Abstract
Background:
Our study aimed to compare real-world healthcare resource utilization (HRU) and healthcare cost (HC) of Medicare-insured patients (≥65 years old) with Dupuytren’s contracture (DC) treated with Clostridium histolyticum (collagenase) or fasciectomy.
Methods:
DC patients treated with collagenase or fasciectomy between July 2011 and June 2017 were identified using the IBM MarketScan Medicare Supplemental Database. The index date was the date of the first procedure. Demographic characteristics were captured on the index date, and comorbidities were assessed during the 24-month preindex period. HRU and HC were analyzed throughout the 12-month postindex period. Patients were matched using propensity score weights. Gamma log-linked generalized linear models were used to evaluate HC drivers.
Results:
Out of 37,374 DC patients, 2911 received collagenase, while 6258 underwent fasciectomy. Postmatching, the total average annual HC was similar between collagenase and fasciectomy ($7271 versus $6220, P = 0.357). When HCs were stratified by the service provider, outpatient facility and physician office costs were lower in the collagenase cohort ($850 versus $1284, P = 0.047 and $546 versus $1001, P < 0.001). The costs of professional services were significantly higher than in the fasciectomy cohort due to the cost of collagenase injection ($1682 versus $629, P < 0.001). The HRU was similar between cohorts, except for more frequent outpatient facility visits in fasciectomy patients (12.3 versus 22.9, P < 0.001). Generalized linear model revealed Charlson comorbidity index, plan type, patients’ residence region, sleep disorder, and hyperlipidemia as significant predictors of total HC.
Conclusion:
This study found comparable total annual HC and HRU between collagenase- and fasciectomy-treated Medicare patients.
Takeaways
Question: Is there a difference in healthcare resource utilization and costs among collagenase- and fasciectomy-treated Medicare-insured patients with Dupuytren’s disease?
Findings: The retrospective analysis of Medicare claims identified 2911 patients receiving collagenase and 6258 undergoing fasciectomy for Dupuytren’s disease. Overall, healthcare resource utilization and related annual costs ($7271 versus $6220, P = 0.357) were similar between the matched cohorts, apart from outpatient facility visits, which were more frequent with fasciectomy (12.3 versus 22.9, P < 0.001).
Meaning: Based on real-world data, the financial burden of treating older Medicare-insured patients with Dupuytren’s disease with less invasive collagenase or surgery is similar.
INTRODUCTION
Dupuytren’s disease is a proliferative fibroplasia of subcutaneous palmar tissue, causing an irreversible flexion of the proximal interphalangeal and metacarpophalangeal joints. Although Dupuytren’s disease per se is typically painless, the progression of the disease causes visible and functional deformity of the affected hand.1 Advanced contractures affect patients’ quality of life as daily activities become burdensome, requiring treatment initiation.2
The global prevalence of Dupuytren’s contracture (DC) increases with age, affecting predominantly men older than 65.3 According to the US Census Bureau, the proportion of older adults will likely double in the next 30 years.4 Considering this trend, together with a high DC prevalence estimated to be around 5% according to the US National Library of Medicine,5 the need for DC treatments is expected to rise substantially.
A common treatment of advanced DC is excisional surgery of the affected tissue, open fasciectomy, recommended for functionally impaired patients with high degrees of contracture.6,7 However, many disadvantages have been associated with fasciectomy, such as frequent postoperative complications and prolonged rehabilitation after surgery.7 Additionally, surgical interventions might not be an appropriate treatment for older DC patients, who tend to be less tolerant of anesthesia, often having multiple coexisting diseases and a prolonged convalescence time.8
The first and only nonsurgical treatment approved by the U.S. Food and Drug Administration in February 2010 is Xiaflex, an injection of Clostridium histolyticum collagenase enzyme.9 Shifting the standard surgical treatment for DC to less invasive nonsurgical alternatives might have a valuable impact in terms of both cost and effectiveness, especially for older patients.9
The effectiveness of collagenase injection in treatment of DC has been studied in several randomized placebo-controlled trials.9–11 However, because of conflicting and sparse evidence on the comparative effectiveness of fasciectomy and collagenase, it is hard to make general recommendations for their use.6 A recently published study explored the real-world costs of collagenase in comparison to fasciectomy and concluded that treatment with the less invasive treatment option, collagenase, may lead to statistically significant cost savings.12 Given that this study concerned commercially insured working-age patients, those results may not be generalizable to the Medicare-covered patients older than 65. Thus, the relative costs of collagenase utilization for managing DC in the geriatric subpopulation are not yet determined.13,14 The generalizability of findings from randomized controlled trials is limited by short follow-ups and homogenous study populations.15 Real-world evidence obtained from a retrospective claims analysis may provide new insights into the health-economic impact of interventions, adding an important aspect to any healthcare decision-making process.
