Abstract
Trichuris spp. are common helminths in NHP, and benzimidazoles and avermectins have both been used to treat these intestinal parasites. The current study compared the efficacy of fenbendazole and ivermectin against natural infection of Trichuris spp. in African green monkeys (Chlorocebus sabaeus). Anthelmintic-naive animals (n = 65) were randomly assigned to 4 groups: an untreated control group, and 3 groups treated with either fenbendazole, ivermectin, or both compounds. Fecal samples were collected before treatment and on days 7, 14, 28, and 60 after treatment, and fecal egg counts (FEC) were determined by using fecal flotation. The mean percentages of FEC reduction at day 60 were 100%, 86%, and 100% for treatment with fenbendazole, ivermectin, and both compounds, respectively. Analyzing the time series of FEC by using a Bayesian generalized linear model showed no significant difference in the proportional reduction in FEC among the 3 treatment groups, although all FEC from treated groups were significantly lower than the FEC of the control group. In contrast, the probability of shedding was highest in the ivermectin group and the lowest in the animals treated with both compounds. The probability of shedding differed significantly between the fenbendazole and ivermectin groups and between the ivermectin and combined-treatment groups. In conclusion, both fenbendazole and ivermectin are effective anthelmintics in treating Trichuris spp. infection in African green monkeys. All treatment groups showed significant reductions in FEC when compared with baseline counts and control animals; however, fenbendazole may be more effective than ivermectin when used solely or in combination with other anthelmintic treatments.
Abbreviations: AGM, African green monkey; FEC, fecal egg count; FECR, fecal egg count reduction; MCMC, Markov chain Monte Carlo
Trichuris spp. (whipworms) from the family Trichuridae are common soil-transmitted gastrointestinal nematodes with a worldwide distribution in mammals.7 Trichuris trichiura is the whipworm species that commonly infects humans and NHP,18 but morphologic and biometrical studies have yielded results suggesting that other Trichuris species may also be present in NHP.6,11,53
Trichuris spp. have a direct life cycle, with eggs transmitted via the fecal–oral route with infection occurring from ingestion of embryonated eggs in contaminated food, soil, and water.48 Under optimal environmental conditions, Trichuris spp. eggs can remain viable in infected soil for years.23 After ingestion, the released L1 larvae molt and travel to the cecum and colon, where they bury into the epithelia and develop into adults. After mating, the unembryonated eggs are released from the female and are deposited into the environment in the host feces.7,13 Light infection is asymptomatic; however severe infection produces clinical signs such as severe enteritis, anorexia, gray mucoid diarrhea, and sometimes death.5
The prevalence of Trichuris spp. (whipworms) in NHP usually is high,49 and species in this genus are commonly found in Old World NHP, including African green monkeys (AGM), baboons, macaques, and sykes.39,54 High prevalence rates ranging from 60% to 98% have been recorded in wild populations,30,39,42 and prevalence rates ranging from 30% to 79% have been found in captive colonies of NHP in zoological parks and research facilities.1,26,39,50 In the Caribbean, a study of captive AGM in St Kitts54 revealed a prevalence of 91%, whereas 53% was recorded in recently captured wild AGM in Barbados.40
AGM (Chlorocebus sabaeus) are the only wild NHP species found on the island of Barbados. These animals were introduced into the island soon after it was colonized in 1627.3 Populations are also found on the islands of St Kitts-Nevis, St Maarten/Martin, and Tortola.14 AGM are used widely in biomedical research25 for studies on hypertension,15 neurologic disease20,34 and vaccine production.36,52 In a research setting, NHP should be parasite-free24 for the sake of the animal’s health (which in turn affects research outcomes) and to eliminate possible risk of transfer of zoonoses to caregivers and other research facility personnel.
Different classes of drugs have been used to treat Trichuris spp. infection in NHP, including benzimidazoles like fenbendazole, mebendazole, and albendazole,45,46,55 and avermectins.27,51 Studies have been conducted using these drugs in macaques, vervets, baboons, and langurs.21,27,38,44,51 Fenbendazole and ivermectin have been used at various doses in the treatment of Trichuris infection in NHP. Fenbendazole has been administered at doses of 10 to 50 mg/kg PO daily for 3 to 5 d, with or without repeated doses.16,38,44,45 Ivermectin has been administered at doses of 100 to 400 µg SC, IM, or PO as a single dose or at specified intervals.21,24,38,51 Little published data exist regarding the use of these drugs specifically in AGM. The current study was conducted to compare the efficacy of fenbendazole and ivermectin—alone and in combination—against Trichuris spp. in naturally infected AGM in Barbados, West Indies, for clearance of this parasite.
Materials and Methods
Ethics statement.
The study was conducted at the Barbados Primate Research Center (Barbados, West Indies) and approval was granted by the center’s IACUC. Data were collected from December 2017 through February 2019.
Animal selection and husbandry.
All AGM used in this study were wild-caught under the Monkey Crop Damage Control program, which was established in 1979 by the Caribbean Agricultural and Research Development Institute and involves the humane trapping of animals.3 Animals were captured by using mesh-and-wire drop-door traps (30 in. × 36 in. × 24 in.) and downfall traps baited with fruit or produce. After capture, the animals were sedated by using ketamine HCl (10 mg/kg IM; RotexMedica, Trittau, Germany), transferred to a presanitized pet carrier of appropriate size, and transported to the Barbados Primate Research Center. After arrival, ketamine-sedated AGM underwent physical examination, collection of a blood sample for serum banking, intradermal tuberculin testing, and treatment with a pyrethrin-based spray for ectoparasites; no endoparasite treatment was administered. The AGM were allowed to acclimate for at least 1 wk. Subadult and adult AGM (males and nonpregnant females) in good health, with a body condition score9 of greater than 2.5, and weighing more than 2.5 kg were selected for inclusion in the study and were randomly assigned to 4 groups (the control group and the 3 anthelmintic treatment groups) by using a random-number generator (www.randomization.com).
