Abstract
Advances in assisted reproductive technologies in rhesus macaques have allowed the development of valuable models of human disease, particularly when combined with recent techniques for gene editing. While the ability to perform in vitro fertilization (IVF) in rhesus macaques is well established, this procedure has not yet been optimized. Specifically, damage to the sperm caused by cryopreservation (cryodamage) may lead to unsuccessful artificial insemination and low fertilization and blastocyst formation rates in vitro. To address this, we systematically assessed 2 cryopreservation methods and 4 recovery methods in the following 3 interdependent experiments: 1) comparing sperm survival after vitrification or slow-freezing; 2) comparing simple wash (SW), density gradient centrifugation (DGC), swim-up (SU), and glass wool filtration (GWF) for removal of cryoprotectants and isolation of motile sperm after thawing; and 3) evaluating the efficacy for IVF of the 2 best methods of isolating thawed sperm. We found that after vitrification, only 1.2 ± 0.3% of thawed sperm were motile, whereas after slow-freezing, 42 ± 5% of thawed sperm were motile. SW was significantly better than all other isolation methods for the recovery of total sperm and for the recovery of sperm with an intact plasma membrane. The isolation methods had no significant differences in the recovery of motile sperm or sperm with progressive motility. However, IVF of ova with sperm recovered by DGC resulted in 5% more embryos and 25% more blastocysts than did IVF with sperm recovered by SW. Although additional studies are required to optimize sperm cryopreservation in rhesus macaques, our study showed that slow-freezing, coupled with DGC, provided the highest efficacy in providing functional sperm for in vitro use.
Abbreviations: ARTs, Assisted reproductive technologies; DGC, Density gradient centrifugation; GWF, Glass wool filtration; IA, Intact acrosome; IPM, Intact plasma membrane; IVF, in vitro fertilization; LSD, Least Significant Difference; MHV1, Macacine herpesvirus 1; Oregon National Primate Research Center, ONPRC; SIV, Simian immunodeficiency virus; SRV, Simian retrovirus; STLV, simian T-cell leukemia virus; SU, Swim-up; SW, Simple wash; TH3, TALP-HEPES
Nonhuman primates (NHPs) are critical for biomedical research due to their close genetic and physiologic relationship to humans. In addition, according to the classification criteria proposed by the International Union for the Conservation of Nature, approximately 63% of the 702 species and subspecies of NHP in the world are at some level of risk of extinction.21,31 Thus, efficient assisted reproductive technologies (ARTs) are required to 1) understand primate reproductive processes, 2) advance NHP biomedical model development and 3) advance species conservation efforts.
The rhesus macaque (Macaca mulatta) is one of the most commonly used NHP species for research studies relevant to human health and disease.42 Advances in the use of ARTs in this important species include cryopreservation of sperm;41 artificial insemination;34 in vitro fertilization (IVF);3 and intracytoplasmic sperm injection (ICSI).30 Establishing effective and reproducible rhesus macaque ARTs is critical in allowing the birth of live offspring after blastomere nuclear transfer,28 somatic cell nuclear transfer,10 mitochondrial replacement in oocytes,43 and autologous grafting of prepubertal rhesus testis, followed by ICSI and embryo development.16 Moreover, recent advances in gene editing now allow NHP genome manipulation in one cell embryos (zygotes) for the subsequent generation of transgenic models of human disease.11
Genetic resource banking or cryobanking, (the low temperature storage of gametes, embryos, and tissues) is crucial to preserving genetic material for future use in ARTs. Cryobanking allows the preservation of material from genetically valuable individuals independent of the long-term goal for its use (biomedical models, domestic species breeding programs, species conservation, etc.).6 Moreover, cryobanking makes sperm readily available when needed, without the need to maintain live male sperm donors or personnel who are trained to collect fresh sperm.
However, spermatozoa cryopreservation and recovery of viable sperm after thawing is still a major concern in NHP ARTs and would benefit from further improvement.50 Although several studies have investigated the optimal conditions for primate sperm preservation,12-14,25,33,39-41,53,54 major gaps exist in our understanding of what is critical for promoting the survival of sperm after thawing. For example, IVF success using fresh sperm varied between 50% to 61%50,51 with 55% of the embryos reaching the blastocyst stage,51 whereas IVF with cryopreserved sperm resulted in 82 ± 13% of the ova developing into embryos, of which only 39 ± 21% reached the blastocyst stage.41 One process that needs further optimization in rhesus macaques is sperm vitrification. Vitrification is the process of obtaining a glass-like solid to avoid ice crystal formation; it is achieved either by rapid cooling or using additives.52 Although successful vitrification of human sperm has been reported,19,20 the only study that assessed sperm vitrification in rhesus macaques reported no motility after thawing.29 Therefore, further studies are necessary on how to successfully cryopreserve sperm from rhesus macaques.
An important factor to consider with regard to sperm cryopreservation is how to isolate good quality sperm after thawing (that is, progressively motile sperm with an intact plasma membrane and acrosome and low levels of DNA fragmentation). Several studies have compared different sperm preparation methods in various species, including bulls,2,23 humans,15 and dogs,22 but no studies to date have systematically compared various methods in rhesus macaques.
Therefore, the objectives of the current study were to 1) compare 2 methods for cryopreservation of sperm from rhesus macaques with regard to sperm viability characteristics after thawing; 2) compare 4 different methods of removal of cryopreservation reagents and sperm isolation after thawing, and (3) evaluate the overall efficacy of the 2 best sperm isolation methods for use in IVF.
Materials and Methods
Experimental design.
This study was divided into 3 interdependent experiments, as shown in Figure 1.
Figure 1.
Experimental outline.
Animals.
All animals used in this study were Indian-origin rhesus macaques, housed indoors at the Oregon National Primate Research Center (ONPRC), an AAALAC-accredited facility. Monkeys were subject to a 12-h light cycle (0700 to 1900) year-round, with natural humidity and temperatures were kept at 71°F ± 5, per the Animal Welfare Act, with an active monitoring system in place to notify whenever the temperature is outside of that range. All rhesus macaques were fed a commercial monkey chow twice daily (6 to 7 biscuits/meal according to weight; LabDiet 5000, Purina Mills, St Louis, MS) and had ad libitum access to water. In addition, the monkeys participated in the ONPRC Behavioral Management Plan, which includes enrichment items such as toys, foraging devices and enrichment, frozen treats, TV, radio, mirrors, and removable verandas.
This study included 4 adult males (age range 5.5 to 11 y old and weight range 7.6 to 10.6 kg) that were proven to be fertile in previous IVF studies, and 6 adult females (age range 4.5 to 7.5 y old and weight range 5.8 to 7 kg) with regular menstrual cycles. All macaques were born at ONPRC and deemed healthy, with no evidence of preexisting disease. All females and the 2 younger males were housed in pairs. The other 2 males were single-housed due to social incompatibility with other monkeys. Nine of the macaques were considered SPF (SPF) for simian retrovirus (SRV), simian immunodeficiency virus (SIV), simian T-cell leukemia virus (STLV), and macacine herpesvirus 1 (MHV1). The remaining female was considered SPF for SRV and MHV1. All animal procedures were preapproved by the Institutional Animal Care and Use Committee at the Oregon Health and Science University and followed the guidelines established in the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.18
Semen collection and evaluation.
Semen was collected by a routine penile electroejaculation technique commonly used for rhesus macaques. Briefly, pretrained unsedated males were transferred into a restraint chair using the pole-and-collar method. The male was then taken to a separate room where the semen sample was collected by penile electroejaculation using nonmetal electrodes.35 Samples were collected from the 4 males into separate polystyrene vials (Catalog Number 201-5266-05K, Evergreen Scientific, Los Angeles, CA), with a minimum 3-d interval between collections.49 All collections (N = 4 to 9 samples per male; Table 1) were performed by the same experienced technician between March and May 2015, at 0900. Prior to collection, the cage was checked for the presence of semen. If there was evidence that the male had masturbated that morning, then a different male would be used.
