Abstract
The incidence of early-onset (EO) GI cancers occurring in individuals younger than age 50 years has been rising at an alarming rate over the past two decades. Although this rise in incidence among young patients correlates with increased rates of obesity, changes in diet, and alterations in the environment, the effects of these environmental factors on carcinogenesis, metastasis, and treatment response are unknown. Although several unique clinical trends exist among EO-GI cancers and their average-onset GI cancer counterparts, GI cancers are molecularly indistinct between younger and older patients, and no data support distinct treatment paradigms for patients with EO disease. The majority of EO-GI cancers are not explained by germline changes. There remains a critical need for further research to understand the pathogenesis and optimal management of EO-GI cancers. In addition, current screening strategies are not adequate to identify EO-GI cancers, and early biomarkers are needed. Specialized centers, with a focus on psychosocial aspects of cancer management, can address the unique care needs of patients with EO-GI cancers.
INTRODUCTION
The incidence of early-onset (EO) GI cancers before age 50 years has been rising by 1%-2% annually since 20001,2; however, the etiology of this alarming rise in incidence and optimal management is unknown. Although EO-colorectal cancer (CRC) is recognized widely, rates of esophagogastric (EG), pancreatic ductal adenocarcinoma (PDAC), and appendiceal adenocarcinoma among individuals younger than age 50 years are also increasing. This rise in incidence of GI malignancies among young patients correlates with increased rates of obesity, changes in diet,3-12 and alterations in the environment,13-15 although how these environmental factors drive carcinogenesis, metastasis, and treatment response is not known. Thus, there is a critical need for further research to understand the pathogenesis and optimal management of EO-GI cancers.
KEY POINTS
The incidence of early-onset (EO) (age < 50) gastrointestinal (GI) cancers is rising in the United States and worldwide.
EO-GI cancers are clinically and molecularly similar to average-onset (AO)-GI cancers.
Patients with EO-GI cancers may not benefit from intensified therapy based on their age alone.
Specialized centers are needed to address the unique psychosocial and clinical care needs of young patients with GI cancers.
CONTEXT
Key Objective
The incidence of early-onset (EO; age < 50 years) GI cancers is rising in the United States and worldwide. Although providers are inclined to treat younger patients more aggressively, this practice is controversial. This review examines the epidemiologic, genetic, and molecular characteristics of EO-GI cancers compared with average-onset GI cancers and response to standard therapy.
Knowledge Generated
EO-GI cancers are predominantly sporadic, rather than inherited, and do not represent distinct disease entities when compared with average-onset GI cancers. More aggressive chemotherapy may not improve outcomes for patients with EO-GI cancers and is associated with increased toxicity. Patients with EO-GI cancers have unique psychosocial care needs compared with their older counterparts.
Relevance
Patients with EO-GI cancers may not benefit from intensified therapy on the basis of their age alone, and further research may help elucidate the etiology and optimal management of EO-GI cancers. Specialized centers are needed to address the unique psychosocial care needs of these young patients.
Various age cutoffs have been used to define early-onset cancers. On the basis of the historical recommendation by the US Preventive Services Task Force (USPSTF) to screen adults for CRC starting at age 50 years (now age 45 years since May 2021)16 and the American Society for Gastrointestinal Endoscopy recommendation to refer adults over age 50 years with new onset upper GI symptoms (dysphagia or odynophagia, persistent or recurrent reflux despite therapy, suspected chronic blood loss in the context of iron deficiency anemia) for endoscopy,17 we use an age cutoff of 50 years. However, several of the studies discussed here use a younger age cutoff, highlighting the fact that no universal definition of early onset exists and that the age of onset of GI cancers continues to decline.
Here, we review existing data examining the epidemiologic, genetic, and molecular characteristics of EO-GI cancers compared with average-onset (AO) GI cancers, with a focus on CRC, esophagogastric cancer (EGC), and PDAC, as these have been studied most extensively. We also examine response to standard therapy and survival in patients with EO-GI compared with AO-GI cancers and discuss the unique care needs of these patients. We conclude with areas of ongoing research and future directions to better understand the pathogenesis and optimal management of EO-GI cancers.
CRC
Epidemiology and Risk Factors
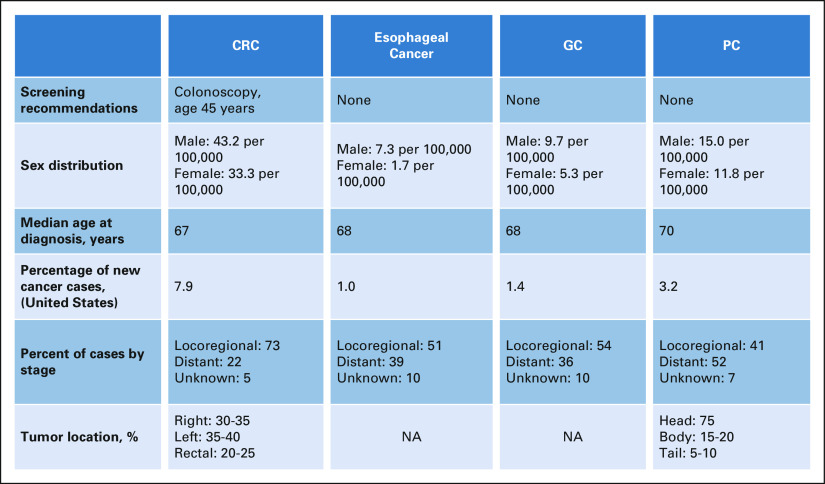
CRC is the third most common cause of cancer-related death in the United States and accounts for 8%-9% of newly diagnosed cancers18,19 (Fig 1). Although screening for CRC among adults age 50 years and older has dramatically reduced the incidence of CRC in this age group, the incidence of CRC overall has continued to increase, driven by the rise in CRC among those younger than age 50 years.18,21
FIG 1.
GI cancer disease characteristics. Overall disease characteristics of CRC, esophageal, GC, and PC in the United States are included. The data presented are from the SEER database.20 CRC, colorectal cancer; GC, gastric cancer; NA, not available; PC, pancreatic cancer.
With regard to race and sex distribution, several studies have demonstrated that the rising prevalence of EO-CRC is disproportionately affecting racial and ethnic minorities and has a male predominance. A study of more than 360,000 CRCs from the SEER and The Cancer Genome Atlas databases demonstrated that EO-CRC is more prevalent in Black (14.6% v 11.0%, P < .001) and Hispanic (14.7% v 8.3%, P < .001) individuals and slightly more prevalent in males (53.7% v 46.4%, P < .001).22 On the basis of the SEER database (1970s-2009), CRC in patients younger than age 50 years represents 6.7% of all CRCs in non-Hispanic White patients, compared with 11.9% in African Americans, 12% in Asians/Pacific Islanders, 15.4% in Hispanic patients, and 16.5% in American Indians/Alaska Natives.23 A 10-year SEER database study also compared Hispanic with White populations (2000-2010) and found that the overall incidence of CRC increased by 48% in Hispanic patients (compared with a decrease in White patients), and there was a 38% increase in incidence of late-onset (LO)-CRC and an 80% increase in EO-CRC.24
The etiology of EO-CRC remains unknown, and studies evaluating risk factors are small and somewhat conflicting. Known risk factors for CRC such as diet high in red meat and low fiber, obesity and excess alcohol consumption, physical inactivity, and smoking do not seem to apply to EO-CRC although some studies have described increased sugar‐sweetened drinks, increased TV watching, and low vitamin D as being potentially linked to EO-CRC.25-29
Germline Predisposition
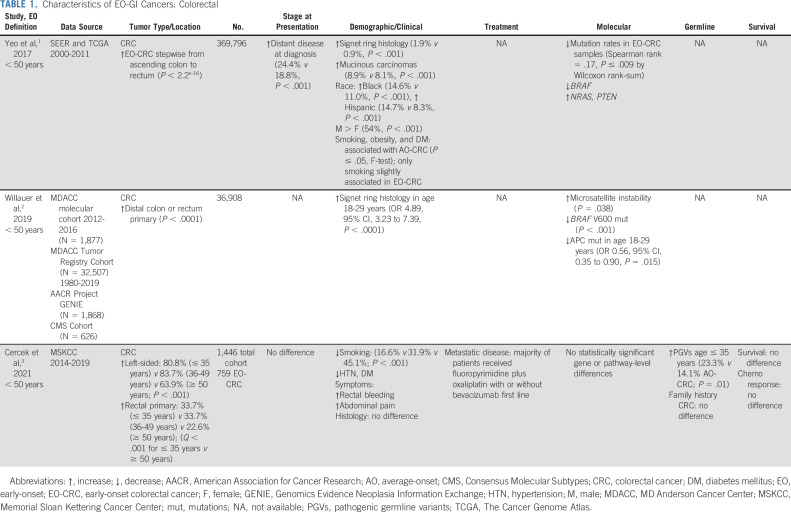
EO-CRC does have a relatively high prevalence of germline variants, and a higher proportion of patients with EO-CRC relative to those with AO-CRC will have mismatch repair deficiency (MMRd)/microsatellite instability (MSI-H) secondary to Lynch syndrome (15%-41% of cases).30-32 However, the rise in EO-CRC is not explained by an inherited genetic predisposition, as the majority of cases are sporadic. Furthermore, in our comprehensive retrospective review of 759 patients with EO-CRC,25 we found that EO-CRCs are more commonly left-sided and present with rectal bleeding and abdominal pain, in contrast to Lynch syndrome–associated CRC, which is most often right-sided. Although we observed the highest prevalence of high-penetrance germline variants in the very early-onset group (age ≤ 35 years; 23.3% compared with 14.1% of the AO cohort, P = .01), similar to the rate of mutations in high- and moderate-penetrance genes in previous publications,33 this is inadequate to explain the overall rise in EO-CRC.25
Clinical Presentation
EO-CRC in the United States and China is more often left-sided compared with AO-CRC, with more than one third of cases associated with rectal primary,22,25,34-38 although a retrospective study from Taiwan showed no difference in tumor location.31 Consistent with the tumor location, patients with EO-CRC are more likely to present with rectal bleeding and abdominal pain and less likely to present with anemia.25
Previous reports have demonstrated that younger patients tend to present with more advanced-stage CRC22,39 and that stage IV EO-CRC is associated with worse survival compared with stage IV AO-CRC36 although many studies do not account for delay in diagnosis.40 In addition, previous studies suggesting more aggressive disease biology were heterogeneous and did not distinguish microsatellite-stable from microsatellite-instable disease, which can present with poorly differentiated tumors that do not necessarily require more aggressive therapy.
