Abstract
Acute systemic inflammation can lead to life-threatening organ dysfunction. In patients with sepsis, systemic inflammation is triggered in response to infection, but in other patients, a systemic inflammatory response syndrome (SIRS) is triggered by non-infectious events. IL-6 is a major mediator of inflammation, including systemic inflammatory responses. In homeostatic conditions, when IL-6 engages its membrane-bound receptor on myeloid cells, it promotes pro-inflammatory cytokine production, phagocytosis, and cell migration. However, under non-physiologic conditions, such as SIRS and sepsis, leucocyte dysfunction could modify the response of these cells to IL-6. So, our aim was to evaluate the response to IL-6 of monocytes from patients diagnosed with SIRS or sepsis. We observed that monocytes from patients with SIRS, but not from patients with sepsis, produced significantly more TNF-α than monocytes from healthy volunteers, after stimulation with IL-6. Monocytes from SIRS patients had a significantly increased baseline phosphorylation of the p65 subunit of NF-κB, with no differences in STAT3 phosphorylation or SOCS3 levels, compared with monocytes from septic patients, and this increased phosphorylation was maintained during the IL-6 activation. We found no significant differences in the expression levels of the membrane-bound IL-6 receptor, or the serum levels of IL-6, soluble IL-6 receptor, or soluble gp130, between patients with SIRS and patients with sepsis. Our results suggest that, during systemic inflammation in the absence of infection, IL-6 promotes TNF-α production by activating NF-κB, and not the canonical STAT3 pathway.
Keywords: systemic inflammatory response syndrome, sepsis, mIL-6R, sIL-6R, NF-κB p-p65
Acute and severe systemic inflammation is found in patients with sepsis and with systemic inflammatory response syndrome (SIRS). Sepsis is a deregulated host response to infection, while SIRS is a deregulated host response to tissue damage. Peripheral blood monocytes from patients with SIRS, but not from patients with sepsis, produced significantly more TNF-α than monocytes from healthy volunteers in response to IL-6, and had a significantly increased baseline phosphorylation of the pro-inflammatory p65 subunit of NF-κB, with no differences in key molecules of the canonical IL-6 signalling pathway. Thus, during systemic inflammation without infection, IL-6 promotes TNF-α production by activating NF-κB in monocytes.
Graphical Abstract
Graphical Abstract.
Introduction
The systemic inflammatory response syndrome (SIRS) can be triggered by non-infectious events, such as surgery, acute pancreatitis, and burn injuries, and is clinically defined by nonspecific criteria (fever or hypothermia, tachycardia, tachypnea, hypoxia and leucocytosis, leukopenia or bandemia) [1, 2]. Biologically, SIRS is a deregulated host response to alarmins or damage-associated molecular patterns (DAMPs) that triggers acute systemic inflammation. On the other hand, sepsis is defined as a deregulated host response to infection that causes life-threatening organ dysfunction [3–5]. Patients with acute and severe systemic inflammation include both patients with sepsis and patients with SIRS [6]. Sepsis is a leading cause of morbidity and mortality in intensive care units worldwide, with 27 to 30 million cases reported annually and 7 to 9 million deaths [7]. In both SIRS and sepsis, systemic inflammation is associated with the activation of endothelial cells and of the complement and the coagulation systems. The systemic effects of pro-inflammatory cytokines can lead to increased capillary permeability and to organ failure, while the anti-inflammatory response can lead to immunosuppression and a higher risk to develop secondary infections. During systemic inflammation, many immune cells undergo apoptosis, including T cells, B cells, NK cells, and dendritic cells, and the remaining T cells express markers of cell exhaustion. In addition, the antimicrobial capacity of neutrophils, monocytes, and macrophages is impaired, and the production of pro-inflammatory cytokines by monocytes and macrophages is reduced [8–12].
IL-6 is produced early in the inflammatory process and causes maturation and activation of neutrophils, maturation of macrophages, and differentiation and maintenance of cytotoxic T cells and NK cells; it also stimulates TNF-α and IL-1β production [13]. Additionally, IL-6 induces the release of acute-phase proteins (C-reactive protein, amyloid A protein, and fibrinogen) by hepatocytes [14]. IL-6 is recognized by a membrane IL-6 receptor (mIL-6R) that is expressed on monocytes, neutrophils, and hepatocytes [15]. Upon IL-6 recognition, mIL-6R associates with two molecules of the signal-transducing glycoprotein 130 (gp130), which activate the JAK-STAT3 pathway and the phosphatidylinositol 3-kinase-dependent pathway, in what is known as the canonical or classic IL-6 signalling [16]. SOCS3 is an inhibitor of the JAK-STAT3 pathway, because this molecule inhibits the JAK-mediated tyrosine phosphorylation of gp130, and thus prevents the binding, phosphorylation, dimerization, and nuclear translocation of STAT3 [17]. The soluble IL-6 receptor (sIL-6R) is generated as a result of the cleavage of mIL-6R by the membrane-bound proteases ADAM10 and ADAM17 [18]. IL-6, in a complex with sIL-6R, can activate cells that lack mIL-6R but express gp130, in what is known as IL-6 trans-signalling [19]. Endothelial cells lack mIL-6R, but are nevertheless activated by IL-6 trans-signalling to release the chemokines IL-8 and MCP-1, amplifying the IL-6-mediated inflammatory response [20]. A soluble form of gp130 binds to the IL-6/sIL-6R complex and inhibits IL-6 trans-signalling, but has no effect on classic IL-6 signalling [21].
The canonical IL-6 signalling induces the acute-phase response in hepatocytes and immune cells, and has protective, regulatory, and anti-apoptotic functions [22]. However, during systemic inflammation, the recognition of IL-6 is associated with inflammatory disorders, since STAT3 increases MyD88- and MAPK-dependent signalling. These two signalling pathways are activated by the recognition of pathogen-associated molecular patterns (PAMPs) and DAMPs and trigger the activation of the transcription factors NF-κB and p38, respectively, thus increasing the release of inflammatory mediators [23, 24]. Serum IL-6- and the IL-6-induced C-reactive protein are increased in patients with sepsis, compared with patients with SIRS [25]. However, it is not currently known if monocytes from patients with sepsis have a different capacity to respond to pro-inflammatory stimuli such as IL-6, compared with monocytes from patients with SIRS. This work aims to characterize how monocytes from patients with sepsis or with SIRS respond to IL-6, analysing the receptors, transcription factors and regulatory molecules that participate in IL-6 classic and trans-signalling.
Materials and methods
Healthy volunteers and patients with SIRS or sepsis
This study was approved by the local Ethics and Scientific Research Committees of the Specialties Hospital UMAE-HE Dr. Bernardo Sepúlveda Gutiérrez of the Mexican Social Security Institute (R-2016-3601-185). All healthy volunteers and patients (or their family members) signed an informed consent, in accordance with the Helsinki declaration. Healthy volunteers were between 20 and 60 years old, clinically healthy and with blood chemistry results within normal values. Patients included all the individuals between 20 and 60 years old that were admitted into the Intensive Care Unit, the Internal Medicine Service or the Gastrointestinal Surgery Service of the Hospital, from January 2016 to December 2019, that were diagnosed with SIRS or with sepsis. Patients were diagnosed with SIRS when they did not show signs of infection but presented at least two of the following signs: fever or hypothermia, tachycardia, tachypnea or hypoxia, and leucocytosis, leukopenia or bandemia; being their main underlying diagnosis acute pancreatitis [2]. Patients were diagnosed with sepsis according to the Third International Consensus Definitions for Sepsis and Septic Shock [4, 5]. All the consecutive patients that met these criteria during the indicated period were included in this study. Individuals were excluded from this study if they were pregnant, had an immune deficiency, an autoimmune disease or were infected with HIV, hepatitis B virus or hepatitis C virus, or if they had received an immunosuppressant or an immunomodulator for more than 3 days immediately before entrance to this study. For each individual, the results of the white blood cell differential and serum lactate concentrations were reported. The quick Sequential Organ Failure Assessment (qSOFA) score and the Acute Physiology and Chronic Health Evaluation (APACHE II) score were also reported.