Table 1.
Results (Mean ± SEM) obtained from the analysis of semen collected by penile electroejaculation from 4 sexually mature male Rhesus macaques (Macaca mulatta). N = number of samples collected; IPM = Intact plasma membrane; IA = Intact acrosome.
| Male | Variable | |||||||
|---|---|---|---|---|---|---|---|---|
| Age (y) | N | Volume (µL) | Total Motility (%) | Progressive motility (%) | Concentration (x106 sperm/mL) | IPM (%) | IA (%) | |
| A | 11 | 4 | 317 ± 98 | 87 ± 2 | 77 ± 2 | 583 ± 164 | 39 ± 5 | 79 ± 3 |
| B | 8.5 | 4 | 1111 ± 117 | 91 ± 1 | 86 ± 2 | 465 ± 114 | 65 ± 7 | 93 ± 3 |
| C | 5.5 | 8 | 77 ± 20 | 59 ± 3 | 40 ± 5 | 5468 ± 647 | 65 ± 3 | 85 ± 2 |
| D | 5.5 | 9 | 56 ± 10 | 47 ± 9 | 34 ± 9 | 2136 ± 357 | 58 ± 6 | 87 ± 3 |
| Average | 7.63 | 6.25 | 273 ± 80 | 64 ± 5 | 51 ± 6 | 2686 ± 475 | 58 ± 3 | 86 ± 2 |
The samples were kept at room temperature (22°C) for 30 min to allow separation of the sperm-rich liquid fraction47 and to allow for the quantification of the sample volume before transfer into a centrifuge tube. Semen processing and all analyses were performed by the same person. Ten microliters of sperm were added to 190 µL of prewarmed TALP-HEPES at 37°C (modified Tyrode solution with albumin (3 mg/mL), lactate, and pyruvate; TH3)4 to assess motility, acrosome integrity, plasma membrane integrity, and concentration, as noted below. The medium was kept in a water bath (Thermo Electric Corporation Precision Microprocessor Controlled 280 Series Water Bath, Thermo Fisher Scientific, Waltham, MA) at 37°C throughout the whole processing time.
To assess total and progressive motility, 10 µL of the diluted sample was placed on a microscope slide under a coverslip and observed on the microscope at 400× magnification. Total motility was defined as the percentage of sperm that showed any tail movement, while progressive motility was considered the percentage of sperm that showed active movement, both linear or in large circles.49 To assess acrosome and plasma membrane integrity,1 3 µL of diluted sperm were mixed with 3 µL of eosin-fast green stain, placed on a slide, air dried on a warm plate at 37°C, and 200 sperm were counted under oil immersion (1000× magnification). Sperm were classified as follows: 1) stained (whole head stained pink), indicating that both the acrosome and the plasma membrane integrity are compromised; 2) half-stained (postacrosomal region stained pink), indicating that the acrosome is intact, but the plasma membrane integrity is compromised; and 3) unstained (whole head is unstained or white), indicating that both the acrosome and the plasma membrane are intact.
To assess sperm concentration, 10 µL of the diluted sample were further diluted with distilled water to immobilize sperm. The dilution factor varied between samples, depending on the concentration of spermatozoa. An initial dilution of 1:20 was performed and if the sample was too concentrated to allow counting, the sample was further diluted until a countable concentration was achieved. Ten µL of diluted sample was added to each chamber of a hemocytometer, and the number of sperm present in 5 squares of the center grid were counted. This number was used to calculate total sperm concentration.
After the initial analysis of motility, acrosome and plasma membrane integrity, and sperm concentration, the semen sample was divided into 2 equal fractions for cryopreservation by either slow-freezing or vitrification. Poor quality samples (that is, samples with low volume, low concentration, low motility, or a high number of defective cells) were discarded because they did not provide enough viable sperm to allow 2 treatment groups at a concentration of 50 × 106 motile sperm/mL
Oocyte collection, evaluation, and preparation.
Controlled ovarian stimulation and oocyte recovery was performed as previously described.32 Briefly, female rhesus macaques were checked daily for menses. Once menses was observed (Day 1 of the cycle), follicular stimulation was started between cycle days 1 and 4. The stimulation protocol consisted of twice-daily injections of recombinant human follicle stimulating hormone (FSH) (30 IU intramuscularly; Merck and Co.-Organon, West Orange, NJ) for 8 consecutive days, as well as recombinant human luteinizing hormone (LH) (30 IU, intramuscularly; Merck-Serono Reproductive Biology Institute, Rockland, MA) twice daily on Days 7 and 8, and one dose of recombinant human chorionic gonadotropin (hCG) on Day 8 (1000 IU, intramuscularly; Novarel, Ferring Pharmaceuticals, Parsippany, NJ). Circulating estradiol levels were measured by the ONPRC Endocrine Technology Core (ETC) daily during the course of the stimulation protocol by analysis of serum obtained from blood collected by femoral venipuncture. For blood collections, females were pretrained for awake collections using a restraint tower. Serum samples were analyzed using a chemiluminescence-based automatic clinical platform (Roche Diagnostics Cobas e411, Indianapolis, IN). When estradiol was above 200 ng/dL, animals also received one injection of Antide, a GnRH antagonist (0.5 mg/kg, subcutaneously; Salk Institute for Biologic Studies, La Jolla, CA), to prevent a spontaneous LH surge. Each animal may undergo up to 5 follicle aspirations with at least one complete menstrual cycle of rest between each collection. The number of follicle aspirations depends on the individual response to the ovarian stimulation and the surgeon’s assessment at the time of surgery.
The stimulations and surgeries were performed between the months of October 2015 and January 2016. Follicle aspirations were carried out in the morning, 36 h after the hCG injection, by video laparoscopy (5 mm RW rigid endoscope model number 8935.441; camera/camera box 3CCD Endocam 5516; Insufflator Laparo CO2-PNEU 2232; Light source Endolight LED1.1 – Richard Wolf, Vernon Hills, IL). Food was withheld from animals for approximately 12 h (overnight) before surgery. Prior to surgery, animals received a combination of ketamine (8 to 20 mg/kg) and glycopyrrolate (0.01 to 0.2 mg/kg) administered intramuscularly. After sedation, animals received a local block consisting of a combination of 0.5% bupivacaine (0.4 mL) and 1% lidocaine (0.1 mL) administered intradermally at the incision sites. Animals also received hydromorphone HCl (0.025 to 0.2 mg/kg) intravenously before and after surgery. For anesthesia, animals were endotracheally intubated (size 4.0 to 6.0 endotracheal tube), induced to surgical depth with 3% isoflurane and maintained with 1% to 2% isoflurane, as needed (inhalant anesthesia machine, Aestiva/5 Datex-Ohmeda, Madison, WI). Postoperative analgesia consisted of hydromorphone HCl (0.05-0.4 mg/kg, intramuscularly, 3 times a day) and buprenorphine (0.01 to 0.1 mg/kg, intramuscularly, once a day) for 48 to 72 h at the discretion of the surgical staff.
Cumulus-oocyte complexes were aspirated from ovarian follicles into warm TH3 medium at 37°C using a 22 gauge SS needle at 60 to 110 mm Hg vacuum. Tubes containing follicular aspirates were placed in a portable incubator at 37°C (Ref: 19180/0001, Minitube USA, Delavan, WI) for transportation to the laboratory. Hyaluronidase (0.5 mg/mL in TH3) was added directly to the tubes containing aspirates and incubated at 37°C for 30 s with gentle agitation using a serological pipette to disrupt the cumulus-oocyte complexes. The aspirate was then poured through a 0.2 µm cell strainer. Oocytes were retained in the strainer mesh, while blood, cumulus, and granulosa cells passed through the filter. The strainer was immediately backwashed with TH3 and the medium containing oocytes was collected in a culture dish. Residual cumulus cells surrounding oocytes were removed with a small-bore pipette before examination to determine developmental stage and quality.