Molecular Characteristics
Investigation into the molecular differences between EO-CRC and AO-CRC has produced conflicting results. Several studies have demonstrated decreased mutation rates in CRC samples with decreasing age,22 consistent with the increased proportion of genomically stable/signet ring cell subtype seen in younger patients.22,41 By contrast, in a pan-cancer study that profiled more than 6,000 cases of 14 cancer types, EO tumors were associated with increased chromosomal instability with fewer genomically stable cases although only 324 cases of colon adenocarcinoma were included.42 Although some data suggest that there are distinct genomic alterations in EO-CRC compared with AO-CRC (eg, decreased BRAF alteration frequency), these studies did not account for tumor sidedness and were conflicting with regard to RAS mutation frequency.22,41-43 Although an association between somatic mutations in PTEN has been associated with EO-CRC, this association was not present when the analysis was adjusted for the confounding effect of hypermutator phenotypes.42
In a retrospective analysis of 1,134 colorectal adenocarcinomas, the right-sided primary site in patients with metastatic CRC was associated with enrichment of oncogenic alterations in KRAS, BRAF, PIK3CA, AKT1, RNF43, and SMAD4, whereas left-sided tumors had no genetic alterations in mitogenic signaling, overall suggesting that the molecular alterations underlying tumorigenesis differ in right-sided compared with left-sided microsatellite-stable CRC.44 This is particularly relevant given the left-sided and specifically rectal subtype seen in EO-CRC. Thus, the predominance of left-sided tumors in previous molecular studies that did not account for sidedness likely explains the differences in tumor genomics between EO-CRC and AO-CRC. In our analysis, which to date represents the largest comprehensive clinical and genomic comparison of EO-CRC and AO-CRC, no significant differences in genomic alterations were noted once the analysis was adjusted for sidedness. The lack of difference was consistent even in the very young (age ≤ 35 years) subgroup. Overall, there are no convincing data to suggest that age inherently affects the molecular profile of these tumors although the left-sided predominance may be relevant in understanding the pathogenesis.
Treatment Recommendations and Outcomes
The above data outlining the disease biology in EO-CRC compared with AO-CRC are crucial in defining the optimal treatment for patients with EO-CRC to avoid overtreatment with increased toxicity and limited additional efficacy. Patients with EO-CRC are more likely to receive multiagent chemotherapy with combination fluoropyrimidine and oxaliplatin or irinotecan rather than single-agent chemotherapy with a fluoropyrimidine alone in the postoperative setting at all stages (I-IV) although this does not translate to improved stage-specific survival outcomes relative to those with AO-CRC who were more likely to receive single-agent chemotherapy.45,46
In fact, in the follow-up analyses from the IDEA study, patients with high-risk stage III EO-CRC (T4/N2) had significantly lower 3-year relapse-free survival (RFS; 54% v 65%, hazard ratio [HR] 1.33, P < .001) despite more intensive treatment and greater GI toxicity.46 On the basis of recently updated data from the IDEA database, when patients with EO-CRC and AO-CRC receive comparable treatment regimens for nonmetastatic disease, the overall rate of adverse events (AEs) is not significantly different although the profile of specific AEs is distinct.47 Among patients with stage II and III CRC receiving adjuvant combination fluoropyrimidine and oxaliplatin, the rate of GI symptoms of any grade was higher in the EO compared with the AO group (68.7% v 64.6%, P = .0436) driven by higher incidence of grade 1-2 nausea and vomiting. By contrast, the rate of hematologic toxicity of any grade was significantly higher in the AO group compared with the EO group (69.4% v 62.2%, P = .0002), with higher rates of febrile neutropenia (2.8% v 1.4%).
Similarly, in the CALGB/SWOG 80405 study of patients with metastatic CRC, those with EO-CRC were less likely to experience grade 3 or higher AEs compared with their older counterparts (30.7% v 43.8%, P < .001) and were less likely to experience grade 3 or higher neutropenia (24.7% v 33.6%, P < .001).48 Although they were also less likely to experience grade 3 or higher nausea (1.2% v 3.7%, P = .004), the overall rates of GI and hematologic toxicity of any grade were not reported.
Although some providers are inclined to intensify treatment with triplet chemotherapy in the metastatic setting, this strategy is associated with increased toxicity, and there are no data to support this practice. The TRIBE (triplet plus bevacizumab) study was a phase III, randomized, open-label, multicenter trial in which previously untreated patients age 18-75 years with unresectable metastatic CRC were randomly assigned to fluorouracil, leucovorin, oxaliplatin, and irinotecan plus bevacizumab or fluorouracil, leucovorin, and irinotecan plus bevacizumab.49,50 The triplet regimen was associated not only with significantly prolonged overall survival (OS; median 29.8 v 25.8, P = .03) but also with significantly higher incidences of grade 3 or higher neurotoxicity, stomatitis, diarrhea, and neutropenia.50 Given that this study did not include a subset analysis by age, it remains unclear whether the triplet regimen is specifically more effective for patients with EO-CRC. It is our practice to treat with either infusional fluorouracil, leucovorin, and oxaliplatin or fluorouracil, leucovorin, and irinotecan to reduce toxicity and reserve the third agent for the second-line setting, given similar efficacy in our experience.
The data regarding outcomes for patients with EO-CRC and AO-CRC treated with similar strategies remain somewhat unclear. Although the IDEA study demonstrated lower RFS, in an analysis of individual patient data of 35,713 patients with stage III colon cancer from 25 randomized studies in the Adjuvant Colon Cancer End point database (ACCENT), there was no significant difference in OS, DFS, or survival after recurrence between EO-CRC and AO-CRC after adjusting for molecular markers of tumor biology.51 Similarly, in the metastatic setting, CALGB/SWOG 80405 was a multicenter, randomized trial of first-line chemotherapy plus biologics in 2,326 patients with CRC, which demonstrated that there was no statistically significant difference in PFS or OS between patients with EO-CRC and AO-CRC.48 Consistently, these studies demonstrated that increased chemotherapy dosing and duration did not result in improved outcomes, but did lead to increased toxicity.
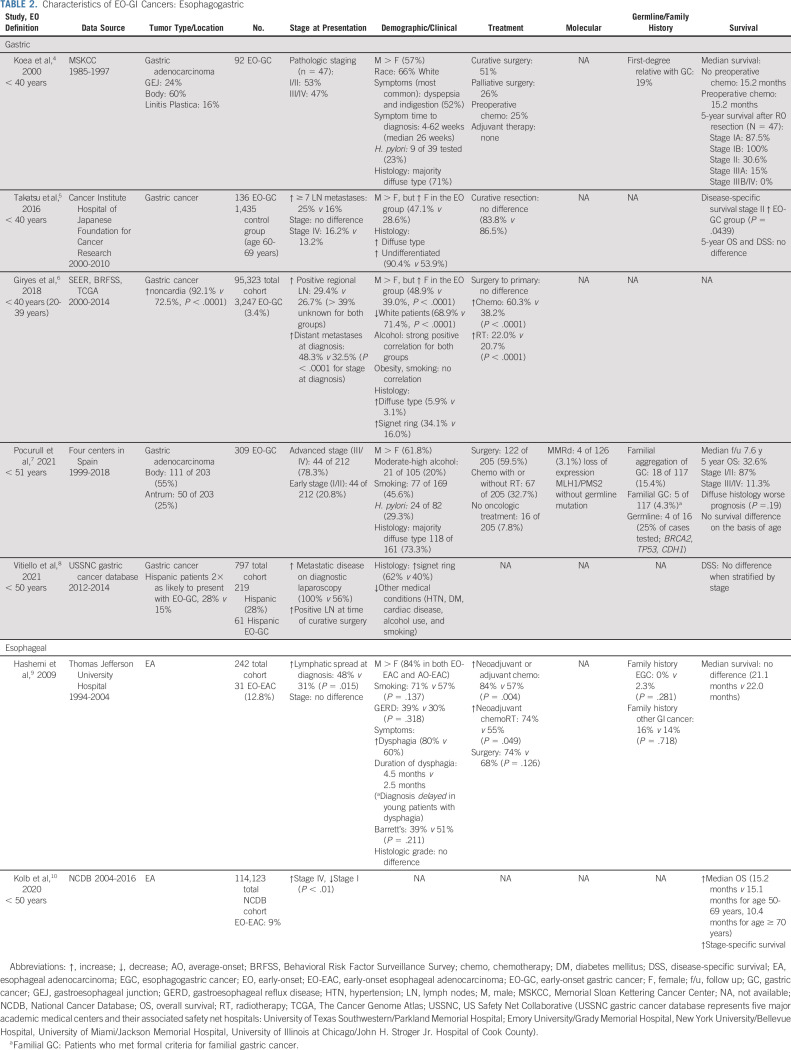
EGC
Epidemiology and Risk Factors
Gastric and esophageal cancers are the third and sixth most common causes of cancer-related mortality worldwide1,52 (Fig 1). The rates of gastric and esophageal adenocarcinoma (EA) among individuals younger than age 50 years have increased by 30% and 50%, respectively, over the past three decades.53,54 Although this trend follows the rising national incidence rates for esophageal cancer, the incidence of gastric cancer (GC) in the overall US population has decreased by 35% during this time period, suggesting an etiology that is specific to younger individuals. Among patients younger than age 50 years, the estimated number of deaths worldwide in 2018 from GC was > 65,000 and that from esophageal cancer was > 39,000.1,52
There is significant heterogeneity in the incidence of EGC by geographical location, with the highest incidence rates historically observed in Eastern Asia and in Eastern and Southern Africa; however, recently, there has been a marked increase in EA incidence rates in the United States and a few European countries, with a concurrent rise in EA among young patients.54-56 Younger patients tend to present with more advanced-stage disease,57-60 and there seems to be a lower ratio of males to females with EGC among those younger than age 30 years (ratio 0.85:1) compared with those over age 30 years (1.45:1).61
Previous data demonstrated that Hispanic patients are 50%-60% more likely to develop GC compared with non-Hispanic White patients,62,63 and in a recent study of 797 patients with GC in the US Safety Net Collaborative (2012-2014) retrospective cohort, 28% of patients were Hispanic. The Hispanic patients were twice as likely to present with EO-GC (age < 50 years; 28% v 15%).58 Similarly, in a study of 95,323 patients with GC, EO-GC was less prevalent in males (51.1% v 61.0%) and White patients (68.9 v 71.4%).57
The majority of EGCs are sporadic, and the increased incidence of GC among higher-income individuals and change in geographic distribution may suggest that the underlying etiology is multifactorial, with a role for both genetic susceptibility and obesity, gastroesophageal reflux disease (GERD), diet, chronic infection, and other environmental factors in the pathogenesis and response to treatment.56 A strong positive correlation has been noted for both EO-GC and AO-GC with heavy drinking, but no correlation between obesity or smoking in either age group has been noted.57
Several studies have identified a strong and statistically significant effect of birth cohort on EA incidence in the United States, interpreted as being because of changes in the prevalence of exposure to causal factors, which differ across successive generations.64-66 Previous studies have also suggested that obesity-induced inflammation, insulin resistance, and oxidative stress may be involved in carcinogenesis and that changes in adipocyte-derived mediators in obesity (increased leptin and decreased adiponectin) may independently increase the risk of Barrett's esophagus and EA.67-70 In a study of pooled individual participant data from eight population-based case-control studies within the international Barrett's and Esophageal Adenocarcinoma Consortium (BEACON) including 1,363 patients with EA, 1,472 patients with gastroesophageal junction (GEJ) adenocarcinoma, and 5,728 controls, EO-EA (age < 50 years) was strongly associated with GERD and body mass index (BMI) relative to older groups.71 Although similar age-specific associations in the GEJ group were also observed, these were not statistically significant.