For the duration of the investigation, the animals were singly housed in stainless-steel cages elevated above concrete flooring to allow monitoring and collection of individual fecal samples. Animals were kept outside in covered enclosures, subject to ambient temperatures, as they would experience in the wild. Housing and care of all animals was in accordance with the Guide for the Care and Use of Laboratory Animals.22 Animals were fed commercial pellets and produce (fruit, vegetables, root crops) and provided water ad libitum.
Fecal samples were collected 4 and 7 d before treatment and on the day treatment began, and the mean of these counts was considered to be the baseline fecal egg counts (FEC), which for analysis purposes represent the day 0 count. AGM not shedding Trichuris spp. eggs during the week prior to treatment were not included in the investigation. All samples were analyzed by using modified McMaster flotation method10 using a saturated sugar solution (specific gravity, 1.27). Each analysis used 3 g (wet weight) of feces. FEC were performed by using McMaster slides (Paracount EPG Kit, Chalex, Park City, UT). Slides were read at 100× magnification. Trichuris spp. eggs were identified as dark, barrel-shaped structures with bipolar hyaline plugs at each end (Figure 1). The eggs were identified to the genus level. The number of Trichuris spp. eggs in each chamber was counted, and the number of eggs per gram was calculated as follows:
where n is the total number of eggs in both chambers.
Figure 1.
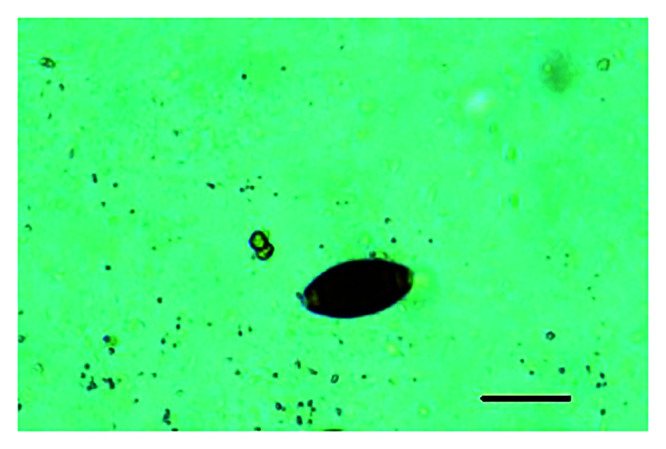
Trichuris spp. egg (magnification, 100×), showing characteristic barrel shape and bipolar plugs. Scale bar, 50 µm.
Treatment groups.
Fenbendazole was administered at 50 mg/kg PO daily for 4 d; ivermectin was provided as a single dose of 200 µg SC. Animals that were treated with fenbendazole received a powdered formulation of fenbendazole (40 mg/g; Hunter 4 Feed Premix, Anglian Nutrition Products, Suffolk, United Kingdom) that was provided on fruit on days 0, 1, 2, and 3. Animals that were treated with an injectable formulation of ivermectin (10 mg/mL; Ivermectin 10, Anglian Nutrition Products) received an injection of 200 µg/kg SC on day 0. For the combined treatment (fenbendazole plus ivermectin), animals received fenbendazole on days 0, 1, 2, and 3 and ivermectin on day 0 as described for the single-compound treatment groups. Animals in the control group did not receive either anthelmintic.
Fecal samples were collected from each animal in each group at approximately the same time of day on days 7, 14, 28, and 60 after treatment, and FEC were calculated for each sample as described earlier. To improve the accuracy of the FEC, 3 separate FEC were performed on each sample collected (before and after treatment), and the mean value of these FEC was used in the analysis.
Statistical analysis.
We summarized the FEC among the treatment and the control groups by presenting the mean FEC and calculating the FEC reduction (FECR)10 over time. The baseline (day 0 count) FEC was used as the before-treatment value to determine the FECR percentage at each sampling point. The FECR percentage was calculated as 100% × [1 – (t2/t1)],29 where t1 is the mean FEC before treatment, and t2 is the mean FEC after treatment; when the FEC increased after treatment, the FECR was recorded as 0%.28 The data were summarized in this way to allow comparison with previous studies; however, we estimated the treatment efficacy within and between treated animals by using the generalized linear model described below because it permits more rigorous evaluation of treatment effects.
A Bayesian zero-inflated, negative binomial model and Markov Chain Monte Carlo (MCMC) techniques were used to estimate the treatment effects of fenbendazole, ivermectin, and the combined therapy on FEC compared with the FEC of untreated animals. The treatment effects were compared with each other to assess the relative efficacy of each treatment. The negative binomial allows for over-dispersion in count data, which is clearly evident in the FEC. A zero-inflated version of this model was used because effective treatment should eliminate shedding completely, resulting in a larger proportion of zero FEC for treatment groups, which then can be accommodated structurally by the negative binomial distribution. Thus, the response to treatment was examined by using 2 different metrics, the probability of shedding and the amount of shedding by an individual animal, under the hypothesis that the most effective treatment will have the lowest probability of shedding and the lowest FEC. In addition, individual AGM and sampling-day effects were included within our model structure to account for interindividual variation in FEC and the correlation associated with serial sampling and to allow the estimated probability of shedding and FEC to vary through time. Specifically, the mean FEC was estimated as follows:
where μi,j is the natural log of the expected FEC for the ith individual AGM during the jth sampling period, βk,o is the baseline effect for the kth treatment group prior to treatment, βk is the effect of the kth treatment, trti,j is the treatment for the ith individual AGM during the jth sampling period (note that trti,j = 0 for all individuals prior to treatment and for the control group), is an individual effect for each AGM to account for interindividual variation and correlation arising from serial sampling, and tj,k is the sampling period effect for the kth treatment to allow FEC to vary through time. The same model structure and a logit link were used to describe the probability of shedding:
where pi,j is the probability of shedding for the ith individual AGM during the jth sampling period, βp,k,o is the effect for the kth treatment group prior to treatment, βp,k is the effect of the kth treatment, trtp,i,j is the treatment for the ith individual AGM at the jth sampling point (note that trtp,i,j = 0 for all individual prior to treatment and for the control group), indivp,i is an individual effect for each AGM to account for correlation arising from serial sampling, and tp,j,k is the sampling period effect for the kth treatment to allow FEC to vary through time.
