Abstract
A putative operon of four genes implicated in the synthesis of the chromophore moiety of the Pseudomonas aeruginosa siderophore pyoverdine, dubbed pvcABCD (where pvc stands for pyoverdine chromophore), was cloned and sequenced. Mutational inactivation of the pvc genes abrogated pyoverdine biosynthesis, consistent with their involvement in the biosynthesis of this siderophore. pvcABCD expression was negatively regulated by iron and positively regulated by both PvdS, the alternate sigma factor required for pyoverdine biosynthesis, and PtxR, a LysR family activator previously implicated in exotoxin A regulation.
Although iron is an essential nutrient for most bacteria, the low solubility and, thus, bioavailability of this element in nature complicates bacterial iron acquisition (32). Many bacteria deal with this problem by synthesizing high-affinity iron-chelating molecules, termed siderophores (31), which function coordinately with cell surface receptors specific for the iron-siderophore complexes (30, 31) to transport iron into the cell. Pathogenic organisms also encounter an iron-limited environment in the host (41), and siderophore-mediated mechanisms of iron acquisition are important contributors to in vivo growth and, thus, pathogenesis of many disease-causing bacteria (e.g., see references 7 and 16).
Pseudomonas aeruginosa is an opportunistic human pathogen which produces two known siderophores, pyoverdine (9) and pyochelin (8). Production of pyoverdine in vivo has been documented (17), consistent with a demonstrated role for this siderophore in promoting in vivo growth and pathogenesis (27). This mixed hydroxymate-catecholate siderophore is characterized by a conserved hydroxyquinoline chromophore bound to an amino acid tail of variable length and composition (6). Synthesis of the chromophore is hypothesized to involve a condensation of d-tyrosine and l-2,4-diaminobutyric acid (DAB) (6), while synthesis of the peptide moiety apparently involves a nonribosomal mechanism (23).
Genes for the synthesis of pyoverdine have been mapped to three regions of the P. aeruginosa PAO chromosome, at 23, 47 (20), and 66 to 70 (44) min on the recalibrated PAO map. A 103-kb fragment of chromosomal DNA originating from the 47-min region has been cloned. Referred to as the pvd region (48), this DNA carries several genes shown to be involved in pyoverdine biosynthesis. DNA originating from the 66- to 70-min region of the PAO chromosome has also been cloned. Responsible for the synthesis of a chromophore-like molecule dubbed pseudoverdine, a gene(s) in this region is required for pyoverdine biosynthesis (44), presumably for the chromophore moiety.
Pyoverdine synthesis is dependent upon an alternate sigma factor, PvdS, required for gene expression from a variety of pvd promoters (10, 28). PvdS is negatively regulated by Fur (10, 28, 34), a repressor protein which mediates the iron-regulated expression of a number of genes, providing a likely explanation for the iron-regulated production of pyoverdine in P. aeruginosa.
In the present report we describe the sequencing of the pseudoverdine gene cluster and the identification and regulation of an operon of four genes (pvcABCD) required for pyoverdine (chromophore) production.
Methods.
Bacterial strains and plasmids used in this study are listed in Table 1. Luria-Bertani (LB) (Difco), brain heart infusion (BHI) (BDH) and the iron-deficient King’s B (KB) (22) or succinate minimal (24) media have been described previously. Strains cultivated for the purpose of extracting RNA for use in RNase protection assays were grown in low-iron Trypticase soy broth dialysate with (iron replete) or without (iron deficient) FeCl3 supplementation (39 μM) (3). Minimal medium was supplemented with amino acids (1 mM) and adenosine (2 mM) as required. The following antibiotics were included in the growth media as required at the indicated concentrations: ampicillin, 100 μg/ml; kanamycin, 100 μg/ml; carbenicillin, 400 μg/ml; streptomycin, 500 μg/ml; chloramphenicol, 50 μg/ml (for Escherichia coli and P. aeruginosa K1081), 200 μg/ml (for P. aeruginosa ML5087), or 600 μg/ml (for P. aeruginosa PAO1); gentamicin, 75 μg/ml; Irgasan DP-300 (Ciba-Geigy), 50 μg/ml; and HgCl2, 50 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Characteristic(s)a | Source or reference |
|---|---|---|
| E. coli | ||
| GM2163 | hsdR2 mcrB1 dam-13::Tn9 dcm-6 | New England Biolabs |
| S17-1 | thi pro hsdR recA Tra+ | 43 |
| P. aeruginosa | ||
| PAO1 | Prototroph | |
| PAO1 ΔptxR | This work | |
| PAO1 ΔpvdS | 34 | |
| ML5087 | ilv-220 thr-9001 leu-9001 met-9001 pur-67 aphA | 36 |
| K1081 | ML5087ΔptxR | This work |
| Plasmids | ||
| pAS16 | pK18mobsacB plasmid containing a 1-kb BamHI-HindIII fragment from pAS20 encompassing the mutated ptxR gene | This work |
| pHP45ΩHg | pUC18 derivative carrying an Hgr cassette | 12 |
| pK18mobsacB | Gene replacement vector derived from plasmid pK18; Mob+ KmrsacB | 42 |
| pKT240 | Broad-host-range cloning vector; IncP-4 Mob+ Kmr Cbr | 2 |
| pMAL-c2 | MalE fusion vector; Apr | Pharmacia |
| pPYP177 | pKT240 derivative carrying a 7.8-kb SacI-HindIII DNA fragment of the pPYP180 insert | 44 |
| pPYP180 | pKT240 derivative carrying a 10.8-kb SacI-ClaI DNA fragment from PAO1 which directs expression of pseudoverdine | 44 |
| pVLT31 | Broad-host-range expression vector; lacI plac Tcr | 11 |
| pPTX990 | pVLT31::ptxR | This work |
| pRK2013 | Broad-host-range helper vector; Tra+ Kmr | 14 |
| pSUP203 | Gene replacement vector; Mob+ Tcr | 43 |
| pUC18 | Cloning and sequencing vector; Apr | 40 |
| pUC19 | Cloning and sequencing vector; Apr | 40 |
| pUC-Gm | pUC18::Gm; Apr Gmr | 33 |
Gmr, gentamicin resistant; Apr, ampicillin resistant; Cbr, carbenicillin resistant; Kmr, kanamycin resistant; Cmr, chloramphenicol resistant; Smr, streptomycin resistant; Tcr, tetracycline resistant; Mob+, mobilizable.
