Abstract
Objective
To determine long-term survival and reoperation rate in patients with a bicuspid aortic valve (BAV) and patients with a tricuspid aortic valve (TAV) after stentless aortic valve replacement (AVR)/aortic root replacement (ARR).
Methods
Between 1992 and 2014, 1293 patients underwent first AVR/ARR with a stentless aortic valve using the modified inclusion operating technique, including 741 patients with a TAV and 552 with a BAV. Using propensity scoring with 26 variables, 330 matched pairs were identified with AVR with or without ascending aorta/arch replacement. Data were obtained through chart review, surveys, and the National Death Index.
Results
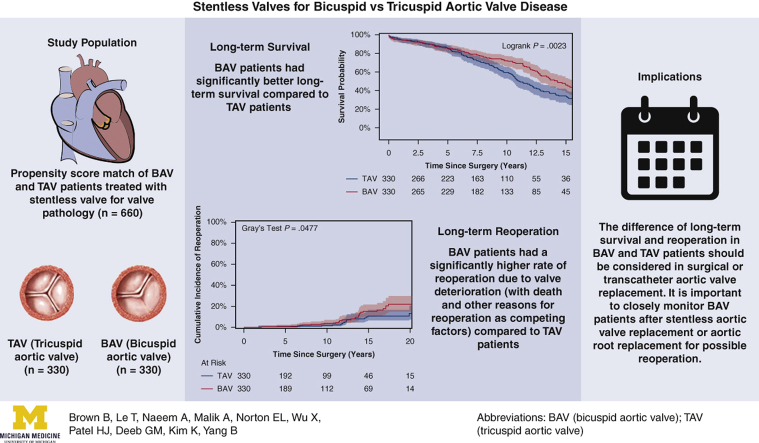
Patient demographics were similar in the propensity score–matched groups. Both groups had similar cardiopulmonary bypass, cross-clamp, and hypothermia circulatory arrest times, cerebral protection strategies, and rate of aortic arch replacement. The median size of implanted valves was similar (BAV: 27 mm [range, 25-29 mm] vs TAV: 27 mm [range, 25-27 mm]). Compared with the TAV group, the BAV group had a shorter hospital stay (6 days vs 7 days; P = .001) but similar 30-day mortality (1.8% vs 1.2%). The BAV group had better long-term (15-year) survival (46% vs 33%; P = .002) but a higher cumulative incidence of reoperation for structural valve deterioration (15-year: 15% vs 11%; P = .048). Cox proportional hazard analysis identified a BAV as a protective factor for long-term mortality (hazard ratio [HR], 0.71; 95% CI, 0.56-0.91; P = .006), but a risk factor for reoperation due to structural valve deterioration (HR, 1.4 [95% CI, 0.8-2.6; P = .27] in the matched cohort and 2.2 [95% CI, 1.3-3.7; P = .004] in the unmatched cohort).
Conclusions
The BAV patients had better long-term survival but a higher reoperation rate compared with TAV patients after stentless AVR. Our findings suggest caution in the use of bioprostheses for BAV patients.
Key Words: bicuspid aortic valve, stentless valve, bioprosthesis, aortic valve replacement, long-term survival, reoperation
Abbreviations and Acronyms: AVR, aortic valve replacement; BAV, bicuspid aortic valve; CI, confidence interval; HR, hazard ratio; NDI, National Death Index; OR, odds ratio; SAVR, surgical aortic valve replacement; TAV, tricuspid aortic valve; TAVR, transcatheter aortic valve replacement
Graphical abstract
Patients with a BAV had better long-term survival but a higher rate of reoperation than patients with a TAV after stentless AVR.
Central Message.
After aortic valve/root replacement with a stentless valve, patients with a bicuspid aortic valve had better long-term survival but a higher reoperation rate compared with patients with a tricuspid aortic valve.
Perspective.
After aortic valve replacement (AVR) with a stentless valve, patients with a bicuspid aortic valve (BAV) had better long-term survival but a higher cumulative incidence of reoperation compared with those with a tricuspid aortic valve (TAV). Our findings suggest that patients with a BAV behave differently from those with a TAV after AVR, and thus surgeons should be cautious when considering bioprosthetic surgical or transcatheter AVR for BAV patients.
The influence of aortic valve morphology—bicuspid aortic valve (BAV) and tricuspid aortic valve (TAV)—on postsurgical clinical outcomes after aortic valve replacement (AVR) with a bioprosthesis, including long-term survival and late reoperations, has been poorly studied. Yet these data would be critically important to guide surgical AVR (SAVR) or transcatheter AVR (TAVR) in BAV patients, especially in young BAV patients. The big question is whether we can treat BAV patients just like TAV patients with AVR.
To bolster the evidence analyzing the effects of preoperative aortic valve morphology on long-term survival and reoperation after stentless AVR, we report a comparison of perioperative and long-term clinical outcomes in 330 propensity score–matched pairs of BAV and TAV patients undergoing AVR with a stentless bioprosthetic valve using a modified inclusion operative technique. In addition, we report the long-term analysis of unmatched 1293 BAV and TAV patients undergoing the same procedures. We hypothesized that the BAV and TAV patients would have similar long-term survival, longevity, and reoperation rate after AVR using a stentless valve.
Methods
Patient Selection and Data Collection
This study was approved by the Institutional Review Board at Michigan Medicine (HUM00076499; July 10, 2013), which provided a waiver of informed consent. Between 1992 and 2014, 1293 patients, including 741 patients with a native TAV and 552 with a native BAV, underwent first AVR for aortic stenosis with a stentless valve using the modified inclusion operative technique. Patients with active endocarditis were excluded, as an active valve infection presents a distinct etiology of pathology and long-term patient outcomes. Using propensity score matching analysis, 330 matched pairs were identified based on 26 preoperative variables that are incorporated into the current (2018) Society of Thoracic Surgeons operative mortality model: age, sex, creatinine concentration, ejection fraction, diabetes, New York Heart Association class 3-4, chronic obstructive pulmonary disease, home oxygen requirement, hypertension, peripheral vascular disease, aortic insufficiency, aortic stenosis, atrial fibrillation, stroke, previous cardiac surgery, immunosuppressive therapy, previous coronary artery bypass grafting surgery, previous mitral valve surgery, previous aortic valve surgery, tricuspid insufficiency, mitral insufficiency, renal failure requiring dialysis, liver disease, mediastinal radiation, surgery status (elective, urgent, emergent), and concomitant ascending aorta/arch procedure. We excluded patients with root aneurysm needing total root replacement, those with aortic dissection, and those needing coronary artery bypass, mitral valve surgery, or tricuspid valve surgery in the matched cohort. All the patients included in the matched cohort underwent the same operation (modified inclusion) for AVR with or without ascending aorta/arch replacement, which was also matched. We followed the American Association of Thoracic Surgery/American Heart Association/American College of Cardiology guidelines to perform concomitant replacement of ascending aorta/arch if it was >4.5 cm. Data was obtained through the Society of Thoracic Surgery Data Warehouse at the University of Michigan to identify the relevant cohort and to determine the preoperative, operative, and postoperative variables. These data were supplemented through medical chart review for specified variables.