Recovered oocytes were then placed in 8-well IVF dishes (Universal GPS Dish, CE 0086, LifeGlobal Group, LLC –) containing 100 µl of preequilibrated TALP (TALP + 0.3 g BSA + 0.006 g pyruvate/100 mL solution equilibrated for at least 2 h at 37°C and 5% CO2) per well, covered with approximately 9 mL of paraffin oil (OVOIL-100, Vitrolife, Englewood, CO), and incubated at 37°C in 5% CO2 (NUAIRE NU-5510 Series 6, Plymouth, MN) for 5 to 7 h before being inseminated.3 Each well held 3 to 5 eggs, depending on the number of eggs collected.
Experiment 1 – Comparison of Two Different Freezing Methods.
This experiment compared a slow-freezing method and a vitrification method for the cryopreservation of spermatozoa from rhesus macaques.
The slow-freezing method was performed based on previously detailed protocols.12-14,24,45 Specifically for this study, all semen samples were first diluted with TEST-yolk buffer without glycerol45 (kindly provided by Dr Catherine A VandeVoort from the California National Primate Research Center of University of California, Davis) to a final concentration of 100 × 106 motile sperm/mL. TEST-yolk buffer containing 6% glycerol was then added at a rate of 1:1, to obtain final concentrations of 3% glycerol and 50 × 106 motile sperm/mL. The diluted semen was loaded into 0.25 mL French straws and sealed with polyvinyl powder. The sealed straws were placed in a 600-mL glass beaker containing 500 mL of distilled water at room temperature and equilibrated at 4°C for 2 h, resulting in a cooling rate of -0.5°C/min from 22°C to 4°C.24 After equilibration, samples were submitted to nitrogen vapor for 10 min, with a cooling rate of approximately -220°C/min from -10°C to -70°C, by placing the straws on a 1-cm thick Styrofoam “boat,” placed in a Styrofoam box (inside dimensions: 20.5 × 15.5 × 21.3 cm) with 4 cm of liquid nitrogen.14 The straws were then plunged into liquid nitrogen and stored until analysis after thawing.
The vitrification method was adapted from a previous study.19 The semen sample was diluted with HTF-HSA medium (Human Tubal Fluid medium with 1% Human Serum Albumin) to a final concentration of 100 × 106 sperm/mL. The sperm sample was then diluted 1:1 with a solution of 0.5 M sucrose, to obtain a final concentration of 0.25 M sucrose19 and 50 × 106 sperm/mL.14 For the vitrification procedure, a 0.25-mL straw was cut down to an 8-cm length, and filled with 100 µL of semen solution. The straw was kept in a horizontal position until frozen to prevent semen from flowing out of the straw. The cut straw was then placed in a 0.5 mL packaging straw, cut down to a 10-cm length and sealed on one end. The remaining end of the packaging straw was sealed and the straw packaging system was submerged directly in liquid nitrogen for 5 s and stored until analysis after thawing.
The samples cryopreserved by slow-freezing were removed from the liquid nitrogen, immediately immersed in a water bath (Thermo Electric Corporation Precision Microprocessor Controlled 280 Series Water Bath, Thermo Fisher Scientific, Waltham, MA) at 37°C for 30 s, and then transferred into a centrifuge tube. For warming of the vitrified samples, the top of the straw packaging system was lifted from the nitrogen and the end of the packaging straw was cut. Then the straw with the sample was removed from the packaging straw by using a 200 µL pipette tip and was immediately immersed in a plastic centrifuge tube containing 1 mL of prewarmed TH3 (adapted from the previous study19). After thawing, samples from both freeze techniques were analyzed as described above.
Experiment 2 - Comparison of Preparation Methods for Thawed Sperm.
Sperm from Experiment 1 that had the best yield after thawing (slow-freezing method) was subjected to 4 different sperm preparation methods after thawing, including 1) simple washing, 2) direct swim-up, 3) density gradient centrifugation, and 4) glass wool filtration. Semen from all 4 males was pooled to reduce biologic variation due to possible differences in the sperm tolerance to freezing among the males, a phenomenon that has been previously described in both rhesus macaques and other species.24,53 Pooling the samples prevents individual differences in sperm tolerance to freezing from affecting the results of each preparation method. One sample from each male was thawed and pooled into a 15-mL conical tube. The pooled sample was immediately diluted 1:1 with TH3 to minimize cryoprotectant toxicity and was analyzed as detailed above for total and progressive motility, plasma membrane integrity, acrosome integrity, and sperm concentration. The pooled sample was then divided into 4 aliquots and submitted to the different preparation protocols. A total of 5 replicates were done using different samples. Samples were again analyzed as described above. For comparison of the preparation methods, we calculated the actual number of sperm with each characteristic by multiplying the percentage of sperm observed and the concentration of sperm in each sample. We also calculated the recovery rate of sperm with each characteristic by dividing the number of sperm after isolation by the number of sperm in the original pool. The protocols for simple washing, direct swim-up, and density gradient centrifugation were adapted from the WHO manual.49 The protocol for glass wool filtration was adapted from a previous study.15
For the simple washing (SW) method, the frozen-thawed semen was diluted with 2 mL of warm TH3 medium (37°C) in a 15-mL conical tube and centrifuged at 300 g for 5 min. Supernatant was discarded and another 2 mL of TH3 was added. The sample was centrifuged a second time and the supernatant (1 mL) was discarded.
For the direct swim-up (SU) method, warm TH3 (37°C; 2 mL) was gently layered over the diluted sperm in a 15-mL conical tube, which was tightly sealed to avoid gas exchange and placed in an incubator (NU-5510, Nuaire, Plymouth, MN) at 37°C for 30 min at a 45° angle. After incubation, the uppermost 0.8 mL was transferred to a new tube and washed once with TH3 at 300 g for 5 min. The supernatant was discarded and the sperm pellet was diluted with TH3 to a final volume of 1 mL.
The density gradient centrifugation (DGC) protocol involved placing 1 mL of ISolate Stock Solution (90% density gradient medium, Catalog ID 99275; Irvine Scientific, CA) in a 15-mL conical tube and layering 1 mL of 45% ISolate (Isolate Stock Solution diluted 1:1 with TH3) on top. Diluted semen (1 mL) was layered on top of the second layer. The sample was then centrifuged at 300 g for 20 min (Allegra 6RCentrifuge, Beckman). After centrifugation, the supernatant (2 mL) was discarded and the sample was washed twice with 5 mL TH3 and centrifuged at 200 g for 5 min. The supernatant (5 mL) was discarded after each wash, leaving 1 mL of diluted sperm.
For the glass wool filtration (GWF) method, a 1-mL plastic syringe was cut at the 0.6-mL mark and 15 mg of glass wool (Microstrand glass microfiber code 112, Johns Manville, OH) was inserted in the syringe to a height of 6 mm. The glass wool was washed with 3 mL of TH3 to remove any debris and the tip of the syringe was inserted through the cap of a 1.5-mL centrifuge tube. The diluted semen was layered on top of the glass wool and incubated for 5 min at 37°C. The filtrate was recovered from the bottom of the centrifuge tube and transferred to a 15-mL conical tube. The sample was then washed by centrifugation with 2 mL TH3 at 300 g for 5 min, after which the supernatant was discarded. The recovered sperm pellet was diluted with TH3 to a final volume of 1 mL.
Experiment 3 – Evaluation of Frozen-Thawed Sperm Quality by IVF.
