Abstract
Objective
To complete the first in-human study of the automated line clearance Thoraguard chest tube system. The study focuses on the viability and efficacy of the device in comparison with conventional models as well as secondary matters such as patient experience and ease of use.
Methods
This was a single-center, prospective, open-label study involving adult patients (n = 27) who underwent nonemergent, first-time, cardiac surgery. Patients received automated clearance chest tubes for surgical drainage in both the mediastinal and pleural spaces. The control group was retrospective (n = 80); individuals received conventional chest tubes placed and secured in locations determined at the surgeon's discretion.
Results
The automated-clearance tubes exhibited a similar drainage profile at 1, 3, 6, 12, and 24 hours compared with the conventional chest tubes. The final output at the time of tube removal was also similar (1150 [750-1590] vs 1289 [766.3-1890] mL, respectively, P = .76). The number of patients readmitted for drainage of an effusion was similar in both groups (1/27 [3.7%] vs 3/80 [3.75%], P > .99).
Conclusions
This study has shown that the Centese Thoraguard chest tube system is a viable option for surgical chest drainage and effective when used in routine cardiac surgery operations.
Key Words: cardiac surgery, chest tube, perioperative, digital, innovation
Abbreviations and Acronyms: AVR, aortic valve repair; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; ERAS, Enhanced Recovery After Surgery; MVr, mitral valve repair; RBS, retained blood syndrome
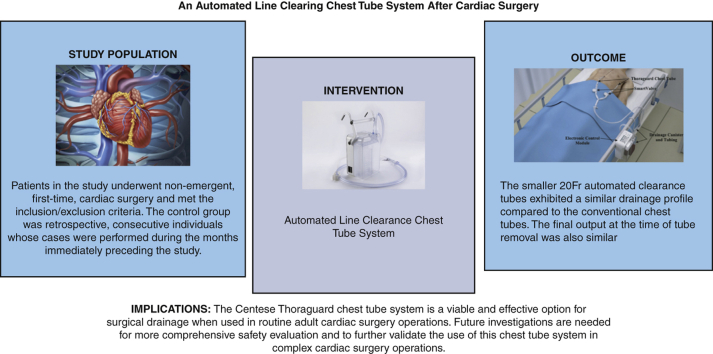
Graphical abstract
Graphical Abstract depicting the study's methods and selection criteria, results, and implications.

Centese Thoraguard Chest Tube System.
Central Message.
This study has shown that the Centese Thoraguard chest tube system is a viable and effective option for surgical drainage when used in routine adult cardiac surgery operations.
Perspective.
This is the first-in-human study of the Thoraguard chest tube system. The occurrence of blood clots within chest tubes has previously been addressed by milking and/or stripping tubes. ERAS guidelines recommend against these strategies. This study shows that the Centese Thoraguard chest tube system is a viable and effective option for surgical drainage in the adult cardiac surgery patient population.
See Commentary on page 254.
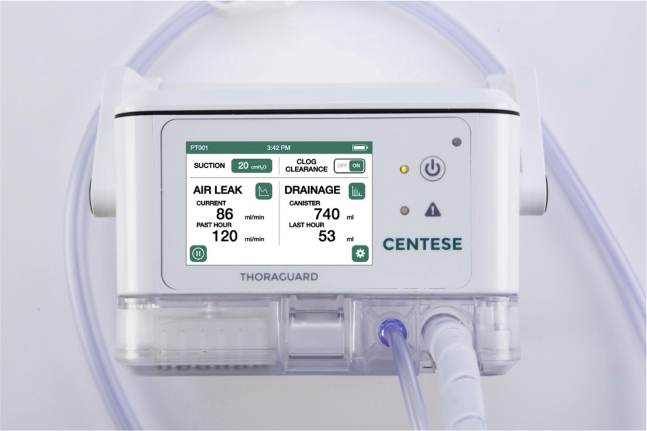
Chest tube drainage is an essential component of postoperative management in cardiac surgery. Adequate drainage from the mediastinum can prevent cardiac tamponade whereas drainage from the pleural space improves respiratory status and decreases the work of breathing.1, 2, 3 Moreover, incomplete thoracic drainage can result in retained blood syndrome (RBS) and lead to a host of acute, subacute, and chronic postoperative complications. Retained blood is associated with increases in transfusion, acute kidney injury, time on mechanical ventilation, length of stay, and mortality. Recent studies have estimated that 13.8% to 22.7% of patients who undergo cardiac surgery develop some form of this syndrome.4 Conversely, protracted in-dwelling of chest tubes increases the risk infections, mechanical irritation, and likelihood of patient discomfort.5, 6, 7 Maintaining tube patency is critical, as it helps to prevent pneumothorax, RBS, development of large pleural effusions, and the pooling of fluid around the heart. Unfortunately, drains used to clear mediastinal blood are prone to clogging, with clotted blood in up to 36% of patients.8, 9, 10 Historically, techniques such as milking and stripping the chest tube have been employed to maintain tube patency. However, recent Enhanced Recovery After Surgery (ERAS) guidelines have called into question the safety and efficacy of these strategies and recommend against their use.10,11 Studies have shown these tactics do not significantly reduce clogging or enhance drainage. Instead, the high negative pressures generated by their use have the potential to cause tissue injury.12,13 Another technique commonly used to maintain patency is to break the sterile field to access the inside of chest tubes using a smaller tube to suction the clot out. This technique is not recommended, as it can increase infection risk and potentially damage internal structures.10,12,13 To maintain chest tube patency while adhering to the ERAS guidelines, the Centese Thoraguard System (Figure 1) employs an automated chest tube-clearance system that clears the chest tube every 5 minutes without clinician manipulation.