These process models were linked to the data with the following zero-inflated, negative binomial model:
where ofeci,j is the observed FEC, dpois represents the probability density function (pdf) for the Poisson distribution, ρi,j is a mixing parameter with a γ distribution for which the shape and scale parameters are equal, sheddingi,j is an indicator of whether an individual was shedding at the jth sampling point, and sheddingi,j ∼ dbinom(pi,j) indicating sheddingi,j is a binomial random variable.
A normal (0,σ2 =1) prior was specified for the In(β0) parameter, and a normal (0,σ2 =25) prior was used for the βp,0,βk,βp,k Vκ For the individual AGM effects on the mean FEC and probability of shedding, zero-mean normal priors were specified with precisions that were assigned γ (1, 1) hyperpriors. Similarly, for the sampling period effects zero-mean normal priors were used with precisions-assigned γ (1, 1) hyperpriors. Finally, a γ (α,α) prior was assigned to, ρi,j and In (α) was given a uniform (0, 5) prior.
Three MCMC chains were run with dispersed starting values for 1,000,000 repetitions after a burn-in of similar length and a thinning interval of 2 due to computer memory limitations. Graphical plots and the Gelman–Rubin diagnostic statistic19 were used to look for evidence of nonconvergence of the chains. Prior distributions were varied to assess model sensitivity to their specification. Lastly, the goodness-of-fit of the model was examined by using several posterior predictive checks.19 First, the posterior distribution of the mean of the FEC across all sampling occasions was determined for the control group and all AGM prior to treatment and the associated Bayesian P value19 calculated. This metric allowed assessment of how well the model was able to fit FEC for the untreated animals. To determine the ability of the model to capture the treatment effects, the posterior distributions for the probability of zero FEC for each treatment group were estimated, and the Bayesian P values calculated for these metrics. All missing values were imputed during the analysis from the posterior predictive distributions. All analyses were conducted in program R version 4.0.143 by using the nimble package,12 and statistical significance was evaluated at α = 0.05.
Results
Data summary.
We initially selected 68 AGM for inclusion in the study, but 3 animals were removed due to lack of shedding of eggs during the pretreatment sampling period. Data were collected for 65 animals; 16 in the fenbendazole group, 16 in the ivermectin group, 15 in the combined treatment group, and 18 in the untreated control group. In total, 51 adults and 14 subadults were used, consisting of 35 males and 30 females. The anthelmintics were well tolerated for all animals and treatment groups, and no adverse reactions were seen. Microscopic examination revealed eggs from hookworms and Strongyloides spp., in addition to eggs from Trichuris spp. Only the EPG counts for Trichuris spp. were recorded.
A total of 317 observations were used in the analysis. Seven observations had missing FEC values due to failure of animals to produce an adequate fecal sample at that sampling time point. Among the different groups, the pretreatment FEC ranged from a minimum of 11 to a maximum of more than 22,000 EPG (Table 1).
Table 1.
Summary statistics for each treatment group prior to treatment
| Group | No. of animals | FEC (EPG) | Pretreatment shedding probability | |||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Maximum | Mean | Median | Mean | LCB | UCB | ||
| Fenbendazole | 16 | 0 | 22000 | 1810 | 250 | 0.982 | 0.902 | 0.999 |
| Ivermectin | 16 | 0 | 9580 | 1420 | 400 | 0.983 | 0.910 | 0.999 |
| Fenbendazole+ivermectin | 15 | 0 | 5380 | 728 | 250 | 0.979 | 0.591 | 0.999 |
| Control | 18 | 0 | 3250 | 402 | 150 | 0.934 | 0.848 | 0.984 |
Data are given as FEC in EPG. The lower and upper bounds of the 95% CI (LCB and UCB, respectively) for pretreatment shedding probability are provided.
FECR.
In all 3 treatment groups, the FEC decreased by day 7 during the 60-d posttreatment sampling period. Groups treated with fenbendazole alone or with the combined therapy showed 100% reduction from day 28 to 60. AGM receiving ivermectin alone showed an initial mean FECR that exceeded 90% at the first 2 sampling points and a mean FECR of 86% on day 60 (Table 2).
Table 2.
Summary of raw data by using mean FECR% for each treatment group for each sampling period.
| Group | No. of animals | Day 7 | Day 14 | Day 28 | Day 60 |
|---|---|---|---|---|---|
| Fenbendazole | 16 | 81 | 91 (13/16)a | 100 | 100 |
| Ivermectin | 16 | 90 | 97 | 89 (15/16)a | 86 (15/16)a |
| Fenbendazole+ivermectin | 15 | 100 | 93 | 100 (13/15)a | 100 |
| Control | 18 | 49 | 43 | 37 | 56 |
No. of animals sampled/total no. in group when feces could not be collected from the entire group for a particular sampling point.
Analysis results.
Comparison of groups prior to treatment.
We did not detect a significant difference (that is, all 95% credible intervals [CI] of differences contained zero; minimum P, 0.08) in the pretreatment shedding levels between the various groups, with shedding probabilities of 0.934 or greater for all groups (Table 1). The control group had a significantly lower estimated mean FEC than did the treatment groups (that is, all 95% CI differences did not contain zero; maximum P, 0.02; Figure 2). No differences were detected between the treatment groups (that is, all 95% CI of differences contained zero; minimum P, 0.16).
Figure 2.
Estimated mean fecal egg counts (FEC) for the fenbendazole (FBZ), ivermectin (IVM), and combined (FBZ+IVM) treatment groups and the control group over time. Note that day 0 values are pretreatment mean FEC. Error bars represent 95% CI.
Treatment effects on probability of shedding.