The fertilizing potential of frozen-thawed sperm isolated by the 2 overall best preparation methods from Experiment 2 (SW and DGC) was compared by IVF. Oocytes were inseminated with a pool of frozen-thawed sperm prepared using the 2 different methods. A fresh sperm sample from one of the 2 older males (A or B) was used as control for standard IVF. Insemination was performed with both metaphase I (MI) and metaphase II (MII) stage oocytes, as MI oocytes may mature to MIIs during IVF culture. Before IVF, the oocytes were distributed between treatments based on the stage of maturation. When possible, the distribution was even among all 3 treatments. For calculation of fertilization rates and statistics, only MIs that had matured to MIIs by the next morning were included because those are the only ones that can be fertilized.
Fresh sperm were processed as previously described with slight modifications.51 Briefly, semen was kept at room temperature (22°C) for 30 min to allow separation of the liquid fraction. The liquid fraction was transferred to a 15 mL conical tube and washed twice in 12 mL of TH3 by centrifugation at 400 g for 5 min. After the second wash, the supernatant was discarded, leaving 1 mL of diluted sperm. Concentration and motility were evaluated. A fraction containing 40 × 106 motile spermatozoa was transferred to a new tube and diluted with TALP to a final volume of 2.0 mL (final concentration of 20 x106 sperm/mL). The diluted sperm were kept in an incubator (NUAIRE NU-5510 Series 6, Plymouth, MN) at 37°C and 5% CO2 for 4 to 6 h. Shortly before IVF, the sperm were activated by adding 100 µl of a solution containing 1 mM of dibutyryl cyclic adenosine monophosphate (dbcAMP) and 1mM of caffeine in saline to 900 µL of diluted sperm.9 This resulted in a concentration of 18 × 106 motile sperm/mL.
Straws containing frozen sperm were thawed at 37°C for 30 s and motility was evaluated. Only samples with at least 30% progressive motility were used. After determination of the initial motility, samples were pooled and divided into 2 equal fractions. Each fraction was submitted to SW or DGC, as described above. After sperm preparation, samples were diluted in TALP5 at a concentration of 20 × 106 motile sperm/mL. For activation, sperm were diluted 9:1 (sperm: activator) with saline solution containing 1 mM of dibutyryl cyclic adenosine monophosphate (dbcAMP) and 1mM of caffeine. This resulted in a sperm concentration of 18 × 106 motile sperm/mL.
Before exposure to sperm, oocytes were reevaluated and redistributed into the IVF dish, based on their maturation stage, so that each treatment group had approximately the same number of MI and MII oocytes, depending on the numbers collected. When the oocyte numbers were not divisible by 3, preference was given to the frozen-thawed sperm samples, rather than the fresh sperm samples. One microliter of activated sperm was added to each well, resulting in 18,000 sperm per well or 3,600 to 6,000 motile sperm per egg, depending on the number of eggs (3 to 5 per well). The dishes were then returned to the incubator. After 16 to 18 h of incubation, oocytes were examined for the formation of the pronuclei. Embryo culture was performed as described previously,32,50 with the fertilized oocytes being transferred to fresh drops containing 100 µL of Global embryo culture medium (CE 0086; LifeGlobal Group). Embryos were examined every 24 h and transferred to fresh medium every 48 h, until they reached the blastocyst stage (7 to 8 d).
Statistical Analysis.
Data were analyzed by ANOVA and the Least Significant Difference (LSD) test, using the statistical software SAS System for Windows (SAS Institute – Cary, NC - EUA, 2000). The significance threshold was set at P < 0.05.
Results
Initial Semen Analysis.
A total of 25 semen samples were collected, out of which 9 were deemed unsuitable for freezing due to poor quality (low motility and high percentage of sperm with morphologic defects). Table 1 shows the characteristics of fresh semen samples collected from each of the males used in the current study. As expected, variability was observed between males and between samples from the same male, with semen volumes among all samples ranging from 15 µL to 1450 µL, and sperm concentrations ranging from 249 to 7688 × 106 sperm/mL Total motility among the 4 males ranged from 1% to 90%, while progressive motility ranged from 0% to 90%. Plasma membrane integrity ranged from 27% to 80%, whereas acrosome integrity ranged from 72% to 100%.
Experiment 1 – Comparison of Two Different Freezing Methods.
A total of 16 semen samples were used for this experiment. Figure 2 shows the comparison of results obtained from the analysis of fresh compared with frozen-thawed sperm using 2 different cryopreservation methods (slow freezing and vitrification). Frozen-thawed sperm had significantly lower quality (P < 0.0001) in all parameters evaluated, including percent total motility, progressive motility, intact plasma membrane, and intact acrosome, as compared with fresh sperm, regardless of the freezing method. However, the slow-freezing method yielded significantly better sperm quality (P < 0.0001) for all parameters evaluated as compared with the vitrification method.
Figure 2.

Mean percentage of total motility, progressive motility, intact plasma membrane (IPM), and intact acrosome (IA) in fresh sperm compared with frozen-thawed sperm from rhesus macaques (Macaca mulatta) cryopreserved by slow freezing or vitrification. Error bars represent the standard error of the mean. Different letters above bars indicate significant differences (P < 0.05) between fresh semen and each freezing protocol group per the Least Significant Difference test.
Experiment 2 – Comparison of Sperm Preparation Methods after Thawing.
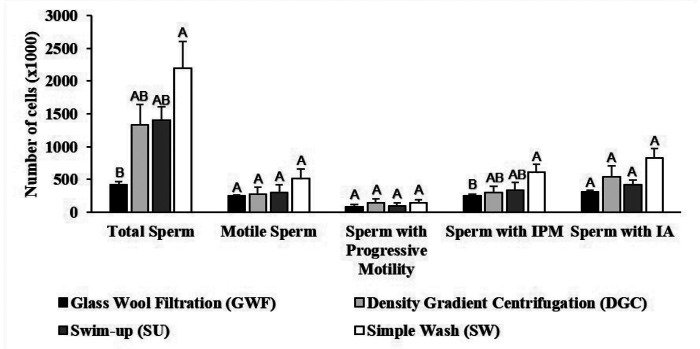
Figure 3 shows the comparison of the sperm preparation methods based on the number of sperm with each characteristic. SW was significantly better than GWF and did not significantly differ from DGC or SU for the total number of sperm and the number of sperm with an intact plasma membrane. The preparation methods did not differ in terms of the number of motile sperm, percentage of sperm with progressive motility, or number of sperm with an intact acrosome.
Figure 3.
Motility and integrity of frozen-thawed sperm prepared by 4 different methods. Error bars represent the standard error of the mean. IPM = Intact Plasma Membrane; IA = Intact Acrosome. Different letters above bars indicate significant differences (P < 0.05) between treatments according to the Least Significant Difference test.
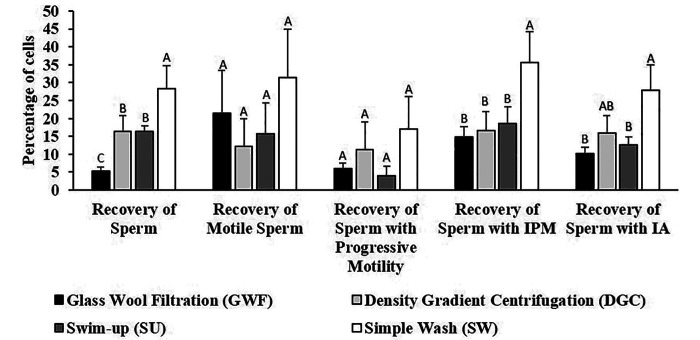
Figure 4 shows the comparison of the sperm preparation methods based on the recovery of sperm with each characteristic. The SW method was significantly better (P < 0.05) than all the other preparation methods for the total recovery of sperm and the recovery of sperm with an intact plasma membrane. There was no significant difference between preparation methods with regard to the recovery of motile sperm and sperm with progressive motility. DGC did not significantly differ from SU for any of the parameters and was significantly better than GWF only for recovery of sperm in total. Based on these results, we chose SW and DGC for comparison of sperm function by IVF (Experiment 3).
