Abstract
Sarcoma is a rare and heterogeneous class of mesenchymal malignancies with poor prognosis. Panobinostat (LBH589) as one of histone deacetylase (HDAC) inhibitors has demonstrated anti-tumor activity in patients with sarcoma, but its mechanisms remains unclear. Here, we found that LBH589 alone inhibited the proliferation and colony formation of soft tissue sarcoma (STS) cell lines. Transcriptome analysis showed that treatment with LBH589 augmented the NK cell-mediated cytotoxicity. Quantitative real-time PCR and flow cytometric analysis (FACS) further confirmed that LBH589 increased the expression of NKG2D ligands MICA/MICB. Mechanistically, LBH589 activated the Wnt/β-catenin pathway by upregulating the histone acetylation in β-catenin promoter. In vitro co-culture experiments and in vivo animal experiments showed that LBH589 increased the cytotoxicity of natural killer (NK) cells while Wnt/β-catenin inhibitor decreased the effects. Our findings suggest that LBH589 facilitates the anti-tumor effect of NK cells, highlights LBH589 an effective assistance drug in NK cell-based immunotherapies.
Keywords: panobinostat, LBH589, NKG2D, Wnt/β-catenin, NK cell-related cytotoxicity
Introduction
Sarcomas are rare and heterogeneous mesenchymal malignancies, of which soft tissue sarcomas (STS) are the most common pathologic type of sarcomas, accounting for about 80% [1]. Due to the insidious symptoms of soft tissue sarcoma, it is confirmed to be in the advanced stage of the disease, and the traditional treatment regimen, including surgery, chemotherapy and radiotherapy, is prone to recurrence or metastasis, so there is still no effective treatment to improve the prognosis of advanced STS. To address these issues, more and more new therapeutic strategies are applied in STS, such as targeted therapy, immunotherapy, and minimally invasive surgery [2–4].
Though anti-CTLA-4 and PD-1/PD-L1 antibodies lead to durable clinical responses in many solid tumor, these immune checkpoint blockade therapy in sarcoma is inefficacious because of the lower ratios of tumor-infiltrating lymphocytes (TILs) infiltration [5–7]. NK cell-based immunotherapy is promising with its cytotoxic ability to kill tumor cells without MHC (“non-missing self”) recognition. Recent preclinical studies suggest that adoptive NK cell therapy could be readily available and broadly applicable [8–11]. The adoption of NK cell immunotherapy has demonstrated its feasibility in osteosarcoma and Ewing sarcoma [12–15]. In addition to strategies augmenting NK cell function such as adoptive NK cell therapy and CAR-NK therapy, the NK cell-based immunotherapies also include strategies sensitizing cancer cells to NK cell-mediated lysis [16, 17].
The immune effects of NK cells are dependent on the natural killer group 2D (NKG2D)-mediated cell kill, and the efficiency of NKG2D-mediated cytotoxicity has been shown to correlate with the expression levels of NKG2D ligands (NKG2DLs), which includes MICA, MICB, ULBP 1–6, on the cancer cells [11, 18]. However, tumor cells are able to escape from NKG2D-mediated immune surveillance by shedding MHC class I chain-related molecules (MICs) from the tumor cell membrane [19]. Therefore, restoring the expression of NK cell-activating ligands on tumor cells would have a significant impact on the efficacy of NK cell-mediated immunotherapy.
Enhancing NK cell antitumor activity can be achieved through various priming strategies and genetic modifications. Histone acetylation and deacetylation of chromatin is important in regulating the activity of transcription, which is controlled by histone acetylases (HAT) and histone deacetylases (HDAC). HDACs critically contribute to tumor pathogenesis with the correlative data showing that HDAC function and/or expression is perturbed in a variety of cancers and is often associated with poor prognosis [20–25].
HDAC inhibitors (HDACi) were discovered through empirical screens for agents that induced tumor cell differentiation [26]. Due to the broad and complex functions of the HDAC enzymes, HDACi have demonstrated the effects on gene expression, apoptosis, cell cycle progression, aspects of the tumor micro-environment and so on [27–30]. Several studies have reported the effect of HDAC inhibitors on immune modulation. HDAC inhibitors are known to upregulate the expression of NKG2D ligands [30–32], which mediated cytotoxicity of NK cells. For example, non-selective HDAC inhibitors valproic acid (VPA) as well as romidepsin have been reported to inhibit tumor by upregulating cytotoxicity-activating ligands (NKG2DL) in different tumor cell lines [33–37], including colon cancer, lymphoma, and liver carcinoma cell lines, thus enhancing NK cell-mediated lysis [38, 39]. Previous studies suggested HDAC inhibitors as possible therapeutic agents for STS [40–42], and a phase I trial of panobinostat(LBH589) and epirubicin in sarcoma also confirmed this [43]. However, the effect and mechanisms action of LBH589 in sarcoma remain unclear.
In order to explore whether LBH589 has potential as a treatment for STS, we examined the effects and mechanism of LBH589 on the expression of MICA and MICB in human STS cells. Our data demonstrated that LBH589 enhances the susceptibility of sarcoma cells to NK cell-mediated cytotoxicity both in vitro and in vivo by upregulating the expression of MICA and MICB via activation of the Wnt/β-catenin pathway.
Materials and Methods
Cell lines
HT1080 (Fibrosarcoma cells), SK-LMS-1 (Leiomyosarcoma cells), SW872 (Liposarcoma cells), U2197 (Pleomorphic sarcoma/malignant fibrous histiocytoma cells), and NK cell line NK92 used in the experiment were obtained from the American Type Culture Collection (ATCC). NK-92 cells are an IL-2-dependent NK cell lines derived from peripheral blood of a 50-year-old white male with aggressive non-Hodgkin’s lymphoma. NK-92 cells had the following characteristics:CD2, CD7, CD11a, CD28, CD45, CD54, CD56bright were positive; negative surface markers for CD1, CD3, CD4, CD5, CD8, CD10, CD14, CD16, CD19, CD20, CD23, CD34 and HLA-DR. HT1080, SK-LMS-1, SW872 and U2197 were cultured in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal bovine serum. NK cell line NK92 was cultured in MEM α (procell, PM150421) +0.2 mM inositol + 0.1 mM β-mercaptoethanol(procell, PB180633) + 0.02 mM folic acid + 12.5%horse serum (procell,164215-100) + 12.5% FBS (procell, 164210-500) + 1%P/S (procell, PB180120), and 200 U/mL rhIL-2.
MTT assay
A total of 800–1200 cells in 200 μL of medium were seeded per well in 96-well plates (five replicates of each sample). Next, 20 μL of MTT (5 mg/mL, BD Biosciences) was added per well on the indicated day (days 0, 1, 2, 3, 4, or 5) and incubated for 4 h at 37 °C. Then, the supernatants were discarded, and 200 μL of dimethylsulfoxide (DMSO) was added per well to dissolve the crystals. A spectrophotometric plate reader (BioTek ELX 800, USA) was used to measure the absorbance at 490 nm.
Colony formation assay
Briefly, cells were plated in six-well plates and cultured with DMEM in the presence or absence of LBH589 for 10 days. Then the colonies were fixed with methanol and stained with 0.5% crystal violet. Three independent wells were established for each treatment concentration.
Preparation of peripheral blood NK cells and activation
Peripheral blood mononuclear cells (PBMCs) of healthy donors were isolated using Ficoll by density gradient centrifugation. PBMCs were rested overnight in RPMI 1640 medium (Gibco) and activated by recombinant human IL-15 (PeproTech, 200-15-10) 50 ng/mL, recombinant human IL-2 (PeproTech, 200-02-50) 200 U/ml with the RPMI1640 medium for 3 days before an in vitro cytotoxicity assay. The NK Cell Isolation Kit (Miltenyi Biotec, 130-092-657) was used for the isolation of primary NK cells from human PBMCs.
