Abstract
Objective
Statins have been shown to delay the inevitable progression of atherosclerosis in native coronaries and saphenous vein grafts, thereby reducing ischemic events after surgical coronary revascularization. However, there is significant controversy as to whether titrating statin therapy to concrete cholesterol targets is appropriate.
Methods
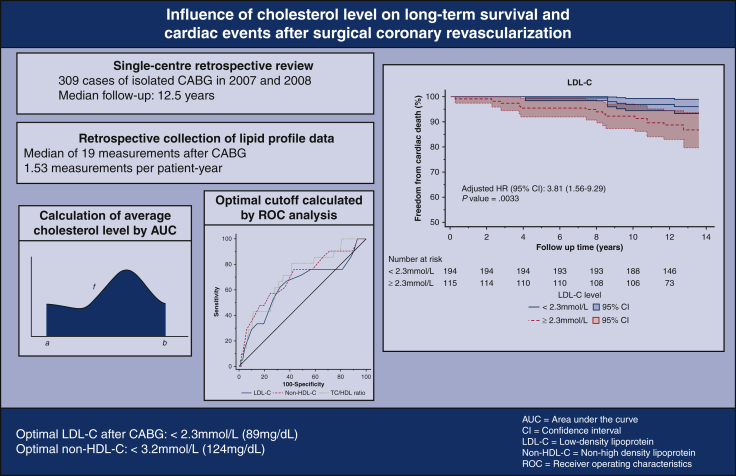
A single-center retrospective analysis of 309 consecutive patients who underwent isolated coronary artery bypass graft in 2007 and 2008 was performed. Measurements of lipid profile subcomponents, namely total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglycerides, in mmol/L, were obtained by retrospective review of electronic health records. The primary end point was cardiac death. The secondary end point was the composite of cardiac events, including cardiac death, nonfatal myocardial infarction, hospitalization for unstable angina, and target lesion revascularization. Database lock date was August 15, 2020.
Results
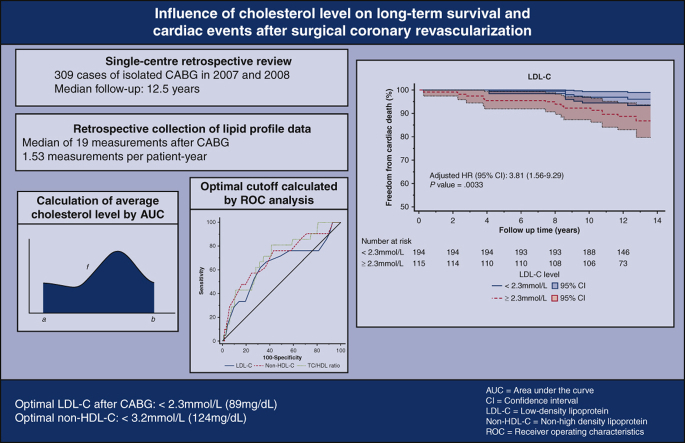
The median follow-up duration was 12.5 years. Cardiac death occurred in 6.8% of the cohort. Cardiac events occurred in 21.7% of the cohort. New-onset myocardial infarction occurred in 8.7% (n = 27), of which 48.1% (n = 13) underwent repeat revascularization. A 2-level nested Cox proportional hazards regression model was constructed to determine whether cholesterol target attainment was independently associated with cardiac events. After risk adjustment, LDL-C, non–HDL-C, total cholesterol (TC), and TC/HDL-C ratio were independently associated with cardiac death. In receiver operating characteristics analyses, the optimal cut-off values for non–HDL-C, LDL-C, and TC/HDL-C ratio were 3.2 mmol/L, 2.3 mmol/L, and 3.5, respectively.
Conclusions
Exposure to elevated LDL-C and non–HDL-C cholesterol levels independently predicted long-term cardiac death after coronary artery bypass graft.
Key Words: CABG, statin, dyslipidemia, LDL, non-HDL
Abbreviations and Acronyms: CABG, coronary artery bypass graft; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; non–HDL-C, non–high-density lipoprotein cholesterol; ROC, receiver operating characteristic; TC, total cholesterol; TC/HDL-C, total cholesterol-to-high-density lipoprotein cholesterol ratio; TG, triglycerides
Graphical abstract
Freedom from cardiac death after CABG at the LDL-C threshold of 2.3 mmol/L.
Central Message.
Persistent lipid abnormalities are associated with late cardiac death after surgical revascularization. The optimal LDL-C and non–HDL-C levels are below 2.3 mmol/L and 3.2 mmol/L, respectively.
Perspective.
Lipid targets do matter after surgical revascularization. Persistent lipid abnormalities are associated with late cardiac death after surgical revascularization. The optimal LDL-C and non–HDL-C levels after CABG are below 2.3 mmol/L (89 mg/dL) and 3.2 mmol/L (124 mg/dL), respectively.
See Commentary on page 204.
In patients with multivessel coronary artery disease who have undergone surgical revascularization, statins are demonstrated to delay the inevitable progression of atherosclerosis in native coronaries and saphenous vein grafts, thereby reducing angiographic stenosis and ischemic events in the short- to medium-term.1
Although consensus exists regarding the benefits of statins for patients with established atherosclerotic cardiovascular disease, a philosophical divide remains between the American Heart Association and European Society of Cardiology as to whether “treating to target” is appropriate. Current American guidelines do not recommend titration of low-density lipoprotein cholesterol (LDL-C) to target values, whereas European guidelines recommend an LDL-C goal of less than 1.8 mmol/L (70 mg/dL) for patients with established atherosclerotic cardiovascular disease.