Chromosomal DNA was prepared by using a modification of the Blin and Stafford procedure as described in reference 40. Large-scale plasmid DNA was prepared with a Plasmid Midi kit (Qiagen, Inc., Chatsworth, Calif.). Standard methods were used for the preparation of small-scale plasmid DNA, enzyme digestions, ligations, agarose gel electrophoresis (40), and transformation of E. coli (40) and P. aeruginosa (4). DNA fragments were purified from agarose gels with a Prep-a-gene kit (Bio-Rad Labs, Mississauga, Canada). Southern hybridizations were carried out as described previously (40), with a digoxigenin labelling kit (Boehringer, Mannheim, Germany) to process the hybridizations.
A region of pPYP180 responsible for pseudoverdine and necessary for pyoverdine synthesis was sequenced following the generation of a number of subclones (in pUC19) from which nested deletions were constructed by using a double-stranded nested deletion kit (Pharmacia Biotech). Plasmid DNA for sequencing was purified as described previously (1) and sequenced by Cortec DNA Services Laboratory Inc., using the M13 universal forward primer. The sequence overlapping the boundaries of the various subclones was obtained by using defined oligonucleotide primers and plasmid pPYP177 DNA as the template. Sequence analysis was carried out with the PCGENE software package (Intelligentics Inc., Mountain View, Calif.). The ptxR gene was cloned as a 990-bp promoterless gene which was generated by PCR using Deep Vent DNA polymerase (New England Biolabs) and the primers 5′-TCTAGACCCGTCCGGACCCACTTC-3′ (XbaI site underlined) and 5′-AAGCTTGCCCAGCCTCATTCGCTCTG-3′ (HindIII site underlined). Following cloning into pCR-blunt (Invitrogen Corporation, Carlsbad, Calif.), the fragment was directionally cloned as an XbaI-HindIII fragment into pVLT31 to yield pPTX990.
Two approaches were taken to generate ΔptxR derivatives of P. aeruginosa. Initially, the 1.4-kb BamHI-BglII DNA fragment of pPYP177 was cloned into the BamHI site of pMAL-c2 (Pharmacia) in the same orientation as the lac promoter of this vector. The recombinant vector was introduced into and subsequently prepared from E. coli GM2163 before being digested with ClaI and NruI to release a 0.4-kb fragment from the ptxR coding region. Following purification of the vector free of this 0.4-kb fragment, the ClaI 5′ end was blunt ended with Klenow fragment and the plasmid was recircularized with T4 DNA ligase. The ptxR coding region with the deletion was excised from the plasmid on a 1-kb BamHI-HindIII DNA fragment and cloned into plasmid pK18mobsacB. The resultant vector, pAS16, was then introduced into E. coli S17-1 and mobilized into P. aeruginosa ML5087 via conjugation as described previously (37). Recipients carrying pAS16 in the chromosome were selected on LB agar containing kanamycin (100 μg/ml) and tetracycline (10 μg/ml). Kanamycin-resistant colonies appearing after 24 h of growth at 37°C were streaked onto LB agar containing sucrose (10%, wt/vol) (42). Sucrose-resistant colonies carrying the ptxR region with the deletion (e.g., K1081) were identified following amplification of the ptxR gene by using Taq polymerase and primers ptxR3 (5′-CAGGACTTCGTCAAGTGGCA-3′) and ptxR4 (5′-AGCTCTTCGAGAAC-GGCCTG-3′). Reaction mixtures were formulated as described previously (38) and subjected to 1 min at 94°C followed by 30 cycles of 40 s at 94°C, 50 s at 50°C, and 3 min at 72°C before finishing with 10 min at 72°C. A ptxR deletion mutant of PAO1 was subsequently generated following the tagging of a 0.8-kb deletion in this gene with a gentamicin resistance cartridge. Briefly, the 5′ and 3′ flanking regions of ptxR were amplified with the primer pair ptx247H (5′-AGGAAGCTTGTCCAATACTTGAG-3′, harboring a HindIII site) and ptx594X (5′-AGGTCTAGATGATTCAATCGCTCC-3′, harboring an XbaI site) or ptx1389K (5′-CCCGGTACCCCTCGGCGCGCTAC-3′, harboring a KpnI site) and ptx1866E (5′-GCGGAATTCCTGGCAACCCAGTTGC-3′, harboring an EcoRI site), respectively. PCR was performed on chromosomal PAO1 DNA with Taq polymerase and 30 cycles of 1 min at 94°C, 1 min at 55°C, and 40 s at 72°C. The PCR fragments were cloned into pCRII-2.1 (Invitrogen) and sequenced by using M13 primers and Sequenase (Amersham Life Science). The flanking regions were directionally transferred as 348-bp HindIII-XbaI and 478-bp KpnI-EcoRI fragments into pUC-Gm which had been previously obtained by placing a 1.7-kb Gmr cartridge (34) in the SmaI site of the pUC18 polylinker (33). The resulting plasmid, pUCΔptxR::Gm, was linearized with EcoRI and ligated to EcoRI-cut pSUP203 (43), yielding pSUPΔptxR::Gm. This vector was then mobilized into P. aeruginosa PAO1 via triparental mating (50), with E. coli HB101(pRK2013) as the helper strain. Gmr transconjugants were isolated on BHI agar containing gentamicin and Irgasan DP-300 (for counterselection). Individual colonies were patched onto BHI agar containing tetracycline to screen for loss of the pSUP203 plasmid-borne tetracycline resistance gene, and candidate PAO1ΔptxR::Gm mutants (Gmr Tcs) were screened for the deletion by Southern blot analysis (data not shown).