Figure 4.
Mean recovery of sperm (%) in relation to the original sample (pool) of frozen-thawed sperm prepared by 4 different methods. Error bars represent the standard error of the mean. IPM = Intact Plasma Membrane; IA = Intact Acrosome. Different letters above bars indicate significant differences (P < 0.05) between treatments according to the Least Significant Difference test.
Experiment 3 – Evaluation of Frozen-Thawed Sperm Quality by IVF.
A total of 7 oocyte collections were obtained from the 6 females. The fertilization outcome after IVF was determined using a fresh sperm sample (control) or a pool of frozen-thawed sperm isolated by either SW or DGC. Three out of 16 samples were rejected for having fewer than 30% motile sperm. Two of the 3 rejected samples were from male C and one was from male A. Statistical analyses revealed a significant interaction (P = 0.0385) between treatment and oocyte stage at the time of insemination with regard to the blastocyst formation rate, thus requiring separate statistical analyses for comparison of fertilization rates of MI and MII oocytes. Oocyte stage had no significant influence on the number of oocytes that cleaved (P = 0.0206) and the number of embryos that became morulas (P = 0.0080), compact morulas (P = 0.0074), and blastocysts (P = 0.0076). Therefore, we performed separate statistical analyses for each oocyte stage (MI and MII) at the time of insemination, as shown on Table 2.
Table 2.
Results of in vitro fertilization (IVF; n = 7 oocyte collections from 6 animals) outcomes using fresh compared with frozen-thawed sperm prepared with 2 different methods. Means in the same row, followed by different letters are significantly different (P < 0.05) according to the LSD test. SEM = standard error of the mean; MI = metaphase I; MII = metaphase II.
| Oocyte stage at IVF | Variable | Fresh sperm | Frozen-thawed sperm | ANOVA P value | ||||
|---|---|---|---|---|---|---|---|---|
| Density gradient Centrifugation | Simple washing | |||||||
| Total | Mean (SEM) | Total | Mean (SEM) | Total | Mean (SEM) | |||
| MI | Number of oocytes | 24 | 4.0 (1.7)A | 16 | 2.3 (0.9)A | 21 | 3.0 (0.9)A | 0.5955 |
| Number that cleaved | 13 | 2.2 (0.9)A | 6 | 0.9 (0.5)AB | 1 | 0.1 (0.1)B | 0.0528 | |
| Number of blastocysts | 3 | 0.5 (0.3)A | 2 | 0.3 (0.2)A | 0 | 0.0A | 0.2697 | |
| Fertilization rate | 13/24 (54%) | 43.5 (16.4)A | 6/16 (38%) | 25.1 (14.2)AB | 1/21 (5%) | 2.0 (2.0)B | 0.0845 | |
| Blastocyst rate | 3/13 (23%) | 10.8 (7.1)A | 2/6 (33%) | 11.9 (7.9)A | 0/1 (0%) | 0.0 (0%)A | 0.3193 | |
| MII | Number of oocytes | 77 | 11.0 (4.6)A | 86 | 12.3 (4.3)A | 84 | 12.0 (4.5)A | 0.9776 |
| Number that cleaved | 27 | 3.9 (1.1)A | 14 | 2.0 (0.6)AB | 9 | 1.3 (0.6)B | 0.0992 | |
| Number of blastocysts | 14 | 2.0 (0.5)A | 5 | 0.7 (0.3)B | 1 | 0.1 (0.1)B | 0.0056 | |
| Fertilization rate | 27/77 (35%) | 41.4 (3.6)A | 14/86 (16%) | 18.4 (6.9)B | 9/84 (11%) | 12.7 (7.7)B | 0.0113 | |
| Blastocyst rate | 14/27 (52%) | 64.7 (13.6)A | 5/14 (36%) | 30.9 (13.8)AB | 1/9 (11%) | 4.7 (4.7)B | 0.0062 | |
For MI stage oocytes at the time of insemination that had become MIIs by the time of the first assessment after insemination, fresh sperm had a significantly higher fertilization rate than SW, but did not significantly differ from DGC (P < 0.05). The blastocyst rate did not differ significantly between treatments. For the oocytes that were at the MII stage at the time of insemination, the fertilization rate for fresh sperm was significantly higher (P < 0.05) than that of frozen-thawed sperm regardless of the treatment used. The blastocyst rate for fresh sperm was significantly higher (P < 0.05) than that of SW, but the SW blastocyst formation rate was not significantly different from the DGC blastocyst formation rate.
Discussion
Initial Semen Analysis.
When considering the individual means shown in the results for fresh semen analysis (Table 1), males C and D clearly had lower quality semen with regards to volume, total and progressive motility, and plasma membrane integrity as compared with males A and B. In addition, although sperm morphology was not part of the analyses performed, in general the semen samples from the younger animals (males C and D - 5.5 y old) had higher number of sperm with morphologic defects than did the older animals (A – 11 y old and B – 8.5 y old). Macaques reach sexual maturity before morphologic sperm maturity and a few years may be necessary until both are attained.46 Sexual maturity occurs in male rhesus macaques around 3 to 4 y of age,17 while the full bodyweight is attained around 8 y of age.7 Although the younger males are considered sexually mature because they are 5.5 y old and both produce ejaculates containing spermatozoa,26 these males have not reached full adult size, and sperm producing potential may be hampered by their relatively young age. Other factors that can influence semen quality and quantity include the time since the last ejaculation, differing technical skill in the animal care staff involved in sample collection, and individual responses to electrostimulation. The time since the last sample collection and operator variability are considered to be minor factors in this study because all animals had a minimum of 3 d rest periods between collections, and the same staff performed the electroejaculation procedure.
Experiment 1 – Comparison of Two Different Freezing Methods.
The results of Experiment 1 (Figure 2) clearly show that the vitrification method used in this study was not an efficient method for cryopreservation of sperm from rhesus macaques. Possibly, the volume of diluted sperm used for vitrification (100 µL) may have been too large,52 or the straw packaging system may have hindered heat transfer, allowing the formation of ice crystals that injure the sperm cells. A possible remedy would be to use smaller volumes of sperm solution in the straw packaging system, as previously described for human sperm.19 On the other hand, a previous study found that dropped microdroplets (40 µL) of sperm diluted in TH3 onto aluminum foil precooled to 160°C over liquid nitrogen vapor also resulted in the unsuccessful vitrification for rhesus macaque sperm, with a yield of 0% motility after thawing.29 The results of these studies indicate that rhesus sperm do not respond to vitrification the same way as human sperm.
