Abstract
Oligopeptides are transported into Bacillus subtilis by two ABC transport systems, App and Opp. Transcription of the operon encoding the Opp system was found to occur during exponential growth, whereas the app operon was induced at the onset of stationary phase. Transcription of both operons was completely curtailed by overproduction of the ScoC regulator from a multicopy plasmid and was enhanced in strains with the scoC locus deleted. ScoC, a member of the MarR family of transcription regulators, is known from previous studies to be a negative regulator of sporulation and of protease production that acts by binding directly to the promoters of the genes it regulates. Since peptide transport is essential for inactivation of the negative regulation of sporulation by Rap phosphatases, the control of ScoC transcription repression activity plays a crucial role in the initiation of sporulation.
Initiation of sporulation is regulated by the cellular level of the phosphorylated Spo0A transcription factor produced by the phosphorelay signal transduction system (3). Regulation of the Spo0A phosphorylation level occurs by a combination of transcription control of key components of the phosphorelay and regulation of the flow of phosphate through the system (9). Control of phosphate flow through the phosphorelay depends on a myriad of regulatory circuits which involve kinases and phosphatases (19, 22).
Mutations in the opp oligopeptide transport system result in decreased sporulation, suggesting that a peptide molecule of some kind must be transported from outside the cell to complete a regulatory circuit involved in the initiation of sporulation (25, 28). In studies designed to identify the target of the transported peptide, suppressors restoring sporulation in a strain with a deletion in the opp operon were sought (12). The suppressors revealed the existence of genes for a second oligopeptide transport system, app, on the Bacillus subtilis chromosome. The App system is inactive in the common laboratory strain BS168 because of a frameshift mutation in the gene for the peptide-binding protein AppA. The App and Opp transport systems show some specificity differences; the Opp system, but not the App system, can transport tripeptides. Otherwise, there seem to be no specificity differences between the two systems for transport of tetra- or pentapeptides or the peptides involved in sporulation or competence.
The targets of the peptides involved in sporulation have been clearly delineated as the Rap phosphatases (22–24). Pentapeptides exported from the cell need to be reimported into the cell to complete the regulatory circuit controlling Rap phosphatases, which prevent sporulation by dephosphorylating the Spo0F∼P phosphorelay component (21). The sporulation defect of opp mutants of BS168 results from the inability to transport the controlling pentapeptides. Thus, oligopeptide transport systems are essential for regulatory functions, as well as serving to nonspecifically transport peptides for anabolic and catabolic purposes.
In the study reported here, the opp and app operons were found to be differentially controlled by growth phase and coregulated by the ScoC transcription factor. scoC was originally described as a sporulation control locus (5) and has also been known as hpr (8) and cat (10).
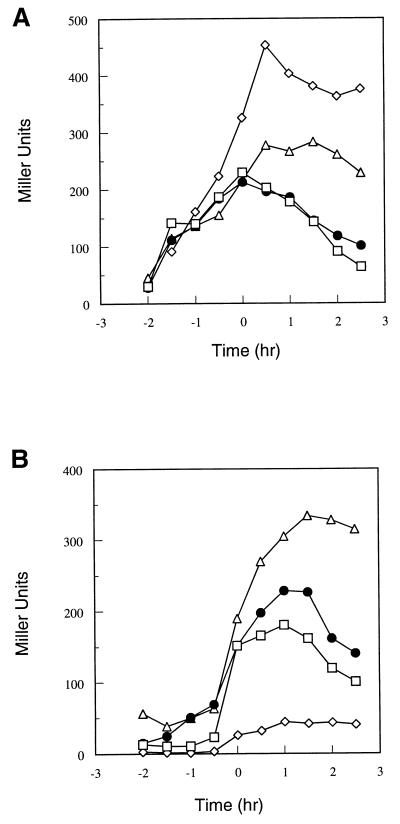
In order to study the timing and level of transcription of both the app and opp operons, promoter-bearing restriction fragments from both operons were cloned in front of a promoterless β-galactosidase gene and integrated into the amylase gene. The strains used are described in Table 1. Cultures were grown in Schaeffer’s sporulation medium and sampled at hourly intervals for determination of growth and β-galactosidase activity. Transcription from the opp promoter was observed to increase during the growth phase up to T0, or the beginning of stationary phase (Fig. 1A). A spo0A mutation was found to elevate the final level of transcription, and this stimulation was due (at least partially) to AbrB, since a spo0A abrB double mutant showed a lower rate than a spo0A mutant.