The specific needs and unpredictable outcomes of the geriatric population represent a great challenge to policymakers, payers, and clinicians worldwide. Therefore, generating real-world economic evaluation of DC treatments in this population can provide further insights into treatment options and may help inform optimal patient care delivery.
Using a representative US integrated claims database, the present study examines collagenase- and fasciectomy-related healthcare resource utilization (HRU) and associated healthcare cost (HC) in a population of older DC patients insured through the Medicare Supplemental plan. Moreover, we aim to identify key drivers of total direct HC in older DC patients.
MATERIAL AND METHODS
Data Sources
Enrollment records and inpatient, outpatient, ancillary, and drug claims were extracted from the IBM MarketScan Medicare Supplemental and Coordination of Benefits Database. The Medicare Supplemental database captures the healthcare experience of individuals with Medicare commercial supplemental insurance paid by their employers. In compliance with the Health Insurance Portability and Accountability Act of 1996, all patient data used in this study were deidentified, and thus, institutional review board approval was not required.
Study Design
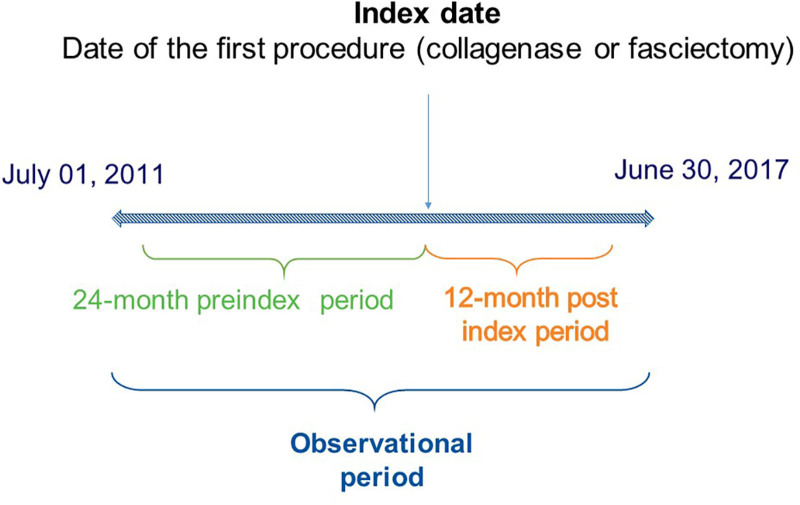
This study was a retrospective administrative claims analysis of beneficiaries in a US Medicare-insured population. Eligible patients were selected from the Medicare Supplemental database during the time period starting on July 1, 2011, and ending on June 30, 2017. Based on initial treatment, patients were assigned to either a collagenase cohort or a fasciectomy cohort, and the date of the first procedure was defined as the index date. Patients were observed within the observational period that comprised 24 months before (preindex period) and 12 months after the index date (postindex period) (Fig. 1).
Fig. 1.
Study design.
Eligible patients had at least one outpatient claim with a diagnosis code for DC (ie, International Classification of Diseases Ninth or Tenth Revision codes 728.6 or M72.0). During the observational period, patients were required to have continuous healthcare and pharmacy coverage. Patients were excluded if they had any record of Peyronie’s disease (ie, a claim coded with International Classification of Diseases Ninth or Tenth Revision 607.85 or N48.6) during the observational period. As we aimed to identify treatment-naive DC patients, no other type of DC treatment was allowed during the observational period.
Patients were included in the collagenase cohort if they satisfied the following criteria: at least one claim for collagenase medication identified through the Healthcare Common Procedure Coding System code J0775 or the National Drug Code 66887-0003-01; medication injection in the palm tissue within 90 days after the medication claim, identified through the Current Procedural Terminology code 20527; and a DC diagnosis obtained on the procedure date. Fasciectomy-treated patients had at least one claim with a Current Procedural Terminology code for fasciectomy (26121, 26123, and 26125) and a DC diagnosis on the same date.
Outcomes Measures
Demographic variables were captured on the index date. Clinical characteristics, including the Charlson comorbidity index (CCI)16 and other comorbid conditions, were measured during the preindex period. The following additional comorbidities associated with DC are considered to be potential confounders in this study and were assessed over the preindex period: hypertension, hyperlipidemia, alcohol use, tobacco use disorder, complex regional pain syndrome, carpal tunnel syndrome, trigger finger syndrome, epilepsy, and gout.
The primary outcomes of the study were all-cause and DC-related HC and HRU evaluated over the 12-month postindex period from the payer’s perspective. Total direct costs were composed of medical and pharmacy costs.