The riboprobes used for the RNase protection assays were generated following PCR amplification of selected regions of the genes of interest and cloning of the PCR fragments into the pCRII vector (Invitrogen). RNA probes were then generated from these cloned fragments by runoff transcription from the T7 promoter by using a Riboprobe kit (Promega), and the RNase protection assay was carried out as described previously (3). Autoradiographs of the dried gels were scanned and imported into Adobe Photoshop (version 4.0), and quantitative analysis was performed by using NIH Image software (version 1.55). The pvcAB probe (bp 1567 to 2015), covers 350 bp of pvcA, the pvcA-pvcB intergenic region, and 30 bp of pvcB. The pvcBC probe (bp 2640 to 3104) covers 180 bp of pvcB, the pvcB-pvcC intergenic region, and 132 bp of pvcC. Finally, the pvcCD probe (bp 3991 to 4367) covers 379 bp of pvcC and 125 bp of pvcD.
Identification and nucleotide sequence of the pvc pyoverdine biosynthetic gene cluster.
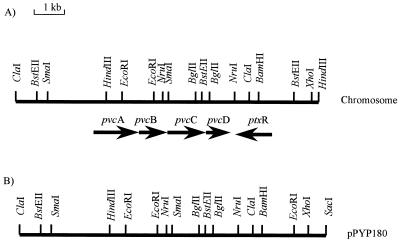
The cloning of a 10.8-kb ClaI-SacI DNA fragment of the 66- to 70-min region of the P. aeruginosa PAO1 chromosome (in pPYP180), which carries a gene(s) involved in pyoverdine biosynthesis, was previously described (44). This DNA fragment promoted the production of a pyoverdine chromophore-related fluorescent compound, termed pseudoverdine, in pyoverdine-deficient strains of P. aeruginosa, suggesting a role in the biosynthesis of the chromophore portion of the pyoverdine molecule (44). Almost 6 kb of the insert DNA present in pPYP180 was sequenced (deposited with the GenBank databases under accession no. AF002222), revealing a set of four open reading frames, designated pvcA, pvcB, pvcC, and pvcD (where pvc stands for pyoverdine chromophore), which comprise a putative operon. A fifth open reading frame was identified downstream of and in the opposite orientation to the pvcABCD genes (Fig. 1) and was subsequently identified as the ptxR gene described by Hamood et al. (18). This LysR family regulator is implicated in exotoxin A production (18). Deletion of a 500-bp BglII fragment, now known to encompass the 3′ end of pvcC and the 5′ end of pvcD (Fig. 1), completely abrogated pyoverdine production (44), confirming the involvement of the pvcABCD operon in pyoverdine biosynthesis.
FIG. 1.
Physical map of the pvcABCD-ptxR region of the P. aeruginosa PAO1 chromosome (A) and plasmid pPYP180 (B). Restriction mapping to the right of ptxR revealed differences between pPYP180 and the chromosome, indicating that some DNA rearrangement had occurred during the cloning of the pvc locus.
The pvcA gene encodes a putative protein (37,019 Da) similar to the Dit1 protein of Saccharomyces cerevisiae (Table 2) (5). Dit1 catalyzes the formation of an uncharacterized tyrosine-containing precursor for a spore wall-specific dityrosine-containing macromolecule (5). As such, PvcD may function in the condensation of tyrosine and DAB, a proposed step in pyoverdine biosynthesis (6, 21). The second gene, pvcB (876 bp), encodes a putative protein (33,165 Da) exhibiting the greatest similarity to the TfdA proteins (oxygenases) of Alcaligenes eutrophus (Ralstonia eutropha) JMP134 (46) and Burkholderia sp. strain RASC (47) (Table 2). The third gene, pvcC (1,500 bp) encodes a predicted product (55,812 Da) which shows substantial similarity to the Klebsiella pneumoniae HpaA and the E. coli HpaB proteins (hydroxylases) (Table 2). It is tempting to suggest, then, that PvcB and PvcC play roles in the two proposed hydroxylation steps of pyoverdine chromophore biosynthesis (6). Finally, the pvcD gene (644 bp) encodes a putative product (23,076 Da) which exhibits similarity to proteins of the cytochrome c family (Table 2). The implied involvement of a c cytochrome in pyoverdine production in P. aeruginosa is reminiscent of an earlier observation that a cytochrome c4 mutant of Azotobacter vinelandii lost its capacity to produce azotobactin (45), a pyoverdine-like siderophore. The recently described cytochrome c biogenesis protein CytA of Pseudomonas fluorescens ATCC 17400 also plays a role, hitherto unknown, in pyoverdine production in this organism (15). Intriguingly, a number of multicomponent aromatic amino acid hydroxylases of mammalian origin utilize components of electron transport to carry out the hydroxylation reaction (19), and PvcD may, therefore, assist PvcBC-mediated hydroxylation.