There is an evidence gap in the literature regarding how stringent cholesterol levels should be after surgical revascularization. Furthermore, it is unclear which lipid profile subcomponent best prognosticates future cardiac events. The aim of the study is to ascertain which lipid profile subcomponent correlated best with long-term outcomes as well as the optimal levels to be achieved.
Methodology
Study Design
This was a single-center retrospective, observational study of all consecutive patients who underwent isolated coronary artery bypass graft (CABG) for left main or triple-vessel disease from January 1, 2007, to December 31, 2008, at Prince of Wales Hospital, Hong Kong, conducted using the Hospital Authority Clinical Management System. Our unit is a tertiary referral center receiving referrals from 5 cardiology units in the neighboring districts with a catchment population of 2.5 million Hong Kong residents.
The Hospital Authority is a statutory organization managing the public health care system in Hong Kong. The organization first introduced a city-wide computerized Clinical Management System in 1991. All patients attending health care services managed by the Hospital Authority are registered in the Hong Kong Patient Master Index. Starting from 2005, all historical electronic health records can be accessed by both public and private health care practitioners on a need-to-know basis, irrespective of the location of health care provision within the city. Baseline demographics, comorbidities, operative details, as well as postoperative complications and cardiac events were all obtained by review of the electronic health records.
Subjects were excluded if they met the following criteria: (1) single-vessel off-pump left internal mammary artery-to-left anterior descending anastomosis only, (2) lost to follow-up, and (3) in-hospital death.
Patients were censored in the actuarial survival calculations at the date of the event or date of last clinical follow-up. Follow-up began at the time of surgery for each patient and ended at the date of death or date of last contact.
The study was authorized by the institutional research ethics board (Joint Chinese University of Hong Kong—New Territories East Cluster Clinical Research Ethics Committee) on December 20, 2021 (reference number: 2021.569). Consent was waived in view of its retrospective nature. Database lock date was August 15, 2020.
Definition of Risk Factors and Cardiac Events
Standard European System for Cardiac Operative Risk Evaluation II definitions of cardiovascular risk factors were used. In adherence with Valve Academic Research Consortium-2 (VARC-2) consensus document, procedural mortality was defined as follows: deaths from all causes within 30 days after discharge or deaths during the index procedure hospitalization if the postoperative stay is longer than 30 days.
The cause of late death was adjudicated as definite cardiac, definite noncardiac, or undetermined. Undetermined cases were conservatively classified as cardiac. The primary end point was cardiac death. Cardiac death was defined as death attributable to myocardial infarction, heart failure, or cardiac arrest because of other or unknown cause. The secondary end point was the composite of cardiac events, including cardiac death, nonfatal myocardial infarction, hospitalization for unstable angina, and repeat revascularization. Due to resource constraints in the public health care system in Hong Kong, angiography after CABG was not routine, but symptom-driven.
Definition of Statin Therapy Intensity and Cholesterol Target Attainment
Statin intensity was defined by the 2013 American College of Cardiology/American Heart Association guidelines.2 Our institutional LDL-C targets for secondary prevention of cardiovascular disease were initially defined by the 2002 National Cholesterol Education Program Adult Treatment Panel III guidelines.3 With concrete cholesterol targets falling out of favor in North America, targets specified by the European Society of Cardiology guidelines have been adopted since 2011.4, 5, 6 Historical cholesterol targets adopted at our institution are shown in Table 1.
Table 1.
Lipid subcomponent thresholds over the years
| Lipid subcomponent | 2002 NCEP-ATP III3 | 2011 ESC4 | 2016 ESC5 | 2019 ESC6 |
|---|---|---|---|---|
| LDL-C | ≥25% reduction from baseline, and <2.6 mmol/L | ≥50% reduction from baseline, and <1.8 mmol/L | ≥50% reduction from baseline <1.8 mmol/L | ≥50% reduction from baseline <1.4 mmol/L |
| Percentage of patients with lifetime average levels below recommended LDL-C threshold | 81.9% | 24.3% | 24.3% | 5.8% |
| Non–HDL-C | No specific goal | No specific goal | No specific goal | <2.2 mmol/L |
| TG | No specific goal | No specific goal | No specific goal | No specific goal Aim <1.7 mmol/L |
| TC | No specific goal | No specific goal | No specific goal | No specific goal |
NCEP-ATP III, National Cholesterol Education Program Adult Treatment Panel III; ESC, European Society of Cardiology; LDL-C, low-density lipoprotein cholesterol; non–HDL-C, non–high-density lipoprotein cholesterol; TG, triglycerides; TC, total cholesterol.
Data Collection and Calculation of Average Cholesterol Level
Measurements of lipid profile subcomponents, namely LDL-C, high-density lipoprotein cholesterol (HDL-C), total cholesterol (TC), and triglycerides (TG) were obtained by retrospective review of electronic health records. All results after the date of operation were acquired from the Clinical Management System. A plot of lipid profile subcomponent measurements against time was compiled, and the area under the curve was calculated to estimate gross exposure after CABG. Average lipid exposure was calculated by dividing the gross exposure by the number of days lapsed between the first and last lipid profile measurements. The calculation was repeated for each patient and for each lipid profile subcomponent. Furthermore, baseline LDL-C before initiation of statin therapy was collected to determine the average percentage LDL-C reduction from baseline.
