Abstract
Pathogenesis of viruses or other agents that are infectious to humans is frequently studied in vivo using natural or genetically modified animals. Depending on the risk group of the pathogen, the majority of such experimental studies are performed at least under biosafety level 2 (BSL-2) conditions. Biosafety considerations are therefore critical at all steps of research involving potentially infectious pathogens. Inactivation of pathogens studied using in vitro experiments is usually performed using moist heat sterilization. However, few standardized and validated protocols are currently available for the thermal inactivation of carcasses from laboratory animals infected with such human pathogens. To comply with laboratory biologic safety rules and requirements imposed by regulatory authorities, documentation of appropriate inactivation conditions or use of a validated procedure according to national or international standards is critical. In the current study, we evaluated inactivation protocols in a standard laboratory autoclave for carcasses of either frozen mice or recently terminated rabbits, which were placed inside autoclave bags with bedding material in stainless steel containers. Temperature sensors were placed into different tissues of the carcasses to continuously record temperature in situ and in real-time, and a reference sensor was placed in the autoclave. To achieve pathogen inactivation, autoclaving protocols had to be optimized for both species. Frozen mice required 2 different fractionated prevacuum stages, whereas recently terminated rabbits required 3 different fractionated prevacuum stages. This study provides a template for an evaluation procedure to safely and effectively inactivate mice and rabbits infected with risk group 2 to 4 pathogens.
Abbreviations: BSL, biosafety level; FLUAV, human influenza viruses; HIV, human immunodeficiency virus; IVC, individually ventilated cage; NHP, nonhuman primates; SAL, sterility assurance level; SARS-CoV2, severe acute respiratory syndrome coronavirus type 2; SOPF, specific and opportunistic pathogen-free; SPF, specific pathogen-free
Animals provide important model organisms in which to study the biology of pathogens, including viruses, parasites, fungi, and bacteria. The use of live animals allows researchers to investigate pathogenesis, transmission, prevention and potential treatments for infectious diseases in vivo, and give insight into fundamental aspects of host-pathogen interactions. Yet, due to the potential for infectious exposure, animal facilities at BSL-2 containment level or higher are required for most human pathogens. For example, in vitro tissue culture research with clinically important, replication-competent viruses affecting humans, like human immunodeficiency viruses (HIV), human influenza viruses (FLUAV) or the novel coronavirus (SARS-CoV2) require BSL-3 containment. According to the national guidelines in Germany, thermal inactivation of the majority of infectious agents is achieved using moist heat sterilization at 121°C for 20 min.10,19,24 Sterilization at this temperature for 15 to 30 min is also recommended by international guidelines.3,4,6 However, these standard procedures with simple prevacuum stages are not suitable to inactivate infected animal carcasses.15,18 Depending on the size of the animal being sterilized, standard cycle times may not be sufficient to reach the required sterilization temperature throughout the whole body, which would result in incomplete or ineffective sterilization. Before infected animal carcasses can leave a containment facility, laboratory biologic safety rules and requirements imposed by regulatory authorities must be followed. Depending on the facility, incineration may be the method of choice to treat large volumes of infectious waste or animal carcasses.
Methods for disposal of animal carcasses on a farm include burial, commercial landfills, rendering, and alkaline hydrolysis.5,14,22 However, these techniques are typically not appropriate for infected laboratory animals. Alkaline hydrolysis and alkaline hydrolysis-based tissue dissolvers were recently proposed as an alternative to decontaminate/inactivate infected animals in a BSL-3 facility.5,11,13,23 However, this might not be suitable for every laboratory working with animals that are infected with human pathogens due to either issues of safety or insufficient space. On the other hand, an autoclave is a prerequisite for a containment facility, and thus readily available.
Few studies have been published that validate decontamination protocols of biomass from containment animal facilities. One group validated a steam sterilization protocol using nonhuman primates (NHP),1 while another used turkeys and Cornish hens as surrogates for NHPs and guinea pigs.20 Recently, a third group established and validated protocols for fresh mouse carcasses using different packaging material and protocols.18 All 3 studies used biologic indicators or temperature sensors/loggers to monitor the autoclaving process. While temperature sensors or loggers collect real-time information about the coldest spots inside the carcass, a biologic indicator, usually spores of Geobacillus stearothermophilus, indicates whether the sterilizing time and temperature were sufficient to kill the microbial organism. The D-value describes the time that is required to kill 90% of the microbial population. This value is between 1.5 and 3 min for G. stearothermophilus at 121°C. Sterilization processes with an exposure time of 20 min at 121°C thus provide to a sterility assurance level (SAL) of 10−4, assuming that 106 spores were initially used.8 However, individual pathogen-specific SAL values may not be not applicable to different species of infected animal carcasses. While the initial number of pathogenic organisms used to infect animals is provided by the experimental design, the SAL values might be an under- or overestimation due to the distribution and replication preferences of the specific pathogen. Achieving verifiable thermal inactivation of infected animal carcasses may require modification of standard sterilization procedures. Testing G. stearothermophilus, a temperature-stable bioindicator, offers a sound approach to validating established sterilization processes.
Here, we established and validated protocols to inactivate pathogenic organisms in frozen mouse carcasses and recently terminated rabbits. Frozen mouse carcasses were chosen for this study because the inactivation of frozen mouse carcasses might represent a safer alternative than thawing potentially infectious carcasses that may have been stored before inactivation. Our protocols will provide a reference for other laboratories working with mice or rabbits under BSL-2 to -4 conditions.
Materials and Methods
Animals.
Four 19-wk-old female New Zealand white rabbits (Oryctolagus cuniculus) (3.6 to 4.6 kg) were ordered from Charles River (France). In addition, three 57-d old female New Zealand white rabbits were ordered from Charles River (France) and housed for an additional 8 wk for unrelated behavioral studies. At the time of the study, the rabbits weighed 3.23 to 3.77 kg. All rabbits were housed in a specific pathogen-free (SPF)-facility. The rabbits were terminated by slow injection of a lethal dose of sodium-pentobarbital (Narcoren, Boehringer Ingelheim, Ingelheim am Rhein, Germany) IV (100 mg/kg). Spleens were then removed for unrelated studies, mimicking a realistic experimental workflow for studying infected rabbits, with subsequent analysis of infection levels in specific organs. The use of rabbits was in accordance with the local government authorities (Az.0-85-2016).
Adult C57BL/6J mice (Mus musculus) (18.3 to 45.8 g; n = 312), which were excess animals produced at the breeding facility of the Center for Neuropathology and Prion Research (LMU Munich), were terminated by cervical dislocation without anesthesia and carcasses were frozen at -20°C. The mice were healthy and had no pathologic findings. They were removed from the breeding facility because they lacked the required genotypes for other unrelated experiments.