TABLE 2.
Identification of proteins exhibiting similarity to deduced PvcABCD proteinsa
| Pvc protein (size [amino acids]) | Similar protein | Strain | Size (amino acids) | Alignment scoreb | Function |
|---|---|---|---|---|---|
| PvcA (327) | Dit1 | S. cerevisiae | 536 | 14.693 | Spore wall maturation |
| PvcB (289) | TfdA | A. eutrophus (R. eutropha) | 297 | 6.294 | 2,4-Dichlorophenoxyacetate α-ketoglutarate dioxygenase |
| PvcC (500) | HpaA | K. pneumoniae | 520 | 40.258 | 4-Hydroxyphenylacetate 3-hydroxylase |
| HpaB | E. coli | 520 | 39.115 | 4-Hydroxyphenylacetic hydroxylase | |
| PvcD (215) | CC4 | Pseudomonas stutzeri | 210 | 13.977 | Cytochrome c4 |
| Cyc1 | Thiobacillus ferrooxidans | 213 | 13.437 | Cytochrome c-552 | |
| CycA | A. vinelandii | 210 | 10.997 | Cytochrome c4 | |
| CycC4 | P. aeruginosa | 181 | 10.544 | Cytochrome c4 |
Proteins exhibiting sequence similarity to the deduced PvcABCD polypeptides were identified by using the network services offered by the National Center for Biotechnology Information (Bethesda, Md.) and the Swiss-Protein sequence database.
Protein sequences exhibiting similarity to PvcA, PvcB, PvcC, or PvcD were individually aligned to the corresponding Pvc protein by using the PCOMPARE program available with the PCGENE software package. The alignment score was obtained by the method of Needleman and Wunsch (29) as implemented by Feng et al. (13), using the structure-genetic matrix with a gap penalty of 6 and a bias parameter of 0. A score above 3.0 is indicative of significant similarity.
Regulation of pvcABCD expression.
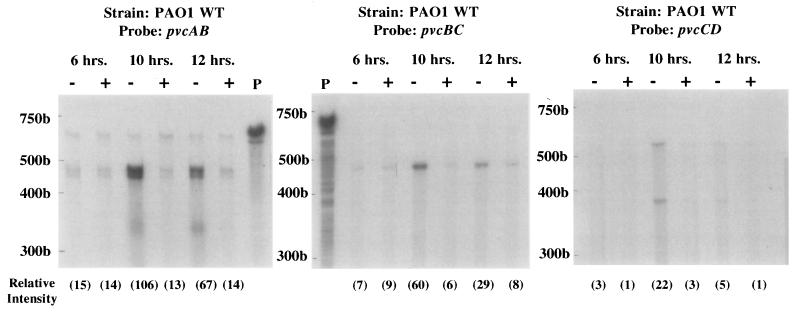
Pyoverdine production is iron regulated, increasing inversely with the concentration of external iron (25). To determine whether pvcABCD expression was iron regulated, RNase protection assays using riboprobes derived from the pvc genes were performed. By using a pvcAB riboprobe an mRNA fragment of the expected size was protected in cells cultured under iron-limiting conditions (Fig. 2). Expression of pvc increased with time of growth in iron-limited medium, showing a maximum at 10 h, at which time it was eightfold higher in iron-limited cells than in iron-replete cells (Fig. 2). Similarly, RNase protection assays with the pvcBC and pvcCD probes demonstrated that iron-limited cells expressed ca. 8- to 10-fold-higher levels of pvc mRNA than did their iron-replete counterparts after 10 h of growth (Fig. 2). Assays carried out with a riboprobe for a gene whose expression is known not to be iron regulated, omlA (35) (see the legend to Fig. 2) confirmed that differences seen with or without iron were not attributable to variations in total RNA used in the assays above.
FIG. 2.
RNase protection analysis of pvcABCD expression in wild-type strain PAO1. RNA samples were extracted from cells grown continuously for 6, 10, or 12 h in medium that was either iron deficient (−) or iron replete (+), and protection against RNase digestion was afforded by the pvcAB, pvcBC, or pvcCD probes assessed as described in Materials and Methods. Undigested 32P-labelled probes (P) are also shown. 32P-labelled RNA standards are shown to the left of each gel. The gels shown in this figure were exposed for 16 h. The relative intensities (in parentheses) of the major band in each lane were determined by using NIH Image software (version 1.55). Riboprobing with omlA (not iron regulated) yielded protected fragments with relative intensities of 155 (6 h, iron deficient), 155 (6 h, iron replete), 175 (10 h, iron deficient), 173 (10 h, iron replete), 175 (12 h, iron deficient), and 190 (12 h, iron replete).