To assess the healthcare expenditures particularly related to DC, we analyzed disease-specific costs and resource consumption, in the form of ‘‘DC-related’’ outcomes. The DC-related cost components were identified as the cost of a medical claim with a DC diagnosis.
All-cause and DC-related HRU were analyzed based on the number of outpatient visits, inpatient hospital admissions, the number of emergency department (ED) visits, and the money-equivalent of provided services in submitted claims. Additionally, HRU was subcategorized based on the service provider.
Statistical Analyses
Continuous variables were summarized as mean and standard deviation, while categorical variables were summarized as numbers and proportions of the sample. The independent t test (continuous variables) and Chi-square test (categorical variables) were used to calculate differences in these variables across the cohorts.
Propensity score matching was used to adjust for baseline differences between the collagenase and fasciectomy cohorts.17,18 Predictor variables included in the logistic regression model, based on a significant correlation with treatment choice, were CCI, geographical region of the patients’ residence, health insurance plan type, gender, hypertension, hyperlipidemia, carpal tunnel syndrome, trigger finger syndrome, depression, and sleep disorder. Using propensity scores estimates throughout the logistic regression model, collagenase patients were matched 1:1 to fasciectomy patients based on the nearest neighbor approach, without replacement. Outcomes were assessed among propensity score-matched patients.
Cost data are typically highly skewed, and thus, generalized linear models (GLMs) with log link and gamma distribution evaluated the relationship between treatment cohorts and total all-cause HC. Statistical analyses were performed using SPSS software ver. 23.0 (IBM, Armonk, N.Y.).
RESULTS
Demographic and Clinical Characteristics
Out of 37,608 patients with DC, 2911 received collagenase injection as primary treatment, while a higher number of patients (6258) underwent fasciectomy. After applying selection criteria, patients were categorized into study groups: 627 patients in the collagenase cohort and 1080 patients in the fasciectomy cohort. (See chart, Supplemental Digital Content 1, which displays the patient selection flow diagram, http://links.lww.com/PRSGO/C142.)
The assessment of patients’ demographic characteristics revealed significant differences in gender distribution across groups (Table 1). In general, the study population comprised older patients with a mean age of 74. A higher proportion of collagenase-treated patients were located in the Northeast, while fasciectomy patients were situated predominantly in the North Central region. A slightly higher proportion of collagenase-treated patients were covered with the preferred provider organization health plan type. When observing comorbid conditions, patients were mildly comorbid with the comparable mean CCI score (Table 1). However, differences were identified in the proportion of patients with anxiety, as well as DC-related comorbidities, such as carpal tunnel and trigger finger syndrome. The cohorts were matched on the following variables: carpal tunnel syndrome, trigger finger syndrome, hypertension, hyperlipidemia, CCI, plan type, gender, region, depression, and sleep disorders. These differences were balanced during the propensity-score matching process. Postmatching, 492 patients remained in each cohort (Table 1).
Table 1.
Demographic and Clinical Characteristics of the Initial Nonmatched Study Sample
| Variable | Collagenase (N = 627) | Fasciectomy (N = 1080) | P * |
|---|---|---|---|
| Age, years, mean ± SD | 74.3 ± 6.1 | 73.8 ± 5.8 | 0.080 |
| Men, n (%) | 480 (76.6) | 733 (67.9) | <0.001 |
| Geographic region, n (%) | |||
| Northeast | 217 (34.6) | 195 (18.1) | <0.001 |
| North Central | 162 (25.8) | 447 (41.4) | <0.001 |
| South | 170 (27.1) | 324 (30.0) | 0.205 |
| West | 77 (12.3) | 110 (10.2) | 0.181 |
| Unknown region | 1 (0.2) | 4 (0.4) | 0.658 |
| Health plan type, n (%) | |||
| Preferred provider Organization | 332 (53.0) | 496 (45.9) | 0.005 |
| Comprehensive | 215 (34.3) | 421 (39.0) | 0.053 |
| Other† | 80 (12.8) | 163 (15.1) | 0.183 |
| CCI, mean ± SD | 1.8 ± 2.2 | 1.9 ± 2.2 | 0.477 |
| Mental health disorders, n (%) | |||
| Anxiety | 45 (7.2) | 108 (10.0) | 0.049 |
| Psychosis | 3 (0.5) | 13 (1.2) | 0.192 |
| Bipolar disorder | 0 (0.0) | 3 (0.3) | 0.302 |
| Depression | 54 (8.6) | 108 (10.0) | 0.346 |
| Sleep disorders | 51 (8.1) | 111 (10.3) | 0.145 |
| Posttraumatic stress disorder | 5 (0.8) | 6 (0.6) | 0.545 |
| DC-related comorbidities, n (%) | |||
| Hypertension | 427 (68.1) | 770 (71.3) | 0.165 |
| Hyperlipidemia | 366 (58.4) | 643 (59.5) | 0.637 |
| Alcohol use | 12 (1.9) | 27 (2.5) | 0.435 |
| Tobacco use disorder | 59 (9.4) | 119 (11.0) | 0.294 |
| Complex regional pain syndrome | 1 (0.2) | 1 (0.1) | 1.000 |
| Carpal tunnel syndrome | 30 (4.8) | 130 (12.0) | <0.001 |
| Trigger finger syndrome | 48 (7.7) | 231 (21.4) | <0.001 |
| Epilepsy | 9 (1.4) | 10 (0.9) | 0.333 |
| Gout | 33 (5.3) | 66 (6.1) | 0.470 |
*Chi-square test or independent t test was performed.