The specific and opportunistic pathogen-free (SOPF)-mouse facility is controlled and registered by the Department of Public Order (KVR-I/221) with an approved animal welfare license (KVR Nr. # 04 to 20). The facility and all animal experiments are in accordance with official animal protection standards and reviewed and approved by an Institutional Animal Care and Use Committee and the office for public health and consumer protection of Upper Bavaria’s government (Nr. # ROB-55.2Vet-2532).
Autoclave.
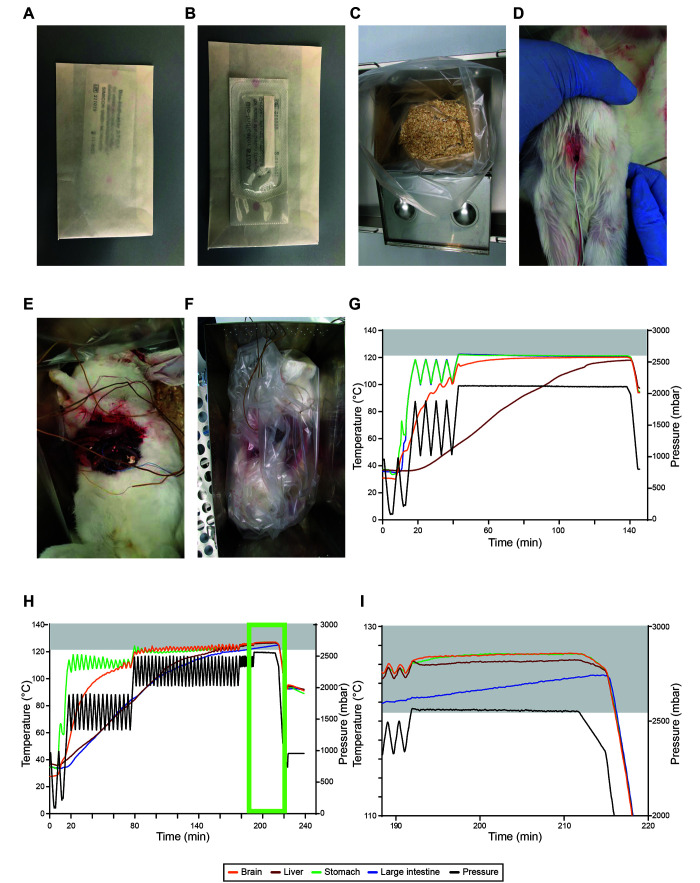
A Ventilab PL-969-2 H R moist heat sterilizer from MMM (Münchener Medizin Mechanik GmbH, https://www.mmmgroup.com/de/produkte/life-science/sterilization, Planegg, Germany) was used. The inner size of the autoclave chamber has dimensions of 1120 mm × 650 mm × 990 mm, and its total capacity is 721 L. The 2S system from PcVue was used to validate the sterilization protocols (ValSys 24, PcVue GmbH, Altdorf, Germany). The reference sensor for pressure measurement dTrans p20 (0 to 4000 mbar) was from JUMO (JUMO GmbH and KG, Fulda, Germany). The reference sensor for temperature measurement “Pt100 Laborfühler” (0 to 150°C) was from TP-temperature products (TP-Temperatur Produkte Koloczek, Hanau, Germany). Nickel chrome (NiCr-N) thermo-elements #901305/99 from JUMO (JUMO GmbH and KG, Fulda, Germany) were used to record the temperature within the carcasses. Sterilization processes must be validated before use and routinely tested as recommended by national and international standards3,6 and recently published.1,2 Furthermore, the efficiency of the liquid and solid waste programs were validated every 6 mo using bioindicator glass ampoules (#110274, Sterikon plus bioindicator, Merck, Darmstadt, Germany) or biologic indicator strips (Bioindicator Simicon ST/DA, G. stearothermophilus, 105 spores, Simicon GmbH, München, Germany) (Figure 1 A and B).
Figure 1.
Establishing inactivation of recently terminated rabbits at 121°C for 20 min. A–B, images of the biologic indicator strip from the front (A) and back (B) side. (C) image of the metal autoclave boxes with bedding material-filled autoclave bags. D–E images of the first rabbit carcass with a hole in the skull to place a temperature sensor into the brain (D) and with sensors placed into the interior of the carcass (E). (F) image of the first rabbit carcass fitted into the metal autoclave box. G–H, temperature (°C, left) and pressure (mbar, right) profiles of individual runs with a 3.8 kg (G) and 3.6 kg (H) rabbit carcass. I, zoom-in image of the temperature profile of H (light green box). Gray boxes indicate temperatures ≥ 121°C.
Inoculation of appropriate growth media at 56°C for up to 7 d and readout were performed by the certified microbiology laboratory of the Max von Pettenkofer Institute at the LMU München. Control biologic indicators that were not autoclaved were always run and evaluated in parallel.
Autoclaving of mouse and rabbit carcasses.
To autoclave frozen mice or freshly terminated rabbit carcasses, an autoclave bag (700 × 1120 mm, Sarstedt, Nümbrecht, Germany) was placed inside a stainless-steel container (27 cm × 40.5 cm × 27 cm, Kuhnle Laborbedarf, Karlsruhe, Germany). 133 to 216 g of aspen chip bedding material (LTE E-001 L-10, Abedd, Kalnciems, Latvia) was placed inside the autoclave bag to soak up any liquid from the autoclaved carcasses. The amount of bedding material was considered to be negligible and was not considered to have any adverse effect on the efficiency of sterilization.
Rabbit carcasses used in this study were freshly terminated and never frozen. The spleens of the rabbits were removed for unrelated experiments via an abdominal incision. Incisions were performed to mimic real experimental protocols for studying infectious disease, in which selected organs are removed for further analyses, including pathology, infectious titer of pathogens and other parameters. A small hole was drilled into the skull of the rabbits using a screwdriver. This hole was small enough to fix the temperature sensor about one centimeter inside the brain. The freshly terminated rabbits were then placed inside the autoclave bags with bedding material in stainless-steel containers. Using the abdominal incision, temperature sensors were placed below the stomach, the liver and the large intestine, where they were secured to ensure that they did not move or change position during autoclaving (Figure 1 F). Prior to loading, the autoclave bag was loosely wrapped to allow steam penetration and the stainless-steel box closed. An autoclave indicator strip (#H590.1, Carl Roth, Karlsruhe, Germany) was placed on the outside of the stainless-steel box to monitor whether autoclaving conditions were fulfilled in that location. For further validation, 2 biologic indicator strips per rabbit were placed both inside the carcass and between the carcass and the bedding material. Our most challenging condition consisted of 3 rabbits (3.2 to 3.8 kg) that were autoclaved in 3 separate stainless-steel containers at the same time in one autoclave run. All animals were equipped with 4 sensors as described above to individually monitor temperatures.