Figure 1.
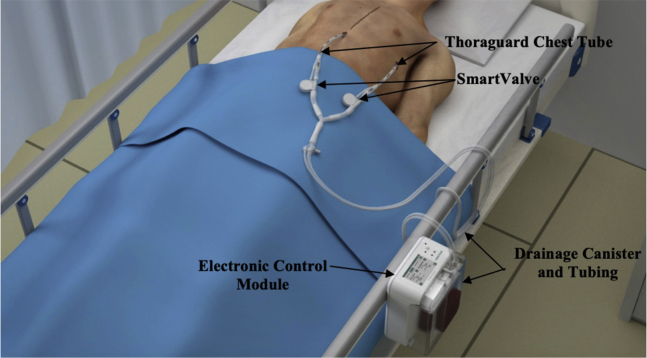
The Centese Thoraguard System. It is an electronic chest drain and chest tube system that uses integrated suction and an automatic air sweep mechanism to maintain chest tube patency and digitally capture drainage output. The system consists of 3 separate components: the Thoraguard Control Module, Thoraguard Drainage Kit, and the Thoraguard Chest Tube Kit.
We report the first-in-human study of this automated-clearance chest tube system and hypothesize this chest tube system will have a similar drainage profile to conventional chest tubes. Furthermore, the Thoraguard may have secondary benefits of improved patient pain control and more precise measurements due to its digital system.
Methods
Study Design
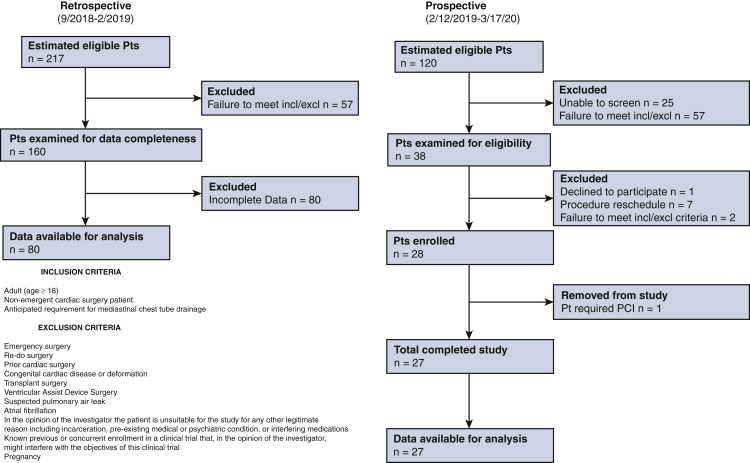
All patients provided consent, and the study was compliant with the Stanford institutional review board protocol (registration #47453; date of approval February 11, 2019). Patients provided informed written consent for publication of their study data. This study was a single-center, prospective, open-label study involving adult patients (n = 27) who underwent nonemergent, first-time, cardiac surgery between February 12, 2019, and March 17, 2020. These included 19 isolated coronary artery bypass grafting (CABG) operations, 3 isolated aortic valve replacements (AVRs), 3 CABG + AVR, 1 mitral valve repair and myomectomy (MVr + myomectomy), and 1 AVR + aortic operation (Table 1). All cases were performed between February and October 2019. Study patients received automated clearance chest tubes for surgical drainage in both the mediastinal and pleural spaces. Tubes were inserted and secured according to the surgeon's preference. The control group was retrospective and contained adult patients (n = 80) (Table 1) who underwent similar first-time cardiac operations. These included 50 isolated CABG operations, 6 CABG + AVR, 2 CABG + MVr, 2 CABG + AVR + aortic operation, 8 isolated AVR, 2 AVR + aortic operation, 6 MVr, 1 AVR + MVr, 1 MVr + tricuspid valve repair, 1 isolated tricuspid valve replacement, and 1 coronary translocation (Table 1). These cases were performed during the months immediately preceding the study (September 2018-February 2019). Control patients received conventional chest tubes placed and secured in locations determined at the surgeon's discretion. Patients were excluded from the study if they had the following: emergency surgery, re-do surgery, congenital cardiac disease, transplant surgery, ventricular assist device surgery, suspected pulmonary air leak, atrial fibrillation, pregnancy, or known previous or concurrent enrollment in a clinical trial that might interfere with the objectives of this study or incarceration (Figure E1). Data are presented as counts with percentages (compared using the Fisher exact test) or as median with interquartile range (compared using the Mann–Whitney U test). Drainage profile, number of occlusion events, and additional interventions (eg, thoracentesis or pericardiocentesis) were considered primary end points. The study methods, intervention, and outcome are depicted in Figure 2.
Table 1.
An automated line-clearing chest tube system after cardiac surgery: operative case breakdown
