Devendra K. Agrawal, PhD (Biochem), PhD (Med Sci), MBA, MS (ITM), and Vikrant Rai, MBBS, MS, PhD
Central Message.
Preconditioning of mesenchymal stem cells and using mesenchymal-stem-cell–derived extracellular vesicles or exosomes via intravascular route improve recovery in spinal cord ischemia–reperfusion injury.
See Article page 23.
Spinal cord ischemic-reperfusion injury (SIRI) during thoracoabdominal aneurysm repair can lead to changes in motor, sensory, and autonomic functions resulting in neurological deficiency and disability. Minimally invasive surgical procedures limit the ischemic injury, but SIRI remains a distressing complication manifested by paraplegia or paraparesis.1 The changing microenvironment after SIRI inhibits axonal regeneration. Bone-marrow–derived mesenchymal stem cells (BM-MSCs), due to their plasticity, can be used as potential therapeutics by modulating the microenvironment. Therapeutic role of MSCs in spinal cord injury is via regulating gliosis, antiapoptosis, inflammation, oxidative stress, angiogenesis, differentiation to neural and glial cells, axonal regeneration, and secretion of growth factors, cytokines, and chemokines.2,3 MSCs are administered intracranially/intrathecally or intravascularly with better results for larger lesions via the intravascular route.4 Retro-orbital injection of MSCs after ischemic injury plays a protective role in repairing SIRI in rats by preventing autophagy and promoting neurite growth and regeneration.3 Preemptive intrathecal injection of MSCs also plays a protective role by stabilizing the blood–spinal cord barrier integrity after SIRI via matrix metallopeptidase 9 and tumor necrosis factor-α inhibition.5 Increased number of neurons and decreased damage to neurons in animal models and mixed results of improvement in motor activity and sphincter control in some patients, whereas no improvement in others, support the notion of therapeutic use of MSCs; however, warrants an in-depth understanding of the repair mechanisms to enhance therapeutic efficacy, efficiency, reproducibility, and to promote clinical use of MSCs.2,6,7
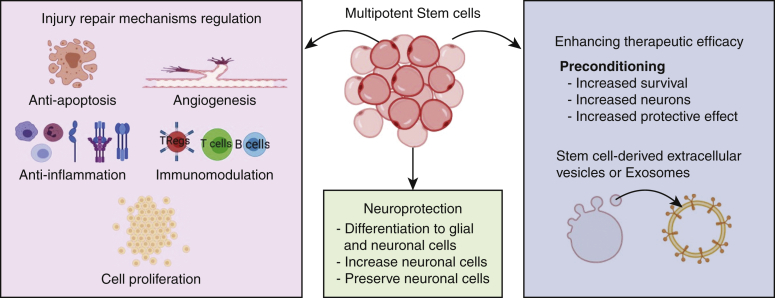
Nakai and colleagues8 report improved hindlimb motor function with significantly preserved motor neurons in mice with SIRI and injected intravenously with human BM-MSCs by promoting angiogenesis and antiapoptosis and inhibition of proinflammatory cytokines. Using Dil (1,1'-Dioctadecyl-3,3,3',3'-Tetramethylindocarbocyanine Perchlorate)-labeled human BM-MSCs revealed localization of MSCs in the ventral horn of the spinal cord; however, the number of surviving MSCs was not evaluated. This is important because apoptosis of transplanted MSCs is a limitation in MSC-based therapy. Preconditioning of MSC with hypoxia effectively increases the survival rate of BM-MSCs via increased HIF-1α (hypoxia-inducible factor 1-alpha), neurologic function, blood–spinal cord barrier, and tissue damage along with apoptosis inhibition after SIRI.9 Administration of simulated microgravity-cultured MSCs improves motor recovery after SIRI in rats.10 Recently, the protective effect of MSCs has been attributed to the paracrine effect of MSC-derived extracellular vesicles (EVs)/exosomes,7 and cell-free therapy using MSC-exosomes is an exciting novel therapy in spinal injury. However, the source for the most potent EVs with therapeutic efficacy needs to be determined. The route of injecting MSCs may have different effects and Nakai and colleagues8 have the advantage of using commercially available allogeneic and autologous human BM-MSCs intravenously compared with previous studies11 using autologous MSCs administered intrathecally. Investigating the acute and long-term effects of MSC-based therapy on the number of neurons and axonal regeneration is also important. These findings support the feasibility of therapeutic use of MSCs (Figure 1); however, the route of administration, preconditioning of MSCs to enhance survival for longer duration, using MSC-derived EVs, and investigating the long-term effect on neuronal number; generation of action potential; and motor, sensory, and autonomic function; microenvironment of the ischemia–reperfusion injury site; host-graft interactions; and the feasibility of therapeutic cell delivery using 3-dimesional scaffolds should be the focus of the follow-up research.