TABLE 1.
B. subtilis strains used in this study
| Straina | Relevant genotype |
|---|---|
| JH642 | trpC2 phe-1 |
| JH11134 | amyE::app-lacZ::aphc ΔsinR::Phlrb |
| JH14270 | amyE::app-lacZ::aphc |
| JH14271 | amyE::app-lacZ::aph ΔscoC::cat |
| JH14272 | amyE::opp-lacZ::aph ΔscoC::cat |
| JH14279 | amyE::opp-lacZ::aphd |
| JH17280 | amyE::opp-lacZ::aph spo0A12 |
| JH14281 | amyE::app-lacZ::aph spo0A12 |
| JH14282 | amyE::opp-lacZ::aph abrB::Tn917mls |
| JH14283 | amyE::app-lacZ::aph abrB::Tn917mls |
| JH14285 | amyE::app-lacZ::aph spo0A12 abrB::Tn917mls |
| JH14286 | amyE::opp-lacZ::aph spo0A12 abrB::Tn917mls |
All JH strains are derivatives of JH642 and therefore carry the trpC2 phe-1 auxotrophic markers and the app frameshift mutation (12).
From strain IS720 (14).
Obtained by transformation of strain JH642 with linearized plasmid pJA143.
Obtained by transformation of strain JH642 with linearized plasmid pJM6178. Escherichia coli DH5α competent cells (Bethesda Research Laboratories) were used for plasmid construction and propagation. The app-lacZ fusion vector pJA143 is a pJM115 derivative carrying the 1.3-kb EcoRI-BglII fragment from pJH6186 (12). The opp-lacZ fusion plasmid pJM6178 contains a 1.2-kb HindIII-ClaI blunt-ended fragment carrying the oppA promoter cloned in pJM115 (25). pJM115 is a transcriptional fusion vector derivative of pDH32 in which the chloramphenicol resistance gene has been replaced with the kanamycin resistance gene (20). The multicopy plasmids pJM2491 and pJM2493 carrying the scoC locus or the scoC promoter region only, respectively, were constructed in the multicopy vector pBS19 and were described by Perego and Hoch (26). Plasmid DNA was prepared by the procedure of Birnboim and Doly (2). DNA manipulations were performed by standard methods (15). B. subtilis chromosomal DNA was prepared by using the Marmur method (16). Transformations were performed by the method of Anagnostopoulos and Spizizen (1) with 1 μg of plasmid DNA or 0.1 μg of chromosomal DNA. Schaeffer’s agar plates (29) supplemented with 5 μg of chloramphenicol, ml−1, 1 μg of erythromycin ml−1, 2 μg of kanamycin ml−1, or 2.5 μg of phleomycin ml−1 were used for selection.
FIG. 1.
(A) Time course of β-galactosidase activity of the opp-lacZ fusion construct (representative of a typical experiment) in the following strains: parental strain JH14279 (□), spo0A12 mutant strain JH14280 (◊), abrB::Tn917 mutant strain JH14282 (●), and spo0A12 abrB::Tn917 mutant strain JH14286 (▵). Time zero is the time of transition from vegetative growth to stationary phase. β-Galactosidase activity in strains harboring lacZ fusion constructs was assayed as previously described (6). Specific activity is expressed in Miller units (17). (B) Time course of β-galactosidase activity of the app-lacZ fusion construct (representative of a typical experiment) in the following strains: parental strain JH14270 (□), spo0A12 mutant strain JH14281 (◊), abrB::Tn917 mutant strain JH14283 (●), and spo0A12 abrB::Tn917 mutant strain JH14285 (▵).