Collection of peripheral blood samples
A single peripheral blood sample was taken by venepuncture from the healthy volunteers. A single peripheral blood sample was taken from the patients, within the first 72 h after the diagnosis of SIRS or sepsis, as has been done in previous studies of patients with these pathologies [26]. The sample was taken from a central catheter with prior asepsis and drainage of the area. At least 6 mL of peripheral blood was collected in tubes with lithium heparin (17 IU/mL, BD Vacutainer, BD Biosciences, San José, CA, reference 367884) and processed for immunophenotyping and functional analysis within 4 h after sampling. In addition, 6 mL of peripheral blood was collected in tubes without anticoagulant; these tubes were centrifuged at 2500 rpm for 10 min and the serum was stored at −70°C until use.
Quantification of serum cytokines and cytokine receptors
Serum cytokines were quantified with a Cytometric Bead Array (BD Biosciences) that measures IL-1β, IL-6, IL-8, IL-10, and TNF-α (detection limits: 7.2, 2.5, 3.6, 3.3, and 3.7 pg/mL, respectively). The samples were acquired with a FACSAria flow cytometer (BD Biosciences). Log-transformed data were used to construct standard curves fitted to 10 points using a 4-parameter logistic model. The concentrations in the test samples were calculated by interpolations in their corresponding standard curves.
The serum concentrations of IL-1Ra, IL-6/ soluble IL-6 receptor (sIL-6R) complex, and soluble gp130 (sgp130) were measured with the IL-1Ra/IL-1F3 ELISA kit (R&D Systems, Minneapolis, MN), the IL-6/sIL-6R DuoSet ELISA kit (R&D Systems) and the sgp130 ELISA kit (R&D Systems), respectively (detection limits: 18.3, 2.0, and 100 pg/mL).
Quantification of serum lipopolysaccharide
Lipopolysaccharide (LPS) presence in the serum samples was evaluated with both the PYROGENT Gel Clot Limulus Amebocyte Lysate assay and the Kinetic Chromogenic Limulus Amebocyte Lysate Assay (Lonza Bioscience, Basel, Switzerland). Serum samples were diluted 1:10 in LPS-free water and inactivated for 15 min at 70°C. A 1:20 dilution of this preparation was quantified by interpolation in a standard curve. The sensitivity of the Kinetic Chromogenic Limulus Amebocyte Lysate assay is 0.0100 EU/mL.
Detection of membrane IL-6 receptor (mIL-6R) on circulating monocytes
One hundred and fifty microlitres of whole blood were mixed with 1 mL of erythrocyte lysis solution (0.15 M ammonium chloride). After 10 min, 1 mL of phosphate-buffered saline (PBS) was added, and the cells were centrifuged at 350×g for 5 min. Cells were resuspended in 150 μL of PBS, and 50 μL of this cell suspension (250 000 cells/mL) were mixed with the following antibodies: anti-CD45/Pacific Blue (BioLegend, clone J.33), anti-CD14/PECy7 (BioLegend, clone MSE2), anti-CD16/APC-Cy7 (BioLegend, clone 3G8), and anti-mIL-6R/APC (BioLegend, clone B-R6). After 15 min, 250 µL of BD FACS lysing solution were added. After 10 min, the cells were washed with 1 mL of PBS 1×, and acquired in a FACSAria flow cytometer (BD Biosciences). Data were analysed with Infinicyt software (Cytognos, Salamanca, Spain). Monocytes were identified with the following phenotype: FSCmed SSCmed CD45+ CD14+. At least 5000 events from the monocyte gate were acquired for each sample. In some experiments, monocyte subsets were identified with the following phenotypes: FSCmed SSCmed CD45+ CD14+ CD16- for classic monocytes, FSCmed SSCmed CD45+ CD14+ CD16+ for intermediate monocytes, and FSCmed SSCmed CD45+ CD14− CD16+ for non-classic monocytes (Fig. S1a).
Preparation of recombinant IL-6
Human recombinant IL-6 was purchased from Gibco (Thermo Fisher Scientific, Waltham, MA), with a purity >95% and <0.1 ng/µg of LPS. IL-6 was resuspended in 100 mM acetic acid to a concentration of 0.5 mg/mL and stored in PBS with 0.5% foetal bovine serum, in single-use aliquots, at −20°C.
Determination of intracellular TNF-α by multiparametric flow cytometry
A whole-blood stimulation assay was used to evaluate TNF-α production. Five hundred microliters of blood were mixed with 500 µL of RPMI culture medium from Gibco 1640 (Thermo Fisher Scientific, Waltham, MA), and incubated at 37°C and 5% CO2. The culture medium was supplemented with IL-6 (100 ng/mL) and brefeldin A (BioLegend) (5 μg/mL); with LPS from Escherichia coli O111:B4 (Sigma Aldrich, St. Louis, MO) (250 ng/mL) and brefeldin A (5 μg/mL); or with IL-6 (100 ng/mL), followed by LPS (250 ng/mL) and brefeldin A (5 μg/mL). After the indicated incubation times, the following antibodies were added: anti-CD45/Pacific Blue (BioLegend, clone J.33), anti-CD14/PE-Cy7 (BioLegend, clone MSE2) and anti-CD16/APC-Cy7 (BioLegend, clone 3G8). After 15 min, 50 µL of Buffer A of the Fix & Perm cell fixation and cell permeabilization kit (Thermo Fisher Scientific) were added. After 20 min, 1 mL of PBS was added, and the cells were centrifuged at 350 xg for 5 min. The cells were resuspended in 50 µL of Buffer B that contained anti-TNF-α/PE antibody (BioLegend, clone MAb11) and incubated for 30 min. The cells were washed with 1 mL of PBS and acquired in a FACSAria flow cytometer (BD Biosciences). Data was analysed with Infinicyt software (Cytognos).
Determination of p-STAT3, SOCS3 and NF-κB p-p65
Fifty microlitres of whole blood were stimulated with IL-6 (100 ng/mL) and incubated at 37°C and 5% CO2 for 15, 30, and 60 min. After this incubation, 500 μL of the BD Phosflow Lyse/Fix Buffer (BD Biosciences) were added. After 15 min, 1 mL of PBS was added, the cells were centrifuged at 350 × g for 5 min and the supernatant was discarded. 500 μL of 70% methanol were added and the cells were incubated for 30 min on ice. The cells were washed twice with 1.5 mL of PBS, and the following antibodies were added: anti-CD45/Pacific Blue (BioLegend, clone J.33), anti-CD14/PE-Cy7 (BioLegend, clone MSE2), anti-CD16/APC-Cy7 (BioLegend, clone 3G8), anti-p-STAT-3/Alexa Fluor 488 (BD Biosciences, clone 4/P-STAT3, anti pY705), anti-SOCS3/PE (Biorbyt, Cambridge, UK, clone ORB491176), and anti-NF-κB p-p65 Alexa Fluor 647 (BD Biosciences, clone K10-895.12.50, anti-pS529). After 30 min, the cells were washed with 1 mL of PBS and acquired in a FACSAria flow cytometer (BD Biosciences). Data were analysed with Infinicyt software (Cytognos).
Statistical analysis
Statistical analyses were performed with GraphPad Prism 8.0 software (GraphPad Software, San Diego, CA). The underlying disease origins were compared with Fisher´s test. Kruskal–Wallis test with Dunn’s multiple comparisons test was used to compare all other data between groups. P < 0.05 was considered statistically significant. Exploratory correspondence analysis was performed in R Core Team [27]. The principal components were calculated from the original data matrix. The missing and outlier data were replaced centring the mean in 0 and the variance in 1 for each variable. Principal component analysis (PCA) was conducted with FactoMineR [28].