With regard to infectious etiologies, in a study of 100 gastric tissue samples from patients who underwent gastric resection for adenocarcinoma in Grand Casablanca, Epstein Barr virus and Helicobacter pylori (H. pylori) coinfection was associated with younger age at diagnosis, although the cutoff was age 58 years, higher than that in most other studies.72 Further investigation into the tumoral genetics, patient-specific resident microbial environment, obesity trends, diet habits, and other environmental factors is necessary to better understand and halt this trend.
Germline Predisposition
Although the majority of EGCs are sporadic, GC can arise within the context of heritable cancer predisposition syndromes, including hereditary diffuse GC (associated with CDH1 mutation), hereditary breast and ovarian cancer (BRCA), and Lynch syndrome (MMRd), and patients with EO disease are more likely to have identifiable pathogenic germline variants (PGVs).
In a single-institution study of 515 patients with EGC (median age 59 years, range 18-87 years), including 243 patients with gastric, 111 patients with GEJ, and 161 with esophageal cancers, 81 patients (48 with GC, 16 with GEJ, and 17 with esophageal cancer) were found to have likely pathogenic (LP) or pathogenic (P) germline variants.73 Those with EO disease (age ≤ 50 years) were more likely to harbor LP/P germline variants compared with those with LO disease. Most notably, ATM LP/P variants occurred in six patients with EO-EGC compared with five patients with EO-EGC. In a smaller study in which only 16 patients underwent germline testing, mutations were found in four patients (25%, BRCA2, TP53, and CDH1).74 The high frequency of positive family history reported in young patients suggests that this is a high-risk population in which screening may be beneficial61 although given the significant heterogeneity and the likelihood that many LP/P variants are yet to be identified, it is important to consider family history as well.
Clinical Presentation
Several retrospective reviews have been published delineating the clinicopathologic characteristics that distinguish EO-EGC from AO-EGC although it remains unclear whether EO-EGC is a distinct disease entity. The reported rate of H. pylori among patients with EO-GC of 20%-25% is similar to that expected in the population.61 With regard to tumor location, younger patients tend to present with more distal gastric tumors rather than proximal/GEJ cancers.61
The majority of previous studies have included patients with EO-gastric rather than esophageal cancers (Tables 1-4). Nearly 80% of patients with EO-GC will be diagnosed with advanced-stage disease,74 and younger patients are more likely to have metastatic disease on diagnostic laparoscopy (100% v 56%) and to have pathologically positive lymph nodes at the time of curative-intent surgery.58 Most notably, the histology of EO-GC is distinct from that of AO-GC. The rates of poorly differentiated, Lauren diffuse type or signet ring subtype are higher among those with EO-GC (62%-73%; defined as age < 40 years or age < 50 years depending on the study) compared with those with AO-GC (50%-58%).58,61,74
TABLE 1.
Characteristics of EO-GI Cancers: Colorectal
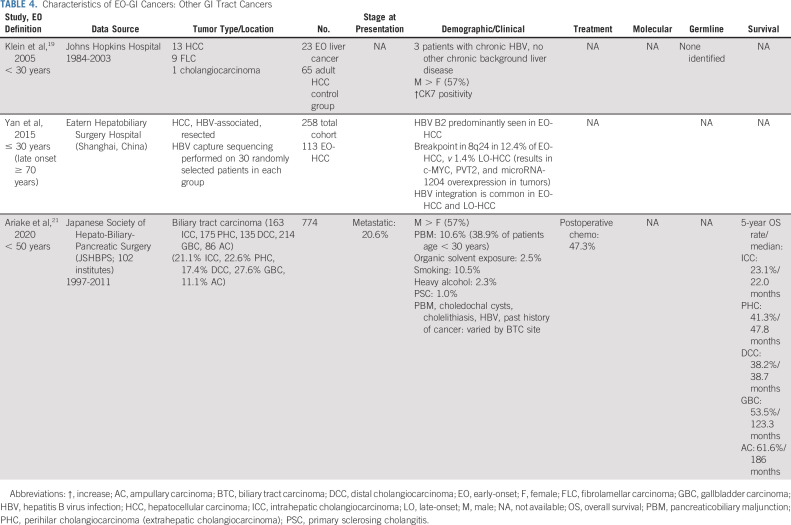
TABLE 4.
Characteristics of EO-GI Cancers: Other GI Tract Cancers
Presenting symptoms are less well defined, but several studies have attempted to characterize the duration of symptoms from onset to diagnosis and the specific symptoms that are more common among EO patients. In one study of 92 patients with EO-GC age 40 years or younger (prospective database of all patients with GC who presented to the Memorial Sloan Kettering Cancer Center [MSKCC] starting in 1985-2000), the duration of symptoms from onset to diagnosis ranged from 4 weeks to 62 weeks (median 26 weeks).61 In this cohort, the most common presenting symptoms were dyspepsia and indigestion (48 patients, 52%) and other common signs/symptoms were anemia, progressive weight loss, and decreased energy.
In a separate cohort of 242 cases of EA, including 31 with EO-EA (age < 50 years), the majority of patients were male and White, with no difference in gender distribution, smoking history, family history of cancer, or GERD between the EO and AO groups.59 There were no differences in primary tumor stage, but patients younger than age 50 years were more likely to have lymphatic spread at diagnosis (48% v 31%). Patients age ≤ 50 years were more likely to present with dysphagia (80% v 60%). There was no difference in median survival between the two groups although early endoscopic evaluation for younger patients with dysphagia may be indicated to avoid delays in diagnosis. However, the criteria that should prompt endoscopic evaluation in patients younger than age 50 years remain unknown.