Frozen mice were prepared by drilling a small hole with a screwdriver and fixing of the temperature sensor about one centimeter inside the body. Temperature sensors were placed in different parts of the frozen body, including the skull (brain tissue), abdominal cavity, rectum and dorsal muscles. Between 2 and 10 frozen mice were separately placed inside the autoclave bag with bedding material. The final steps before autoclaving were performed as described above. For further validation, one biologic indicator strip per stainless steel container was placed between one frozen mouse and the bedding material. For our most challenging condition for successful sterilization, 10 frozen mice were well separated in the stainless-steel container as described above, and a total number of 6 containers were autoclaved in one run (n = 60). Here, 4 mice per box were equipped with sensors as described above.
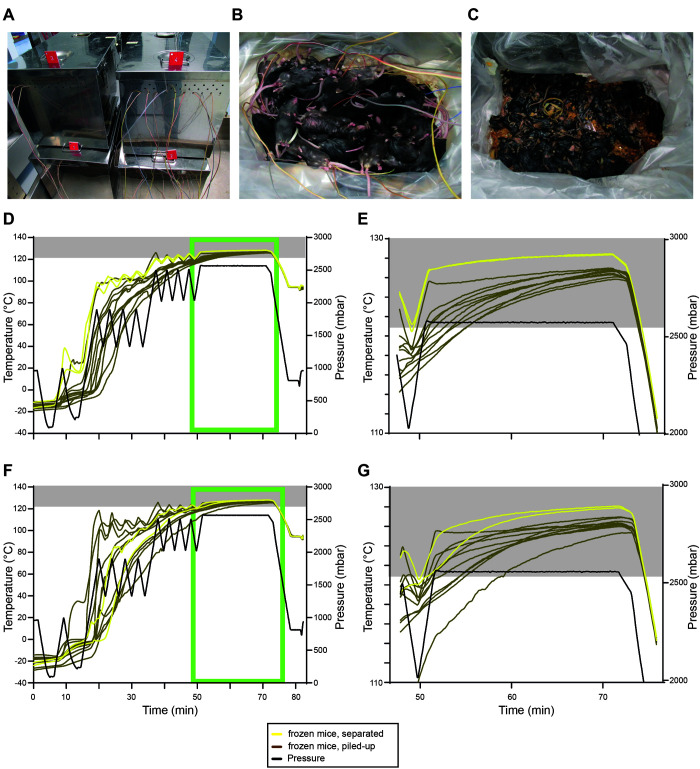
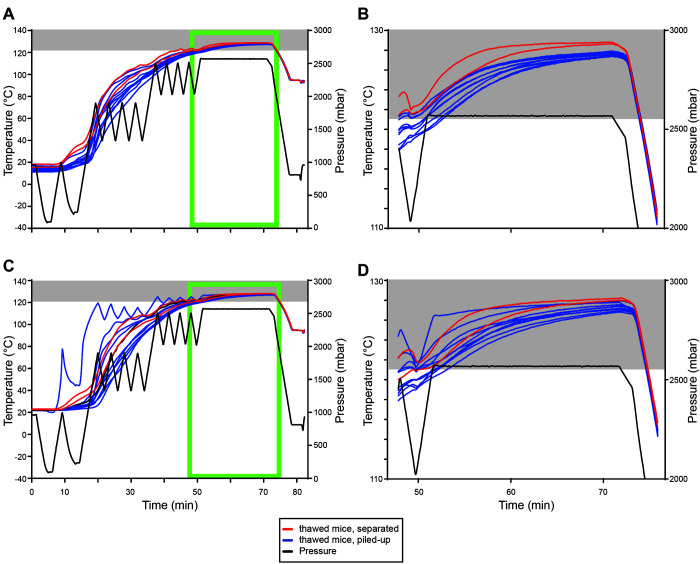
The validated sterilization protocol was further tested with both thawed mice and piled mouse carcasses. Two separate runs were performed using four stainless steel containers (Figure 2 A). In the first container, 10 separately placed frozen mice were used as controls. In the second container, 10 thawed mice were separately placed on the bedding material. For both conditions, 2 mice were equipped with temperature sensors as described above. In addition, one biologic indicator strip was placed below a mouse carcass in each container (Figure 1 A and B). The third and fourth containers contained 50 frozen or thawed mice, piled up (Figure 2 B). Here, 10 mice per box were equipped with temperature sensors as described above; these mice were either placed at the bottom of the container or in the pile. In addition, 2 to 3 biologic indicator strips per box were placed below the lowest mice in the pile.
Figure 2.
Using the validated inactivation protocol for separately placed and piled-up thawed as well as frozen mice. (A) image showing the 4 steel containers with the different conditions run at the same time: no.1 - thawed separately placed; number 2 – thawed piled-up; no.3: frozen separately placed; number 4 frozen piled-up. B–C, images showing 50 thawed mice before (B) and after autoclaving (C). (D) temperature (°C, left) and pressure (mbar, right) profiles of the first run containing autoclave boxes with 10 separately placed frozen mice (yellow) and with 50 piled-up frozen mice (brown). (E) zoom-in image of the temperature profile of D (light green box). (F) temperature (°C, left) and pressure (mbar, right) profiles of the second run containing autoclave boxes with 10 separately placed frozen mice (yellow) and with 50 piled-up frozen mice (brown). (G) zoom-in image of the temperature profile of F (light green box). Gray boxes indicate temperatures ≥ 121°C.
Results
Establishing an inactivation protocol for recently terminated rabbits.
Recently terminated New Zealand white rabbits were placed in an autoclave bag with bedding material in metal autoclave boxes (Figure 1 C). A temperature sensor was placed into the brain tissue after drilling a hole into the skull, the others underneath the liver, the stomach and the large intestine (Figure 1 D and E). The first carcass was a female rabbit with a body weight of 3.8 kg. This carcass received only one fractionated prevacuum stage with four overpressure pulses. With this arrangement, the coldest spots in the rabbit carcass were the liver and the brain (Figure 1 G). The sensor placed underneath the liver reached a temperature of only 117°C, whereas the one located in the brain reached 120°C within the timeframe of 133 min (Figure 1 G). The hole drilled into the brain (Figure 1 D) was substantially larger than in the subsequent trials and even under this condition, the brain did not reach 121°C.