All living patients with a known address were mailed a questionnaire or contacted by phone through December 2015. Survival and reoperation data were collected by medical record review, questionnaire response, and National Death Index (NDI) data through December 31, 2018.1 In patients who had undergone reoperation, the reason for the reoperation, including valve dysfunction, valve infection, valve thrombosis, or other (eg, aortic aneurysm or pseudoaneurysm), was elicited as well. Only reoperation for valve dysfunction was counted as reoperation for structural valve deterioration.
For the matched sample, the median follow-up time for long-term survival was 7.4 years (interquartile range [IQR], 3.7-11 years) in the TAV group and 8.1 years (IQR, 3.8-13 years) in the BAV group. The median follow-up time for reoperation in the matched cohort was 7.4 years (IQR, 3.6-11 years) in the TAV group and 7.8 years (IQR, 3.7-12 years) in the BAV group. Completeness of follow-up for death and reoperation for the matched cohort was calculated based on the ratio of the observed person-time and potential person-time follow-up in the study.2
Operative Technique
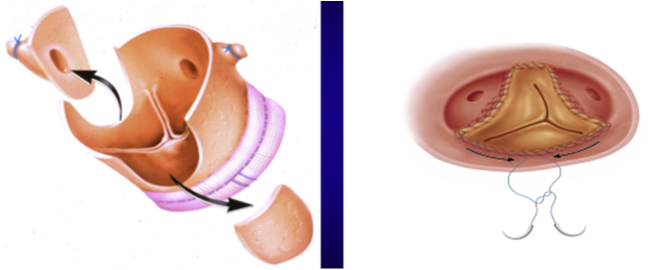
All patients underwent AVR with a stentless bioprosthesis conduit (Freestyle porcine aortic root; Medtronic, Dublin, Ireland) for the first time. The modified inclusion technique for operative implantation of the stentless bioprosthesis valve was used in all patients from both groups. This technique involved scalloping the left and right coronary sinuses of the stentless bioprosthesis, which corresponded to the right and left main coronary ostia, and a side-to-side anastomosis was performed with a running 5-0 Prolene suture, which was also used to anastomose the noncoronary sinus of the stentless valve to the native aortic root at the sinotubular junction as the distal suture line. The proximal suture line was interrupted 2-0 Ethibond (Ethicon, Cincinnati, Ohio) to secure the sewing ring of stentless valve to the aortic root at the basal ring level (Figure E1).
Figure E1.
Illustration of the modified inclusion operative technique. Different from the figure, in our cohort, we scalloped the left and right coronary sinus of the Freestyle porcine aortic root instead of the noncoronary sinus.
Statistical Analysis
Continuous data are presented as median (IQR), and categorical data are presented number (%). Univariate comparisons between groups were performed using the χ2 test for categorical data. The Kolmogorov–Smirnov and Cramer–von Mises tests were used to test the normality of the data. The Wilcoxon rank-sum test was performed for continuous data. Survival analysis was analyzed by Kaplan–Meier methods, with log-rank testing. The Cox proportional hazards regression model was used to calculate the adjusted hazard ratios (HRs) for long-term mortality among the matched sample since surgery, adjusting for group (BAV vs TAV), age (modeled as a categorical variable), sex, renal failure, coronary artery disease, congestive heart failure, preoperative atrial fibrillation, and previous cardiac surgery based on model diagnostics. The variables included in the Cox model were based on earlier reports in the literature and on clinical practice. Cumulative incidence function curves were adjusted for death and other indications for reoperation besides structural valve degeneration as competing risks (i.e. endocarditis, thrombosis, aortic aneurysm, or aortic dissection) using the Fine and Gray subdistribution method to assess the incidence of reoperation over time. The Gray test was used to test the differences in cumulative incidence function curves between groups. The cause-specific hazards regression model was used to model the reoperation due to structural valve deterioration for the matched cohort, with death and the other reasons for reoperation as competing risks. The adjusted HRs for group (BAV vs TAV), age, sex, and concomitant ascending aorta/arch procedure are reported. All results with a P value < .05 were considered statistically significant. Statistical calculations were performed with SAS 9.4 (SAS Institute, Cary, NC).
Results
Demographics and Preoperative Data
The demographics were unbalanced in the unmatched cohort (Table E1). After propensity score matching, the BAV and TAV groups had very balanced demographics, with a standard mean difference <0.1 for all preoperative variables (Table 1).
Table 1.
Preoperative demographics and characteristics of the propensity score–matched groups
| Variable | BAV group (N = 330) | TAV group (N = 330) | SMD∗ |
|---|---|---|---|
| Age, y, median (IQR) | 62 (52-71) | 63 (52-72) | 0.020 |
| Female sex, n (%) | 97 (29) | 96 (29) | 0.043 |
| Creatinine, mg/dL, median (IQR) | 1.0 (0.9-1.1) | 1.0 (0.9-1.2) | 0.085 |
| Ejection fraction, %, median (IQR) | 55 (50-60) | 55 (50-62) | 0.009 |
| Diabetes, n (%) | 55 (17) | 55 (17) | 0 |
| NYHA class 3-4, n (%) | 98 (30) | 97 (29) | 0.007 |
| COPD, n (%) | 50 (15) | 58 (18) | 0.066 |
| Home oxygen use, n (%) | 0 (0) | 0 (0) | 0 |
| Hypertension, n (%) | 196 (59) | 200 (61) | 0.025 |
| Peripheral vascular disease, n (%) | 18 (5.5) | 18 (5.5) | 0 |
| Aortic insufficiency, n (%) | 136 (41) | 137 (42) | 0.006 |
| Aortic stenosis, n (%) | 220 (67) | 219 (66) | 0.006 |
| Atrial fibrillation, n (%) | 28 (8.5) | 24 (7.3) | 0.045 |
| Stroke, n (%) | 15 (4.6) | 17 (5.2) | 0.028 |
| Previous cardiac surgery, n (%) | 39 (12) | 41 (12) | 0.019 |
| Immunosuppressive therapy, n (%) | 6 (1.8) | 5 (1.5) | 0.024 |
| Previous CABG, n (%) | 13 (3.9) | 16 (4.9) | 0.044 |
| Previous mitral valve surgery, n (%) | 4 (1.2) | 3 (0.9) | 0.030 |
| Previous aortic valve surgery, n (%) | 26 (2.9) | 27 (8.2) | 0.011 |
| Tricuspid insufficiency, n (%) | 91 (68) | 101 (65) | 0.055 |
| Mitral insufficiency, n (%) | 135 (84) | 144 (87) | 0.055 |
| Renal failure requiring dialysis, n (%) | 2 (0.6) | 4 (1.2) | 0.064 |
| Liver disease, n (%) | 1 (0.3) | 1 (0.3) | 0 |
| Mediastinal radiation, n (%) | 0 (0) | 0 (0) | 0 |
| Surgical status, n (%) | |||
| Elective | 289 (88) | 282 (85) | 0.062 |
| Urgent | 34 (10) | 37 (11) | 0.029 |
| Emergent | 7 (2.1) | 11 (3.3) | 0.075 |
| Concomitant ascending aorta/arch procedure, n (%) | 136 (41) | 143 (43) | 0.043 |
BAV, Bicuspid aortic valve; TAV, tricuspid aortic valve; SMD, standardized mean difference; IQR, interquartile range; NYHA, New York Heart Association; COPD, chronic obstructive pulmonary disease; CABG, coronary artery bypass grafting.