All 3 treatments reduced shedding of Trichuris spp. eggs in feces. The highest mean individual shedding probability (that is, average shedding probability over all treatment time points) occurred for animals treated with ivermectin (mean probability of 0.40; 95% CI, 0.20 to 0.61), followed by the fenbendazole group (mean probability of 0.21; 95% CI, 0.05 to 0.43), and lastly the combined-treatment group (mean probability of 0.07; 95% CI, 0.01 to 0.19; Table 3). Ivermectin had a significantly higher shedding probability than the combined treatment, with an estimated difference of 0.32 (95% CI, 0.01 to 0.57; P = 0.003). Fenbendazole alone did not differ significantly from the combined treatment group (0.14; 95% CI, –0.05 to 0.38; P = 0.074). The estimated mean shedding probability was significantly higher for the control than for all treatment groups (that is, all 95% CI differences did not contain zero; maximum P < 0.00001; Table 3).
Table 3.
Posterior means of proportional reduction in FEC compared with untreated animals and the probability of shedding for each treatment group, with associated lower and upper bounds of the 95% CI (LCB and UCB, respectively)
| Group | No. of animals | Mean | LCB | UCB | P c |
|---|---|---|---|---|---|
| Proportional reduction in FEC | |||||
| Fenbendazole | 16 | 0.04 | 0.01 | 0.14a | 0.0001a |
| Ivermectin | 16 | 0.07 | 0.02 | 0.18a | 0.00006a |
| Fenbendazole+ivermectin | 15 | 0.12 | 0.02 | 0.40a | 0.0018a |
| Control | 18 | 1b | NA | NA | NA |
| Probability of shedding | |||||
| Fenbendazole | 16 | 0.21 | 0.06 | 0.43a | <0.00001a |
| Ivermectin | 16 | 0.40 | 0.20 | 0.61a | <0.00001a |
| Fenbendazole+ivermectin | 15 | 0.07 | 0.01 | 0.19a | <0.00001a |
| Control | 18 | 0.93 | 0.85 | 0.98 | NA |
NA, not applicable.
Value for treatment group is significantly different than that for the control group.
Proportional reduction in FEC is defined as 1 because proportion is calculated relative to the control group.
P values indicate the probability that each treatment did not reduce FEC or shedding probability according to the posterior distribution of each treatment effect.
The shedding dynamics over time for the 3 treatment groups and controls are shown in Figure 3. Animals treated with fenbendazole showed a rapid decline in shedding probability, with negligible shedding by 28 d after treatment. Those treated with ivermectin had a consistent decline in shedding throughout the sampling period, but it was not as rapid or dramatic as the other 2 treatment groups. Finally, the group treated with both anthelmintics had low shedding probability across all sampling points, with the greatest shedding probability occurring 14 d after treatment. The shedding dynamics of the fenbendazole and ivermectin treatments did not differ significantly from the control group on the first sampling period after treatment (that is, all 95% CI differences contained zero, with P = 0.10 and 0.06, respectively; Figure 3). However shedding in the combined-treatment group was significantly lower than for other groups, with an estimated difference of –0.78 (95% CI, –0.94 to –0.53; P < 0.00001). The shedding probabilities from day 14 after treatment until the end of the study remained significantly higher for the control group as compared with all 3 treatment groups (that is, all 95% CI differences did not contain zero; maximum P, < 0.00005; Figure 3). When comparing the treatment groups, on day 28, shedding with fenbendazole was significantly lower than for ivermectin, with an estimated difference of –0.35 (95% CI, –0.66 to –0.09; P = 0.0036). The treatment groups were not significantly different for any of the remaining sampling periods (that is, all 95% CI of differences did not contain zero; minimum P = 0.07; Figure 3).
Figure 3.
Estimates of individual mean shedding probability for the fenbendazole (FBZ), ivermectin (IVM), and combined (FBZ+IVM) treatment groups and the control group over time. Note that day 0 values are pretreatment shedding probabilities. Error bars represent 95% CI.
Treatment effects on FEC.
The treatments significantly reduced the mean FEC as compared with the FEC values of each group before treatment and with those of the control group (Table 2). Among all the treatment groups, animals treated with both fenbendazole and ivermectin had the lowest estimated mean FEC (5 EPG; 95% CI, 0 to 25) across all sampling points, whereas those treated solely with ivermectin had the highest; 28 EPG [6 to 83] (Table 4). The treatment effects were not significantly different among groups, with the P value of these differences estimated as 0.19 and all 95% CI for these differences including zero. The control group had an estimated mean FEC of 262 EPG (95% CI, 158 to 427). Figure 2 shows the estimated mean FEC dynamics.
Table 4.
Posterior means of FEC (given as EPG) for each group across all posttreatment sampling time points and associated lower and upper bounds of the 95% CI (LCB and UCB, respectively)
| Group | No. of animals | Mean | LCB | UCB | P b |
|---|---|---|---|---|---|
| Fenbendazole | 16 | 10 | 21 | 39a | 0.00008a |
| Ivermectin | 16 | 28 | 6 | 83a | 0.00006a |
| Fenbendazole+ivermectin | 15 | 5 | 0 | 25a | 0.0017a |
| Control | 18 | 262 | 158 | 427 | — |
Value for the treatment group is significantly different than that for the control group.
P values are the probability that each treatment did not reduce FEC according to the posterior distribution of each treatment effect.
For individual animals in each group that were still shedding after treatment, the estimated proportions of mean FEC remaining relative to the FEC before treatment are shown in Table 3. The fenbendazole–ivermectin group had the highest proportion of eggs remaining (0.12; 95% CI, 0.02, 0.40), whereas the greatest reduction occurred in the fenbendazole group with a proportion of 0.04 (95% CI, 0.009 to 0.14); however, the treatment groups were not significantly different with the minimum P value, which was estimated as 0.19, and all 95% CI for these differences included zero.
Model evaluation.