Statistical Analysis
Descriptive statistics were reported as mean with standard deviation or median with interquartile range for continuous variables, and as frequencies and percentages for categorical variables. Difference between means was compared using the Student t-test or one-way analysis of variance, after verifying equality of variances with the Levene test and normality of distribution with Shapiro–Wilk Test. If the Levene test was violated, Welch and Games–Howell tests were used. Categorical variables were compared using the χ2 test when the minimum number of observations in a category was over 5; otherwise, likelihood ratios G-tests were used.
A 2-level nested Cox proportional hazards regression model was constructed to calculate the association between cholesterol target attainment and cardiac death using generalized linear mixed models. Biochemical, clinical, and operative covariates were included in the first level of the multivariate model if the univariate association with combined cardiac death and events was significant to a P value less than .2 and removed using the backwards stepwise elimination procedure. Lipid profile subcomponents were incorporated in the second layer of the regression model separately to avoid multicollinearity.
Survival function was generated using the Kaplan–Meier method. For composite outcomes, survival models were developed based on time to the earliest event. Cox proportional hazards regression was used to calculate hazards ratio. Overall model significance was assessed using log-rank test. Long-term survival was compared between the 2 groups by calculation of the 95% confidence intervals.
The discriminatory ability of lipid profile subcomponents for late cardiac death and events outcomes were evaluated using area under the receiver operating characteristic (ROC) curves. Area under the ROC curves were compared using the Hanley–McNeil method. The optimal cut-off value was determined by Youden index, defined as the value at which the value of [sensitivity + specificity – 1] is maximal.
A predetermined alpha value of 0.05 was used as the threshold for statistical significance. Bonferroni adjustment was applied to account for multiple comparisons (5 different lipid profile subcomponents) (overall significance level set to P ≤ .05; Bonferroni-corrected level of significance was set to P ≤ .01). Unless otherwise specified, all analyses were performed on an intention-to-treat basis, which meant that patients were analyzed according to the statin intensity they were originally prescribed, regardless of subsequent crossover. Statistical analysis was performed with IBM SPSS, version 23.0 (IBM Corp).
Results
Demographics and Baseline Characteristics
We identified 330 patients who underwent CABG in the years 2007 and 2008. There were 4 in-hospital deaths (1.2%). Off-pump single-vessel left internal mammary artery-to-left anterior descending anastomosis was performed in 8 patients. Follow-up was complete in 97.2%. As such, 309 patients in total were included in the analysis.
Patient demographics and baseline characteristics are shown in Table 2. Most patients were male (79%). The mean age of the cohort at the index operation was 62.3 ± 9.0 years. The incidence of diabetes mellitus was 48.9%. Significant left main stenosis was present in 42.4%. More than 60% had a history of acute coronary syndrome, and 15.5% had a history of percutaneous coronary intervention. Urgent operations due to unstable angina or cardiogenic shock accounted for 3.2% of all cases.
Table 2.
Demographics and baseline characteristics
| Variable | n (%) or mean ± SD or median [IQR] | Univariate analysis P value |
|---|---|---|
| Age, y | 62.3 ± 9.0 | .595 |
| Male | 244 (79%) | .401 |
| Mode of presentation∗ | .064† | |
| Asymptomatic | 5 (1.6%) | |
| Stable angina | 99 (32.0%) | |
| History of ACS | ||
| Unstable angina | 45 (14.6%) | |
| NSTEMI | 103 (33.3%) | |
| STEMI | 39 (12.6%) | |
| Cardiogenic shock | 27 (2.6%) | |
| Ischemic cardiomyopathy | 10 (3.2%) | |
| Hypertension | 211 (68.3%) | .862 |
| Diabetes mellitus | 151 (48.9%) | .049† |
| On insulin | 23 (7.4%) | |
| On oral hypoglycemic agents | 115 (37.2%) | |
| Diet only | 13 (4.2%) | |
| Smoking | 158 (51.1%) | .734 |
| Active smoker | 18 (5.8%) | |
| Ex-smoker | 140 (45.3%) | |
| Extracardiac arteriopathy | 27 (8.7%) | .351 |
| Chronic pulmonary disease | 21 (6.8%) | .439 |
| Renal function abnormality‡ | 10 (3.1%) | .809 |
| Creatinine over 200 μmol/L | 7 (1.9%) | |
| Dialysis required | 3 (1.0%) | |
| Poor mobility | 2 (0.6%) | N/A |
| Previous PCI | 48 (15.5%) | .870 |
| Left main disease | 131 (42.4%) | .184† |
| LV function | <.001† | |
| LVEF ≥50% | 220 (71.2%) | |
| LVEF 31%-49% | 73 (23.6%) | |
| LVEF ≤30% | 16 (5.2%) | |
| Pulmonary hypertension | N/A | |
| Moderate 31-54 mm Hg | 7 (1.9%) | |
| Severe ≥55 mm Hg | 0 | |
| Number of grafts | 3 [IQR = 0] | .691 |
| Off-pump | 13 (4.2%) | N/A |
| Aspirin at discharge | 307 (99.3%) | N/A |
| Dual antiplatelet therapy at discharge | 66 (21.4%) | .789 |
| Statin at discharge | 295 (95.5%) | N/A |
| Beta-blocker at discharge | 208 (67.3%) | .789 |
| ACEi or ARB at discharge | 138 (44.7%) | .303 |
SD, Standard deviation; IQR, interquartile range; ACS, acute coronary syndrome; NSTEMI, non–ST elevation myocardial infarction; STEMI, ST elevation myocardial infarction; N/A, not available; PCI, percutaneous coronary intervention; LV, left ventricular; LVEF, left ventricular ejection fraction; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Comparison between those with history of ACS and those without.