| Operation | Automated clearance | Conventional | Total |
|---|---|---|---|
| CABG | 19 | 50 | 69 |
| CABG AVR | 3 | 6 | 9 |
| CABG MVR | 0 | 2 | 2 |
| CABG AVR + aorta | 0 | 2 | 2 |
| AVR | 3 | 8 | 11 |
| AVR + aorta | 1 | 2 | 3 |
| MVr + myomectomy | 1 | 0 | 1 |
| MVr | 0 | 6 | 6 |
| AVR MVr | 0 | 1 | 1 |
| MVr TVr | 0 | 1 | 1 |
| TVR | 0 | 1 | 1 |
| Coronary translocation | 0 | 1 | 1 |
| Total | 27 | 80 | 107 |
CABG, Coronary artery bypass grafting; AVR, aortic valve replacement; MVR, mitral valve replacement; MVr, mitral valve repair; TVr, tricuspid valve repair; TVR, tricuspid valve replacement.
Figure E1.
An automated line-clearing chest tube system after cardiac surgery: patient selection CONSORT Diagram with inclusion and exclusion criteria.
Figure 2.
Shown are the study's methods and selection criteria, results, and implications.
Thoraguard Automated Chest Drainage System
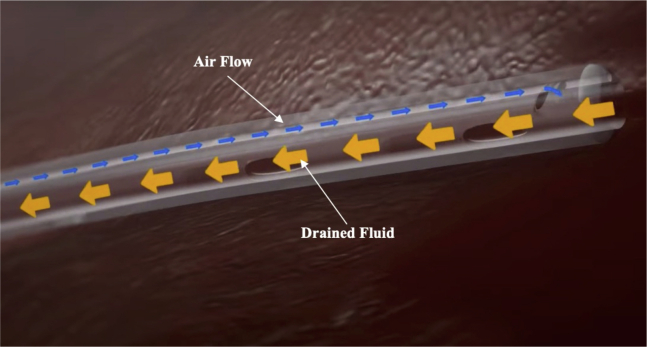
The Thoraguard System is a medical device cleared by the Food and Drug Administration and authorized for use in the study population. It was cleared via the 510K process, which did not require human clinical data. The ClinicalTrials.gov Identifier for this study is NCT03860363. The system, made by Centese (Omaha, Neb), is an electronic chest drain and chest tube system that uses integrated suction and an automatic air sweep mechanism to maintain chest tube patency and digitally capture drainage output. The system consists of 3 separate components: the Thoraguard Drainage Kit, Thoraguard Control Module, (Figures 3 and 4), and the Thoraguard Chest Tube Kit (Figure 5), all showcased in Video 1. The control module is an electronic monitor that provides the core functionality of the Thoraguard System. It incorporates an integrated pump, battery-power, sensors for suction regulation, and digital measurements of fluid output and air leak rate. The drainage kit includes a 1200-mL drainage canister and drainage tubing, which connects the drainage canister to chest tube. The chest tube kit consists of a chest tube for insertion and SmartValve filter for use during the automated clearance function. The chest tube is soft, 20-French (Fr) dual-lumen drainage catheter with one lumen for drainage and one for venting air flow during clog clearance (Figure 5). To ensure proper drainage, the chest tube is automatically cleared every 5 minutes. In doing this, suction is elevated from the baseline setting (–20 cmH2O) to –100 cmH2O. The elevated suction pulls open a normally closed valve on the SmartValve. Ambient air is pulled across a sterilization-grade filter on the SmartValve and into the venting lumen of the chest tube, which joins the primary drainage lumen at the proximal tip of the chest tube. The air bolus then sweeps fluids stagnating within the chest tube into the drainage canister. Upon completion of the clearance cycle, suction is automatically restored to baseline and the SmartValve is resealed. At no time is the pressurized air introduced into the drainage system or thoracic cavity as the sweeping mechanism is only activated in the presence of suction. The entire clearance sequence takes approximately 30 seconds to 2 minutes to complete.
