Abstract
Since its approval in 2018 by the US Food and Drug Administration, peptide receptor radionuclide therapy (PRRT) has become a mainstay in the treatment of neuroendocrine tumors. Lutetium-177-DOTATATE, the only approved agent, is indicated for the treatment of gastroenteropancreatic-neuroendocrine tumors. Although patient selection appears straightforward with somatostatin receptor-positron emission tomography, there is considerable complexity when deciding which patients to treat and when to start PRRT. Herein, we review the many factors that affect patient selection, focusing on the optimal patients to treat. Although significant effort has been expended to determine which patients benefit the most from PRRT, a validated predictive biomarker remains elusive. Although PRRT has been used for more than 2 decades in Europe and standards of care exist for safe treatment, there remain numerous questions regarding when PRRT should be used relative to other treatments. It is important to remember that multidisciplinary discussions are essential. Currently, there are a number of ongoing studies looking to assess the efficacy of PRRT compared with other treatment options and to optimize treatment through combination therapy, different dosing strategies, or use of different radionuclides and radioligands.
INTRODUCTION
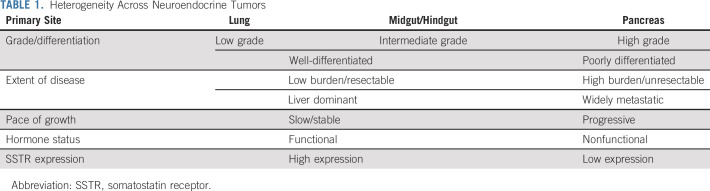
Neuroendocrine neoplasms comprise a highly diverse spectrum of tumors that are classified on the basis of their primary site of origin and their pathology (differentiation and grading; Table 1). Neuroendocrine neoplasms are subclassified into well-differentiated (neuroendocrine tumors [NETs]) and poorly differentiated (neuroendocrine carcinomas) neoplasms, with well-differentiated tumors subclassified on the basis of their Ki-67 proliferation index and/or mitotic rate (MR) into grade 1 (G1) (Ki-67 between 1%-2%; MR < 2 per 10 high powered fields), grade 2 (G2) (Ki-67 between 3%-20%, MR between 2-20), and grade 3 (G3) (Ki-67 > 20%; MR > 20). In 2017 and 2018, the WHO classification was updated to include well-differentiated gastroenteropancreatic G3 tumors (WDG3), whereas previously, G3 NETs and neuroendocrine carcinomas were grouped together.1,2 In patients with metastatic disease, the most common primary sites are the pancreas (Pan-NETs) and small bowel (SB-NETs), followed by the lung. Each of these tumors, depending on primary site and classification, has different behaviors and outcomes. In addition to their histological features and anatomic site, tumors can be functional, secreting a variety of bioactive compounds such as serotonin or peptide hormones (gastrin, insulin, vasoactive intestinal peptide, and others).
TABLE 1.
Heterogeneity Across Neuroendocrine Tumors
KEY POINTS
Lutetium-177-DOTATATE is approved for the treatment of somatostatin receptor–positive neuroendocrine tumors (NETs).
Patient selection for peptide receptor radionuclide therapy is primarily based on somatostatin receptor-positron emission tomography.
NETs vary on the basis of primary site, extent of disease, pace of growth, and other characteristics, and the appropriate sequence of therapies is complex and remains in flux.
Multidisciplinary discussions are essential when choosing between therapeutic options for NET.
CONTEXT
Key Objective
How do we select patients optimally for peptide receptor radionuclide therapy?
Knowledge Generated
Neuroendocrine tumors vary on the basis of primary site, extent of disease, pace of growth, and other characteristics, and the appropriate sequence of therapies is complex and remains in flux. Systemic therapies include targeted agents such as everolimus and sunitinib, chemotherapies such as capecitabine/temozolomide, and somatostatin analogs. Debulking strategies include surgery and liver-directed therapies. A number of clinical trials are ongoing, focused both on how to improve upon peptide receptor radionuclide therapy and to better understand how to sequence therapies.
Relevance
Somatostatin receptor-positron emission tomography is currently used for patient selection, but a validated predictive biomarker remains elusive. Multidisciplinary discussions are essential when choosing between therapeutic options for neuroendocrine tumor.
In patients with unresectable advanced disease, systemic treatment typically begins with somatostatin analogs (SSAs, octreotide, or lanreotide), which are also used for control of symptoms related to hypersecretion of serotonin or hormones. However, as outlined in a number of current treatment guidelines, the precise sequence of therapy must be individualized, on the basis of a variety of factors, including symptoms, comorbidities, prior therapy, tumor characteristics, and whether stability or shrinkage is acceptable.3-5 In Pan-NETs, chemotherapy (either temozolomide- or streptozotocin-based) and targeted therapies including everolimus and sunitinib are often used.6-9 In SB-NETs, SSAs and everolimus are approved, and everolimus is also approved for use in bronchial NETs.10,11 Of note, the tyrosine kinase inhibitor surufatinib is approved in China for the treatment of advanced panNETs and extrapancreatic NETs, but has not been approved by the US Food and Drug Administration (FDA).12,13 Beyond systemic agents, given the predilection for the liver, liver-directed therapies (LDTs), such as bland embolization and chemoembolization, are commonly used. In selected patients, ablative therapies (radiofrequency or microwave ablation) are used.
The most recently approved therapy is peptide receptor radionuclide therapy (PRRT), which targets the somatostatin receptor (SSTR) using SSAs labeled with radioactivity. Yttrium-90 (90Y)–labeled compounds have been largely replaced by lutetium-177 (177Lu)–labeled compounds, in part because of the higher rates of renal toxicity with 90Y-labeled compounds.14 In the only phase III trial (NETTER-1), 177Lu-DOTATATE (Lutathera) plus octreotide long-acting release (LAR) 30 mg once every 4 weeks was compared with high-dose octreotide LAR (60 mg once every 4 weeks) in patients with adequate renal function who had advanced SSTR-positive midgut NETs (Ki67 ≤ 20%) that were progressive despite standard dose octreotide LAR.15 SSTR expression was defined using SSTR scintigraphy with 111In-pentetreotide (octreoscan). Treatment was shown to prolong progression-free survival (PFS) and improve patient quality of life.15,16 The median PFS was 8.4 months (95% CI, 5.8 to 9.1) with octreotide LAR alone and was not reached in the PRRT arm (P < .001; hazard ratio [HR] = 0.21; 95% CI, 0.13 to 0.33), although the overall response rate (ORR) with 177Lu-DOTATATE was only 13%.15,17 There was also a trend toward increased overall survival (OS), although the difference was not statistically significant (P = .30).18 177Lu-DOTATATE was given in four cycles of a fixed 200 mCi administered activity intravenous over 30 minutes every 8 weeks; further details on administration can be found elsewhere.19
The NETTER-1 trial was performed in midgut NET (mostly SB-NETs), but the final FDA approval was for gastroenteropancreatic (GEP)-NETs and included Pan-NETs on the basis of prospective single-arm European data.20 The authors reported a 55% ORR, median PFS of 30 months, and median OS of 71 months in Pan-NETs, although the ORR in the 177Lu-DOTATATE prescribing information for the same population was only 16% in GEP-NETs.17,20
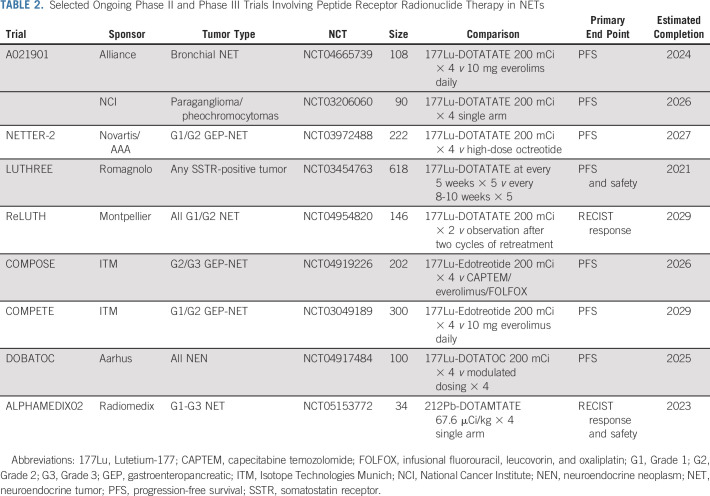
National Comprehensive Cancer Network guidelines include PRRT as a potential therapy for SB-NETs, Pan-NETs, bronchial NETs, and paraganglioma/pheochromocytomas.21 In SB-NETs, Society of Nuclear Medicine and Molecular Imaging/North American Neuroendocrine Tumor Society guidelines place PRRT before everolimus as a second-line therapy while European Society for Medical Oncology guidelines place PRRT before everolimus when the Ki-67 is < 10% and everolimus before PRRT when the Ki-67 is > 10% and European Neuroendocrine Tumour Society guidelines place both everolimus and PRRT as second-line options.3,22-24 In Pan-NETs, European Society for Medical Oncology and European Neuroendocrine Tumour Society guidelines place PRRT after capecitabine/temozolomide while Society of Nuclear Medicine and Molecular Imaging/North American Neuroendocrine Tumor Society guidelines placed both as second-line options. Overall, PRRT is recognized routinely in published guidelines, but treatment sequencing varies by society and primary site. Ongoing comparative trials (Table 2) will provide more evidence on sequencing of therapies as discussed below.