P < .2, to be entered into multivariate model.
Creatinine clearance used in univariate analysis.
Operative Technique and Early Postoperative Outcomes
All operations were performed through a median sternotomy. Our default strategy was on-pump with the heart arrested, with 95.8% of all operations were performed in this manner. An off-pump strategy was required in the remaining cases primarily due to significant ascending aortic calcification. The left internal mammary artery was anastomosed to the left anterior descending artery in 95.8% of all cases. The radial artery was used in 13 patients (4.2%). The default second conduit was the great saphenous vein. In total, 563 vein grafts were deployed. The median number of coronary grafts was 3. Three-quarters of the cohort received 2 or more vein grafts.
All patients received aspirin starting postoperative day 1. In total, 21.4% of the cohort received dual antiplatelet therapy, with the commonest indication being urgent inpatient CABG for acute coronary syndrome.
Late Cardiac Death and Events
The median follow-up duration was 12.5 years. On database lock date, 31.7% (n = 98) of the cohort had died. Cardiac death occurred in 6.8% (n = 21) of the cohort and accounted for one-fifth of all deaths. The cause of death was adjudicated as definite cardiac in 12.2% (n = 12), definite noncardiac in 78.5 (n = 77), and undetermined in 9.1% (n = 9). Freedom from cardiac death for all patients was 98.0% at 5 years and 94.8% at 10 years.
The secondary composite outcome of cardiac events, including cardiac death, myocardial infarction, hospitalization for unstable angina, and repeat revascularization, occurred in 21.7% (n = 67) of the cohort. New-onset myocardial infarction occurred in 8.7% (n = 27), of which 48.1% (n = 13) underwent repeat revascularization.
Recurrent angina occurred in 13.3% (n = 41) of patients and accounted for 61.2% of all cardiac events. Repeat revascularization was performed in 41.5% (n = 17) of patients with recurrent angina.
Intensity of Statin Therapy and Crossover
Baseline LDL-C levels were known for 85% of the patients. Before surgery, statin therapy had been prescribed to 94% of the cohort. On discharge, all but 2 patients were receiving statins. Three months after surgery, low-, moderate-, and high-intensity statin therapies were prescribed to 35.5%, 58.6%, and 5.9% of the cohort, respectively. In the years following CABG, significant crossover between treatment intensity groups occurred. In total, 38.2% of patients received statin therapy of greater intensity than originally prescribed, primarily due to suboptimal cholesterol target attainment. 3.9% received statin therapy of lesser intensity due to statin intolerance. On the date of censorship, low-, moderate-, and high-intensity therapies were being prescribed to 24.1%, 46.9%, and 29.0%, respectively.
There was no significant difference in percentage of reduction in LDL-C from baseline between those who received low-, moderate-, and high-intensity therapies (F [3, 309] = 1.2, P = .31). However, the high-intensity group experienced a greater absolute reduction in LDL-C from baseline (1.72 ± 1.37 mmol/L) compared with both moderate- (1.31 ± 0.92 mmol/L) and low-intensity groups (1.05 ± 0.74 mmol/L) (FWelch [3, 309] = 6.329, P = .003).
To ascertain whether treatment bias was present, the baseline clinical and biochemical characteristics of patients who received low-, moderate-, and high-intensity therapies were compared. Patients who received high-intensity therapy were significantly younger than other patients by a mean of 5.9 years. Furthermore, there was a statistically significant difference in baseline LDL-C between the 3 subgroups (FWelch [2, 43.6] = 10.5, P < .001). Post-hoc comparison using the Games–Howell test indicated that patients who were prescribed high-intensity therapy had a greater baseline LDL-C level when compared with those prescribed low- and moderate-intensity therapy. These results suggested that physicians were more likely to prescribe high-intensity therapy to patients with greater baseline LDL-C levels.
Prevalence of Persistent Lipid Abnormalities
The total number of lipid profile measurements taken in this 309-patient cohort was 5774, which translated to 1.53 measurements per patient-year follow-up. Using historical targets listed in Table 1 as reference, 81.9% of patients achieved an average LDL-C below 2.6 mmol/L. However, less than one-quarter of patients were able to attain an LDL-C below 1.8 mmol/L. Even fewer patients (5.8%) were able to attain an average LDL-C below 1.4 mmol/L. For non–HDL-C, merely 1 in 10 patients achieved the modern average of less than 2.2 mmol/L. For TG, more than 75% attained the soft target of less than 1.7 mmol/L.
Effect of Cholesterol Target Attainment on Long-Term Clinical Outcomes
Univariate analysis identified left main disease, diabetes mellitus, history of percutaneous coronary intervention, and left ventricular systolic dysfunction as potential confounding covariates for cardiac death and events. These variables were entered in the first layer of the nested regression model.
Results of the hierarchical regression analysis were shown in Table 3. After we controlled for confounding, there remained a statistically significant association between diabetes mellitus and left ventricular systolic dysfunction (χ2 [3, N = 309] = 36.2, P < .001) and cardiac death. Furthermore, LDL-C, non–HDL-C, TC, and TC/HDL-C ratio were independently associated with cardiac death. For every mmol/L increment in LDL-C, the risk of cardiac death increases by 2.3% per annum.
Table 3.
