Abstract
Objective:
Antibiotics are widely used by all specialties in the hospital setting. We evaluated previously defined high-risk antibiotic use in relation to Clostridioides difficile infections (CDIs).
Methods:
We analyzed 2016–2017 data from 171 hospitals. High-risk antibiotics included second-, third-, and fourth-generation cephalosporins, fluoroquinolones, carbapenems, and lincosamides. A CDI case was a positive stool C. difficile toxin or molecular assay result from a patient without a positive result in the previous 8 weeks. Hospital-associated (HA) CDI cases included specimens collected >3 calendar days after admission or ≤3 calendar days from a patient with a prior same-hospital discharge within 28 days. We used the multivariable Poisson regression model to estimate the relative risk (RR) of high-risk antibiotic use on HA CDI, controlling for confounders.
Results:
The median days of therapy for high-risk antibiotic use was 241.2 (interquartile range [IQR], 192.6–295.2) per 1,000 days present; the overall HA CDI rate was 33 (IQR, 24–43) per 10,000 admissions. The overall correlation of high-risk antibiotic use and HA CDI was 0.22 (P = .003), and higher correlation was observed in teaching hospitals (0.38; P = .002). For every 100-day (per 1,000 days present) increase in high-risk antibiotic therapy, there was a 12% increase in HA CDI (RR, 1.12; 95% CI, 1.04–1.21; P = .002) after adjusting for confounders.
Conclusions:
High-risk antibiotic use is an independent predictor of HA CDI. This assessment of poststewardship implementation in the United States highlights the importance of tracking trends of antimicrobial use over time as it relates to CDI.
Clostridioides difficile remains the most commonly identified cause of healthcare-associated infection and diarrhea in adults in the United States.1,2 Clostridioides difficile infections (CDIs) affect nearly 500,000 patients annually, with a mortality of ~30,000 per year in the United States alone.3 Multiple risk factors have been associated with CDI, the most modifiable being antimicrobial use. Literature suggests that certain classes of antibiotics are associated with different levels of risk for developing CDIs.1,4-9
Antibiotic overuse has come under scrutiny as a major driver of CDI, with ~50% of inpatients prescribed an antibiotic during hospitalization and 30% of these antibiotics being potentially unnecessary.10 Changes in intestinal anaerobic microbiota are thought to mediate C. difficile spore activation and infection.1,4 In addition to the direct effects of antibiotics on CDI risk, antibiotics mediate carriage of C. difficile spores by asymptomatic carriers (including both those never previously infected with C. difficile and in post-CDI patients) and are sources of transmission that may further increase the CDI burden in acute-care settings.5,11-14 Together, these community- and hospital-level influences have made CDI a focus for both outpatient and inpatient appropriate antimicrobial use initiatives.15
Extensive research has shown the association of patient-level antibiotic use and CDI risk.1,4-7 Moreover, a CDI practice guideline highlights antibiotics at particularly high risk of causing CDI.1 However, 2 multicenter studies evaluating the relationship of facility-level antibiotic use and CDI rate showed mixed results. One earlier study on Veterans’ Affairs (VA) facilities found that facility-level antibiotic use was an independent predictor only for long-term care facilities but not for acute-care facilities.5 In contrast, a recent study using claims data from >500 non-VA acute-care hospitals demonstrated significant associations with hospital-onset (HO) CDI for overall and for some individual antibiotic classes when evaluated separately.16 In this study, we evaluated population-level strength of association of the antibiotics highlighted in a recent CDI practice guideline1 on hospital-associated (HA) CDI, using microbiological laboratory test results for the CDI case identification and hospital pharmacy data as an exposure assessment, from a large number of community and teaching hospitals. In this analysis, we controlled for confounding factors such as community CDI pressure, hospital length of stay, the proportion of elderly patient admissions, and hospital characteristics. In addition, we investigated the relationship of hospital-level proton pump inhibiter (PPI) use to CDI rate, an area of controversy in the research community.17-20
Methods
Data source
We analyzed de-identified electronic microbiological and pharmacy data from July 1, 2016 through June 30, 2017, in the BD Insights Research Database (Becton, Dickinson and Co., Franklin Lakes, NJ). The BD Insights Research Database has been continuously updated and used for aggregated epidemiology studies.8,21,22 This study was approved as a limited dataset for retrospective study and was exempted from consent by the New England Institutional Review Board/Human Subjects Research Committee (Wellesley, MA). This study was conducted in compliance with the Health Insurance Portability and Accountability Act (HIPAA).
