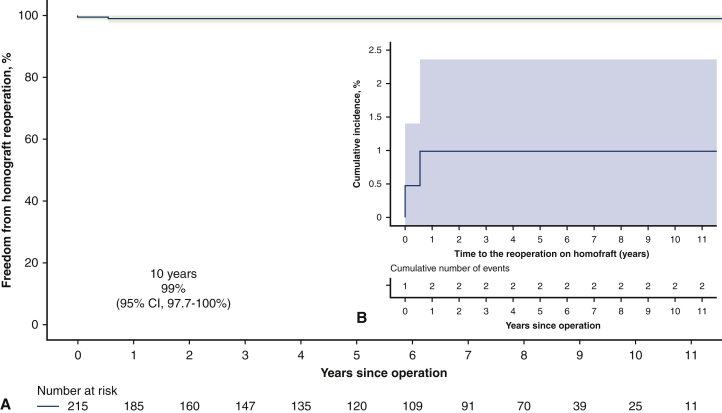
During the follow-up, 11 patients (5.1%) required reoperation due to pulmonary autograft (9 patients) and pulmonary homograft (2 patients) dysfunction. The indications for autograft reoperation were severe aortic insufficiency due to dilatation of the neoaortic root or the ascending aorta dilatation. There was one case of severe aortic regurgitation due to the noncoronary cusp prolapse. In 5 cases, the autograft was repaired using David operation; in 2 patients, the neoaortic roots were preserved by aortic annuloplasty; and the other 2 patients required aortic valve replacement (AVR) with mechanical prostheses. In addition, 7 patients required ascending aorta replacement at the time of the AV repair or replacement and none of these patients had aortic dilatation (ascending aorta ≥45 mm) before first intervention. The pulmonary homografts were reoperated due to thrombosis in the first case at 5.5 months and infective endocarditis in the second case at 7 years of the follow-up. As to the patient with early pulmonary homograft thrombosis, he had no risk factors for hypercoagulability and homograft type used in his case was the fresh one. At 11 years, freedom from reoperation was 88.2% (95% CI, 80.3%-97.0%). Freedom from reoperation as well as cumulative incidence of reoperation, either on autograft or homograft, are shown in Figures 2 and 3. There were no deaths at reoperative intervention, and all reoperated patients were alive as of May 2021.
Figure 2.
A, Freedom from autograft reoperation after the Ross procedure (shading represents 95% confidence interval). B, Cumulative incidence of reoperation on pulmonary autograft considering death as competing factor (shading represents 95% confidence interval [CI]).
Figure 3.
A, Freedom from pulmonary homograft reoperation after the Ross procedure (shading represents 95% confidence interval). B, Cumulative incidence of reoperation on pulmonary homograft considering death as competing factor (shading represents 95% confidence interval [CI]).
Echocardiographic Results
All patients underwent echocardiographic assessment before discharge, whereas only 157 patients had echocardiographic data available at the end of follow-up. The last performed echocardiograms before patient censoring showed no or trace aortic insufficiency in 27 (17.2%) patients, mild aortic insufficiency in 110 (70.1%) patients, moderate aortic insufficiency in 14 (8.9%) patients, and severe aortic insufficiency in 6 (3.8%) patients.
There was no significant change in the transaortic gradients at the follow-up (P = .86), whereas the gradients on pulmonary homograft did increase significantly (P < .01). Pulmonary autograft root showed a statistically significant increase in diameters during the follow-up (P < .001). The median of autograft root diameter at discharge was 36 mm (IQR, 7 mm), and at the follow-up, it was 38 mm (IQR, 6 mm). There were 22 (13.9%) patients with autograft root diameter ≥45 mm. The diameters of the ascending aorta also increased during the follow-up (P < .04). The median of the ascending aorta diameter at discharge was 36 mm (IQR, 9 mm) and at the end of follow-up it was 38 mm (IQR, 6.8 mm). There were 18 (7%) patients with the ascending aorta diameter ≥45 mm.