Two-level nested hierarchical Cox proportional hazards regression model of predictors for cardiac death after CABG
| Variable | Cardiac death |
|
|---|---|---|
| Hazards ratio (95% CI) | P value | |
| First layer—significant variables from univariate analysis | ||
| Diabetes mellitus | 3.759 (1.244-11.357) | .019∗ |
| LVEF (>50% vs 30%-50% vs <30%) | 2.325 (1.314-4.116) | .004∗ |
| Left main disease | 0.589 (0.228-1.525) | .276 |
| History of PCI | 1.044 (0.303-3.598) | .946 |
| Second layer—lipid profile subcomponents (calculated separately) | ||
| LDL-C | 2.297 (1.234-4.277) | .009∗ |
| Non–HDL-C | 2.557 (1.461-4.474) | .001∗ |
| HDL-C | 0.343 (0.051-2.322) | .273 |
| TC/HDL-C ratio | 1.740 (1.187-2.552) | .005∗ |
| Triglycerides | 1.686 (1.043-2.725) | .033 |
CI, Confidence interval; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; LDL-C, low-density lipoprotein cholesterol; non–HDL-C, non–high-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TC, total cholesterol.
P ≤ .01, statistically significant.
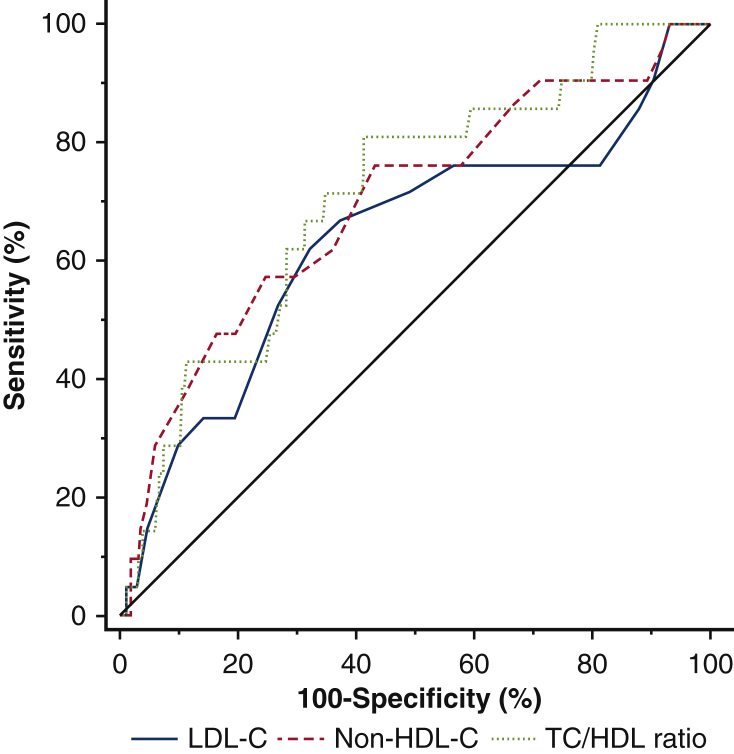
In ROC analyses, all 3 parameters exhibited fair discriminative ability with AUCs in the range of 0.63 to 0.70, with no statistically significant difference in discriminative ability based on the Hanley–McNeil method (Figure 1).
Figure 1.
Receiver operating curves of different lipid subcomponents at prognosticating long-term cardiac death after CABG. There is no difference in discriminatory ability between TC/HDL ratio, non–HDL-C, and LDL-C (AUC mean ± SD = 0.710 ± 0.056 vs 0.692 ± 0.064 vs 0.638 ± 0.070). The diagonal line represents no discriminatory power (area under the receiver operating characteristic curve of 0.5). Green line: TC/HDL-C ratio. Red line: non–HDL-C. Blue line: LDL-C. LDL-C, Low-density lipoprotein cholesterol; non–HDL-C, non–high-density lipoprotein cholesterol; TC/HDL, total cholesterol-to-high density lipoprotein.