Results
The levels of IL-6 and its receptors are similar among SIRS and sepsis patients
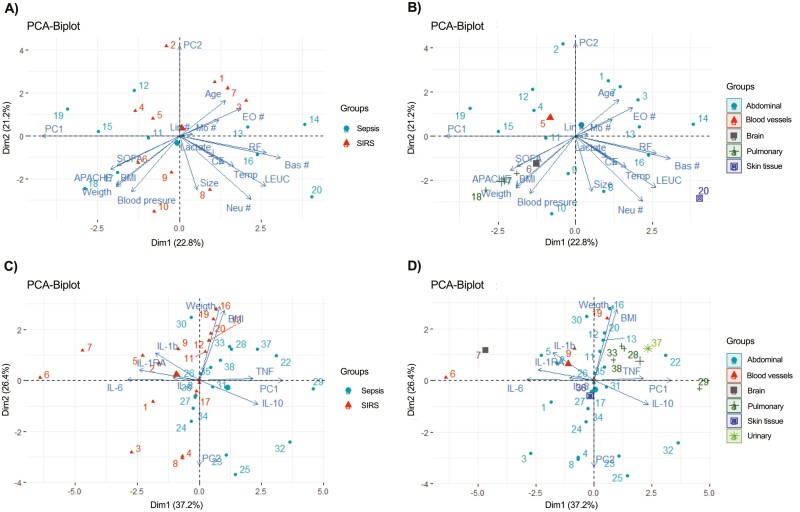
The effector functions triggered by IL-6 depend on the expression of mIL-6R on the target cells, and also on the available sIL-6R, to activate IL-6 classic or trans-signalling [29]. Here we analysed 38 patients (17 with sepsis and 21 with SIRS) and 15 healthy volunteers. As shown in Table 1, there were no significant differences in age or gender, respiratory rates, blood pressures, and temperatures among groups. Both the SIRS and sepsis groups had significant increases in heart rate, compared with the healthy volunteers. Also, the SIRS and sepsis groups presented leucocytosis with neutrophilia, although no significant differences were observed in their lymphocyte counts, compared with the healthy volunteers. As expected, SIRS and sepsis patients had higher qSOFA scores than healthy volunteers. We performed an exploratory component analysis to determine whether differences in the variables indicated in Table 1, in addition to weight, height, and body mass index (BMI), were independent of the diagnosis (SIRS or sepsis) and/or the underlying disease origin. The principal component 1 described 84.7% of the data variation, and indicated that height is the strongest contributing variable (98%) to the observed distribution. In the corresponding biplots, patients were scattered throughout the graphs, without clustering according to their diagnosis (SIRS or sepsis) (Fig. 1a) or their underlying disease origin (Fig. 1b), indicating that the clinical and biochemical variables analysed in this study were largely independent of the diagnosis and the disease origin of the patients.
Table 1.
Demographic and clinical data of the healthy volunteers and the SIRS and sepsis patients
| Healthy (n = 15) |
SIRS (n = 21) |
Sepsis (n = 17) |
|
|---|---|---|---|
| Age (years) | 40 ± 4 | 45 ± 17 | 38 ± 13 |
| Gender (F:M) | 10:5 | 14:7 | 8:9 |
| Survival after 28 days | NA | 100% | 100% |
| Severity scores | |||
| qSOFA (0–3) | 0 | 2.0 ± 4 | 2.0 ± 3.5 |
| APACHE II (0–71) | NA | 4.0 ± 9 | 9.0 ± 10 |
| Underlying disease origin | |||
| Abdominal | NA | 14 (66.7%) | 11 (64.7%) |
| Blood vessels | NA | 6 (28.6%) | 0 (0%)+ |
| Brain | NA | 1 (4.8%) | 0 (0%) |
| Pulmonary | NA | 0 (0%) | 4 (23.5%)+ |
| Skin tissue | NA | 0 (0%) | 1 (5.9%) |
| Urinary | NA | 0 (0%) | 1(5.9%) |
| Clinical signs | |||
| Heart rate (beats/min) | 77 ± 9 | 88 ± 28* | 94 ± 26* |
| Respiratory rate (breaths/min) | 19 ± 2 | 17 ± 2.6 | 18 ± 5 |
| Blood pressure (mmHg) | 108/70 | 122/78 | 109/65 |
| Temperature (°C) | 36 ± 0.3 | 36.5 ± 0.5 | 36 ± 0.4 |
| Blood cell count (×10 6 /mm 3 ) | |||
| Leukocytes | 6.9 ± 1.5 | 14.4 ± 3.5* | 14.7 ± 5.6* |
| Neutrophils | 4.0 ± 1.3 | 13.5 ± 3.5* | 22 ± 3.1* |
| Monocytes | 0.9 ± 0.1 | 0.9 ± 0.4 | 1.5 ± 0.5 |
| Lymphocytes | 3.4 ± 5.2 | 4.1 ± 1.1 | 9.2 ± 3.3 |
| Biochemical inflammation marker | |||
| Lactate (mM) | 0.5 | 1.9 ± 1.5 | 2.0 ± 1.1 |
APACHE II, Acute Physiology and Chronic Health Evaluation. NA, not applicable. qSOFA, quick Sequential Organ Failure Assessment. Data represent n, n (%) or mean ± standard error of the mean.
*P < 0.05 between SIRS or sepsis patients and healthy volunteers, Kruskal–Wallis test with Dunn’s multiple comparisons test.
+P < 0.05 between SIRS and sepsis patients, Fisher’s test.
There were no significant differences between SIRS and sepsis patients in their ages, gender distribution, severity scores, clinical signs, blood cell counts or lactate concentrations.
Figure 1:
principal component analysis of clinical and biochemical variables, and serum cytokines, in patients with SIRS and sepsis. (A) Principal component analysis (PCA) of clinical and biochemical variables, for patients grouped into SIRS and sepsis. (B) PCA of clinical and biochemical variables, for patients grouped according to their underlying disease origin. (C) PCA of serum cytokines, for patients grouped into SIRS and sepsis. (D) PCA of serum cytokines, for patients grouped according to their underlying disease origin; the “urinary” cluster is not shown in (B) because it does not influence the first component in this PCA. The explained percentage of the total variance for each component is shown in parentheses.
The serological concentrations of the pro-inflammatory cytokines TNF-α, IL-1β, and IL-8 were increased in both SIRS and sepsis patients, compared to healthy volunteers, as were the anti-inflammatory cytokines IL-10 and IL-1Ra. However, no significant differences were observed in the serum cytokine concentrations between SIRS and sepsis patients. We did not find any differences in serum LPS levels in the analysed groups (Table 2). We performed an exploratory component analysis to determine whether the serum cytokine concentrations were independent of the diagnosis (SIRS or sepsis) and/ or the underlying disease origin. The corresponding biplots indicated that patients did not cluster according to these variables (Fig. 1c and d).
Table 2:
serum markers in the healthy volunteers and patients
| Serological concentration | Healthy (n = 8) |
SIRS (n = 7) |
Sepsis (n = 12) |
|---|---|---|---|
| TNF-α (pg/mL) | 3.6 ± 0.4 | 13.7 ± 8* | 15.5 ± 9* |
| IL-1β (pg/mL) | 4.5 ± 0.1 | 28.8 ± 5* | 26.5 ± 6* |
| IL-8 (pg/mL) | 15.9 ± 8.4 | 70 ± 30* | 61.8 ± 50* |
| IL-10 (pg/mL) | 3.6 ± 0.1 | 20.6 ± 5* | 54.5 ± 30* |
| IL-1Ra(pg/mL) | 134.7 ± 60.4 | 2360 ± 220* | 2353 ± 233* |
| LPS(EU/mL) | < 0.01 | < 0.01 | < 0.01 |
TNF-α, tumour necrosis factor-alpha; IL, interleukin; LPS, lipopolysaccharide.
Data represent mean ± standard error of the mean.
*P < 0.05 between SIRS or sepsis patients and healthy volunteers, Kruskal–Wallis test with Dunn’s multiple comparisons test.
There were no significant differences in the cytokine or LPS concentrations between SIRS and sepsis patients.