Figure 3.
Thoraguard Drainage Kit. It includes a 1200-mL drainage canister and drainage tubing, which connects the drainage canister to chest tube.
Figure 4.
Thoraguard Control Module. An electronic monitor that provides the core functionality of the Thoraguard System. It incorporates an integrated pump, battery-power, sensors for suction regulation, and digital measurements of fluid output and air leak rate.
Figure 5.
Thoraguard Chest Tube Kit. The kit consists of a chest tube for insertion and SmartValve filter for use during the automated clearance function. The chest tube is soft, 20-Fr dual-lumen drainage catheter with one lumen for drainage and one for venting air flow during clog clearance. To ensure proper drainage, the chest tube is automatically cleared every 5 minutes.
Conventional Chest Tube System
The Atrium Oasis Dry Suction Water Seal Chest Drain was uniformly used as the conventional chest tube system in this study and employed in the historical patient cohort. The chest drain is manufactured by Getinge. Covidien chest tubes were used and ranged from 24 Fr to 40 Fr in size.
Results
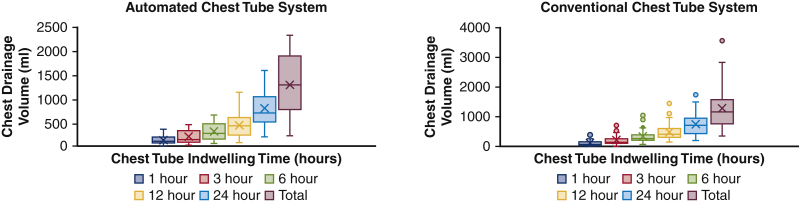
A total of 57 automated-clearance tubes were used in the experimental group (2.6 [2.0-3.0] tubes per patient), and 135 conventional chest tubes were used in the control group (2.6 [2.0-3.0] tubes per patient), P = .64 (Table 2). The automated-clearance tubes were significantly smaller (20.0 [20.0-20.0] vs 28 Fr [IQR 24.0-36.0Fr]), but exhibited a similar drainage profile at 1, 3, 6, 12, and 24 hours compared with the conventional chest tubes (Figure 6). The final output at the time of tube removal was also similar (1150 [750-1590] vs 1289 [766.3-1890] mL, respectively, P = .76). Chest tubes were kept in place for 75.6 [65.4-98.2] versus 76.5 [61.1-95.0] hours, respectively (P = .49) (Table 3). Only 1 automated-clearance chest tube was occluded upon removal. No additional interventions were required during the hospitalization of patients in either group for postoperative pneumothorax or pleural/pericardial effusion. The number of patients readmitted for drainage of an effusion was similar in both groups (1/27 [3.7%] vs 3/80 [3.75%], P > .99). For these cases, drainage was performed using thoracentesis. The adjusted odds ratio for hospital readmission for a patient with a Thoraguard chest tube, after adjusting for age, body mass index, cardiopulmonary bypass time, crossclamp time, and preoperative ejection fraction, was 7.08 (95% confidence interval, 0.17-1319.13), P = .338. Per recommendations by Westreich and Greenland,14 we do not include the estimates of the other variables included in the multivariable model to prevent incorrect interpretation of effects, also known as the table 2 fallacy. A multivariable analysis is included in Table E1. We decided not to include preoperative Society of Thoracic Surgeons (STS) score in the model because including it into any variation of a multivariable model to control for the effect of group (conventional vs automated line clearance) on outcome (hospital admission) caused errors with convergence in the logistic regression. The average time in the cardiovascular intensive care unit for the automated clearance group was 68.4 hours versus 67 hours for the conventional chest tube group. While in place, neither group had patients who suffered damage to surrounding structures attributable to the chest tubes. The average postoperative pain score for patients in the automated-clearance chest tube study group was 1.24 (standard deviation 0.96) and 1.63 (standard deviation 1.04) for patients in the conventional chest tube group, P = .14. Pain scores were obtained directly from patients using a standard 0 to 10 pain scale.