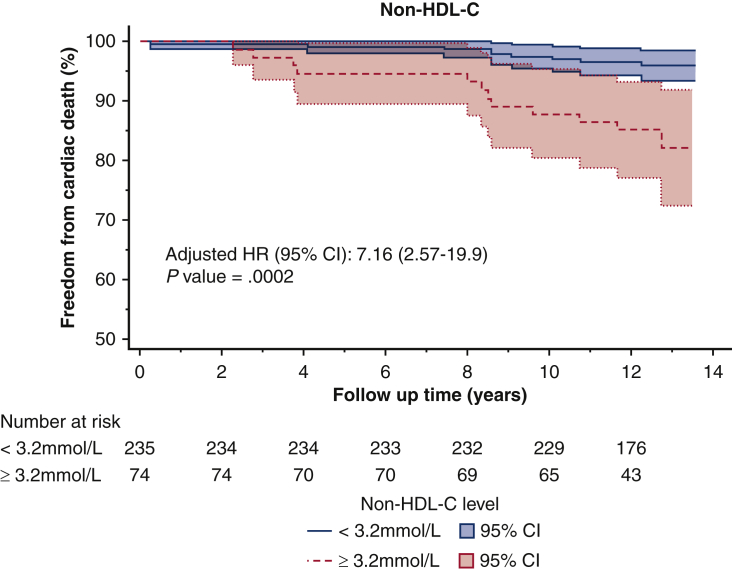
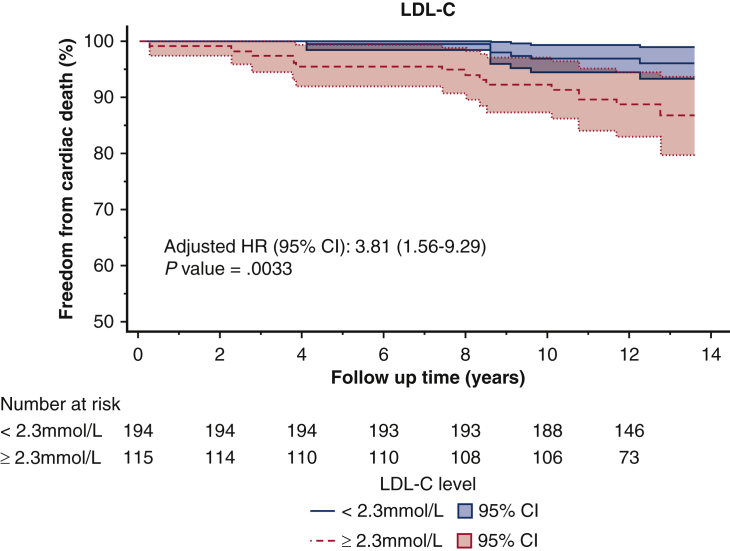
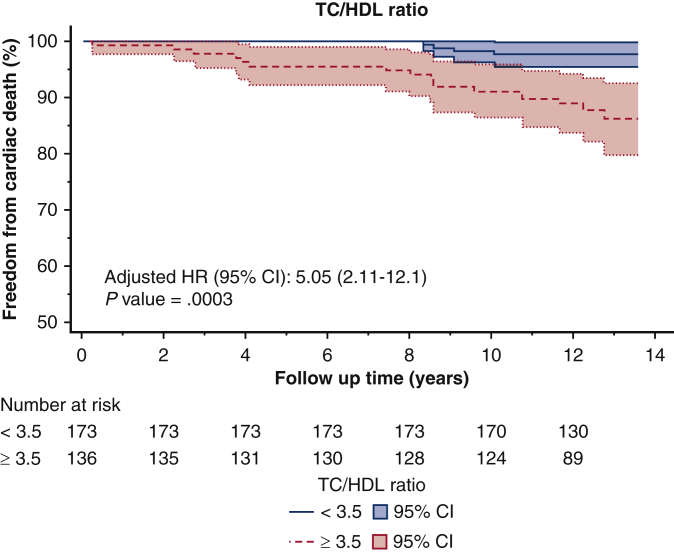
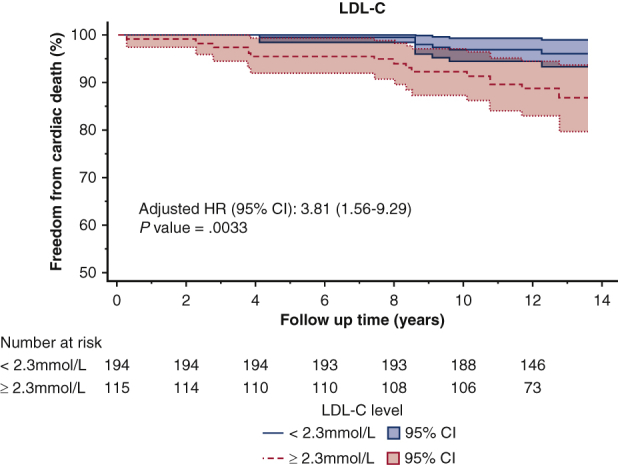
The optimal cut-off values for non–HDL-C, LDL-C, and TC/HDL-C ratio, as determined by the Youden Index, were 3.2 mmol/L (124 mg/dL), 2.3 mmol/L (88 mg/dL), and 3.5, respectively. For patients with an average non–HDL-C below 3.2 mmol/L, freedom from cardiac death was 97% at 10 years, compared with 87% in those with a non–HDL-C above 3.2 mmol/L (Figure 2, Figure 3, Figure 4) (P < .001) (Video 1).
Figure 2.
Kaplan–Meier survival curves of freedom from cardiac death after CABG based on the non–HDL-C threshold of 3.2 mmol/L identified in receiver operating curve analysis. non–HDL-C, Non–high-density lipoprotein cholesterol; HR, hazards ratio; CI, confidence interval.
Figure 3.
Kaplan–Meier survival curves of freedom from cardiac death after CABG based on the LDL-C threshold of 2.3 mmol/L identified in receiver operating curve analysis. LDL-C, Low-density lipoprotein cholesterol; HR, hazards ratio; CI, confidence interval.
Figure 4.
Kaplan–Meier survival curves of freedom from cardiac death after CABG based on the TC/HDL-C threshold of 3.5 identified in receiver operating curve analysis. TC/HDL, Total cholesterol-to-high density lipoprotein; HR, hazards ratio; CI, confidence interval.
The analysis was repeated for the secondary outcome of composite cardiac events (Table 4). On multivariate analysis, there remains a statistically significant association between left ventricular systolic dysfunction (χ2 [1, N = 309] = 12.4, P < .001). After risk adjustment, TG was the only lipid subcomponent independently associated with cardiac events.
Table 4.