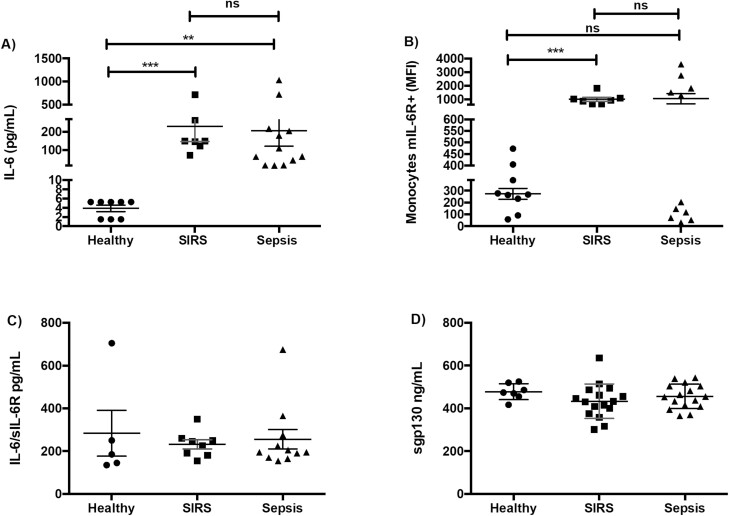
In agreement with previous reports [30], we found, compared with healthy volunteers, increased levels of IL-6 in the serum from patients with SIRS and sepsis, with no differences between them (Fig. 2a). The expression levels of mIL-6R, which initiates classic IL-6 signalling, on peripheral blood monocytes, and the serum concentrations of the soluble IL-6 receptor (sIL-6R), which initiates IL-6 trans-signalling, were not significantly different between the SIRS and sepsis groups (Fig. 2b and c), and the same was observed with soluble gp130, a negative regulator of IL-6 trans-signalling (Fig. 2d). The percentages of the classic, intermediate and non-classic monocyte subsets were not significantly different between our three study groups (Fig. S1b), and the expression levels of mIL-6R were not significantly different between the monocyte subsets in any of the study groups (data not shown).
Figure 2:
The levels of serum IL-6 and its receptors are similar in patients with SIRS or sepsis. (A) Serum concentration of IL-6 in healthy volunteers (n = 8), patients with SIRS (n = 7) and patients with sepsis (n = 12). (B) Mean fluorescence intensity (MFI) of the IL-6 membrane receptor (mIL-6R) on peripheral blood monocytes (FSCmed SSCmed CD45+ CD14+) of healthy volunteers (n = 9), patients with SIRS (n = 7) and patients with sepsis (n = 11). (C) Serum concentration of the soluble IL-6 receptor (sIL-6R) in healthy volunteers (n = 5), patients with SIRS (n = 8) and patients with sepsis (n = 11). (D) Serum concentration of the soluble gp130 receptor (sgp130) in healthy volunteers (n = 7), patients with SIRS (n = 16) and patients with sepsis (n = 16). Graphs represent individual data (each symbol represents data from one individual), mean and standard error of the mean. ns, not significant, **P < 0.01, ***P < 0.001, Kruskal–Wallis test with Dunn’s multiple comparisons test.
The response to IL-6 of monocytes from SIRS or sepsis patients is associated with differences in NF-κB activation
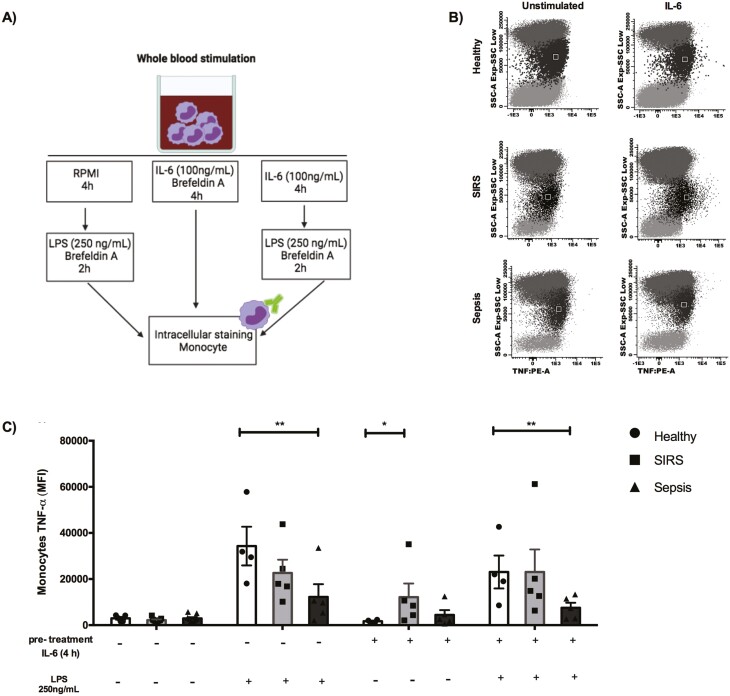
Since peripheral blood monocytes from SIRS and sepsis patients can have a differential functional phenotype [31], we hypothesized that the response to IL-6 would be different in monocytes from patients with SIRS than in monocytes from patients with sepsis. Blood from healthy volunteers and from patients with SIRS or sepsis was treated with IL-6, with LPS (a TLR4 ligand), or with IL-6 followed by LPS, to evaluate if IL-6 pre-treatment modified the monocyte response to LPS in each of the study groups (Fig. 3a), since IL-6 has been shown to increase PAMP-triggered MyD88-dependent TLR signalling [19]. Monocytes from patients with SIRS produced significantly more TNF-α than monocytes from healthy volunteers after 4 h of treatment with IL-6 (Fig. 3b and c). In fact, after this IL-6 treatment, TNF-α production by monocytes from SIRS patients increased 7-fold, compared with monocytes from healthy volunteers, while TNF-α production by monocytes from septic patients was similar to the production by monocytes from healthy volunteers (Fig. S2).
Figure 3:
IL-6-induced TNF-α is increased in monocytes from patients with SIRS. (A) Representation of the experimental conditions. (B) Dot plots representing TNF-α production in peripheral blood leukocytes from a healthy volunteer, a patient with SIRS and a patient with sepsis, with or without stimulation with IL-6 (100 ng/mL) for 4 h. The black region in the dot plots represents the monocytes (SSCmed). (C) Peripheral blood was cultured with RPMI alone, or with RPMI supplemented with IL-6 (100 ng/mL) and brefeldin A (5 μg/mL) for 4 h; with LPS (250 ng/mL) and brefeldin A (5 μg/mL) for 2 h; or with IL-6 (100 ng/mL) for 4 h, followed by LPS (250 ng/mL) and brefeldin A (5 μg/mL) for 2 h [healthy volunteers (circles), n = 4; patients with SIRS (squares), n = 5; patients with sepsis (triangles), n = 5]. Production of TNF-α by monocytes (FSCmed SSCmed CD45+ CD14+) was analysed by flow cytometry. The mean fluorescence intensity (MFI) of TNF-α is shown. Graphs represent individual data (each symbol represents data from one individual), mean and standard error of the mean. Data from healthy volunteers, patients with SIRS and patients with sepsis with the same treatment were analysed with Kruskal–Wallis test and Dunn’s multiple comparisons test. *P < 0.05, **P < 0.01.
Monocytes from septic patients produced significantly less TNF-α in response to LPS than monocytes from healthy volunteers (Fig. 3c). After LPS activation, TNF-α production by monocytes from healthy volunteers increased 25-fold, compared with non-stimulated cells, while the increase observed in monocytes from sepsis patients was only 5-fold (Fig. S2). However, pre-treatment with IL-6 did not affect the levels of TNF-α produced in response to LPS by monocytes from healthy volunteers, from patients with SIRS or from patients with sepsis; under this experimental condition, the levels of TNF-α produced by monocytes from sepsis patients after LPS activation remained significantly lower than the levels produced by monocytes from healthy volunteers (Fig. 3c and Fig. S2).