Table 2.
An automated line-clearing chest tube system after cardiac surgery: baseline patient characteristics
| Automated clearance | Conventional | P value | |
|---|---|---|---|
| Number of patients, n | 27 | 80 | |
| Age, mean [IQR] | 66.0 [56.0-71.0] | 66.5 [57.3-74.0] | .42 |
| Male/female | 19/8 | 63/17 | .43 |
| BMI [IQR] | 28.9 [24.8-32.6] | 28.1 [24.6-30.4] | .48 |
| CPB time, min | 100.0 [85.0-130.5] | 105.5 [83.5-137.5] | .40 |
| Crossclamp time, min | 75.0 [63.5-100.0] | 72.0 [63.0-94.8] | .97 |
| No. chest tubes per patient [IQR] | 2.6 [2.0-3.0] | 2.6 [2.0-3.0] | .64 |
| Total chest tubes | 57 | 135 | |
| Dialysis, n (%) | 4 (0.148) | 6 (0.075) | .27 |
| Diabetes, n (%) | 16 (0.593) | 35 (0.438) | .19 |
| Chronic lung disease, n (%) | 4 (0.148) | 14 (0.175) | .27 |
| Ejection fraction, % [IQR] | 60.0 [45.4-66.7] | 57.0 [42.3-61.5] | .07 |
| Heart failure, n (%) | 3 (0.11) | 15 (0.1875) | .55 |
| Hypertension, n (%) | 24 (0.889) | 76 (0.962) | .35 |
| Preoperative STS score, %, mean (SD) | 0.016 (0.0123) | 0.0224 (0.0294) | .35 |
| Average postoperative pain score (SD) | 1.24 (0.96) | 1.63 (1.04) | .14 |
IQR, Interquartile range; BMI, body mass index; CPB, cardiopulmonary bypass time; STS, Society of Thoracic Surgeons; SD, standard deviation.
Figure 6.
Although smaller in caliber, the automated clearance chest tubes produced a similar drainage profile as conventional control chest tubes. The final output at the time of tube removal was also similar (1150 [750-1590] vs 1289 [766.3-1890] mL, respectively, P = .76). In this box-and whiskers dot plot, the upper and lower borders of the box represent the upper and lower quartiles. The middle horizontal line represents the median. The upper and lower horizontal lines represent the maximum and minimum values of nonoutliers.
Table 3.
An automated line-clearing chest tube system after cardiac surgery: chest tube related complication and drainage comparison
| Automated clearance | Conventional patients | P value | |
|---|---|---|---|
| Tube duration, h | 76.5 [61.1-95.0] | 75.6 [65.4-98.2] | .49 |
| 1-h output | 105 [71.3-195] | 60 [35.5-155.5] | .33 |
| 6-h output | 270 [153.8-470] | 256.5 [207.5-377.5] | .92 |
| 24-h output | 700.0 [512.3-1038] | 710 [435-935] | .67 |
| Final output | 1289 [766.3-1890] | 1150 [750-1590] | .76 |
| Takeback for bleeding | 0 [0%] | 0 [0%] | .57 |
| Pneumothorax | 0 [0%] | 0 [0%] | N/A |
| Pleura effusion drained | 0 [0%] | 0 [0%] | N/A |
| Pericardiocentesis | 0 [0%] | 0 [0%] | N/A |
| Readmit of effusion | 1 [3.7%] | 3 [3.75%] | >.99 |
N/A, Not available. Values are reported as median [IQR] except those labeled with mean.