For the slow-freezing method, total motility ranged from 10% to 70%, with a mean of 42 ± 5%, similar to the previously reported mean of 45 ± 20%53 and lower than the previously reported means of 56%27 and 64 ± 3%.38 Progressive motility ranged from 1% to 60%, with a mean of 26 ± 5%, similar to the previously reported mean of 30%.27 Plasma membrane integrity ranged from 9% to 48%, with a mean of 21 ± 3%, lower than the published mean of 50%.27 Acrosome integrity ranged from 23% to 75%, with a mean of 43 ± 4%, lower than the reported means of 85 ± 4%38 and 71 ± 1%.53
A previous study used a technique known as directional freezing in which, in contrast to conventional freezing, different gradients of heat transfer are applied to precisely control ice propagation and avoid injury due to intracellular ice crystal formation.38 That study used 5% glycerol, rather than the 3% used in the present study. Their protocol yielded better total motility (64 ± 3% as compared with 42 ± 5% in the present study) and acrosome integrity (85 ± 4% as compared with 43 ± 4% in this study). Although that study did not evaluate plasma membrane integrity, the directional freezing and higher glycerol concentration might have better maintained plasma membrane integrity, which would explain the better results obtained as compared with our study. Sperm with a damaged plasma membrane lose the ability to control fluid and small molecule transport, which can result in loss of both the acrosome and motility.48 Another study that used a slow-freezing protocol with 5% glycerol compared different cooling rates for the equilibration phase (from 37°C to 4°C) and the effect of seminal plasma on cold shock resistance and cryosurvival.53 Slow cooling (-0.4°C/min) yielded better results than did rapid cooling (-16°C/min). The presence of seminal plasma did not generally affect the resistance to cold shock or cryosurvival. However, the presence of seminal plasma did improve motility and acrosome integrity after thawing in individual males. The difference in acrosome integrity cannot be explained by the presence of seminal plasma because our sperm samples also contained seminal plasma. A possible explanation is that the higher glycerol concentration might have been beneficial for the maintenance of acrosome integrity after thawing.
Another study compared the effect of different suprazero (22°C to 0°C) and subzero (0°C to -110°C) cooling rates on sperm quality after thawing, using the same extender (Test-yolk buffer with 3% glycerol) used in the current study.27 That study found that suprazero cooling rates have a greater influence on quality after thawing as compared with subzero cooling rates. In addition, the study found that slower suprazero freezing rates lead to less plasma membrane injury and less lipid peroxidation caused by temperature shock as compared with faster cooling rates. In our study, the cooling rate from 22 to 4°C was approximately 0.5°C/min, the same rate as the slow cooling rate of the referenced study.27 Although the freezing protocols and extenders were the same, our initial analyses revealed that we started our studies with an inferior semen quality; this would explain the lower quality we obtained after thawing.
Experiment 2 – Comparison of Post-Thaw Sperm Preparation Methods.
Due to the higher sperm survival, the slow freezing method was chosen for the comparison of the different preparation methods of thawed sperm. When comparing the sperm preparation methods, if we had considered only the initial percentages, as in previous studies,2,15,22,23 we would arrive at the false conclusion that GWF was the most efficient method because the percentages do not take into account the actual number of sperm. For example, the mean total motility in the sperm pool was 35 ± 11%, while the mean total motility obtained by GWF was 62 ± 9%, giving the impression that GWF has more motile sperm than the original pool of sperm, which is not possible. However, comparing the actual number of sperm with total motility in the pool (3431.40 × 103 sperm) with the number obtained by GWF (251.60 × 103 sperm) reveals that using only the percentage can be deceiving. Therefore, comparing the actual number of sperm (Figure 3) and the recovery rates (Figure 4) revealed that the glass wool was retaining not only defective and dead sperm, but also healthy sperm. Thus, according to the analyses performed, the most efficient method was SW and the GWF method was less efficient. The recovery rate of sperm with intact acrosome was not significantly different between DGC and SU.
Experiment 3 – Evaluation of Frozen-Thawed Sperm Quality by IVF.
Usually, IVF success in rhesus macaques is determined by combining the results of both MI and MII oocytes because ultimately, they must become MIIs to be fertilized. However, because of a significant interaction between treatment and oocyte stage at the time of insemination, we performed separate statistical analyses for comparison of rates of MI and MII oocytes (Table 2). Studies of IVF in rhesus macaques using fresh sperm report fertilization rates from as low as 50%51 to as high as 88%36 and 89%55 and blastocyst rates from 48% to 61%.36 The fertilization rates obtained in our study were 44 ± 16% (MI) and 41 ± 4% (MII), which are similar to each other, but are significantly lower than in the aforementioned reports, despite using similar protocols. We could not determine the cause for our low IVF rate because the animals used in this study had previously been used in other studies that yielded higher fertilization rates. On the other hand, the blastocyst rates were 11 ± 7% (MI) and 65 ± 14% (MII). The rates we obtained for MII oocytes were comparable to those reported previously,36 as opposed to those we obtained for MIs, which were low. The same outcomes occurred when we used frozen-thawed sperm. The immature oocytes (GVs and MIs) possibly were not fully competent for blastocyst development due to incomplete or inadequate cytoplasmic maturation or the transition through embryonic genome activation (EGA).37 This could explain why even though we obtained high fertilization rates for MI oocytes, their blastocyst formation rate for both fresh and frozen-thawed sperm was lower than what was observed with MII oocytes.
The literature contains 3 reports on IVF using frozen-thawed sperm from rhesus macaques. One study compared 2 sperm cryopreservation protocols and performed IVF using sperm from the protocol with the best results. The selected protocol (TES, Tris, egg yolk base; TTE) differed from our protocol (TEST-yolk buffer) in the following aspects: it used lactose and raffinose in addition to glucose, 5% glycerol instead of 3%; and an equilibration period of 30 min instead of 2 h. Samples were thawed using the same protocol in both cases. The reported fertilization rate was 82 ± 13% and the blastocyst rate was 39 ± 21%.41 A second study used a Tris-egg yolk-based extender with 5% glycerol to pellet-freeze sperm from rhesus macaques. The ability of the frozen-thawed sperm to fertilize oocytes was then evaluated in vivo (artificial insemination) and in vitro (IVF). The in vitro fertilization rate was 64%; blastocyst rates were not reported.34 The third study also compared 2 sperm freezing methods – conventional freezing and directional freezing for use in IVF. The fertilization rates were 79 ± 13% (conventional freezing), and 79 ± 10% (directional freezing), while the blastocyst rates were 34 ± 7% (conventional freezing), and 42 ± 15% (directional freezing).38 All fertilization rates reported above were higher than those we obtained in the current study, which were 25 ± 14% (MI) and 18 ± 7% (MII), reflecting the same results observed with fresh sperm. Our blastocyst rate for MIIs (31 ± 14%) was similar to that obtained for sperm frozen using TTE and conventional freezing and lower than what was obtained with directional freezing. However, our blastocyst rate for MIs (12 ± 8%) was lower than all of the above. The first study described above was performed using immature GV oocytes matured in vitro.41 Although the study found a high fertilization rate, the blastocyst rate was relatively low, which corroborates our previous suggestion that in vitro matured oocytes have inferior developmental competence as compared with in vivo matured oocytes, as has also been described for bovine oocytes.8
This study allows the following conclusions. The vitrification protocol used in this study is not a viable option for cryopreservation of sperm from rhesus macaques and, therefore, the standard slow freezing protocol remains the best option for long term storage of sperm from this species. Based on our direct and controlled comparison of sperm preparation methods after thawing, the SW and the DGC methods were adequate for sperm isolation as compared with the GWF and the SU methods. Sperm isolation by DGC yielded sperm with greater capacity for IVF as compared with SW. Finally, frozen-thawed sperm were able to fertilize oocytes, with the resultant embryos capable of developing to the blastocyst stage. More work is needed to achieve comparable IVF results for frozen-thawed and fresh sperm. For example, according to a recent review,44 new vitrification systems are constantly being developed and must be assessed for their ability to effectively cryopreserve rhesus macaque sperm. Moreover, future studies are necessary to assess individual variables of cryopreservation to improve the efficiency of each step, including variables of sample volume, sperm concentration, cryoprotectant concentration, and rates of freezing and thawing temperature changes. Although many aspects of rhesus macaque sperm cryopreservation require continued refinement, this study provides the first direct comparison of vitrification and separation methods in terms of sperm structure-function.