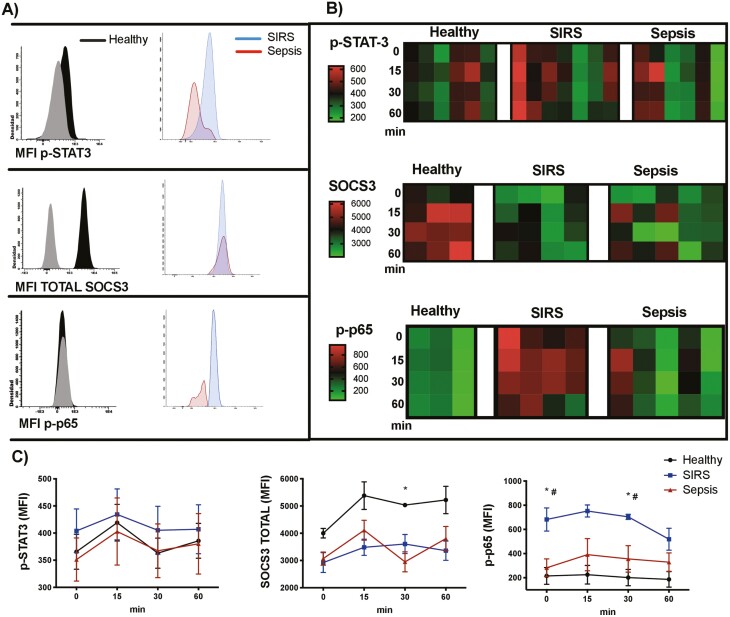
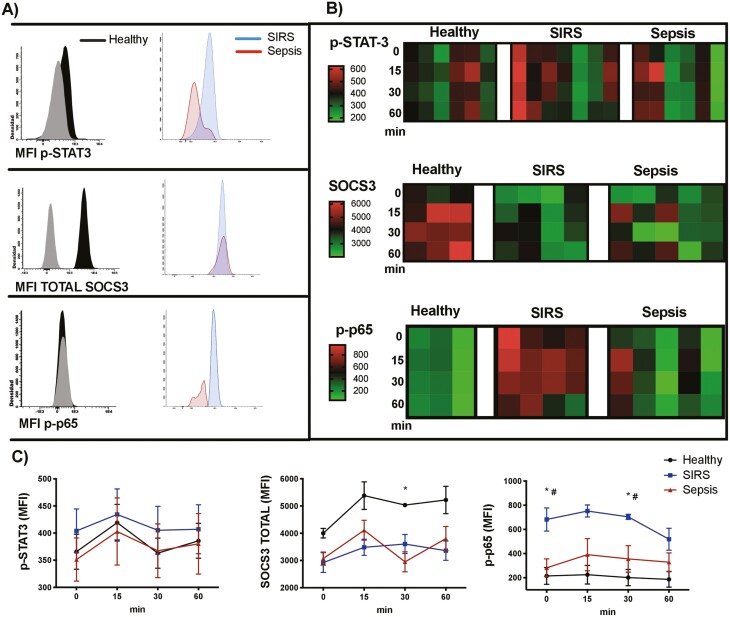
Because STAT3 is the canonical pathway activated by IL-6, we evaluated STAT3 phosphorylation (p-STAT3) after stimulating the monocytes from SIRS or sepsis patients and from healthy volunteers with IL-6. After 15, 30, and 60 min of treatment with IL-6, STAT3 phosphorylation showed no significant differences among the analysed groups. SOCS3, the major negative regulator of IL-6, increased its levels in monocytes from healthy volunteers after 30 min of IL-6 treatment, but was not modified in monocytes from patients with SIRS or from patients with sepsis (Fig. 4a, b and c). We then evaluated the phosphorylation of p65 (p-p65), the pro-inflammatory subunit of NF-κB [32]. We found that monocytes from SIRS patients had a significantly increased baseline phosphorylation of p65, compared with monocytes from sepsis patients and from healthy volunteers, and this increased phosphorylation was maintained after 30 min of IL-6 treatment. In monocytes from patients with sepsis and from healthy volunteers, no significant differences in p-p65 were observed after IL-6 treatment (Fig. 4a, b and c).
Figure 4:
NF-κB activation, but not the canonical STAT3 IL-6 pathway, is increased in patients with SIRS. (A) Histograms showing fluorescence minus one controls (grey) and p-STAT3, SOCS3 and p-p65 (NF-κB) staining in monocytes (FSCmed SSCmed CD45+ CD14+) from healthy volunteers, patients with SIRS and patients with sepsis, after stimulation with IL-6 (100 ng/mL) for 30 min (the histograms represent merged data from all the individuals in each group). (B) Heat maps representing the mean fluorescence intensity (MFI) of p-STAT3, SOCS3, and p-p65 (NF-κB) in monocytes, after activation of peripheral blood with 100 ng/mL of IL-6 for 15, 30, and 60 min (each column represents the results of a healthy volunteer, a patient with SIRS or a patient with sepsis). (C) Activation kinetics of p-STAT-3, SOCS3, and p-p65 (NF-κB) in monocytes, after activation of peripheral blood with 100 ng/mL of IL-6; the graphs represent means and standard error of the mean. Kruskal–Wallis test with Dunn’s multiple comparisons test. For SOCS3: *P < 0.05 in healthy volunteers vs. patients with sepsis at 30 min. For p-p65 (NF-κB): *P < 0.05 in healthy volunteers vs. patients with SIRS at 0 and 30 min. #P < 0.05 in patients with SIRS vs. patients with sepsis at 0 and 30 min.
Discussion
SIRS and sepsis are leading causes of morbidity and mortality in intensive care units worldwide. In a previous study, we found that patients with SIRS or with sepsis have similar clinical parameters, symptoms, concentrations of pro- and anti-inflammatory cytokines, percentages of circulating immune cells, and expression levels of molecules associated with cellular activation (CD69, HLA-DR) and phagocytosis (CD16) [33]. However, septic patients have an increased prevalence of blood neutrophils expressing CD11c and CD64, compared with SIRS patients [26]. The SIRS and sepsis patients included in this study had no significant differences in their clinical parameters (such as heart rate, respiratory rate, blood leukocytes or qSOFA score or APACHE II score). The serum levels of LPS, a TLR4 ligand that is derived from Gram-negative bacteria, were not significantly increased in the sepsis patients. In addition, principal component analysis indicated that the clinical and biochemical variables analysed in this study were largely independent of the diagnosis (SIRS or sepsis) or the disease origin of these patients.
The SIRS and sepsis included in this study had leucocytosis with neutrophilia, but no significant differences were observed in their lymphocyte counts, compared to the healthy volunteers. This is in contrast with previous studies that report apoptosis-induced lymphopenia in patients with SIRS and sepsis [34]. However, this lymphopenia is usually associated with poor clinical outcomes [34]. All of the patients included in this study had a positive clinical outcome (survival after 28 days), which could explain why they did not have lymphopenia within the first 72 h after the diagnosis of SIRS or sepsis.
Both groups of patients had increased serum levels of pro- and anti-inflammatory cytokines, and this mixed cytokine environment has been described as mixed antagonist response syndrome [35, 36]. We found no differences in the serum IL-6 concentrations between SIRS and sepsis patients. Selberg et al. reported that the plasma concentrations of procalcitonin and C3a (a key product of complement activation) are significantly higher in patients with sepsis than in patients with SIRS [25]. These authors also found that IL-6 levels are increased in patients with sepsis, compared to patients with SIRS; however, their sepsis group includes patients with septic shock. Our study included patients that were diagnosed with sepsis according to the Third International Consensus Definitions for Sepsis and Septic Shock [4, 5], but did not include patients with septic shock, which could explain the absence of increased serum IL-6 concentration in our patients with sepsis, compared with those with SIRS.
In addition to changes in serological markers, functional differences have been reported in the peripheral blood monocytes from SIRS and sepsis patients. Reyes et al. identified eight genes (PLAC8, CLU, RETN, CD63, ALOX5AP, SEC61G, TXN, and MT1X) whose co-expression in peripheral blood monocytes allowed the discrimination of patients with sepsis from patients with sterile inflammation [31]. Here we investigated if peripheral blood monocytes from patients with SIRS and from patients with sepsis had a differential response to IL-6, and we analysed the receptors (soluble or membrane-bound), transcription factors and regulatory molecules involved in this response. We found no differences in mIL-6R expression levels on monocytes, or in the serum concentrations of IL-6/ sIL-6R complex and sgp130, between the patients with SIRS and the patients with sepsis. Plasma or serum IL-6 concentrations have been reported to be very low in healthy volunteers (1–10 pg/mL). In contrast, sIL-6R levels range from 25 to 75 ng/mL and sgp130 levels range from 100 to 400 ng/mL in healthy volunteers. In acute or chronic systemic inflammatory processes, the levels of sIL-6R and sgp130 do not change significantly, while IL-6 levels increase [29], which is in agreement with what we report regarding the levels of these molecules. These results indicate that the main molecules involved in IL-6 recognition are found at similar level in patients with SIRS and sepsis. In the sepsis group, the levels of mIL-6R expression defined two clusters of patients (Fig. 2b). However, these levels of mIL-6R did not correlate with the amount of TNF-α that was produced in response to IL-6, or with the phosphorylation of the p65 subunit of NF-κB in response to IL-6 (Spearman’s rank correlation coefficient <0.6, P > 0.05).