We optimized the inactivation protocol by using 2 additional female rabbits (3.9 kg and 4.6 kg). With up to 3 fractionated prevacuum stages and 30 overpressure pulses, we were still unable to reach 121°C (data not shown). We realized that fractionated prevacuum stages with high deltas for the pressure injection and release negatively impacted temperature stability and may have been the reason that temperatures consistently failed to reach 121°C in all tissues.
Thus, for the fourth female rabbit (3.6 kg), we modified the protocol once more by using additional, shorter, fractionated prevacuum stages to further stabilize the temperature increase. With this protocol, all sensors reached the required temperature of 121°C (Figure 1 H and I (light green box)) for at least 20 min. Here, the sensors placed below the liver and the large intestine required 158 min and 180 min, respectively, to reach the sterilization temperature of 121°C. The sterilization phase of the program, hereafter referred to as sterilization, started 191 min after initiating the autoclaving procedure. Thus, the rabbit carcass maintained the sterilization temperature of 121°C for at least 31 min holding time. The final protocol is summarized in Table 1, indicating the number of fractionated prevacuum stages that are necessary to reach 121°C for at least 20 min in all body compartments to effectively inactivate all potential pathogens inside the carcass of a rabbit.
Table 1.
Sterilization conditions for freshly terminated rabbits based on EN ISO 17665-1.
| Conditions | Temperature | Duration | Pressure | ||
|---|---|---|---|---|---|
| Advanced sterilization | 1.0 Kelvin | ||||
| Fractionated prevacuum stages | Stage 1 | Stage 2 | Stage 3 | Stage 4 | Stage 5 |
| Number of repetitions | 1 | 1 | 15 | 30 | 10 |
| Pressure steam injection | 600 mbar /8.7 psi | 1000 mbar /14.5 psi | 1900 mbar /27.6 psi | 2500 mbar /36.3 psi | 2500 mbar /36.3 psi |
| Pressure steam flush II | 90 mbar /1.3 psi | 90 mbar /1.3 psi | 1300 mbar /18.9 psi | 2000 mbar /29 psi | 2300 mbar /33.4 psi |
| Flow steam flush II | 100% | 100% | 100% | 100% | 100% |
| Pressure evacuation | 75 mbar /1.1 psi | 200 mbar /2.9 psi | 1300 mbar /18.9 psi | 2000 mbar /29 psi | 2300 mbar /33.4 psi |
| Pressure steam flush I | 90 mbar /1.3 psi | 215 mbar /3.1 psi | 1300 mbar /18.9 psi | 2000 mbar /29 psi | 2300 mbar /33.4 psi |
| Time steam flush I | 60 s | 60 s | — | — | — |
| Flow steam flush I | 10% | 10% | 100% | 100% | 100% |
| Sterilization | 127 °C | 20 min | 2500 mbar / 36.3 psi | ||
| Drying /Airflow | . | 3 min | 800 mbar / 11.6 psi | ||
| Ventilation pressure | — | — | 1100 mbar / 16 psi | ||
Validation of an inactivation protocol for recently terminated rabbits.
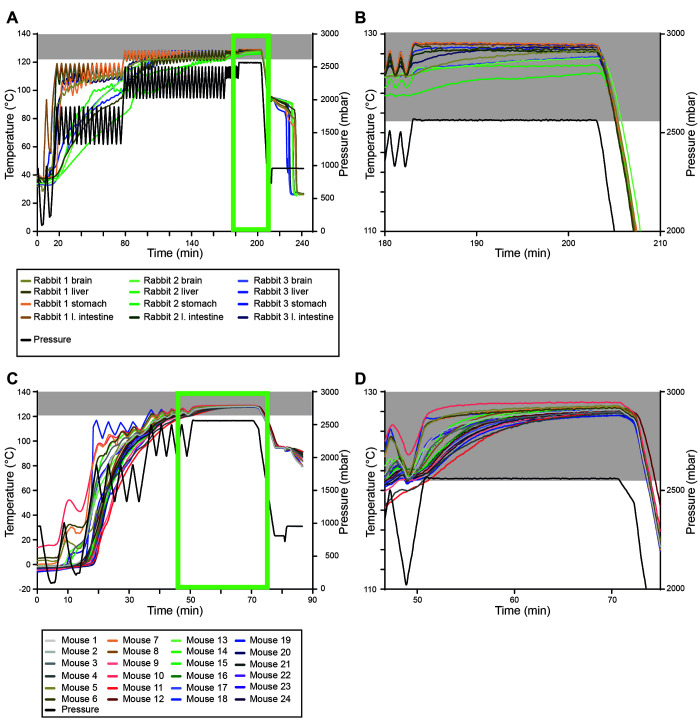
To validate the protocol just described, we autoclaved 3 recently terminated rabbits (3.2 to 3.8 kg) in 3 separate stainless-steel containers simultaneously in one run. All carcasses had temperature sensors. As an additional indicator for a successful autoclaving process, we placed one biologic indicator strip inside each rabbit carcass and one between the carcass and the bedding material. The first rabbit (3.8 kg) reached 121°C after 130 min, with the liver being the coldest spot. The second rabbit (3.7 kg) required 152 min before the sterilization temperature was reached below the stomach, while the third rabbit (3.2 kg) required 124 min for the subhepatic sensor to reach 121°C (Figure 3 A and B is an enlarged image of A (light green box)). Sterilization began only after 183 min, resulting in an optimal sterilization period of at least 51 min. The inoculated spore test strips inside and below the carcass did not show signs of spore growth, indicating that our protocol is also sufficient to kill G. stearothermophilus (data not shown). In summary, we validated our protocol to inactivate rabbit carcasses below a certain weight with an open abdominal cavity.
Figure 3.
Efficient inactivation of recently terminated rabbits and frozen mice at 121°C for 20 min. (A) temperature (°C, left) and pressure (mbar, right) profiles of the validation run using 3.77 kg, 3.7 kg and 3.23 kg rabbit carcasses in parallel. (B) zoom-in image of the temperature profile of (A, light green box). (C) temperature (°C, left) and pressure (mbar, right) profiles of the validation run using 60 mouse carcasses (18.3–45.8 g) in parallel. (D) zoom-in image of the temperature profile of C (light green box). Gray boxes indicate temperatures ≥ 121°C.
Establishing an inactivation protocol for frozen mice.