†Includes health maintenance organization, noncapitated point-of-service, consumer-driven health plan, exclusive provider organization, point-of-service with capitation, high deductible health plan, and unknown.
SD, standard deviation.
Total Annual Healthcare Resource Utilization and Costs
The comparison of the mean annual number of all-cause and DC-associated prescription fills, physician office visits, hospitalizations, and ED visits revealed no significant differences between treatments (Table 2).
Table 2.
Healthcare Resource Utilization Categorized by the Healthcare Setting in Collagenase versus Fasciectomy-matched Patients
| Healthcare Services | Collagenase (N = 492) | Fasciectomy (N = 492) | P * |
|---|---|---|---|
| Prescriptions, mean ± SD | 26.0 ± 22.3 | 26.5 ± 20.9 | 0.686 |
| Physician office visits | |||
| Patients with outpatient service, n (%) | 492 (100.0) | 492 (100.0) | — |
| Outpatient visits, mean ± SD | 24.0 ± 17.8 | 23.8 ± 15.7 | 0.828 |
| DC-associated outpatient visits, mean ± SD | 5.1 ± 4.6 | 5.8 ± 8.3 | 0.095 |
| Hospitalization | |||
| Hospitalized patients, n (%) | 62.0 (12.6) | 61.0 (12.4) | 0.923 |
| Hospitalizations, mean ± SD | 0.6 ± 2.2 | 0.7 ± 2.6 | 0.542 |
| Length of hospitalization in days, mean ± SD | 0.5 ± 1.6 | 0.5 ± 2.1 | 0.476 |
| Length of DC-associated hospitalization, in days, mean ± SD | 0.0 ± 0.0 | 0.0 ± 1.0 | 0.318 |
| ED visits | |||
| Patients with ED visit, n (%) | 114 (23.2) | 123 (25.0) | 0.502 |
| ED visits, mean ± SD | 0.4 ± 0.9 | 0.5 ± 1.3 | 0.226 |
| DC-associated ED visits, mean ± SD | 0.0 ± 0.0 | 0.0 ± 0.1 | 0.317 |
*Chi-square test or independent t test was performed.
SD, standard deviation.
With HRU subcategorized based on a service provider, a significant difference was observed between the compared groups in the average number of outpatient facility visits that were less frequent in the collagenase versus fasciectomy cohort (mean, 12.3 versus 22.9, P < 0.001; Table 3) during the 12-month postindex period.
Table 3.
Healthcare Resource Utilization Categorized by the Service Provider in Collagenase versus Fasciectomy-matched Patients
| Service Provider, mean ± SD | Collagenase (N = 492) | Fasciectomy (N = 492) | P * |
|---|---|---|---|
| Outpatient pharmacy | 26.0 ± 22.3 | 26.5 ± 20.9 | 0.686 |
| Inpatient facility | 3.7 ± 11.4 | 3.9 ± 12.4 | 0.831 |
| Outpatient facility | 12.3 ± 18.5 | 22.9 ± 27.6 | <0.001 |
| Outpatient laboratory | 6.9 ± 10.6 | 7.5 ± 10.0 | 0.391 |
| Mental health substance abuse center | 0.7 ± 4.5 | 0.4 ± 2.3 | 0.158 |
| Radiology | 3.9 ± 7.9 | 4.2 ± 11.0 | 0.642 |
| Physician in an outpatient setting | 22.0 ± 19.4 | 22.3 ± 17.1 | 0.794 |
| Physician in an inpatient setting | 2.1 ± 7.4 | 2.7 ± 12.2 | 0.392 |
| Other professional services | 23.9 ± 29.7 | 25.4 ± 32.6 | 0.467 |
| Other† | 0.0 ± 0.2 | 0.1 ± 0.6 | 0.027 |
*Chi-square test or independent t test was performed.
†Category “Other” includes dental services and services related to vision and hearing.
SD, standard deviation.