Monocytes are essential effector cells during inflammation. During acute systemic inflammation, monocytes decrease their phagocytic capacity [33] and their capacity to produce pro-inflammatory cytokines in response to TLR ligands [37]. A first exposure to a pro-inflammatory stimulus induces chromatin modifications and changes in microRNAs that lead to a reduced production of pro-inflammatory cytokines after a second exposure to the pro-inflammatory stimulus. This observation was first reported for LPS, which is a TLR4 ligand, and so it is known as endotoxin tolerance [37]. However, monocyte tolerance also occurs in response to other TLR agonists and to cytokines, such as IL-1β [37, 38]. TNF-α is considered as one of the best markers of endotoxin tolerance, although other cytokines, including IL-6 and IL-1β, behave similarly [39, 40]. Pre-exposure of monocytes to infectious microorganisms not only alters their subsequent response to TLR ligands: it also decreases the activation of signalling pathways associated with cytokines, such as STAT3 and STAT1 [41]. However, it is currently unknown if monocytes from patients with SIRS and from patients with sepsis respond differentially to IL-6. Here we report that monocytes from patients with SIRS, but not from patients with sepsis, produced TNF-α after a 4-h activation with IL-6. The monocytes from both groups of patients were functional, since they produced TNF-α after activation with LPS, and the monocytes from the patients with sepsis produced less TNF-α than the monocytes from the healthy volunteers in response to this TLR4 ligand, which is in accordance with previous reports of tolerance to LPS in monocytes from patients with sepsis [42].
To establish if the canonical pathway activated by IL-6 is involved in the differential production of TNF-α by monocytes from patients with SIRS that we observed, we evaluated p-STAT3, SOCS3 (its mayor negative regulator) and the p-p65 subunit of NF-κB. No differences were observed in the phosphorylation levels of STAT3 in monocytes from healthy volunteers or from patients with SIRS or sepsis, but the levels of SOCS3 were increased in healthy volunteers, which indicates that classic IL-6 signalling was tightly regulated in these individuals. The baseline phosphorylation of p65 was increased in monocytes from patients with SIRS, compared with patients with sepsis and healthy volunteers, and this phosphorylation was maintained during IL-6 treatment. The p65 subunit of NF-κB binds to the TNF-α promoter and induces its transcription [43]. During acute systemic inflammation, STAT3 can increase PAMP-triggered MyD88-dependent TLR signalling [19, 23] and, at least in hepatocyte-derived cell lines, NF-κB and STAT3 can interact directly and induce a distinct transcription profile [44]. Our results suggest that the IL-6 activation of monocytes from patients with SIRS, but not from patients with sepsis, could lead to TNF-α production through the activation of NF-κB.
A limitation of this study is the timing of blood sampling, which occurred within the first 72 h after the diagnosis of SIRS or sepsis. Since the serum concentration of cytokines and the expression levels of immunological markers on leukocytes can be highly dynamical in these pathologies [45, 46], the immune status of a patient 12 h after diagnosis could differ from the status of the same patient 72 h after diagnosis. However, principal component analysis of the SIRS or sepsis patients included in this study indicated that they did not cluster according to their serum cytokine concentrations, suggesting that the timing of blood sampling was equally spread between the two groups of patients. The patients included in this study are relatively young, with mean ages (45 for SIRS patients and 38 for septic patients) that are associated with the lowest occurrence of sepsis-related deaths worldwide (according to the principal component analysis, age is independent of the SIRS or sepsis diagnosis). Sepsis-related deaths increase sharply after 65 years [7], so it will be interesting to analyse the effects of IL-6 on monocytes from older patients, which tend to have increased systemic chronic inflammation [47] and would be expected to have higher baseline phosphorylation of p65 than younger patients.
The uncontrolled release of cytokines during systemic inflammation is associated with activation of the coagulation pathway and of vascular endothelial cells, leading to increased mortality. As a result, the neutralization of these cytokines has been explored as a therapeutic strategy for these patients [48]. As of January 31, 2021, there were seven anti-IL-6 or anti-IL-6R monoclonal antibodies and seven small-molecule inhibitors of the JAK-STAT3 pathway, in phase 2 or phase 3 study, or already approved for the treatment of chronic diseases, including rheumatoid arthritis [49]. One of these antibodies is Tocilizumab, which acts as an IL-6R antagonist and has been explored as a potential treatment for patients with SIRS, with sepsis and with severe COVID-19 [50, 51]. In a rat model of sepsis, Tocilizumab attenuates acute lung and kidney injuries and increases survival, mainly by inhibiting NF-B activation and JNK signalling [52], and in patients hospitalized for COVID-19, Tocilizumab decreases mortality, especially if it is used with corticosteroids [53]. Understanding the differential role of IL-6 in patients with SIRS and in patients with sepsis is important not only to clarify the physiopathology of these syndromes but also to assess the effectiveness of therapies that block this cytokine or its receptors, and to avoid their future indistinct use in SIRS and sepsis patients.
Supplementary data
Supplementary data is available at Clinical and Experimental Immunology online.
Supplementary Figure 1. Analysis algorithm for monocytes, and proportion of monocyte subsets in patients with systemic inflammation. (A) For the identification of total leukocytes, we used three dot plots: size-height (FSC-H) vs. size-area (FSC-A), which allows us to distinguish the events that were individually acquired by the cytometer (singlets); complexity-area (SSC-A) vs. size-area (FSC-A), in which we can select the events that present the classic size and complexity of viable human peripheral blood leukocytes; and complexity-area (SSC-A) vs. CD45 expression, with which we can discriminate leukocytes (CD45+, to different degrees) from the remaining erythrocytes (CD45-). Monocytes were identified as FSCmed SSCmed (black region in the dot plots) CD45+ CD14+; mIL-6R expression and TNF-α production were determined in these cells. In some experiments, the FSCmed SSCmed CD45+ events were selected, and from these events, monocyte subsets were identified as follows: classic monocytes (FSCmed SSCmed CD45+ CD14+ CD16-), intermediate monocytes (FSCmed SSCmed CD45med CD14+ CD16+), and non-classic monocytes (FSCmed SSCmed CD45+ CD14- CD16+). (B) Comparison of the percentages of each monocyte subset between healthy volunteers (n = 9) and patients with SIRS (n = 8) or sepsis (n = 11) is shown. Graphs represent individual data, mean, and standard error of the mean. Kruskal–Wallis test with Dunn’s multiple comparisons test.
Supplementary Figure 2. IL-6-induced TNF-α is increased in monocytes from patients with SIRS. Peripheral blood was cultured with RPMI alone, or with RPMI supplemented with IL-6 (100 ng/mL) and brefeldin A (5 μg/mL) for 4 h; with LPS (250 ng/mL) and brefeldin A (5 μg/mL) for 2 h; or with IL-6 (100 ng/mL) for 4 h, followed by LPS (250 ng/mL) and brefeldin A (5 μg/mL) for 2 h [healthy volunteers (circles), n = 4; patients with SIRS (squares), n = 5; patients with sepsis (triangles), n = 5]. Production of TNF-α by monocytes (FSCmed SSCmed CD45+ CD14+) was analysed by flow cytometry. The fold-increase of TNF-α production (normalized to non-stimulated blood samples) is shown. Graphs represent individual data, mean, and standard error of the mean. *P < 0.05, **P < 0.01, Kruskal–Wallis test with Dunn’s multiple comparisons test.
Acknowledgments
The authors acknowledge the Flow Cytometry core facility from Coordinación de Investigación en Salud at Centro Medico Nacional “Siglo XXI”, Instituto Mexicano del Seguro Social, Mexico City, Mexico, for instrumentation.