Western blot analysis and reagents
Cells were lysed in RIPA buffer, and proteins (20–40 µg) were resolved using SDS–PAGE, and then transferred onto a polyvinylidene difluoride (PVDF) membrane. After the blocking procedure with 5% skim milk for 1 h at room temperature, membranes were incubated with primary antibodies (1:1000) and HRP-conjugated secondary antibodies (1:5000), and visualized in Imager (Bio-Rad) using ECL system (Termo Fisher Scientifc, 34095). The antibodies used in this study are listed below in the format of name (application, supplier, catalogue): MICA/MICB(F, Biolegend, 320906), MICA/MICB(WB&IHC, Abcam, ab224702), PI3K(WB, CST, 4252S), p-Akt (WB, CST, 4060S), AKT(WB, CST, 9272S), p-p38 (WB, CST, 4511S), ERK(WB, CST, 4695S), p-Erk (CST, 4370S), JAK1 (WB, upstate, 06-665), p-JAK1 (WB, upstate, 07-849), Stat3 (WB, CST, 30835S), p-Stat3 (WB, CST, 9145S), β-catenin (WB, CST, 8480s), vimentin (WB, CST, 5741S), c-Jun (WB, CST, 9165S), β-actin (WB, CST, 3700S), acetyl-histone H3 (Lys27) (ChIP, CST, 8173S).
RNA sequencing analysis
Cells were treated with LBH589 or DMSO control for 48 h. Then, total RNA was extracted from cells using TRIzol. Library construction was performed with the generated 100 bp paired-end reads. The libraries were sequenced on an Illumina HiSeq 2500 platform by Annoroad Gene Technology (Beijing, China).
Quantitative PCR with reverse transcription (qRT–PCR)
Total RNA was isolated by using the TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. RNA concentration and quality were determined with NanoDrop spectrophotometer (Thermo Scientific, MA, USA). cDNA was synthesized using 1 μg of the total RNA according to a reverse transcriptase kit (Invitrogen). Expression was assessed by quantitative real-time PCR using SYBR Green on a Bio-Ran CFX96 system. Resultant data were assessed by Bio-Rad CFX software and calculated by the formula 2^ (−(∆∆Ct) for relative mRNA expression. Sequences of all primers used in qPCR are presented in Table S1
Flow cytometric analysis
For MICA/MICB analysis, cultured cells were treated for 24 h with the indicated HDAC pan-inhibitor LBH589 or DMSO control. After the treatment, cells were harvested and stained for MICA/MICB and data were acquired by flow cytometry. Autofluorescence MFI values were subtracted and the adjusted MFI values are evaluated.
Chromatin immunoprecipitation (Ch-IP) assays
Ch-IP assays were performed as described by Zeng et al [44]. Briefly, 5 × 106 HT1080 cells were treated with or without LBH589 for 24 h. The cells were scraped in PBS and resuspended in lysis buffer, and the nuclei were isolated and sonicated to shear the DNA to 500 bp–1 kb fragment (verified by agarose gel electrophoresis). Equal aliquots of chromatin supernatants were subjected to overnight immunoprecipitation (IP) with anti-acetylated-histone H3K27 from Cell Signaling and IgG control from R&D Systems which were used as negative controls. Immunoprecipitated chromatin was analyzed by q-PCR using primers targeting individual regions in the human β-catenin promoter. The primer sequences used for ChIP-qPCR are listed in Table S2. PCR conditions were set according to the instructions provided in SYBR Green Kit (Roche).
Cytotoxicity assay
The cytotoxicity of different experimental groups of NK92 cells or primary NK cells was determined using the Lactate Dehydrogenase (LDH) release assay using a CytoTox 96 Non-Radioactive Cytotoxicity Assay Kit following the manufacturer’s protocol. The target HT1080 and SK-LMS-1 cells were incubated with or without 20 nM LBH589 or ICG001 5 μM for 24 h, washed with PBS, then NK-92 cells or primary NK cells were added to the target cells as effector cells, and the cells were co-cultured for 4 h at 37 °C. The experimental LDH release values were corrected by subtraction of the spontaneous LDH release values of effector cells at the same dilution. Percentage lysis was calculated as: (corrected experimental LDH release − spontaneous LDH release)/(maximum LDH release – spontaneous LDH release) × 100%.
In Vivo Experiments
Four-week-old female NOD/SCID mice bearing LUC-positive HT1080 cells were randomly divided into four groups (n = 8 per group). Group A received PBS treatment, group B received LBH589, group C received NK-92 cells (5 × 106), group D received LBH589 followed by NK92 cells (5 × 106). Mice were monitored daily for tumor growth by visual examination and palpation. NOD/SCID mice were further monitored by means of bioluminescence imaging (BLI) (n = 3). Mice in groups B and D received 10 mg/kg of ip LBH589 once per week for 3 weeks (on days 7, 14, and 21). Mice in group D received IV injection of NK92 cells (5 × 106) 24 h after receiving each dose of LBH589 through the tail vein. Tumor volume was calculated every week using the formula: (width2 × length)/2. The mice were sacrificed 3 weeks after the initial injection and the xenografts were excised and subjected to further analysis.
All animal studies were approved by the Ethics Committee of Animal Experiments of Sun Yat-sen University Cancer Center.
Immunohistochemistry
Tumors from mouse models were carefully dissected, fixed with formalin, and embedded with paraffin. Paraffin blocks were cut into 3 mm sections and some were stained with hematoxylin and eosin (H&E). For immunohistochemical staining, each section was dewaxed and rehydrated using xylene and then washed with a graded alcohol series. Antigen retrieval was performed for 2.5 min at high pressure using sodium citrate. The sections were incubated with against MICA/MICB primary antibody at a dilution of 1:150 overnight at 4 °C and then with goat secondary antibody against rabbit at a dilution of 1:200 for 30 min at 37°C. MICA and MICB expression were scored semi-quantitatively on the basis of the staining intensity and percentage of positive cells.
Dataset analyses
The expression level of HDACs in pan-cancer was determined by Oncomine database analysis (https://www.oncomine.org/resource/login.html, 11 November 2021, data last accessed) (Rhodes et al., 2007). The thresholds (P ≤ 0.0001, fold change: 2, and gene rank: Top 10%) were considered statistically significant.
The TCGA-SARC cohort’s RNA sequencing (RNA-seq) profile was retrieved through the GDC API (https://portal.gdc.cancer.gov/repository). Before therapy, 263 STS samples and two normal soft tissue samples were taken from this group. Data on 864 normal tissues (386 subcutaneous fatty tissue and 478 skeletal muscle) was gathered from the GTEx database. To match the normal and tumor samples, the RNA-sequencing were merged and normalized using the “limma” R package (V4.0).
Statistical analysis
Data were presented as the mean ± standard deviation for flow cytometry, quantitative real-time PCR, cellular cytotoxicity assay, and xenograft assay, analyzed by unpaired, two-tailed t-test. A significance threshold of P < 0.05 was used. Data were performed using GraphPad Prism V.6.0 software and SPSS V.19.0.