The all-cause and DC-related HC were similar between the cohorts across all healthcare settings (Table 4). The mean annual DC-related cost was approximately $1500 per treated patient regardless of the DC treatment option selected. The mean annual DC-related cost accounted for approximately 20% of the total direct medical cost among older patients with DC.
Table 4.
All-cause and DC-related Annual Healthcare Costs Categorized by the Healthcare Setting in Collagenase versus Fasciectomy-matched Patients
| Healthcare Costs, Mean ± SD | Collagenase (N = 492) | Fasciectomy (N = 492) | P * |
|---|---|---|---|
| Prescription fill costs | $3260 ± 21,329 | $2621 ± 7877 | 0.533 |
| Outpatient costs | $3406 ± 6967 | $3111 ± 5970 | 0.476 |
| DC-related† outpatient costs | $1511 ± 2068 | $1375 ± 2263 | 0.325 |
| Inpatient costs | $530 ± 2758 | $381 ± 1760 | 0.315 |
| DC-related inpatient costs | $0 ± 0 | $0 ± 0 | — |
| ED costs | $75 ± 301 | $107 ± 436 | 0.187 |
| DC-related ED costs | $0 ± 3 | $1 ± 17 | 0.256 |
| Total costs | $7271 ± 23,117 | $6220 ± 10,221 | 0.357 |
| DC-related total healthcare costs excluding pharmacy setting | $1512 ± 2068 | $1377 ± 2262 | 0.329 |
*Independent t test was performed.
†DC-related costs represent the sum of costs for all claims with a DC diagnosis. Note that the diagnosis codes were not available in the pharmacy claims database.
SD, standard deviation.
Costs of services provided in an outpatient facility and by a physician in the outpatient setting were significantly lower in the collagenase versus fasciectomy cohort ($850 versus $1284, P = 0.047; $546 versus $1001, P < 0.001, respectively; Table 5). Meanwhile, costs of services provided by healthcare professionals were lower in the fasciectomy cohort (Table 5).
Table 5.
All-cause Healthcare Costs Categorized by the Service Provider in Collagenase versus Fasciectomy-matched Patients
| Service Provider, Mean ± SD | Collagenase (N = 492) | Fasciectomy (N = 492) | P * |
|---|---|---|---|
| Outpatient pharmacy | $3260 ± 21,329 | $2621 ± 7877 | 0.533 |
| Inpatient facility | $507 ± 2752 | $373 ± 1641 | 0.357 |
| Outpatient facility | $850 ± 3918 | $1284 ± 2821 | 0.047 |
| Outpatient laboratory | $59 ± 161 | $72 ± 226 | 0.297 |
| Mental health substance abuse center | $21 ± 201 | $12 ± 101 | 0.360 |
| Radiology | $254 ± 2706 | $116 ± 445 | 0.263 |
| Physician in an outpatient setting | $546 ± 973 | $1001 ± 2133 | <0.001 |
| Physician in an inpatient setting | $86 ± 334 | $86 ± 327 | 0.983 |
| Other professional services | $1682 ± 2676 | $629 ± 3260 | <0.001 |
| Other† | $6 ± 91 | $26 ± 204 | 0.047 |
| Total cost | $7271 ± 23,117 | $6220 ± 10,221 | 0.357 |
*Independent t test was performed.
†Category “Other” includes dental services and services related to vision and hearing.
SD, standard deviation.
Key Drivers of Healthcare Costs
Key drivers of total all-cause HC in the matched population were identified using a GLM (Table 6). Key drivers of the total HC were certain health insurance plan types (comprehensive plan and health maintenance organization in comparison to preferred provider organization), CCI, hyperlipidemia, sleep disorder, and North East and South region of patients’ residence in reference to North Central. Total all-cause HC adjusted for confounders did not differ between groups ($5944 in the collagenase versus $5930 in the fasciectomy cohort, P = 0.947).
Table 6.
Key Drivers of the Total All-cause Healthcare Costs in Collagenase versus Fasciectomy-matched Patients—Results of the GLM
| Parameters | Adjusted Odds Ratio | Significance |
|---|---|---|
| Charlson comorbidity score (ref: the sum of weights 0) | ||
| Charlson comorbidity score weight sum 1 | 1.49 | <0.001 |
| Charlson comorbidity score weight sum 2 | 1.35 | <0.001 |
| Charlson comorbidity score weight sum 3 | 1.49 | <0.001 |
| Charlson comorbidity score weight sum 4 and over | 2.23 | <0.001 |
| Sleep disorder (ref: none) | 1.35 | 0.027 |
| Hyperlipidemia (ref: none) | 0.90 | 0.027 |
| Type of insurance plan (ref: preferred provider organization) | ||
| Comprehensive | 0.74 | <0.001 |
| Health maintenance Organization | 1.82 | <0.001 |
| Noncapitated point-of-service | 0.82 | 0.358 |
| Other | 0.67 | 0.254 |
| Region (ref: North Central) | ||
| North East | 0.55 | <0.001 |
| South | 0.74 | <0.001 |
| West | 0.82 | 0.134 |
| Unknown | 1.00 | 0.958 |
SD, standard deviation.