Discussion
The main role for a chest tube in the postoperative setting is to evacuate air and surgical fluid without becoming occluded. Failure to fulfill this purpose can result in cardiac tamponade, hemodynamic instability, and respiratory compromise.15 Studies have shown that the occurrence of blood clots, particularly in mediastinal chest tubes, is significantly elevated.6, 7, 8 This problem has been addressed in the past by milking, stripping, and sometimes breaking the sterile barrier to suction out clot from within chest tubes. Recent ERAS guidelines recommend against these strategies and suggest the automated-clearance chest tube systems may reduce the occurrence of RBS8,16 while avoiding the severe spikes in intrathoracic pressure that occur during milking and stripping of chest tubes and the increased risk of infection that is associated with breaking the sterile barrier to suction within the chest tube. Recent efforts have been made to develop chest tube systems that maintain tube patency without requiring external manipulation.17,18 The Centese Thoraguard uses an automated clearance system to achieve tube patency.
The Thoraguard System differs from the PleuraFlow (ClearFlow, Inc) system, which is a commercially available chest tube clearance apparatus that uses an internal guidewire with a small loop at the end to mechanically remove clots by moving the wire in and out of the chest tube. The guidewire is advanced and retracted manually by the bedside nurse on a predefined activation schedule (0-8 hours postoperative: every 15 minutes, 8-24 hours postoperative: every 30 minutes, 24+ hours postoperative: every 60 minutes). Clotted material is then pulled into a traditional chest drain connected to wall suction. Perrault and colleagues17 describe in their study the first clinical experience study suggesting positive incorporation into the workflow. In the study, it was noted that the intensivist in the intensive care unit observed nonobstructive clot on the guidewire in 13% (2/15) of the PleuraFlow chest tubes compared with 33% (5/15) of the standard chest tubes. Respiratory variation was present upon removal of all PleuraFlow chest tubes. One notable observation made during the study was that nurses frequently relied on visible evidence of clotting to initiate efforts to maintain patency. Although the active line clearance mechanism was effective in removing clots, it is potentially limited by the fact that requires the user to manually actuate the inner wire and therefore first notice that the tube is obstructed. The Thoraguard automated system automatically sweeps the chest tube every 5 minutes and addresses occlusions that occur in the proximal portion of the chest tube, not visible to those at the bedside.
Of note, the Thoraguard System's integrated suction feature was found to be beneficial while transporting patients from the operating room to the intensive care unit. This was because we were able to maintain suction, if appropriate, without the need for an additional vacuum source. This feature was particularly useful in the early postoperative period when mobilizing the patient. The Thoraguard has a suction range of 0 to 100 cmH2O. This range of operation is larger than traditional chest drains, which are regularly limited to –40 cmH2O. Stable, elevated suction likely assists with improved drainage without damage to surrounding structures.19,20
The Thoraguard system is also equipped with safety alarms to safeguard against system malfunctions, such as suction loss, leaks, or drainage tube obstructions and to alert the provider to irregular output levels. Chest drains with digital control have been present in the field of cardiothoracic surgery for some time. The Medela Thopaz+ system is a commercially available digital chest drainage system. The Thopaz+ system has been shown to have utility in thoracic surgery for air leak quantification.21 Recent investigation of its use in cardiac surgery has shown benefit.22 Thoraguard offers similar functions as the Thopaz+ system in thoracic surgery, such as digital air leak measurement and facilitation of early ambulation under suction. However, a thoracic surgery population was not included as part of this experience. The Thopaz+ does not offer any chest tube clearance functionality that the authors are aware of.
Variation exists between surgeons when it comes to chest tube management. The complexity of the operation significantly impacts the size of the tube and the duration for which it is left in place. Accurate measurements are important for clinical decision-making and codifying postoperative chest tube management. The Thoraguard control module provided precise and accurate output measurements consistent with volume seen in the drainage containers throughout the study. Moreover, the soft 20-Fr chest tubes were associated with decreased pain at the insertion site.
Nevertheless, the findings of this study must be considered in the context of some limitations. This was a first-in-human study with a primary aim to evaluate the viability of the automated line clearing chest tube system and compare its efficacy with conventional chest tube systems. While our results demonstrate that the automated line clearing chest tube is viable and functionally comparable with the conventional chest tube drainage system, a larger sample size is needed in future studies to comprehensively evaluate safety and demonstrate the advantages of this chest drainage model. Furthermore, our control group is retrospective, and although consecutive patients were included in the study, there was no randomization of patients into the automated and conventional chest tube study arms. Future investigations are needed for more comprehensive safety evaluation and to further validate the use of this chest tube system in complex cardiac surgery operations.