No evidence of nonconvergence of the MCMC chains was found for our generalized linear model via graphical checks, and the univariate and multivariate potential scale reduction factors all had 95% CI in which the upper limit was 1.12 or less. We also found no evidence of prior sensitivity, although the rate of chain mixing was variable across the various prior distributions examined. The priors described earlier were chosen to maximize chain mixing and rate of convergence. No significant problems were identified with the goodness-of-fit of the model, with all Bayesian P values equaling 0.49 or greater.
Discussion
In this study, both fenbendazole alone and combined with ivermectin proved to be more effective than use of ivermectin alone for the treatment of Trichuris spp. in AGM. Concurrent treatment with fenbendazole and ivermectin appeared to be the most effective treatment, based on the rapid decrease in shedding rates and FEC. Studies in other Old World primate species similarly show that avermectins are less effective than benzimidazoles in treating Trichuris spp. infections.
An investigation in Kenya27 compared the use of albendazole and ivermectin in vervets (Chlorocebus aethiops) over a 28-d period. The results showed that albendazole alone and in combination with ivermectin was more effective than ivermectin alone. In addition, treatment with albendazole alone seemed to be less effective than using fenbendazole, with a 75% FECR compared with the 100% achieved our current study at a similar time point. In the previous study,27 ivermectin (dosed at 300 µg/kg SC daily for 3 d) had no effect on Trichuris spp. infection, with a 0% FECR reported at day 28 compared with the 89% demonstrated in the current study, which used a smaller dose (200 µg/kg) that was administered only once. The previous study27 covered a shorter time period (28 d) and used fewer animals (only 6 per group), compared with the 15 or 16 that we used here).
Other authors51 compared ivermectin and mebendazole in rhesus macaques (Macaca mulatta) and reported FECR of 97% and 100%, respectively, at 11 d after treatment. Olive baboons (Papio cynocephalus anubis) treated with fenbendazole stopped shedding eggs within 6 d of treatment and remained negative for as long as 65 d afterward, whereas those treated with milbemycin oxime (a second-generation avermectin) showed a reduction in the FEC, but shedding was not eliminated even after collection of samples 30 d apart.45 The study did not include a combination treatment of milbemycin oxime and fenbendazole.
A study38 that used fenbendazole at a dose of 10 mg/kg PO for 3 d to treat Trichuris infection in 4 species of captive NHP achieved a FECR of only 40% at 21 d after treatment. The authors achieved a 94% FECR when using the same dose and increasing the number of treatment days to 5. Our present study achieved high FECR results using a higher dose of fenbendazole for 4 treatment days.
The primary action of benzimidazoles, of which fenbendazole is a second-generation compound, is to bind the cytoskeletal protein tubulin, thereby blocking the formation of the microtubule matrix necessary for functioning of eukaryotic cells.32 These anthelmintics appear to have a higher affinity for parasitic tubulin than vertebrate tubulin17 and thus are not as toxic toward host cells. Fenbendazole is poorly absorbed via the gastrointestinal tract after oral administration and is effective against adult, immature, arrested larval, and egg stages of Trichuris spp. and other parasites.2 The poor absorption from the alimentary tract likely allows for prolonged exposure of susceptible gastrointestinal parasites to the drug.
Avermectins are 16-membered macrocyclic lactones that possess anthelmintic, insecticidal and acaricidal activity.4,41 Macrocyclic lactones affect various life stages of nematode and arthropod species by modulating the Cys-loop family of ligand-gated ion channels, including glutamate-gated chloride ion channels.37 The resulting influx of chloride ions leads to long-lasting hyperpolarization, which decreases the formation of action potentials and blocks further functions.36 This affects motor neurons, interneurons, and muscle cells, leading to inhibition of pharyngeal pumping, motility, and egg laying in parasitic nematodes.31 Ivermectin is a chemically modified derivative of naturally occurring avermectin B1, which has potent activity against a broad spectrum of parasitic nematodes after both oral and parenteral administration.33
We used FEC as the response variable for comparison in our study; this index gives no information regarding worm burden but does indicate the presence of ovulating females and at least one male in the gastrointestinal tract. FEC reveals whether ovulating female Trichuris spp. are present in the gastrointestinal tract. The time from host infection with infective eggs to adult helminth development is approximately 60 to 70 d.23
The animals were sampled over a 60-d period, thus allowing time for any infective L1 larvae present to mature. The AGM in this study were housed in elevated stainless-steel cages, which were cleaned daily to minimize possibility of autoinfection. Given the mode of action, both fenbendazole and ivermectin likely are effective against all stages of Trichuris spp. from L1 to adults. Ivermectin treatment alone was unsuccessful in eliminating all the Trichuris spp. present. The lack of shedding with fenbendazole alone and in combination with ivermectin at days 28 and 60 indicates that no egg-producing females were present in the gastrointestinal tracts of the study animals. Several factors can depress worm ovulation, including host immunity, density-dependent factors, and environmental conditions.8,47 Extending the sampling period past 60 d would provide an assessment of whether any ovulating female Trichuris had survived.
In addition, more frequent sampling during the first 2 wk, perhaps every other day or daily, may enable better comparison of the short-term effectiveness of each treatment group by showing how quickly the anthelmintics worked. One could also investigate whether oral administration of ivermectin would be more effective. In our current study, fenbendazole used alone at a dose of 50 mg/kg PO daily for 4 d appeared to be effective in significantly reducing shedding of Trichuris spp. in AGM for a period of at least 60 d. According to these results, repeat dosing with fenbendazole may not be necessary in AGM for 7 to 8 wk after the initial treatment. Unlike fenbendazole, ivermectin used alone as a single dose at 200 µg/kg SC did not eliminate shedding, meaning that ovulating females were still present. Another dose after 14 d may be necessary to eliminate those adults. The AGM were all in good health throughout the investigation, and a good outcome was achieved. No clinical signs were seen that might have implied a high worm burden. FEC cannot quantify the number of adults present in the gastrointestinal tract. Even if the animals were showing a high FEC, their actual number of adults may have been low. Consideration must be given that the treatment regimens of fenbendazole and ivermectin used in the study may have a different effect on AGM carrying a heavy parasite burden.