Although all probes used provided evidence of iron regulation of pvcABCD expression, the levels of pvc mRNA protected declined as the riboprobes used moved from the 5′ end to the 3′ end of the operon. Indeed, levels protected by the pvcBC and pvcCD probes were only 60 and 20%, respectively, of that protected by the pvcAB probe (Fig. 2), indicating that the pvcCD genes were underrepresented in the pvc mRNA population. Intriguingly, a sequence capable of forming a stem-loop structure was identified (CGCCGGCCGGTGCGCGCCACGGCCGGCG; ΔG = −30.4 kcal) within the 51-bp intergenic region between pvcB and pvcC. Given the absence of an obvious promoter sequence in this region, this likely has an attenuating effect on expression of the pvcC and pvcD genes from a promoter upstream of pvcA. Consistent with this, the insertion of suicide vector pSUP202 sequences between wild-type copies of pvcB and pvcC in the chromosome of P. aeruginosa ML5087 (during an unsuccessful attempt at constructing a pvcB mutant) abrogated pyoverdine biosynthesis, despite the fact that the complete pvcABCD genes were present in this strain (data not shown). The separation of pvcCD from pvcAB by pSUP202 sequences likely leads to a lack of pvcCD expression, owing to the uncoupling of the latter from the promoter upstream of pvcA. Finally, the riboprobes used in this study invariably spanned portions of two adjacent genes, and the fact that fragments of the expected size were protected in each case supports the contention that these genes are encoded on the same (i.e., polycistronic) message.
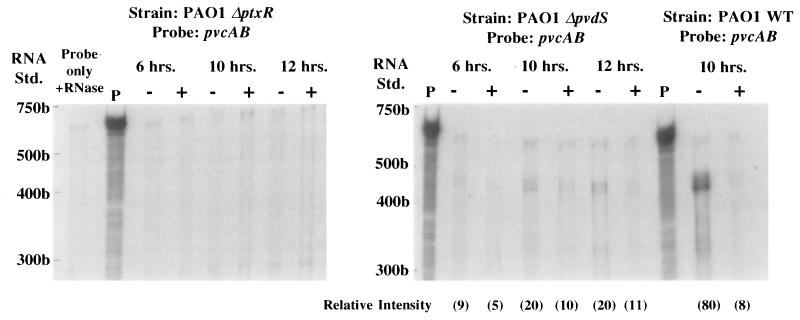
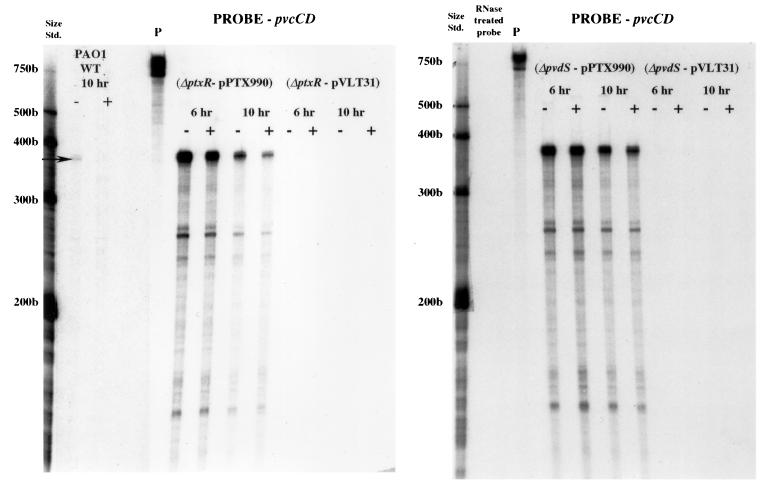
Given the proximity of the ptxR gene to the pvcABCD operon it seemed possible that PtxR plays a role in the expression of pvcABCD and, thus, pyoverdine. Consistent with this, a mutant carrying a ptxR deletion (K1081) was examined for pyoverdine production. K1081 lacked visible pyoverdine (based on the pigmentation and fluorescence of spent culture supernatants). The involvement of ptxR in pvc expression was confirmed by the RNase protection assay with the pvcAB probe. As seen in Fig. 3, the ptxR deletion in strain PAO1 completely abrogated pvc expression, with no pvc mRNA detected under iron-limited or iron-replete conditions. Similar results were observed when the pvcCD probe was used (data not shown). Again, control experiments using omlA as the riboprobe confirmed that the decline in pvc expression was not due to a decrease in RNA (see the legend to Fig. 3). Finally, constitutive ptxR expression from a multicopy plasmid (pPTX990) promoted high-level pvc expression, irrespective of iron availability in the growth medium (Fig. 4). Thus, PtxR activates pvc gene expression, and iron regulation of this putative operon is not mediated at the level of the pvcABCD genes.
FIG. 3.
RNase protection analysis of pvcABCD gene expression in the PAO1 parental strain (PAO1 WT) and PAO1 strains which carry deletion mutations in the ptxR (PAO1 ΔptxR) or pvdS (PAO1 ΔpvdS) genes. RNA samples were extracted from cells grown continuously for 6, 10, or 12 h in medium that was either iron deficient (−) or iron replete (+), and protection against RNase digestion was afforded by the pvcAB probe assessed as described in Materials and Methods. Undigested (P) and RNase treated (Probe only + RNase) 32P-labelled probes are also shown. The positions of 32P-labelled RNA standards (Std.) are shown to the left of each gel. The gels shown in this figure were exposed for 16 h. The relative intensity of the major band in each lane (in parentheses) was determined by using NIH Image software (version 1.55). As no pvcAB-protected fragment (400 to 500 bp range) was observable in the PAO ΔptxR lanes, relative intensities were not assessed. Riboprobing of total RNA from PAO1 ΔptxR with omlA (not iron regulated) yielded protected fragments with relative intensities of 155 (6 h, iron deficient), 158 (6 h, iron replete), 170 (10 h, iron deficient), 136 (10 h, iron replete), 176 (12 h, iron deficient), and 184 (12 h, iron replete). Riboprobing of total RNA from PAO1 ΔpvdS with omlA yielded protected fragments with relative intensities of 189 (6 h, iron deficient), 192 (6 h, iron replete), 174 (10 h, iron deficient), 143 (10 h, iron replete), 175 (12 h, iron deficient), and 176 (12 h, iron replete).