TABLE 2.
Selected Ongoing Phase II and Phase III Trials Involving Peptide Receptor Radionuclide Therapy in NETs
SELECTION OF PATIENTS FOR PRRT USING SSTR-POSITRON EMISSION TOMOGRAPHY
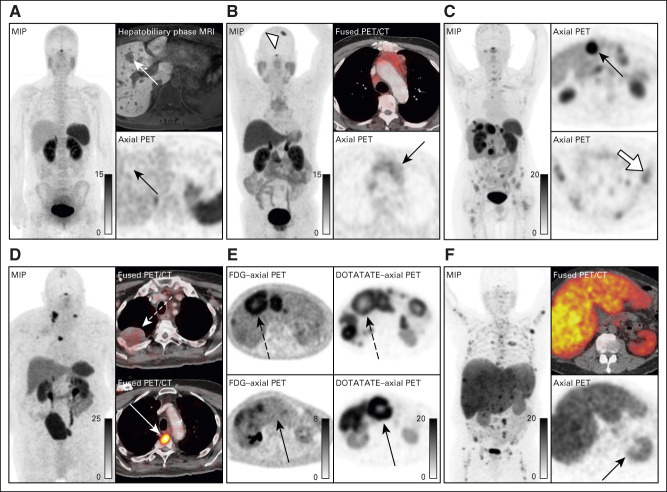
In NETs, SSTR imaging is used to select patients for PRRT. Historically, this was performed using SSTR scintigraphy with 111In-pentetreotide. Assessment of intensity of SSTR expression used the Krenning score,25 a qualitative five-point score from 0 (Fig 1A, no uptake) to 4 (Figs 1C, uptake greater than the spleen). In NETTER-1, uptake greater than or equal to the liver (Krenning score of 2) was used as the inclusion criteria. It should be noted that 111In-pentetreotide has been mostly replaced with SSTR-positron emission tomography (PET) using one of the three FDA-approved agents (68Ga-DOTATATE, 68Ga-DOTATOC, and 64Cu-DOTATATE). SSTR-PET is a marked improvement over 111In-pentetreotide in regards to lesion detection.26 Although there are slight differences in the three SSTR-PET radiopharmaceuticals, they are treated as equivalent for patient selection for PRRT. Krenning scores applied to SSTR-PET (often termed as modified Krenning scores) are not equivalent to 111In-pentetreotide, and SSTR-PET typically results in higher scores particularly in patients with smaller lesions.25,27 Of note, disease can technically have uptake on SSTR-PET, but not have high enough uptake for PRRT (Fig 1B).
FIG 1.
Issues with patient selection in SSTR-PET. (A) Patient A demonstrates no uptake in the liver lesions seen on hepatobiliary phase MRI (white arrow, Krenning 0) and is not a candidate for PRRT. (B) Patient B demonstrates uptake in the mediastinal mass above blood pool but less than the liver (black arrow, Krenning 1), which although technically has uptake, it is not adequate for treatment. There are benign causes of uptake on SSTR-PET, as seen in a meningioma in patient B (open arrowhead). (C) Patient C has uptake in some lesions greater than the liver and spleen (black arrow; SUVmax of 47, Krenning 4), but the bone lesions have uptake less than the liver (white arrow; SUVmax of 7.7, Krenning 2). Given that the bone lesions were the site of progression, PRRT is not a good option. (D) Patient D has lesions with high uptake in the thoracic spine (dotted white arrow) while other sites of disease, for example the pulmonary nodule, have no uptake on SSTR-PET (solid white arrow). Therefore, the patient is not a candidate for PRRT. (E) Patient E has disease that is heterogeneous when comparing FDG with SSTR-PET. Some lesions have uptake on both FDG and SSTR (dotted arrows) while other lesions are positive on SSTR and negative on FDG (solid arrows). In this case, the patient maybe a candidate for PRRT. (F) Patient F has uptake greater than the liver and spleen (SUVmax of 12), although all uptakes are diminished because of the large volume of tumor and kidney uptake is relatively decreased (black arrow). This patient meets criteria for treatment but has a poor prognosis because of the large tumor volume. CT, computed tomography; FDG, fluorodeoxyglucose; MIP, maximum intensity projection; MRI, magnetic resonance imaging; PET, positron emission tomography; PRRT, peptide receptor radionuclide therapy; SSTR, somatostatin receptor; SUVmax, maximum standardized uptake value.
Uptake on PET can be quantitatively measured using the standardized uptake value (SUV), which corrects measured activity in an individual volume for the mass of the patient. SUVs are typically reported as the maximum SUV, which is the voxel with the highest measured uptake in the lesion of interest. Unfortunately, SUVs are affected by more than just receptor density, for example high-volume tumor can serve as sink for the radioligand decreasing measured uptake across tissues (Fig 1D). Another important issue with SSTR-PET is the presence of heterogeneous disease. Within patients, there can be disease that is both SSTR-positive (Krenning 3 and 4) and SSTR-negative (Krenning 0-2; Fig 1C).
SSTR-PET AS A PREDICTOR OF RESPONSE
There has been a number of efforts to predict response to PRRT using baseline SSTR-PET images, but before going further, it is important to review the difference between prognostic and predictive biomarkers.28 A prognostic biomarker will identify the likelihood that a patient will have a more or less favorable outcome, regardless of therapy. An example of a prognostic biomarker is tumor growth rate (TGR), and as expected, a higher TGR correlates with progression.29 A predictive biomarker will separate similar individuals into those that are more or less likely to respond to a specific intervention or experience a certain toxicity. SSTR-PET is both a predictive and prognostic biomarker, but it should be noted that developing predictive biomarkers typically requires evaluation in a population of patients (with and without the biomarker) treated with two different therapies. A predictive marker must also predict response reliably enough to affect treatment choices. One confounding factor with SSTR-PET is that uptake is related to proliferative rate and differentiation, with more aggressive tumors having lower uptake.30 For example, in patients treated with SSAs, higher SUVs correlate with longer PFS,31 but whether or not this is due to SSAs being more effective in patients with higher SSTR-PET uptake or because of patients who are likely to have better outcomes is not clear.
A number of studies have correlated higher pretreatment SSTR-PET uptake to better PRRT outcomes.32,33 The only randomized trial with PRRT (NETTER-1) used 111In-pentetreotide for baseline imaging.15 There was no difference in the HR of patients who were Krenning 4 versus those with lower uptake (HR = 0.23 v 0.18), suggesting that we should be careful when using SUVs to select which patients to treat with PRRT and that further work needs to be performed to determine how SSTR-PET should be used as a predictive biomarker.15 In spite of this, there appears to be benefit to having higher uptake on SSTR-PET before PRRT as pretreatment uptake correlates with subsequent measured dose to the tumor,34 and the mechanism of action is dependent on dose delivery with higher doses having more response.35 Therefore, although there are no absolute cutoffs on which to make clinical decisions, when faced with multiple treatment options, higher uptake on SSTR-PET may sway one toward PRRT.
In addition to uptake on SSTR-PET, heterogeneity may be equally as important for patient selection. Patients with heterogeneous disease on SSTR-PET have worse outcomes, including OS.36,37 SSTR-negative lesions will not respond and are associated with primary treatment resistance because of the absence of the target.38
An alternative to pretreatment cutoffs is to use post-treatment imaging to evaluate response during therapy. In addition to emitting an electron, 177Lu emits gamma photons that can be imaged using a single-photon emission computed tomography camera. Using quantitative techniques, one can calculate absorbed dose in organs and lesions, which may become more feasible with newly described single time point imaging techniques.39,40 Ultimately, radiographic response takes into account both intrinsic radiation sensitivity and dose to the tumor and can allow an evaluation of treatment efficacy during treatment. Figure 2 shows a patient with relatively low uptake on SSTR-PET (maximum SUV of 13.6), who demonstrated an impressive response after only one cycle of 177Lu-DOTATATE.
FIG 2.
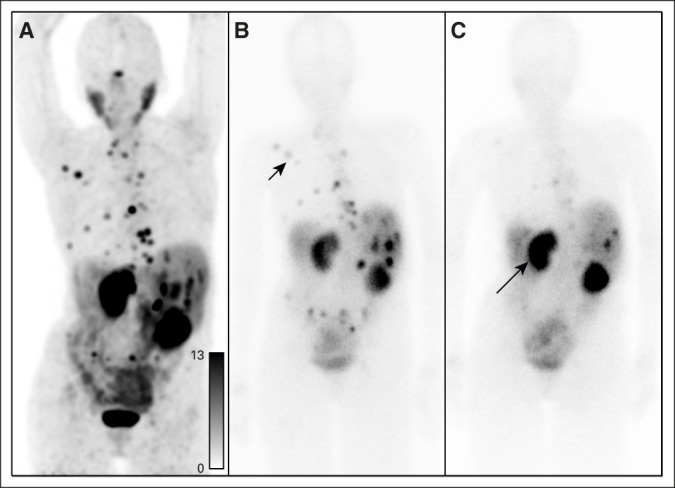
Post-treatment imaging for evaluation of response. A 70-year-old woman with pancreatic neuroendocrine tumor treated with two cycles of 177Lu-DOTATATE. (A) Pretreatment 68Ga-DOTATATE PET demonstrates SSTR-positive disease, with standardized uptake values up to 13.6. (B) Postcycle 1 planar gamma camera imaging demonstrates uptake in the osseous and hepatic disease (black arrowhead). (C) Postcycle 2 planar gamma camera imaging demonstrates increased uptake in the kidneys (black arrow) and significant reduction in uptake in the previously visualized disease consistent with response. PET, positron emission tomography; SSTR, somatostatin receptor.
DEVELOPING ROLE OF CIRCULATING BIOMARKERS
Nonhormonal tumor markers, particularly chromogranin A, are frequently used,41 but rarely affect patient management.42 Although chromogranin A at baseline was shown to be prognostic for PFS and OS in multiple studies including the RADIANT trials,10,43 it has not shown to be predictive of response to PRRT or other treatments most likely because of its high false-positive and false-negative rates.44 Taken together, there is no clear role for chromogranin A as a biomarker for patient selection for PRRT.
The primary issue of using either SSTR-PET or nonhormonal tumor markers to predict response to SSTR-PET is that they do not take into account tumor-specific factors, primarily the radiosensitivity of the tumor. Circulating biomarkers can potentially measure the intrinsic sensitivity of a tumor, which could be used as a predictive biomarker for PRRT. Blood-based genomic markers include the NETest and the PRRT Predictive Quotient (PPQ). The NETest, a 51 gene assay of circulating transcripts, is under study as a biomarker of PRRT response.45 The NETest has shown prognostic value in for predicting outcomes after curative surgery or systemic therapies including PRRT. PPQ combines eight blood gene transcripts with the Ki-67 of the tumor to create a binary output of response to PRRT.46 Two studies assessing the PPQ in non-PRRT cohorts suggest that it might be a predictive biomarker46,47; however, data from randomized trials are not available. Although not evaluated in the setting of PRRT to date, the presence and increased fractions of circulating tumor DNA were associated with poorer PFS.48 Further work using circulating tumor cells and DNA may add additional value in understanding who will benefit the most from PRRT.
LOW-VOLUME DISEASE
The decision of when to start systemic therapy and particularly 177Lu-DOTATATE is not clear. In patients with low-volume disease, especially slow-growing disease, systemic therapy is often best postponed or a treatment with low toxicity such as SSA may be considered.11,49 Overall, NET patients with low-volume disease do better than patients with higher-volume disease; for example, in the PROMID trial of octreotide versus placebo in midgut NET, the median time to progression on the treatment arm decreased from 29.4 months in patients with 0%-10% liver involvement to 4.6 months in patients with > 50% liver involvement.49 Similarly, indolent disease course was demonstrated in the CLARINET trial, a placebo controlled trial of lanreotide in GEP-NET.11
One issue with this approach is defining low-volume disease. In PROMID, low-volume disease was considered as patients with < 10% liver involvement, whereas in CLARINET, it was 25% liver involvement.11,49 Another limitation is that the assessment of tumor volume from published trials is restricted to the liver and does not consider disease spread to other sites such as the bone, lung, or peritoneum. Given the difference in detection rate between 111In-pentetreotide and SSTR-PET, one might consider lesion sizes < 2 cm as low-volume disease.27 In addition to volume, tumor grade is important. In the CLARINET study, the median PFS for placebo decreased from 18.3 months in G1 tumors to 12.1 months in G2 tumors.11
If time to progression on octreotide is as long as 29 months in low-grade midgut NET with low-volume disease, might it be appropriate to postpone the onset of systemic therapy? Given that PRRT can have significant long-term toxicities, primarily bone marrow and renal, delaying treatment is appealing. In GEP-NET patients with low-volume disease and a slow pace of growth, alternative strategies might include local therapies, higher-dose SSA, or even an oral agent (eg, everolimus or sunitinib) although potential toxicity should be factored in. Although not routine, emerging data suggest that treatment with SSAs may be appropriate beyond progression with dose intensification.15,50 In short, low-volume disease presents a unique challenge given a lack of data to guide therapy decisions and the potential for toxicities in patients who might otherwise have a relatively good prognosis.
HIGH-VOLUME DISEASE
The higher the volume of disease, the worse the outcome.51 Many prognostic factors including > 50% liver involvement, more than five bone metastases, or a highly elevated chromogranin A all correlate with poor outcomes after PRRT.52 In patients with high-volume disease treated with 177Lu-DOTATATE, tumor sink results in decreased uptake and, therefore, lower efficacy.53 In a secondary analysis from the NETTER-1 trial, higher tumor volume was associated with poorer outcomes, but only the presence of liver lesions > 3 cm was predictive of a worse outcome.54 Therefore, it may be optimal to debulk patients before PRRT, which could be performed with surgery, LDT, or chemotherapy. LDT is effective in debulking larger hepatic lesions, and the RETNET trial (ClinicalTrials.gov identifier: NCT02724540) will help us understand which type of embolic therapy is optimal. In particular, radioembolization should be reserved for patients with SSTR-negative tumors or those who have localized large SSTR-positive tumors, where selective arterial administration can be used to spare normal liver.55 In patients with high-volume Pan-NET, treatment with capecitabine/temozolomide can be used given the 33% ORR observed in the E2211 study, which showed a median PFS for temozolomide of 14.4 months versus 22.7 months with capecitabine/temozolomide (HR = 0.58, P = .023).7 Two important studies, A022001 (177Lu-DOTATATE versus capecitabine/temozolomide in Pan-NETs) and COMPOSE (177Lu-Edotreotide versus best standard of care in GEP-NETs; Table 2) will help to address the question of sequence chemotherapy and PRRT.56
One important consideration with LDT is that relatively fast debulking can be beneficial in symptomatic patients or those who have a high urine 5-HIAA or hypersecretion of peptide hormones. However, PRRT can also be effective in hormonal symptom control as well, with one series showing 71% of functional Pan-NET patients with uncontrolled symptoms at baseline had improvement.57 In SB-NET, symptomatic improvement is often seen without a radiographic response. For example, with Y90-DOTATOC, only 4% of patients had a radiographic response while 42% had improvement in diarrhea,14 and in the NETTER-1 study, only 13% had a radiographic response while 48% had improvement in diarrhea.16
COMPLIMENTARY ROLES OF SSTR AND FLUORODEOXYGLUCOSE-PET IN HIGHER-GRADE NETs
The majority of evidence for PRRT is in G1/G2 NETs, although PRRT is beneficial in patients with G3 NETs.58-60 The largest retrospective multicenter study evaluated 149 patients with G3 NETs and demonstrated a 42% ORR, 14-month PFS, and 29-month OS.59 The higher the Ki-67, the worse the outcome with PFS falling from 16 months to 6 months when the Ki-67 was > 55%. Compared with G1/G2 NETs, WDG3 NETs have a higher ORR, but shorter PFS.
Imaging is important when selecting higher-grade NETs for PRRT. 18F-fluorodeoxyglucose (FDG) is a marker of tumor metabolism and uptake increases with more aggressive tumors. Converse to SSTR-PET, higher uptake on FDG-PET correlates with worse outcomes and has been shown to outperform pathologic grading.61 Although often FDG and SSTR-PET uptake are inversely correlated, they can be unrelated and, therefore, the NETPET score was developed to take into account differing uptakes.62 In general, patients with higher uptake on FDG-PET have a higher score, have a poorer outcome, and are less suitable candidates for PRRT.63 As discussed above, heterogeneous SSTR expression is a poor prognostic factor, and the combination of FDG and SSTR-PET can help to further elucidate variation across metastases. It is important to make sure that there are not lesions that are FDG-positive and SSTR-PET–negative as these sites of disease will not be successfully treated with PRRT. Although less commonly used in the United States, FDG-PET is recommended by the European Association of Nuclear Medicine not only in G3 cases but also in patients with rapidly progressive disease and those with SSTR-negative disease on computed tomography.64
As FDG-PET is a marker of metabolism, uptake likely relates to both TGR and proliferation rate, and both higher FDG uptake and TGR are poor prognostic factors.29,61 As a simplification of radiation sensitivity and a modern application of the law of Bergonié and Tribondeau, it is often considered that tumors with higher TGR, Ki-67, and uptake on FDG (all markers of higher proliferation rates) are more sensitive to radiation.65 Although this may be true, patients with WDG3 tumors who have higher Ki-67 and higher uptake on FDG-PET have lower ORR, PFS, and OS after treatment with PRRT.60 Although PRRT is approved in patients with progressive disease, patients without documented tumor progression who have high-volume disease or higher-grade disease may be considered for treatment, as is being evaluated in COMPOSE and NETTER-2 (ClinicalTrials.gov identifiers: NCT03972488 and NCT04919226).
LIMITATIONS AND CHALLENGES
There are many challenges for patient selection. Pre-existing liver, renal, and bone marrow dysfunction can be worsened with treatment. In patients with liver injury, it is difficult to decide if PRRT is safe. One study showed a high rate of toxicity in heavily pretreated liver-dominant patients after receiving PRRT with nearly 60% of patients developing ascites66 while other studies have shown that regional hepatic embolization is safe before PRRT.67 Although there are no data on the use of PRRT after Y90-radioembolization, it appears that Y90-radioembolization is safe after PRRT.68,69 Limited LDT before PRRT is safe, yet PRRT may worsen liver injury in patients with ascites or other signs of liver failure.
Renal injury has been demonstrated with Y90-based treatments,70 but it appears that the rate of renal toxicity is lower with 177Lu-labeled compounds, and it is not clear what the rate of renal injury is with 177Lu-DOTATATE. The NETTER-1 trial did not demonstrate any toxicity related to PRRT in patients with mild renal dysfunction.71 Renal toxicity is likely more an issue in the setting of repeat PRRT because of the cumulative kidney dose, although in one study of 168 patients receiving repeat PRRT, there were no cases of grade III or IV renal toxicity.72 One difficulty with renal toxicity is that it develops months to years after treatment, and, therefore, one cannot dynamically evaluate for toxicity during the 6-month course of therapy. The delay in development of renal toxicity also limits the reports of renal injury in the literature. Although renal injury appears uncommon with PRRT, as repeat treatments become more common, we will have to address cumulative injury to the kidneys.
Bone marrow toxicity is typically acute, and evaluating for cytopenias between cycles is straightforward. In contrast, long-term bone marrow toxicity (leukemia and myelodysplasia) occurs in 2%-3% of patients typically 1-4 years after treatment.18,20,70 There are no predictive biomarkers, and myeloid neoplasms are typically predated by the development of thrombocytopenia.73 The risk appears to be higher when concurrent chemotherapy is administered, limiting the value of this approach.74,75 Early work suggests that clonal hematopoiesis may relate to the development of cytopenias and potentially secondary myeloid neoplasms.73,76 In patients with advanced NET, the median survival is measured in years, with a subset of patients living decades, and, therefore, the risk of secondary bone marrow malignancies can be the biggest concern.
Additionally, how to treat patients with mesenteric and peritoneal disease, who may develop subsequent bowel obstructions, has yet to be determined.77,78 It is generally agreed upon that steroids should be used in patients with mesenteric or peritoneal disease to prevent complications. Importantly, PRRT is not effective in decreasing the size of mesenteric masses in SB-NETs and is likely not beneficial in treating bowel complications.79
One last issue relates to patients with poor performance status (PS). Anecdotally, patients can have poor outcomes from PRRT if they have a poor baseline PS, and poor PS has been shown to be an independent prognostic factor for OS after PRRT.80 However, precise information is lacking as such patients are not candidates for clinical trials.
OPTIMIZING PRRT
The current implementation of PRRT is to administer a unit dose of 200 mCi per cycle for a total of four cycles. There is over a 10-fold variation tumor absorbed dose with PRRT because of the widely different extents of disease and varying tumor uptake.81 A patient-specific dosing protocol would be optimal, but consensus on how this should be performed remains elusive. A handful of trials (Table 2) are being performed by modulating the administered activity on the basis of measured kidney absorbed dose,82,83 but none to date are targeting an optimal tumor absorbed dose. Significant work needs to be performed to determine the optimal number of cycles, frequency of cycles, and administered activity moving forward.
In liver-dominant patients, a method to increase the efficacy of PRRT is to administer the activity intra-arterially rather than intravenously. Initial work using 68Ga-DOTATOC demonstrated an over three-fold increase in tumor uptake in hepatic lesions when administered intra-arterially,84 although a subsequent study with 90Y-DOTATOC did not reproduce such impressive results.85 There are currently multiple trials looking at the benefit of intra-arterial administration (ClinicalTrials.gov identifiers: NCT03590119 and NCT03590119).
One other approach to improving the efficacy of PRRT is to use combination therapy approaches. One of the first approaches was to combine PRRT with chemotherapy such as capecitabine/temozolomide (ClinicalTrials.gov identifier: NCT02358356), although early reports from a prospective RCT and long-term follow-up data from a phase II study indicate that the rate of marrow toxicity, including myelodysplastic syndrome and acute leukemia, is unacceptably high.74,75 Other trials are studying the combination of therapies that impair DNA repair such as olaparib (ClinicalTrials.gov identifiers: NCT04375267 and NCT04086485) and triapine (ClinicalTrials.gov identifier: NCT04234568), although there may be similar concerns with marrow toxicity.86 Finally, although single-agent check point inhibitors have not been successful in NETs,87,88 combinations with PRRT are being evaluated (ClinicalTrials.gov identifier: NCT03457948); preclinical data showing antitumor immune responses by PRRT with 177Lu-DOTATATE in a murine model of human NET support this strategy.89
FUTURE DIRECTIONS
There are currently a number of phase II/III trials in NETs (Table 2), primarily focused on new indications (eg, bronchial NET and paraganglioma/pheochromocytomas), radioligands (DOTATOC and Edotreotide), radionuclides (212Pb), retreatment, modulated dosing, and assessing treatment earlier in the disease course. With the approval of 177Lu-DOTATATE, the field continues to move quickly to adapt to the introduction of this new treatment modality.
In March 2021, the National Cancer Institute Gastrointestinal Steering Committee convened a clinical trials planning meeting focused on NETs.56 There were two immediate term concepts that were discussed: the role of retreatment with PRRT and modified PRRT on the basis of lesional absorbed dose. Additionally, combination trials with immunotherapy and DNA repair–targeted therapies were considered. Although not discussed at the NET clinical trials planning meeting because of feasibility concerns, there is a considerable interest in the use of alpha particle therapy in NETs. Alpha particles (a helium atom) are much larger than beta particles (an electron) and so deposit their energy over a much shorter distance (50-60 m v 1-2 mm). A single-center phase I study evaluating 212Pd-DOTAMTATE demonstrated an 80% ORR in patients treated at the recommended phase II dose.90 Early work has also shown efficacy with 225Ac-DOTATATE and 225Ac-DOTATOC but has been limited to single-center series to date.91,92
NETs are a heterogeneous disease with many unanswered questions. Although the selection of patients for PRRT is based on uptake on an imaging biomarker, we lack a predictive biomarker to help select which patients are most likely to benefit from PRRT. Overall, there is likely a sweet spot for PRRT where patients with low-volume disease are followed with observation or treated with SSAs, whereas high-volume patients or those with a fast TGR may be best treated with chemotherapy or other debulking approaches (Fig 3). Everything being equal, higher uptake on SSTR-PET would make one consider PRRT over other options, although no strict cutoffs exist. This is similar in concept to that proposed by Hofman and Hicks93 previously, which focused on the integration of information from functional imaging, SSTR, and FDG-PET on one side and proliferative activity on the other. We acknowledge that this approach is overly simplified and urge the use of multidisciplinary discussions to evaluate treatment options for individual patients, in order integrate key factors such as functionality, TGR, prior therapies, patients features, and comorbidities. We are fortunate that the field is rapidly moving forward, and many opportunities to optimize and improve upon PRRT are being evaluated.
FIG 3.
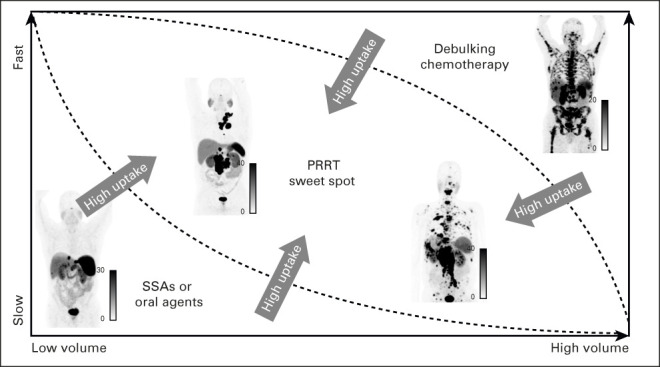
Optimal setting for PRRT. Patients with low-volume disease that is relatively stable may be better treated with SSAs, oral-targeted agents, or even observation. Patients with high-volume disease may benefit from debulking therapies before PRRT or chemotherapy. If disease is faster pace or higher-grade, PRRT may be appropriate in lower-volume patients. Additionally, the higher the uptake on somatostatin receptor-positron emission tomography, the better an option PRRT becomes relative to other therapies. Overall, patient selection remains complex, and multidisciplinary tumor board discussions are needed to determine optimal treatment strategies. PRRT, peptide receptor radionuclide therapy; SSA, somatostatin analog.
DISCLAIMER
This is a US Government work. There are no restrictions on its use.
SUPPORT
T.A.H. is supported by NIH: R01CA212148 and R01CA235741.
Thomas A. Hope
Stock and Other Ownership Interests: RayzeBio
Honoraria: GE Healthcare
Consulting or Advisory Role: Ipsen, Curium Pharma, Blue Earth Diagnostics
Research Funding: GE Healthcare (Inst), Philips Healthcare (Inst), Advanced Accelerator Applications (Inst), Clovis Oncology (Inst)
Travel, Accommodations, Expenses: GE Healthcare
Marianne Pavel
Honoraria: Ipsen, Hutchison MediPharma, Advanced Accelerator Applications, Riemser, Boehringer Ingelheim, Lilly
Consulting or Advisory Role: Advanced Accelerator Applications, Ipsen
Travel, Accommodations, Expenses: Ipsen, Hutchison MediPharma
Emily K. Bergsland
Stock and Other Ownership Interests: More Health, Exai Bio
Consulting or Advisory Role: More Health
Research Funding: Merck
Patents, Royalties, Other Intellectual Property: UpToDate
Uncompensated Relationships: Amgen
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Thomas A. Hope
Data analysis and interpretation: Thomas A. Hope
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Neuroendocrine Tumors and Peptide Receptor Radionuclide Therapy: When Is the Right Time?
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Thomas A. Hope
Stock and Other Ownership Interests: RayzeBio
Honoraria: GE Healthcare
Consulting or Advisory Role: Ipsen, Curium Pharma, Blue Earth Diagnostics
Research Funding: GE Healthcare (Inst), Philips Healthcare (Inst), Advanced Accelerator Applications (Inst), Clovis Oncology (Inst)
Travel, Accommodations, Expenses: GE Healthcare
Marianne Pavel
Honoraria: Ipsen, Hutchison MediPharma, Advanced Accelerator Applications, Riemser, Boehringer Ingelheim, Lilly
Consulting or Advisory Role: Advanced Accelerator Applications, Ipsen
Travel, Accommodations, Expenses: Ipsen, Hutchison MediPharma
Emily K. Bergsland
Stock and Other Ownership Interests: More Health, Exai Bio
Consulting or Advisory Role: More Health
Research Funding: Merck
Patents, Royalties, Other Intellectual Property: UpToDate
Uncompensated Relationships: Amgen
No other potential conflicts of interest were reported.
REFERENCES
- 1.Organisation mondiale de la santé, Centre international de recherche sur le cancer (eds): WHO Classification of Tumours of Endocrine Organs (ed 4). Lyon, France, International Agency for Research on Cancer, 2017 [Google Scholar]
- 2.Rindi G, Klimstra DS, Abedi-Ardekani B, et al. : A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol 31:1770-1786, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hicks RJ, Kwekkeboom DJ, Krenning E, et al. : ENETS consensus guidelines for the standards of care in neuroendocrine neoplasia: Peptide receptor radionuclide therapy with radiolabeled somatostatin analogues. Neuroendocrinology 105:295-309, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Halfdanarson TR, Strosberg JR, Tang L, et al. : The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of pancreatic neuroendocrine tumors. Pancreas 49:863-881, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Carbonero R, Rinke A, Valle JW, et al. : ENETS consensus guidelines for the standards of care in neuroendocrine neoplasms: Systemic therapy—Chemotherapy. Neuroendocrinology 105:281-294, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Yao JC, Shah MH, Ito T, et al. : Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 364:514-523, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunz PL, Catalano PJ, Nimeiri H, et al. : A randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors: A trial of the ECOG-ACRIN Cancer Research Group (E2211). J Clin Oncol 36, 2018. (suppl 15; abstr 4004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kouvaraki MA, Ajani JA, Hoff P, et al. : Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol 22:4762-4771, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Raymond E, Dahan L, Raoul J-L, et al. : Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 364:501-513, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Yao JC, Fazio N, Singh S, et al. : Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet 387:968-977, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caplin ME, Pavel M, Ćwikła JB, et al. : Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 371:224-233, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Xu J, Shen L, Bai C, et al. : Surufatinib in advanced pancreatic neuroendocrine tumours (SANET-p): A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 21:1489-1499, 2020 [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Shen L, Zhou Z, et al. : Surufatinib in advanced extrapancreatic neuroendocrine tumours (SANET-ep): A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 21:1500-1512, 2020 [DOI] [PubMed] [Google Scholar]
- 14.Bushnell DL, O'Dorisio TM, O'Dorisio MS, et al. : 90Y-Edotreotide for metastatic carcinoid refractory to octreotide. J Clin Oncol 28:1652-1659, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strosberg J, El-Haddad G, Wolin E, et al. : Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med 376:125-135, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strosberg J, Wolin E, Chasen B, et al. : Health-related quality of life in patients with progressive midgut neuroendocrine tumors treated with 177Lu-dotatate in the phase III NETTER-1 trial. J Clin Oncol 36:2578-2584, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Food and Drug Administration : Lutathera Package Insert. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208700s000lbl.pdf [Google Scholar]
- 18.Strosberg JR, Caplin ME, Kunz PL, et al. : 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol 22:1752-1763, 2021 [DOI] [PubMed] [Google Scholar]
- 19.Hope TA, Abbott A, Colucci K, et al. : NANETS/SNMMI procedure standard for somatostatin receptor-based peptide receptor radionuclide therapy with 177Lu-DOTATATE. J Nucl Med 60:937-943, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Brabander T, van der Zwan WA, Teunissen JJM, et al. : Long-term efficacy, survival, and safety of [(177)Lu-DOTA(0),Tyr(3)]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin Cancer Res 23:4617-4624, 2017 [DOI] [PubMed] [Google Scholar]
- 21.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Neuroendocrine and Adrenal Tumors, Version 4.2021. 2021. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1448 [DOI] [PubMed] [Google Scholar]
- 22.Hope TA, Bodei L, Chan JA, et al. : NANETS/SNMMI consensus statement on patient selection and appropriate use of 177Lu-DOTATATE peptide receptor radionuclide therapy. J Nucl Med 61:222-227, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavel M, Öberg K, Falconi M, et al. : Gastroenteropancreatic neuroendocrine neoplasms: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 31:844-860, 2020 [DOI] [PubMed] [Google Scholar]
- 24.Pavel M, O'Toole D, Costa F, et al. : ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology 103:172-185, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Hofman MS, Lau WFE, Hicks RJ: Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: Clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics 35:500-516, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Sadowski SM, Neychev V, Millo C, et al. : Prospective study of 68Ga-DOTATATE positron emission tomography/computed tomography for detecting gastro-entero-pancreatic neuroendocrine tumors and unknown primary sites. J Clin Oncol 34:588-596, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hope TA, Calais J, Zhang L, et al. : 111In-Pentetreotide scintigraphy versus 68Ga-DOTATATE PET: Impact on Krenning scores and effect of tumor burden. J Nucl Med 60:1266-1269, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.FDA-NIH Biomarker Working Group : Understanding prognostic versus predictive biomarkers, in BEST (Biomarkers, EndpointS, and Other Tools) Resource. Silver Spring, MD, Food and Drug Administration, 2016, pp 58. [PubMed] [Google Scholar]
- 29.Dromain C, Pavel ME, Ruszniewski P, et al. : Tumor growth rate as a metric of progression, response, and prognosis in pancreatic and intestinal neuroendocrine tumors. BMC Cancer 19:66-69, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kayani I, Bomanji JB, Groves A, et al. : Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (DOTA-DPhe1,Tyr3-octreotate) and 18F-FDG. Cancer 112:2447-2455, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Koch W, Auernhammer CJ, Geisler J, et al. : Treatment with octreotide in patients with well-differentiated neuroendocrine tumors of the ileum: Prognostic stratification with Ga-68-DOTA-TATE positron emission tomography. Mol Imaging 13:1-10, 2014 [PubMed] [Google Scholar]
- 32.Öksüz MÖ, Winter L, Pfannenberg C, et al. : Peptide receptor radionuclide therapy of neuroendocrine tumors with (90)Y-DOTATOC: Is treatment response predictable by pre-therapeutic uptake of (68)Ga-DOTATOC? Diagn Interv Imaging 95:289-300, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Ambrosini V, Campana D, Polverari G, et al. : Prognostic value of 68Ga-DOTANOC PET/CT SUVmax in patients with neuroendocrine tumors of the pancreas. J Nucl Med 56:1843-1848, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Ezziddin S, Lohmar J, Yong-Hing CJ, et al. : Does the pretherapeutic tumor SUV in 68Ga DOTATOC PET predict the absorbed dose of 177Lu octreotate? Clin Nucl Med 37:e141-e147, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Ilan E, Sandström M, Wassberg C, et al. : Dose response of pancreatic neuroendocrine tumors treated with peptide receptor radionuclide therapy using 177Lu-DOTATATE. J Nucl Med 56:177-182, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Werner RA, Ilhan H, Lehner S, et al. : Pre-therapy somatostatin receptor-based heterogeneity predicts overall survival in pancreatic neuroendocrine tumor patients undergoing peptide receptor radionuclide therapy. Mol Imaging Biol 21:582-590, 2019 [DOI] [PubMed] [Google Scholar]
- 37.Graf J, Pape U-F, Jann H, et al. : Prognostic significance of somatostatin receptor heterogeneity in progressive neuroendocrine tumor treated with Lu-177 DOTATOC or Lu-177 DOTATATE. Eur J Nucl Med Mol Imaging 47:881-894, 2020 [DOI] [PubMed] [Google Scholar]
- 38.Puranik AD, Dromain C, Fleshner N, et al. : Target heterogeneity in oncology: The best predictor for differential response to radioligand therapy in neuroendocrine tumors and prostate cancer. Cancers (Basel) 13:3607, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devasia TP, Dewaraja YK, Frey KA, et al. : A novel time–activity information-sharing approach using nonlinear mixed models for patient-specific dosimetry with reduced imaging time points: Application in SPECT/CT after 177Lu-DOTATATE. J Nucl Med 62:1118-1125, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawhn-Heath C, Hope TA, Martinez J, et al. : Dosimetry in radionuclide therapy: The clinical role of measuring radiation dose. Lancet Oncol 23:e75-e87, 2022 [DOI] [PubMed] [Google Scholar]
- 41.Chan DL, Moody L, Segelov E, et al. : Follow-up for resected gastroenteropancreatic neuroendocrine tumours: A practice survey of the Commonwealth Neuroendocrine Tumour Collaboration (CommNETS) and the North American Neuroendocrine Tumor Society (NANETS). Neuroendocrinology 107:32-41, 2018 [DOI] [PubMed] [Google Scholar]
- 42.Strosberg JR, Halfdanarson TR, Bellizzi AM, et al. : The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas 46:707-714, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao JC, Pavel M, Phan AT, et al. : Chromogranin A and neuron-specific enolase as prognostic markers in patients with advanced pNET treated with everolimus. J Clin Endocrinol Metab 96:3741-3749, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Papantoniou D, Grönberg M, Landerholm K, et al. : Assessment of hormonal levels as prognostic markers and of their optimal cut-offs in small intestinal neuroendocrine tumours grade 2. Endocrine 72:893-904, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bodei L, Kidd MS, Singh A, et al. : PRRT neuroendocrine tumor response monitored using circulating transcript analysis: The NETest. Eur J Nucl Med Mol Imaging 47:895-906, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bodei L, Kidd MS, Singh A, et al. : PRRT genomic signature in blood for prediction of 177Lu-octreotate efficacy. Eur J Nucl Med Mol Imaging 45:1155-1169, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bodei L, Kidd M, Modlin IM, et al. : Measurement of circulating transcripts and gene cluster analysis predicts and defines therapeutic efficacy of peptide receptor radionuclide therapy (PRRT) in neuroendocrine tumors. Eur J Nucl Med Mol Imaging 43:839-851, 2016 [DOI] [PubMed] [Google Scholar]
- 48.Boons G, Vandamme T, Mariën L, et al. : Longitudinal copy-number alteration analysis in plasma cell-free DNA of neuroendocrine neoplasms is a novel specific biomarker for diagnosis, prognosis, and follow-up. Clin Cancer Res 28:338-349, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rinke A, Müller H-H, Schade-Brittinger C, et al. : Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID study group. J Clin Oncol 27:4656-4663, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Pavel M, Ćwikła JB, Lombard-Bohas C, et al. : Efficacy and safety of high-dose lanreotide autogel in patients with progressive pancreatic or midgut neuroendocrine tumours: CLARINET FORTE phase 2 study results. Eur J Cancer 157:403-414, 2021 [DOI] [PubMed] [Google Scholar]
- 51.Tirosh A, Papadakis GZ, Millo C, et al. : Prognostic utility of total 68Ga-DOTATATE-Avid tumor volume in patients with neuroendocrine tumors. Gastroenterology 154:998-1008.e1, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swiha MM, Sutherland DEK, Sistani G, et al. : Survival predictors of 177Lu-Dotatate peptide receptor radionuclide therapy (PRRT) in patients with progressive well-differentiated neuroendocrine tumors (NETS). J Cancer Res Clin Oncol 148:225-236, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beauregard J-M, Hofman MS, Kong G, et al. : The tumour sink effect on the biodistribution of 68Ga-DOTA-octreotate: Implications for peptide receptor radionuclide therapy. Eur J Nucl Med Mol Imaging 39:50-56, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Strosberg J, Kunz PL, Hendifar A, et al. : Impact of liver tumour burden, alkaline phosphatase elevation, and target lesion size on treatment outcomes with 177Lu-Dotatate: An analysis of the NETTER-1 study. Eur J Nucl Med Mol Imaging 47:2372-2382, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jonathan S, El-Haddad G, Al-Toubah T, et al. : Radioembolization versus bland or chemoembolization for liver-dominant neuroendocrine tumors: Is it an either/or question? J Nucl Med 62:1669-1671, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hope TA, Kunz P, Singh S, et al. Neuroendocrine tumor clinical trials planning meeting: Treatment in the era of peptide receptor radionuclide therapy. Neuroendocrine tumor PRRT CTPM. https://www.cancer.gov/about-nci/organization/ccct/steering-committees/nctn/gastrointestinal/gisc-net-prrt-ctpm-execsum.pdf
- 57.Zandee WT, Brabander T, Blažević A, et al. : Symptomatic and radiological response to 177Lu-DOTATATE for the treatment of functioning pancreatic neuroendocrine tumors. J Clin Endocrinol Metab 104:1336-1344, 2019 [DOI] [PubMed] [Google Scholar]
- 58.Thang SP, Lung MS, Kong G, et al. : Peptide receptor radionuclide therapy (PRRT) in European Neuroendocrine Tumour Society (ENETS) grade 3 (G3) neuroendocrine neoplasia (NEN)—A single-institution retrospective analysis. Eur J Nucl Med Mol Imaging 45:262-277, 2018 [DOI] [PubMed] [Google Scholar]
- 59.Carlsen EA, Fazio N, Granberg D, et al. : Peptide receptor radionuclide therapy in gastroenteropancreatic NEN G3: A multicenter cohort study. Endocr Relat Cancer 26:227-239, 2019 [DOI] [PubMed] [Google Scholar]
- 60.Zhang J, Kulkarni HR, Singh A, et al. : Peptide receptor radionuclide therapy in grade 3 neuroendocrine neoplasms: Safety and survival analysis in 69 patients. J Nucl Med 60:377-385, 2019 [DOI] [PubMed] [Google Scholar]
- 61.Binderup T, Knigge U, Johnbeck CB, et al. : 18F-FDG PET is superior to WHO grading as a prognostic tool in neuroendocrine neoplasms and useful in guiding PRRT: A prospective 10-year follow-up study. J Nucl Med 62:808-815, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chan DL, Pavlakis N, Schembri GP, et al. : Dual somatostatin receptor/FDG PET/CT imaging in metastatic neuroendocrine tumours: Proposal for a novel grading scheme with prognostic significance. Theranostics 7:1149-1158, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chan DL, Ulaner GA, Pattison D, et al. : Dual PET imaging in bronchial neuroendocrine neoplasms: The NETPET score as a prognostic biomarker. J Nucl Med 62:1278-1284, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ambrosini V, Kunikowska J, Baudin E, et al. : Consensus on molecular imaging and theranostics in neuroendocrine neoplasms. Eur J Cancer 146:56-73, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vogin G, Foray N: The law of Bergonié and Tribondeau: A nice formula for a first approximation. Int J Radiat Biol 89:2-8, 2013 [DOI] [PubMed] [Google Scholar]
- 66.Riff BP, Yang Y-X, Soulen MC, et al. : Peptide receptor radionuclide therapy-induced hepatotoxicity in patients with metastatic neuroendocrine tumors. Clin Nucl Med 40:845-850, 2015 [DOI] [PubMed] [Google Scholar]
- 67.Hamiditabar M, Ali M, Bolek L, et al. : Safety and effectiveness of 177Lu-DOTATATE peptide receptor radionuclide therapy after regional hepatic embolization in patients with somatostatin-expressing neuroendocrine tumors. Clin Nucl Med 42:822-828, 2017 [DOI] [PubMed] [Google Scholar]
- 68.Braat AJAT, Ahmadzadehfar H, Kappadath SC, et al. : Radioembolization with 90Y resin microspheres of neuroendocrine liver metastases after initial peptide receptor radionuclide therapy. Cardiovasc Intervent Radiol 43:246-253, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ezziddin S, Meyer C, Kahancova S, et al. : 90Y radioembolization after radiation exposure from peptide receptor radionuclide therapy. J Nucl Med 53:1663-1669, 2012 [DOI] [PubMed] [Google Scholar]
- 70.Bodei L, Kidd M, Paganelli G, et al. : Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: The value and limitations of clinical factors. Eur J Nucl Med Mol Imaging 42:5-19, 2015 [DOI] [PubMed] [Google Scholar]
- 71.Strosberg JR, Wolin EM, Chasen BA, et al. : Clinical outcomes in patients with baseline renal dysfunction in the NETTER-1 study: 177Lu-Dotatate vs. high dose octreotide in progressive midgut neuroendocrine tumors. J Clin Oncol 36, 2018. (abstr 4102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van der Zwan WA, Brabander T, Kam BLR, et al. : Salvage peptide receptor radionuclide therapy with [177Lu-DOTA,Tyr3]octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging 46:704-717, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goncalves I, Burbury K, Michael M, et al. : Characteristics and outcomes of therapy-related myeloid neoplasms after peptide receptor radionuclide/chemoradionuclide therapy (PRRT/PRCRT) for metastatic neuroendocrine neoplasia: A single-institution series. Eur J Nucl Med Mol Imaging 46:1902-1910, 2019 [DOI] [PubMed] [Google Scholar]
- 74.Pavlakis N, Ransom DT, Wyld D, et al. : Australasian Gastrointestinal Trials Group (AGITG) CONTROL NET Study: Phase II study evaluating the activity of 177Lu-Octreotate peptide receptor radionuclide therapy (LuTate PRRT) and capecitabine, temozolomide CAPTEM)—First results for pancreas and updated midgut neuroendocrine tumors (pNETS, mNETS). J Clin Oncol 38, 2020. (abstr 4608) [Google Scholar]
- 75.Kesavan M, Grover P, Lam W, et al. : Long-term hematologic toxicity of 177Lu-octreotate-capecitabine-temozolomide therapy of GEPNET. Endocrine-Related Cancer 28:521-527, 2021 [DOI] [PubMed] [Google Scholar]
- 76.Singh A, Mencia-Trinchant N, Griffiths EA, et al. : Mutant PPM1D- and TP53-Driven hematopoiesis populates the hematopoietic compartment in response to peptide receptor radionuclide therapy. JCO Precis Oncol 10.1200/PO.21.00309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Merola E, Prasad V, Pascher A, et al. : Peritoneal carcinomatosis in gastro-entero-pancreatic neuroendocrine neoplasms: Clinical impact and effectiveness of the available therapeutic options. Neuroendocrinology 110:517-524, 2020 [DOI] [PubMed] [Google Scholar]
- 78.Strosberg JR, Al-Toubah T, Pellè E, et al. : Risk of bowel obstruction in patients with mesenteric or peritoneal disease receiving peptide receptor radionuclide therapy. J Nucl Med 62:69-72, 2021 [DOI] [PubMed] [Google Scholar]
- 79.Blažević A, Brabander T, Zandee WT, et al. : Evolution of the mesenteric mass in small intestinal neuroendocrine tumours. Cancers (Basel) 13:443, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ezziddin S, Khalaf F, Vanezi M, et al. : Outcome of peptide receptor radionuclide therapy with 177Lu-octreotate in advanced grade 1/2 pancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging 41:925-933, 2014 [DOI] [PubMed] [Google Scholar]
- 81.Roth D, Gustafsson J, Warfvinge CF, et al. : Dosimetric quantities in neuroendocrine tumors over treatment cycles with 177Lu-DOTATATE. J Nucl Med 63:399-405, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Del Prete M, Buteau F-A, Arsenault F, et al. : Personalized 177Lu-octreotate peptide receptor radionuclide therapy of neuroendocrine tumours: Initial results from the P-PRRT trial. Eur J Nucl Med Mol Imaging 46:728-742, 2019 [DOI] [PubMed] [Google Scholar]
- 83.Garske-Román U, Sandström M, Fröss Baron K, et al. : Prospective observational study of 177Lu-DOTA-octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (NETs): Feasibility and impact of a dosimetry-guided study protocol on outcome and toxicity. Eur J Nucl Med Mol Imaging 45:970-988, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kratochwil C, Giesel FL, López-Benítez R, et al. : Intraindividual comparison of selective arterial versus venous 68Ga-DOTATOC PET/CT in patients with gastroenteropancreatic neuroendocrine tumors. Clin Cancer Res 16:2899-2905, 2010 [DOI] [PubMed] [Google Scholar]
- 85.Lawhn-Heath C, Fidelman N, Chee B, et al. : Intraarterial peptide receptor radionuclide therapy using 90Y-DOTATOC for hepatic metastases of neuroendocrine tumors. J Nucl Med 62:221-227, 2021 [DOI] [PubMed] [Google Scholar]
- 86.Cullinane C, Waldeck K, Kirby L, et al. : Enhancing the anti-tumour activity of 177Lu-DOTA-octreotate radionuclide therapy in somatostatin receptor-2 expressing tumour models by targeting PARP. Sci Rep 10:10196, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yao JC, Strosberg J, Fazio N, et al. : Spartalizumab in metastatic, well/poorly differentiated neuroendocrine neoplasms. Endocr Relat Cancer 28:161-172, 2021 [DOI] [PubMed] [Google Scholar]
- 88.Strosberg J, Mizuno N, Doi T, et al. : Efficacy and safety of pembrolizumab in previously treated advanced neuroendocrine tumors: Results from the phase II KEYNOTE-158 study. Clin Cancer Res 26:2124-2130, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu Y, Pfeifer A, Myschetzky R, et al. : Induction of anti-tumor immune responses by peptide receptor radionuclide therapy with 177Lu-DOTATATE in a murine model of a human neuroendocrine tumor. Diagnostics 3:344-355, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Delpassand ES, Tworowska I, Esfandiari R, et al. : Targeted alpha-emitter therapy with 212Pb-DOTAMTATE for the treatment of metastatic SSTR-expressing neuroendocrine tumors: First-in-human, dose-escalation clinical trial. J Nucl Med 10.2967/jnumed.121.263230 [epub ahead of print on January 6, 2022] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ballal S, Yadav MP, Bal C, et al. : Broadening horizons with 225Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to 177Lu-DOTATATE PRRT: First clinical experience on the efficacy and safety. Eur J Nucl Med Mol Imaging 47:934-946, 2020 [DOI] [PubMed] [Google Scholar]
- 92.Kratochwil C, Apostolidis L, Rathke H, et al. : Dosing 225Ac-DOTATOC in patients with somatostatin-receptor-positive solid tumors: 5-Year follow-up of hematological and renal toxicity. Eur J Nucl Med Mol Imaging 49:54-63, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hofman MS, Hicks RJ: Changing paradigms with molecular imaging of neuroendocrine tumors. Discov Med 14:71-81, 2012 [PubMed] [Google Scholar]