Our data clearly show individual and temporal variability in Trichuris spp. shedding rates, as evidenced by the wide distribution of FEC documented from animals before treatment and in the control group. Similarly, the FEC fluctuate through time in the absence of treatment (Figures 2 and 3). We included an untreated group in our study specifically to control for these issues. Despite randomly assigning animals to treatment groups, a difference was present between our control group and the 3 treatment groups. Although the groups had similar individual shedding probabilities, the control group had lower baseline FEC prior to treatment, compared with animals in the anthelmintic treatment groups. We do not believe this difference affected the study’s findings, because each of the anthelmintic treatments reduced the shedding probability and the FEC to levels well below those found in the control group. In addition, our approach allowed us to compare pre- and posttreatment shedding probabilities and FEC within each group and to account for the differences between groups.
In conclusion, fenbendazole and ivermectin significantly reduced EPG output in all the anthelmintic treatment groups in this study as compared with pretreatment values and the control group. Fenbendazole and ivermectin can be used to effectively treat Trichuris infection in AGM; however, fenbendazole may be more effective when used solely or in combination with other anthelmintic treatments.
Acknowledgments
We thank David Elcock (Barbados Veterinary Services Laboratory) for providing some of the equipment used in the study; Jean Baulu, Genevieve Marsh, and the staff of the Barbados Primate Research Center; and Eric Trotman who assisted in sample analysis. Any use of trade, product, or firm names does not imply endorsement by the US Government.
References
- 1.Anderson J, Upadhayay R, Sudimack D, Nair S, Leland M, Williams JT, Anderson TJC. 2012. Trichuris sp. and Strongyloides sp. infections in a free-ranging baboon colony. J Parasitol 98:205–208. 10.1645/GE-2493.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogan J, Armour J. 1987. Anthelmintics for ruminants. Int J Parasitol 17:483–491. 10.1016/0020-7519(87)90124-X. [DOI] [PubMed] [Google Scholar]
- 3.Boulton A, Horrocks JA, Baulu J. 1996. The Barbados vervet monkey (Cercopithecus aethiops sabaeus): Changes in population size and crop damage, 1980–1994. Int J Primatol 17:831–844. 10.1007/BF02735267. [DOI] [Google Scholar]
- 4.Burg RW, Miller BM, Baker EE, Birnbaum J, Curries JA, Harman R, Kong VL, Monaghan RL, Olson G, Putter I, Tunac JB, Wallick H, Stapley EO, Oiwa R, Omura S. 1979. Avermectins, new family of potent anthelmintic agents: producing organism and fermentation. Antimicrob Agents Chemother 15:361–367. 10.1128/AAC.15.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calle PP, Joslin JO. 2014. New world and old world monkeys. p 301–335. Chapter 37. In: Fowler ME, Miller RE, editors. Zoo and wild animal medicine, 8th ed. St Louis (MO): Elsevier Saunders. [Google Scholar]
- 6.Callejón R, Halajian A, Cutillas C. 2017. Description of a new species, Trichuris ursinus n. sp.(Nematoda: Trichuridae) from Papio ursinus Keer, 1792 from South Africa. Infect Genet Evol 51:182–193. 10.1016/j.meegid.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Cavallero S, Nejsum P, Cutillas C, Callejón R, Doležalová J, Modrý D, D’Amelio S. 2019. Insights into the molecular systematics of Trichuris infecting captive primates based on mitochondrial DNA analysis. Vet Parasitol 272:23–30. 10.1016/j.vetpar.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Christensen CM, Barnes EH, Nansen P, Roepstorff A, Slotved HC. 1995. Experimental Oesophagostomum dentatum infection in the pig: worm populations resulting from single infections with three doses of larvae. Int J Parasitol 25:1491–1498. 10.1016/0020-7519(95)00085-2. [DOI] [PubMed] [Google Scholar]
- 9.Clingerman KJ, Summers L. 2005. Development of a body condition scoring system for nonhuman primates using Macaca mulatta as a model. Lab Anim (NY) 34:31–36. 10.1038/laban0505-31. [DOI] [PubMed] [Google Scholar]
- 10.Coles GC, Bauer C, Borgsteede FHM, Geerts S, Klei TR, Taylor MA, Waller PJ. 1992. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol 44:35–44. 10.1016/0304-4017(92)90141-U. [DOI] [PubMed] [Google Scholar]
- 11.Cutillas C, de Rojas M, Zurita A, Oliveros R, Callejón R. 2014. Trichuris colobae n. sp.(Nematoda: Trichuridae), a new species of Trichuris from Colobus guereza kikuyensis. Parasitol Res 113:2725–2732. 10.1007/s00436-014-3933-6. [DOI] [PubMed] [Google Scholar]
- 12.de Valpine P, Turek D, Paciorek CJ, Anderson-Bergman C, Temple Lang D, Bodik R. 2017. Programming with models: Writing statistical algorithms for general model structures with nimble. J Comput Graph Stat 26:403–413. 10.1080/10618600.2016.1172487. [DOI] [Google Scholar]
- 13.Despommier DD, Gwadz RW, Hotez PJ. 2012. Parasitic diseases. New York (NY): Springer–Verlag. [Google Scholar]
- 14.Dore KM. 2016. Vervets in the Caribbean. p 1–3. In: The international encyclopedia of primatology. 10.1002/9781119179313.wbprim0358. John Wiley and Sons. [DOI] [Google Scholar]
- 15.Ervin F, Palmour R. 2003. Primates for 21st century biomedicine: The St. Kitts vervet (Chlorocebus aethiops, SK). p 49–53. In: International Perspectives: The future of nonhuman primate resources. Washington (DC): National Research Council. [Google Scholar]
- 16.Feeser P, White F. 1992. Medical management of Lemur catta, Varecia varegata, and Propithecus verreauxi in natural habitat enclosures. p 320–323. In: Proceedings of the annual meeting of the american association of zoo veterinarians.