Results
LBH589 exhibits antitumor activity in STS in vitro
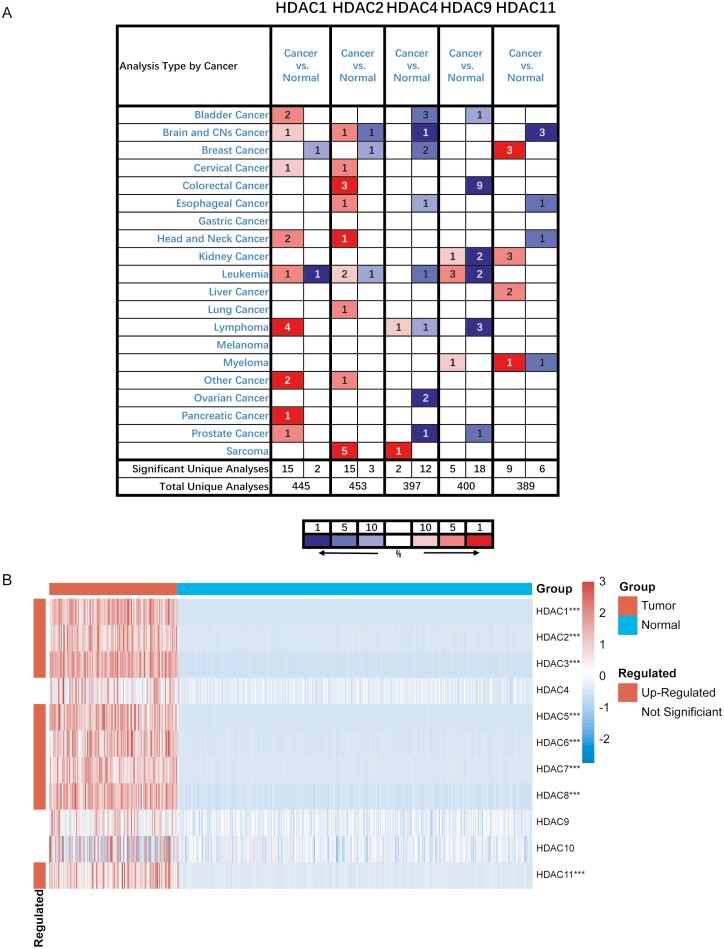
We first analyzed the mRNA expression levels of HDACs family in several type tumor tissues and corresponding normal tissues in the Oncomine database (Fig. 1A) There is a significant overexpression of the HDAC1 gene in lymphoma, HDAC2 gene in colorectal cancer and sarcoma, HDAC9 gene in leukemia and HDAC11 gene in breast cancer, expression of other HDACs showed little difference between tumor and normal tissues. Moreover, the expression of HDACs in sarcoma tissues from TCGA database with the normal tissue from GTEx database was compared, which appeared that most of HDACs were highly expressed in patients with STS (Fig. 1B). In particular, this finding also holds true in leiomyosarcoma and liposarcoma, which are two most common subtypes in STS (Supplementary Fig. S1).
Figure 1:
HDAC gene family was extensively higher expressed in pan-cancer. A. The transcription expression level of HDAC gene family members was higher in most cancer types in Oncomine database. The numbers in the table represent the quantities of datasets with relatively higher expressed (red) or lower expressed (blue) of target genes. The following criteria were used: analysis type: cancer vs. normal tissue; P-value < 0.0001, fold change > 2, and gene rank= top 10%. B. HDAC1/2/3/5/6/7/8/11 was highly expressed in tumor compared to normal tissues in TCGA+GETx database in STS. All histological subtypes in TCGA cohort have been included in the analysis. Red squares indicate upregulated genes, blue squares indicate downregulated genes, and white squares indicate genes without difference. The P values were showed as: ***P < 0.001.
Based on these data, we proposed that HDACi would be a potential effective inhibitor in sarcoma. Then the effect of pan-HDAC inhibitor LBH589 in sarcoma was tested.
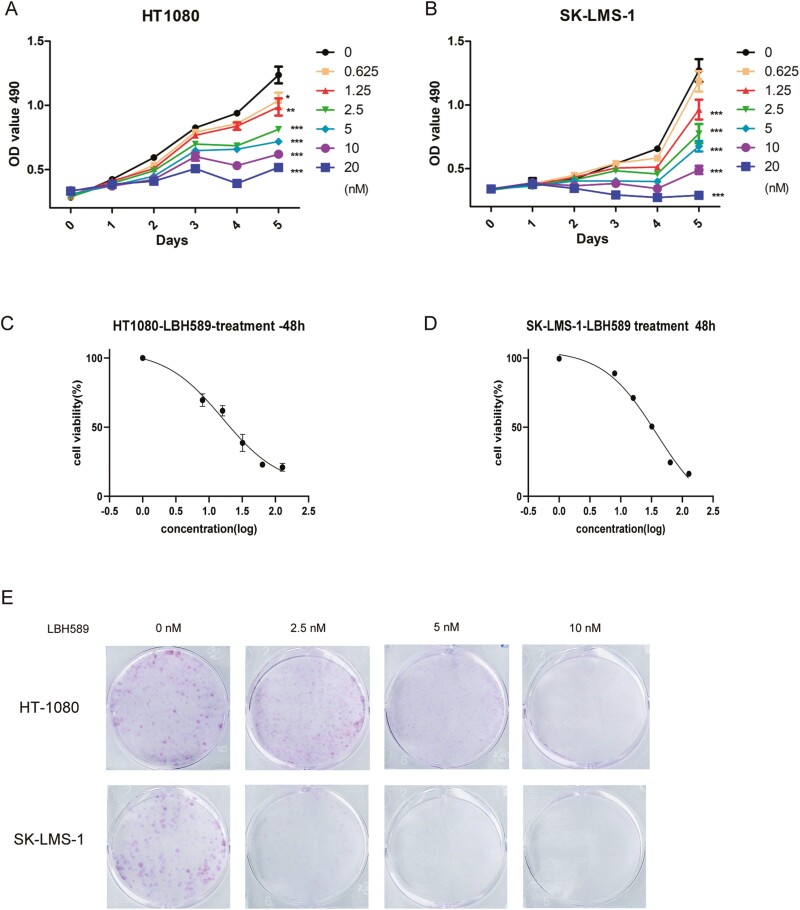
To assess the effect of LBH589 on soft tissue sarcoma cell lines, we initially treated diverse cancer cell lines with 0-20 nM LBH589. MTT assay showed LBH589 remarkably suppressed cell viability of HT1080 and SK-LMS-1 cells in a dose- and time-dependent manner (Fig. 2A and B). As presented in Fig. 2, LBH589 at concentration of 2.5 nM or higher significantly suppressed the proliferation of HT1080 and SK-LMS-1 cells with estimated IC50 values of 16.1 and 38.1 nM, respectively (Fig. 2C and D). Colony formation assay also revealed that LBH589 dramatically reduced colony numbers of HT1080 and SK-LMS-1 cells in a dose-dependent manner (Fig. 2E). Next, we detected whether LBH589 may exert similar effects on another two sarcoma cell lines SW872 and U2197. The results showed that LBH589 also repressed the proliferation and colony formation of SW872 and U2197 with estimated IC50 values of 31.57 and 29.04 nM, respectively (Supplementary Fig. S2).
Figure 2:
LBH589 suppressed the proliferation of STS cells in vitro. (A, B) Human soft tissue sarcoma cell lines HT1080 and SK-LMS-1 were treated with LBH589 (0, 0.625, 1.25, 2.5, 5, 10, and 20 nM) for 5 days. (C, D) Cell viability was measured by MTT assays. (E) Colony formation assays were performed using HT1080 and SK-LMS-1 cells treated with LBH589 (0, 2.5, 5, and 10 nM) to assess proliferation. Colonies were imaged.