P values <0.05 were considered statistically significant.
DISCUSSION
This retrospective analysis of Medicare claims data provides important insights into the DC treatment pathways in a real-world setting, by exploring HRU and HC through an observational study over a 1-year period following either collagenase or fasciectomy treatment of patients with DC older than 65 years.
A previous study aimed to address the lack of comparison of health economic aspects of different treatment options for DC targeting the commercially insured working-age population.12 Results showed that costs for collagenase-treated patients were significantly lower than for patients treated with fasciectomy mainly due to the lower rate of outpatient visits. The current study explored the economic burden in Medicare-Advantage covered older patients, where no significant difference in healthcare costs of collagenase-treated individuals compared with fasciectomy-treated individuals was observed. Notably, the difference in outpatient visits was also not observed. These findings may be explained by the fragility and comorbidity burdens of the older population who require closer postprocedural monitoring and careful follow-up,19 which may impact earlier recovery and lower outpatient visit rates observed in the previous studies.12,20
The increasing prevalence of DC with age3 and the substantial growth of the older population worldwide21 underline the importance of evaluation of DC treatment pathways in this population. However, a few studies have evaluated the benefits of the use of less invasive treatment procedures for treating DC in the older population. A systematic literature review aiming to provide evidence about the treatment of contractures with a particular reference to the older population emphasized that there are very few studies explicitly conducted in older people and dealing with conditions relevant to this population.22 The use of less invasive procedures might be more suitable for older patients with DC, due to the possible risks and complications related to procedures performed under general anesthesia.19 Around 10% of older adults in nursing homes have a fixed flexion deformity of fingers.19 Almost two-thirds of older patients in nursing homes had at least one joint contracture, with hand and fingers affected in about 20% of cases.23 Because of all these interesting findings, we considered it an opportunity for this study to shed light on this population.
The current study indicates that a larger proportion of patients with DC were treated with fasciectomy compared with collagenase during the observed time period, although we can only speculate per underlying reasons for higher referral to fasciectomy in comparison to collagenase treatment. A comparable trend was observed in Canada, where less invasive procedures were performed less often than open surgery, for treatment of DC.24 The authors acknowledged that they were surprised by the results and discussed that it remained unclear whether this was observed because of underreporting or a genuinely smaller number of procedures offered and performed in centers across the country.24
A small retrospective study conducted in Sweden suggested that the treatment cost of collagenase injection was 33% lower than the cost of fasciectomy ($1418 versus $2103, respectively).13 This study found that the posttreatment median (IQR) of total extension deficit was 10 (0–30) for the collagenase group and 10 (0–34) for the fasciectomy group. Although the study population was similar to our study sample, comparing the outcomes was not possible due to different time horizons, perspectives, and country-specific parameters.
Compared with fasciectomy, treatment with collagenase was reported to require fewer postoperative follow-up outpatient visits to either doctor, nurse, or physiotherapist.13,25 We found comparable rates of DC-associated overall number of outpatient visits. However, when observing HRU by service provider category, our study revealed that collagenase treatment is associated with considerably lower posttreatment outpatient facility visits in comparison to fasciectomy, on an annual basis.
A recently published study showed that indirect costs related to the productivity loss observed during the 12-month follow-up are lower in patients treated with collagenase, due to the shorter disability leave.20 Although the current analysis dealt with older people, and productivity loss was not assessed, shorter recovery after the intervention would likely help activities of daily living.
Our study did not detect a significant change in the 12-month total HC between collagenase and fasciectomy treatment when controlling for confounders. These findings are comparable to recently published research, which assessed annual change in overall disease-specific costs concluding there was no significant difference in the observed outcome with collagenase injection versus fasciectomy.14 This study also found that the treatment modality (collagenase or fasciectomy) was not a significant predictor of total healthcare cost among older patients (P = 0.068), which is confirmed by our study findings.14
Limitations
This study has several limitations inherent to the design of retrospective claims analyses. We analyzed data primarily collected for reimbursement purposes to assess the real-world consumption of healthcare resources and associated costs. Databases might lack some medically relevant information due to imprecise diagnostic or procedural coding stemming from potential human error or omission.