SMD <0.10 indicates balanced variables between the 2 groups.
Intraoperative Data
A similar proportion of patients in the 2 groups underwent ascending aorta/arch replacement (21% for the BAV group vs 20% for the TAV group). The respective times for hypothermic circulatory arrest (29 minutes vs 31 minutes), cross-clamping (130 minutes vs 120 minutes), and cardiopulmonary bypass (162 minutes vs 166 minutes) were very similar in the 2 groups. The strategy for cerebral protection, including cerebral perfusion and lowest bladder temperature, was also similar in the 2 groups. Median valve size was similar (27 mm [IQR, 25-29 mm] for the BAV group vs 27 mm [IQR, 25-27 mm] for the TAV group); however, the BAV group had more patients with a 29-mm valve implant, and the TAV group had more patients with a 27-mm valve (Table 2).
Table 2.
Intraoperative results for the propensity score–matched groups
| Variable | BAV group (N = 330) | TAV group (N = 330) | P value |
|---|---|---|---|
| CPB time, min, median (IQR) | 162 (138-194) | 166 (140-199) | .42 |
| Clamp time, min, median (IQR) | 130 (107-155) | 120 (111-155) | .79 |
| Valve size, mm, n (%) | |||
| 19 | 0 (0) | 1 (0.3) | 1.0 |
| 21 | 10 (3.0) | 14 (4.3) | .41 |
| 23 | 50 (15) | 50 (15) | 1.0 |
| 25 | 78 (24) | 85 (26) | .53 |
| 27 | 90 (27) | 104 (32) | .23 |
| 29 | 102 (31) | 76 (23) | .03 |
| Median size, mm, median (IQR) | 27 (25-29) | 27 (25-27) | .10 |
| PRBC transfusion, units, median (IQR) | 2.0 (0.0-4.0) | 2.0 (1.0-4.0) | .05 |
| Arch procedure, n (%) | 69 (21) | 67 (20) | .85 |
| HCA, n (%) | 69 (21) | 67 (20) | .85 |
| HCA time, min, median (IQR) | 29 (25-34) | 31 (24-37) | .44 |
| Cerebral perfusion, n (%) | |||
| Antegrade | 3 (4.3) | 2 (3.0) | .67 |
| Retrograde | 29 (42) | 29 (43) | .88 |
| Both antegrade and retrograde | 37 (54) | 36 (54) | .99 |
| Lowest bladder temperature, °C, median (IQR) | 18 (18-19) | 18 (18-19) | .96 |
P value indicates the difference in the incidence rate between the BAV and TAV groups. BAV, Bicuspid aortic valve; TAV, tricuspid aortic valve; CPB, cardiopulmonary bypass; IQR, interquartile range; PRBCs, packed red blood cells; HCA, hypothermic circulatory arrest.
Perioperative Outcomes
There were no significant differences in most perioperative complications and mortality between the matched BAV and TAV groups, including in-hospital mortality (1.8% vs 1.0%) and 30-day mortality (1.8% vs 1.2%); however, the BAV group had a shorter hospital stay after surgery (6 days vs 7 days; P = .001) (Table 3).
Table 3.
Postoperative outcomes of propensity score–matched groups
| Variable | BAV group (N = 330) | TAV group (N = 330) | P value |
|---|---|---|---|
| Atrial fibrillation | 113 (34) | 121 (37) | .52 |
| Complete heart block or pacemaker | 7 (2.1) | 12 (3.6) | .24 |
| Myocardial infarction | 1 (0.3) | 0 (0) | 1.0 |
| Stroke | 3 (0.9) | 5 (1.5) | .72 |
| Reoperation for bleeding | 7 (2.1) | 11 (3.3) | .34 |
| Creatinine, mg/dL | 1.1 (0.9, 1.2) | 1.1 (0.9, 1.3) | .24 |
| Time to extubation, h | 8.1 (4.4, 17) | 8.9 (4.6, 17) | .72 |
| Blood transfusion | 108 (33) | 98 (30) | .41 |
| RBC transfusion, units | 2.0 (2.0, 4.0) | 2.0 (2.0, 4.0) | .99 |
| New-onset renal failure | 2 (0.6) | 5 (1.5) | .45 |
| Hospital stay, d | 6 (5, 9) | 7 (5, 11) | .001 |
| In-hospital mortality | 6 (1.8) | 3 (1.0) | .51 |
| 30-d mortality | 6 (1.8) | 4 (1.2) | .52 |
P value indicates the difference in the incidence rate between the BAV and TAV groups. Significant P values are in bold type. BAV, Bicuspid aortic valve; TAV, tricuspid aortic valve; RBC, red blood cell.
Long-Term Outcomes
Our study had 100% completeness of follow-up for long-term mortality through December 31, 2018, using NDI data. Completeness of follow-up for reoperation was 80%.