- 17.Friedman PA, Platzer EG. 1980. Interaction of anthelmintic benzimidazoles with AscarisSuum embryonic tubulin. Biochim Biophys Acta 630:271–278. 10.1016/0304-4165(80)90431-6. [DOI] [PubMed] [Google Scholar]
- 18.García-Sánchez AM, Rivero J, Callejón R, Zurita A, Reguera-Gomez M, Valero MA, Cutillas C. 2019. Differentiation of Trichuris species using a morphometric approach. Int J Parasitol Parasites Wildl 9:218–223. 10.1016/j.ijppaw.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gelman A, Carlin J, Stern H, Dunson D, Vehtari A, Rubin D. 2015. Bayesian data analysis. New York (NY): Chapman and Hall/CRC. 10.1201/b16018 [DOI] [Google Scholar]
- 20.Harvey DC, Laćan G, Melega WP. 2000. Regional heterogeneity of dopaminergic deficits in vervet monkey striatum and substantia nigra after methamphetamine exposure. Exp Brain Res 133:349–358. 10.1007/s002210000386. [DOI] [PubMed] [Google Scholar]
- 21.Hartmann C, Göttling J, Nadler T, Streicher U. 2015. Effect of orally applied ivermectin on gastrointestinal nematodes in douc langurs (Pygathrix spp.). Vietnamese J Primatol 2:39–44. [Google Scholar]
- 22.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 23.Izurieta R, Reina-Ortiz M, Ochoa-Capello T. [Internet]. 2018. Trichuris trichiura. [Cited 8 June 2020] Available at: www.waterpathogens.org/book/trichuris-trichiura. 10.14321/waterpathogens.43 [DOI] [Google Scholar]
- 24.Johnson-Delaney CA. 2009. Parasites of captive nonhuman primates. Vet Clin North Am Exot Anim Pract 12:563–581. 10.1016/j.cvex.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Jorgensen MJ, Lambert KR, Breaux SD, Baker KC, Snively BM, Weed JL. 2017. Pair housing of vervets/African green monkeys for biomedical research. Am J Primatol 79:1–10. 10.1002/ajp.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joshua K, Yidawi JP, Sada A, Msheliza EG, Turaki UA. 2020. Prevalence and morphotype diversity of Trichuris species and other soil-transmitted helminths in captive non-human primates in northern Nigeria. J Threat Taxa 12:16239–16244. 10.11609/jott.4552.12.10.16239-16244. [DOI] [Google Scholar]
- 27.Kagira JM, Oluoch G, Waititu K, Mulei I, Maingi N, Ngotho M. 2011. High efficacy of combined albendazole and ivermectin treatment against gastrointestinal nematodes in vervet monkeys and baboons. Scand J Lab Anim Sci 38:187–193. 10.23675/sjlas.v38i3.239 [DOI] [Google Scholar]
- 28.Kaplan RM, Klei TR, Lyons ET, Lester G, Courtney CH, French DD, Tolliver SC, Vidyashankar AN, Zhao Y. 2004. Prevalence of anthelmintic-resistant cyathostomes on horse farms. J Am Vet Med Assoc 225:903–910. 10.2460/javma.2004.225.903. [DOI] [PubMed] [Google Scholar]
- 29.Kochapakdee S, Pandey VS, Pralomkarn W, Choldumrongkul S, Ngampongsai W, Lawpetchara A. 1995. Anthelmintic resistance in goats in southern Thailand. Vet Rec 137:124–125. 10.1136/vr.137.5.124. [DOI] [PubMed] [Google Scholar]
- 30.Kouassi RYW, McGraw SW, Yao PK, Abou-Bacar A, Brunet J, Pesson B, Bonfoh B, N’goran EK, Candolfi E. 2015. Diversity and prevalence of gastrointestinal parasites in seven non-human primates of the Taï National Park, Côte d’Ivoire. Parasite 22:1–12. 10.1051/parasite/2015001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotze AC, Hines BM, Ruffell AP. 2011. A reappraisal of the relative sensitivity of nematode pharyngeal and somatic musculature to macrocyclic lactone drugs. Int J Parasitol Drugs Drug Resist 2:29–35. 10.1016/j.ijpddr.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lacey E, Gill JH. 1994. Biochemistry of benzimidazole resistance. Acta Trop 56:245–262. 10.1016/0001-706X(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 33.Laing R, Gillan V, Devaney E. 2017. Ivermectin–old drug, new tricks? Trends Parasitol 33:463–472. 10.1016/j.pt.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemere CA, Beierschmitt A, Iglesias M, Spooner ET, Bloom JK, Leverone JF, Zheng JB, Seabrook TJ, Louard D, Li D, Selkoe DJ, Palmour RM, Ervin FR. 2004. Alzheimer’s disease Aβ vaccine reduces central nervous system Aβ levels in a non-human primate, the Caribbean vervet. Am J Pathol 165:283–297. 10.1016/S0002-9440(10)63296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martín J, Hermida L, Castro J, Lazo L, Martínez R, Gil L, Romero Y, Puente P, Zaragoza S, Cosme K, Guzmán MG, Cardosa J, Guillén G. 2009. Viremia and antibody response in green monkeys (Chlorocebus aethiops sabaeus) infected with dengue virus type 2: a potential model for vaccine testing. Microbiol Immunol 53:216–223. 10.1111/j.1348-0421.2009.00112.x. [DOI] [PubMed] [Google Scholar]
- 36.Ménez C, Sutra JF, Prichard R, Lespine A. 2012. Relative neurotoxicity of ivermectin and moxidectin in Mdr1ab (–/–) mice and effects on mammalian GABA (A) channel activity. PLoS Negl Trop Dis 6:1–10. 10.1371/journal.pntd.0001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyers JI, Gray M, Kuklinski W, Johnson LB, Snow CD, Black WC, Partin KM, Foy BD. 2015. Characterization of the target of ivermectin, the glutamate-gated chloride channel, from Anopheles gambiae. J Exp Biol 218:1478–1486. 10.1242/jeb.118570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moudgil AD, Singla LD. 2018. Molecular confirmation and anthelmintic efficacy assessment against natural trichurid infections in zoo-housed non-human primates. J Med Primatol 47:388–392. 10.1111/jmp.12358. [DOI] [PubMed] [Google Scholar]