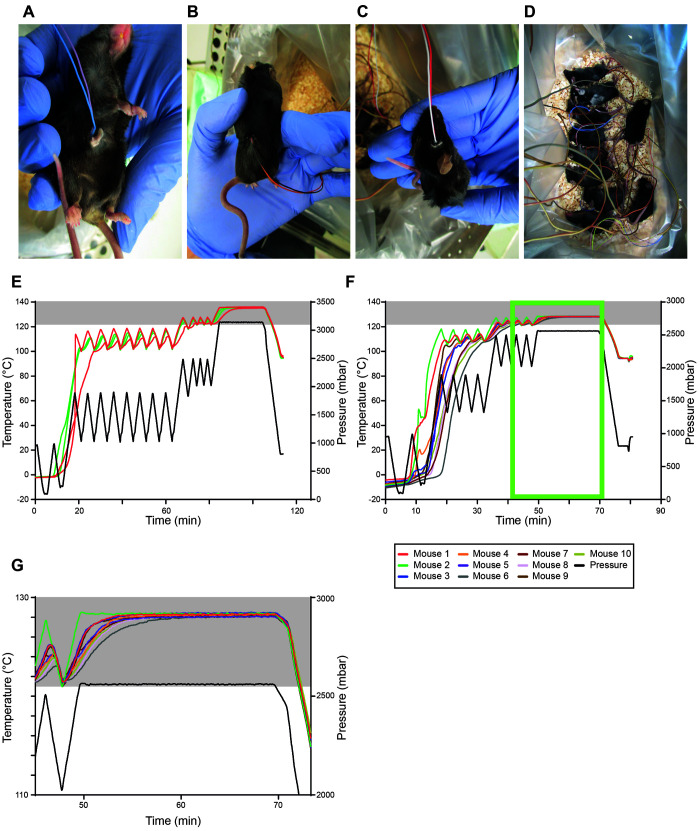
Small holes were drilled into different parts of the frozen body to position temperature sensors - for example, skull (brain tissue), abdominal cavity, rectum and dorsal muscles (Figure 4 A through C). Due to their small body size and frozen condition, we were only able to position one sensor per mouse. Frozen adult C57BL/6J mice were individually placed into autoclave bags with bedding material in metal autoclave boxes (Figure 4 D). For the first run, 2 mice were autoclaved using 2 fractionated prevacuum stages with 8 and 4 overpressure pulses, respectively (Figure 4 E). Here, we first fixed 2 sensors into one hole to monitor at least one of them efficiently. Both mouse carcasses had reached the required temperature of 121°C by the start of the second fractionated prevacuum stage. Thus, in the next round, we reduced the cycle number of the first fractionated prevacuum stage, this time using 10 mice. Regardless of where the sensors were placed, all mouse carcasses reached 121°C for holding times of more than 20 min (Figure 4 F and G is an enlarged image of F (light green box)). Mouse #1 required only 36 min to reach the sterilization temperature of 121°C, whereas mouse #6 required 42 min. Sterilization began after 49 min; thus the mouse carcasses maintained 121°C for at least 27 min. Collectively, these trials established a protocol to effectively sterilize frozen mouse carcasses by autoclaving. The final protocol is summarized in Table 2.
Figure 4.
Establishing inactivation of frozen mice at 121°C for 20 min. A–C, image of frozen mouse carcasses with temperature sensors placed into the abdominal cavity (A), into the dorsal muscles (B), and into the skull (brain) (C). (D) 10 mice were separately placed onto the bedding material. E–F, temperature (°C, left) and pressure (mbar, right) profiles of individual runs for 2 (E) and 10 mice (F). (G) zoom-in image of the temperature profile of F (light green box). Gray boxes indicate temperatures ≥ 121°C.
Table 2.
Sterilization conditions for frozen mice based on EN ISO 17665-1.
| Conditions | Temperature | Duration | Pressure | |
|---|---|---|---|---|
| Advanced sterilization | 1.0 Kelvin | |||
| Fractionated prevacuum stages | Stage 1 | Stage 2 | Stage 3 | Stage 4 |
| Number of repetitions | 1 | 1 | 4 | 4 |
| Pressure steam injection | 600 mbar /8.7 psi | 1000 mbar /14.5 psi | 1900 mbar /27.6 psi | 2500 mbar /36.3 psi |
| Pressure steam flush II | 90 mbar /1.3 psi | 90 mbar /1.3 psi | 1300 mbar /18.9 psi | 2000 mbar /29 psi |
| Flow steam flush II | 100% | 100% | 100% | 100% |
| Pressure evacuation | 75 mbar /1.1 psi | 200 mbar /2.9 psi | 1300 mbar /18.9 psi | 2000 mbar /29 psi |
| Pressure steam flush I | 90 mbar /1.3 psi | 215 mbar /3.1 psi | 1300 mbar /18.9 psi | 2000 mbar /29 psi |
| Time steam flush I | 60 s | 60 s | — | — |
| Flow steam flush I | 10% | 10% | 100% | 100% |
| Sterilization | 127 °C | 20 min | 2500 mbar / 36.3 psi | |
| Drying /Airflow | . | 3 min | 800 mbar / 11.6 psi | |
| Ventilation pressure | — | — | 1100 mbar / 16 psi | |
I: Evacuation and flow pressure used for lower pressure levels
II: Evacuation and flow pressure used for higher pressure levels
Advanced sterilization: This value is added to the set the sterilization temperature
Flow steam flush I: Opening percentage of the vacuum valve
Flow steam flush II: Opening percentage of the vacuum valve
Number of repetitions: This value indicates how often the process element (stage) is repeated with an identical procedure
Pressure steam injection: Set pressure for steam injection
Pressure evacuation: Set pressure for evacuation
Pressure steam flush II: Set pressure for steam flush II
Pressure steam flush I: Set pressure for steam flush I
Time steam flush I: Holding time at steam flush pressure I
Validation of an inactivation protocol for frozen mice.
To validate the protocol we established for frozen mice, we simultaneously autoclaved 6 stainless steel boxes with 10 mice per box in a single run (n = 60). In each box, 4 of the 10 mice were instrumented with temperature sensors (n = 24). As the mice were still frozen, we could not place a biologic indicator strip inside the body. Instead, we placed one biologic indicator strip per box between a frozen mouse and the bedding material, assuming that this would be the most difficult spot to sterilize in the box. The first mouse reached 121°C within 39 min (mouse #10), while the last mouse required 51 min (mouse #11) (Figure 3 C and D is a magnified image of C (light green box)). Both were located in the same steel container. Sterilization began about 50 min after initiating the run. In theory, this would mean that the last mouse (mouse #11) did not reach our sterilization requirements of 121°C and 20 min holding time. The temperature of mouse #11 dropped below 121°C only after 73 min, indicating that the temperature within the carcass lags behind the ventilation of the autoclave. The inoculated spore test strips below the carcass did not show any spore growth, indicating that our protocol is also sufficient to kill G. stearothermophilus (data not shown). Collectively, we validated our protocol to inactivate separately placed, frozen mouse carcasses.