Acknowledgments
The authors would like to thank the São Paulo Research Foundation (FAPESP, Grant no.2014/15847-1) and NIH P51 OD011092 (Support for National Primate Research Center and Cores) for their financial support. We would also like to thank the Division of Comparative Medicine, the Endocrine Core and the Assisted Reproductive Technologies (ART) Core at the Oregon National Primate Research Center for their services and support. We are also grateful for the contribution of the Fast Green-Eosin dye from the late Dr Naida Loskutoff previously from the Henry Doorly Zoo in Omaha, Nebraska as well as for the Test-yolk buffer provided by Dr Catherine A VandeVoort from the California National Primate Research Center of the University of California, Davis.
References
- 1.Aalseth EP, Saacke RG. 1986. Vital staining and acrosomal evaluation of bovine sperm. Gamete Res 15:73–81. 10.1002/mrd.1120150108. [DOI] [Google Scholar]
- 2.Arzondo MM, Caballero JN, Marin-Briggiler CI, Dalvit G, Cetica PD, Vazquez-Levin MH. 2012. Glass wool filtration of bull cryopreserved semen: a rapid and effective method to obtain a high percentage of functional sperm. Theriogenology 78:201–209. 10.1016/j.theriogenology.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Bavister BD, Boatman DE, Collins K, Dierschke DJ, Eisele SG. 1984. Birth of rhesus monkey infant after in vitro fertilization and nonsurgical embryo transfer. Proc Natl Acad Sci USA 81:2218–2222. 10.1073/pnas.81.7.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bavister BD, Leibfried ML, Lieberman G. 1983. Development of preimplantation embryos of the golden hamster in a defined culture medium. Biol Reprod 28:235–247. 10.1095/biolreprod28.1.235. [DOI] [PubMed] [Google Scholar]
- 5.Bavister BD, Yanagimachi R. 1977. The effects of sperm extracts and energy sources on the motility and acrosome reaction of hamster spermatozoa in vitro. Biol Reprod 16:228–237. 10.1095/biolreprod16.2.228. [DOI] [PubMed] [Google Scholar]
- 6.Bennet PM. 2001. Establishing animal germplasm resource banks for wildlife conservation: genetic, population, and evolutionary aspects, p 47–67. Chapter 4. In: Watson PF, Holt WV, editors. Cryobanking the genetic resource: wildlife conservation for the future? London: Taylor and Francis. [Google Scholar]
- 7.Bercovitch FB, Widdig A, Trefilov A, Kessler MJ, Berard JD, Schmidtke J, Nürnberg P, Krawczak M. 2003. A longitudinal study of age-specific reproductive output and body condition among male rhesus macaques, Macaca mulatta. Naturwissenschaften 90:309–312. 10.1007/s00114-003-0436-1. [DOI] [PubMed] [Google Scholar]
- 8.Blanco MR, Demyda S, Millán MM, Genero E. 2011. Developmental competence of in vivo and in vitro matured oocytes: a review. Biotechnol Mol Biol Rev 6:155–165. [Google Scholar]
- 9.Boatman DE, Bavister BD. 1984. Stimulation of rhesus monkey sperm capacitation by cyclic nucleotide mediators. J Reprod Fertil 71:357–366. 10.1530/jrf.0.0710357. [DOI] [PubMed] [Google Scholar]
- 10.Byrne JA, Pedersen DA, Clepper LL, Nelson M, Sanger WG, Gokhale S, Wolf DP, Mitalipov SM. 2007. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature 450:497–502. 10.1038/nature06357. Erratum in Nature 2014. 516:276. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Niu Y, Ji W. 2016. Genome editing in nonhuman primates: approach to generating human disease models. J Intern Med 280:246–251. 10.1111/joim.12469. [DOI] [PubMed] [Google Scholar]
- 12.Dong Q, Correa LM, VandeVoort CA. 2009. Rhesus monkey sperm cryopreservation with TEST-yolk extender in the absence of permeable cryoprotectant. Cryobiology 58:20–27. 10.1016/j.cryobiol.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong Q, Hill D, VandeVoort CA. 2009. Interactions among pre-cooling, cryoprotectant, cooling, and thawing for sperm cryopreservation in rhesus monkeys. Cryobiology 59:268–274. 10.1016/j.cryobiol.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong Q, Rodenburg SE, Huang C, VandeVoort CA. 2008. Effect of pre-freezing conditions on semen cryopreservation in rhesus monkeys. Theriogenology 70:61–69. 10.1016/j.theriogenology.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engel S, Weber H, Petzoldt R, Seidl B, Wiehe W, Sperl J. 2001. An improved method of sperm selection by glass wool filtration. Andrologia 33:223–230. 10.1046/j.1439-0272.2001.00434.x. [DOI] [PubMed] [Google Scholar]
- 16.Fayomi AP, Peters K, Sukhwani M, Valli-Pulaski H, Shetty G, Meistrich ML, Houser L, Robertson N, Roberts V, Ramsey C, Hanna C, Hennebold JD, Dobrinski I, Orwig KE. 2019. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science 363:1314–1319. 10.1126/science.aav2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fortman JD, Hewett TA, Bennett BT. 2002. The laboratory nonhuman primate. Boca Raton (FL): CRC Press. [Google Scholar]
- 18.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 19.Isachenko E, Isachenko V, Sanchez R, Katlov II, Kreinberg R. 2011. Cryopreservation of spermatozoa, p 176–198. Chapter 14. In: Donnez J, Kim SS, editors. Principles and practice of fertility preservation. New York (NY): Cambridge University Press. [Google Scholar]
- 20.Isachenko V, Isachenko E, Petrunkina AM, Sanchez R. 2012. Human spermatozoa vitrified in the absence of permeable cryoprotectants: birth of two healthy babies. Reprod Fertil Dev 24:323–326. 10.1071/RD11061. [DOI] [PubMed] [Google Scholar]
- 21.IUCN. [Internet]. 2012. IUCN red list categories and criteria. [Cited 16 December 2019]. Available at: https://www.iucnredlist.org/resources/categories-and-criteria
- 22.Kim SH, Yu DH, Kim YJ. 2010. Apoptosis-like change, ROS, and DNA status in cryopreserved canine sperm recovered by glass wool filtration and Percoll gradient centrifugation techniques. Anim Reprod Sci 119:106–114. 10.1016/j.anireprosci.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Lee H-L, Kim S-H, Ji D-B, Kim Y-J. 2009. A comparative study of Sephadex, glass wool and Percoll separation techniques on sperm quality and IVF results for cryopreserved bovine semen. J Vet Sci 10:249–255. 10.4142/jvs.2009.10.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leibo SP, Kubisch HM, Schramm RD, Harrison RM, VandeVoort CA. 2007. Male-to-male differences in post-thaw motility of rhesus spermatozoa after cryopreservation of replicate ejaculates. J Med Primatol 36:151–163. 10.1111/j.1600-0684.2006.00194.x. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Cai K, Li J, Dinnyes A, Ji W. 2006. Comparative studies with six extenders for sperm cryopreservation in the cynomolgus monkey (Macaca fascicularis) and rhesus monkey (Macaca mulatta). Am J Primatol 68:39–49. 10.1002/ajp.20205. [DOI] [PubMed] [Google Scholar]