Glossary
Abbreviations
- APACHE II score
acute physiology and chronic health evaluation score
- DAMPs
damage-associated molecular patterns
- gp130
glycoprotein 130
- IL
interleukin
- LPS
lipopolysaccharide
- MFI
mean fluorescence intensity
- mIL-6R
membrane IL-6 receptor
- p-p65
phosphorylated p65 subunit of NF-κB
- p-STAT3
phosphorylated STAT3
- PAMPs
pathogen-associated molecular patterns
- PBS
phosphate-buffered saline
- PCA
principal component analysis
- qSOFA
quick Sequential Organ Failure Assessment
- sgp130
soluble glycoprotein 130
- sIL-6R
soluble IL-6 receptor
- SIRS
systemic inflammatory response syndrome
- SOCS3
suppressor of cytokine signalling 3
- STAT3
signal transducer and activator of transcription 3
- TNF-α
tumour necrosis factor-alpha
Contributor Information
Graciela L Cabrera-Rivera, Unidad de Investigación Médica en Inmunoquímica, Centro Medico Nacional “Siglo XXI”, Instituto Mexicano del Seguro Social, Mexico City, Mexico; Departamento de Inmunología, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, Mexico City, Mexico.
Ruth L Madera-Sandoval, Unidad de Investigación Médica en Inmunoquímica, Centro Medico Nacional “Siglo XXI”, Instituto Mexicano del Seguro Social, Mexico City, Mexico.
José Israel León-Pedroza, Coordinación de Investigación, Unidad 401-C, Urgencias Médicas, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico; Coordinación de Ciclos Básicos, Universidad Anáhuac, Mexico City, Mexico.
Eduardo Ferat-Osorio, Unidad de Investigación Médica en Inmunoquímica, Centro Medico Nacional “Siglo XXI”, Instituto Mexicano del Seguro Social, Mexico City, Mexico; División de Investigación en Salud, UMAE Hospital de Especialidades “Dr. Bernardo Sepúlveda Gutiérrez”, Centro Médico Nacional “Siglo XXI”, Instituto Mexicano del Seguro Social, Mexico City, Mexico.
Enrique Salazar-Rios, Unidad de Investigación Médica en Inmunoquímica, Centro Medico Nacional “Siglo XXI”, Instituto Mexicano del Seguro Social, Mexico City, Mexico; Facultad de Medicina, Universidad Autónoma del Estado de Morelos, Mexico City, Mexico.
Juan A Hernández-Aceves, Unidad de Investigación Médica en Inmunoquímica, Centro Medico Nacional “Siglo XXI”, Instituto Mexicano del Seguro Social, Mexico City, Mexico; Facultad de Química, Universidad Nacional Autónoma de México, Mexico City, Mexico.
Uriel Guadarrama-Aranda, Unidad de Investigación Médica en Inmunoquímica, Centro Medico Nacional “Siglo XXI”, Instituto Mexicano del Seguro Social, Mexico City, Mexico; Facultad de Medicina, Universidad Nacional Autónoma de México, Mexico City, Mexico.
Constantino López-Macías, Unidad de Investigación Médica en Inmunoquímica, Centro Medico Nacional “Siglo XXI”, Instituto Mexicano del Seguro Social, Mexico City, Mexico; Visiting Professor of Immunology, Nuffield Department of Medicine, University of Oxford, UK.
Isabel Wong-Baeza, Departamento de Inmunología, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, Mexico City, Mexico.
Lourdes A Arriaga-Pizano, Unidad de Investigación Médica en Inmunoquímica, Centro Medico Nacional “Siglo XXI”, Instituto Mexicano del Seguro Social, Mexico City, Mexico.
Funding
Funding was provided by Coordinación de Investigación en Salud, Instituto Mexicano del Seguro Social, Mexico City, Mexico (grant FIS/IMSS/PROT/G15/1484, awarded to L.A.A.P.) and Fondo Sectorial de Investigación en Salud y Seguridad Social (SS/IMSS/ISSSTE/CONACYT, grant SALUD-2013-01-202621, awarded to L.A.A.P.). Funding was also provided by Consejo Nacional de Ciencia y Tecnología (CONACYT) (SEP-CONACYT, grant CB-2015-256402, awarded to C.L.M.). G.L.C.R. was recipient of a CONACYT fellowship (633116). I.W.B. is fellow of Estímulo al Desempeño de los Investigadores, Instituto Politécnico Nacional. The funders had no direct role in the study design, data collection/analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author contributions
L.A.A.P. conceived and designed the work. G.L.C.R., E.S.R., J.A.H.A., U.G.A. performed experiments. G.L.C.R., E.F.O., C.L.M., and I.W.B. analysed and interpreted data. J.I.L.P., E.F.O., E.S.R., J.A.H.A., U.G.A. enrolled patients and collected clinical data. RLMS performed statistical analysis. G.L.C.R., I.W.B., and L.A.A.P. wrote the manuscript. All authors contributed to the article, critically revised the manuscript and approved the manuscript for submission. All contributors to this study are listed as co-authors.
Ethical approval
This study involving human participants was reviewed and approved by the local Ethics and Scientific Research Committees of the Specialties Hospital UMAE-HE Dr. Bernardo Sepúlveda Gutiérrez of the Mexican Social Security Institute (R-2016-3601-185).
Patient consent
All participants provided their written informed consent to participate in this study.
Data availability
The data supporting the conclusions of this article will be made available by the authors upon reasonable request, without undue reservation.
References
- 1. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101, 1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 2. Nyström PO. The systemic inflammatory response syndrome: definitions and aetiology. J Antimicrob Chemother 1998, 41, 1–7. doi: 10.1093/jac/41.suppl_1.1. [DOI] [PubMed] [Google Scholar]
- 3. Nduka OO, Parrillo JE.. The pathophysiology of septic shock. Crit Care Nurs Clin North Am 2011, 23, 41–66. doi: 10.1016/j.ccell.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 4. Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–74. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bergmann CB, Beckmann N, Salyer CE, Hanschen M, Crisologo PA, Caldwell CC.. Potential targets to mitigate trauma- or sepsis-induced immune suppression. Front Immunol 2021, 12, 622601. doi: 10.3389/fimmu.2021.622601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–11. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abe T, Kubo K, Izumoto S, Shimazu S, Goan A, Tanaka T, et al. Complement activation in human sepsis is related to sepsis-induced disseminated intravascular coagulation. Shock 2020, 54, 198–204. doi: 10.1097/SHK.0000000000001504. [DOI] [PubMed] [Google Scholar]
- 9. Hotchkiss RS, Monneret G, Payen D.. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 2013, 13, 862–74. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Markiewski MM, DeAngelis RA, Lambris JD.. Complexity of complement activation in sepsis. J Cell Mol Med 2008, 12, 2245–54. doi: 10.1111/j.1582-4934.2008.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Mourik JA, Boertjes R, Huisveld IA, Fijnvandraat K, Pajkrt D, van Genderen PJ, et al. von Willebrand factor propeptide in vascular disorders: a tool to distinguish between acute and chronic endothelial cell perturbation. Blood 1999, 94, 179–85. [PubMed] [Google Scholar]
- 12. Zonneveld R, Martinelli R, Shapiro NI, Kuijpers TW, Plötz FB, Carman CV.. Soluble adhesion molecules as markers for sepsis and the potential pathophysiological discrepancy in neonates, children and adults. Crit Care 2014, 18, 204. doi: 10.1186/cc13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M.. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 2012, 122, 143–59. doi: 10.1042/CS20110340. [DOI] [PubMed] [Google Scholar]
- 14. Heinrich PC, Castell JV, Andus T.. Interleukin-6 and the acute phase response. Biochem J 1990, 265, 621–36. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Müllberg J, Geib T, Jostock T, Hoischen SH, Vollmer P, Voltz N, et al. IL-6 receptor independent stimulation of human gp130 by viral IL-6. J Immunol 2000, 164, 4672–7. doi: 10.4049/jimmunol.164.9.4672. [DOI] [PubMed] [Google Scholar]
- 16. Yawata H, Yasukawa K, Natsuka S, Murakami M, Yamasaki K, Hibi M, et al. Structure-function analysis of human IL-6 receptor: dissociation of amino acid residues required for IL-6-binding and for IL-6 signal transduction through gp130. EMBO J 1993, 12, 1705–12. doi: 10.1002/j.1460-2075.1993.tb05815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rose-John S, Waetzig GH, Scheller J, Grötzinger J, Seegert D.. The IL-6/sIL-6R complex as a novel target for therapeutic approaches. Expert Opin Ther Targets 2007, 11, 613–24. doi: 10.1517/14728222.11.5.613. [DOI] [PubMed] [Google Scholar]