In contrast to opp, the app operon was not transcribed during the growth phase but was sharply induced about 30 min before T0 (Fig. 1B). A spo0A mutation in this case drastically reduced this induction, and expression in a spo0A abrB double mutant was stimulated above the wild-type level. This is the now-classical pattern of a promoter repressed by AbrB.
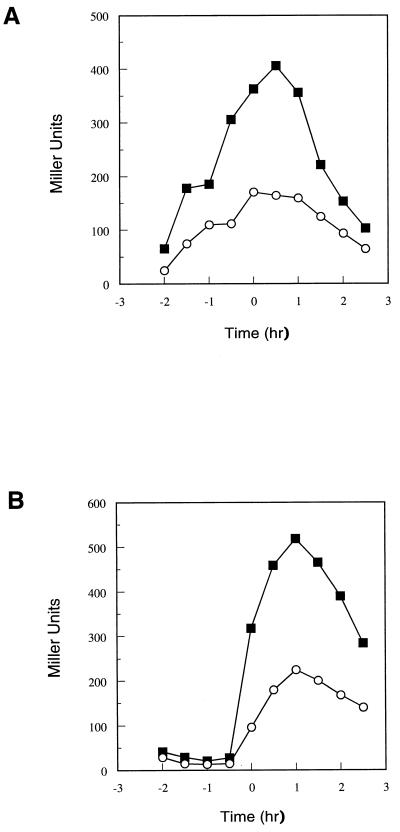
The effects of several sporulation genes on the transcription of the opp operon were tested with little or no evidence of a consistent pattern. However, a mutation in the scoC gene was found to stimulate transcription of the opp and app operons approximately twofold without affecting the timing of the transcription (Fig. 2).
FIG. 2.
Effect of scoC deletion on opp (A) or app (B) transcription. Symbols: ○, parental strain JH14279 or JH14270; ■, scoC::cat mutant strain JH14272 or JH14271.
The scoC mutation utilized here inactivates the functions of ScoC, suggesting that ScoC may repress transcription of the operon. To test this notion, expression of the opp operon was assayed in a strain bearing the scoC gene on a multicopy plasmid. In this strain, the expression of the opp operon was shut off and the strain bearing a similar plasmid containing only the promoter for scoC but not an intact scoC gene was unaffected in opp expression (Fig. 3A). This result mimics the effects of these plasmids on sporulation and protease production (26).
FIG. 3.
Effect of scoC overexpression on opp (A) or app (B) transcription. Strain JH14279 (A) or JH14270 (B) carrying replicative plasmid pBS19 (○; vector only), pJM2491 (□); scoC), or pJM2493 (⧫; scoC promoter) was grown in Schaeffer’s sporulation medium containing chloramphenicol at 5 μg/ml. β-Galactosidase activity was measured at the indicated times.
Identical experiments were carried out with a strain carrying an app-lacZ fusion. The scoC multicopy plasmid completely shut off expression of the app genes (Fig. 3B). The promoter-alone plasmid still expressed the app operon but, in this case, showed twofold less transcription than was observed in the strain with only the vector. Thus, ScoC is highly effective in repressing both of the oligopeptide permease operons.