Discussion
In different studies, there is a wide variation in hospital mortality rate after the Ross procedure.5,6 In our series, the mortality early after the operation was 0.9%, which is quite acceptable after such a complex procedure. It is important to note that the rate of the operative mortality after isolated AVR is about 1.2% in our center. The Ross procedures were led by one surgeon (I.C.) who already had a substantial experience with this operation before starting the Ross program in our center. Also, the procedure was implemented in selected patients. Those facts can potentially explain the acceptable rate of early mortality.7
To date, there seem to be no AV substitute that can provide an anticipated life expectancy with a comparable quality of life in the young adult except for the pulmonary autograft. It was proven in numerous studies that the Ross procedure offers an excellent long-term survival, which is comparable with the expected survival in the sex- and age-matched general population.5,8, 9, 10 Our study has similar results; the long-term survival after AVR with pulmonary autograft was not statistically different from the expected survival in the age- and sex-matched general population.
The reoperation due to the pulmonary autograft dysfunction is the Achilles' heel of the Ross procedure. The rate of autograft reinterventions differs between studies, and common indications for reoperation include the neoaortic root dilatation, infective endocarditis, technical failures, and others.6,8,11,12 In the current study, the freedom from reoperation for the autograft was 89.1% (95% CI, 81.2%-97.8%) at 11 years. The main causes of autograft reintervention were dilatation of the neoaortic root and the ascending aorta. There was no case of reoperation due to infective endocarditis of the pulmonary autograft. The frequently reported predictors of autograft reintervention are dilated aortic annulus, preoperative aortic insufficiency, age at operation, bicuspid AV, and male sex4,8,13,14; however, our uni- and multivariate analysis did not identify clear predictors of autograft reoperation.
The modified variants of the Ross procedure were proposed as the options to prevent late autograft dilatation and dysfunction and hence to reduce the rates of reoperations.4,15, 16, 17 For the first 5 years, we used the full-root replacement technique. Since February 2014, we have employed the modified techniques of the Ross procedure, involving Dacron graft or autologous aortic wall. Since then, there was only one case of reoperation among the patients operated on after February 2014, but the experience is relatively short (7 years), and a longer follow-up is needed.
Many different ways exist to reconstruct the RVOT,8,14,18 but pulmonary homografts seem to have been the best option.13,14 In our series, we used only fresh and cryopreserved pulmonary homografts, and they were deliberately oversized to decrease the risk of stenosis at the sites of anastomoses. There were only 2 reoperations during the follow-up with the freedom from homograft reintervention of 99% (95% CI 97.7%-100%) at 11 years.
The pulmonary autograft tends to fail over time. The commonly reported risk factors for autograft dysfunction include age at operation, male sex, preoperative aortic insufficiency, bicuspid AV and aortic annulus dilatation.8,19,20 In the current study, we did not identify clear predictors of autograft dysfunction via Cox regression analysis.
David and colleagues19 proposed that the dilated aortic annulus is probably a resultant marker of connective tissue disorders and the annulus reduction does not provide any benefit in the long term. Chauvette and colleagues,21 however, thought that an external support could be beneficial in selected patients. Based on the literature data and our experience, we think the lack of support for the autograft may play a crucial role in late autograft failure and reoperation. In fact, the use of modified technique using the reduction and re-enforcement of the aortic annulus was decided on in our latest years of practice for this main reason, and our early-term results are encouraging. Nevertheless, we have not found the annulus support to be statistically meaningful in terms of prevention from late pulmonary autograft dysfunction or reoperation.
There was a relatively large portion of patients with infective endocarditis undergoing AVR with pulmonary autograft (Table 1). Duke criteria were used for the diagnosis, and the extent of the infective process should be confined to aortic leaflets and annulus but should not involve the aortic root or cause an aortic root abscess to undergo the Ross procedure. Moreover, we still exploit the modified techniques of the Ross procedure for patients with infective endocarditis, and the rationale is that the synthetic material is not in direct contact with the blood stream; hence the risk of recurrence of the infection with adequate antibiotic therapy is very low. Presence of IE was initially included in the Cox regression analysis as a potential factor influencing survival or autograft long-term performance but it failed to prove any association.