FIG. 4.
RNase protection analysis of pvcABCD gene expression in the PAO1 ΔptxR and PAO1 ΔpvdS strains harboring vectors pVLT31 and pVLT31::ptxR (pPTX990). RNA samples were extracted from cells grown continuously for 6 or 10 h in medium that was either iron deficient (−) or iron replete (+), and protection against RNase digestion was afforded by the pvcCD probe assessed as described in Materials and Methods. Results for the plasmid-free PAO1 wild-type strain are also shown. Undigested (P) 32P-labelled probe and the positions of 32P-labelled RNA standards (Std.) are shown to the left of each gel. The gels shown in this figure were exposed for 16 h.
The PvdS alternative sigma factor is required for expression of pyoverdine biosynthetic genes of the pvd locus (10, 28). To determine if PvdS was similarly required for pvcABCD expression, RNase protections assays were also carried out with the pvcAB probe and a pvdS deletion derivative of PAO1. As seen in Fig. 3, elimination of pvdS reduced pvc mRNA levels substantially (with no effect on omlA mRNA [see the legend to Fig. 3]), although, in contrast to the ptxR mutant, some pvc expression was still detectable in the pvdS mutant and it was iron-regulated (twofold increase in iron-limited cells). Again, control assays run using the omlA riboprobe did not show any variation related to iron levels in the growth medium (see the legend to Fig. 3), confirming that this modest effect of iron on pvc expression was real. The constitutively expressed cloned ptxR gene restored expression of pvcABCD to high levels in the pvdS deletion strain, indicating that PvdS does not act directly on pvcABCD expression. This is consistent with the absence of a putative PvdS binding site (39) upstream of the pvc genes and with the recent demonstration that PvdS acts on ptxR (49). Thus, PtxR mediates the PvdS effect on pvcABCD expression. This involvement of PvdS in pvcABCD expression also likely explains the iron-regulation of pvc expression, as pvdS is iron-regulated by the Fur repressor (26). Still, the observation that a pvdS deletion yields detectable, iron-regulated pvc gene expression while a ptxR deletion completely abrogates pvc expression suggests that some PtxR-mediated pvc expression can occur in the absence of pvdS and that it is still iron regulated.
The ptxR gene shown here to be linked to and involved in the expression of the pvcABCD operon and, hence, pyoverdine production was described previously (18) and was shown to play a positive regulatory role in the expression of exotoxin A. Hamood et al. (18) also indicated that the gene influenced siderophore production, an observation we have confirmed here. This connection between exotoxin A production and pyoverdine biosynthesis, first suggested by observations that PvdS plays a positive role in expression of exotoxin A (34), is intriguing, although its significance is unclear.
Acknowledgments
We thank G. Seyer for technical assistance and A. N. Hamood for providing strains and plasmids.
A.S. and J.-M.M acknowledge the financial support of the Association Française de Lutte contre la Mucoviscidose. Work in K.P.’s laboratory was supported by an operating grant from the Medical Research Council of Canada. Work in M.L.V.’s laboratory was supported by a grant (AI15940) from the National Institutes of Allergy and Infectious Diseases. The Support of NATO in the form of a collaborative research award to support travel between the laboratories of K.P. and J.-M.M. is also gratefully acknowledged. A.S. is the recipient of an EAITC (Academic Relations Division of Foreign Affairs and International Trade Canada) fellowship. K.P. is a Natural Sciences and Engineering Research Council University Research Fellow.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Short protocols in molecular biology. 2nd ed. New York, N.Y: John Wiley & Sons, Inc.; 1992. [Google Scholar]
- 2.Bagdasarian M M, Amann E, Lurz R, Rueckert B, Bagdasarian M. Activity of the hybrid trp-lac (tac) promoter of Escherichia coli in Pseudomonas putida. Construction of broad-host-range, controlled expression vectors. Gene. 1983;26:273–282. doi: 10.1016/0378-1119(83)90197-x. [DOI] [PubMed] [Google Scholar]
- 3.Barton H A, Johnson Z, Vasil A I, Vasil M L. Ferric uptake regulator mutants of Pseudomonas aeruginosa with distinct alterations in the iron-dependent repression of exotoxin A and siderophores in aerobic and microaerobic environments. Mol Microbiol. 1996;21:1001–1017. doi: 10.1046/j.1365-2958.1996.381426.x. [DOI] [PubMed] [Google Scholar]
- 4.Berry D, Kropinski A M. Effect of lipopolysaccharide mutations and temperature on plasmid transformation efficiency in Pseudomonas aeruginosa. Can J Microbiol. 1986;32:436–438. doi: 10.1139/m86-082. [DOI] [PubMed] [Google Scholar]
- 5.Briza P, Eckerstorfer M, Breitenbach M. The sporulation-specific enzymes encoded by the DIT1 and DIT2 genes catalyze a two-step reaction leading to a soluble LL-dityrosine-containing precursor of the yeast spore wall. Proc Natl Acad Sci USA. 1994;91:4524–4528. doi: 10.1073/pnas.91.10.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Budzikiewicz H. Secondary metabolites from fluorescent pseudomonads. FEMS Microbiol Rev. 1993;104:209–228. doi: 10.1111/j.1574-6968.1993.tb05868.x. [DOI] [PubMed] [Google Scholar]