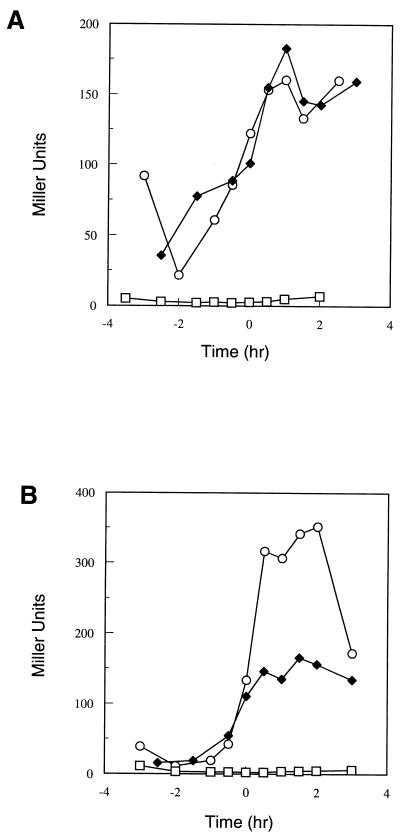
The phenotypic effects of multicopy scoC on protease production and sporulation are reminiscent of the phenotypes resulting from SinR repression activity (7). SinR is known to repress sporulation when overexpressed, and it was shown to bind specifically to the spo0A promoter and to inhibit transcription of the spoIIG gene (4, 13). ScoC is known to bind to the promoter of SinI, which serves to regulate the activity of SinR (11). Therefore, multicopy scoC could exert its effects on the app and opp operons through SinR by repressing SinI expression. In order to test this possibility, app-lacZ and opp-lacZ fusions were constructed in a sinR mutant strain into which the multicopy scoC plasmid had been transformed. Both app expression (Fig. 4) and opp expression (data not shown) were unchanged by the sinR mutation.
FIG. 4.
Time course of β-galactosidase activity of the app-lacZ construct in parental strain JH14270 (□) and sinR mutant strain JH11134 (●).
In view of the importance of oligopeptide transport in the regulation of sporulation, it was of interest to understand the mechanisms controlling the expression of peptide uptake systems. The oligopeptide permease operon was expressed during the exponential phase of growth, reaching a maximum value at the beginning of stationary phase and decreasing thereafter. Expression was enhanced by the spo0A mutation, and some of the observed enhancement was lost in an abrB spo0A strain. In contrast, app transcription was induced at the end of growth and this expression was repressed by AbrB. Since AbrB levels are repressed by Spo0A∼P, the level of phosphorylation of this sporulation regulator is a factor in determining whether the app operon is expressed. However, both operons must respond to other regulators to fully explain the growth phase dependence of each.
Deletion of the scoC locus results in enhanced expression of the app and opp operons, while a multicopy scoC plasmid represses both operons. Therefore ScoC is a repressor of oligopeptide uptake. The promoter regions of the app and opp operons contain consensus ScoC binding sites (11), but no promoter binding studies have been carried out to prove a direct interaction. Other, unidentified, regulators must be responsible for the timing of the expression of these operons and the observed growth phase dependence of their expression.
ScoC is a member of the MarR family of negative regulators named for their control of multiple antibiotic resistance genes in gram-negative bacteria (18). This family contains a conserved sequence of amino acid residues consistent with a similar tertiary structure for all members. Extrapolating from other members of the family, it appears that these regulators act in a negative fashion and are inactivated by small-molecule effectors. This would be consistent with the observed effects of scoC mutations on sporulation and on expression of proteases and oligopeptide permeases. In the absence of hypothetical effectors, ScoC is a repressor of sporulation acting in at least two ways: by repressing peptide permease synthesis, thereby allowing Rap phosphatases to short circuit the phosphorelay, and by repressing sinI transcription to allow uninhibited SinR to repress some sporulation genes. The end result is a deficiency of Spo0A∼P needed to induce sporulation.
A sinR mutation does not overcome the sporulation deficiency of multicopy ScoC, indicating that SinR is not the major or only reason for the phenotype (11a). Loss of the oligopeptide transport systems due to a multicopy scoC plasmid predominantly affects the activity of the RapA phosphatase, since the sporulation deficiency of an opp app mutant is suppressed by a rapA mutation (27). However, a rapA deletion does not suppress sporulation in a strain bearing a multicopy scoC plasmid, indicating that ScoC has effects on other, unidentified sporulation genes (22a).
A major question left unanswered is the nature of the hypothetical effector molecule for ScoC. Based on the reactivity of certain members of the MarR family with phenolic compounds, it has been postulated that naturally occurring toxic compounds may be the effector molecules for this family of regulators (18). Mutations in scoC are known to relieve catabolite repression of sporulation, suggesting that catabolites may repress sporulation by preventing formation of the effector molecule for ScoC. There are no obvious candidates for this hypothetical effector of ScoC.