The use of insurance claims for the current analysis prevented us from assessing the severity of DC, although it would be beneficial to evaluate the effect of the disease severity on the treatment choice and, subsequently, HRU and HC. Also, it prevented us from reaching any evidence-based conclusion on the reasons behind the higher referral to fasciectomy in comparison to collagenase treatment observed in our study sample. We had to assume that the severity of the disease was comparable between groups and, thus, was not a factor in the cost analysis. It has to be denoted that there are different forms of DC, and each of them requires a personalized treatment approach, so it may not be the same if we are comparing patients treating palmar contracture with multiple fingers and joints involved and the single joint treatment. Still, acknowledging insurance claims’ genuine limitations, we tended to diminish the possibility of bias by selecting the patients at their initial visit (no prior surgical interventions of any kind in the 24-month preindex period) and also by providing balance between all patient comorbidities, risk factors, and demographic characteristics that we were able to evaluate in the database.
As the observational design is susceptible to patients’ selection bias, to estimate the treatment effect while controlling for confounders, we performed propensity score matching using a greedy algorithm, an optimization technique recommended as a good research practice by the International Society for Pharmacoeconomics and Outcomes Research.26 Moreover, to address highly positively skewed cost data, a common issue in claims database research, gamma log-linked GLM was performed to identify key drivers of total HC.27
Another important study aspect is the choice of the comparators. Our study aimed to compare collagenase versus fasciectomy HC. Still, a single-surgeon retrospective study demonstrated that percutaneous needle aponeurotomy cost was substantially lower than both other interventions when observing the initial procedure costs and also cumulative costs accounting for the reinterventions.28
Finally, we evaluated the outcomes throughout the 12-month period following the initial treatment of DC. The most recent systematic literature review acknowledged that inconsistency in follow-up may lead to substantial variation in reported clinical outcomes, particularly level of contracture extension, and recurrence.29 Thus, assessing the budgetary impact of evaluated treatments over a longer time horizon may lead to different conclusions than those presented in the current study. A recent meta-analysis reported higher rates of recurrence with collagenase versus fasciectomy (6.8% versus 2.3%), but at a cost of a higher rate of complications with surgical procedures.30 A retrospective review published by Leafblad et al28 reported cumulative costs of collagenase versus fasciectomy treatment for DC to be lower at 1 year ($4189 versus $5291) and similar at 5-year follow-up ($5952 versus $5507).
Further prospective randomized studies or real-world evidence analysis would be required to confirm our findings, while future analyses should address patient-reported outcomes regarding preferable treatment option, patient satisfaction, and quality of life.
CONCLUSIONS
In this comprehensive claims analysis of Medicare-insured patients with DC requiring treatment, overall, collagenase was similar to fasciectomy in terms of annual healthcare resource use and related total healthcare costs, with significantly fewer outpatient facility follow-up visits in the collagenase study group.
Supplementary Material
Footnotes
Published online 18 August 2022.
Presented in part at the Annual Meeting of American Association for Hand Surgery, January 2020, Fort Lauderdale, Florida.
Disclosure: V. Zah, F. Stanicic, and D. Vukicevic are employees of ZRx Outcomes Research. ZRx Outcomes Research received funding from Endo Pharmaceuticals for conducting the research. J. Ruby was employed by Endo Pharmaceuticals Inc. at the time of the study. D. Hurley is an employee of Endo Pharmaceuticals. This research was supported by Endo Pharmaceuticals Inc. (Malvern, Pa.). The source of funding had no influence on the study design, analysis, or interpretation of data.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Bayat A, Cunliffe EJ, McGrouther DA. Assessment of clinical severity in Dupuytren’s disease. Br J Hosp Med (Lond). 2007;68:604–609. [DOI] [PubMed] [Google Scholar]
- 2.Wilburn J, McKenna SP, Perry-Hinsley D, et al. The impact of Dupuytren disease on patient activity and quality of life. J Hand Surg Am. 2013;38:1209–1214. [DOI] [PubMed] [Google Scholar]
- 3.Hindocha S, McGrouther DA, Bayat A. Epidemiological evaluation of Dupuytren’s disease incidence and prevalence rates in relation to etiology. Hand (N Y). 2009;4:256–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. 2015. United States Census Bureau, Economics and Statistics Administration, US Department of Commerce. Available at https://time.com/wp-content/uploads/2015/01/p25-1140.pdf. Accessed 24 July, 2018.
- 5.National Institutes of Health (NIH). Dupuytren contracture. 2019. Available at https://ghr.nlm.nih.gov/condition/dupuytren-contracture#statistics. Accessed July 24, 2019.