- 7.Bullen J J, Ward C G, Wallis S N. Virulence and the role of iron in Pseudomonas aeruginosa infection. Infect Immun. 1974;10:443–450. doi: 10.1128/iai.10.3.443-450.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox C D. Iron uptake with ferripyochelin and ferric citrate by Pseudomonas aeruginosa. J Bacteriol. 1980;142:581–587. doi: 10.1128/jb.142.2.581-587.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox C D, Adams P. Siderophore activity of pyoverdin for Pseudomonas aeruginosa. Infect Immun. 1985;48:130–138. doi: 10.1128/iai.48.1.130-138.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunliffe H E, Merriman T R, Lamont I L. Cloning and characterization of pvdS, a gene required for pyoverdine synthesis in Pseudomonas aeruginosa: PvdS is probably an alternate sigma factor. J Bacteriol. 1995;177:2744–2750. doi: 10.1128/jb.177.10.2744-2750.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Lorenzo V, Eltis L, Kessler B, Timmis K. Analysis of Pseudomonas gene products using lacIq/Ptrp-lac plasmids and transposons that confer conditional phenotypes. Gene. 1993;123:17–24. doi: 10.1016/0378-1119(93)90533-9. [DOI] [PubMed] [Google Scholar]
- 12.Fellay R, Frey J, Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of Gram-negative bacteria. Gene. 1987;52:147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- 13.Feng D F, Johnson M S, Doolittle R F. Aligning amino acid sequences: comparison of commonly used methods. J Mol Evol. 1985;21:112–125. doi: 10.1007/BF02100085. [DOI] [PubMed] [Google Scholar]
- 14.Figurski D H, Helinski E R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaballa A, Koedam N, Cornelis P. A cytochrome c biogenesis gene involved in pyoverdine production in Pseudomonas fluorescens ATCC 17400. Mol Microbiol. 1996;21:777–785. doi: 10.1046/j.1365-2958.1996.391399.x. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths E. The iron-uptake systems of pathogenic bacteria. In: Bullen J J, Griffiths E, editors. Iron and infection. New York, N.Y: John Wiley & Sons; 1987. pp. 69–137. [Google Scholar]
- 17.Haas B, Kraut J, Marks J, Zanker S C, Castignetti D. Siderophore presence in sputa of cystic fibrosis patients. Infect Immun. 1991;59:3997–4000. doi: 10.1128/iai.59.11.3997-4000.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamood A N, Colmer J A, Ochsner U A, Vasil M L. Isolation and characterization of a Pseudomonas aeruginosa gene, ptxR, which positively regulates exotoxin A production. Mol Microbiol. 1996;21:97–110. doi: 10.1046/j.1365-2958.1996.6251337.x. [DOI] [PubMed] [Google Scholar]
- 19.Harayama S, Kok M. Functional and evolutionary relationships among diverse oxygenases. Annu Rev Microbiol. 1992;46:565–601. doi: 10.1146/annurev.mi.46.100192.003025. [DOI] [PubMed] [Google Scholar]
- 20.Hohnadel D, Haas D, Meyer J-M. Mapping of mutations affecting pyoverdine production in Pseudomonas aeruginosa. FEMS Microbiol Lett. 1986;36:195–199. [Google Scholar]
- 21.Jacques P, Ongena M, Gwose I, Seinsche D, Schroeder H, Delfosse P, Thonart P, Taraz K, Budzikiewicz H. Structure and characterization of isopyoverdin from Pseudomonas putida BTP1 and its relation to the biogenetic pathway leading to pyoverdins. Z Naturforsch Sect C. 1995;50:622–629. doi: 10.1515/znc-1995-9-1005. [DOI] [PubMed] [Google Scholar]
- 22.King E O, Ward M K, Raney D F. Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med. 1954;44:301–307. [PubMed] [Google Scholar]
- 23.Merriman T R, Merriman M E, Lamont I L. Nucleotide sequence of pvdD, a pyoverdine biosynthetic gene from Pseudomonas aeruginosa: PvdD has similarity to peptide synthetases. J Bacteriol. 1995;177:252–258. doi: 10.1128/jb.177.1.252-258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer J-M, Abdallah M A. The fluorescent pigment of Pseudomonas fluorescens: biosynthesis, purification and physiochemical properties. J Gen Microbiol. 1978;107:319–328. [Google Scholar]
- 25.Meyer J-M, Halle F, Hohnadel D, Lemanceau P, Ratefiarivelo H. Siderophores of Pseudomonas—biological properties. In: Winkelmann G, van der Helm D, Neilands J B, editors. Iron transport in microbes, plants and animals. Weinheim, Germany: VCH Verlagsgesellschaft mbH; 1987. pp. 189–205. [Google Scholar]
- 26.Meyer J-M, Hohnadel D, Halle F. Cepabactin from Pseudomonas cepacia, a new type of siderophore. J Gen Microbiol. 1989;135:1479–1487. doi: 10.1099/00221287-135-6-1479. [DOI] [PubMed] [Google Scholar]
- 27.Meyer J-M, Neely A, Stintzi A, Georges C, Holder I A. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect Immun. 1996;64:518–523. doi: 10.1128/iai.64.2.518-523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyazaki H, Kato H, Nakazawa T, Tsuda M. A positive regulatory gene, pvdS, for expression of pyoverdin biosynthetic genes in Pseudomonas aeruginosa PAO. Mol Gen Genet. 1995;248:17–24. doi: 10.1007/BF02456609. [DOI] [PubMed] [Google Scholar]
- 29.Needleman S B, Wunsch C D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970;48:443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- 30.Neilands J B. Iron absorption and transport in microorganisms. Annu Rev Nutr. 1981;1:27–46. doi: 10.1146/annurev.nu.01.070181.000331. [DOI] [PubMed] [Google Scholar]
- 31.Neilands J B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- 32.Neilands J B, Konopka K, Schwyn B, Coy M, Francis R T, Paw B H, Bagg A. Comparative biochemistry of microbial iron assimilation. In: Winkelmann G, van der Helm D, Neilands J B, editors. Iron transport in microbes, plants and animals. Weinheim, Germany: VCH Verlagsgesellschaft mbH; 1987. pp. 3–33. [Google Scholar]
- 33.Ochsner, U. 1999. Unpublished data.