The reason this bacterium has two separate systems for oligopeptide uptake remains elusive. While the BS168 strain carries a frameshift mutation in the app operon, this is a peculiarity of the laboratory strain, as sequence studies of other B. subtilis strains (e.g., Bacillus natto) revealed the absence of this mutation (11a). Structural studies of the periplasmic oligopeptide binding protein OppA indicates there is little or no basis for amino acid sequence specificity in the active site of this protein (30). While substrate specificity may not be the reason for the redundancy in seemingly identical systems, regulation of transcription is different in the two systems, suggesting a requirement under all conditions of growth.
Acknowledgments
This research was supported, in part, by grants GM19416 and GM55594 from the National Institute of General Medical Sciences, National Institutes of Health, USPHS.
We thank Mark Strauch for pointing out putative ScoC binding sites in the app promoter region.
Footnotes
Publication 12189-MEM from the Department of Molecular and Experimental Medicine at The Scripps Research Institute.
REFERENCES
- 1.Anagnostopoulos C, Spizizen J. Requirements for transformation in Bacillus subtilis. J Bacteriol. 1961;81:741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burbulys D, Trach K A, Hoch J A. The initiation of sporulation in Bacillus subtilis is controlled by a multicomponent phosphorelay. Cell. 1991;64:545–552. doi: 10.1016/0092-8674(91)90238-t. [DOI] [PubMed] [Google Scholar]
- 4.Cervin M A, Lewis R J, Brannigan J A, Spiegelman G B. The Bacillus subtilis regulator SinR inhibits spoIIG promoter transcription in vitro without displacing RNA polymerase. Nucleic Acids Res. 1998;26:3806–3812. doi: 10.1093/nar/26.16.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dod B, Balassa G. Spore control (Sco) mutations in Bacillus subtilis. III. Regulation of extracellular protease synthesis in the spore control mutations Sco C. Mol Gen Genet. 1978;163:57–63. [Google Scholar]
- 6.Ferrari E, Henner D J, Perego M, Hoch J A. Transcription of Bacillus subtilis subtilisin and expression of subtilisin in sporulation mutants. J Bacteriol. 1988;170:289–295. doi: 10.1128/jb.170.1.289-295.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaur N K, Dubnau E, Smith I. Characterization of a cloned Bacillus subtilis gene that inhibits sporulation in multiple copies. J Bacteriol. 1986;168:860–869. doi: 10.1128/jb.168.2.860-869.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higerd T B, Hoch J A, Spizizen J. Hyperprotease-producing mutants of Bacillus subtilis. J Bacteriol. 1972;112:1026–1028. doi: 10.1128/jb.112.2.1026-1028.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoch J A. Regulation of the phosphorelay and the initiation of sporulation in Bacillus subtilis. Annu Rev Microbiol. 1993;47:441–465. doi: 10.1146/annurev.mi.47.100193.002301. [DOI] [PubMed] [Google Scholar]
- 10.Ito J, Spizizen J. Genetic studies of catabolite repression insensitive sporulation mutants of Bacillus subtilis. Colloq Int Cent Nat Rech Sci. 1973;227:81–82. [Google Scholar]
- 11.Kallio P T, Fagelson J E, Hoch J A, Strauch M A. The transition state regulator Hpr of Bacillus subtilis is a DNA-binding protein. J Biol Chem. 1991;266:13411–13417. [PubMed] [Google Scholar]
- 11a.Koide, A. Unpublished data.