Molecular Characteristics
Although MMRd/MSI-H is classically associated with Lynch syndrome, MMRd/MSI-H is uncommon among patients with EO-EGC.74,75 With regard to other immunohistochemical biomarkers, on the basis of the tissue microarray of 108 cases of EO-GC and 91 cases of AO-GC, EO-GCs have a significantly lower frequency of human epidermal growth factor receptor 2 (HER2) amplification (2%) and overexpression (0%) than AO-GCs (8% and 7%, respectively).76 Examining the molecular characteristics of EO-GC, there are several distinct features including losses of CDH1 and TP53 with corresponding aberrant protein staining, which may suggest that these genomic changes confer a functional role.75
Treatment Recommendations and Outcomes
Many studies are conflicting about age as a prognostic indicator for patients with EGC, with some citing better prognosis among younger patients and others reporting younger age at diagnosis as a negative prognostic marker. Most of these studies have also included patients with squamous cell carcinoma of the esophagus, whereas the trend toward increased incidence of esophageal cancer among young patients is specific to adenocarcinoma. Median survival time and disease-specific 5-year survival seem to be similar when adjusted for tumor stage.58,61
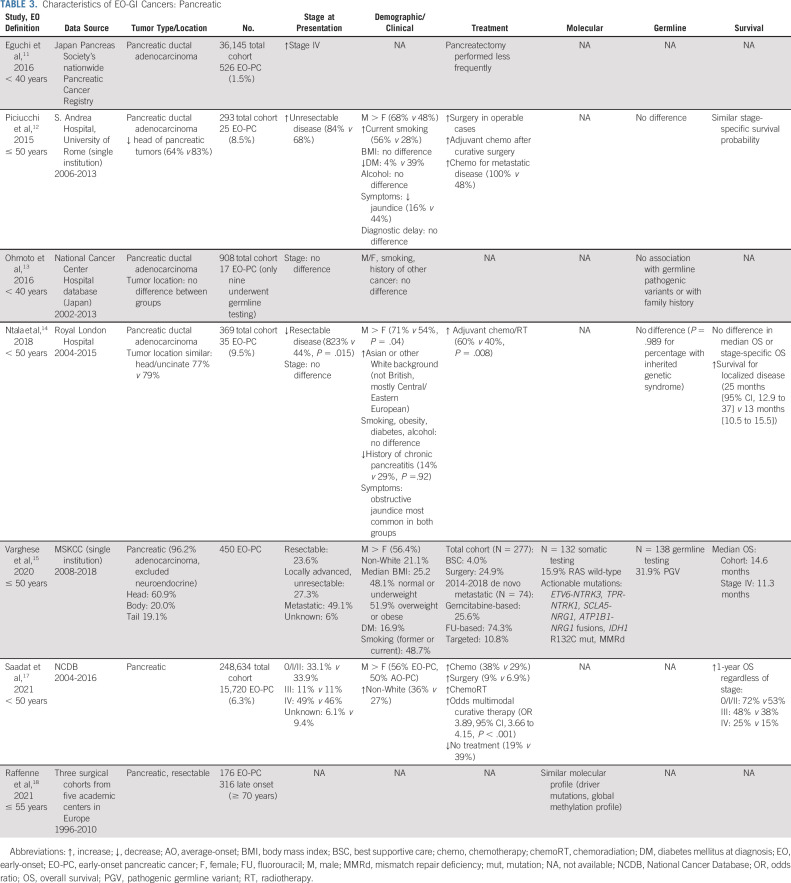
PANCREATIC CANCER
Pancreatic adenocarcinoma is the fifth most common cause of cancer death (CDC; Fig 1), and rates of pancreatic adenocarcinoma are rising in individuals younger than age 50 years with an estimated 1.6% annual increase in incidence on the basis of data from 2000 to 2017 (SEER) and 5%-10% of cases occurring among individuals younger than age 50 years.77
On the basis of the results of a large SEER database query that identified patients with pancreatic ductal adenocarcinoma during 2004-2016, EO-pancreatic cancer (PC) was associated with more advanced American Joint Committee on Cancer stage at diagnosis (P < .001) and poorer 5-year OS (6.1% v 8.6%), although with limitations in this retrospective large database analysis.78
It remains unclear whether the risk factors and tumor biology of EO-PC differ from those of AO-PC, because of a lack of data and only recent recognition of the trend. Although cigarette smoking,79,80 high alcohol intake, and familial genetic disorders81 have been proposed as primary risk factors for EO-PC, the reason for the recent increase in cases remains unclear. Similarly, previous studies have suggested that the molecular biology of EO-PC and LO-PC is similar although potential distinctive features such as lower rates of KRAS mutations are yet to be fully elucidated.82,83
Epidemiology and Risk Factors
Among patients with EO-PC compared with AO-PC, there are a higher proportion of males and a higher proportion of non-White, composed of a mix of Black, Asian, and Hispanic individuals.84,85 Data from the WHO mortality database from 1995 to 1999 also demonstrated a higher incidence of EO-PC in males and found that the highest PC rate ratios were found in Central/Eastern European countries (2.4-4.5 for males and 1.6-4.3 for females).80 Several other studies have corroborated the male predominance of EO-PC.79,84-86 However, a retrospective study of more than 280,000 cases of PC from the SEER database found that the average annual percentage change in incidence rate was significantly greater among women (1.93%) compared with men (0.77%) younger than 55 years (P = .002), indicating that although the incidence is higher among men, incidence rates are rising more rapidly among women in this age group.87
A significant correlation was identified between the PC rate ratio and early lung cancer mortality in the WHO database, suggesting, though not establishing, an association with smoking.80 This study was limited in its assumption that the male/female ratio was a surrogate measure of smoking prevalence. However, an association with former or current smoking has been demonstrated in other retrospective studies.79,86
With regard to other risk factors, there is no difference in BMI or alcohol use in patients with EO-PC compared with AO-PC,79,85,86 and the rates of pre-existing diabetes mellitus and chronic pancreatitis seem to be lower in patients with EO-PC.79,85
Clinical Presentation
A large cohort study in the United States noted no significant difference in distribution of stage at time of presentation,84 whereas a cohort study in Japan88 and smaller retrospective studies have noted increased rates of stage IV and/or unresectable disease at diagnosis among younger patients.79,85 In a single-institution study of EO-PC (N = 450) without an AO-PC comparison group, 23.6% of patients had resectable disease at diagnosis, compared with 27.3% of locally advanced or unresectable and 49.1% of metastatic at diagnosis.86
In a single-institution study at MSKCC (N = 450) including only 132 patients with EO-PC without a comparison group, 15.9% of cases were RAS wild-type by somatic mutation analysis compared with 5.4% of pancreatic ductal adenocarcinomas in the overall institutional cohort and 10% on the basis of The Cancer Genome Atlas for PDAC.89-91 Targetable somatic alterations were identified in eight of the patients, allowing four of these individuals to receive therapies targeted at MMRd, NTRK, HER2/3, and IDH1.86
In a retrospective study of three surgical cohorts from five academic centers in Europe of resectable PC in which 176 patients with EO-PC (defined as ≤ 55 years) were compared with 316 patients with LO-PC (defined as ≥ 70 years), a similar prevalence of driver mutations and global methylation profiles were observed in both groups.92
Germline Predisposition
Greater than 30% of patients with EO-PC have been demonstrated to have a PGV, the majority of which are BRCA 1/2 mutations (54.4%), followed by PALB2 (9.1%), ATM (6.8%), and CHEK2 (4.5%).86 Interestingly, in a single-institution study, patients with EO-PC and PGVs had a significantly reduced risk of all-cause mortality compared with those without a PGV (HR = 0.42). An increase in germline predisposition has not been established in other studies.79,85,93
Treatment Recommendations and Outcomes
On the basis of retrospective data in the United States, patients with EO-PC are more likely to receive treatment with chemotherapy, surgery, and/or chemoradiation compared with those with AO-PC and have improved 1-year OS regardless of stage.84 However, the specific type and duration of chemotherapy were not available from this study, and it remains unknown whether older individuals have more aggressive disease biology or instead are more likely to have poorer functional status and thus receive suboptimal therapy.
OTHER GI CANCERS
Despite a concurrent increase in the incidence of EO liver, biliary tract, appendiceal, and small bowel cancers, these have been less well studied. Similar to PC, EO-liver cases have been associated with a male predominance.94
In a retrospective analysis of 23 patients with EO (age < 30 years) hepatocellular carcinoma (HCC), fibrolamellar carcinoma (FLC), or intrahepatic cholangiocarcinoma compared with 65 patients in the AO (age > 40 years) HCC or hepatoblastoma control group, only three EO patients with typical HCC had chronic hepatitis B virus (HBV) infection and no other patients had background chronic liver disease, compared with 25 patients in the age > 40 years cohort with background liver disease.94
Considering biliary tract carcinoma (BTC), on the basis of the Japanese cancer registry (1975-2013), 3.3% of patients with gallbladder and bile duct cancer are younger than age 50 years.95 In a cohort of 774 patients with EO-BTC, a male predominance was again noted (57%).96 However, the ratio of females was higher in gallbladder cancer (1:1.25). Of the risk factors examined, pancreaticobiliary maljunction was present at the highest frequency (10.6% of cohort, 38.9% of patients < age 30 years). Although organic solvent exposure has been associated with biliary malignancies in Japan,97 only 2.5% of cases were associated with organic solvent exposure.
Although appendiceal cancer is rare, one study examined 1,652 cases diagnosed in patients younger than age 50 years from the SEER database (2000-2011) and characterized the clinical characteristics and survival among those with EO-appendiceal cancer.98 With regard to histology, the largest proportion of patients had mucinous appendiceal adenocarcinoma (34%) compared with nonmucinous appendiceal adenocarcinoma (17%), goblet cell (29%), carcinoid (13.8%), and signet ring cell (7%) carcinomas. There was a significant difference in outcomes on the basis of race, as non-Hispanic Black patients had significantly poorer OS at 5 years compared with non-Hispanic White patients (63% v 76%; 75% among Hispanic individuals; P = .001). However, this study did not directly compare patients with EO-appendiceal cancer and AO-appendiceal cancer.
The clinical presentation, molecular characteristics, risk of germline predisposition, and treatment recommendations among EO-HCC, EO-BTC, and EO-appendiceal cancer have not been previously described.
SPECIALIZED SUPPORT SERVICES
Psychosocial Needs
Several recent studies have defined the unique psychosocial burden and care needs affecting young adults with cancer. A multicenter cross-sectional survey across six hospitals in England found that the most common psychological issues were uncertainty about the future and fear of cancer recurrence; this study also highlighted the unmet need for support with changes in sexual function and relationships.99 It importantly found that patients who self-identified with these needs had lower health-related quality of life, more helplessness, and more unmet service needs. A challenge identified in this study and in others is the low response rate of young patients to these interventions,100 highlighting the need to identify more efficient and effective ways to engage young patients in available support services.
Fertility and Sexual Health
Treatment-related infertility is an increasing concern as patients with EO-GI cancers are more likely to be of reproductive potential compared with those over age 50 years. This is of particular concern for survivors of CRC who are at increased risk of infertility because of tumor location and the need for multimodality therapy. The results of an online, cross-sectional survey administered to 234 young adult survivors of CRC (age < 50 years) revealed that > 50% of respondents reported no discussion with their provider about fertility before treatment, 75% did not bank eggs/embryos or sperm, and > 20% reported that they did not know that fertility preservation was an option.101 On the basis of recent ASCO guidelines, sperm, embryo, and oocyte cryopreservation is a standard strategy for fertility preservation and should be discussed with patients and documented early in the treatment process.102
Targeted Interventions
An emerging model to address the needs of patients with EO-GI cancers is the establishment of a dedicated center focusing on ancillary services including social work, fertility, sexual health, and nutrition.100 The Center for Young Onset Colorectal and Gastrointestinal Cancers at the Memorial Sloan Kettering Cancer Center is the first of its kind worldwide and was established in 2018, on the basis of needs identified from patient and caregiver survey data from the Colorectal Cancer Alliance103 with two primary goals: (1) to meet the unique clinical needs of patients diagnosed with GI cancers before age 50 years and (2) to create the necessary infrastructure to study the etiology and distinct features of EO disease.100 The clinical infrastructure consists of a multidisciplinary support service, which includes a dedicated social worker who provides early psychosocial resources; integrated GI oncology, radiation oncology, and surgical consultation; sexual health and fertility; and genetic counseling. The research database includes a comprehensive risk assessment questionnaire, fecal microbiome analysis, somatic mutation analysis, and germline testing for all patients who choose to participate. Further research is needed to understand the appropriate timing and methods for incorporating these services into patient care.