Sterilization of piled-up thawed or frozen mice.
In a subsequent trial, we tested the established and validated protocol for its ability to sterilize thawed and piled-up frozen and thawed mice. Both runs (Figure 2 D through G show frozen mice; E and G are enlarged images of D (first run) and F (second run) (light green boxes); Figure 5 A through D show thawed mice; B and D are enlarged images of A (first run) and C (second run) (light green boxes)) contained 4 stainless steel containers (Figure 2 A) including (a) 10 separately placed frozen mice (yellow, Figure 2 D through G), (b) 10 separately placed thawed mice (red, Figure 5 A through D), (c) 50 piled frozen mice (brown, Figure 2 D through G) and (d) 50 piled thawed mice (blue, Figure 5 A through D; Figure 2 B). In this trial, well-separated frozen and thawed mice reached 121°C before the sterilization process started in both runs (Figure 2 D through G; Figure 5 A through D). No detectable growth of G. stearothermophilus was detected beneath the mice, presumably the coldest spot. Piling of mice, either frozen or thawed, reduced the efficiency of our established and validated protocol as 7 out of 10 (first run) and 5 out of 10 (second run) frozen mice, as well as 3 out of 10 (first run) and 4 out of 10 (second run) thawed mice did not reach 121°C before the sterilization process started. However, holding times of at least 20 min at 121°C were achieved for all thawed mice in both runs, while only 16 (first run) and 13 (second run) minutes minimal holding times at 121°C were achieved for all frozen mice in both runs. Growth of G. stearothermophilus was detected beneath the lowest mouse for 1 out of 3 (thawed) and 1 out of 3 (frozen) biologic indicator strips in the first run and zero of 2 (thawed) and zero of 2 (frozen) in the second run. Thus, our validated protocol is only suitable for separately placed thawed or frozen mice, and additional safety margins must be considered when piling up thawed or frozen mice.
Figure 5.
Using the validated inactivation protocol for separately placed and piled-up thawed mice. (A) temperature (°C, left) and pressure (mbar, right) profiles of the first run containing autoclave boxes with 10 separately placed thawed mice (red) and with 50 piled-up thawed mice (blue). (B) zoom-in image of the temperature profile of A (light green box). (C) temperature (°C, left) and pressure (mbar, right) profiles of the second run containing autoclave boxes with 10 separately placed thawed mice (red) and with 50 piled-up thawed mice (blue). (D) zoom-in image of the temperature profile of C (light green box). Gray boxes indicate temperatures ≥ 121°C.
Discussion
Infection studies with animals usually require facilities that operate at least at BSL-2. Mice and rabbits in particular are commonly used to study pathogenesis of infectious agents in vivo.9,16,17,21 Prior to the start of animal experiments, a process for the safe disposal of infected animal carcasses must be established to comply with both laboratory biologic safety rules and requirements imposed by national or local regulatory authorities. Few published studies have addressed this critical step in work with infectious disease models. Depending on the animal species, the specific autoclave size and total volume must also be considered. Autoclaving of smaller carcasses can be performed in standard-size autoclaves, while the carcasses of larger animals like cows will require more volume. Alternatives to autoclaving infectious carcasses, such as incineration or alkaline hydrolysis, are unlikely to be a suitable standard operating procedure for most laboratories.5,11,13,23
Although a previous study established a total inactivation time of 180 min at 150°C using alkaline hydrolysis,23 a procedure that requires a pressure vessel and the use of the dangerous irritant potassium hydroxide, in our protocol the total inactivation time for rabbits was about 240 min and for mice only about 86 min. The most significant difference between the 2 studies is that the previous study used dissected animal tissues only, while we examined largely intact carcasses. Thus, the suggestion that alkaline hydrolysis of a whole rabbit carcass would provide an effective inactivation within 180 min may be inaccurate. Furthermore, our validated thermal inactivation approach does not require potentially toxic chemicals or additional equipment but simply relies on the existing autoclave in the biosafety unit.
Previously, thermal inactivation protocols have been published for turkeys and Cornish hens, NHPs and freshly terminated mice.1,18,20 In the current study, we established and validated protocols based on a porous goods program7 for thermal inactivation of mouse and rabbit carcasses using moist heat sterilization at 121°C for 20 min. The documentation of adequate temperature profiles in hard-to-penetrate tissue compartments of the carcass is critical for the effective inactivation of risk group 2 to 4 pathogens. Tissues like muscle, fat, or skin, the undigested food within the intestine or the animal’s fur may act as insulators, and the body’s water content may also influence the autoclaving efficiency. Saturated steam may condense on the surface of the carcass, with heating occurring through thermal conduction. In this case, the pulsing process, which leads to quick changes in the pressure level, might cause mechanical movements inside the carcass. Similar to mixing, this would improve the heat transfer, and the sterilization temperature could be efficiently achieved.
Alternatively, steam penetration into the carcass could be improved by the pulsing process. Pulsed vacuum processes are commonly used for sterilization of medical devices to effectively remove air from porous or hollow loads and replace it with steam to allow for effective steam penetration.12 In that case, the steam could directly condense inside the carcass. Further, one could hypothesize that steam penetration primarily occurs via body orifices rather than via the skin. Whether one situation or the other prevails or whether a mixture of both leads to the temperature increase inside the carcass cannot be quantified.
Another group used 2 biologic indicators per NHP of less than 10 kg body weight and a wireless temperature data logger in the abdominal cavity, which recorded one temperature reading per minute.1 Using a sterilization program based on liquids, they recorded peak core temperatures of the carcasses between 110°C and 115°C, which were nevertheless lethal for the spores.1 Although NHPs and rabbits cannot be directly compared, differences in body core temperatures were notable. Another group validated their sterilization programs by using biologic indicators. Based on their study, a sterilization for 2 to 4 h at 121°C effectively killed the spores.20 In contrast to these 2 studies, we performed sterilization using a program designed and optimized for porous goods. The advantage of this is that the sterilization process was regulated via the temperature of the inner autoclave chamber and validated with temperature sensors. Thus, standardized and reproducible processes could be created. Furthermore, programs for porous goods use fractionated prevacuum cycles, which remove air pockets in porous or hollow loads and maximize steam penetration. The first 2 cycles remove the air pockets and the fractionated prevacuum cycles allow quick attainment of the sterilization temperature. Liquid programs do not have fractionated prevacuum pulses, which would otherwise lead to evaporation of liquids. For liquid programs, the sterilization process is controlled by the temperature of the reference sensor placed into bottles containing equivalent amounts of liquid. Thus, the sterilization time depends on the temperature of the reference sensor.