- 12.Koide A, Hoch J A. Identification of a second oligopeptide transport system in Bacillus subtilis and determination of its role in sporulation. Mol Microbiol. 1994;13:417–426. doi: 10.1111/j.1365-2958.1994.tb00436.x. [DOI] [PubMed] [Google Scholar]
- 13.Mandic-Mulec I, Doukhan L, Smith I. The Bacillus subtilis SinR protein is a repressor of the key sporulation gene spo0A. J Bacteriol. 1998;177:4619–4627. doi: 10.1128/jb.177.16.4619-4627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandic-Mulec I, Gaur N, Bai U, Smith I. Sin, a stage-specific repressor of cellular differentiation. J Bacteriol. 1992;174:3561–3569. doi: 10.1128/jb.174.11.3561-3569.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 16.Marmur J. A procedure for the isolation of deoxyribonucleic acid from microorganisms. J Mol Biol. 1961;3:208–218. [Google Scholar]
- 17.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 352–355. [Google Scholar]
- 18.Miller P F, Sulavik M C. Overlaps and parallels in the regulation of intrinsic multiple-antibiotic resistance in Escherichia coli. Mol Microbiol. 1996;21:441–448. doi: 10.1111/j.1365-2958.1996.tb02553.x. [DOI] [PubMed] [Google Scholar]
- 19.Ohlsen K L, Grimsley J K, Hoch J A. Deactivation of the sporulation transcription factor Spo0A by the Spo0E protein phosphatase. Proc Natl Acad Sci USA. 1994;91:1756–1760. doi: 10.1073/pnas.91.5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perego M. Integrational vectors for genetic manipulation in Bacillus subtilis. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C: American Society for Microbiology; 1993. pp. 615–624. [Google Scholar]
- 21.Perego M. A peptide export-import control circuit modulating bacterial development regulates protein phosphatases of the phosphorelay. Proc Natl Acad Sci USA. 1997;94:8612–8617. doi: 10.1073/pnas.94.16.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perego M. Kinase-phosphatase competition regulates Bacillus subtilis development. Trends Microbiol. 1998;6:366–370. doi: 10.1016/s0966-842x(98)01350-x. [DOI] [PubMed] [Google Scholar]
- 22a.Perego, M. Unpublished data.
- 23.Perego M, Glaser P, Hoch J A. Aspartyl-phosphate phosphatases deactivate the response regulator components of the sporulation signal transduction system in Bacillus subtilis. Mol Microbiol. 1996;19:1151–1157. doi: 10.1111/j.1365-2958.1996.tb02460.x. [DOI] [PubMed] [Google Scholar]
- 24.Perego M, Hanstein C G, Welsh K M, Djavakhishvili T, Glaser P, Hoch J A. Multiple protein aspartate phosphatases provide a mechanism for the integration of diverse signals in the control of development in Bacillus subtilis. Cell. 1994;79:1047–1055. doi: 10.1016/0092-8674(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 25.Perego M, Higgins C F, Pearce S R, Gallagher M P, Hoch J A. The oligopeptide transport system of Bacillus subtilis plays a role in the initiation of sporulation. Mol Microbiol. 1991;5:173–185. doi: 10.1111/j.1365-2958.1991.tb01838.x. [DOI] [PubMed] [Google Scholar]
- 26.Perego M, Hoch J A. Sequence analysis and regulation of the hpr locus, a regulatory gene for protease production and sporulation in Bacillus subtilis. J Bacteriol. 1988;170:2560–2567. doi: 10.1128/jb.170.6.2560-2567.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perego M, Hoch J A. Cell-cell communication regulates the effects of protein aspartate phosphatases on the phosphorelay controlling development in Bacillus subtilis. Proc Natl Acad Sci USA. 1996;93:1549–1553. doi: 10.1073/pnas.93.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudner D Z, Ladeaux J R, Breton K, Grossman A D. The spo0K locus of Bacillus subtilis is homologous to the oligopeptide permease locus and is required for sporulation and competence. J Bacteriol. 1991;173:1388–1398. doi: 10.1128/jb.173.4.1388-1398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaeffer P, Millet J, Aubert J P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci USA. 1965;54:701–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tame J R H, Murshudov G N, Dodson E J, Neil T K, Dodson G G, Higgins C F, Wilkinson A J. The structural basis of sequence-independent peptide binding by OppA protein. Science. 1994;264:1578–1581. doi: 10.1126/science.8202710. [DOI] [PubMed] [Google Scholar]