FUTURE DIRECTIONS
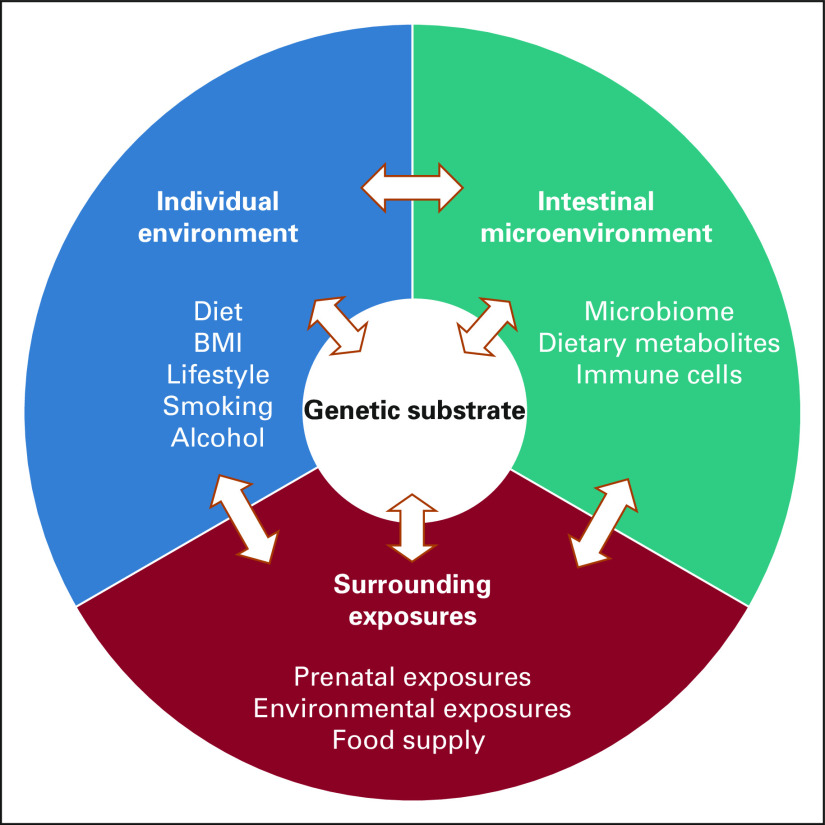
Patients with EO-GI cancers represent a group in which clinical differences exist, but among whom a distinct underlying pathway for tumorigenesis and thus specific biomarkers of disease and strategies for early detection are not yet defined (Fig 2).
FIG 2.
Schema representing mechanisms underlying early-onset GI cancers. BMI, body mass index.
Early Detection
Although the screening guidelines for CRC have recently changed to include patients age 45-49 years, we know that many patients are diagnosed with CRC before age 45 years and that early detection and screening programs for other GI cancers do not exist. This is largely since symptoms of other GI cancers are often nonspecific. One of the most important lessons learned from the above studies is that new and persistent symptoms in young patients should not be ignored and warrant further consideration and possible evaluation with imaging and/or endoscopy.59 Future directions in the arena of early detection may include use of artificial intelligence to risk stratify patients for screening on the basis of history and symptoms104 and exploration of the intestinal microbiome as a novel biomarker.
Intestinal Microenvironment
Several microbes including Fusobacterium nucleatum, Bacteroides fragilis, and pks + Escherichia coli105 have been identified as potential drivers of CRC although only a few associations have been linked specifically to EO-CRC.106,107 In addition, it is unclear if shifts in the microbial community structure are causative or an effect of the presence of the tumor. One large meta-analysis that analyzed gut microbiome differences in a data set of more than 2,500 individuals (age 20-89 years) demonstrated CRC-specific patterns in the gut microbiome that differed by age group and specifically noted that younger age groups tend to gain microbial taxa including F. nucleatum.108 In a small cohort that included 137 patients with EO-CRC and 278 with AO-CRC, the intratumoral microbiome alpha diversity was significantly higher in the EO group and the diversity of genera as measured by beta diversity was significantly different between groups although the investigators did not control for tumor sidedness.109 Further prospective data would be needed to confirm these associations.110
Several studies have raised the question of whether the microbiome is implicated in the pathogenesis of EGC and PC although have not specifically examined the microbiome in EO disease. Transformation of the microbiome has been identified from precursor states, such as reflux esophagitis and Barrett’s metaplasia, to a distinct profile in EA.111 With regard to gastric adenocarcinoma specifically, several studies have shown that Helicobacter pylori, in addition to directly promoting adenocarcinoma, changes the gastric mucosa into a hypochlorhydric environment, which allows specific other microbes to colonize the stomach,112 and several bacterial genera have been reported to be increased in patients with gastric adenocarcinoma.113 However, there is no evidence that eradication of bacteria other than H. pylori will have an impact on gastric adenocarcinoma progression and no trends have been identified among EO-EGCs compared with AO-EGCs. In PC, studies have identified a potential role for several microbes, including H. pylori, Enterobacter spp. Enterococcus spp., and the oral microbiota related to periodontal disease (P. gingivalis, Fusobacterium, N. elongate, and S. mitis) in the pathogenesis of pancreatic adenocarcinoma114 although again these have not been associated with age of onset.
Obesity and Diet
The effects of diet and obesity on EO-GI cancer risk and response to treatment to date have been underexplored. On the basis of data from the Nurses’ Health Study II (NHS II), obesity and weight gain are associated with an increased risk of EO-CRC among women, with a relative risk of 1.63 for women with a BMI of 23.0 kg/m2 or greater at age 18 years compared with those with a BMI of 18.5 to 20.9 kg/m2.115 Similarly, the relative risk for women gaining 40.0 kg or more was 2.15 compared with those who had gained < 5.0 kg or had lost weight. However, this study included only 114 cases of EO-CRC among the > 85,000 participants, and a causal relationship similarly has not been established. Obesity and body weight trajectories starting in young adulthood have also been associated with risk of esophageal and gastric cardia adenocarcinomas (GCA), although not specifically with development of these cancers before age 50 years.116 In a pooled analysis of two prospective cohort studies including 409,796 individuals, 633 with EA and 415 with GCA, overweight BMI (≥ 25-30 kg/m2) at age 20 years with subsequent obesity (BMI ≥ 30 kg/m2) by age 50 years was associated with increased risks of both EA and GCA.116 Further research is needed to assess the association of BMI and weight gain and EO-CRC in men and to elucidate the role in EO-EGC and EO-PC.
On the basis of a report from the Nurses’ Health Study, which included 70,450 individuals, participants who reported the behavior of eating at any time (presumed surrogate for unrestrained eating behavior) had an increased risk of digestive system cancer (1.22; 95% CI, 1.10 to 1.35) and of GI tract cancer (HR 1.33; 95% CI, 1.18 to 1.50) compared with those not reporting this behavior.117 In addition, on the basis of a combined analysis of 45,816 men (Health Professionals Follow-up Study, 1986-2012) and 74,191 women (Nurses’ Health Study, 1984-2012), there is a direct association between higher insulinemic potential of diet or lifestyle and the risk of developing digestive system cancers in men and women.118 However, these observations have not been proven to have a causal effect and appropriate strategies to modulate dietary intake to lower risk of EO-GI cancers and their progression are unknown.
Physical Activity
Sedentary TV viewing time has been used as a surrogate for sedentary behavior and was prospectively associated with EO-CRC, specifically rectal cancer, among women in the Nurses' Health Study II.27 Although this finding was independent of exercise and obesity, overweight and obese participants might have increased susceptibility to the effects of sedentary behavior, and the results might have been confounded by other unhealthy lifestyle factors or environmental effects that were not measured. In a population-based case-control study in Canada including 175 cases of EO-CRC, longer sedentary time (≥ 10 hours/day compared with < 5 hours/day) was associated with an increased risk of EO-CRC (odds ratio 1.93, 95% CI, 1.02 to 3.65).119 Given that previous studies have demonstrated that physical activity is associated with decreased risk of CRC, we do agree that a more active and less sedentary lifestyle is recommended although its benefit may not be specific to preventing EO-CRC.120,121 Although physical activity has also been associated with a decreased risk of EGC122 and PC,123 this association is not specific to EO-GI cancers and the optimal dose and intensity of exercise have not been defined.
In summary, although several unique features exist among EO-GI cancers compared with their AO-GI cancer counterparts, there are no data at this time to support distinct treatment paradigms for these patients. Specialized centers, with a focus on psychosocial aspects of cancer management, are essential to address the unique care needs of patients with EO-GI cancers (Fig 3).
FIG 3.
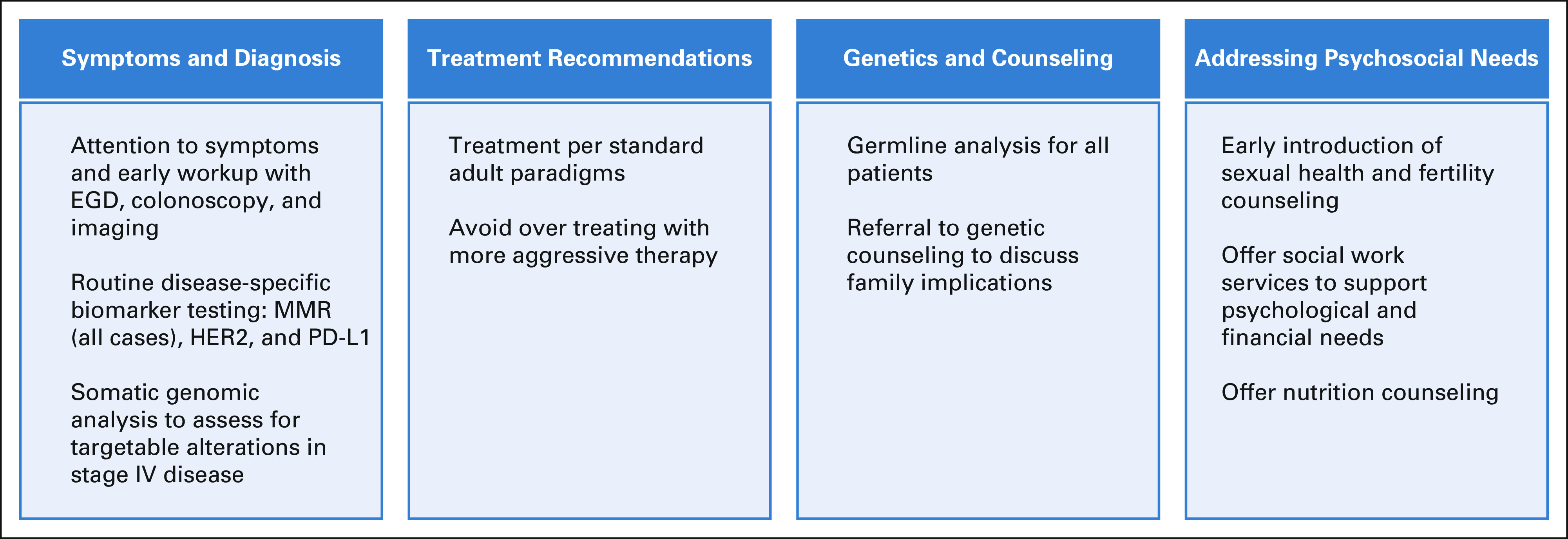
Practical considerations for treating patients with EO-GI cancers. EGD, esophagogastroduodenoscopy; EO, early-onset; HER2, human epidermal growth factor receptor 2; MMR, mismatch repair; PD-L1, programmed death-ligand 1.