LBH589 induces NK cell-mediated cytotoxicity by upregulation of genes related to NKG2D
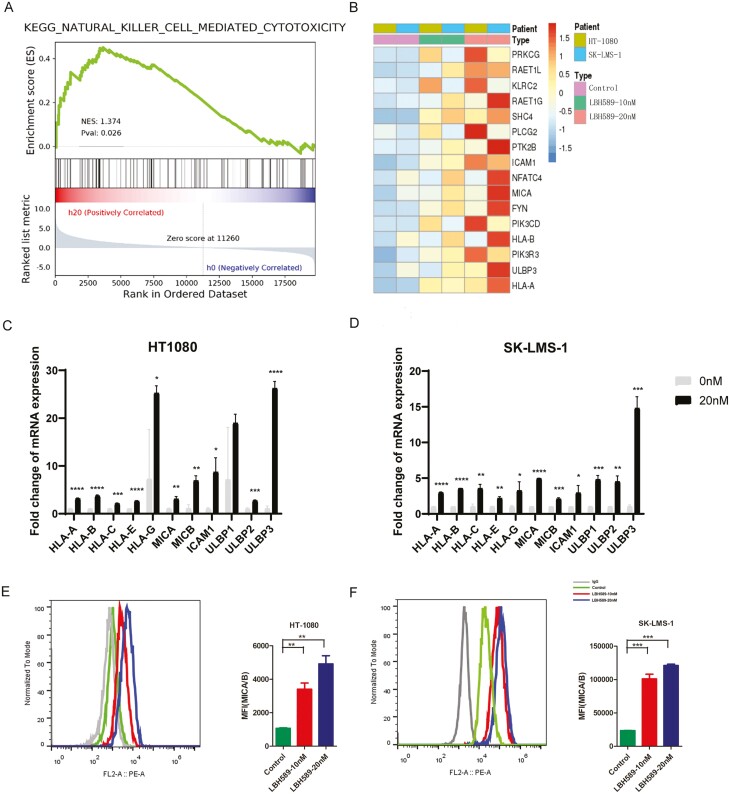
Assessment of drug effects on immune-response-related pathways yields important insight alterations that may relate to immune involved combination therapy. The epigenetic therapy is potential to increase the effect of immunotherapies. To investigate the impact of LBH589 on the sarcoma cell lines at the molecular level, we carried out transcriptome analysis (RNA-seq). Gene set enrichment analysis identified some significantly induced pathways related to immune signaling. The natural killer cells related cytotoxicity pathway was the most of these altered pathways (Fig. 3A). The notable differential expression was the upregulation genes related to NKG2D and antigen presentation, suggesting that LBH589 to be an effective approach in terms of induction of natural killer cell-mediated cytotoxicity pathway (Fig. 3B).
Figure 3:
LBH589 increased NK cell-mediated cytotoxicity. (A) The GSEA result indicated an enrichment of gene sets related to NK cell-related cytotoxicity. (B) Heatmap showed the differentially expressed genes following LBH589 treatment. (C, D) Real-time q-PCR analysis showed the effects of LBH589 treatment on the expression of NKG2DL in STS cells (mean ± SD, n = 3). (E, F) Flow cytometry analysis of surface levels of MICA/B on LBH589(10 and 20 nM) treatment HT1080 cells (left) and SK-LMS-1 cells (right) and quantification. Data were mean ± SD of three independent experiments. *P < 0.05, ** P < 0.01, *** P < 0.001 (two-tailed unpaired t-test).
To confirm the effect of LBH589 on the expression of ligands and receptors related to NK cell mediated cytotoxicity pathway, we performed real-time PCR on two sarcoma cell lines (HT1080 and SK-LMS-1). We noted a significant increase on surface mRNA expression of MICA/MICB and ULBP1/2/3 after exposure to LBH589 (Fig. 3C and D). Consistent with the results of mRNA expression, elevation of the MICA/MICB protein expression levels following LBH589 treatment was observed in FACS assay. Upregulation of expression of MICA/MICB was further confirmed by flow cytometry (Fig. 3E and F). We noted that LBH589 did not significantly affect expression of NK checkpoints, such as after co-cultured with HT1080 and SK-LMS-1 (data not shown). Moreover, we also detected the expression of these NK cell-mediated checkpoints after treatment with LBH589 in SW872 and U2197, and we found similar changes (Supplementary Fig. S3). These data demonstrated that HDAC inhibitor LBH589 is able to enhance the expression of NKG2D ligands including MICA/MICB in sarcoma cell lines.
Wnt signaling pathway was most responsible for NK cell-related cytotoxicity in sarcoma cell lines
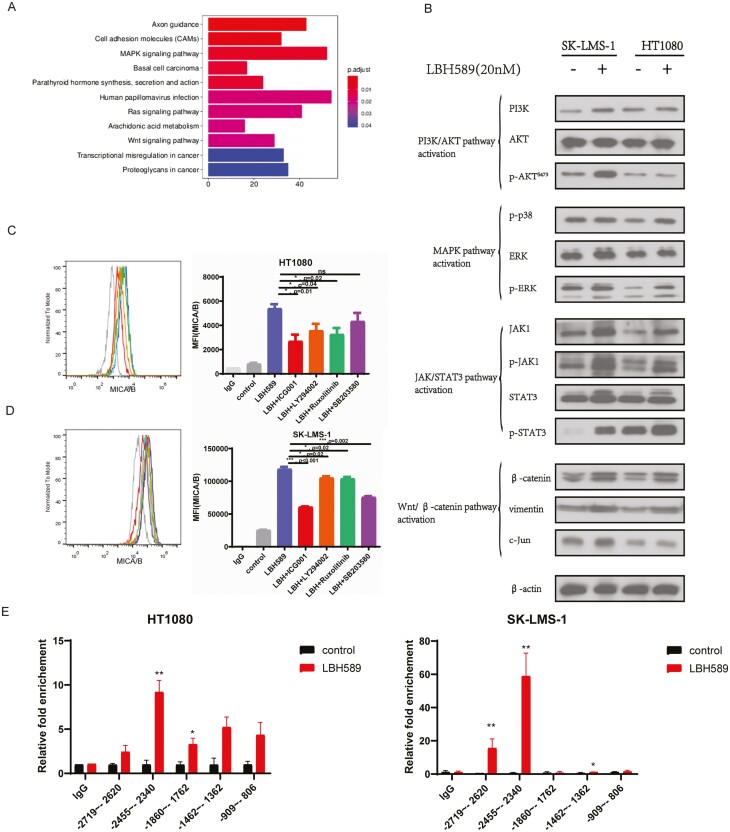
Furthermore, the putative signaling pathways involved in NK cell-related cytotoxicity in sarcoma cell lines were identified. We performed enrichment analysis on significantly up- and downregulated genes, and KEGG defined significant enrichment as p.adj < 0.05. The results of the enrichment are depicted in Fig. 4A. Then, using western blot analysis, we discovered that LBH589 stimulation activated practically all of these signaling pathways, including PI3K/AKT, MAPK/ERK, JAK/Stat3, and Wnt/-catenin pathways (Fig. 4B). Then, we examined whether inhibiting potential signaling pathways in sarcoma cells could result in a decrease in constitutively produced MICA/MICB. As illustrated in Fig. 4C and D, treatment with inhibitors of the JAK/STAT3 (Ruxolitinib), MAPK (SB203580), Wnt/β-catenin (ICG001), and PI3K/AKT (LY294002) signaling pathways all decreased the MICA/MICB level in LBH589 treated sarcoma cells, but the most significant effect was observed with the Wnt/β-catenin inhibitor. To further understand the role of LBH589 on promoter-driven expression of β-catenin, we designed primers targeting the β-catenin promoter for ChIP-qPCR. We observed a significant enrichment of H3K27ac in the upstream 2455-2340 bp 5ʹ-flanking region of the β-catenin promoter compared with negative control (Fig. 4E). Taken together, these data revealed that LBH589 enhanced the expression of β-catenin by acetylation of its promoter, resulting in constitutive upregulation of MICA/MICB in sarcoma cells.