As the body weight of rabbits is markedly higher than that of mice, a more complex protocol was required to consistently reach 121°C for more than 20 min throughout the carcass. The first 2 fractionated prevacuum stages increased the body temperature up to 120°C. However, the temperatures plateaued and 121°C was never reached. Thus, direct steam penetration of the brain was problematic, as even the brain of the first rabbit that had a larger hole in the skull (Figure 1 D) did not reach the required temperature. Thus, we implemented a fractionated prevacuum stage with smaller pressure differences (Table 1) to keep the temperature increase constant. With this additional parameter, we were able to reach 121°C in all tissues monitored.
In order to establish protocols suitable for animal work with risk group 2 to 4 pathogens, we had to document the required sterilization temperature and time in the carcasses. In our validation run, we also used biologic indicators to document that our program was able to kill all spores. Indeed, the spores were inactivated with our protocol, regardless of whether they were placed inside or below the rabbit carcass. Further, we were able to show that, depending on the rabbit, the brain, stomach, or liver were some of the coldest spots in the carcass and took the longest time to reach 121°C. As temperature increase was very slow in the brain, the effect of drilled hole seems to be negligible and did not appear to have disturbed or affected the sterilization process, as compared with an intact carcass. To demonstrate that our protocol was compliant with biosafety rules, we showed that the sterilization temperature was maintained for at least 20 min at all measured anatomical locations.
Our validation run indicated that the number of rabbits autoclaved at the same time does not appear to affect the efficiency of the sterilization process. Thus, our protocol is suitable for rabbits with similar or lower body weight. Autoclaving of heavier rabbits would require reevaluation, as the time to reach the sterilization temperature might be longer than needed in the suggested protocol. If studies were performed that resulted in intact rabbit carcasses, biologic indicators should be placed into the freshly terminated rabbits and the incision closed.
In terms of biosafety considerations, sterilization protocols for frozen mice should preclude the need to thaw carcasses prior to autoclaving. As the frozen adult mice had a body weight between 18.3 and 45.8 g, moist heat sterilization was faster and much more efficient than autoclaving a rabbit with about 4 kg. The first test round was able to successfully reach 121°C throughout the entire carcass. Thus, we were even able to reduce the pulses of the first fractionated prevacuum stage. In contrast, using free steam phases for inactivation would require a very long time to reach the sterilization temperature, if it were reached at all. The fractionated prevacuum stage pulses alternatively caused the injection of steam and removal of exhaust air, which leads to effective and even distribution of heat within the load and was thus our preferred method. A critical parameter for autoclaving frozen carcasses of mice was to separately place them on the bedding material, barely touching each other. This arrangement allowed a uniform autoclaving process.
While all mice maintained 121°C for at least 20 min, we realized that the pressure was already released even though the last mice did not yet fulfill our sterilization requirements. As a safety margin, one could thus either include one additional shorter, fractionated prevacuum stage, similar to the rabbits, to steadily increase the temperature in the mouse carcasses or extend the holding time during the sterilization process.
Recently, one group validated different settings to autoclave freshly terminated mice.18 They used temperature sensors and biologic indicators implanted in the peritoneal cavity to validate their established protocols. Their final recommendation as a starting point for efficient sterilization of freshly terminated mice to institutions that may want to decontaminate large volumes of mouse carcasses was to pile up to 10 kg of mouse carcasses in autoclave bags inside stainless steel containers and start with a liquid program including 6 h preheating and sterilization at 121°C for one hour.18 This study provides a good basis for larger throughput experiments in which immediate autoclaving of the mouse carcasses is possible. However, this group placed the reference sensor in a 5 L bottle of water,18 which might not accurately represent mouse carcasses based on their composition.
In contrast, small or medium-sized mouse experiments may require short-term storage of the mouse carcasses in the freezer in order to autoclave a larger number of mice at once. Here, we showed that autoclaving well-separated frozen mouse carcasses does not cause any loss of sterilization efficacy. The number of mice in a stainless-steel container should not affect sterilization as long as they are well separated to allow adequate steam penetration. Increasing the number of boxes from one to 6 did not affect sterilization. In our validation run, we used biologic indicators to monitor that our established program was able to kill all spores placed beneath the frozen mice, and the spores were inactivated with our protocol (Table 2). However, it should be stated that we could not place bioindicator strips into mouse carcasses prior to freezing as the strips would have been destroyed during the freezing process.
We further showed that the validated protocol is suitable for thawed mice placed separately on bedding material. Piled mice, either frozen or thawed, also reached 121°C. However, additional safety margins should be taken into account as in both conditions, all 10 mice probed with temperature sensors had not reached the necessary sterilization temperature prior to the start of the sterilization process and not all biologic indicator strips remained negative. For these conditions, increased sterilization time would be required.
Different published sterilization protocols require up to 360 min to reach 121°C.1,18,20 Furthermore, one group validated inactivation of 5 to 7 fresh mouse carcasses in an individually ventilated cage (IVC) using their “dirty cages program” (60 min for preheating).18
In contrast to previous publications, we were able to reach 121°C within 180 min for rabbits and 51 min for mice, perhaps because we used a porous goods program rather than a liquid waste program. The advantage of a porous goods program is the inclusion of fractionated prevacuum, which allow steam penetration into porous and hollow goods. In consideration of economic and ecologic reasons to save the lifespan and maintenance of autoclaves, porous goods programs might be advantageous for the inactivation of infectious pathogens in rabbit and mouse carcasses. Because a similarly detailed thermal inactivation protocol as shown in Table 1 and 2 is missing in other recent publications, we cannot compare the specific differences between factors that may underlie these discrepancies. Adapting or predicting effective inactivation conditions across species is difficult based on our experience.
In summary, we established and validated protocols for moist heat sterilization of mouse and rabbit carcasses. Our protocol can be used by steam sterilizers that allow a flexible adjustment of the relevant cycle parameters, and can also be used as a template for others to develop protocols to sterilize frozen mice or freshly terminated rabbits infected with risk group 2 to 4 pathogens. We recommend that other investigators validate their sterilization cycles based on our detailed approach and choose, based on the pathogen of interest, appropriate bioindicators, temperature profiles and holding times for the sterilization processes used in their facilities.
Acknowledgments
We thank Fabian Kriesel and Dr Christopher Carrie for critical reading of the manuscript. We are grateful for the technical assistance of Christian Riske and Bianca Stahr from the Biomedical Center, LMU München. H-M.B. acknowledges funding from the Else-Kröner-Fresenius Foundation (2017_A97). SW and MPB are employees at MMM.