- 6.Sweet S, Blackmore S. Surgical and therapy update on the management of Dupuytren’s disease. J Hand Ther. 2014;27:77–83. [DOI] [PubMed] [Google Scholar]
- 7.Denkler K. Surgical complications associated with fasciectomy for Dupuytren’s disease: a 20-year review of the English literature. Eplasty. 2010;10:e15. [PMC free article] [PubMed] [Google Scholar]
- 8.Kanonidou Z, Karystianou G. Anesthesia for the elderly. Hippokratia. 2007;11:175–177. [PMC free article] [PubMed] [Google Scholar]
- 9.Warwick D, Arandes-Renú JM, Pajardi G, et al. Collagenase clostridium histolyticum: emerging practice patterns and treatment advances. J Plast Surg Hand Surg. 2016;50:251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurst LC, Badalamente MA, Hentz VR, et al. ; CORD I Study Group. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361:968–979. [DOI] [PubMed] [Google Scholar]
- 11.Gilpin D, Coleman S, Hall S, et al. Injectable collagenase clostridium histolyticum: a new nonsurgical treatment for Dupuytren’s disease. J Hand Surg Am. 2010;35:2027–38.e1. [DOI] [PubMed] [Google Scholar]
- 12.Zah V, Pelivanovic J, Tatovic S, et al. Healthcare costs and resource use of patients with Dupuytren contracture treated with collagenase clostridium histolyticum or fasciectomy: a propensity matching analysis. Clinicoecon Outcomes Res. 2020;12:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atroshi I, Strandberg E, Lauritzson A, et al. Costs for collagenase injections compared with fasciectomy in the treatment of Dupuytren’s contracture: a retrospective cohort study. BMJ Open. 2014;4:e004166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donga P, DeKoven M, Kaplan F, et al. Costs of collagenase clostridium histolyticum and fasciectomy for Dupuytren’s contracture. Am J Pharm Benefits. 2015;7:24–31. [Google Scholar]
- 15.Sanson-Fisher RW, Bonevski B, Green LW, et al. Limitations of the randomized controlled trial in evaluating population-based health interventions. Am J Prev Med. 2007;33:155–161. [DOI] [PubMed] [Google Scholar]
- 16.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 17.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33:1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schnitzler A, Diebold A, Parratte B, et al. An alternative treatment for contractures of the elderly institutionalized persons: microinvasive percutaneous needle tenotomy of the finger flexors. Ann Phys Rehabil Med. 2016;59:83–86. [DOI] [PubMed] [Google Scholar]
- 20.Vukicevic D, Tatovic S, Zah V, et al. The burden of productivity loss of U.S. commercially insured patients diagnosed with Dupuytren’s disease undergoing collagenase versus fasciectomy treatment. Expert Rev Pharmacoecon Outcomes Res. 2021;21:127–136. [DOI] [PubMed] [Google Scholar]
- 21.United Nations. World population ageing 2013. 2014. New York: The Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat. Available at: https://www.un.org/en/development/desa/population/publications/ageing/WorldPopulationAgeing2013.asp. Accessed July 24 2018. [Google Scholar]
- 22.Offenbächer M, Sauer S, Rieß J, et al. Contractures with special reference in elderly: definition and risk factors—a systematic review with practical implications. Disabil Rehabil. 2014;36:529–538. [DOI] [PubMed] [Google Scholar]
- 23.Wagner LM, Capezuti E, Brush BL, et al. Contractures in frail nursing home residents. Geriatr Nurs. 2008;29:259–266. [DOI] [PubMed] [Google Scholar]
- 24.Liu W, O’Gorman DB, Gan BS. Operative trends and physician treatment costs associated with Dupuytren’s disease in Canada. Can J Plast Surg. 2013;21:229–233. [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta S, Belcher HJ. A single-centre cost comparison analysis of collagenase injection versus surgical fasciectomy for Dupuytren’s contracture of the hand. J Plast Reconstr Aesthet Surg. 2014;67:368–372. [DOI] [PubMed] [Google Scholar]
- 26.Berger ML, Sox H, Willke RJ, et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: recommendations from the joint ISPOR-ISPE Special Task Force on real-world evidence in health care decision making. Pharmacoepidemiol Drug Saf. 2017;26:1033–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barber J, Thompson S. Multiple regression of cost data: use of generalised linear models. J Health Serv Res Policy. 2004;9:197–204. [DOI] [PubMed] [Google Scholar]
- 28.Leafblad ND, Wagner E, Wanderman NR, et al. Outcomes and direct costs of needle aponeurotomy, collagenase injection, and fasciectomy in the treatment of Dupuytren contracture. J Hand Surg Am. 2019;44:919–927. [DOI] [PubMed] [Google Scholar]
- 29.Wong CR, Huynh MNQ, Fageeh R, et al. Outcomes of management of recurrent Dupuytren contracture: a systematic review and meta-analysis. Hand (N Y). 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper TB, Poonit K, Yao C, et al. The efficacies and limitations of fasciectomy and collagenase clostridium histolyticum in Dupuytren’s contracture management: a meta-analysis. J Orthop Surg (Hong Kong). 2020;28:2309499020921747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.