- 34.Ochsner U A, Johnson Z, Lamont I L, Cunliffe H E, Vasil M L. Exotoxin A production in Pseudomonas aeruginosa requires the iron-regulated pvdS gene encoding an alternative sigma factor. Mol Microbiol. 1996;21:1019–1028. doi: 10.1046/j.1365-2958.1996.481425.x. [DOI] [PubMed] [Google Scholar]
- 35.Ochsner U A, Vasil A I, Johnson Z, Vasil M L. Pseudomonas aeruginosa fur overlaps with a gene encoding a novel outer membrane lipoprotein, OmlA. J Bacteriol. 1998;181:1099–1109. doi: 10.1128/jb.181.4.1099-1109.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okii M, Iyobe S, Mitsuhashi S. Mapping of the gene specifying aminoglycoside 3′-phosphotransferase II on the Pseudomonas aeruginosa chromosome. J Bacteriol. 1983;155:643–649. doi: 10.1128/jb.155.2.643-649.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poole K, Heinrichs D E, Neshat S. Cloning and sequence analysis of an EnvCD homologue in Pseudomonas aeruginosa: regulation by iron and possible involvement in the secretion of the siderophore pyoverdine. Mol Microbiol. 1993;10:529–544. doi: 10.1111/j.1365-2958.1993.tb00925.x. [DOI] [PubMed] [Google Scholar]
- 38.Poole K, Zhao Q, Neshat S, Heinrichs D E, Dean C R. The tonB gene of Pseudomonas aeruginosa encodes a novel TonB protein. Microbiology. 1996;142:1449–1458. doi: 10.1099/13500872-142-6-1449. [DOI] [PubMed] [Google Scholar]
- 39.Rombel I T, McMorran B J, Lamont I L. Identification of a DNA sequence motif required for expression of iron-regulated genes in pseudomonads. Mol Gen Genet. 1995;246:519–528. doi: 10.1007/BF00290456. [DOI] [PubMed] [Google Scholar]
- 40.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 41.Sawatzki G. The role of iron binding proteins in bacterial infection. In: Winkelmann G, van der Helm D, Neilands J B, editors. Iron transport in microbes, plants and animals. Weinheim, Germany: VCH Verlagsgesellschaft mbH; 1987. pp. 477–489. [Google Scholar]
- 42.Schaefer A, Tauch A, Jaeger W, Kalinowski J, Thierbach G, Puehler A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene. 1994;145:69–73. doi: 10.1016/0378-1119(94)90324-7. [DOI] [PubMed] [Google Scholar]
- 43.Simon R, Priefer U, Puehler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 44.Stintzi A, Cornelis P, Hohnadel D, Meyer J-M, Dean C, Poole K, Kourambas S, Krishnapillai V. Novel pyoverdine biosynthesis gene(s) of Pseudomonas aeruginosa PAO. Microbiology. 1996;142:1181–1190. doi: 10.1099/13500872-142-5-1181. [DOI] [PubMed] [Google Scholar]
- 45.Stintzi, A., and J.-M. Meyer. 1997. Unpublished results.
- 46.Streber W R, Timmis K N, Zenk M H. Analysis, cloning, and high-level expression of 2,4-dichlorophenoxyacetate monooxygenase gene tfdA of Alcaligenes eutrophus JMP134. J Bacteriol. 1987;169:2950–2955. doi: 10.1128/jb.169.7.2950-2955.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suwa Y, Wright A D, Fukimori F, Nummy K A, Hausinger R P, Holben W E, Forney L J. Characterization of a chromosomally encoded 2,4-dichlorophenoxyacetic acid/α-ketoglutarate dioxygenase from Burkholderia sp. strain RASC. Appl Environ Microbiol. 1996;62:2464–2469. doi: 10.1128/aem.62.7.2464-2469.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsuda M, Miyazaki H, Nakazawa T. Genetic and physical mapping of genes involved in pyoverdin production in Pseudomonas aeruginosa PAO. J Bacteriol. 1995;177:423–431. doi: 10.1128/jb.177.2.423-431.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vasil M L, Ochsner U A, Johnson Z, Colmer J A, Hamood A N. The Fur-regulated gene encoding the alternative sigma factor, PvdS, is required for the iron-dependent expression of the LysR-type regulator, PtxR, in Pseudomonas aeruginosa. J Bacteriol. 1998;180:6784–6788. doi: 10.1128/jb.180.24.6784-6788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao Q, Li X-Z, Mistry A, Srikumar R, Zhang L, Lomovskaya O, Poole K. Influence of the TonB energy-coupling protein on efflux-mediated multidrug resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1998;42:2225–2231. doi: 10.1128/aac.42.9.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]