- 39.Munene E, Otsyula M, Mbaabu DA, Mutahi WT, Muriuki SM, Muchemi GM. 1998. Helminth and protozoan gastrointestinal tract parasites in captive and wild-trapped African non-human primates. Vet Parasitol 78:195–201. 10.1016/S0304-4017(98)00143-5. [DOI] [PubMed] [Google Scholar]
- 40.Mutani A, Rhynd K, Brown G. 2003. A preliminary investigation on the gastrointestinal helminths of the Barbados green monkey, Cercopithecus aethiops sabaeus. Rev Inst Med Trop São Paulo 45:193–195. 10.1590/S0036-46652003000400003. [DOI] [PubMed] [Google Scholar]
- 41.Pitterna T, Cassayre J, Hüter OF, Jung PM, Maienfisch P, Kessabi FM, Quaranta L, Tobler H. 2009. New ventures in the chemistry of avermectins. Bioorg Med Chem 17:4085–4095. 10.1016/j.bmc.2008.12.069. [DOI] [PubMed] [Google Scholar]
- 42.Ravasi DF, O’Riain MJ, Davids F, Illing N. 2012. Phylogenetic evidence that two distinct Trichuris genotypes infect both humans and non-human primates. PLoS One 7:1–8. 10.1371/journal.pone.0044187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.R Core Team. [Internet]. 2019. R: A language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing. [Cited 18 November 2019.] Available at: https://www.R-project.org/. [Google Scholar]
- 44.Reichard MV, Thomas JE, Chavez-Suarez M, Cullin CO, White GL, Wydysh EC, Wolf RF. 2017. Pilot study to assess the efficacy of ivermectin and fenbendazole for treating captive-born Olive Baboons (Papio anubis) coinfected with Strongyloides fülleborni and Trichuris trichiura. J Am Assoc Lab Anim Sci 56:52–56. [PMC free article] [PubMed] [Google Scholar]
- 45.Reichard MV, Wolf RF, Carey DW, Jane Garrett J, Briscoe HA. 2007. Efficacy of fenbendazole and milbemycin oxime for treating baboons (Papio cynocephalus anubis) infected with Trichuris trichiura. J Am Assoc Lab Anim Sci 46:42–45. [PubMed] [Google Scholar]
- 46.Reichard MV, Wolf RF, Clingenpeel LC, Doan SK, Jones AN, Gray KM. 2008. Efficacy of fenbendazole formulated in a commercial primate diet for treating specific pathogen-free baboons (Papio cynocephalus anubis) infected with Trichuris trichiura. J Am Assoc Lab Anim Sci 47:51–55. [PMC free article] [PubMed] [Google Scholar]
- 47.Stear MJ, Bishop SC, Doligalska M, Duncan JL, Holmes PH, Irvine J, McCririe L, McKellar QA, Sinski E, Murray MAX. 1995. Regulation of egg production, worm burden, worm length and worm fecundity by host responses in sheep infected with Ostertagia circumcincta. Parasite Immunol 17:643–652. 10.1111/j.1365-3024.1995.tb01010.x. [DOI] [PubMed] [Google Scholar]
- 48.Stephenson LS, Holland CV, Cooper ES. 2000. The public health significance of Trichuris trichiura. Parasitology 121 Suppl:S73–S95. 10.1017/S0031182000006867. [DOI] [PubMed] [Google Scholar]
- 49.Strait K, Else JG, Eberhard ML. 2012. Parasitic diseases of nonhuman primates. p 243–244. In: Abee CR, Mansfield K, Tardif SD, Morris T, editors. Nonhuman primates in biomedical research: diseases, 2nd ed., London. Elsevier Academic. [Google Scholar]
- 50.Tabasshum T, Mukutmoni M, Begum A. 2018. Occurrence of gastrointestinal Helminths in captive rhesus macaques (Macaca mulatta). Bangladesh J Zool 46:231–237. 10.3329/bjz.v46i2.39065. [DOI] [Google Scholar]
- 51.Wang T, Yang GY, Yan HJ, Wang S, Bian Y, Chen AC, Bi FJ. 2008. Comparison of efficacy of selamectin, ivermectin and mebendazole for the control of gastrointestinal nematodes in rhesus macaques, China. Vet Parasitol 153:121–125. 10.1016/j.vetpar.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 52.Westcott MM, Smedberg J, Jorgensen MJ, Puckett S, Lyles DS. 2018. Immunogenicity in African green monkeys of m protein mutant vesicular stomatitis virus vectors and contribution of vector-encoded flagellin. Vaccines (Basel) 6:1–13. 10.3390/vaccines6010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie Y, Zhao B, Hoberg EP, Li M, Zhou X, Gu X, Lai W, Peng X, Yang G. 2018. Genetic characterisation and phylogenetic status of whipworms (Trichuris spp.) from captive non-human primates in China, determined by nuclear and mitochondrial sequencing. Parasit Vectors 11:1–16. 10.1186/s13071-018-3100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yao C, Walkush J, Shim D, Cruz K, Ketzis J. 2018. Molecular species identification of Trichuris trichiura in African green monkey on St. Kitts, West Indies. Vet Parasitol Reg Stud Reports 11:22–26. 10.1016/j.vprsr.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Zhao B, Li M, Wang Q, Niu L, Deng J, Yan H, Yang G. 2016. Anthelmintic effect of Mebendazole and fenbendazole on Trichuris infection in primates. Sichuan J Zool 35:249–251.[[Article in Chinese]]. [Google Scholar]