Two-level nested hierarchical Cox proportional hazards regression model of predictors for cardiac events after CABG
| Variable | Cardiac events |
|
|---|---|---|
| Hazards ratio (95% CI) | P value | |
| First layer—significant variables from univariate analysis | ||
| Diabetes mellitus | 1.123 (0.689-1.832) | .641 |
| LVEF (>50% vs 30%-50% vs <30%) | 1.930 (1.354-2.751) | <.001∗ |
| Left main disease | 0.790 (0.480-1.298) | .352 |
| History of PCI | 0.727 (0.346-1.524) | .398 |
| Second layer—lipid profile subcomponents (calculated separately) | ||
| LDL-C | 1.199 (0.835-1.723) | .325 |
| Non–HDL-C | 1.358 (0.987-1.867) | .060 |
| HDL-C | 0.563 (0.218-1.456) | .236 |
| TC/HDL-C ratio | 1.269 (0.997-1.615) | .053 |
| Triglycerides | 1.523 (1.131-2.053) | .006∗ |
CI, Confidence interval; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; LDL-C, low-density lipoprotein cholesterol; non–HDL-C, non–high-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TC, total cholesterol.
P ≤ .01, statistically significant.
Discussion
Clinical Relevance
Despite the survival benefits afforded by total arterial revascularization, the great saphenous vein remains the most commonly deployed conduit during coronary artery bypass.7 Beyond the first year after bypass graft surgery, vein graft failure is heavily driven by accelerated atherosclerosis,8 for which hyperlipidemia is a strong predisposing factor.9 In Campeau's landmark angiographic study, merely 60% of saphenous vein grafts remained patent by 10 years after surgery, and one-half of those that were patent exhibited clinically important stenosis.10 Remarkably, arterial grafts were resistant to dyslipidemia even up to 9.4 years of angiographic follow-up.11
These angiographic studies paved the way for the Post-CABG and Treating to New Targets (TNT) trials. These were the 2 largest large randomized controlled trials to date that assessed the relationship between lipid targets and medium-term clinical outcomes after CABG.1,12 In the Post-CABG trial, an intensive LDL-C–lowering strategy (average 2.4 mmol/L) was compared with a lenient strategy (average 3.5 mmol/L). After 7.5 years of follow-up, patients receiving intensive therapy were 30% less likely to require further revascularization, although there was no significant difference in cardiac death.1 The TNT trial complemented the Post-CABG trial by evaluating for any incremental benefit of further lowering LDL-C to 2.0 mmol/L compared with the conventional target of 2.6 mmol/L stipulated in the 2007 European Society of Cardiology guidelines. After 4.5 years of follow-up, the former group of patients were 27% less likely to require further revascularization. However, no significant difference in cardiac death was demonstrated.12
The Achilles' heel of both post-CABG and TNT trials was that patients were enrolled at various intervals after surgical revascularization. Notably, some patients were recruited 11 years after surgery in the post-CABG trial. This is in stark contrast to our cohort, in which almost all patients received statins within the first week of surgery. As demonstrated by Kulik and colleagues,13 statin therapy initiated in the first month of surgery significantly improved long-term survival.
The first key message of this study is that lipid targets do matter. Elevated LDL-C after surgical revascularization was independently associated with cardiac death. The target LDL-C of less than 2.3 mmol/L (88 mg/dL) identified in our study reaffirmed the target used in the TNT trial. This lends further support to the use of intensive lipid-lowering for secondary prevention after surgical revascularization, specifically the use of high-intensity statins.
The second key message is that persistent lipid abnormalities are often overlooked. Less than one-quarter of patients treated with statins in this cohort were able to attain an LDL-C below 1.8 mmol/L, highlighting a significant gap between guidelines and clinical practice. As shown in the Dyslipidemia International Study, this is not a phenomenon unique to Hong Kong.14
The final message is that lesser-known lipid profile subcomponents may be more accurate indices of cardiovascular risk when compared with LDL-C. With regard to non–HDL-C, the 2021 Canadian Cardiovascular Society guidelines actually recommended non–HDL-C over LDL-C as the preferred therapeutic target in particular for patients with elevated triglyceride levels.15 The rationale for this recommendation is that there is more cholesterol in the very LDL fraction in patients with elevated TG. As such, non–HDL-C appears to be a more accurate index of vascular risk than LDL-C because it is a better surrogate for LDL particle number (Figure 5).16
Figure 5.
Shown are the study's methods, results, and conclusions. CABG, Coronary artery bypass graft; LDL-C, low-density lipoprotein cholesterol; AUC, area under the curve; ROC, receiver operating characteristics; HR, hazards ratio; CI, confidence interval; non–HDL-C, non–high-density lipoprotein cholesterol; TC/HDL, total cholesterol-to-high density lipoprotein.
With regard to TC/HDL-C ratio, in the Quebec Cardiovascular study, an elevated TC/HDL-C ratio is observed among overweight hyperinsulinemic individuals with hypertriglyceridemia.17 In fact, the triad consisting of high LDL-C or TC, low HDL-C, and high TG was described historically as atherogenic dyslipidemia.18
Limitations
The sample size was small. The study was retrospective in nature and suffered from weaknesses inherent to all retrospective studies, including selection bias and confounding. It was evident that younger patients and those with a greater baseline LDL-C were more likely to receive high-intensity statin therapy. This reflected the prevailing public health care sector policy in the late 2000s and early 2010s, which restricted access to high-intensity statins for patients with high cardiovascular risk. Due to significant crossover between statin intensity groups, cholesterol target attainment is the only quantifiable measure of dyslipidemia control for most patients, and this is the avenue we pursued.
Due to the retrospective nature of the study, the frequency of lipid profile measurements was determined by the referring institutions, typically based on the patient's individual cardiovascular risk, adherence to pharmacotherapy, and target attainment. With 1.53 measurements per patient-year follow-up, the average cholesterol level derived is representative of the dyslipidemic milieu in most patients.