In our series, 10 patients required urgent coronary artery bypass grafting due to myocardial infarction that was caused by kinking or angulation of the right coronary artery when reimplanting its button in most cases. To avoid these pitfalls in future, we have implemented the following techniques: (1) we form larger coronary buttons than before; (2) we reimplant the right coronary button the last, ie, after suturing the proximal end of the autograft followed by reimplantation of the left coronary button and the distal end of the autograft, we fill the neoaortic root with blood and identify the reimplantation spot for the right coronary button on the autograft and complete reimplantation; and (3) we suture the coronary buttons only to the autograft itself and do not include a Dacron graft or autologous aorta, to avoid displacement of the buttons from their intended position. These tips and tricks have proved very effective since they had been used.
Study Limitations
This is a retrospective analysis of prospectively collected data with an intermediate duration of clinical follow-up (median 6.1 years). Moreover, echocardiographic follow-up was not 100% complete, with only 157 echocardiograms available at the end. In addition, all operations were performed by one experienced surgeon.
Conclusions
The Ross procedure is a safe alternative to AVR in experienced hands with the acceptably low rate of early mortality. The long-term survival after this operation is not statistically different from the expected survival in an age- and sex-matched general population. The autograft dysfunction is the Achilles' heel of the Ross procedure, and the modified techniques can potentially decrease the rates of reintervention on the neoaortic root. Pulmonary homografts used to reconstruct the RVOT demonstrate excellent durability with 99% freedom from reoperation at 11 years. The study's main findings are summarized in Figure 4.
Figure 4.
Summary of methods, results, and implications.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Supplementary Data
Autologous inclusion technique of Ross procedure. Video available at: https://www.jtcvs.org/article/S2666-2736(22)00189-9/fulltext.
References
- 1.Ross D.N. Replacement of aortic and mitral valves with a pulmonary autograft. Lancet. 1967;2:956–958. doi: 10.1016/s0140-6736(67)90794-5. [DOI] [PubMed] [Google Scholar]
- 2.Vahanian A., Beyersdorf F., Praz F., Milojevic M., Baldus S., Bauersachs J., et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg. 2021;00:1–74. doi: 10.1093/ejcts/ezab389. [DOI] [Google Scholar]
- 3.Otto C.M., Nishimura R.A., Bonow R.O., Carabello B.A., Erwin J.P., III, Gentile F., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2021;143:e72–e227. doi: 10.1161/CIR.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 4.Skillington P.D., Mokhles M.M., Takkenberg J.J.M., Larobina M., O'Keefe M., Wynne R., et al. The Ross procedure using autologous support of the pulmonary autograft: techniques and late results. J Thorac Cardiovasc Surg. 2015;149:S46–S52. doi: 10.1016/j.jtcvs.2014.08.068. [DOI] [PubMed] [Google Scholar]
- 5.Etnel J.R.G., Grashuis P., Huygens S.A., Pekbay B., Papageorgiou G., Helbing W.A., et al. The Ross procedure: a systematic review, meta-analysis, and microsimulation. Circ Cardiovasc Qual Outcomes. 2018;11:e004748. doi: 10.1161/CIRCOUTCOMES.118.004748. [DOI] [PubMed] [Google Scholar]
- 6.Sibilio S., Koziarz A., Belley-Côté E.P., McClure G.R., MacIsaac S., Reza S.J., et al. Outcomes after Ross procedure in adult patients: a meta-analysis and microsimulation. J Card Surg. 2019;34:285–292. doi: 10.1111/jocs.14020. [DOI] [PubMed] [Google Scholar]
- 7.Bouhout I., Ghoneim A., Poirier N., Cartier R., Demers P., Perrault L.P., et al. Impact of the learning curve on early outcomes following the Ross procedure. Can J Cardiol. 2017;33:493–500. doi: 10.1016/j.cjca.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Karaskov A., Sharifulin R., Zheleznev S., Demin I., Lenko E., Bogachev-Prokophiev A. Results of the Ross procedure in adults: a single-centre experience of 741 operations. Eur J Cardiothorac Surg. 2016;49:e97–e104. doi: 10.1093/ejcts/ezw047. [DOI] [PubMed] [Google Scholar]