Figure 4:
Wnt/β-catenin pathway was critically implicated in NK cell-related cytotoxicity induced by LBH589. (A) KEGG analysis of the enriched pathways in STS cells with LBH589 treatment. (B) Proteins in the PI3K/AKT pathway, MAPK pathway, JAK/STAT3 pathway, and Wnt/β-catenin pathway were analyzed by Western blot after STS cell lines treated with (+) or without (−) LBH589 (20 nM). The results showed that LBH589 activated Wnt/β-catenin pathway in HT1080 and SK-LMS-1 cells. (C, D) Flow cytometry analysis of surface levels of MICA/B on LBH589 and inhibitors of JAK/STAT3 (Ruxolitinib), MAPK (SB203580), Wnt/β-catenin (ICG001), and PI3K/AKT (LY294002) signaling pathways treated HT1080 cells (C) and SK-LMS-1 cells (D) and quantification. Data were mean ± SD of three independent experiments. *P < 0.05, ***P < 0.001 (two-tailed unpaired t-test). (E) Histone acetylation in β-catenin promoter. HT1080 cells and SK-LMS-1 cells were treated with LBH589 (20 nM) for 24 h and subjected to ChIP assay using anti-acetyl-histone H3K27(Ac-H3K27) antibody followed by q-PCR analysis using primers targeting β-catenin promoter region.
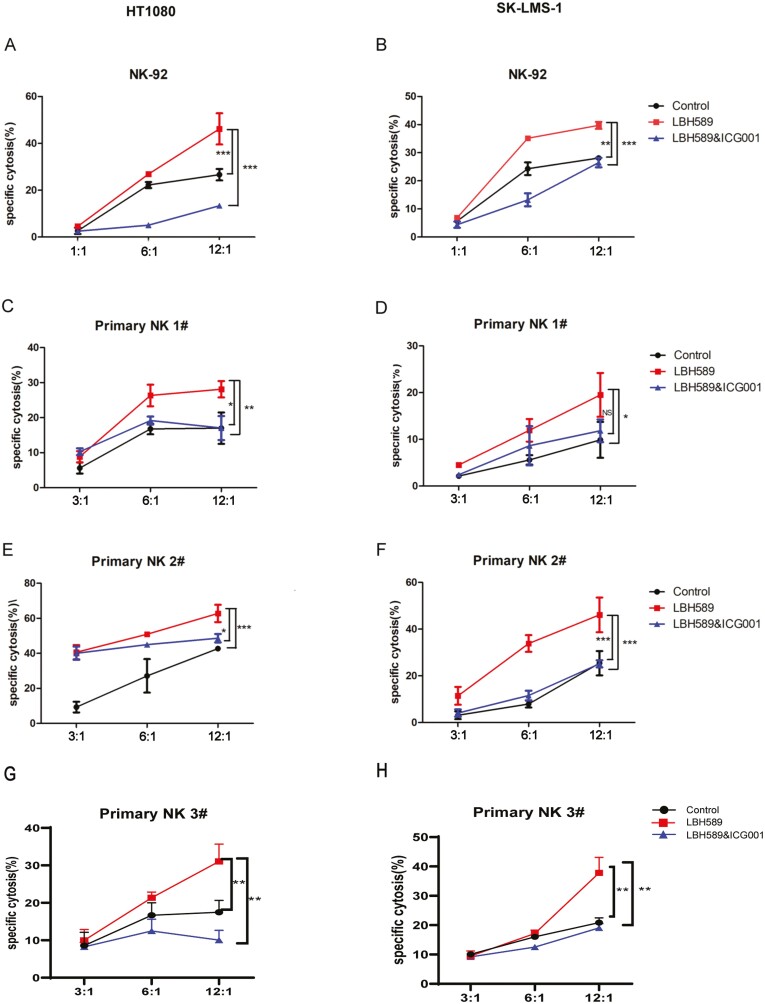
Inhibition of Wnt/β-catenin pathway weaken the effect of increased NK cell-induced lysis of sarcoma cells by LBH589 stimulation
To demonstrate the role of the Wnt/β-catenin pathway, we first investigated the effect of LBH589 on NK cell-mediated kill of sarcoma cells. HT1080 and SK-LMS-1 cells were incubated with LBH589 or LBH589&ICG001 for 24 h. The control group was treated with DMSO. The cytotoxicity of NK cells was investigated using two NK cell sources for the experiments. The established NK92 cell line was known to exhibit high NK cytotoxicity and has been widely used in in vitro and mouse studies [45, 46]. The primary NK cells isolated from the peripheral blood mononuclear cells (PBMCs) had a purity of higher than 90% of CD3− CD56+ NK cell markers, which had been confirmed by flow cytometric analyses (data not shown). The lactate dehydrogenase (LDH) release-based NK cytotoxicity test was applied to detect NK cell-mediated cytotoxicity. As shown in Fig. 5, treatment of sarcoma cells with LBH589 showed higher sensitivity to NK cell lysis than untreated control cells (mean ± SD: 56 ± 3% vs. 31 ± 4% at E/T 8:1, P < 0.05). The enhancing effect of LBH589 was blocked by Wnt pathway inhibitor ICG001. These findings support that LBH589 activated the NK cell through the Wnt/β-catenin signaling pathway in soft tissue sarcoma in vitro.
Figure 5:
Inhibition of Wnt/β-catenin pathway reversed the enhanced NK cell cytotoxicity induced by LBH589. STS cell lines were co-cultured with NK-92 cells and primary natural killer (pNK) cells from healthy donors at effector/target (E/T) ratios: 12:1, 6:1, and 3:1. Red, black, and blue lines indicate the cytotoxicity of NK cells alone, NK cells with Wnt/β-catenin inhibitor (ICG001), and control group, respectively. NK cytotoxicity to STS cell lines was assessed by LDH-release assay. All data are compared at *P < 0.05, **P < 0.01, ***P < 0.001. All data were presented as the means ± SD in three independent experiments.
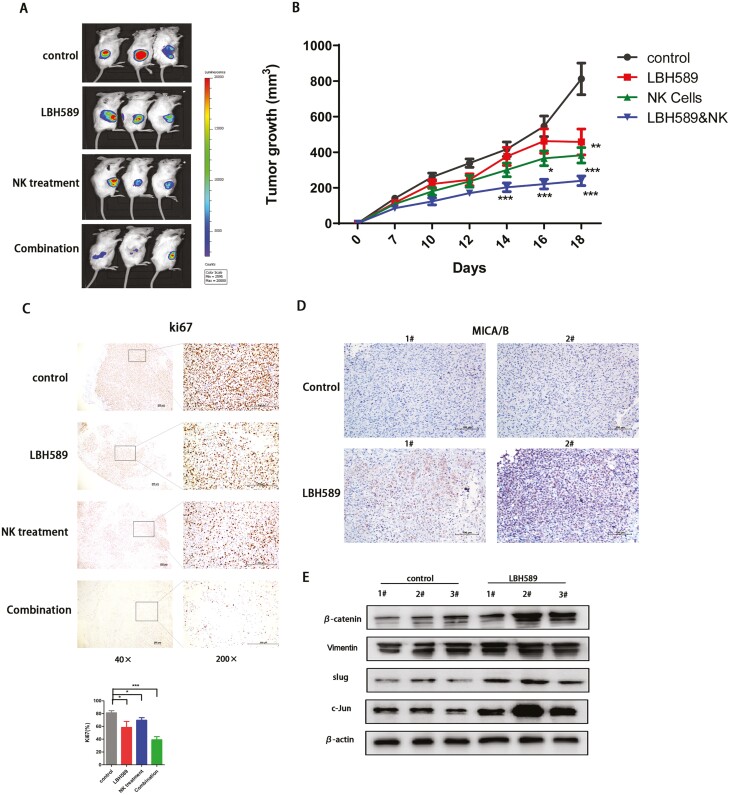
LBH589 improves the anti-tumor effects of NK-92 cells in bearing HT1080 murine models
NOD/SCID mice bearing HT1080 sarcoma cells were utilized to determine the therapeutic efficacy of LBH589 combined with NK-92 cells. HT1080 cells were subcutaneously injected into NOD/SCID mice on day 0. Approximately 7 days later, mice were randomized divided into four groups (n = 8 per group), including group did not receive any treatment but A. PBS control, B. group consisted of xenografted mice that receive LBH589 (10 mg/kg weekly, three doses on days 7, 14, 21), C. group consisted of mice that received NK-92 cells (107 cells weekly, three doses on days 7, 14, and 21), and D. combination group received both LBH589 and NK-92 cells. Engraftment of HT1080 cells in NOD/SCID mice was confirmed by BLI on day 14. As shown in Fig. 6A and B, treatment with LBH589 significantly enhanced the ability of NK-92 cells to inhibit the growth of tumors. The combination group showed a significantly decreased tumor burden (p < 0.001) compared with that of the PBS control group (Fig. 6B). Furthermore, immunohistochemical analysis revealed that LBH589 significantly reduced tumor cells proliferation compared with the control group and group treated with NK-92 cells alone (Fig. 6C) and upregulated expression of MICA/MICB (Fig. 6D). Moreover, western blot of related downstream molecules of the Wnt/β-catenin signaling pathway was performed to confirm the activation of this pathway in the group treated with LBH589 compared with the control group in vivo (Fig. 6E).