Regarding confounding, adherence to pharmacotherapy was unclear from the medical records. Significant recall bias was expected even if telephone interviews were conducted due to the long period of follow-up. Practice variations across institutions means that patients may deviate from guideline-directed medical therapy. Due to resource constraints, there was no routine angiographic assessment of graft patency. The absence of routine angiography means that the residual SYNTAX score was unknown and cannot be controlled for in the multivariate analysis.
Conclusions
Lipid targets do matter after surgical revascularization. Persistent lipid abnormalities are associated with late cardiac death after surgical revascularization. The optimal LDL-C and non–HDL-C levels after CABG were below 2.3 mmol/L (89 mg/dL) and 3.2 mmol/L (124 mg/dL), respectively.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Supplementary Data
Description of the study design and key findings by the authors. Video available at: https://www.jtcvs.org/article/S2666-2736(22)00086-9/fulltext.
References
- 1.Knatterud G.L., Rosenberg Y., Campeau L., Geller N.L., Hunninghake D.B., Forman S.A., et al. Long-term effects on clinical outcomes of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation in the post coronary artery bypass graft trial. Circulation. 2000;102:157–165. doi: 10.1161/01.CIR.102.2.157. [DOI] [PubMed] [Google Scholar]
- 2.Stone N.J., Robinson J.G., Lichtenstein A.H., Bairey Merz C.N., Blum C.B., Eckel R.H., et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63(25 PART B):2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. doi: 10.4135/9781412952576.n154. [DOI] [PubMed] [Google Scholar]
- 4.European Association for Cardiovascular Prevention & Rehabilitation. Reiner Z., Catapano A.L., De Backer G., Graham I., Taskinen M.R., Wiklund O., et al. ESC/EAS Guidelines for the management of dyslipidaemias. Eur Heart J. 2011;32:1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 5.Catapano A.L., Graham I., De Backer G., Wiklund O., Chapman M.J., Drexel H., et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 6.Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L., et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 7.Catarino P., Black E., Taggart D.P. Why do UK cardiac surgeons not perform their first choice operation for coronary artery bypass graft. Heart. 2002;88:643–644. doi: 10.1136/heart.88.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motwani J.G., Topol E.J. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97:916–931. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- 9.Hiroyuki D., Hisashi Y., Hiroshi M., Mokuno H., Satoh H., Kottke T.E., et al. Relation of saphenous vein graft obstruction to serum cholesterol levels. J Am Coll Cardiol. 1995;25:193–197. doi: 10.1016/0735-1097(94)00341-M. [DOI] [PubMed] [Google Scholar]
- 10.Lucien C., Enjalbert M., Lespérance J., Bourassa M.G., Kwiterovich P., Jr., Wacholder S., et al. The relation of risk factors to the development of atherosclerosis in saphenous-vein bypass grafts and the progression of disease in the native circulation: a study 10 years after aortocoronary bypass surgery. N Engl J Med. 1984;311:1329–1332. doi: 10.1056/NEJM198411223112101. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Y.Y., Hayward P.A.R., Hare D.L., Reid C., Stewart A.G., Buxton B.F. Effect of lipid exposure on graft patency and clinical outcomes: arteries and veins are different. Eur J Cardiothorac Surg. 2014;45:323–328. doi: 10.1093/ejcts/ezt261. [DOI] [PubMed] [Google Scholar]
- 12.Shah S.J., Waters D.D., Barter P., Kastelein J.J., Shepherd J., Wenger N.K., et al. Intensive lipid-lowering with atorvastatin for secondary prevention in patients after coronary artery bypass surgery. J Am Coll Cardiol. 2008;51:1938–1943. doi: 10.1016/j.jacc.2007.12.054. [DOI] [PubMed] [Google Scholar]
- 13.Kulik A., Brookhart M.A., Levin R., Ruel M., Solomon D.H., Choudhry N.K. Impact of statin use on outcomes after coronary artery bypass graft surgery. Circulation. 2008;118:1785–1792. doi: 10.1161/CIRCULATIONAHA.108.799445. [DOI] [PubMed] [Google Scholar]
- 14.Goodman S.G., Langer A., Bastien N.R., McPherson R., Francis G.A., Genest J.J., et al. Prevalence of dyslipidemia in statin-treated patients in Canada: results of the DYSlipidemia International Study (DYSIS) Can J Cardiol. 2010;26(9):e330–e335. doi: 10.1016/s0828-282x(10)70454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson G.J., Thanassoulis G., Anderson T.J., Barry A.R., Couture P., Dayan N., et al. 2021 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can J Cardiol. 2021;37:1129–1150. doi: 10.1016/j.cjca.2021.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Sniderman A.D., Williams K., Contois J.H., Monroe H.M., McQueen M.J., de Graaf J., et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4:337–345. doi: 10.1161/CIRCOUTCOMES.110.959247. [DOI] [PubMed] [Google Scholar]
- 17.Lemieux I., Lamarche B., Couillard C., Pascot A., Cantin B., Bergeron J., et al. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men. Arch Intern Med. 2001;161:2685–2692. doi: 10.1001/archinte.161.22.2685. [DOI] [PubMed] [Google Scholar]
- 18.Grundy S.M. Hypertriglyceridemia, atherogenic dyslipidemia, and the metabolic syndrome. Am J Cardiol. 1998;81(4A):18B–25B. doi: 10.1016/s0002-9149(98)00033-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of the study design and key findings by the authors. Video available at: https://www.jtcvs.org/article/S2666-2736(22)00086-9/fulltext.