With an exceptionally low occlusion and reintervention rate and a similar drainage profile as larger-caliber thoracostomy tubes, our results suggest that the automated-clearance chest tube system from Centese is a viable and effective option for surgical drainage after routine adult cardiac surgery. Our analysis shows the smaller 20-Fr Thoraguard chest tubes provided comparable fluid drainage with the larger conventional chest tubes. Further studies regarding the Thoraguard system should be done focusing on cost analysis.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
We thank Shari Miller, Mina Liu, Rhodalene Benjamin-Addy, Tiffany K Koyano, Scott Kronenberg, and Kokil Bakshi for coordinating this study and assistance with data collection. We thank Matthew Leipzig for assistance with biostatistics.
Footnotes
Centese (Omaha, Neb), which developed the Thoraguard Automated Clearance System, provided the chest tube systems without cost to Stanford Health Care and provided funding for the study. This material is based upon work supported by the National Science Foundation under Grant No. 1660238. Oluwatomisin Obafemi currently receives funding from an R38 training grant No. R38HL14361501.
Appendix E1
Table E1.
An automated line-clearing chest tube system after cardiac surgery: multivariable analysis
| Predictors | Hospital readmission |
|
|---|---|---|
| Odds ratios (CI) | P value | |
| Group | 7.08 (0.17-1319.13) | .338 |
| Age | 0.93 (0.72-1.12) | .486 |
| BMI | 0.74 (0.40-1.03) | .157 |
| CPB time | 1.02 (0.76-1.18) | .769 |
| XC time | 0.98 (0.85-1.38) | .839 |
| Preoperative EF | 1.45 (1.06-3.39) | .140 |
CI, Confidence interval; BMI, body mass index; CPB, cardiopulmonary bypass time; XC, crossclamp, EF, ejection fraction.
Supplementary Data
The video presentation showcases the components of the Thoraguard Chest Tube Kit, Drainage Kit, and Control Module. Installation of the chest tube system is demonstrated postcardiac surgery and an example of an integrated safety alarm for tube obstruction or kinking is shown. Video available at: https://www.jtcvs.org/article/S2666-2736(22)00084-5/fulltext.
References
- 1.Duncan C.R., Erickson R.S., Weigel R.M. Effect of chest tube management on drainage after cardiac surgery. Heart Lung. 1987;16:1–9. [PubMed] [Google Scholar]
- 2.Light R.W., Rogers J.T., Moyers J.P., Lee Y.C., Rodriguez R.M., Alford W.C., Jr., et al. Prevalence and clinical course of pleural effusions at 30 days after coronary artery and cardiac surgery. Am J Respir Crit Care Med. 2002;166:1567–1571. doi: 10.1164/rccm.200203-184OC. [DOI] [PubMed] [Google Scholar]
- 3.Munnell E.R. Thoracic drainage. Ann Thorac Surg. 1997;63:1497–1502. doi: 10.1016/s0003-4975(97)00082-9. [DOI] [PubMed] [Google Scholar]
- 4.Boyle E.M., Jr., Gillinov A.M., Cohn W.E., Ley S.J., Fischlein T., Perrault L.P. Retained blood syndrome after cardiac surgery: a new look at an old problem. Innovations. 2015;10:296–303. doi: 10.1097/IMI.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 5.Kwiatt M., Tarbox A., Seamon M.J., Swaroop M., Cipolla J., Allen C., et al. Thoracostomy tubes: a comprehensive review of complications and related topics. Int J Crit Illn Inj Sci. 2014;4:143–155. doi: 10.4103/2229-5151.134182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller K.S., Sahn S.A. Chest tubes. Indications, technique, management and complications. Chest. 1987;91:258–264. doi: 10.1378/chest.91.2.258. [DOI] [PubMed] [Google Scholar]
- 7.Mirmohammad-Sadeghi M., Etesampour A., Gharipour M., Shariat Z., Nilforoush P., Saeidi M., et al. Early chest tube removal after coronary artery bypass graft surgery. N Am J Med Sci. 2009;1:333–337. doi: 10.4297/najms.2009.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karimov J.H., Gillinov A.M., Schenck L., Cook M., Kosty Sweeney D., Boyle E.M., et al. Incidence of chest tube clogging after cardiac surgery: a single-center prospective observational study. Eur J Cardiothorac Surg. 2013;44:1029–1036. doi: 10.1093/ejcts/ezt140. [DOI] [PubMed] [Google Scholar]