Figure 6:
Treatment with LBH589 facilitated the anti-tumor effect of NK cells. (A) Bioluminescent images and (B) Tumor burden of HT1080-bearing NOD/SCID mice on specific day after treatment with PBS, LBH589, NK-92 cells, and LBH589 combination with NK-92 cells. All data were compared at *P < 0.05 and presented as the means ± SD in three independent experiments. (C) IHC staining of Ki67 indicated the anti-tumor effect of LBH589 and NK-92 cells, and combination treatment showed the most significant effect. Quantification of Ki67 staining in different groups was shown in the bar graph. Data were analyzed using one-way ANOVA. *P < 0.05, **P < 0.01, and ***P < 0.01 (D) MICA/B was assessed by immunohistochemistry after treatment with LBH589. (E) Western blot analysis of proteins in Wnt/β-catenin pathway in PBS and LBH589 treated tumor tissues. Visualization was conducted using light microscopy (40× and 200×).
Discussion
The present study was conducted to elucidate the precise mechanism via which LBH589 modulates the NK-cell based cytotoxicity and to determine whether LBH589 is effective as an anticancer agent in STS. The present results showed that LBH589 increased the expression of MICA/MICB by activating Wnt/β-catenin signaling pathway. The upregulation of MICA/MICB enhanced the NK cell-induced lysis of sarcoma cells in vitro and in vivo. Therefore, our results suggest that LBH589 reduces STS progression and that it is a potential anticancer agent for the treatment of STS as well as a promising adjuvant for NK cell-based immunotherapy in STS.
The histone deacetylases (HDACs) have been approved as promising therapeutic targets as their inhibition may reverse dysregulated epigenetic states associated with cancer [23, 47]. Various studies have reported that HDAC gene family was extensively amplified in different types of cancer and their corresponding specific inhibitors showed potential anticancer effect [22, 48]. In this project, we reported that expression of HDAC gene family was increased at different levels in many types of cancers including sarcoma, indicating that pan-HDAC inhibitors could be potential antitumor agents.
Panobinostat (LBH589), a non-selective HDAC inhibitor, has demonstrated anti-tumor activity in lymphoma, prostate cancer, breast cancer, and other solid hematological malignancies [49–51]. We found that LBH589 effectively inhibited STS cell growth in a dose-dependent manner. Moreover, colony formation ability was significantly suppressed in STS cells. These observations are consistent with those previous studies reporting the role of LBH589 in antitumor activity [52, 53].
As an important component of innate immune response, natural killer (NK) cells play an important role in anti-tumor immune response and several studies have shown that NK cell-mediated immunotherapy may be a promising option for the treatment of solid tumor [8]. It has been proved that HDAC inhibitors (HDACi) could upregulated the expression of NKG2D ligands (NKG2DLs) on cancer cells to enhance the NK cell-mediated cytotoxicity [30–32, 34–36]. In our study, transcriptional genes expression alteration on LBH589 treatment showed that genes related to NK cell-mediated lysis were transcriptionally modulated. Real-time PCR and FACS analysis were performed to confirm that MICA/MICB was most significantly upregulated by LBH589. Previous research found that HLA class I allotypes enhance the NK cell cytotoxicity [54–56]. In our study, in addition to MICA/B, HLA-A, -B was increased by LBH589 in STS cell lines. HLA-C could also be upregulated in HT1080, SK-LMS-1, and U2197, but not in SW872. We analyzed that this difference might be due to the heterogeneity of the cells, the different pathways in which HLA-C was regulated. To further investigate the underlying pathways of LBH589 on upregulation of MICA/MICB, we performed KEGG analysis of the enriched pathways and measured associated protein expression levels. Together with the Ch-IP assay, these results indicated that LBH589 enhanced the expression of MICA/MICB via upregulation of Wnt/β-catenin pathway, though a previous study showed that LBH589 inhibits Wnt/β-catenin signaling pathway [51]. Based on the above results, we tested the effect of LBH589 on cytotoxicity of NK cell targeting STS cell lines in vitro and in vivo. LBH589 improved the anti-tumor effects of NK-92 cells in bearing HT1080 murine models while inhibition of Wnt/β-catenin pathway weaken the effect of increased NK cell-induced lysis of sarcoma cells by LBH589 stimulation, which has not been investigated in other researches. These results implicated combination LBH589 with adoptive NK cell therapy as potential treatment for STS. However, the mechanism in which β-catenin regulates the expression of MICA/MICB, and whether LBH589 inhibits tumor in vivo by enhancing the cytotoxicity of NK cell in mice with normal immunity, requires further studies. Therefore, the precise mechanism that LBH589 enhance the cytotoxicity of NK cell via Wnt/β-catenin signaling in STS requires further investigation.
Supplementary Material
Glossary
Abbreviations
- ATCC
American Type Culture Collection
- BLI
bioluminescence imaging
- Ch-IP
chromatin immunoprecipitation
- DMEM
Dulbecco’s modified eagle medium
- DMSO
dimethylsulfoxide
- FACS
flow cytometric analysis
- H&E
hematoxylin and eosin
- HAT
histone acetylases
- HDAC
histone deacetylase
- HDACi
histone deacetylase inhibitors
- LDH
lactate dehydrogenase
- MICs
MHC class I chain-related molecules
- NK
natural killer
- NKG2D
natural killer group 2D
- NKG2DLs
natural killer group 2D ligands
- PBMCs
peripheral blood mononuclear cells
- PVDF
polyvinylidene difluoride
- qRT-PCR
quantitative real-time PCR
- STS
soft tissue sarcoma
- TILs
tumor-infiltrating lymphocytes
- VPA
valproic acid
Contributor Information
Xiuxia Lu, Melanoma and Sarcoma Medical Oncology Unit, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, 651 Dongfeng Road East, Guangzhou 510060, PR China.
Mengmeng Liu, Melanoma and Sarcoma Medical Oncology Unit, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, 651 Dongfeng Road East, Guangzhou 510060, PR China.
Jing Yang, Melanoma and Sarcoma Medical Oncology Unit, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, 651 Dongfeng Road East, Guangzhou 510060, PR China.