Long-Term Survival
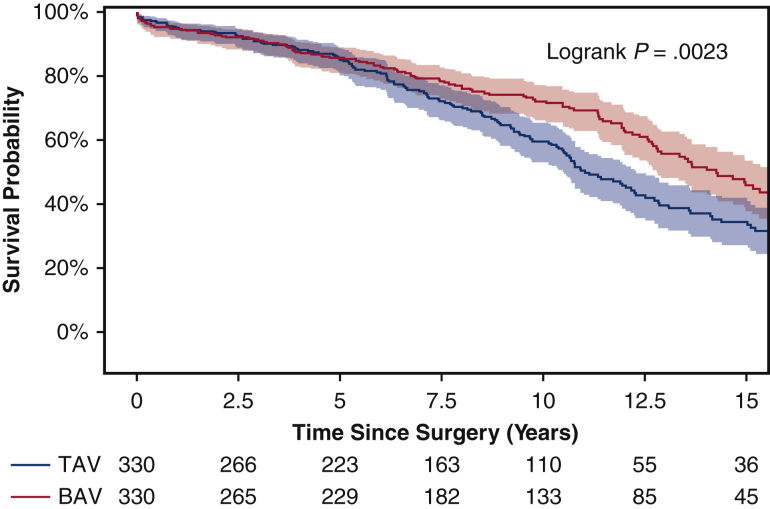
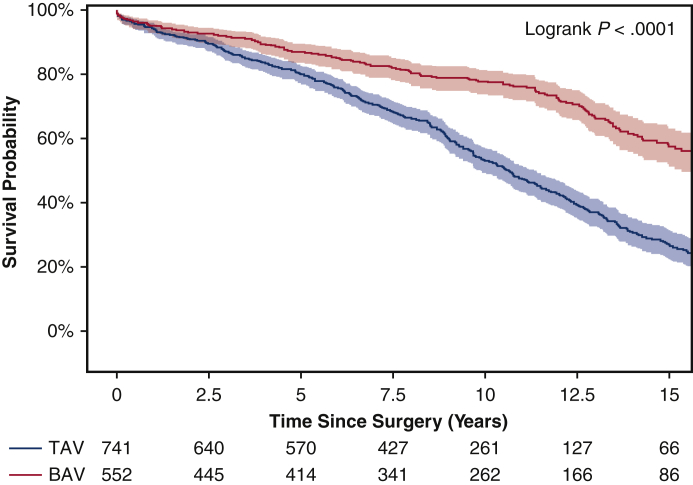
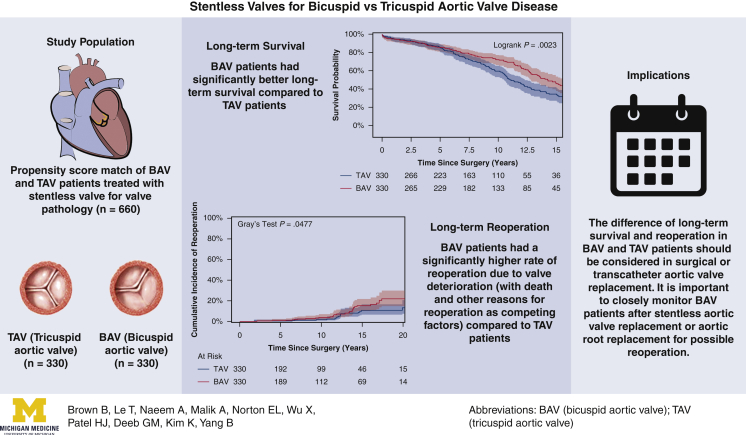
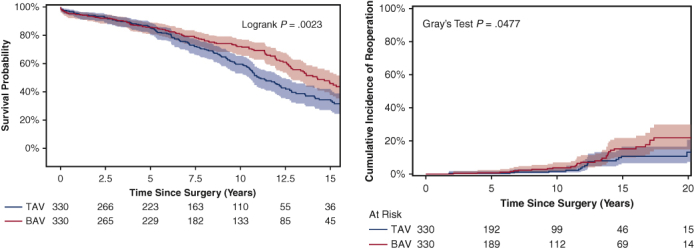
Among the matched population, the overall median duration of follow-up for long-term survival was 7.8 years (IQR, 3.7-12 years), with a median follow-up of 8.1 years (IQR, 3.8-13 years) for the BAV group and 7.4 years (IQR, 3.7-11 years) for the TAV group. Long-term survival was better in the BAV group than the TAV group (10-year: 72% vs 59%; 15-year: 46% vs 33%; P = .0023) (Figure 1). This finding held true in the unmatched cohorts (Figure E2). Significant risk factors for long-term mortality included age (HR, 1.06; 95% CI, 1.04-1.07; P < .0001), coronary artery disease (HR, 1.33; 95% CI, 1.02-1.75; P = .04), and previous cardiac surgery (HR, 1.54; 95% CI, 1.09-2.18; P = .02). BAV was a significant independent protective factor for long-term mortality compared with TAV (HR, 0.71; 95% CI, 0.56-0.91; P = .006) (Table 4). The protective effect of BAV persisted in the Cox model with the unmatched cohort (Table E2).
Figure 1.
Kaplan–Meier long-term survival of propensity score–matched bicuspid aortic valve (BAV) and tricuspid aortic valve (TAV) patients. Ten-year survival was 72% (95% confidence interval [CI], 65%-77%) for the BAV group versus 59% (95% CI, 52%-65%) for the TAV group. Fifteen-year survival was significantly better in the BAV group (46% [95% CI, 38%-54%] vs 33% [95% CI, 26%-41%]).
Figure E2.
Kaplan–Meier long-term survival of the entire unmatched cohort of bicuspid aortic valve (BAV) and tricuspid aortic valve (TAV) patients. The 10-year survival was 78% (95% confidence interval [CI], 74%-81%) for the BAV group versus 53% (95% CI, 49%-57%) for the TAV group. The 15-year survival was significantly better in the BAV group compared with the TAV group (57% [95% CI, 51%-63%] vs 27% [95% CI, 22%-31%]).
Table 4.
Cox proportional hazards regression for long-term mortality in the propensity score–matched sample
| Variable | HR (95% Wald CI) | P value |
|---|---|---|
| Age | 1.06 (1.04-1.07) | <.0001 |
| Male sex | 0.92 (0.72-1.18) | .51 |
| BAV | 0.71 (0.56-0.91) | .006 |
| Renal failure | 1.28 (0.29-5.64) | .75 |
| Coronary artery disease | 1.33 (1.02-1.75) | .04 |
| Congestive heart failure | 0.88 (0.69-1.11) | .28 |
| Preoperative atrial fibrillation | 1.13 (0.71-1.79) | .61 |
| Previous cardiac surgery | 1.54 (1.09-2.18) | .02 |
Significant P values are in bold type. HR, Hazard ratio; CI, confidence interval; BAV, bicuspid aortic valve.
Chronic obstructive pulmonary disease violated the proportional hazards assumption and thus was treated as strata in the analysis.
Reoperation due to Structural Valve Deterioration
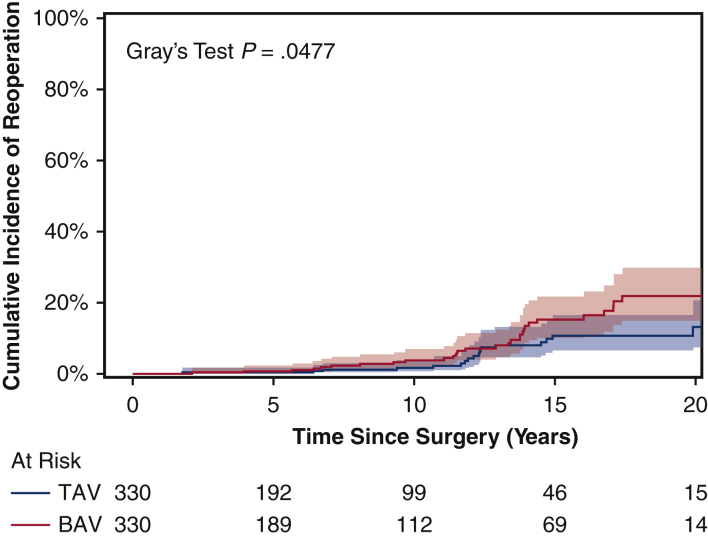
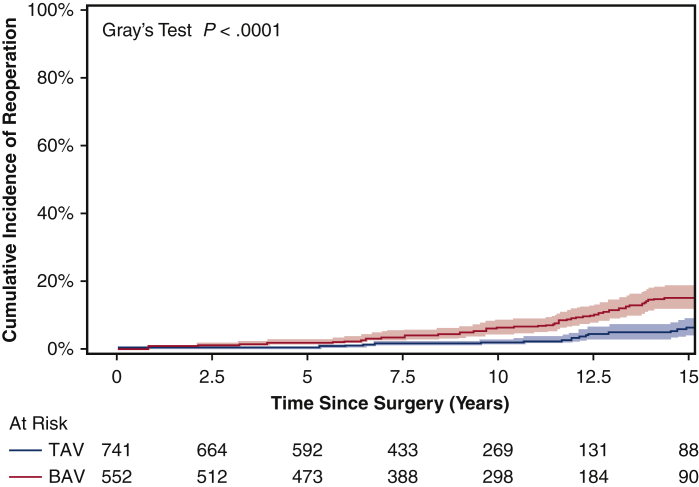
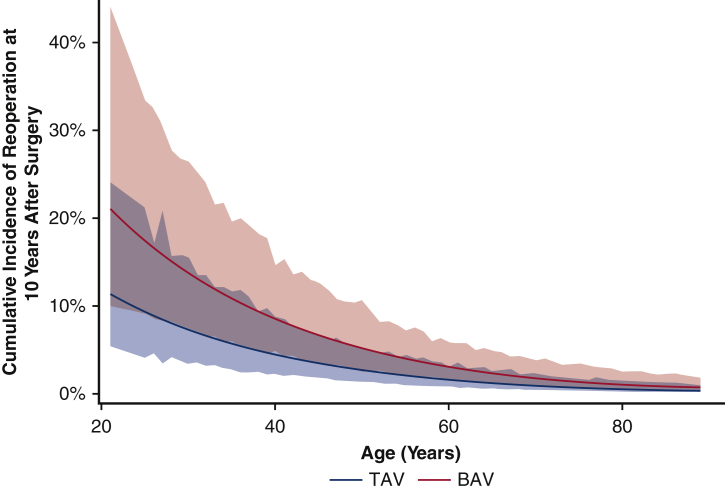
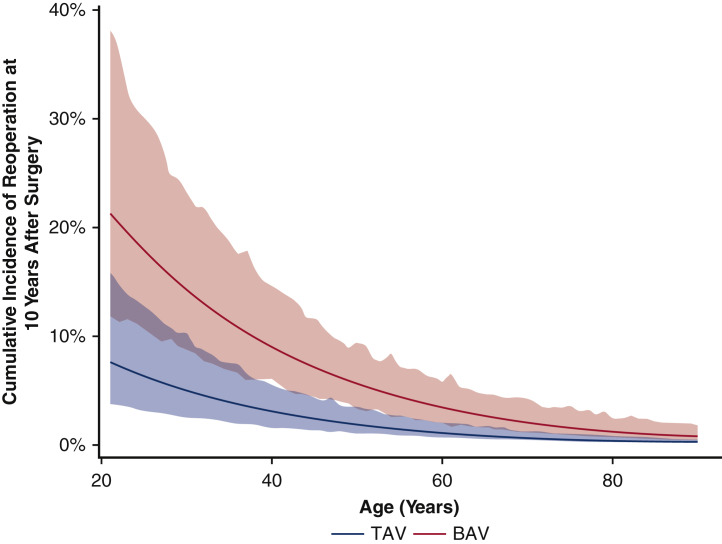
For the matched cohort, the overall median duration of follow-up for reoperation due to structural valve deterioration was 7.6 years (IQR, 3.7-11 years), with a median follow-up of 7.8 years (IQR, 3.7-12 years) for the BAV group and 7.4 years (IQR, 3.6-11 years) for the TAV group. A total of 64 patients underwent a reoperation; the TAV group had a lower cumulative incidence of reoperation for valve deterioration adjusting for death and other reasons for reoperation as competing factors at 10 years (2.3% vs 3.8%) and 15 years (11% vs 15%) (Figure 2). This finding held true in the unmatched cohorts (Figure E3). Age was an independent protective factor for reoperation due to structural valve deterioration (HR, 0.97; 95% CI, 0.95-0.99; P = .01) (Table 5). The HR of BAV compared with TAV for reoperation for valve deterioration was 1.4 (95% CI, 0.8-2.6; P = .27) in the matched cohort (Table 5) and 2.2 (95% CI, 1.3-3.7; P = .004) in the unmatched cohort (Table E3). Across all age groups, the 10-year cumulative incidence of reoperation was higher in the BAV group compared with the TAV group in both the matched cohort (Figure 3) and the unmatched cohort (Figure E4).
Figure 2.
Cumulative incidence of reoperation for valve deterioration (with death and other reasons for reoperation as competing factors) among propensity score–matched bicuspid aortic valve (BAV) and tricuspid aortic valve (TAV) patients following aortic valve replacement. The 15-year cumulative incidence of reoperation was 15% (95% confidence interval [CI], 10%-22%) for the BAV group versus 11% (95% CI, 6.4%-17%) for the TAV group.
Figure E3.
Cumulative incidence of reoperation for valve deterioration (with death and other reasons for reoperation as competing factors) in the whole unmatched cohort of bicuspid aortic valve (BAV) and tricuspid aortic valve (TAV) patients following aortic valve replacement. The 15-year cumulative incidence of reoperation was 15% (95% confidence interval (CI), 12%-19%) for the BAV group versus 6.0% (95% CI, 3.8%-9.0%) for the TAV group.
Table 5.
Cox proportional hazards regression for reoperation due to structural valve deterioration with death and other indications for reoperation as competing risks, for the propensity score–matched samples
| Variable | HR (95% Wald CI) | P value |
|---|---|---|
| Age | 0.97 (0.95-0.99) | .01 |
| Male sex | 0.95 (0.50-1.79) | .86 |
| BAV | 1.41 (0.76-2.58) | .27 |
| Concomitant ascending aorta/arch procedure | 1.16 (0.61-2.23) | .65 |
Significant P values are in bold type. HR, Hazard ratio; CI, confidence interval; BAV, bicuspid aortic valve.
Figure 3.
Ten-year cumulative incidence of reoperation with 95% confidence interval (CI), adjusting for death and other causes of reoperation besides structural factors as competing factors (see Methods), owing to structural valve deterioration at 10 years by age for bicuspid aortic valve (BAV) and tricuspid aortic valve (TAV) patients in the propensity score–matched cohort.
Figure E4.
Ten-year cumulative incidence of reoperation with 95% confidence intervals (CI), adjusting for death and other competing factors (see Methods), owing to structural valve deterioration at different ages for bicuspid aortic valve (BAV) and tricuspid aortic valve (TAV) patients in the whole unmatched cohort.
Discussion
In our 22-year retrospective study using one-to-one propensity score matching with 26 preoperative and demographic criteria, we found that the BAV group had better 15-year survival compared with the TAV group (46% vs 33%; HR, 0.71; 95% CI, 0.56-0.91) (Figure 1, Table 4) but a higher rate of reoperation due to structural valve deterioration at 15 years (15% vs 11%) (Figure 2).
Few previous studies have examined the short- and long-term outcomes of BAV patients after surgical AVR.3,4 Our short-term results from the propensity-matched sample suggest that the difference in native valve morphology between a BAV and a TAV does not affect the risk of most postoperative complications or short-term survival. Specifically, our study found no difference in the rate of complete heart block or need for pacemaker implantation after stentless AVR in both the BAV and TAV groups. This differs from the findings of a recent study by Haunschild and colleagues,4 who reported a higher pacemaker implantation rate in BAV patients compared with TAV patients (5.1% vs 4.4%) after isolated AVR with a bioprosthesis (86%) or mechanical valve (14%) for aortic stenosis.4 Interestingly, the surgical technique was modified aortic root implantation for all of our patients, and the pacemaker implantation rate of 2.1% in the BAV group is lower than the rate reported by Haunschild and colleagues (5.1%), whereas our pacemaker implantation rate in TAV patients was similar to the rate reported in that study (3.6% vs 4.4%). Using a modified inclusion root replacement technique, we placed the bottom suture line at the basal ring level of the root with an interrupted nonpledgeted suture, and the vast majority of those cases were performed by 2 or 3 aortic surgeons. This procedure could explain our study's lower rate of postoperative pacemaker implantation in BAV patients. In contrast, the reported pacemaker implantation rate in TAVR for BAV patients ranges from 9.1% to 15.4%.5,6 A 2.1% pacemaker implantation rate would be a high-reach goal for TAVR if we would like to use TAVR as a first option for BAV patients, especially in low-risk, younger patients who are excellent candidates for surgical AVR.
The differences in native valve morphology also affected the long-term results in our matched samples. Kaplan–Meier analysis of the propensity score–matched BAV and TAV groups found a significant survival improvement in the BAV group throughout follow-up at 10 years (BAV, 72%; TAV, 59%) and 15 years (BAV, 46%; TAV, 33%). This was confirmed by multivariable Cox proportional hazards regression showing the protective effect of BAV with an HR of 0.71 (Table 4).
Our results are consistent with the findings reported from 2 studies of matched BAV and TAV patients with isolated AVR.4,7 Both of those studies and our present study showed better Kaplan–Meier survival in BAV patients and similar HRs using a Cox proportional hazards model (0.71 in our study vs 0.56 in those studies). Based on these 3 large propensity score matching studies, we can confidently say that BAV patients have better long-term survival than TAV patients after AVR. As other studies have suggested,4,7 and as we agree, because BAV patients survive longer and present with aortic stenosis at a younger age, and because the longevity of TAVR valves remains unknown, we should use TAVR in BAV patients with caution.
The underlying mechanisms for better long-term survival in BAV patients after stentless valve implantation is unclear. The BAV develops calcification and aortic stenosis owing to the intrinsic anatomy of the valve.8 We speculate that the aortic valve and associated proximal aortic pathology may have been the sole pathology presented by BAV patients. Thus, replacing BAV morphology with a stentless TAV conduit with or without ascending aorta/arch replacement could relieve BAV strain and improve long-term valve function and patient longevity, given that the new valve conduit would not present an abnormal valve anatomy. Conversely, TAV patients were born with normal trileaflet aortic valve anatomy and gradually developed aortic stenosis or regurgitation. The valvular disease in TAV patients could indicate some underlying whole-body disease of unknown cause rather than an aortic valve–intrinsic cause. When the diseased TAV is replaced with a stentless TAV conduit, the underlying, unresolved cause of the initial calcification problem remains, causing long-term strain on the body and decreased lifespan.
Interestingly, after stentless valve implantation, the BAV patients experienced more valve deterioration, resulting in significantly more reoperations (15-year cumulative reoperation rate: 15% vs 11% in the TAV group) (Figures 2 and E3). This higher rate of reoperation for structural valve deterioration in BAV patients compared with TAV patients was seen in every age group at 10 years postsurgery (Figures 3 and E4). The main mechanism of stentless valve failure was fracture of the cusps in both the BAV and TAV groups.9 In our experience, almost all structural valve deterioration in stentless valves is related to leaflet fracture, except in some smaller stentless valves (19-21 mm), which can cause aortic valve stenosis from growth of pannus around the sewing ring. In some cases, calcifications of the stentless valve were seen, which could be a precursor of leaflet fracture. The BAV and TAV groups underwent AVR using the same stentless valve implantation technique (modified inclusion) and had a similar proportion of ascending aorta/arch replacement operations (Tables 1 and 2). The modified inclusion technique prevents dilation of the sinotubular junction.
The surgical technique and possible remaining ascending aortic dilation in BAV patients were not the reasons for the higher reoperation rate. It seems that BAV patients still had some undetermined factor contributing to earlier porcine valve deterioration compared with TAV patients, given that the BAV and TAV groups were propensity score matched using comparable demographics and preoperative comorbidities. The lower long-term survival rate in TAV patients also could have contributed to the lower reoperation rate in recipients of stentless valves, because patients could have died of other causes before stentless valve deterioration occurred. This again differed than the findings reported by Haunschild and colleagues,4 which showed no difference in freedom from long-term reoperation. Because Haunschild and colleagues used freedom from reoperation without adjusting for death as a competing factor, whereas we used the cumulative incidence of reoperation adjusting death and other reasons for reoperation as competing factors, we found a difference in the cumulative incidence of reoperation in BAV patients, as TAV patients had significantly lower long-term survival (Figures 1 and E2).
The study of Haunschild and colleagues also included 14% of patients implanted with mechanical AVR, with the remaining 86% of patients implanted with bioprostheses of different types. Our study included only one type of valve, the Freestyle stentless valve, implanted using the same technique in all patients. The combination of better long-term survival and a higher cumulative incidence of reoperation after stentless AVR in BAV patients strongly advocates caution when considering using bioprostheses for BAV patients who need AVR, including SAVR and TAVR. Approximately 40% of our BAV patients required an ascending aorta/arch procedure at the time of their operation, which could not be addressed with TAVR but could be addressed by SAVR. This is another reason to be cautious when applying TAVR to BAV patients.
After our massive survey, we did not add more recent patients to the study, for several reasons (1) the survey of 1293 patients took a considerable amount of time, and the most current data from NDI ended in December 31, 2018; (2) our study already involved a large sample size, and primary outcomes focused on long-term outcomes; and (3) we used more stented valves and fewer stentless valves in recent years, so adding a few more stentless valve cases would not add considerable value to the long-term outcomes of our study. Reasons for our decreasing use of stentless valves include (1) stented valves are easier to implant, (2) there is no survival benefit of same-size stentless valves versus stented valves (abstract was presented at The Society of Thoracic Surgeons Virtual Annual Meeting on January 29, 2021 - January 31, 2021), and (3) larger stented valves are easier for future valve-in-valve TAVR. We have developed a new technique for aortic root enlargement using a “Y” incision and a rectangular patch, which allows us to enlarge the aortic annulus by 3 to 4 valve sizes to accommodate large stented valves.10, 11, 12
Although we conducted a robust propensity score matching analysis with a large sample size, our study limitations are consistent with those of traditional retrospective studies. The follow-up for reoperation was not 100%. We could have underestimated the cumulative incidence of reoperation in both groups. The majority of patients who underwent stentless valve implantation at our institution were followed at our aortic clinic. They were informed of the durability of the Freestyle valve and subsequently received earlier valve degeneration diagnoses and reoperation. We performed most, if not all, of the reoperations for stentless valves, which is an uncommon operation at many hospitals. The rate of reoperation used in this study underestimated the rate of structural valve deterioration of the Freestyle valve, given the lack of follow-up echocardiographic data for our patients. We did not have information from the NDI on the causes of death for the BAV and TAV patients. In addition, the matched cohort had a mean age of 62 years in the BAV group, most of whom had aortic stenosis. Younger patients with a BAV/aortic insufficiency might benefit from a completely different treatment approach, such as valve repair, mechanical AVR, or a Ross procedure. Our study included only patients with AVR of stentless valves (Figure 4).
Figure 4.
Summary of the study describing the propensity score–matched cohort of bicuspid aortic valve (BAV) and tricuspid aortic valve (TAV) patients undergoing stentless aortic valve replacement, with long-term survival and reoperation outcomes and implications. Long-term survival was significantly better in the BAV patients; however, they also had higher rates of reoperation.
Conclusions
BAV patients had better long-term survival but a higher rate of reoperation compared with TAV patients after stentless AVR. Our findings suggest the need for caution when surgeons considering bioprosthesis in BAV patients (Video 1).
Conflict of Interest Statement
Dr Patel reported financial relationships with Medtronic, W. L. Gore, and Edwards Lifesciences. Dr Deeb reported a financial relationship with Medtronic. The other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
We thank our colleagues in the Department of Cardiac Surgery, including Drs Rich Prager, Steve Bolling, Frank Pagani, Jonathan Haft, Paul Tang, Matthew Romano, and Karen Kim, along with Linda Farhat, MS, Alexander Makkinejad, BS, Emma St. Pierre, BS, and Mohamed-Ali Sareini, as well as our Aortic Research Team.
Footnotes
Dr Yang is supported by the National Heart, Lung, and Blood Institute (Grants K08 HL130614, R01 HL141891, and R01 HL151776) and the Phil Jenkins and Darlene & Stephen J. Szatmari Funds. Dr Patel is supported by the Joe D. Morris Collegiate Professorship, the David Hamilton Fund, and the Phil Jenkins Breakthrough Fund in Cardiac Surgery. Dr Deeb is supported by the Herbert Sloan Collegiate Professorship, Jamie Buhr Fund, and Richard Nerod Fund.
B. Brown, T. Le, and A. Naeem contributed equally as co–first authors.
Appendix E1
Table E1.
Demographics and characteristics of the unmatched cohort
| Variable | BAV group (N = 552) | TAV group (N = 741) | SMD∗ |
|---|---|---|---|
| Age, y, median (IQR) | 57 (47-67) | 68 (59-76) | 0.734 |
| Female sex, n (%) | 144 (26) | 258 (35) | 0.043 |
| Creatinine, mg/dL, median (IQR) | 1.0 (0.9-1.1) | 1.0 (0.9-1.2) | 0.134 |
| Ejection fraction, %, median (IQR) | 55 (50-60) | 55 (50-60) | -0.031 |
| Diabetes, n (%) | 75 (14) | 136 (18) | 0.130 |
| NYHA class 3-4, n (%) | 185 (54) | 160 (22) | 0.269 |
| COPD, n (%) | 61 (11) | 147 (20) | 0.245 |
| Home oxygen use, n (%) | 3 (0.5) | 0 (0) | 0.105 |
| Hypertension, n (%) | 254 (46) | 528 (71) | 0.530 |
| Peripheral vascular disease, n (%) | 32 (5.8) | 61 (8.2) | 0.096 |
| Aortic insufficiency, n (%) | 240 (43) | 292 (39) | 0.083 |
| Aortic stenosis, n (%) | 398 (72) | 446 (60) | 0.254 |
| Atrial fibrillation, n (%) | 29 (5.3) | 111 (15) | 0.327 |
| Stroke, n (%) | 19 (3.4) | 42 (5.7) | 0.107 |
| Previous cardiac surgery, n (%) | 48 (8.7) | 171 (23) | 0.401 |
| Previous CABG, n (%) | 13 (2.4) | 82 (11) | 0.354 |
| Previous mitral valve surgery, n (%) | 5 (0.9) | 12 (1.6) | 0.064 |
| Previous aortic valve surgery, n (%) | 35 (6.3) | 104 (14) | 0.257 |
| Tricuspid insufficiency, n (%) | 142 (67) | 240 (69) | 0.074 |
| Mitral insufficiency, n (%) | 213 (84) | 341 (86) | 0.151 |
| Immunosuppressive therapy, n (%) | 7 (1.3) | 36 (4.9) | 0.210 |
| Renal failure requiring dialysis, n (%) | 4 (0.7) | 14 (1.9) | 0.103 |
| Liver disease, n (%) | 1 (0.2) | 4 (0.5) | 0.060 |
| Mediastinal radiation, n (%) | 0 (0) | 2 (0.3) | 0.074 |
| Surgical status, n (%) | |||
| Elective | 480 (87) | 589 (79) | 0.257 |
| Urgent | 56 (10) | 126 (17) | 0.257 |
| Emergent | 13 (2.4) | 20 (2.7) | 0.257 |
| Concomitant ascending aorta/arch procedure, n (%) | 267 (48) | 247 (33) | 0.310 |
BAV, Bicuspid aortic valve; TAV, tricuspid aortic valve; SMD, standardized mean difference; IQR, interquartile range; NYHA, New York Heart Association; COPD, chronic obstructive pulmonary disease; CABG, coronary artery bypass grafting.
SMD <0.10 indicates balanced variables between the 2 groups.
Table E2.
Cox proportional hazards regression for long-term mortality for entire unmatched cohort
| Variable | HR (95% Wald CI) | P value |
|---|---|---|
| Age | 1.04 (1.03-1.05) | <.0001 |
| Male sex | 1.06 (0.89-1.26) | .50 |
| BAV | 0.71 (0.58-0.86) | .0006 |
| Renal failure | 4.02 (2.23-7.24) | <.0001 |
| Coronary artery disease | 1.15 (0.95-1.39) | .15 |
| Congestive heart failure | 0.96 (0.81-1.13) | .62 |
| COPD | 1.94 (1.59-2.38) | <.0001 |
| Preoperative atrial fibrillation | 1.22 (0.94-1.59) | .14 |
| Previous cardiac surgery | 1.71 (1.39-2.10) | <.0001 |
Significant P values are in bold type. HR, Hazard ratio; CI, confidence interval; BAV, bicuspid aortic valve; COPD, chronic obstructive pulmonary disease.
Table E3.
Cox proportional hazard regression for reoperation due to structural valve deterioration with death and other indications for reoperation as competing risks, for the entire unmatched cohort
| Variable | HR (95% Wald CI) | P value |
|---|---|---|
| Age | 0.97 (0.95-0.98) | <.0001 |
| Male sex | 0.76 (0.46-1.25) | .27 |
| BAV | 2.17 (1.29-3.66) | .004 |
| Concomitant ascending aorta/arch procedure | 1.11 (0.71-1.74) | .65 |
Significant P values are in bold type. HR, Hazard ratio; CI, confidence interval; BAV, bicuspid aortic valve.
Supplementary Data
Discussion of the long-term outcomes after stentless aortic valve replacement in patients with a bicuspid aortic valve and patients with a tricuspid aortic valve. Video available at: https://www.jtcvs.org/article/S2666-2736(21)00333-8/fulltext.
References
- 1.Centers for Disease Control and Prevention National Center for Health Statistics. National death index. https://www.cdc.gov/nchs/ndi/index.htm Available at:
- 2.Clark T.G., Altman D.G., De Stavola B.L. Quantification of the completeness of follow-up. Lancet. 2002;359:1309–1310. doi: 10.1016/s0140-6736(02)08272-7. [DOI] [PubMed] [Google Scholar]
- 3.Ali A., Patel A., Ali Z.A., Abu-Omar Y., Freed D., Sheikh A.Y., et al. Stentless aortic valve replacement in patients with bicuspid aortic valve disease: clinical outcome and aortic diameter changes during follow-up. Eur J Cardiothorac Surg. 2010;38:134–140. doi: 10.1016/j.ejcts.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 4.Haunschild J., Misfeld M., Schroeter T., Lindemann F., Davierwala P., von Aspern K., et al. Prevalence of permanent pacemaker implantation after conventional aortic valve replacement—a propensity-matched analysis in patients with a bicuspid or tricuspid aortic valve: a benchmark for transcatheter aortic valve replacement. Eur J Cardiothorac Surg. 2020;58:130–137. doi: 10.1093/ejcts/ezaa053. [DOI] [PubMed] [Google Scholar]
- 5.Makkar R.R., Yoon S.H., Leon M.B., Chakravarty T., Rinaldi M., Shah P.B., et al. Association between transcatheter aortic valve replacement for bicuspid vs tricuspid aortic stenosis and mortality or stroke. JAMA. 2019;321:2193–2202. doi: 10.1001/jama.2019.7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoon S.H., Bleiziffer S., De Backer O., Delgado V., Arai T., Ziegelmueller J., et al. Outcomes in transcatheter aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Am Coll Cardiol. 2017;69:2579–2589. doi: 10.1016/j.jacc.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Holmgren A., Enger T.B., Näslund U., Videm V., Valle S., Evjemo K.J.D., et al. Long-term results after aortic valve replacement for bicuspid or tricuspid valve morphology in a Swedish population. Eur J Cardiothorac Surg. 2021;59:570–576. doi: 10.1093/ejcts/ezaa348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theodoris C.V., Li M., White M.P., Liu L., He D., Pollard K.S., et al. Human disease modeling reveals integrated transcriptional and epigenetic mechanisms of NOTCH1 haploinsufficiency. Cell. 2015;160:1072–1086. doi: 10.1016/j.cell.2015.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang B., Patel H.J., Norton E.L., Debenedictus C., Farhat L., Wu X., et al. Aortic valve reoperation following stentless bioprosthesis: short- and long-term outcomes. Ann Thorac Surg. 2018;106:521–525. doi: 10.1016/j.athoracsur.2018.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang B., Naeem A. A “Y” incision/rectangular patch to enlarge the aortic annulus by 4 valve sizes in TAV and BAV patients. April 13, 2021. https://www.ctsnet.org/article/%E2%80%9Cy%E2%80%9D-incisionrectangular-patch-enlarge-aortic-annulus-4-valve-sizes-tav-and-bav-patients Available at: [DOI] [PMC free article] [PubMed]
- 11.Yang B. A novel simple technique to enlarge the aortic annulus by two valve sizes. JTCVS Tech. 2021;5:13–16. doi: 10.1016/j.xjtc.2020.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang B., Naeem A. A Y-incision and rectangular patch to enlarge the aortic annulus by 3 valve sizes. Ann Thorac Surg. 2021;112:e139–e141. doi: 10.1016/j.athoracsur.2021.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Discussion of the long-term outcomes after stentless aortic valve replacement in patients with a bicuspid aortic valve and patients with a tricuspid aortic valve. Video available at: https://www.jtcvs.org/article/S2666-2736(21)00333-8/fulltext.