- 9.Balzer F., von Heymann C., Boyle E.M., Wernecke K.D., Grubitzsch H., Sander M. Impact of retained blood requiring reintervention on outcomes after cardiac surgery. J Thorac Cardiovasc Surg. 2016;152:595–601.e4. doi: 10.1016/j.jtcvs.2016.03.086. [DOI] [PubMed] [Google Scholar]
- 10.Gregory A.J., Grant M.C., Manning M.W., Cheung A.T., Ender J., Sander M., et al. Enhanced recovery after cardiac surgery (ERAS Cardiac) recommendations: an important first step-but there is much work to be done. J Cardiothorac Vasc Anesth. 2020;34:39–47. doi: 10.1053/j.jvca.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Engelman D.T., Ben Ali W., Williams J.B., Perrault L.P., Reddy V.S., Arora R.C., et al. Guidelines for perioperative care in cardiac surgery: enhanced recovery after surgery society recommendations. JAMA Surg. 2019;154:755–766. doi: 10.1001/jamasurg.2019.1153. [DOI] [PubMed] [Google Scholar]
- 12.Halm M.A. To strip or not to strip? physiological effects of chest tube manipulation. Am J Crit Care. 2007;16:609–612. [PubMed] [Google Scholar]
- 13.Day T.G., Perring R.R., Gofton K. Is manipulation of mediastinal chest drains useful or harmful after cardiac surgery? Interact Cardiovasc Thorac Surg. 2008;7:888–890. doi: 10.1510/icvts.2008.185413. [DOI] [PubMed] [Google Scholar]
- 14.Westreich D., Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol. 2013;177:292–298. doi: 10.1093/aje/kws412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shalli S., Saeed D., Fukamachi K., Gillinov A.M., Cohn W.E., Perrault L.P., et al. Chest tube selection in cardiac and thoracic surgery: a survey of chest tube-related complications and their management. J Card Surg. 2009;24:503–509. doi: 10.1111/j.1540-8191.2009.00905.x. [DOI] [PubMed] [Google Scholar]
- 16.Sirch J., Ledwon M., Püski T., Boyle E.M., Pfeiffer S., Fischlein T. Active clearance of chest drainage catheters reduces retained blood. J Thorac Cardiovasc Surg. 2016;151:832–838. doi: 10.1016/j.jtcvs.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Perrault L.P., Pellerin M., Carrier M., Cartier R., Bouchard D., Demers P., et al. The PleuraFlow Active Chest Tube Clearance System: initial clinical experience in adult cardiac surgery. Innovations. 2012;7:354–358. doi: 10.1097/IMI.0b013e31827e2b4d. [DOI] [PubMed] [Google Scholar]
- 18.Shalli S., Boyle E.M., Saeed D., Fukamachi K., Cohn W.E., Gillinov A.M. The active tube clearance system: a novel bedside chest-tube clearance device. Innovations. 2010;5:42–47. doi: 10.1097/IMI.0b013e3181cf7ce3. [DOI] [PubMed] [Google Scholar]
- 19.Farhat F., Ginon I., Lefevre M., Lu Z., Andre-Fouët X., Mikaeloff P., et al. Prospective randomized comparison between redon catheters and chest tubes in drainage after cardiac surgery. J Cardiovasc Surg (Torino) 2003;44:179–186. [PubMed] [Google Scholar]
- 20.Newcomb A.E., Alphonso N., Nørgaard M.A., Cochrane A.D., Karl T.R., Brizard C.P. High-vacuum drains rival conventional underwater-seal drains after pediatric heart surgery. Eur J Cardiothorac Surg. 2005;27:395–399. doi: 10.1016/j.ejcts.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Pompili C., Detterbeck F., Papagiannopoulos K., Sihoe A., Vachlas K., Maxfield M.W., et al. Multicenter international randomized comparison of objective and subjective outcomes between electronic and traditional chest drainage systems. Ann Thorac Surg. 2014;98:490–496. doi: 10.1016/j.athoracsur.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 22.Van Linden A., Hecker F., Courvoisier D.S., Arsalan M., Köhne J., Brei C., et al. Reduction of drainage-associated complications in cardiac surgery with a digital drainage system: a randomized controlled trial. J Thorac Dis. 2019;11:5177–5186. doi: 10.21037/jtd.2019.12.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The video presentation showcases the components of the Thoraguard Chest Tube Kit, Drainage Kit, and Control Module. Installation of the chest tube system is demonstrated postcardiac surgery and an example of an integrated safety alarm for tube obstruction or kinking is shown. Video available at: https://www.jtcvs.org/article/S2666-2736(22)00084-5/fulltext.