TABLE 2.
Characteristics of EO-GI Cancers: Esophagogastric
TABLE 3.
Characteristics of EO-GI Cancers: Pancreatic
Andrea Cercek
Consulting or Advisory Role: Bayer, GlaxoSmithKline, Incyte, Merck, Janssen, Seattle Genetics
Research Funding: Seattle Genetics, Rgenix (Inst), GlaxoSmithKline
No other potential conflicts of interest were reported.
SUPPORT
Supported by NCI-P30 CA 008748.
AUTHOR CONTRIBUTIONS
Conception and design: Andrea Cercek
Collection and assembly of data: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Practical Considerations in Diagnosing and Managing Early-Onset GI Cancers
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Andrea Cercek
Consulting or Advisory Role: Bayer, GlaxoSmithKline, Incyte, Merck, Janssen, Seattle Genetics
Research Funding: Seattle Genetics, Rgenix (Inst), GlaxoSmithKline
No other potential conflicts of interest were reported.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results: SEER*Explorer Application. https://seer.cancer.gov/explorer/application.html?site=18&data_type=1&graph_type=2&compareBy=sex&chk_sex_1=1&chk_sex_3=3&chk_sex_2=2&race=1&age_range=9&stage=101&rate_type=2&advopt_precision=1&advopt_display=2#tableWrap [Google Scholar]
- 3. Cross AJ, Leitzmann MF, Gail MH, et al. A prospective study of red and processed meat intake in relation to cancer risk. PloS Med. 2007;4:e325. doi: 10.1371/journal.pmed.0040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gaard M, Tretli S, Løken EB. Dietary factors and risk of colon cancer: A prospective study of 50,535 young Norwegian men and women. Eur J Cancer Prev. 1996;5:445–454. [PubMed] [Google Scholar]
- 5. Larsson SC, Wolk A. Meat consumption and risk of colorectal cancer: A meta-analysis of prospective studies. Int J Cancer. 2006;119:2657–2664. doi: 10.1002/ijc.22170. [DOI] [PubMed] [Google Scholar]
- 6. Martínez ME, Jacobs ET, Ashbeck EL, et al. Meat intake, preparation methods, mutagens and colorectal adenoma recurrence. Carcinogenesis. 2007;28:2019–2027. doi: 10.1093/carcin/bgm179. [DOI] [PubMed] [Google Scholar]
- 7. Norat T, Lukanova A, Ferrari P, et al. Meat consumption and colorectal cancer risk: Dose-response meta-analysis of epidemiological studies. Int J Cancer. 2002;98:241–256. doi: 10.1002/ijc.10126. [DOI] [PubMed] [Google Scholar]
- 8. Shin A, Shrubsole MJ, Ness RM, et al. Meat and meat-mutagen intake, doneness preference and the risk of colorectal polyps: The Tennessee colorectal polyp study. Int J Cancer. 2007;121:136–142. doi: 10.1002/ijc.22664. [DOI] [PubMed] [Google Scholar]
- 9. Willett WC, Stampfer MJ, Colditz GA, et al. Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women. N Engl J Med. 1990;323:1664–1672. doi: 10.1056/NEJM199012133232404. [DOI] [PubMed] [Google Scholar]
- 10. Cross AJ, Ferrucci LM, Risch A, et al. A large prospective study of meat consumption and colorectal cancer risk: An investigation of potential mechanisms underlying this association. Cancer Res. 2010;70:2406–2414. doi: 10.1158/0008-5472.CAN-09-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benito E, Cabeza E, Moreno V, et al. Diet and colorectal adenomas: A case‐control study in Majorca. Int J Cancer. 1993;55:213–219. doi: 10.1002/ijc.2910550208. [DOI] [PubMed] [Google Scholar]
- 12. Bostick RM, Potter JD, McKenzie DR, et al. Reduced risk of colon cancer with high intake of vitamin E: The Iowa Women’s Health Study. Cancer Res. 1993;53:4230–4237. [PubMed] [Google Scholar]
- 13. Siegert S, Hampe J, Schafmayer C, et al. Genome-wide investigation of gene-environment interactions in colorectal cancer. Hum Genet. 2013;132:219–231. doi: 10.1007/s00439-012-1239-2. [DOI] [PubMed] [Google Scholar]
- 14. Hutter CM, Chang-Claude J, Slattery ML, et al. Characterization of gene-environment interactions for colorectal cancer susceptibility loci. Cancer Res. 2012;72:2036–2044. doi: 10.1158/0008-5472.CAN-11-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heavey PM, McKenna D, Rowland IR. Colorectal cancer and the relationship between genes and the environment. Nutr Cancer. 2004;48:124–141. doi: 10.1207/s15327914nc4802_2. [DOI] [PubMed] [Google Scholar]
- 16. US Preventive Services Task Force. Davidson KW, Barry MJ, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965–1977. doi: 10.1001/jama.2021.6238. [DOI] [PubMed] [Google Scholar]
- 17. Early DS, Early DS, Ben-Menachem T, et al. Appropriate use of GI endoscopy. Gastrointest Endosc. 2012;75:1127–1131. doi: 10.1016/j.gie.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 18. Shah RR, Millien VO, da Costa WL, et al. Trends in the incidence of early-onset colorectal cancer in all 50 United States from 2001 through 2017. Cancer. 2022;128:299–310. doi: 10.1002/cncr.33916. [DOI] [PubMed] [Google Scholar]
- 19. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 20.Surveillance, Epidemiology, and End Results (SEER): Cancer Stat Facts. https://seer.cancer.gov/statfacts/index.html [Google Scholar]
- 21. Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109:djw322. doi: 10.1093/jnci/djw322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yeo H, Betel D, Abelson JS, et al. Early-onset colorectal cancer is distinct from traditional colorectal cancer. Clin Colorectal Cancer. 2017;16:293–e6. doi: 10.1016/j.clcc.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 23. Rahman R, Schmaltz C, Jackson CS, et al. Increased risk for colorectal cancer under age 50 in racial and ethnic minorities living in the United States. Cancer Med. 2015;4:1863–1870. doi: 10.1002/cam4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koblinski J, Jandova J, Nfonsam V. Disparities in incidence of early- and late-onset colorectal cancer between Hispanics and Whites: A 10-year SEER database study. Am J Surg. 2018;215:581–585. doi: 10.1016/j.amjsurg.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 25. Cercek A, Chatila WK, Yaeger R, et al. A comprehensive comparison of early-onset and average-onset colorectal cancers. J Natl Cancer Inst. 2021;113:1683–1692. doi: 10.1093/jnci/djab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Siegel RL, Torre LA, Soerjomataram I, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019;68:2179–2185. doi: 10.1136/gutjnl-2019-319511. [DOI] [PubMed] [Google Scholar]
- 27. Nguyen LH, Liu PH, Zheng X, et al. Sedentary behaviors, TV viewing time, and risk of young-onset colorectal cancer. JNCI Cancer Spectr. 2018;2:pky073. doi: 10.1093/jncics/pky073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim H, Lipsyc-Sharf M, Zong X, et al. Total vitamin D intake and risks of early-onset colorectal cancer and precursors. Gastroenterology. 2021;161:1208–1217.e9. doi: 10.1053/j.gastro.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hur J, Otegbeye E, Joh HK, et al. Sugar-sweetened beverage intake in adulthood and adolescence and risk of early-onset colorectal cancer among women. Gut. 2021;70:2330–2336. doi: 10.1136/gutjnl-2020-323450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Silla IO, Rueda D, Rodríguez Y, et al. Early-onset colorectal cancer: A separate subset of colorectal cancer. World J Gastroenterol. 2014;20:17288–17296. doi: 10.3748/wjg.v20.i46.17288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liang JT, Huang KC, Cheng AL, et al. Clinicopathological and molecular biological features of colorectal cancer in patients less than 40 years of age. Br J Surg. 2003;90:205–214. doi: 10.1002/bjs.4015. [DOI] [PubMed] [Google Scholar]
- 32. Losi L, Di Gregorio C, Pedroni M, et al. Molecular genetic alterations and clinical features in early-onset colorectal carcinomas and their role for the recognition of hereditary cancer syndromes. Am J Gastroenterol. 2005;100:2280–2287. doi: 10.1111/j.1572-0241.2005.00223.x. [DOI] [PubMed] [Google Scholar]
- 33. Gupta S, Provenzale D, Regenbogen SE, et al. NCCN guidelines® insights: Genetic/Familial High-Risk Assessment: Colorectal, version 3.2017 featured updates to the NCCN Guidelines. J Natl Compr Cancer Netw. 2017;15:1465–1475. doi: 10.6004/jnccn.2017.0176. [DOI] [PubMed] [Google Scholar]
- 34. Meyer JE, Narang T, Schnoll-Sussman FH, et al. Increasing incidence of rectal cancer in patients aged younger than 40 years: An analysis of the surveillance, epidemiology, and end results database. Cancer. 2010;116:4354–4359. doi: 10.1002/cncr.25432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Glover M, Mansoor E, Panhwar M, et al. Epidemiology of colorectal cancer in average risk adults 20–39 years of age: A population-based national study. Dig Dis Sci. 2019;64:3602–3609. doi: 10.1007/s10620-019-05690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fu J, Yang J, Tan Y, et al. Young patients (≤ 35 years old) with colorectal cancer have worse outcomes due to more advanced disease: A 30-year retrospective review. Medicine (Baltimore) 2014;93:e135. doi: 10.1097/MD.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yantiss RK, Goodarzi M, Zhou XK, et al. Clinical, pathologic, and molecular features of early-onset colorectal carcinoma. Am J Surg Pathol. 2009;33:572–582. doi: 10.1097/PAS.0b013e31818afd6b. [DOI] [PubMed] [Google Scholar]
- 38. Chang DT, Pai RK, Rybicki LA, et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: An adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod Pathol. 2012;25:1128–1139. doi: 10.1038/modpathol.2012.61. [DOI] [PubMed] [Google Scholar]
- 39. Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150:17–22. doi: 10.1001/jamasurg.2014.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yarden RI, Newcomer KL, Board NTYA. Young onset colorectal cancer patients are diagnosed with advanced disease after multiple misdiagnoses. Cancer Res. 2019;79 abstr 3347. [Google Scholar]