- 26.Luetjens CM, Weinbauer GF. 2012. Functional assessment of sexual maturity in male macaques (Macaca fascicularis). Regul Toxicol Pharmacol 63:391–400. 10.1016/j.yrtph.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Martorana K, Klooster K, Meyers S. 2014. Suprazero cooling rate, rather than freezing rate, determines post thaw quality of rhesus macaque sperm. Theriogenology 81:381–388. 10.1016/j.theriogenology.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meng L, Ely JJ, Stouffer RL, Wolf DP. 1997. Rhesus monkeys produced by nuclear transfer. Biol Reprod 57:454–459. 10.1095/biolreprod57.2.454. [DOI] [PubMed] [Google Scholar]
- 29.Nichols SM, Bavister BD. 2006. Comparison of protocols for cryopreservation of rhesus monkey spermatozoa by post-thaw motility recovery and hyperactivation. Reprod Fertil Dev 18:777–780. 10.1071/RD06019. [DOI] [PubMed] [Google Scholar]
- 30.Nusser KD, Mitalipov S, Widmann A, Gerami-Naini B, Yeoman RR, Wolf DP. 2001. Developmental competence of oocytes after ICSI in the rhesus monkey. Hum Reprod 16:130–137. 10.1093/humrep/16.1.130. [DOI] [PubMed] [Google Scholar]
- 31.Primate Specialist Group. [Internet]. 2018. Primate diversity by region. [Cited 16 December 2019]. Available at: http://www.primate-sg.org/primate_diversity_by_region/
- 32.Ramsey C, Hanna C. 2019. In vitro culture of rhesus macaque (Macaca mulatta) embryos. Methods Mol Biol 2006: 341–353. doi: 10.1007/978-1-4939-9566-0_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roussel JD, Austin CR. 1967. Preservation of primate spermatozoa by freezing. J Reprod Fertil 13:333–335. 10.1530/jrf.0.0130333. [DOI] [PubMed] [Google Scholar]
- 34.Sánchez-Partida LG, Maginnis G, Dominko T, Martinovich C, McVay B, Fanton J, Schatten G. 2000. Live rhesus offspring by artificial insemination using fresh sperm and cryopreserved sperm. Biol Reprod 63:1092–1097. 10.1095/biolreprod63.4.1092. [DOI] [PubMed] [Google Scholar]
- 35.Sarason RL, VandeVoort CA, Mader DR, Overstreet JW. 1991. The use of nonmetal electrodes in electroejaculation of restrained but unanesthetized macaques. J Med Primatol 20:122–125. 10.1111/j.1600-0684.1991.tb00503.x. [DOI] [PubMed] [Google Scholar]
- 36.Schramm RD, Bavister BD. 1996. Development of in-vitro-fertilized primate embryos into blastocysts in a chemically defined, protein-free culture medium. Hum Reprod 11:1690–1697. 10.1093/oxfordjournals.humrep.a019471. [DOI] [PubMed] [Google Scholar]
- 37.Schramm RD, Bavister BD. 1999. Onset of nucleolar and extranucleolar transcription and expression of fibrillarin in macaque embryos developing in vitro. Biol Reprod 60:721–728. 10.1095/biolreprod60.3.721. [DOI] [PubMed] [Google Scholar]
- 38.Si W, Lu Y, He X, Ji S, Niu Y, Tan T, Ji W. 2010. Directional freezing as an alternative method for cryopreserving rhesus macaque (Macaca mulatta) sperm. Theriogenology 74:1431–1438. 10.1016/j.theriogenology.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 39.Si W, Wang H, Reid C, Hildebrandt TB, Ji W. 2006. Effect of sugar type on the survival of frozen-thawed rhesus monkey (Macaca mulatta) sperm. Am J Primatol 68:103–108. 10.1002/ajp.20209. [DOI] [PubMed] [Google Scholar]
- 40.Si W, Zheng P, Li Y, Dinnyes A, Ji W. 2004. Effect of glycerol and dimethyl sulfoxide on cryopreservation of rhesus monkey (Macaca mulatta) sperm. Am J Primatol 62:301–306. 10.1002/ajp.20023. [DOI] [PubMed] [Google Scholar]
- 41.Si W, Zheng P, Tang X, He X, Wang H, Bavister BD, Ji W. 2000. Cryopreservation of rhesus macaque (Macaca mulatta) spermatozoa and their functional assessment by in vitro fertilization. Cryobiology 41:232–240. 10.1006/cryo.2000.2283. [DOI] [PubMed] [Google Scholar]
- 42.Smith DG. 2012. Taxonomy of nonhuman primates used in biomedical research, p. 57–85. In: Abee C, Mansfield K, Tardif S, Morris T, editors. Nonhuman primates in biomedical research. San Diego (CA): Elsevier. [Google Scholar]
- 43.Tachibana M, Sparman M, Sritanaudomchai H, Ma H, Clepper L, Woodward J, Li Y, Ramsey C, Kolotushkina O, Mitalipov S. 2009. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature 461:367–372. 10.1038/nature08368. Erratum. Nature 2014. 516:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tao Y, Sanger E, Saewu A, Leveille MC. 2020. Human sperm vitrification: the state of the art. Reprod Biol Endocrinol 18:1–10. 10.1186/s12958-020-00580-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tollner TL, VandeVoort CA, Overstreet JW, Drobnis EZ. 1990. Cryopreservation of spermatozoa from cynomolgus monkeys (Macaca fascicularis). J Reprod Fertil 90:347–352. 10.1530/jrf.0.0900347. [DOI] [PubMed] [Google Scholar]
- 46.Turnquist JE, Hong N. 1995.Functional morphology, p 49–76. Chapter 4. In: Bennett BT, Abee CR, Henrickson R, editors. Nonhuman primates in biomedical research: biology and management, vol. 1. San Diego (CA): Academic Press. [Google Scholar]
- 47.VandeVoort CA. 2004. High quality sperm for nonhuman primate ART: production and assessment. Reprod Biol Endocrinol 2:1–5. 10.1186/1477-7827-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watson PF. 2000. The causes of reduced fertility with cryopreserved semen. Animal Reproduction Science 60–61:481–492. 10.1016/S0378-4320(00)00099-3. [DOI] [PubMed] [Google Scholar]
- 49.World Health Organization. 2010. WHO laboratory manual for the examination and processing of human semen, 5the ed. Switzerland: World Health Organization. [Google Scholar]
- 50.Wolf DP, Thormahlen S, Ramsey C, Yeoman RR, Fanton J, Mitalipov S. 2004. Use of assisted reproductive technologies in the propagation of rhesus macaque offspring. Biol Reprod 71:486–493. 10.1095/biolreprod.103.025932. [DOI] [PubMed] [Google Scholar]
- 51.Wolf DP, Vandevoort CA, Meyer-Haas GR, Zelinski-Wooten MB, Hess DL, Baughman WL, Stouffer RL. 1989. In vitro fertilization and embryo transfer in the rhesus monkey. Biol Reprod 41:335–346. 10.1095/biolreprod41.2.335. [DOI] [PubMed] [Google Scholar]
- 52.Woods E, Thirumala S, Han X, Critser JK. 2011. Fundamental cryobiology of reproductive cells and tissues, p 129–144. Chapter 11. In: Donnez J, Kim SS, editors. Principles and practice of fertility preservation. New York (NY): Cambridge University Press. [Google Scholar]
- 53.Yang S, Ping S, Ji S, Lu Y, Niu Y, Wang H, Ji W, Si W. 2011. The positive effects of seminal plasma during the freezing process on cryosurvival of sperm with poor freezability in the rhesus macaque (Macaca mulatta). J Reprod Dev 57:737–743. 10.1262/jrd.11-056N. [DOI] [PubMed] [Google Scholar]
- 54.Yeoman RR, Mitalipov S, Gerami-Naini B, Nusser KD, Wolf DP. 2005. Low temperature storage of rhesus monkey spermatozoa and fertility evaluation by intracytoplasmic injection. Theriogenology 63:2356–2371. 10.1016/j.theriogenology.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 55.Zelinski-Wooten MB, Hutchison JS, Hess DL, Wolf DP, Stouffer RL. 1995. Follicle stimulating hormone alone supports follicle growth and oocyte development in gonadotrophin-releasing hormone antagonist-treated monkeys. Hum Reprod 10:1658–1666. 10.1093/oxfordjournals.humrep.a136151. [DOI] [PubMed] [Google Scholar]