Figure 1.
Cellular processes involved in the regulation of injury repair mechanisms, neuroprotection, and enhancement of therapeutic efficacy of stem cells.
Footnotes
Dr Agrawal is supported by research grants R01 HL144125 and R01HL147662 from the National Institutes of Health. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures: The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
References
- 1.Bisdas T., Panuccio G., Sugimoto M., Torsello G., Austermann M. Risk factors for spinal cord ischemia after endovascular repair of thoracoabdominal aortic aneurysms. J Vasc Surg. 2015;61:1408–1416. doi: 10.1016/j.jvs.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 2.Liau L.L., Looi Q.H., Chia W.C., Subramaniam T., Ng M.H., Law J.X. Treatment of spinal cord injury with mesenchymal stem cells. Cell Biosci. 2020;10:1–17. doi: 10.1186/s13578-020-00475-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin F., Meng C., Lu R., Li L., Zhang Y., Chen H., et al. Bone marrow mesenchymal stem cells repair spinal cord ischemia/reperfusion injury by promoting axonal growth and anti-autophagy. Neural Regen Res. 2014;9:1665–1671. doi: 10.4103/1673-5374.141801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bliss T.M., Andres R.H., Steinberg G.K. Optimizing the success of cell transplantation therapy for stroke. Neurobiol Dis. 2010;37:275–283. doi: 10.1016/j.nbd.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang B., Wang H., Sun X.J., Li X.Q., Ai C.Y., Tan W.F., et al. Intrathecal transplantation of bone marrow stromal cells attenuates blood-spinal cord barrier disruption induced by spinal cord ischemia-reperfusion injury in rabbits. J Vasc Surg. 2013;58:1043–1052. doi: 10.1016/j.jvs.2012.11.087. [DOI] [PubMed] [Google Scholar]
- 6.Qu J., Zhang H. Roles of mesenchymal stem cells in spinal cord injury. Stem Cells Int. 2017;2017:5251313. doi: 10.1155/2017/5251313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Y., Wen L.L., Li Y.F., Wu K.M., Duan R.R., Yao Y.B., et al. Exosomes derived from bone marrow mesenchymal stem cells protect the injured spinal cord by inhibiting pericyte pyroptosis. Neural Regen Res. 2022;17:194–202. doi: 10.4103/1673-5374.314323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakai H., Fujita Y., Masuda S., Komatsu M., Tani A., Okita Y., et al. Intravenous injection of adult human bone marrow mesenchymal stromal cells attenuates spinal cord ischemia/reperfusion injury in a murine aortic arch cross-clamping model. J Thorac Cardiovasc Surg Open. 2021;7:23–40. doi: 10.1016/j.xjon.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo Z., Wu F., Xue E., Huang L., Yan P., Pan X., et al. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival by inducing HIF-1alpha in injured neuronal cells derived exosomes culture system. Cell Death Dis. 2019;10:134. doi: 10.1038/s41419-019-1410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurose T., Takahashi S., Otsuka T., Nakagawa K., Imura T., Sueda T., et al. Simulated microgravity-cultured mesenchymal stem cells improve recovery following spinal cord ischemia in rats. Stem Cell Res. 2019;41:101601. doi: 10.1016/j.scr.2019.101601. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z., Fang B., Tan Z., Zhang D., Ma H. Hypoxic preconditioning increases the protective effect of bone marrow mesenchymal stem cells on spinal cord ischemia/reperfusion injury. Mol Med Rep. 2016;13:1953–1960. doi: 10.3892/mmr.2016.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]