- 18. Müllberg J, Schooltink H, Stoyan T, Günther M, Graeve L, Buse G, et al. The soluble interleukin-6 receptor is generated by shedding. Eur J Immunol 1993, 23, 473–80. doi: 10.1002/eji.1830230226. [DOI] [PubMed] [Google Scholar]
- 19. Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci 2012, 8, 1237–47. doi: 10.7150/ijbs.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity 1997, 6, 315–25. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- 21. Jostock T, Müllberg J, Ozbek S, Atreya R, Blinn G, Voltz N, et al. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem 2001, 268, 160–7. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- 22. Hirano T, Ishihara K, Hibi M.. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 2000, 19, 2548–56. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 23. Greenhill CJ, Rose-John S, Lissilaa R, Ferlin W, Ernst M, Hertzog PJ, et al. IL-6 trans-signaling modulates TLR4-dependent inflammatory responses via STAT3. J Immunol 2011, 186, 1199–208. doi: 10.4049/jimmunol.1002971. [DOI] [PubMed] [Google Scholar]
- 24. Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, et al. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell 2009, 15, 283–93. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Selberg O, Hecker H, Martin M, Klos A, Bautsch W, Köhl J.. Discrimination of sepsis and systemic inflammatory response syndrome by determination of circulating plasma concentrations of procalcitonin, protein complement 3a, and interleukin-6. Crit Care Med 2000, 28, 2793–8. doi: 10.1097/00003246-200008000-00019. [DOI] [PubMed] [Google Scholar]
- 26. Lewis SM, Treacher DF, Edgeworth J, Mahalingam G, Brown CS, Mare TA, et al. Expression of CD11c and EMR2 on neutrophils: potential diagnostic biomarkers for sepsis and systemic inflammation. Clin Exp Immunol 2015, 182, 184–94. doi: 10.1111/cei.12679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. R: A language and environment for statistical computing. R Foundation for Statistical Comuting, Vienna, Austria, 2020. https://www.R-project.org/. [Google Scholar]
- 28. Lê S, Josse J, Husson F.. FactoMineR: an R package for multivariate analysis. J Stat Softw 2008, 25, 1–18. [Google Scholar]
- 29. Garbers C, Aparicio-Siegmund S, Rose-John S.. The IL-6/gp130/STAT3 signaling axis: recent advances towards specific inhibition. Curr Opin Immunol 2015, 34, 75–82. doi: 10.1016/j.coi.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 30. Schulte W, Bernhagen J, Bucala R.. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets--an updated view. Mediators Inflamm 2013, 2013, 165974. doi: 10.1155/2013/165974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reyes M, Filbin MR, Bhattacharyya RP, Billman K, Eisenhaure T, Hung DT, et al. An immune-cell signature of bacterial sepsis. Nat Med 2020, 26, 333–40. doi: 10.1038/s41591-020-0752-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang Q, Lenardo MJ, Baltimore D.. 30 years of NF-κB: a blossoming of relevance to human pathobiology. Cell 2017, 168, 37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Flores-Mejía LA, Cabrera-Rivera GL, Ferat-Osorio E, Mancilla-Herrera I, Torres-Rosas R, Boscó-Garate IB, et al. Function is dissociated from activation-related immunophenotype on phagocytes from patients with SIRS/sepsis syndrome. Shock 2019, 52, e68–75. doi: 10.1097/SHK.0000000000001314. [DOI] [PubMed] [Google Scholar]
- 34. Girardot T, Rimmelé T, Venet F, Monneret G.. Apoptosis-induced lymphopenia in sepsis and other severe injuries. Apoptosis 2017, 22, 295–305. doi: 10.1007/s10495-016-1325-3. [DOI] [PubMed] [Google Scholar]
- 35. Ostanin AA, Leplina OY, Shevela CY, Kozhevnikov VS, Chernykh HR.. Inflammatory syndromes (SIRS, MARS, CARS) in patients with surgical infection. Russ J Immunol 2000, 5, 289–300. [PubMed] [Google Scholar]
- 36. Osuchowski MF, Welch K, Siddiqui J, Remick DG.. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol 2006, 177, 1967–74. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 37. Vergadi E, Vaporidi K, Tsatsanis C.. Regulation of endotoxin tolerance and compensatory anti-inflammatory response syndrome by non-coding RNAs. Front Immunol 2018, 9, 2705. doi: 10.3389/fimmu.2018.02705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nahid MA, Satoh M, Chan EK.. Interleukin 1β-responsive microRNA-146a is critical for the cytokine-induced tolerance and cross-tolerance to Toll-like receptor ligands. J Innate Immun 2015, 7, 428–40. doi: 10.1159/000371517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cavaillon JM, Adib-Conquy M.. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit Care 2006, 10, 233. doi: 10.1186/cc5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Foster SL, Hargreaves DC, Medzhitov R.. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 2007, 447, 972–8. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 41. Hotson AN, Hardy JW, Hale MB, Contag CH, Nolan GP.. The T cell STAT signaling network is reprogrammed within hours of bacteremia via secondary signals. J Immunol 2009, 182, 7558–68. doi: 10.4049/jimmunol.0803666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Opal SM, Scannon PJ, Vincent JL, White M, Carroll SF, Palardy JE, et al. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J Infect Dis 1999, 180, 1584–9. doi: 10.1086/315093. [DOI] [PubMed] [Google Scholar]
- 43. Liu T, Zhang L, Joo D, Sun SC.. NF-κB signaling in inflammation. Signal Transduct Target Ther 2017, 2, 17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grivennikov SI, Karin M.. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev 2010, 21, 11–9. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rubio I, Osuchowski MF, Shankar-Hari M, Skirecki T, Winkler MS, Lachmann G, et al. Current gaps in sepsis immunology: new opportunities for translational research. Lancet Infect Dis 2019, 19, e422–36. doi: 10.1016/S1473-3099(19)30567-5. [DOI] [PubMed] [Google Scholar]
- 46. Skirecki T, Drechsler S, Hoser G, Jafarmadar M, Siennicka K, Pojda Z, et al. The fluctuations of leukocytes and circulating cytokines in septic humanized mice vary with outcome. Front Immunol 2019, 10, 1427. doi: 10.3389/fimmu.2019.01427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med 2019, 25, 1822–32. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tuttle K, McDonald M, Anderson E.. Re-evaluating biologic pharmacotherapies that target the host response during sepsis. Int J Mol Sci 2019, 20, 6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McElvaney OJ, Curley GF, Rose-John S, McElvaney NG.. Interleukin-6: obstacles to targeting a complex cytokine in critical illness. Lancet Respir Med 2021, 9, 643–54. doi: 10.1016/S2213-2600(21)00103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tanaka T, Narazaki M, Kishimoto T.. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy 2016, 8, 959–70. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- 51. Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA 2020, 117, 10970–5. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ibrahim YF, Moussa RA, Bayoumi AMA, Ahmed AF.. Tocilizumab attenuates acute lung and kidney injuries and improves survival in a rat model of sepsis via down-regulation of NF-κB/JNK: a possible role of P-glycoprotein. Inflammopharmacology 2020, 28, 215–30. doi: 10.1007/s10787-019-00628-y. [DOI] [PubMed] [Google Scholar]
- 53. Shankar-Hari M, Vale CL, Godolphin PJ, Fisher D, Higgins JPT, Spiga F, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA 2021, 326, 499–518. doi: 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the conclusions of this article will be made available by the authors upon reasonable request, without undue reservation.