References
- 1.Bearss JJ, Honnold SP, Picado ES, Davis NM, Lackemeyer JR. 2017. Validation and verification of steam sterilization procedures for the decontamination of biological waste in a biocontainment laboratory. Appl Biosaf 22:33–37. 10.1177/1535676017694147. [DOI] [Google Scholar]
- 2.Boca BM, Pretorius E, Gochin R, Chapoullie R, Apostolides Z. 2002. An overview of the validation approach for moist heat sterilization, part II. Pharmaceutical technology 26:96–112. [Google Scholar]
- 3.British Pharmacopoeia by the British Pharmacopeia Commission. 2001. Stationary Office Books, Norwich, United Kingdom, Volume II. pp. A332–A335. [Google Scholar]
- 4.CDC. [Internet]. 2008. Guideline for disinfection and sterilization in healthcare facilities. [Cited 02 February 2021]. Available at: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/index.html.
- 5.Council for Agricultural Science and Technology (CAST). 2008. Poultry carcass disposal options for routine and catastrophic mortality, Issue paper 40. Ames (IA): CAST. [Google Scholar]
- 6.Council of Europe. 1997. European Pharmacopoeia, 3rd ed. p 283–285. Strasbourg (France) WorldCat [Google Scholar]
- 7.de Magalhães Brito LF, Magagna D. 2004. Sanitation, p 532–546. Chapter 114. In: Dyro JF, editor. Clinical engineering handbook. Burlington (MA): Academic Press. [Google Scholar]
- 8.Dion M, Parker W. 2013. Steam sterilization principles. Pharmaceutical engineering 33:60–69. [Google Scholar]
- 9.Esteves PJ, Abrantes J, Baldauf HM, BenMohamed L, Chen Y, Christensen N, Gonzalez-Gallego J, Giacani L, Hu J, Kaplan G, Keppler OT, Knight KL, Kong XP, Lanning DK, Le Pendu J, de Matos AL, Liu J, Liu S, Lopes AM, Lu S, Lukehart S, Manabe YC, Neves F, McFadden G, Pan R, Peng X, de Sousa-Pereira P, Pinheiro A, Rahman M, Ruvoen-Clouet N, Subbian S, Tunon MJ, van der Loo W, Vaine M, Via LE, Wang S, Mage R. 2018. The wide utility of rabbits as models of human diseases. Exp Mol Med 50:1–10. 10.1038/s12276-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.German Biosafety Regulations. [Internet]. 2021. (GenTSV)§ 26 (1). [Cited 20 May 2021]. Available in German at: http://www.gesetze-im-internet.de/gentsv_2021/__26.html [Google Scholar]
- 11.Homer LC, Fisher DJ, Heflin DT, Cole KS. 2012. Decontamination and digestion of infectious animal waste using a tissue dissolver in an animal biosafety level 3 facility. Lab Anim (NY) 41:327–335. 10.1038/laban.151. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser U, Goeman J. 1998. Investigation of air removal from hollow devices in steam sterilisation processes. Zentr Steril 6:401–413. [Google Scholar]
- 13.Kaye G, Weber P, Evans A, Venezia R. 1998. Efficacy of alkaline hydrolysis as an alternative method for treatment and disposal of infectious animal waste. Contemp Top Lab Anim Sci 37:43–46. [PubMed] [Google Scholar]
- 14.Koziel JA, Frana TS, Ahn H, Glanville TD, Nguyen LT, van Leeuwen J. 2017. Efficacy of NH(3) as a secondary barrier treatment for inactivation of Salmonella Typhimurium and methicillin-resistant Staphylococcus aureus in digestate of animal carcasses: Proof-of-concept. PLoS One 12:1–17. 10.1371/journal.pone.0176825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGirr R, Sample C, Arwood L, Burch J, Alderman S. 2019. Validating autoclave cycles for carcass disposal in animal biosafety level 2/3 containment laboratories. Appl Biosaf 24:134–140. 10.1177/1535676019856799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mykytyn AZ, Lamers MM, Okba NMA, Breugem TI, Schipper D, van den Doel PB, van Run P, van Amerongen G, de Waal L, Koopmans MPG, Stittelaar KJ, van den Brand JMA, Haagmans BL. 2021. Susceptibility of rabbits to SARS-CoV-2. Emerg Microbes Infect 10:1–7. 10.1080/22221751.2020.1868951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nazerai L, Schøller AS, Rasmussen POS, Buus S, Stryhn A, Christensen JP, Thomsen AR. 2018. A New in vivo model to study protective immunity to zika virus infection in mice with intact type I interferon signaling. Front Immunol 9:1–15. 10.3389/fimmu.2018.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pils MC, Kränzler K, Beyer P, Heise U, Pasche B, Riedesel H. 2019. Validation of an autoclave procedure for sterilization of mouse (Mus musculus) carcasses. J Am Assoc Lab Anim Sci 58:87–91. 10.30802/AALAS-JAALAS-18-000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robert-Koch-Institut. [Internet]. 2017. Liste der vom Robert Koch-Institut geprüften und anerkannten Desinfektionsmittel und -verfahren. [[In German]] Bundesgesundheitsbl 60:1274–1297. [Cited 02 February 2021]. 10.1007/s00103-017-2634-6 [DOI] [PubMed] [Google Scholar]
- 20.Santacroce JC, Swearengen J, Weaver P. 2015. Novel approach for validating autoclave cycles for biomass in BSL-3/-4. Appl Biosaf 20:141–145. 10.1177/153567601502000304. [DOI] [Google Scholar]
- 21.Ueki H, Wang IH, Zhao D, Gunzer M, Kawaoka Y. 2020. Multicolor two-photon imaging of in vivo cellular pathophysiology upon influenza virus infection using the two-photon IMPRESS. Nat Protoc 15:1041–1065. 10.1038/s41596-019-0275-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veterinary Services AaPHIS, US Department of Agriculture.[Internet]. 2012. National animal health emergency management system—Operational guidelines: Disposal. [Cited 02 February 2021] Available at: https://www.aphis.usda.gov/animal_health/emergency_management/downloads/nahems_guidelines/disposal_nahems.pdf.
- 23.Wang T, Wu J, Yi Y, Qi J. 2016. Optimization of process conditions for infected animal tissues by alkaline hydrolysis technology. Procedia Environ Sci 31:366–374. 10.1016/j.proenv.2016.02.049. [DOI] [Google Scholar]
- 24.World Health Organization. [Internet]. 2004. Laboratory biosafety manual. [Cited 02 February 2021]. Available at: https://www.who.int/publications/i/item/9241546506