- 41. Willauer AN, Liu Y, Pereira AAL, et al. Clinical and molecular characterization of early-onset colorectal cancer. Cancer. 2019;125:2002–2010. doi: 10.1002/cncr.31994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee W, Wang Z, Saffern M, et al. Genomic and molecular features distinguish young adult cancer from later-onset cancer. Cell Rep. 2021;37:110005. doi: 10.1016/j.celrep.2021.110005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lieu CH, Golemis EA, Serebriiskii IG, et al. Comprehensive genomic landscapes in early and later onset colorectal cancer. Clin Cancer Res. 2019;25:5852–5858. doi: 10.1158/1078-0432.CCR-19-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yaeger R, Chatila WK, Lipsyc MD, et al. Clinical sequencing defines the genomic landscape of metastatic colorectal cancer. Cancer Cell. 2018;33:125–e3. doi: 10.1016/j.ccell.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kneuertz PJ, Chang GJ, Hu CY, et al. Overtreatment of young adults with colon cancer: More intense treatments with unmatched survival gains. JAMA Surg. 2015;150:402–409. doi: 10.1001/jamasurg.2014.3572. [DOI] [PubMed] [Google Scholar]
- 46. Fontana E, Meyers JP, Sobrero AF, et al. Early-onset stage II/III colorectal adenocarcinoma in the IDEA database: Treatment adherence, toxicities, and outcomes from adjuvant fluoropyrimidine and oxaliplatin. J Clin Oncol. 2021;39 doi: 10.1200/JCO.21.02008. suppl; abstr 3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fontana E, Meyers J, Sobrero A, et al. Early-onset colorectal adenocarcinoma in the IDEA database: Treatment adherence, toxicities, and outcomes with 3 and 6 months of adjuvant fluoropyrimidine and oxaliplatin. J Clin Oncol. 2021;39:4009–4019. doi: 10.1200/JCO.21.02008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lipsyc-Sharf M, Zhang S, Ou FS, et al. Survival in young-onset metastatic colorectal cancer: Findings from Cancer and Leukemia Group B (Alliance)/SWOG 80405. J Natl Cancer Inst. 2022;114:427–435. doi: 10.1093/jnci/djab200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16:1306–1315. doi: 10.1016/S1470-2045(15)00122-9. [DOI] [PubMed] [Google Scholar]
- 50. Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609–1618. doi: 10.1056/NEJMoa1403108. [DOI] [PubMed] [Google Scholar]
- 51. Jin Z, Dixon JG, Fiskum JM, et al. Clinicopathological and molecular characteristics of early-onset stage III colon adenocarcinoma: An analysis of the ACCENT database. J Natl Cancer Inst. 2021;113:1693–1704. doi: 10.1093/jnci/djab123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 53.Howlader N, Noone AM, Krapcho M, et al. Cancer Statistics Review, 1975-2017—SEER Statistics. SEER Cancer Statistics Review, 1975-2017. Bethesda, MD: National Cancer Institute; 2019. [Google Scholar]
- 54. Islami F, DeSantis CE, Jemal A. Incidence trends of esophageal and gastric cancer subtypes by race, ethnicity, and age in the United States, 1997–2014. Clin Gastroenterol Hepatol. 2019;17:429–439. doi: 10.1016/j.cgh.2018.05.044. [DOI] [PubMed] [Google Scholar]
- 55. Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2–9. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 56. Gupta B, Kumar N. Worldwide incidence, mortality and time trends for cancer of the oesophagus. Eur J Cancer Prev. 2017;26:107–118. doi: 10.1097/CEJ.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 57. Giryes A, Oweira H, Mannhart M, et al. Exploring the differences between early-onset gastric cancer and traditional-onset gastric cancer. J Gastrointest Oncol. 2018;9:1157–1163. doi: 10.21037/jgo.2018.06.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vitiello GA, Hani L, Wang A, et al. Clinical presentation patterns and survival outcomes of Hispanic patients with gastric cancer. J Surg Res. 2021;268:606–615. doi: 10.1016/j.jss.2021.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hashemi N, Loren D, DiMarino AJ, et al. Presentation and prognosis of esophageal adenocarcinoma in patients below age 50. Dig Dis Sci. 2008;548:1708–1712. doi: 10.1007/s10620-008-0565-7. [DOI] [PubMed] [Google Scholar]
- 60. Kolb JM, Han S, Scott FI, et al. Early-onset esophageal adenocarcinoma presents with advanced-stage disease but has improved survival compared with older individuals. Gastroenterology. 2020;159:2238–2240.e4. doi: 10.1053/j.gastro.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Koea JB, Karpeh MS, Brennan MF. Gastric cancer in young patients: Demographic, clinicopathological, and prognostic factors in 92 patients. Ann Surg Oncol. 2000;7:346–351. doi: 10.1007/s10434-000-0346-9. [DOI] [PubMed] [Google Scholar]
- 62. Sanjeevaiah A, Cheedella N, Hester C, et al. Gastric cancer: Recent molecular classification advances, racial disparity, and management implications. JCO Oncol Pract. 2018;14:217–224. doi: 10.1200/JOP.17.00025. [DOI] [PubMed] [Google Scholar]
- 63.American Cancer Society: Cancer Facts & Figures for Hispanics/Latinos 2018-2020. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-facts-and-figures-for-hispanics-and-latinos
- 64. Thrift AP, Whiteman DC. The incidence of esophageal adenocarcinoma continues to rise: Analysis of period and birth cohort effects on recent trends. Ann Oncol. 2012;23:3155–3162. doi: 10.1093/annonc/mds181. [DOI] [PubMed] [Google Scholar]
- 65. Zheng T, Mayne ST, Holford TR, et al. Time trend and age-period-cohort effects on incidence of esophageal cancer in Connecticut, 1935-89. Cancer Causes Control. 1992;3:481–492. doi: 10.1007/BF00051361. [DOI] [PubMed] [Google Scholar]
- 66. Olsen CM, Pandeya N, Green AC, et al. Population attributable fractions of adenocarcinoma of the esophagus and gastroesophageal junction. Am J Epidemiol. 2011;174:582–590. doi: 10.1093/aje/kwr117. [DOI] [PubMed] [Google Scholar]
- 67. Kendall BJ, Macdonald GA, Hayward NK, et al. Leptin and the risk of Barrett’s oesophagus. Gut. 2008;57:448–454. doi: 10.1136/gut.2007.131243. [DOI] [PubMed] [Google Scholar]
- 68. Garcia JM, Splenser AE, Kramer J, et al. Circulating inflammatory cytokines and adipokines are associated with increased risk of Barrett’s esophagus: A case-control study. Clin Gastroenterol Hepatol. 2014;12:229–e3. doi: 10.1016/j.cgh.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Duggan C, Onstad L, Hardikar S, et al. Association between markers of obesity and progression from Barrett’s esophagus to esophageal adenocarcinoma. Clin Gastroenterol Hepatol. 2013;11:934–943. doi: 10.1016/j.cgh.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Long E, Beales IL. The role of obesity in oesophageal cancer development. Therap Adv Gastroenterol. 2014;7:247–268. doi: 10.1177/1756283X14538689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Drahos J, Xiao Q, Risch HA, et al. Age-specific risk factor profiles of adenocarcinomas of the esophagus: A pooled analysis from the international BEACON consortium. Int J Cancer. 2016;138:55–64. doi: 10.1002/ijc.29688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rihane FE, Erguibi D, Elyamine O, et al. Helicobacter pylori co-infection with Epstein-Barr virus and the risk of developing gastric adenocarcinoma at an early age: Observational study infectious agents and cancer. Ann Med Surg (Lond) 2021;68:102651. doi: 10.1016/j.amsu.2021.102651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ku GY, Kemel Y, Maron SB, et al. Prevalence of germline alterations on targeted tumor-normal sequencing of esophagogastric cancer. JAMA Netw Open. 2021;4:e2114753. doi: 10.1001/jamanetworkopen.2021.14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pocurull A, Herrera-Pariente C, Carballal S, et al. Clinical, molecular and genetic characteristics of early onset gastric cancer: Analysis of a large multicenter study. Cancers (Basel) 2021;13:3132. doi: 10.3390/cancers13133132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Carvalho R, Milne AN, van Rees BP, et al. Early-onset gastric carcinomas display molecular characteristics distinct from gastric carcinomas ccurring at a later age. J Pathol. 2004;204:75–83. doi: 10.1002/path.1602. [DOI] [PubMed] [Google Scholar]
- 76. Moelans CB, Milne AN, Morsink FH, et al. Low frequency of HER2 amplification and overexpression in early onset gastric cancer. Cell Oncol (Dordr) 2011;34:89–95. doi: 10.1007/s13402-011-0021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:197–209. doi: 10.1016/j.bpg.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 78. Ansari D, Althini C, Ohlsson H, et al. Early-onset pancreatic cancer: A population-based study using the SEER registry. Langenbecks Arch Surg. 2019;404:565–571. doi: 10.1007/s00423-019-01810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Piciucchi M, Capurso G, Valente R, et al. Early onset pancreatic cancer: Risk factors, presentation and outcome. Pancreatology. 2015;15:151–155. doi: 10.1016/j.pan.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 80. Raimondi S, Maisonneuve P, Löhr JM, et al. Early onset pancreatic cancer: Evidence of a major role for smoking and genetic factors. Cancer Epidemiol Biomarkers Prev. 2007;16:1894–1897. doi: 10.1158/1055-9965.EPI-07-0341. [DOI] [PubMed] [Google Scholar]