Yi Que, Department of Pediatric Oncology, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong 510060, PR China.
Xing Zhang, Melanoma and Sarcoma Medical Oncology Unit, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, 651 Dongfeng Road East, Guangzhou 510060, PR China.
Funding
This work was supported by the National Key Laboratory Foundation of China (No. 2021YFC2400601), National Natural Science Foundation of China (No. 82072958) and Guangzhou Science and Technology project (No. 202201011572). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interests
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Author Contributions
X.L. and M.L.: conceived the original ideas of this manuscript and writing the original draft. J.Y.: data curation and formal analysis. Y.Q. and X.Z.: supervision and review editing. All authors read and approved the final manuscript.
Ethical approval
This study was approved by the Institutional Research Ethics Committee of Sun Yat-sen University Cancer Center. All animal studies were approved by the Ethics Committee of Animal Experiments of Sun Yat-sen University Cancer Center.
Data Availability
The datasets presented in this study can be found in online repositories.
Reference
- 1. Gamboa AC, Gronchi A, Cardona K. Soft-tissue sarcoma in adults: an update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J Clin 2020, 70, 200–29. [DOI] [PubMed] [Google Scholar]
- 2. Polito L, Calafato G, Bortolotti M, Chiarelli Olivari C, Maiello S, Bolognesi A. Antibody conjugates for sarcoma therapy: how far along are we? Biomedicines 2021, 9, 978. doi: 10.3390/biomedicines9080978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fordham AM, Ekert PG, Fleuren EDG. Precision medicine and phosphoproteomics for the identification of novel targeted therapeutic avenues in sarcomas. Biochim Biophys Acta Rev Cancer 2021, 1876, 188613. [DOI] [PubMed] [Google Scholar]
- 4. Tang F, Tie Y, Wei YQ, Tu CQ, Wei XW. Targeted and immuno-based therapies in sarcoma: mechanisms and advances in clinical trials. Biochim Biophys Acta Rev Cancer 2021, 1876, 188606. [DOI] [PubMed] [Google Scholar]
- 5. Zambo I, Veselý K. [WHO classification of tumours of soft tissue and bone 2013: the main changes compared to the 3rd edition]. Cesk Patol 2014, 50, 64–70. [PubMed] [Google Scholar]
- 6. D’Angelo SP, Mahoney MR, Van Tine BA, Atkins J, Milhem MM, Jahagirdar BN, et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol 2018, 19, 416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol 2017, 18, 1493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fabian KP, Hodge JW. The emerging role of off-the-shelf engineered natural killer cells in targeted cancer immunotherapy. Mol Ther Oncolytics 2021, 23, 266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang J, Toregrosa-Allen S, Elzey BD, Utturkar S, Lanman NA, Bernal-Crespo V, et al. Multispecific targeting of glioblastoma with tumor microenvironment-responsive multifunctional engineered NK cells. Proc Natl Acad Sci USA 2021, 118, e2107507118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jan CI, Huang SW, Canoll P, Bruce JN, Lin YC, Pan CM, et al. Targeting human leukocyte antigen G with chimeric antigen receptors of natural killer cells convert immunosuppression to ablate solid tumors. J ImmunoTher Cancer 2021, 9, e003050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leivas A, Valeri A, Córdoba L, García-Ortiz A, Ortiz A, Sánchez-Vega L, et al. NKG2D-CAR-transduced natural killer cells efficiently target multiple myeloma. Blood Cancer J 2021, 11, 146. doi: 10.1038/s41408-021-00537-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fernández L, Metais JY, Escudero A, Vela M, Valentín J, Vallcorba I, et al. Memory T cells expressing an NKG2D-CAR efficiently target osteosarcoma cells. Clin Cancer Res 2017, 23, 5824–35. [DOI] [PubMed] [Google Scholar]
- 13. Tullius BP, Setty BA, Lee DA. Natural killer cell immunotherapy for osteosarcoma. Adv Exp Med Biol 2020, 1257, 141–54. [DOI] [PubMed] [Google Scholar]
- 14. Quamine AE, Olsen MR, Cho MM, Capitini CM. Approaches to enhance natural killer cell-based immunotherapy for pediatric solid tumors. Cancers (Basel) 2021, 13, 2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kailayangiri S, Altvater B, Spurny C, Jamitzky S, Schelhaas S, Jacobs AH, et al. Targeting Ewing sarcoma with activated and GD2-specific chimeric antigen receptor-engineered human NK cells induces upregulation of immune-inhibitory HLA-G. Oncoimmunology 2017, 6, e1250050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lachota M, Vincenti M, Winiarska M, Boye K, Zagożdżon R, Malmberg KJ. Prospects for NK Cell therapy of sarcoma. Cancers (Basel) 2020, 12, 3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fortes-Andrade T, Almeida JS, Sousa LM, Santos-Rosa M, Freitas-Tavares P, Casanova JM, et al. The role of natural killer cells in soft tissue sarcoma: prospects for immunotherapy. Cancers (Basel) 2021, 13, 3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu Z, Zhang H, Wu M, Peng G, He Y, Wan N, et al. Targeting the NKG2D/NKG2D-L axis in acute myeloid leukemia. Biomed Pharmacother 2021, 137, 111299. doi: 10.1016/j.biopha.2021.111299. [DOI] [PubMed] [Google Scholar]
- 19. Paczulla AM, Rothfelder K, Raffel S, Konantz M, Steinbacher J, Wang H, et al. Absence of NKG2D ligands defines leukaemia stem cells and mediates their immune evasion. Nature 2019, 572, 254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest 2014, 124, 30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmidt O, Nehls N, Prexler C, von Heyking K, Groll T, Pardon K, et al. Class I histone deacetylases (HDAC) critically contribute to Ewing sarcoma pathogenesis. J Exp Clin Cancer Res 2021, 40, 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Noh JH, Bae HJ, Eun JW, Shen Q, Park SJ, Kim HS, et al. HDAC2 provides a critical support to malignant progression of hepatocellular carcinoma through feedback control of mTORC1 and AKT. Cancer Res 2014, 74, 1728–38. [DOI] [PubMed] [Google Scholar]
- 23. Biersack B, Polat S, Höpfner M. Anticancer properties of chimeric HDAC and kinase inhibitors. Semin Cancer Biol 2022, 83, 472–86. [DOI] [PubMed] [Google Scholar]
- 24. Emmett MJ, Lazar MA. Integrative regulation of physiology by histone deacetylase 3. Nat Rev Mol Cell Biol 2019, 20, 102–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baretti M, Yarchoan M. Epigenetic modifiers synergize with immune-checkpoint blockade to enhance long-lasting antitumor efficacy. J Clin Invest 2021, 131, e151002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Riggs MG, Whittaker RG, Neumann JR, Ingram, VM. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature 1977, 268, 462–4. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- 27. Karagiannis D, Rampias T. HDAC inhibitors: dissecting mechanisms of action to counter tumor heterogeneity. Cancers (Basel) 2021, 13, 3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oliveira T, Hermann E, Lin D, Chowanadisai W, Hull E, Montgomery M. HDAC inhibition induces EMT and alterations in cellular iron homeostasis to augment ferroptosis sensitivity in SW13 cells. Redox Biol 2021, 47, 102149. doi: 10.1016/j.redox.2021.102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xie J, Wang Z, Fan W, Liu Y, Liu F, Wan X, et al. Targeting cancer cell plasticity by HDAC inhibition to reverse EBV-induced dedifferentiation in nasopharyngeal carcinoma. Signal Transduct Target Ther 2021, 6, 333. doi: 10.1038/s41392-021-00702-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hirano M, Imai Y, Kaito Y, Murayama T, Sato K, Ishida T, et al. Small-molecule HDAC and Akt inhibitors suppress tumor growth and enhance immunotherapy in multiple myeloma. J Exp Clin Cancer Res 2021, 40, 110. doi: 10.1186/s13046-021-01909-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ferrari de Andrade L, Kumar S, Luoma AM, Ito Y, Alves da Silva PH, Pan D, et al. Inhibition of MICA and MICB shedding elicits NK-cell-mediated immunity against tumors resistant to cytotoxic T cells. Cancer Immunol Res 2020, 8, 769–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Diermayr S, Himmelreich H, Durovic B, Mathys-Schneeberger A, Siegler U, Langenkamp U, et al. NKG2D ligand expression in AML increases in response to HDAC inhibitor valproic acid and contributes to allorecognition by NK-cell lines with single KIR-HLA class I specificities. Blood 2008, 111, 1428–36. doi: 10.1182/blood-2007-07-101311. [DOI] [PubMed] [Google Scholar]
- 33. Zocchi MR, Catellani S, Canevali P, Tavella S, Garuti A, Villaggio B, et al. High ERp5/ADAM10 expression in lymph node microenvironment and impaired NKG2D ligands recognition in Hodgkin lymphomas. Blood 2012, 119, 1479–89. [DOI] [PubMed] [Google Scholar]
- 34. Wu X, Tao Y, Hou J, Meng X, Shi J. Valproic acid upregulates NKG2D ligand expression through an ERK-dependent mechanism and potentially enhances NK cell-mediated lysis of myeloma. Neoplasia 2012, 14, 1178–89. doi: 10.1593/neo.121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bhat J, Dubin S, Dananberg A, Quabius ES, Fritsch J, Dowds CM, et al. Histone deacetylase inhibitor modulates NKG2D receptor expression and memory phenotype of human Gamma/Delta T cells upon interaction with tumor cells. Front Immunol 2019, 10, 569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cho H, Son WC, Lee YS, Youn EJ, Kang CD, Park YS, et al. Differential effects of histone deacetylases on the expression of NKG2D ligands and NK cell-mediated anticancer immunity in lung cancer cells. Molecules 2021, 26, 3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Satwani P, Bavishi S, Saha A, Zhao F, Ayello J, van de Ven C, et al. Upregulation of NKG2D ligands in acute lymphoblastic leukemia and non-Hodgkin lymphoma cells by romidepsin and enhanced in vitro and in vivo natural killer cell cytotoxicity. Cytotherapy 2014, 16, 1431–40. [DOI] [PubMed] [Google Scholar]
- 38. Bressan A, Bigioni M, Bellarosa D, Nardelli F, Irrissuto C, Maggi CA, et al. Induction of a less aggressive phenotype in human colon carcinoma HCT116 cells by chronic exposure to HDAC inhibitor SAHA. Oncol Rep 2010, 24, 1249–55. [DOI] [PubMed] [Google Scholar]
- 39. Schmudde M, Braun A, Pende D, Sonnemann J, Klier U, Beck JF, et al. Histone deacetylase inhibitors sensitize tumour cells for cytotoxic effects of natural killer cells. Cancer Lett 2008, 272, 110–21. [DOI] [PubMed] [Google Scholar]
- 40. Ghayad SE, Rammal G, Sarkis O, Basma H, Ghamloush F, Fahs A, et al. The histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) as a therapeutic agent in rhabdomyosarcoma. Cancer Biol Ther 2019, 20, 272–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Di Martile M, Desideri M, Tupone MG, Buglioni S, Antoniani B, Mastroiorio C, et al. Histone deacetylase inhibitor ITF2357 leads to apoptosis and enhances doxorubicin cytotoxicity in preclinical models of human sarcoma. Oncogenesis 2018, 7, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lopez G, Liu J, Ren W, Wei W, Wang S, Lahat G, et al. Combining PCI-24781, a novel histone deacetylase inhibitor, with chemotherapy for the treatment of soft tissue sarcoma. Clin Cancer Res 2009, 15, 3472–83. [DOI] [PubMed] [Google Scholar]
- 43. Thomas S, Aggarwal R, Jahan T, Ryan C, Troung T, Cripps AM, et al. A phase I trial of panobinostat and epirubicin in solid tumors with a dose expansion in patients with sarcoma. Ann Oncol 2016, 27, 947–52. doi: 10.1093/annonc/mdw044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zeng H, Qu J, Jin N, Xu J, Lin C, Chen Y, et al. Feedback activation of leukemia inhibitory factor receptor limits response to histone deacetylase inhibitors in breast cancer. Cancer Cell 2016, 30, 459–73. doi: 10.1016/j.ccell.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 45. Tam YK, Miyagawa B, Ho VC, Klingemann HG. Immunotherapy of malignant melanoma in a SCID mouse model using the highly cytotoxic natural killer cell line NK-92. J Hematother 1999, 8, 281–90. doi: 10.1089/106161299320316. [DOI] [PubMed] [Google Scholar]
- 46. Klingemann H, Boissel L, Toneguzzo F. Natural killer cells for immunotherapy - advantages of the NK-92 cell line over blood NK cells. Front Immunol 2016, 7, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Eckschlager T, Plch J, Stiborova M, Hrabeta J. Histone deacetylase inhibitors as anticancer drugs. Int J Mol Sci 2017, 18, 1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Que Y, Zhang XL, Liu ZX, Zhao JJ, Pan QZ, Wen XZ, et al. Frequent amplification of HDAC genes and efficacy of HDAC inhibitor chidamide and PD-1 blockade combination in soft tissue sarcoma. J ImmunoTher Cancer 2021, 9, e001696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Anne M, Sammartino D, Barginear MF, Budman D. Profile of panobinostat and its potential for treatment in solid tumors: an update. Onco Targets Ther 2013, 6, 1613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Klein JM, Henke A, Sauer M, Bessler M, Reiners KS, Engert A, et al. The histone deacetylase inhibitor LBH589 (panobinostat) modulates the crosstalk of lymphocytes with Hodgkin lymphoma cell lines. PLoS One 2013, 8, e79502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Qin G, Li Y, Xu X, Wang X, Zhang K, Tang Y, et al. Panobinostat (LBH589) inhibits Wnt/β-catenin signaling pathway via upregulating APCL expression in breast cancer. Cell Signal 2019, 59, 62–75. doi: 10.1016/j.cellsig.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 52. Lee NR, Kim DY, Jin H, Meng R, Chae OH, Kim SH, et al. Inactivation of the Akt/FOXM1 signaling pathway by panobinostat suppresses the proliferation and metastasis of gastric cancer cells. Int J Mol Sci 2021, 22, 5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Afolabi LO, Bi J, Li X, Adeshakin AO, Adeshakin FO, Wu H, et al. Synergistic tumor cytolysis by NK cells in combination with a pan-HDAC inhibitor, panobinostat. Front Immunol 2021, 12, 701671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sim MJW, Rajagopalan S, Altmann DM, Boyton RJ, Sun PD, Long EO. Human NK cell receptor KIR2DS4 detects a conserved bacterial epitope presented by HLA-C. Proc Natl Acad Sci USA 2019, 116, 12964–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Battin C, Kaufmann G, Leitner J, Tobias J, Wiedermann U, Rölle A, et al. NKG2A-checkpoint inhibition and its blockade critically depends on peptides presented by its ligand HLA-E. Immunology 2022, 166, 507–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Deng Z, Zhen J, Harrison GF, Zhang G, Chen R, Sun G, et al. Adaptive admixture of HLA Class I allotypes enhanced genetically determined strength of natural killer cells in East Asians. Mol Biol Evol 2021, 38, 2582–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories.