Abstract
The peritoneum is a common site of metastasis in advanced gastric cancer (GC). Diagnostic laparoscopy is now routinely performed as part of disease staging, leading to an earlier diagnosis of synchronous peritoneal metastasis (PM). The biology of GCPM is unique and aggressive, leading to a dismal prognosis. These tumors tend to be resistant to traditional systemic therapy, and yet, this remains the current standard-of-care recommended by most international clinical guidelines. As this is an area of unmet clinical need, several translational studies and clinical trials have focused on addressing this specific disease state. Advances in genomic sequencing and molecular profiling have revealed several promising therapeutic targets and elucidated novel biology, particularly on the role of the surrounding tumor microenvironment in GCPM. Peritoneal-specific clinical trials are being designed with a combination of locoregional therapeutic strategies with systemic therapy. In this review, we summarize the new knowledge of cancer biology, advances in surgical techniques, and emergence of novel therapies as an integrated strategy emerges to address GCPM as a distinct clinical entity.
INTRODUCTION
Gastric cancer (GC) is an important cause of cancer mortality and morbidity, being the fifth most frequently diagnosed cancer and the fourth leading cause of cancer death globally.1 The peritoneum is a common site of metastasis for GC, occurring in nearly a third of patients at diagnosis.2 The prognosis of patients with GC peritoneal metastases (PM) remains dismal, with a median survival of less than 1 year.3 Several clinical challenges in the management of GCPM contribute to the poor prognosis. GCPM is difficult to accurately detect and measure using conventional imaging modalities, leading to an increasing use of peritoneal staging modalities such as diagnostic laparoscopy and cytology washings, which have led to earlier diagnosis of PMs.4
KEY POINTS
Gastric cancer peritoneal metastasis (GCPM) is a distinct clinical entity that is common in advanced gastric cancer with dismal prognosis.
We describe 11 biologic hallmarks of GCPM across four categories: tumor-related factors, the peritoneal microenvironment, paracrine factors, and biomechanical forces.
Although systemic therapies may benefit patients with GCPM, the magnitude of benefit is lower.
Therefore, a combination of peritoneal-directed treatment strategies and systemic therapy may be required for the treatment of GCPM.
Unraveling the genomic biology of GCPM offers the opportunity to integrate these treatment strategies, which may lead to improved outcomes.
CONTEXT
Key Objective
Peritoneal metastasis (PM) is common in advanced gastric cancer (GC) and confers a dismal prognosis. Advances in genomic sequencing have provided deeper insights into the biology of GCPM. Concurrently, several clinical studies are evaluating peritoneal-directed strategies to treat GCPM. This review aims to integrate these new data to provide an update on this difficult-to-treat disease.
Knowledge Generated
We review and synthesize recent major genomic studies of GCPM into 11 biologic hallmarks across four categories, including tumor-related factors, the peritoneal microenvironment, paracrine factors, and biomechanical forces. Next, we summarize various peritoneal-directed treatment strategies that are being used to target therapeutic vulnerabilities aimed to prevent the occurrence of GCPM or its treatment.
Relevance
Integration of recent novel genomic biology unraveled in GCPM with peritoneal-specific therapeutic strategies may lead to improved outcomes of this distinct clinical entity.
To date, treatment algorithms and clinical practice guidelines for patients with GCPM are included under the broader umbrella of metastatic (or stage IV) GC, with recommendations largely focused only on systemic therapy. Yet, because of the difficulty in measuring GCPM on conventional imaging modalities, patients with PM-only metastatic disease do not have measurable disease, as per RECIST, a common inclusion criterion for most clinical trials.5 This has led to an under-representation of this subgroup of patients in major trials.
Because of the presence of the peritoneal-plasma barrier and poor cancer tissue vascularity, PMs respond poorly to systemic antineoplastic therapy.6 This has led to the need to develop locoregional (intraperitoneal) treatment strategies such as catheter-based intraperitoneal chemotherapy, hyperthermic intraperitoneal chemotherapy (HIPEC), and pressurized intraperitoneal aerosol chemotherapy (PIPAC). Unless patients with GCPM are enrolled in peritoneal-directed clinical trials, there are limited opportunities for direct access to the PM for tissue sampling, leading to a poor understanding of the biologic components of GCPM, such as the tumor microenvironment (TME). However, recent advances in molecular characterization and genomic sequencing have enabled analysis of various aspects of GCPM, starting with analysis of cells derived from malignant ascites and inferring the role of the TME. This has led to advances in precision oncology in this area, through subclassification of GCPM patients on the basis of gene expression profiles, and identification of novel therapeutic targets.7
These emerging data of the molecular and biologic characteristics of GCPM suggest consideration of targeted (or peritoneal-directed) treatment, distinct from GC with metastases to distant organs. Here, we discuss GCPM as a unique clinical entity, explain the biology of the disease, summarize its natural history, and cover emerging biomarkers and, importantly, the potential application of genomic biology as an integrated strategy to improve existing and future potential therapeutic approaches to GCPM.
DIAGNOSIS AND EPIDEMIOLOGY OF GCPM
The diagnosis of PM made simultaneously with the primary GC is referred to as synchronous GCPM, whereas metachronous GCPM refers to the emergence of PM (usually at least 6 months) after the primary GC diagnosis.
Synchronous GCPM
Patients with synchronous GCPM may present with symptomatic ascites, with confirmation of PM through abdominal paracentesis and cytologic examination of ascitic fluid.8 In asymptomatic patients, synchronous GCPM is often diagnosed via (1) imaging as part of routine staging, (2) staging laparoscopy with or without peritoneal washing cytology, or (3) as an incidental intraoperative finding in patients planned for curative gastrectomy (Fig 1).
FIG 1.
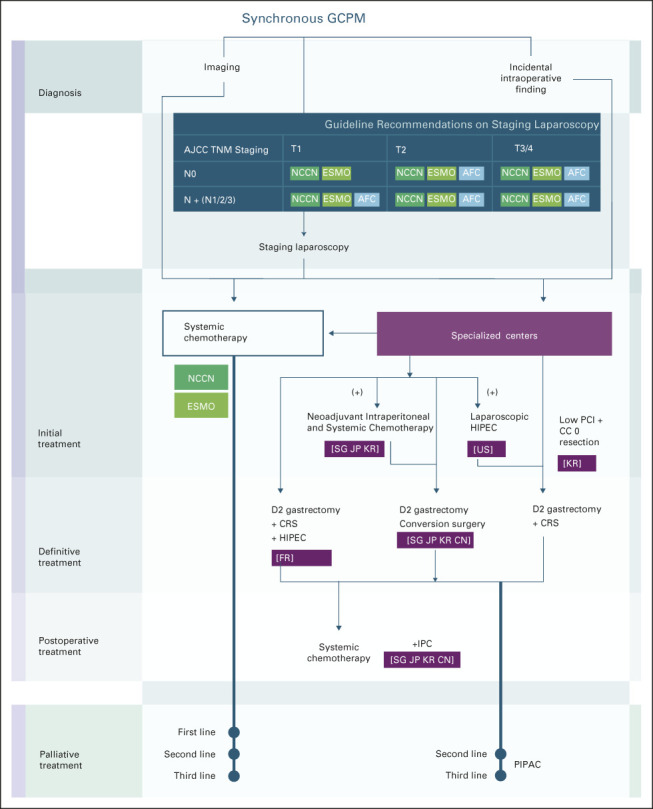
Diagnosis and treatment strategies of GCPM. Various groups across the world use different strategies in the management of GCPM. This figure aims to highlight the most commonly used approaches. Major guidelines (NCCN and ESMO) recommend treatment of GCPM with systemic therapy alone, similar to patients with advanced or inoperable metastatic GC. However, academic and high-volume subspecialized tertiary centers (in SG, JP, KR, CN, FR and the US, within purple boxes) tend to deploy more aggressive and experimental approaches with peritoneal-directed therapies, which are not recommended by either NCCN or ESMO. Guidelines on staging laparoscopy not represented in the figure include those from the JGCA and the SSO. JGCA recommends weakly for staging laparoscopy to decide on the treatment plan for patients with relatively high risk of peritoneal dissemination, referring also to the results of peritoneal lavage cytology using samples that are collected at staging laparoscopy, and for patients with advanced GC (TNM not otherwise specified) who can be indicated for neoadjuvant chemotherapy. SSO guidelines recommend strong consideration for diagnostic laparoscopy before the initiation of systemic chemotherapy in all patients with proven GC. AFC, French Association for Surgery; AJCC, American Joint Committee on Cancer; CN, China; CRS, cytoreductive surgery; ESMO, European Society for Medical Oncology; FR, France; GCPM, gastric cancer peritoneal metastasis; HIPEC, hyperthermic intraperitoneal chemotherapy; IPC, intraperitoneal chemotherapy; JGCA, Japanese Gastric Cancer Association; JP, Japan; KR, Korea; NCCN, National Comprehensive Cancer Network; PCI, Peritoneal Cancer Index; PIPAC, pressurized intraperitoneal aerosol chemotherapy; SG, Singapore; SSO, Society of Surgical Oncology Chicago Consensus 2020.
The imaging modality of choice to evaluate for distant metastases in the staging of GC is a computed tomography (CT) scan of the chest, abdomen, and pelvis.9-11 Although the specificity of CT for the detection of PM is high (97%-99%), the sensitivity is low (28%-51%).12,13 Recently, whole-body diffusion-weighted magnetic resonance imaging has emerged as an alternative imaging modality for the diagnosis of PM.14 Because of the difficulty in diagnosing PM through radiology, methods to standardize reporting have been created.15 A radiomic signature on the basis of CT phenotypes of primary tumors and adjacent peritoneum in patients with GC was developed to improve the predictive capability of CT imaging for occult GCPM.16 Positron emission tomography-CT is not routinely recommended for staging,9-11 in particular, for diffuse-type GC (mucinous and signet ring cell [SRC] histology) that tends to have lower uptake of 18F-fluoro-2-deoxy-d-glucose.17 Fluoro-2-deoxy-d-glucose positron emission tomography-CT scans were found to detect only 3% of occult PM, compared with 19% by diagnostic laparoscopy.18
Diagnostic laparoscopy with or without peritoneal washing cytology is recommended for routine staging of most stage II and III tumors by various international guidelines although slight variations exist in recommendations (Fig 1).9,10,19,20 Staging laparoscopy as a preoperative staging tool has a high sensitivity (85%) and specificity (100%) in the detection of PM not found on imaging.21
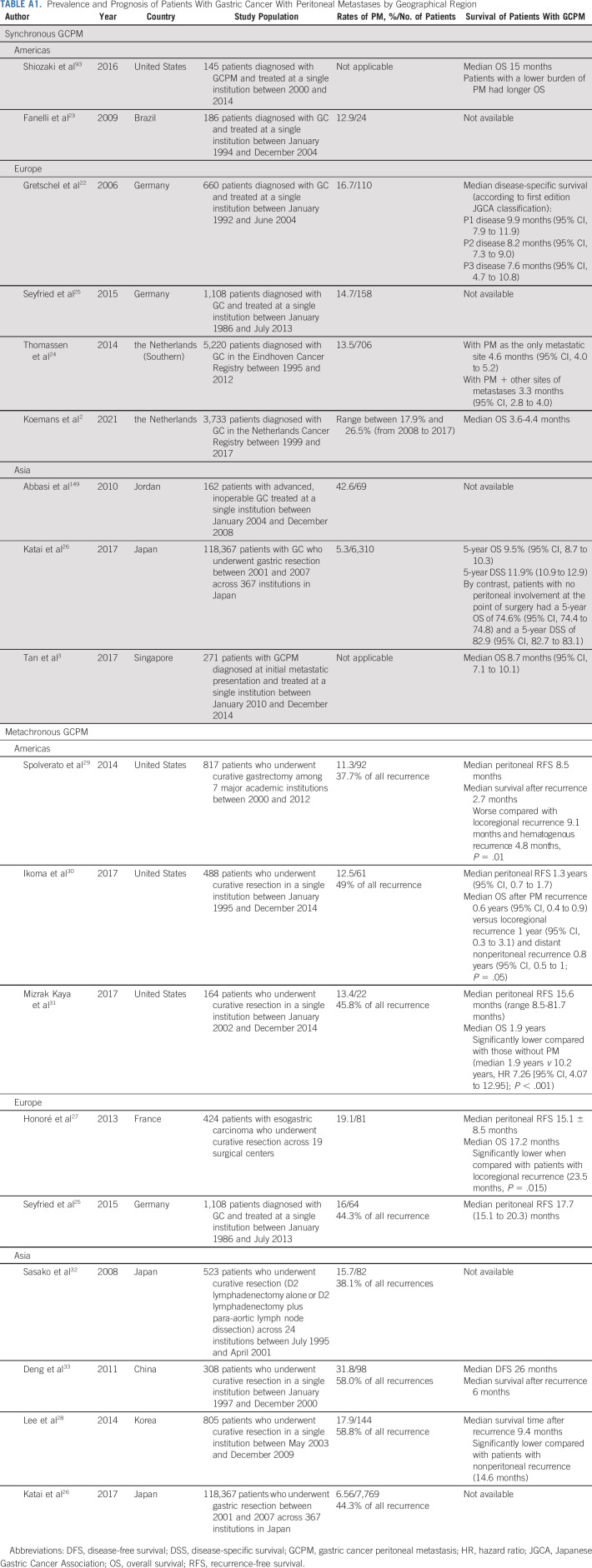
Several studies have shown that the rate of synchronous PM at the time of diagnosis of GC ranges between 12.9% and 26.5% (Appendix Table A1, online only).2,22-25 Incorporation of diagnostic laparoscopy in routine staging of newly diagnosed GC has led to an increase in the diagnosis of synchronous GCPM. A Dutch nationwide cohort study found that the proportion of patients with GC diagnosed with synchronous PM had increased from 18% to 26.5% over a 10-year period from 2008 to 2017.2
Metachronous GCPM
Metachronous GCPM is often diagnosed late when patients present with symptomatic ascites or mass effects, or can be detected on routine surveillance scans in asymptomatic patients. There are currently little data to guide surveillance strategies in patients who have undergone surgical resection of primary tumors for early-stage GC (with or without adjuvant/perioperative chemotherapy). Some clinical guidelines recommend routine annual surveillance CT scan,9 whereas others recommend scans only if patients present with symptoms or elevated serum tumor markers.11 The incidence of metachronous GCPM after curative gastrectomy ranges between 7% and 32%, within a median of 8.5-26 months after surgery in various studies (Appendix Table A1).25-33 Metachronous PM accounts for between one fifth and three fifths of all patients who have metastatic disease recurrence after gastrectomy.25,26,28-33
BIOLOGIC HALLMARKS OF GCPM
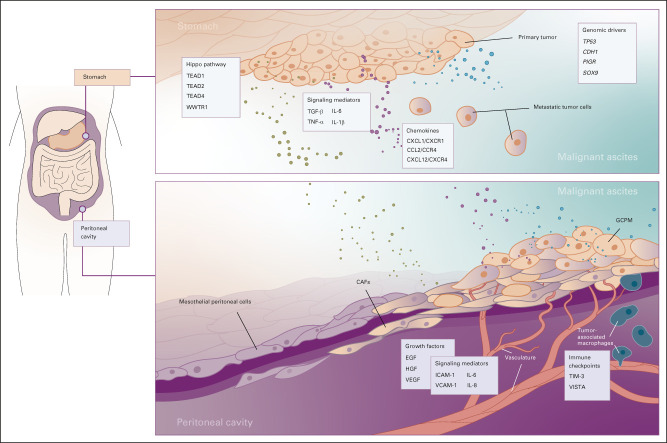
The peritoneum is the largest of three serous cavities (along with the pleura and pericardium), which are known to be immunologic niches, controlled by a diverse range of signaling networks. The peritoneum consists of a basement membrane, mesothelial cells, and connective tissue including hyaluron, collagen, proteoglycans, and interstitial cells (endothelial, fibroblasts, and pericytes).34 In addition to GC, several other tumor types display a propensity to metastasize to the peritoneum, such as colorectal and ovarian tumors. Lobular breast cancer is a unique disease entity with metastatic spread to the peritoneum reported similar to GC.35 This affinity is likely due to specific molecular characteristics of the primary tumor and the interaction with peritoneum during transcoelomic metastases. Malignant ascites often contain various growth factors, cytokines and chemokines, and other soluble factors. The interaction of the niche peritoneal microenvironment and malignant ascites with tumor cells is an area of great interest from a cancer biology point of view, as perturbation of these interactions may form potential therapeutic targets (Figs 2 and 3).
FIG 2.
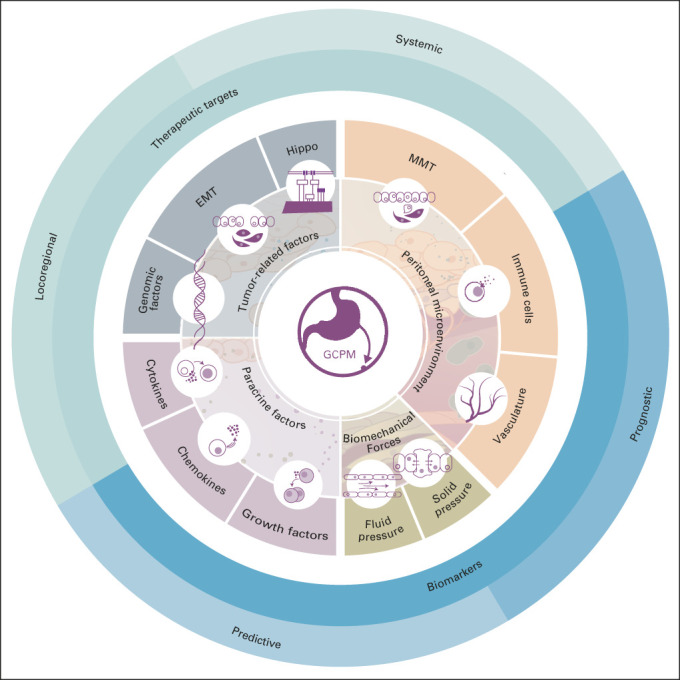
Biologic hallmarks of GCPM. The 11 biologic hallmarks of GCPM derived from four broad factors, including tumor-related factors, peritoneal microenvironment, paracrine factors, and biomechanical forces. Deeper understanding of these hallmarks has led to the development of novel therapeutic strategies and biomarkers. EMT, epithelial-mesenchymal transition; GCPM, gastric cancer peritoneal metastasis; MMT, mesothelial mesenchymal transition.
FIG 3.
Regulators of metastasis to the peritoneum in GC. Metastasis of the GC primary tumor to the peritoneum is a multistep process involving several pathways and networks. In this figure, we highlight some of the key regulators of this process, with factors that determine the metastatic cascade being present in the primary tumor, malignant ascites, and the peritoneal cavity. CAF, cancer-associated fibroblast; EGF, endothelial growth factor; GCPM, gastric cancer peritoneal metastasis; HGF, hepatocyte growth factor; ICAM-1, intercellular adhesion molecule-1; TGF, transforming growth factor; TNF, tumor necrosis factor; VCAM-1, vascular adhesion molecule-1; VEGF, vascular endothelial growth factor.
Tumor-Related Factors
The dissociation of tumor cells from the primary tumor is a multistep process, which involves several pathways and networks being co-opted to enable metastasis to the peritoneal lining.
Epithelial mesenchymal transition.
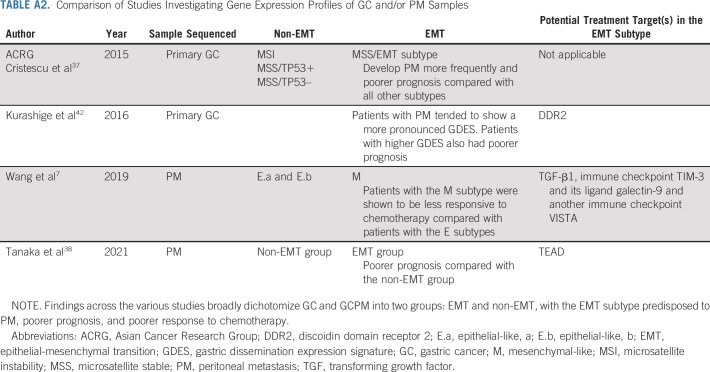
Epithelial mesenchymal transition (EMT) is a process through which epithelial cells undergo a transformation into a mesenchymal phenotype, with increased migratory and invasive capability, resistance to anoikis, and production of extracellular matrix (ECM) components.36 Primary GC tumors of the EMT subtype, identified using the Asian Cancer Research Group classification, were found to develop PM more frequently and had the worst prognosis, compared with all other non-EMT subtypes.37 A large multiomic profiling study of malignant ascites collected from patients with GCPM was recently reported.38 The study was predominantly in diffuse subtype (Lauren classification) tumors, with integrated profiling being performed including bulk whole-genome, whole-transcriptome, and epigenetic profiling (ChIP-Seq and methylation). A key finding in this study was that unsupervised hierarchical clustering of GCPM revealed two distinct molecular subtypes: EMT and non-EMT, with the EMT group associated with diffuse GC and having poorer prognosis. Several studies are pursuing diffuse-type GC-specific treatment strategies.39,40
Downregulation of expression and function of intercellular adhesion molecules, particularly classical cadherins such as E-cadherin, has been associated with EMT and peritoneal carcinomatosis.41 High expression of discoidin domain receptor 2 (DDR2) in primary GC tumors, a type I collagen receptor tyrosine kinase, was found to be significantly associated with EMT and peritoneal dissemination and could potentially be inhibited using dasatinib, a clinically available drug used in leukemia therapy.42
Genomic drivers.
Whole-genome/whole-exome sequencing and whole-transcriptome sequencing (RNA-seq) on tumor cells purified from malignant ascites of patients have started to provide some insight into the genomic determinants of GCPM.7,38 Although TP53 mutations in PM occurred at a rate similar to primary tumors, CDH1 mutations tended to occur more frequently, particularly in the diffuse subtype (Lauren classification) tumors. Novel drivers such as PIGR and SOX9 have also been identified in the tumor cells derived from malignant ascites.38 PIGR encodes the polyimmunoglobulin receptor, which transports polymeric immunoglobulins produced by plasma cells in the lamina propria across the epithelial barrier to be secreted into the luminal space.43 SOX9 is involved in embryonic developmental pathways.44 Clonality analyses suggest that tumor cells in malignant ascites are derived from only a single clone per patient or just a few subclones.38 Somatic copy number analysis has identified amplifications in several potential therapeutic targets such as KRAS, FGFR2, MET, ERBB2, EGFR, and MYC (several of which were found to be actionable in animal models).38 This finding is of clinical importance, as The Cancer Genome Atlas and Asian Cancer Research Group analysis of primary GC tumors report scarce aberrations of the mitogen-activated protein kinase/oncogenic pathways in diffuse GC.37,45 This suggests the importance of profiling the PM to identify potential therapeutic options for patients with refractory disease, which may be missed by profiling the primary tumor alone.
Single-cell RNA sequencing (scRNA-seq) has been recently used to characterize gene expression across thousands of cells simultaneously and provide a more granular understanding of the different cell states. In an scRNA-seq analysis of malignant ascites from 15 patients, tumor cells from different patients broadly clustered separately, reflective of the single clonality analysis described earlier.46 Approximately two thirds of tumor cells mapped to cells of GC origin, such as pit, mucosal, and chief cells. The remaining third mapped to other gastrointestinal organs such as the duodenum and colon. Samples could be classified into two main subtypes, on the basis of tumor cell lineage compositions—gastric-dominant (mainly gastric cell lineages) and GI-mixed (with mixed gastric and colorectal-like cells), although no significant difference was observed in the histopathologic features between these two subtypes. This classification was found to have a strong correlation with patient survival.
Evolutionary Hippo pathway dysregulation.
The Hippo signaling pathway is involved in tissue homeostasis.47 Expression of genes involved in the Hippo pathway, including TEAD1, TEAD2, TEAD4, and WWTR1, was significantly elevated in EMT-associated malignant ascites.38 The role of TEAD inhibition was explored through the administration of K-975, a TEAD inhibitor, resulting in significant PM tumor suppression and improved survival in a mouse model.38
The transforming growth factor-β (TGF-β) superfamily consists of various cytokines and proteins including activins and inhibins, as well as bone morphogenetic proteins, and is downstream of the Hippo pathway.48 The TGF-β pathway is involved in several cellular processes, including EMT, cellular migration and invasion, and ECM remodeling. Excessive production of TGF-β leads to oncogenesis through dysregulation of these cellular processes. Integrative classification of malignant ascites, incorporating DNA, RNA, and clinicopathologic characteristics, has identified a mesenchymal and an epithelial subtype of GCPM.7 The mesenchymal subtype was found to have higher expression of TGF-β pathway genes, less frequent mutations of TP53 and CDH1, a lower level of chromosomal instability, and decreased response to chemotherapy.
Paracrine Factors
Malignant ascites, with its admixture of cytokines, chemokines, and growth factors, has been shown to provide a tumorigenic environment for PM. Ascites also contains multiple ligands, which are upstream regulators of signaling, leading to phenotypic changes to the cell, enhanced tumor cell proliferation and migration, and attenuated drug-induced malignant cell apoptosis.
The TGF-β pathway has been shown to be upregulated within the tumor cells, and elevated levels of the TGF-β1 cytokine have been detected in the peritoneal washings of patients with GCPM.49 Through the Smad pathways, TGF-β upregulates collagen and fibronectin deposition, leading to peritoneal fibrosis and increased GC tumor cell adhesion. TGF-β also increases crosstalk between cancer-associated fibroblasts (CAFs) and endothelial and other stromal cells, sustained through cytokines such as CXCLs, interleukins (ILs), and vascular endothelial growth factors (VEGFs).
Inflammatory cytokines such as tumor necrosis factor-α, interferon-γ, and the IL-6 and IL-1β, found in malignant ascites, increase the expression of adhesion molecules such as intercellular adhesion molecule-1 and vascular adhesion molecule-1 on mesothelial cells.50 GCPM also secretes IL-6 and IL-8, which increase cell growth, invasiveness, motility, and chemoresistance.51
Several chemokines and their axes detected in malignant ascites, including CXCL1/CXCR1, CCL2/CCR4, and CXCL12/CXCR4, have been shown to play an important role in migration, chemotaxis, proliferation, and adhesion of tumor cells.52-54 Growth factors regulating various pathways instrumental to tumor cell metastasis and propagation have been found in malignant ascites of GCPM. These, which include endothelial growth factor, hepatocyte growth factor, and VEGF, induce mesothelial cell contraction, leading to exposure of the peritoneal basement membrane.55 VEGF, an angiogenic growth factor, promotes vascular permeability of the peritoneal microenvironment. Crosstalk between heparin-binding-endothelial growth factor–like growth factor, CXCR4, CXCL12, and tumor necrosis factor-α converting enzyme was shown to stimulate GCPM through an autocrine/paracrine mechanism.56
Notably, some of the paracrine factors described are not unique to GCPM. In ovarian cancer, several studies have demonstrated the possibility of using peritoneal-directed treatment to target specific paracrine factors, such as anti-IL6R (tocilizumab)57 and anti-VEGF (bevacizumab)58 therapies, to control malignant ascites, suggesting a similar potential to target these factors in GCPM.
Peritoneal Microenvironment
The localization of free-floating tumor cells (either transcoelomic or translymphatic) to the peritoneal mesothelial lining is regulated by adhesion molecules such as CD44 and integrin and selectin superfamilies.59 Cancer-associated stem cells have been isolated with a propensity for peritoneal homing and EMT.60 Several cell types within the peritoneal microenvironment then determine the fate and progression of these tumor cells. However, because of lack of tissue availability, detailed analyses of the TME of GCPM are yet to be performed.
Mesothelial mesenchymal transition.
Tumor cells adhere to the mesothelial peritoneal cells and submesothelial connective tissue through interaction of integrins.61 Mesothelial cells secrete adhesion molecules for a variety of basement membrane proteins, including collagen, laminin, and fibronectin. Mesothelial mesenchymal transition, a process well described in the field of peritoneal dialysis for renal failure, has also been reported in PM. Mesothelial cells have been demonstrated to progressively acquire features of CAFs.62
CAFs driven through TGF-β signaling pathways sustain and stimulate tumor proliferation. RHBDF2 expressed by CAFs is induced by inflammatory cytokines present in the malignant ascites and secreted by tumor cells.63 Through TGF-β, RHBDF2 promotes motility of CAFs inducing invasion of the ECM and lymphatic vessels. CAFs have also been associated with secretion of ILs and growth factors.49,54
Immune cell–mediated immunosuppressive niche.
Few studies have been performed directly on the immune cells within the TME of the PM, and this remains an area of intense research. In one study, the TME of GCPM was inferred through bulk RNA-Seq deconvolution to deduce immune cell types and proportions and two major subgroups were identified: T-cell–exclusive and T-cell–exhausted. Immune checkpoint TIM-3, its ligand galectin-9, and VISTA were highly expressed in the T-cell–exhausted (mesenchymal) subtype, as well as TGF-β1, suggesting an immune suppressive microenvironment.7 In addition, GCPM with higher proportions of resting memory CD4 T cells tended to be associated with a more aggressive phenotype.7
Plasma cell homing through epithelial-resident KLF2 in diffuse-type GC tumors was reported in one of the largest scRNA-seq data sets of GC reported to date, including GCPM samples.64 Perturbation of this interaction may present a potential therapeutic target for GCPM. Omental neutrophils have been shown to generate extracellular traps, involving the release of a protein-rich chromatin web that functions as a premetastatic niche.65,66
Macrophages found to be residing in serous cavities such as the peritoneum have been found to have unique characteristics through GATA6-mediated homeostasis.67 Cavity-resident macrophages within the peritoneum have high levels of Tim-4, which has been shown to mediate sequestration of CD8 T cells, thereby limiting antitumor activity in PM.68 This suggests a possible strategy of using the Tim-4 blockade to enhance efficacy of CD8 T-cell–based immunotherapies in the treatment of malignant ascites. In addition, tumor-associated macrophages were found to promote PM via IL-6 and a potential therapeutic vulnerability.69
Vascular microenvironment.
Milky spots are regions of lymphoid tissue found on the omentum in the peritoneal cavity. These tend to have dense capillary networks, forming a proangiogenic habitat for metastases, driven through CD105-positive vessels.70 Oncogenesis may be further propagated by tumor and mesothelial secretion of VEGFs such as VEGF and platelet-derived growth factor, which lead to abnormal, hyperpermeable blood vessel formation. Several studies have associated changes in the tumor vasculature with the immune microenvironment and oncogenic signaling, suggesting an interplay between various biologic hallmarks.71
Physical Factors
As an enclosed space, the peritoneal cavity is subject to biomechanical forces, which were shown to affect tissue homeostasis. Imbalances to this tensional homeostasis have been associated with the pathogenesis of PM. These forces have also been associated with induction of EMT or mesothelial mesenchymal transition.72 Leaky vasculature associated with PM, along with a deficient lymphatic drainage, leads to an elevated fluid pressure in the interstitium. The increased pressures within the fluid and the PM increases epithelial cell shedding and metastasis, leading to decreased diffusion and convection within the tumor and resulting in poor drug penetration.73
Although described individually, the multiple biologic hallmarks are interconnected, with several overlapping biologic programs regulating and signaling pathways. For example, the mesenchymal subtype of GCPM (v epithelial subtype) was found to have a T-cell–exhausted phenotype with increased expression of immune checkpoint TIM-3, its ligand galectin-9, VISTA, and TGF-β1.7 Other groups, sampling either primary GC tumors or GCPM, have also described molecular subgroups. An overarching similarity across these studies is the dichotomization of GCPM into EMT and non-EMT subgroups (Appendix Table A2, online only). Tumors with active EMT tend to have poorer survival, but more importantly, EMT-specific novel and potential therapeutic targets have been identified. Collectively, a deeper understanding of the unique biologic and molecular networks driving GCPM has identified therapeutic vulnerabilities that could be harnessed either through systemic or locoregional therapies or by a combination of both.
MOLECULAR BIOMARKERS PREDICTIVE OF GCPM
Given the poor prognosis of GCPM, significant research efforts were put into identification of biomarkers that predict the emergence of GCPM. Conventional serum tumor markers such as carcinoembryonic antigen, cancer antigen (CA) 19-9, CA 72-4, and CA125 can modestly predict GCPM recurrence.74
High mesothelin protein expression in primary GC tumors, measured by immunohistochemistry, is associated with GCPM recurrence.75 A series of studies, on the basis of bulk RNA-Seq data of primary GC tissue, showed a significant association between higher expression of SYT8,76 SYT13,77 and TNNI278 and the risk of developing metachronous GCPM. Other groups have studied specific patterns of the TME to develop metabolic,79 immune,80 and collagen81 signatures predictive of GCPM. In a more comprehensive, transcriptome-wide analysis, a six-gene panel predictive of both synchronous and metachronous GCPM has been identified.82 This signature consists of genes such as CAVIN2, part of the TGF-β pathway and associated with EMT.
Several studies have tried to identify predictive biomarkers for GCPM recurrence in intraoperative peritoneal lavage samples. Positive SYT13 mRNA in peritoneal lavage fluid was found to be an independent prognostic factor for peritoneal recurrence.83 MMP-7,84 CK20, FABP1, and MUC285 in peritoneal washings have also been identified as potential biomarkers for identifying patients at risk of peritoneal recurrence after gastrectomy. More recently, reduced expression of miR-29s in peritoneal exosomes was identified as a strong risk factor for GCPM development.86 However, most studies evaluating these biomarkers were retrospective in nature, with varying definitions of PM recurrence end points, and further prospective large-scale validation studies are required before these can be incorporated into clinical practice.
RISK FACTORS FOR DEVELOPMENT OF GCPM
Risk Factors
Patient characteristics such as female gender3,24,87 and primary GC tumor characteristics including more advanced T stage,24,25,28,87 nodal involvement,24,25,27,28 and distal gastric (v proximal or gastroesophageal junction) location27,87 have been identified as clinical risk factors for the development of GCPM in both metachronous and synchronous settings.
Diffuse-type GCs by Lauren's classification, most often composed of SRCs, are more biologically aggressive than intestinal-type GCs, and correspondingly, diffuse/mixed type tumors and the presence of SRC histology have been shown to be associated with increased risk of developing PM, in both the synchronous3,23,24 and metachronous settings.25,87
Role of Adjuvant Systemic Therapy in Preventing GCPM Recurrence
Systemic chemotherapy is commonly administered in either the perioperative or adjuvant setting for patients with GC undergoing curative resection.88-92 In the ACTS-GC trial, adjuvant S-1 significantly lowered the PM recurrence rate (15% v 19% in the surgery-alone group, hazard ratio 0.69).89 However, in the CLASSIC trial, adjuvant capecitabine plus oxaliplatin had only a small, nonsignificant effect on PM recurrence,90 whereas the addition of adjuvant docetaxel to S-1 in the JACCRO GC-07 trial did not further lower the incidence of PM recurrence.92 Various cohort studies from both Western and Asian populations found that the use of systemic therapy was not associated with a lower risk of metachronous PM after curative-intent gastrectomy.25,27-29,87 Therefore, although adjuvant chemotherapy in GC prevents distant metastases and prolongs survival, the efficacy of systemic therapy to prevent PM remains uncertain.
PROGNOSIS OF GCPM AND THE EFFECT OF SYSTEMIC THERAPY
Prognosis of GCPM
Both synchronous GCPM and metachronous GCPM portend a poor prognosis. Studies evaluating synchronous GCPM showed a dismal survival, ranging between 3 and 15 months,2,22,24,93 whereas the median survival ranged between 3 and 9 months in patients with metachronous GCPM (Appendix Table A1).28-30,33 Patients with peritoneal recurrence had shorter survival compared with patients with nonperitoneal (distant and locoregional) recurrences.27-31
Patients with synchronous PM as the only metastatic site tend to have marginally better survival compared with patients with PM with concomitant extra-PMs.3,24 The prognosis of patients with GCPM is also dependent on the PM disease burden. The Peritoneal Cancer Index (PCI) and the Japanese Gastric Cancer Association classification are two commonly used metrics to quantify GCPM.22,93 The Japanese Gastric Cancer Association classification94 describes PM as peritoneal lavage cytology–negative (CY0) and peritoneal lavage cytology–positive (CY1) and the absence (P0) or presence of macroscopic PM (P1), whereas the PCI takes into account the extent of PM by calculating the size of PM lesions across 13 pelvic-abdominal regions within the abdominal cavity.95 Regardless of the metric used, survival of patients with synchronous GCPM worsens with increasing PM burden.22,93,96,97
Role of Systemic Therapy in the Treatment of GCPM
Several systemic therapies have been introduced in the past 2 decades for the treatment of metastatic GC, including combinations of chemotherapeutic agents,98-100 targeted therapies such as trastuzumab101 or ramucirumab,102,103 and immune checkpoint inhibitors,104,105 leading to a clinically meaningful improvement in survival.25 Yet, there are several challenges in the use of systemic chemotherapy in the treatment of patients with GCPM. The presence of the plasma-peritoneal barrier and the poor blood supply of PM limit the tissue penetration and therapeutic effect of systemic agents.106,107 Patients with GCPM may also develop complications such as intestinal obstruction and, in turn, poor nutrition and performance status, which may preclude them from systemic treatment.108 Furthermore, because radiologic studies such as CT scans cannot consistently and accurately identify low-volume PM, objective assessment of treatment response remains a challenge.109,110
Within the limited number of randomized controlled trials that performed subset analysis of survival on the basis of the presence or absence of PM, patients with GCPM benefit from systemic therapies such as cisplatin plus S-1 (first line; SPIRIT99), ramucirumab monotherapy (second line; REGARD102), paclitaxel plus ramucirumab (second line; RAINBOW103), TAS-102 (trifluridine/tipiracil) (third line; TAGS111), and nivolumab monotherapy (second line; ATTRACTION-2112), similar to patients with metastatic GC without PM. However, the magnitude of benefit is lower in patients with PM in many of these studies compared with those without PM, confirming that PM is a negative prognostic marker among patients with inoperable metastatic GC.102,103,111,112 Furthermore, two large-scale cohort studies found that the prognosis of GC patients with synchronous and metachronous GCPM has not improved significantly over time, despite an increasing proportion of patients who received systemic therapy in the past 2 decades.2,25 These results suggest that solely using systemic therapy may inadequately treat patients with GCPM. These also highlight the need for better detection, risk stratification, and therapeutic strategies in patients with GC. In particular, patients with early-stage disease, treated with curative intent, may benefit from earlier identification of those at risk for peritoneal recurrence and interventions to prevent or at least delay the development of metachronous GCPM.
INTRAPERITONEAL THERAPEUTIC STRATEGIES FOR GCPM AND THEIR ROLE IN THE PREVENTION AND TREATMENT OF GCPM
Given the dismal prognosis of GCPM, novel peritoneal-directed strategies for the prophylaxis of metachronous GCPM and treatment of synchronous GCPM are areas of active research and clinical trials. Various modalities, in conjunction with surgery and systemic therapy, have been developed with ongoing evaluation to determine their role in the management of patients with GCPM (Fig 4). These strategies have been used as prophylactic strategies to prevent GCPM recurrence or conversion strategies to allow surgical resection of primary tumor and GCPM or incorporated into palliative/disease control approaches with systemic therapy.
FIG 4.
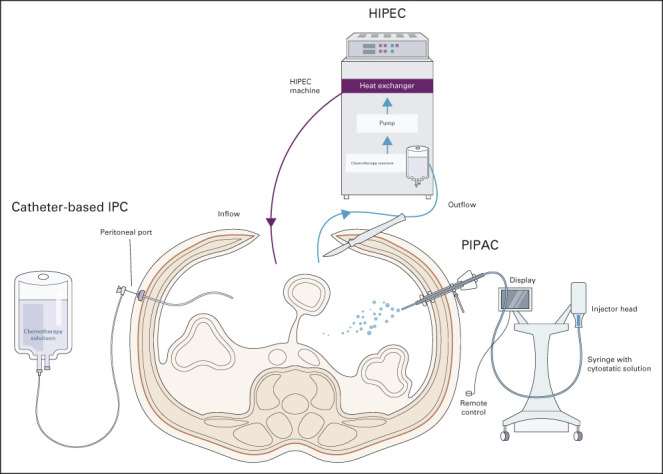
Peritoneal-directed modalities and their roles in treatment and prophylactic strategies of GCPM. Catheter-based IPC in combination has been evaluated in both adjuvant and neoadjuvant settings in the management of GCPM. Adjuvant combination of systemic and intraperitoneal chemotherapy may help downstage PM, allowing for conversion gastrectomy, whereas the role of adjuvant early postoperative intraperitoneal chemotherapy in prevention of metachronous PM remains unclear. HIPEC is most commonly carried out in conjunction with cytoreductive surgery as a potentially curative strategy in patients with low-volume PM and potential for complete cytoreduction. A potential role exists for prophylactic HIPEC in patients with GC undergoing gastrectomy to prevent or reduce metachronous PM recurrence, with ongoing studies underway. Studies of PIPAC have thus far been limited to palliative treatment for patients with PM; the role of PIPAC in treatment of GCPM requires further evaluation and is currently limited to the settings of clinical trial. GCPM, gastric cancer peritoneal metastasis; HIPEC, hyperthermic intraperitoneal chemotherapy; IPC, intraperitoneal chemotherapy; PIPAC, pressurized intraperitoneal aerosol chemotherapy.
Extensive Intraoperative Peritoneal Lavage: Primary Prevention
Since free intraperitoneal cancer cells exfoliate from the primary gastric tumor and result in PM formation, the hypothesis that repeated intraoperative peritoneal lavages (extensive intraoperative peritoneal lavage [EIPL]) with saline solution during primary resection might reduce GCPM was formulated. Three randomized controlled trials failed to demonstrate significant improvement in both overall survival (OS) and peritoneal recurrence-free survival.113-115 Furthermore, patients in the EIPL arm of the EXPEL trial experienced a higher risk of adverse events compared with the standard surgery group.114 Currently, there is no established role for the use of EIPL as a prophylactic strategy in the prevention of metachronous GCPM.
HIPEC: Primary Prevention and/or Conversion to Resectable Disease
Pre-emptive, intraoperative HIPEC (most commonly with oxaliplatin, mitomycin, or cisplatin as single agent or in combination with other drugs) may eliminate progression of peritoneal implantation after curative surgery and reduce metachronous PM recurrence. A meta-analysis evaluating the role of HIPEC in addition to gastrectomy in patients with advanced GC without PM showed a significant reduction in rates of PM recurrence (risk ratio = 0.63) compared with gastrectomy alone. However, HIPEC was associated with significantly higher risk of postoperative complications, in particular, renal dysfunction.116 The ongoing multicenter phase III GASTRICHIP randomized trial (ClinicalTrials.gov identifier: NCT01882933) aims to develop definitive evidence evaluating the role of adjuvant HIPEC with oxaliplatin in patients with locally advanced GC without gross PM undergoing curative gastrectomy.117
HIPEC in addition to cytoreductive surgery (CRS) remains contentious as a treatment strategy for synchronous GCPM. The CYTO-CHIP study, an observational cohort study, demonstrated that patients with GCPM who underwent complete CRS with curative intent with HIPEC (using various agents including oxaliplatin, mitomycin, and cisplatin) had significantly longer survival compared with patients who underwent CRS alone, with similar morbidity rates across both groups.118 In particular, patients with only microscopic PM or positive peritoneal cytology (ie, PCI score 0) who underwent CRS plus HIPEC had a longer median OS than those who underwent CRS alone although the difference was not statistically significant. In a follow-up study, poorly cohesive carcinoma (including SRC histology) was shown to be associated with poorer prognosis. CRS plus HIPEC conferred a longer median OS in this group of patients, compared with CRS alone.119 The GASTRIPEC trial (ClinicalTrials.gov identifier: NCT02158988), which compared CRS plus HIPEC (with mitomycin C and cisplatin) with CRS alone with pre- and postoperative systemic chemotherapy, reported no significant difference in OS nor treatment-related adverse events.120 Subgroup analysis demonstrated a significant improvement in OS in patients in whom complete cytoreduction (CC) was achieved in the HIPEC arm. In addition, progression-free survival was significantly longer in the HIPEC arm compared with that in the non-HIPEC arm (7.1 months v 3.5 months, P = .0472). Importantly, this trial was closed early because of poor patient recruitment and is underpowered for OS. On the other hand, a meta-analysis demonstrated that CRS plus HIPEC, although superior to control, was not superior to systemic chemotherapy alone.116 Furthermore, HIPEC was associated with a significantly higher risk of postoperative complications including respiratory failure and renal dysfunction. The benefits of CRS plus HIPEC need to be balanced against the risks; patients with low-volume PM (by PCI score) and possibility for CC are most likely to benefit from CRS plus HIPEC.118-122
Catheter-Based Intraperitoneal Chemotherapy
The implantation of a peritoneal port is considerably less invasive than HIPEC, allows for repeated IP administration of chemotherapy, and leads to high concentrations of chemotherapeutic drugs in the peritoneal cavity, allowing prolonged direct exposure of free cancer cells or peritoneal deposits.123,124 Therefore, catheter-based intraperitoneal chemotherapy (IPC; most commonly with taxane-based drugs) plus systemic chemotherapy presents a theoretical advantage over HIPEC plus systemic chemotherapy.125
Early postoperative intraperitoneal chemotherapy: Primary prophylaxis.
There are little data on the use of adjuvant early postoperative intraperitoneal chemotherapy (EPIC) after curative gastrectomy in patients at high risk of PM recurrence. A randomized study of EPIC (using mitomycin C and fluorouracil), immediately postgastrectomy, compared with surgery alone, demonstrated a clinically meaningful reduction in the rate of PM recurrence.126 However, there was a significantly higher incidence of postoperative complications in the EPIC group, including intra-abdominal bleeding and sepsis. By contrast, the more recent INPACT trial comparing adjuvant IP paclitaxel versus intravenous paclitaxel demonstrated that postgastrectomy, IP paclitaxel did not confer any survival or PM-recurrence benefit over the intravenous group.127 In view of these findings, the role of EPIC in the prevention of PM recurrence remains uncertain. The ongoing Japanese multicenter, randomized phase III PHOENIX-GC2 trial (JPRN-jRCT2031200087) aims to evaluate the role of IPC, in addition to gastrectomy and systemic chemotherapy, in patients with diffuse GC without distant metastasis or macroscopic PM.128
Combination of systemic and intraperitoneal chemotherapy: Conversion to resectable disease; palliative disease control.
Several phase II trials evaluating systemic and intraperitoneal chemotherapy (SIPC) in patients with GCPM using IP taxanes demonstrated high rates of conversion to negative peritoneal cytology (71%-86%) and 1-year survival rates of more than 70%.125 Although primary analysis of the phase III RCT (PHOENIX-GC) comparing S-1/systemic paclitaxel/IPC paclitaxel versus S-1/systemic cisplatin reported no statistical advantage of the IP paclitaxel group (IP v non-IP group, median OS 18 v 15 months, P = .080), exploratory analysis adjusting for an imbalance in ascites between the two groups demonstrated an adjusted hazard ratio of 0.59, suggesting possible efficacy of the IP regimen.129 Other groups have reported successful downstaging of PM (disappearance of macroscopic GCPM and conversion to negative peritoneal cytology) after combined SIPC, allowing for conversion gastrectomy in those without extra-peritoneal unresectable metastases and leading to a median OS ranging between 21.6 and 34.6 months.130-132 These data suggest a role for combined SIPC and subsequent conversion gastrectomy in the treatment of GCPM in selected patients.
PIPAC: Palliative Disease Control
PIPAC is a novel method of intraperitoneal chemotherapy administration.133 During PIPAC, aerosolized chemotherapy is directly administered to the peritoneum through a laparoscope. Various studies have shown that PIPAC (most commonly using cisplatin, doxorubicin, or oxaliplatin) in combination with systemic chemotherapy is safe and feasible in GCPM and has shown promise in improving outcomes.134-137 Within the limited existing literature, studies on PIPAC are mostly limited to palliative treatment for patients with PM.138 The role of PIPAC in the treatment of GCPM requires further evaluation and should only be performed within the framework of clinical trials. Numerous trials are underway in Europe, Singapore, and the International PIPAC Registry, which will provide more conclusive evidence on the role of PIPAC in GCPM.134,139,140
Geographical Differences in Treatment Strategies of GCPM
Major guidelines around the world consider synchronous GCPM to be metastatic disease and recommend palliative systemic chemotherapy (Fig 1).10,20,141,142 Although there are differences in the management of GCPM across the world, whether this is necessary because of geographical or ethnic variations in GC biology remains an area of controversy.143 In patients who have incidentally discovered GCPM during index surgery, the Korea Gastric Cancer Association guidelines recommend considering radical gastrectomy (gastrectomy with D2 lymphadenectomy) and limited CRS if CC can be achieved.144 If systemic chemotherapy leads to complete resolution of PM, conversion gastrectomy is recommended by guidelines from the National Health Commission of the People's Republic of China and Korea Gastric Cancer Association.11,144 Although catheter-based IPC is administered in combination with systemic chemotherapy by several academic groups in Japan, Singapore, and Korea, it remains experimental.129,131,145,146 In patients with good response to this treatment and minimal residual GCPM, conversion gastrectomy is considered. In specialized centers in the United States, GCPM patients with good response to systemic chemotherapy and low PCI proceed with laparoscopic HIPEC.141 Those with good response to systemic chemotherapy and laparoscopic HIPEC, with a low PCI score, are considered for radical surgery with CRS. By contrast, patients with good response to systemic chemotherapy with low PCI scores are subsequently offered CRS and HIPEC in specialized centers in Europe.20 Of note, European and US guidelines do not require complete resolution of GCPM and see a benefit in limited CRS if CC can be achieved. There is no consensus on the role of intraperitoneal chemotherapy after curative surgery (either conversion surgery or radical gastrectomy with CRS). A number of groups from Asia continue catheter-based IPC in the postoperative period.129,131,145,146
DISCUSSION
In conclusion, we describe GCPM as a distinct clinical entity with significant mortality and morbidity and an area of unmet clinical need. Despite earlier diagnosis of GCPM and the introduction of new systemic treatment agents, outcomes remain poor. Novel modalities of peritoneal-directed therapies are being extensively evaluated and are gradually being adopted in various countries. Concurrently, in addition to conventional clinicohistopathologic risk factors, molecular profiling of GCPM has uncovered subtypes with varying molecular biologies and disease behaviors. Importantly, several novel therapeutic targets specific to GCPM have been identified. These advances will pave the way for the integration of molecular information into prognostication, follow-up, and treatment strategies of GCPM. It is likely that future studies will consider incorporation of peritoneal-directed treatment with systemic therapy. One example is the PIANO study (ClinicalTrials.gov identifier: NCT03172416), which is evaluating the role of PIPAC-delivered oxaliplatin, in combination with systemic nivolumab in patients with GCPM. In principle, immunogenic cell death induced by PIPAC with oxaliplatin in a conventionally immune-cold cancer niche may render lesions hot, thereby inducing a response to systemic immune checkpoint inhibition. It is fathomable that in the future, more sophisticated intratumoral agents (such as STING agonists)147 may also be delivered intraperitoneally through either PIPAC or other methods, opening the door to several other combination treatment strategies that are currently being pursued in other tumor types such as melanoma.148 These integrated combination strategies are the most plausible way through which patients with this dreadful illness may finally have better therapeutic options.
APPENDIX
TABLE A1.
Prevalence and Prognosis of Patients With Gastric Cancer With Peritoneal Metastases by Geographical Region
TABLE A2.
Comparison of Studies Investigating Gene Expression Profiles of GC and/or PM Samples
SUPPORT
R.S. was supported by the National Medical Research Council (NMRC; NMRC/Fellowship/0059/2018, NMRC/MOH/000627). C.-A.J.O. was supported by the National Research Council Transition Award (NMRC/TA/0061/2017). P.T. was supported by Duke-NUS Medical School and the Biomedical Research Council, Agency for Science, Technology and Research. As part of the Singapore Gastric Cancer Consortium, the study was also partially funded by the National Medical Research Council Open Fund-Large Collaborative Grant (OFLCG18May-0023).
Wei Peng Yong
Consulting or Advisory Role: AbbVie/Genentech, Amgen, Bristol Myers Squibb, Ipsen, Novartis, AstraZeneca
Speakers' Bureau: Lilly, Sanofi/Aventis, Taiho Pharmaceutical, Eisai, Bayer, MSD Oncology
Travel, Accommodations, Expenses: Pfizer
Patrick Tan
Stock and Other Ownership Interests: Tempus Healthcare, Auristone Pte Ltd
Patents, Royalties, Other Intellectual Property: Patents related to cancer epigenetics
Raghav Sundar
Honoraria: MSD, Bristol Myers Squibb, Lilly, Roche, Taiho Pharmaceutical, AstraZeneca, AstraZeneca, DKSH
Consulting or Advisory Role: Bristol Myers Squibb, Eisai, Taiho Pharmaceutical, Bayer, Merck, Novartis, MSD
Research Funding: Paxman, MSD
Travel, Accommodations, Expenses: Roche, AstraZeneca, Taiho Pharmaceutical, Eisai
No other potential conflicts of interest were reported.
C.-A.J.O. and R.S. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Yong Xiang Gwee, Daryl Kai Ann Chia, Jimmy So, Wim Ceelen, Wei Peng Yong, Chin-Ann Johnny Ong, Raghav Sundar
Collection and assembly of data: Yong Xiang Gwee, Daryl Kai Ann Chia, Raghav Sundar
Data analysis and interpretation: Yong Xiang Gwee, Daryl Kai Ann Chia, Wim Ceelen, Wei Peng Yong, Patrick Tan, Chin-Ann Johnny Ong, Raghav Sundar
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Integration of Genomic Biology Into Therapeutic Strategies of Gastric Cancer Peritoneal Metastasis
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Wei Peng Yong
Consulting or Advisory Role: AbbVie/Genentech, Amgen, Bristol Myers Squibb, Ipsen, Novartis, AstraZeneca
Speakers' Bureau: Lilly, Sanofi/Aventis, Taiho Pharmaceutical, Eisai, Bayer, MSD Oncology
Travel, Accommodations, Expenses: Pfizer
Patrick Tan
Stock and Other Ownership Interests: Tempus Healthcare, Auristone Pte Ltd
Patents, Royalties, Other Intellectual Property: Patents related to cancer epigenetics
Raghav Sundar
Honoraria: MSD, Bristol Myers Squibb, Lilly, Roche, Taiho Pharmaceutical, AstraZeneca, AstraZeneca, DKSH
Consulting or Advisory Role: Bristol Myers Squibb, Eisai, Taiho Pharmaceutical, Bayer, Merck, Novartis, MSD
Research Funding: Paxman, MSD
Travel, Accommodations, Expenses: Roche, AstraZeneca, Taiho Pharmaceutical, Eisai
No other potential conflicts of interest were reported.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 [DOI] [PubMed] [Google Scholar]
- 2.Koemans WJ, Lurvink RJ, Grootscholten C, et al. : Synchronous peritoneal metastases of gastric cancer origin: Incidence, treatment and survival of a nationwide Dutch cohort. Gastric Cancer 24:800-809, 2021 [DOI] [PubMed] [Google Scholar]
- 3.Tan HL, Chia CS, Tan GH, et al. : Gastric peritoneal carcinomatosis—A retrospective review. World J Gastrointest Oncol 9:121-128, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen CJ, Blumenthaler AN, Das P, et al. : Staging laparoscopy and peritoneal cytology in patients with early stage gastric adenocarcinoma. World J Surg Oncol 18:39, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Sugarbaker PH, Van der Speeten K, Stuart OA: Pharmacologic rationale for treatments of peritoneal surface malignancy from colorectal cancer. World J Gastrointest Oncol 2:19-30, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang R, Song S, Harada K, et al. : Multiplex profiling of peritoneal metastases from gastric adenocarcinoma identified novel targets and molecular subtypes that predict treatment response. Gut 69:18-31, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sangisetty SL, Miner TJ: Malignant ascites: A review of prognostic factors, pathophysiology and therapeutic measures. World J Gastrointest Surg 4:87-95, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network : Gastric cancer (version 5.2021), 2021. https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf [DOI] [PubMed]
- 10.Smyth EC, Verheij M, Allum W, et al. : Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 27:v38-v49, 2016 [DOI] [PubMed] [Google Scholar]
- 11.National Health Commission of the People's Republic of China : Chinese guidelines for diagnosis and treatment of gastric cancer 2018 (English version). Chin J Cancer Res 31:707-737, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yajima K, Kanda T, Ohashi M, et al. : Clinical and diagnostic significance of preoperative computed tomography findings of ascites in patients with advanced gastric cancer. Am J Surg 192:185-190, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Kim SJ, Kim HH, Kim YH, et al. : Peritoneal metastasis: Detection with 16- or 64-detector row CT in patients undergoing surgery for gastric cancer. Radiology 253:407-415, 2009 [DOI] [PubMed] [Google Scholar]
- 14.De Vuysere S, Vandecaveye V, De Bruecker Y, et al. : Accuracy of whole-body diffusion-weighted MRI (WB-DWI/MRI) in diagnosis, staging and follow-up of gastric cancer, in comparison to CT: A pilot study. BMC Med Imaging 21:18, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandramohan A, Thrower A, Smith SA, et al. : “PAUSE”: A method for communicating radiological extent of peritoneal malignancy. Clin Radiol 72:972-980, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Dong D, Tang L, Li ZY, et al. : Development and validation of an individualized nomogram to identify occult peritoneal metastasis in patients with advanced gastric cancer. Ann Oncol 30:431-438, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwee RM, Kwee TC: Modern imaging techniques for preoperative detection of distant metastases in gastric cancer. World J Gastroenterol 21:10502-10509, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gertsen EC, Brenkman HJF, van Hillegersberg R, et al. : 18F-Fludeoxyglucose-positron emission tomography/computed tomography and laparoscopy for staging of locally advanced gastric cancer: A multicenter prospective Dutch cohort study (PLASTIC). JAMA Surg 156:e215340, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Japanese Gastric Cancer Association : Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 24:1-21, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voron T, Romain B, Bergeat D, et al. : Surgical management of gastric adenocarcinoma. Official expert recommendations delivered under the aegis of the French Association of Surgery (AFC). J Visc Surg 157:117-126, 2020 [DOI] [PubMed] [Google Scholar]
- 21.Ramos RF, Scalon FM, Scalon MM, et al. : Staging laparoscopy in gastric cancer to detect peritoneal metastases: A systematic review and meta-analysis. Eur J Surg Oncol 42:1315-1321, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Gretschel S, Siegel R, Estevez-Schwarz L, et al. : Surgical strategies for gastric cancer with synchronous peritoneal carcinomatosis. Br J Surg 93:1530-1535, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Fanelli MF, Silva MJ, de Paiva TF, Jr, et al. : Factors correlated with peritoneal carcinomatosis and survival in patients with gastric cancer treated at a single institution in Brazil. Int J Clin Oncol 14:326-331, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Thomassen I, van Gestel YR, van Ramshorst B, et al. : Peritoneal carcinomatosis of gastric origin: A population-based study on incidence, survival and risk factors. Int J Cancer 134:622-628, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Seyfried F, von Rahden BH, Miras AD, et al. : Incidence, time course and independent risk factors for metachronous peritoneal carcinomatosis of gastric origin—A longitudinal experience from a prospectively collected database of 1108 patients. BMC Cancer 15:73, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katai H, Ishikawa T, Akazawa K, et al. : Five-year survival analysis of surgically resected gastric cancer cases in Japan: A retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001-2007). Gastric Cancer 21:144-154, 2018 [DOI] [PubMed] [Google Scholar]
- 27.Honoré C, Goéré D, Messager M, et al. : Risk factors of peritoneal recurrence in eso-gastric signet ring cell adenocarcinoma: Results of a multicentre retrospective study. Eur J Surg Oncol 39:235-241, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Lee JH, Son SY, Lee CM, et al. : Factors predicting peritoneal recurrence in advanced gastric cancer: Implication for adjuvant intraperitoneal chemotherapy. Gastric Cancer 17:529-536, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Spolverato G, Ejaz A, Kim Y, et al. : Rates and patterns of recurrence after curative intent resection for gastric cancer: A United States multi-institutional analysis. J Am Coll Surg 219:664-675, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Ikoma N, Chen HC, Wang X, et al. : Patterns of initial recurrence in gastric adenocarcinoma in the era of preoperative therapy. Ann Surg Oncol 24:2679-2687, 2017 [DOI] [PubMed] [Google Scholar]
- 31.Mizrak Kaya D, Nogueras-Gonzalez GM, Harada K, et al. : Risk of peritoneal metastases in patients who had negative peritoneal staging and received therapy for localized gastric adenocarcinoma. J Surg Oncol 117:678-684, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasako M, Sano T, Yamamoto S, et al. : D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 359:453-462, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Deng J, Liang H, Wang D, et al. : Investigation of the recurrence patterns of gastric cancer following a curative resection. Surg Today 41:210-215, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Baron MA: Structure of the intestinal peritoneum in man. Am J Anat 69:439-497, 1941 [Google Scholar]
- 35.Cortés-Guiral D, Hübner M, Alyami M, et al. : Primary and metastatic peritoneal surface malignancies. Nat Rev Dis Primers 7:91, 2021 [DOI] [PubMed] [Google Scholar]
- 36.Kalluri R, Weinberg RA: The basics of epithelial-mesenchymal transition. J Clin Invest 119:1420-1428, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cristescu R, Lee J, Nebozhyn M, et al. : Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 21:449-456, 2015 [DOI] [PubMed] [Google Scholar]
- 38.Tanaka Y, Chiwaki F, Kojima S, et al. : Multi-omic profiling of peritoneal metastases in gastric cancer identifies molecular subtypes and therapeutic vulnerabilities. Nat Cancer 2:962-977, 2021 [DOI] [PubMed] [Google Scholar]
- 39.Xu R-H, Wang Z-Q, Shen L, et al. : S-1 plus oxaliplatin versus S-1 plus cisplatin as first-line treatment for advanced diffuse-type or mixed-type gastric/gastroesophageal junction adenocarcinoma: A randomized, phase 3 trial. J Clin Oncol 37, 2019. (abstr 4017) [Google Scholar]
- 40.Perrot-Applanat M, Vacher S, Pimpie C, et al. : Differential gene expression in growth factors, epithelial mesenchymal transition and chemotaxis in the diffuse type compared with the intestinal type of gastric cancer. Oncol Lett 18:674-686, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yonemura Y, Nojima N, Kaji M, et al. : E-cadherin and urokinase-type plasminogen activator tissue status in gastric carcinoma. Cancer 76:941-953, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Kurashige J, Hasegawa T, Niida A, et al. : Integrated molecular profiling of human gastric cancer identifies DDR2 as a potential regulator of peritoneal dissemination. Sci Rep 6:22371, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaetzel CS, Robinson JK, Chintalacharuvu KR, et al. : The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: A local defense function for IgA. Proc Natl Acad Sci USA 88:8796-8800, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong X, Song S, Li Y, et al. : Loss of ARID1A activates mTOR signaling and SOX9 in gastric adenocarcinoma-rationale for targeting ARID1A deficiency. Gut 71:467-478, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cancer Genome Atlas Research Network : Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513:202-209, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang R, Dang M, Harada K, et al. : Single-cell dissection of intratumoral heterogeneity and lineage diversity in metastatic gastric adenocarcinoma. Nat Med 27:141-151, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boopathy GTK, Hong W: Role of Hippo pathway-YAP/TAZ signaling in angiogenesis. Front Cell Dev Biol 7:49, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akhurst RJ, Hata A: Targeting the TGFbeta signalling pathway in disease. Nat Rev Drug Discov 11:790-811, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lv ZD, Na D, Liu FN, et al. : Induction of gastric cancer cell adhesion through transforming growth factor-beta1-mediated peritoneal fibrosis. J Exp Clin Cancer Res 29:139, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Grevenstein WM, Hofland LJ, Jeekel J, et al. : The expression of adhesion molecules and the influence of inflammatory cytokines on the adhesion of human pancreatic carcinoma cells to mesothelial monolayers. Pancreas 32:396-402, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Kersy O, Loewenstein S, Lubezky N, et al. : Omental tissue-mediated tumorigenesis of gastric cancer peritoneal metastases. Front Oncol 9:1267, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gurler Main H, Xie J, Muralidhar GG, et al. : Emergent role of the fractalkine axis in dissemination of peritoneal metastasis from epithelial ovarian carcinoma. Oncogene 36:3025-3036, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao L, Hu X, Zhang J, et al. : The role of the CCL22-CCR4 axis in the metastasis of gastric cancer cells into omental milky spots. J Transl Med 12:267, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cottone L, Capobianco A, Gualteroni C, et al. : Leukocytes recruited by tumor-derived HMGB1 sustain peritoneal carcinomatosis. Oncoimmunology 5:e1122860, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yonemura Y, Endou Y, Nojima M, et al. : A possible role of cytokines in the formation of peritoneal dissemination. Int J Oncol 11:349-358, 1997 [DOI] [PubMed] [Google Scholar]
- 56.Yasumoto K, Yamada T, Kawashima A, et al. : The EGFR ligands amphiregulin and heparin-binding egf-like growth factor promote peritoneal carcinomatosis in CXCR4-expressing gastric cancer. Clin Cancer Res 17:3619-3630, 2011 [DOI] [PubMed] [Google Scholar]
- 57.Pasquier J, Gosset M, Geyl C, et al. : CCL2/CCL5 secreted by the stroma induce IL-6/PYK2 dependent chemoresistance in ovarian cancer. Mol Cancer 17:47, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sjoquist KM, Espinoza D, Mileshkin L, et al. : REZOLVE (ANZGOG-1101): A phase 2 trial of intraperitoneal bevacizumab to treat symptomatic ascites in patients with chemotherapy-resistant, epithelial ovarian cancer. Gynecol Oncol 161:374-381, 2021 [DOI] [PubMed] [Google Scholar]
- 59.Jayne D: Molecular biology of peritoneal carcinomatosis. Cancer Treat Res 134:21-33, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Song XH, Chen XZ, Chen XL, et al. : Peritoneal metastatic cancer stem cells of gastric cancer with partial mesenchymal-epithelial transition and enhanced invasiveness in an intraperitoneal transplantation model. Gastroenterol Res Pract 2020:3256538, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kawamura T, Endo Y, Yonemura Y, et al. : Significance of integrin alpha2/beta1 in peritoneal dissemination of a human gastric cancer xenograft model. Int J Oncol 18:809-815, 2001 [PubMed] [Google Scholar]
- 62.Sugimoto A, Okuno T, Tsujio G, et al. : The clinicopathologic significance of Tks5 expression of peritoneal mesothelial cells in gastric cancer patients. PLoS One 16:e0253702, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ishimoto T, Miyake K, Nandi T, et al. : Activation of transforming growth factor beta 1 signaling in gastric cancer-associated fibroblasts increases their motility, via expression of Rhomboid 5 Homolog 2, and ability to induce invasiveness of gastric cancer cells. Gastroenterology 153:191-204.e16, 2017 [DOI] [PubMed] [Google Scholar]
- 64.Kumar V, Ramnarayanan K, Sundar R, et al. : Single-cell atlas of lineage states, tumor microenvironment and subtype-specific expression programs in gastric cancer. Cancer Discov 12:670-691, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee W, Ko SY, Mohamed MS, et al. : Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J Exp Med 216:176-194, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ceelen W, Ramsay RG, Narasimhan V, et al. : Targeting the tumor microenvironment in colorectal peritoneal metastases. Trends Cancer 6:236-246, 2020 [DOI] [PubMed] [Google Scholar]
- 67.Buechler MB, Kim KW, Onufer EJ, et al. : A stromal niche defined by expression of the transcription factor WT1 mediates programming and homeostasis of cavity-resident macrophages. Immunity 51:119-130.e5, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chow A, Schad S, Green MD, et al. : Tim-4(+) cavity-resident macrophages impair anti-tumor CD8(+) T cell immunity. Cancer Cell 39:973-988.e9, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sakamoto S, Kagawa S, Kuwada K, et al. : Intraperitoneal cancer-immune microenvironment promotes peritoneal dissemination of gastric cancer. Oncoimmunology 8:e1671760, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gerber SA, Rybalko VY, Bigelow CE, et al. : Preferential attachment of peritoneal tumor metastases to omental immune aggregates and possible role of a unique vascular microenvironment in metastatic survival and growth. Am J Pathol 169:1739-1752, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Melichar B, Freedman RS: Immunology of the peritoneal cavity: Relevance for host-tumor relation. Int J Gynecol Cancer 12:3-17, 2002 [DOI] [PubMed] [Google Scholar]
- 72.Northey JJ, Przybyla L, Weaver VM: Tissue force programs cell fate and tumor aggression. Cancer Discov 7:1224-1237, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nia HT, Munn LL, Jain RK: Mapping physical tumor microenvironment and drug delivery. Clin Cancer Res 25:2024-2026, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shimada H, Noie T, Ohashi M, et al. : Clinical significance of serum tumor markers for gastric cancer: A systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer 17:26-33, 2014 [DOI] [PubMed] [Google Scholar]
- 75.Shin SJ, Park S, Kim MH, et al. : Mesothelin expression is a predictive factor for peritoneal recurrence in curatively resected stage III gastric cancer. Oncologist 24:e1108-e1114, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kanda M, Shimizu D, Tanaka H, et al. : Significance of SYT8 for the detection, prediction, and treatment of peritoneal metastasis from gastric cancer. Ann Surg 267:495-503, 2018 [DOI] [PubMed] [Google Scholar]
- 77.Kanda M, Shimizu D, Tanaka H, et al. : Synaptotagmin XIII expression and peritoneal metastasis in gastric cancer. Br J Surg 105:1349-1358, 2018 [DOI] [PubMed] [Google Scholar]
- 78.Sawaki K, Kanda M, Miwa T, et al. : Troponin I2 as a specific biomarker for prediction of peritoneal metastasis in gastric cancer. Ann Surg Oncol 25:2083-2090, 2018 [DOI] [PubMed] [Google Scholar]
- 79.Kaji S, Irino T, Kusuhara M, et al. : Metabolomic profiling of gastric cancer tissues identified potential biomarkers for predicting peritoneal recurrence. Gastric Cancer 23:874-883, 2020 [DOI] [PubMed] [Google Scholar]
- 80.Zhang C, Li D, Yu R, et al. : Immune landscape of gastric carcinoma tumor microenvironment identifies a peritoneal relapse relevant immune signature. Front Immunol 12:651033, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen D, Liu Z, Liu W, et al. : Predicting postoperative peritoneal metastasis in gastric cancer with serosal invasion using a collagen nomogram. Nat Commun 12:179, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee IS, Lee H, Hur H, et al. : Transcriptomic profiling identifies a risk stratification signature for predicting peritoneal recurrence and micrometastasis in gastric cancer. Clin Cancer Res 27:2292-2300, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nakanishi K, Kanda M, Umeda S, et al. : The levels of SYT13 and CEA mRNAs in peritoneal lavages predict the peritoneal recurrence of gastric cancer. Gastric Cancer 22:1143-1152, 2019 [DOI] [PubMed] [Google Scholar]
- 84.Li Z, Zhang D, Zhang H, et al. : Prediction of peritoneal recurrence by the mRNA level of CEA and MMP-7 in peritoneal lavage of gastric cancer patients. Tumour Biol 35:3463-3470, 2014 [DOI] [PubMed] [Google Scholar]
- 85.Satoh Y, Mori K, Kitano K, et al. : Analysis for the combination expression of CK20, FABP1 and MUC2 is sensitive for the prediction of peritoneal recurrence in gastric cancer. Jpn J Clin Oncol 42:148-152, 2012 [DOI] [PubMed] [Google Scholar]
- 86.Ohzawa H, Saito A, Kumagai Y, et al. : Reduced expression of exosomal miR29s in peritoneal fluid is a useful predictor of peritoneal recurrence after curative resection of gastric cancer with serosal involvement. Oncol Rep 43:1081-1088, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee JH, Chang KK, Yoon C, et al. : Lauren histologic type is the most important factor associated with pattern of recurrence following resection of gastric adenocarcinoma. Ann Surg 267:105-113, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cunningham D, Allum WH, Stenning SP, et al. : Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355:11-20, 2006 [DOI] [PubMed] [Google Scholar]
- 89.Sasako M, Sakuramoto S, Katai H, et al. : Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 29:4387-4393, 2011 [DOI] [PubMed] [Google Scholar]
- 90.Noh SH, Park SR, Yang HK, et al. : Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 15:1389-1396, 2014 [DOI] [PubMed] [Google Scholar]
- 91.Al-Batran SE, Homann N, Pauligk C, et al. : Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 393:1948-1957, 2019 [DOI] [PubMed] [Google Scholar]
- 92.Kakeji Y, Yoshida K, Kodera Y, et al. : Three-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 plus docetaxel versus S-1 alone in stage III gastric cancer: JACCRO GC-07. Gastric Cancer 25:188-196, 2022 [DOI] [PubMed] [Google Scholar]
- 93.Shiozaki H, Elimova E, Slack RS, et al. : Prognosis of gastric adenocarcinoma patients with various burdens of peritoneal metastases. J Surg Oncol 113:29-35, 2016 [DOI] [PubMed] [Google Scholar]
- 94.Japanese Gastric Cancer Association : Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14:101-112, 2011 [DOI] [PubMed] [Google Scholar]
- 95.Jacquet P, Sugarbaker PH: Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 82:359-374, 1996 [DOI] [PubMed] [Google Scholar]
- 96.Hioki M, Gotohda N, Konishi M, et al. : Predictive factors improving survival after gastrectomy in gastric cancer patients with peritoneal carcinomatosis. World J Surg 34:555-562, 2010 [DOI] [PubMed] [Google Scholar]
- 97.Lee SD, Ryu KW, Eom BW, et al. : Prognostic significance of peritoneal washing cytology in patients with gastric cancer. Br J Surg 99:397-403, 2012 [DOI] [PubMed] [Google Scholar]
- 98.Cunningham D, Starling N, Rao S, et al. : Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358:36-46, 2008 [DOI] [PubMed] [Google Scholar]
- 99.Koizumi W, Narahara H, Hara T, et al. : S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet Oncol 9:215-221, 2008 [DOI] [PubMed] [Google Scholar]
- 100.Ajani JA, Rodriguez W, Bodoky G, et al. : Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: The FLAGS trial. J Clin Oncol 28:1547-1553, 2010 [DOI] [PubMed] [Google Scholar]
- 101.Bang YJ, Van Cutsem E, Feyereislova A, et al. : Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 376:687-697, 2010 [DOI] [PubMed] [Google Scholar]
- 102.Fuchs CS, Tomasek J, Yong CJ, et al. : Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 383:31-39, 2014 [DOI] [PubMed] [Google Scholar]
- 103.Wilke H, Muro K, Van Cutsem E, et al. : Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol 15:1224-1235, 2014 [DOI] [PubMed] [Google Scholar]
- 104.Shitara K, Van Cutsem E, Bang YJ, et al. : Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: The KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol 6:1571-1580, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Janjigian YY, Shitara K, Moehler M, et al. : First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 398:27-40, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jacquet P, Sugarbaker PH: Peritoneal-plasma barrier. Cancer Treat Res 82:53-63, 1996 [DOI] [PubMed] [Google Scholar]
- 107.Yonemura Y, Endou Y, Sasaki T, et al. : Surgical treatment for peritoneal carcinomatosis from gastric cancer. Eur J Surg Oncol 36:1131-1138, 2010 [DOI] [PubMed] [Google Scholar]
- 108.Sadeghi B, Arvieux C, Glehen O, et al. : Peritoneal carcinomatosis from non-gynecologic malignancies: Results of the EVOCAPE 1 multicentric prospective study. Cancer 88:358-363, 2000 [DOI] [PubMed] [Google Scholar]
- 109.Bozzetti F, Yu W, Baratti D, et al. : Locoregional treatment of peritoneal carcinomatosis from gastric cancer. J Surg Oncol 98:273-276, 2008 [DOI] [PubMed] [Google Scholar]
- 110.Solass W, Sempoux C, Detlefsen S, et al. : Peritoneal sampling and histological assessment of therapeutic response in peritoneal metastasis: Proposal of the peritoneal regression grading score (PRGS). Pleura Peritoneum 1:99-107, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shitara K, Doi T, Dvorkin M, et al. : Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 19:1437-1448, 2018 [DOI] [PubMed] [Google Scholar]
- 112.Kang YK, Boku N, Satoh T, et al. : Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390:2461-2471, 2017 [DOI] [PubMed] [Google Scholar]
- 113.Misawa K, Mochizuki Y, Sakai M, et al. : Randomized clinical trial of extensive intraoperative peritoneal lavage versus standard treatment for resectable advanced gastric cancer (CCOG 1102 trial). Br J Surg 106:1602-1610, 2019 [DOI] [PubMed] [Google Scholar]
- 114.Yang HK, Ji J, Han SU, et al. : Extensive peritoneal lavage with saline after curative gastrectomy for gastric cancer (EXPEL): A multicentre randomised controlled trial. Lancet Gastroenterol Hepatol 6:120-127, 2021 [DOI] [PubMed] [Google Scholar]
- 115.Guo J, Xu A, Sun X, et al. : Three-year outcomes of the randomized phase III SEIPLUS trial of extensive intraoperative peritoneal lavage for locally advanced gastric cancer. Nat Commun 12:6598, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Desiderio J, Chao J, Melstrom L, et al. : The 30-year experience—A meta-analysis of randomised and high-quality non-randomised studies of hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer. Eur J Cancer 79:1-14, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Glehen O, Passot G, Villeneuve L, et al. : GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: A randomized and multicenter phase III study. BMC Cancer 14:183, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bonnot PE, Piessen G, Kepenekian V, et al. : Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastases (CYTO-CHIP study): A propensity score analysis. J Clin Oncol 37:2028-2040, 2019 [DOI] [PubMed] [Google Scholar]
- 119.Bonnot PE, Lintis A, Mercier F, et al. : Prognosis of poorly cohesive gastric cancer after complete cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy (CYTO-CHIP study). Br J Surg 108:1225-1235, 2021 [DOI] [PubMed] [Google Scholar]
- 120.Rau B, Lang H, Königsrainer A, et al. : The effect of hyperthermic intraperitoneal chemotherapy (HIPEC) upon cytoreductive surgery (CRS) in gastric cancer (GC) with synchronous peritoneal metastasis (PM): A randomized multicentre phase III trial (GASTRIPEC-I-trial). Ann Oncol 32, 2021. (suppl 5; abstr 13760) [Google Scholar]
- 121.Glehen O, Gilly FN, Arvieux C, et al. : Peritoneal carcinomatosis from gastric cancer: A multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol 17:2370-2377, 2010 [DOI] [PubMed] [Google Scholar]
- 122.Yang XJ, Huang CQ, Suo T, et al. : Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: Final results of a phase III randomized clinical trial. Ann Surg Oncol 18:1575-1581, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kobayashi D, Kodera Y: Intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis. Gastric Cancer 20:111-121, 2017 [DOI] [PubMed] [Google Scholar]
- 124.Kono K, Yong WP, Okayama H, et al. : Intraperitoneal chemotherapy for gastric cancer with peritoneal disease: Experience from Singapore and Japan. Gastric Cancer 20:122-127, 2017 [DOI] [PubMed] [Google Scholar]
- 125.Kitayama J, Ishigami H, Yamaguchi H, et al. : Treatment of patients with peritoneal metastases from gastric cancer. Ann Gastroenterol Surg 2:116-123, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yu W, Whang I, Chung HY, et al. : Indications for early postoperative intraperitoneal chemotherapy of advanced gastric cancer: Results of a prospective randomized trial. World J Surg 25:985-990, 2001 [DOI] [PubMed] [Google Scholar]
- 127.Takahashi N, Kanda M, Yoshikawa T, et al. : A randomized phase II multicenter trial to explore efficacy of weekly intraperitoneal in comparison with intravenous paclitaxel administered immediately after gastrectomy to the patients with high risk of peritoneal recurrence: Final results of the INPACT trial. Gastric Cancer 21:1014-1023, 2018 [DOI] [PubMed] [Google Scholar]
- 128.Ishigami H, Tsuji Y, Shinohara H, et al. : Intraperitoneal chemotherapy as adjuvant or perioperative chemotherapy for patients with type 4 scirrhous gastric cancer: PHOENIX-GC2 trial. J Clin Med 10:5666, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ishigami H, Fujiwara Y, Fukushima R, et al. : Phase III trial comparing intraperitoneal and intravenous paclitaxel plus S-1 versus cisplatin plus S-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC trial. J Clin Oncol 36:1922-1929, 2018 [DOI] [PubMed] [Google Scholar]
- 130.Kitayama J, Ishigami H, Yamaguchi H, et al. : Salvage gastrectomy after intravenous and intraperitoneal paclitaxel (PTX) administration with oral S-1 for peritoneal dissemination of advanced gastric cancer with malignant ascites. Ann Surg Oncol 21:539-546, 2014 [DOI] [PubMed] [Google Scholar]
- 131.Chan DY, Syn NL, Yap R, et al. : Conversion surgery post-intraperitoneal paclitaxel and systemic chemotherapy for gastric cancer carcinomatosis peritonei. Are we ready? J Gastrointest Surg 21:425-433, 2017 [DOI] [PubMed] [Google Scholar]
- 132.Ishigami H, Yamaguchi H, Yamashita H, et al. : Surgery after intraperitoneal and systemic chemotherapy for gastric cancer with peritoneal metastasis or positive peritoneal cytology findings. Gastric Cancer 20:128-134, 2017 [DOI] [PubMed] [Google Scholar]
- 133.Alyami M, Hubner M, Grass F, et al. : Pressurised intraperitoneal aerosol chemotherapy: Rationale, evidence, and potential indications. Lancet Oncol 20:e368-e377, 2019 [DOI] [PubMed] [Google Scholar]
- 134.Garg PK, Jara M, Alberto M, et al. : The role of pressurized intraPeritoneal aerosol chemotherapy in the management of gastric cancer: A systematic review. Pleura Peritoneum 4:20180127, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tan HL, Kim G, Charles CJ, et al. : Safety, pharmacokinetics and tissue penetration of PIPAC paclitaxel in a swine model. Eur J Surg Oncol 47:1124-1131, 2021 [DOI] [PubMed] [Google Scholar]
- 136.Kim G, Tan HL, Sundar R, et al. : PIPAC-OX: A phase I study of oxaliplatin-based pressurized intraperitoneal aerosol chemotherapy in patients with peritoneal metastases. Clin Cancer Res 27:1875-1881, 2021 [DOI] [PubMed] [Google Scholar]
- 137.Alyami M, Bonnot PE, Mercier F, et al. : Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for unresectable peritoneal metastasis from gastric cancer. Eur J Surg Oncol 47:123-127, 2021 [DOI] [PubMed] [Google Scholar]
- 138.Alberto M, Brandl A, Garg PK, et al. : Pressurized intraperitoneal aerosol chemotherapy and its effect on gastric-cancer-derived peritoneal metastases: An overview. Clin Exp Metastasis 36:1-14, 2019 [DOI] [PubMed] [Google Scholar]
- 139.Oliver Goetze T, Al-Batran SE, Pabst U, et al. : Pressurized intraperitoneal aerosol chemotherapy (PIPAC) in combination with standard of care chemotherapy in primarily untreated chemo naive upper gi-adenocarcinomas with peritoneal seeding—A phase II/III trial of the AIO/CAOGI/ACO. Pleura Peritoneum 3:20180113, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Eveno C, Jouvin I, Pocard M: PIPAC EstoK 01: Pressurized intraPeritoneal aerosol chemotherapy with cisplatin and doxorubicin (PIPAC C/D) in gastric peritoneal metastasis: A randomized and multicenter phase II study. Pleura Peritoneum 3:20180116, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chicago Consensus Working Group : The Chicago consensus on peritoneal surface malignancies: Management of gastric metastases. Ann Surg Oncol 27:1768-1773, 2020 [DOI] [PubMed] [Google Scholar]
- 142.Muro K, Van Cutsem E, Narita Y, et al. : Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with metastatic gastric cancer: A JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol 30:19-33, 2019 [DOI] [PubMed] [Google Scholar]
- 143.Yeoh KG, Tan P: Mapping the genomic diaspora of gastric cancer. Nat Rev Cancer 22:71-84, 2022 [DOI] [PubMed] [Google Scholar]
- 144.Guideline Committee of the Korean Gastric Cancer Association (KGCA), Development Working Group and Review Panel : Korean practice guideline for gastric cancer 2018: An evidence-based, multi-disciplinary approach. J Gastric Cancer 19:1-48, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yonemura Y, Sako S, Wakama S, et al. : History of peritoneal surface malignancy treatment in Japan. Indian J Surg Oncol 10:3-11, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim DW, Jee YS, Kim CH, et al. : Multicenter retrospective analysis of intraperitoneal paclitaxel and systemic chemotherapy for advanced gastric cancer with peritoneal metastasis. J Gastric Cancer 20:50-59, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kwon J, Bakhoum SF: The cytosolic DNA-sensing cGAS-STING pathway in cancer. Cancer Discov 10:26-39, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Meric-Bernstam F, Larkin J, Tabernero J, et al. : Enhancing anti-tumour efficacy with immunotherapy combinations. Lancet 397:1010-1022, 2021 [DOI] [PubMed] [Google Scholar]
- 149.Abbasi SY, Taani HE, Saad A, et al. : Advanced gastric cancer in Jordan from 2004 to 2008: A study of epidemiology and outcomes. Gastrointest Cancer Res 4:122-127, 2011 [PMC free article] [PubMed] [Google Scholar]