- 81. McWilliams RR, Maisonneuve P, Bamlet WR, et al. Risk factors for early-onset and very-early-onset pancreatic adenocarcinoma: A Pancreatic Cancer Case-Control Consortium (PanC4) analysis. Pancreas. 2016;45:311–316. doi: 10.1097/MPA.0000000000000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bergmann F, Aulmann S, Wente MN, et al. Molecular characterisation of pancreatic ductal adenocarcinoma in patients under 40. J Clin Pathol. 2006;59:580–584. doi: 10.1136/jcp.2005.027292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lüttges J, Stigge C, Pacena M, et al. Rare ductal adenocarcinoma of the pancreas in patients younger than age 40 years: An analysis of its features and a literature review. Cancer. 2004;100:173–182. doi: 10.1002/cncr.11860. [DOI] [PubMed] [Google Scholar]
- 84. Saadat LV, Chou JF, Gonen M, et al. Treatment patterns and survival in patients with early-onset pancreatic cancer. Cancer. 2021;127:3566–3578. doi: 10.1002/cncr.33664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ntala C, Debernardi S, Feakins RM, et al. Demographic, clinical, and pathological features of early onset pancreatic cancer patients. BMC Gastroenterol. 2018;18:139. doi: 10.1186/s12876-018-0866-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Varghese AM, Singh I, Singh R, et al. Early-onset pancreas cancer: Clinical descriptors, genomics, and outcomes. J Natl Cancer Inst. 2021;113:1194–1202. doi: 10.1093/jnci/djab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gaddam S, Abboud Y, Oh J, et al. Incidence of pancreatic cancer by age and sex in the US, 2000-2018. JAMA. 2021;326:2075–2077. doi: 10.1001/jama.2021.18859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Eguchi H, Yamaue H, Unno M, et al. Clinicopathological characteristics of young patients with pancreatic cancer. Pancreas. 2016;45:1411–1417. doi: 10.1097/MPA.0000000000000636. [DOI] [PubMed] [Google Scholar]
- 89. Lowery MA, Jordan EJ, Basturk O, et al. Real-time genomic profiling of pancreatic ductal adenocarcinoma: Potential actionability and correlation with clinical phenotype. Clin Cancer Res. 2017;23:6094–6100. doi: 10.1158/1078-0432.CCR-17-0899. [DOI] [PubMed] [Google Scholar]
- 90. Biankin AV, Waddell N, Kassahn KS, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Raffenne J, Martin FA, Nicolle R, et al. Pancreatic ductal adenocarcinoma arising in young and old patients displays similar molecular features. Cancers (Basel) 2021;13:1234. doi: 10.3390/cancers13061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ohmoto A, Yachida S, Kubo E, et al. Clinicopathologic features and germline sequence variants in young patients (≤40 years old) with pancreatic ductal adenocarcinoma. Pancreas. 2016;45:1056–1061. doi: 10.1097/MPA.0000000000000574. [DOI] [PubMed] [Google Scholar]
- 94. Klein WM, Molmenti EP, Colombani PM, et al. Primary liver carcinoma arising in people younger than 30 years. Am J Clin Pathol. 2005;124:512–518. doi: 10.1309/TT0R7KAL32228E99. [DOI] [PubMed] [Google Scholar]
- 95. Hori M, Matsuda T, Shibata A, et al. Cancer incidence and incidence rates in Japan in 2009: A study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2015;45:884–891. doi: 10.1093/jjco/hyv088. [DOI] [PubMed] [Google Scholar]
- 96. Ariake K, Unno M, Yoshida H, et al. Risk factors and characteristics of young patients with the biliary tract carcinoma: Results of a project study for biliary surgery by the Japanese Society of Hepato‐Biliary‐Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2020;27:571–580. doi: 10.1002/jhbp.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kumagai S, Kurumatani N, Arimoto A, et al. Cholangiocarcinoma among offset colour proof-printing workers exposed to 1,2-dichloropropane and/or dichloromethane. Occup Environ Med. 2013;70:508–510. doi: 10.1136/oemed-2012-101246. [DOI] [PubMed] [Google Scholar]
- 98. Holowatyj AN, Washington KM, Salaria SN, et al. Early-onset appendiceal cancer survival by race or ethnicity in the United States. Gastroenterology. 2020;159:1605–1608. doi: 10.1053/j.gastro.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lidington E, Giesinger JM, Janssen SHM, et al. Describing unmet supportive care needs among young adults with cancer (25–39 years) and the relationship with health-related quality of life, psychological distress, and illness cognitions. J Clin Med. 2021;10:4449. doi: 10.3390/jcm10194449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mendelsohn R, Palmaira RL, Lumish M, et al. A coordinated clinical center for young onset colorectal cancer. Oncologist. 2021;26:625–629. doi: 10.1002/onco.13849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Stal J, Yi S, Cohen-Cutler S, et al. Prevalence of fertility discussions between young adult colorectal cancer survivors and their providers J Clin Oncol 39 3518 3518 2021. 34506232 [Google Scholar]
- 102. Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500–2510. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Colorectal Cancer Alliance: Reversing the trend of earlier onset. 2017 Young Onset Report | Colorectal Cancer Alliance, 2018. https://www.ccalliance.org/about/never-too-young/survey/2017-young-onset-colorectal-cancer-survey-report [Google Scholar]
- 104. Kenner B, Chari ST, Kelsen D, et al. Artificial intelligence and early detection of pancreatic cancer: 2020 summative review. Pancreas. 2021;50:251–279. doi: 10.1097/MPA.0000000000001762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Brennan CA, Garrett WS. Gut microbiota, inflammation, and colorectal cancer. Annu Rev Microbiol. 2016;70:395–411. doi: 10.1146/annurev-micro-102215-095513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gao R, Kong C, Huang L, et al. Mucosa-associated microbiota signature in colorectal cancer. Eur J Clin Microbiol Infect Dis. 2017;36:2073–2083. doi: 10.1007/s10096-017-3026-4. [DOI] [PubMed] [Google Scholar]
- 107. Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292–298. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ghosh TS, Das M, Jeffery IB, et al. Adjusting for age improves identification of gut microbiome alterations in multiple diseases. Elife. 2020;9:e50240. doi: 10.7554/eLife.50240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Barot SV, Sangwan N, Nair KG, et al. Tumor microbiome variation in young versus average onset colorectal cancer. J Clin Oncol. 2022;40:144–144. [Google Scholar]
- 110. Abdullah M, Sukartini N, Nursyirwan SA, et al. Gut microbiota profiles in early- and late-onset colorectal cancer: A potential diagnostic biomarker in the future. Digestion. 2021;102:823–832. doi: 10.1159/000516689. [DOI] [PubMed] [Google Scholar]
- 111. Yang L, Lu X, Nossa CW, et al. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology. 2009;137:588–597. doi: 10.1053/j.gastro.2009.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Parsons BN, Ijaz UZ, D’Amore R, et al. Comparison of the human gastric microbiota in hypochlorhydric states arising as a result of Helicobacter pylori-induced atrophic gastritis, autoimmune atrophic gastritis and proton pump inhibitor use. PloS Pathog. 2017;13:e1006653. doi: 10.1371/journal.ppat.1006653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Vinasco K, Mitchell HM, Kaakoush NO, et al. Microbial carcinogenesis: Lactic acid bacteria in gastric cancer. Biochim Biophys Acta Rev Cancer. 2019;1872:188309. doi: 10.1016/j.bbcan.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 114. Wei MY, Shi S, Liang C, et al. The microbiota and microbiome in pancreatic cancer: More influential than expected. Mol Cancer. 2019;18:97. doi: 10.1186/s12943-019-1008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Liu PH, Wu K, Ng K, et al. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol. 2019;5:37–44. doi: 10.1001/jamaoncol.2018.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Petrick JL, Kelly SP, Liao LM, et al. Body weight trajectories and risk of oesophageal and gastric cardia adenocarcinomas: A pooled analysis of NIH-AARP and PLCO studies. Br J Cancer. 2017;116:951–959. doi: 10.1038/bjc.2017.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Zhang Y, Song M, Chan AT, et al. Unrestrained eating behavior and risk of digestive system cancers: A prospective cohort study. Am J Clin Nutr. 2021;114:1612–1624. doi: 10.1093/ajcn/nqab235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wang W, Fung TT, Wang M, et al. Association of the insulinemic potential of diet and lifestyle with risk of digestive system cancers in men and women. JNCI Cancer Spectr. 2018;2:pky080. doi: 10.1093/jncics/pky080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Chang VC, Cotterchio M, De P, et al. Risk factors for early-onset colorectal cancer: A population-based case-control study in Ontario, Canada. Cancer Causes Control. 2021;32:1063–1083. doi: 10.1007/s10552-021-01456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wolin KY, Yan Y, Colditz GA, et al. Physical activity and colon cancer prevention: A meta-analysis. Br J Cancer. 2009;100:611–616. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Morris JS, Bradbury KE, Cross AJ, et al. Physical activity, sedentary behaviour and colorectal cancer risk in the UK Biobank. Br J Cancer. 2018;118:920–929. doi: 10.1038/bjc.2017.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kunzmann AT, Mallon KP, Hunter RF, et al. Physical activity, sedentary behaviour and risk of oesophago-gastric cancer: A prospective cohort study within UK Biobank. United Eur Gastroenterol J. 2018;6:1144–1154. doi: 10.1177/2050640618783558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Michaud DS, Giovannucci E, Willett WC, et al. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA. 2001;286:921–929. doi: 10.1001/jama.286.8.921. [DOI] [PubMed] [Google Scholar]