- 9.Guerreiro S., Madeira M., Ribeiras R., Queiroz E., Melo J., Canada M., et al. Long-term assessment of the Ross procedure in adults: clinical and echocardiographic follow-up at 20 years. Rev Port Cardiol. 2019;38:315–321. doi: 10.1016/j.repc.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Sievers H.-H., Stierle U., Charitos E.I., Takkenberg J.J., Hörer J., Lange R., et al. A multicentre evaluation of the autograft procedure for young patients undergoing aortic valve replacement: update on the German Ross Registry. Eur J Cardiothorac Surg. 2016;49:212–218. doi: 10.1093/ejcts/ezv001. [DOI] [PubMed] [Google Scholar]
- 11.Generali T., Jansen K., Steedman R., De Rita F., Viganò G., McParlin D., et al. Contemporary Ross procedure outcomes: medium- to long-term results in 214 patients. Eur J Cardiothorac Surg. 2021;60:1112–1121. doi: 10.1093/ejcts/ezab193. [DOI] [PubMed] [Google Scholar]
- 12.Pergola V., Di Salvo G., Fadel B., Galzerano D., Al-Shaid M., Al-Admawi M., et al. The long term results of the Ross procedure: the importance of candidate selection. Int J Cardiol. 2020;320:35–41. doi: 10.1016/j.ijcard.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Christ T., Claus B., Woythal N., Dushe S., Falk V., Grubitzsch H. The Ross procedure in adults: long-term results of homografts and stentless xenografts for pulmonary valve replacement. Thorac Cardiovasc Surg. 2017;65:656–661. doi: 10.1055/s-0036-1586157. [DOI] [PubMed] [Google Scholar]
- 14.Oeser C., Uyanik-Uenal K., Kocher A., Laufer G., Andreas M. Long-term performance of pulmonary homografts after the Ross procedure: experience up to 25 years. Eur J Cardiothorac Surg. 2019;55:876–884. doi: 10.1093/ejcts/ezy372. [DOI] [PubMed] [Google Scholar]
- 15.Skillington P.D., Mokhles M.M., Takkenberg J.J.M., O'Keefe M., Grigg L., Wilson W., et al. Twenty-year analysis of autologous support of the pulmonary autograft in the Ross procedure. Ann Thorac Surg. 2013;96:823–829. doi: 10.1016/j.athoracsur.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 16.Juthier F., Banfi C., Vincentelli A., Ennezat P.V., Le Tourneau T., Pinçon C., et al. Modified Ross operation with reinforcement of the pulmonary autograft: six-year results. J Thorac Cardiovasc Surg. 2010;139:1420–1423. doi: 10.1016/j.jtcvs.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 17.Sankhyan L.K., Ghosh R., Kumar S., Chatterjee S., Bhattachariya S., Das S., et al. Outcome of 40 consecutive cases of modified Ross procedure using novel Dacron valved conduit. Indian J Thorac Cardiovasc Surg. 2020;36:28–36. doi: 10.1007/s12055-019-00845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christ T., Paun A.C., Grubitzsch H., Holinski S., Falk V., Dushe S. Long-term results after the Ross procedure with the decellularized AutoTissue Matrix P® bioprosthesis used for pulmonary valve replacement. Eur J Cardiothorac Surg. 2019;55:885–892. doi: 10.1093/ejcts/ezy377. [DOI] [PubMed] [Google Scholar]
- 19.David T.E., Ouzounian M., David C.M., Lafreniere-Roula M., Manlhiot C. Late results of the Ross procedure. J Thorac Cardiovasc Surg. 2019;157:201–208. doi: 10.1016/j.jtcvs.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 20.David T.E., Woo A., Armstrong S., Maganti M. When is the Ross operation a good option to treat aortic valve disease? J Thorac Cardiovasc Surg. 2010;139:68–75. doi: 10.1016/j.jtcvs.2009.09.053. [DOI] [PubMed] [Google Scholar]
- 21.Chauvette V., Chamberland M.-È., El-Hamamsy I. A review of pulmonary autograft external support in the Ross procedure. Expert Rev Med Devices. 2019;16:981–988. doi: 10.1080/17434440.2019.1685380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Autologous inclusion technique of Ross procedure. Video available at: https://www.jtcvs.org/article/S2666-2736(22)00189-9/fulltext.