Definition of high-risk antibiotics
Based on recent guidelines that target specific antibiotics for mitigation of CDI1 and other studies,4-7 we defined 4 antibiotic classes as high risk: cephalosporins (second-, third-, and fourth-generation: cefoxitin, cefprozil, cefaclor, cefotetan, cefuroxime, ceftibuten, cefdinir, cefixime, cefotaxime, cefpodoxime, ceftazidime, ceftriaxone, cefepime, ceftazidime-avibactam, ceftolozane/tazobactam), fluoroquinolones (levofloxacin, moxifloxacin, norfloxacin, ciprofloxacin, ofloxacin), carbapenems (meropenem, imipenem, ertapenem, doripenem), and lincosamides (clindamycin). We evaluated the combined high-risk antibiotic use as well as the 4 classes individually. We also evaluated piperacillin/tazobactam, a frequently used antibiotic, which is considered to be of medium risk for CDI in some studies.5,8 In addition, we compiled all-risk antibiotic use to provide a baseline context of high-risk antibiotic use. All-risk antibiotics included high-risk antibiotics and the following: aminoglycosides, β-lactam-β-lactamase-inhibitor-combinations, first-generation cephalosporins, macrolides, monobactam, and penicillins.
The hospital-level antibiotic use was measured as days of therapy (DOT) per 1,000 days present (DP), consistent with the CDC’s National Healthcare Safety Network (NHSN) antibiotic use (AU) module.23
Definition of CDI
We defined a CDI case as a positive stool C. difficile toxin or molecular assay result from a patient with no positive stool C. difficile result in the previous 8 weeks, according to the CDC NHSN definition.3 To better capture the full impact of inpatient antibiotic use on the epidemiology of CDI, we adopted a previously used epidemiologic classification of cases21 consistent with the most current Clinical Practice Guidelines for CDI.1 Specifically, the outcome for this study was HA CDI, including stool specimens collected >3 calendar days after admission (ie, HO CDI) or stool specimens collected ≤3 calendar days from a patient with documented overnight stay in the same hospital in the prior 28 days (ie, community-onset, hospital-associated CDI or CO-HA CDI). Because of the inclusion of CO-HA CDI in the composite HA CDI rate, we used number of admissions as the denominator. For specimens collected ≤3 calendar days from a patient with no documented overnight stay in the same hospital in the prior 28 days, it was classified as a community-onset non–hospital-associated (CO-NHA) CDI case and was not included in the outcome of HA CDI. Instead, the CO-NHA CDI rate was included as a measure of community CDI pressure, one of the confounders in the multivariable model for the outcome.
Statistical analysis
We used the Pearson correlation coefficient to assess the hospital-level correlation between high-risk antibiotic use and HA CDI rate, χ2 test for categorical variables, and t test or logged t test for continuous variables wherever appropriate. We restricted our analysis to hospitals with at least 10 HA CDI cases. We used the multivariable Poisson regression model to estimate the relative risk (RR) of high-risk antibiotic use on HA CDI, adjusting for potential confounders, including the CO-NHA CDI rate, PPI use, average length of stay, proportion of patients aged 65 or older, geographic location, and hospital teaching status. We retained only significant covariates in the final multivariable model, using P < .05 as a criteria of significance.
Results
Of the 171 study sites meeting inclusion criteria, 66 (39%) were teaching hospitals and 105 were nonteaching hospitals (61%). The geographic location distribution was 26 (15%) in the Northeast region; 48 (28%) in the Midwest region, 78 (46%) in the South region, and 19 (11%) in the West region.
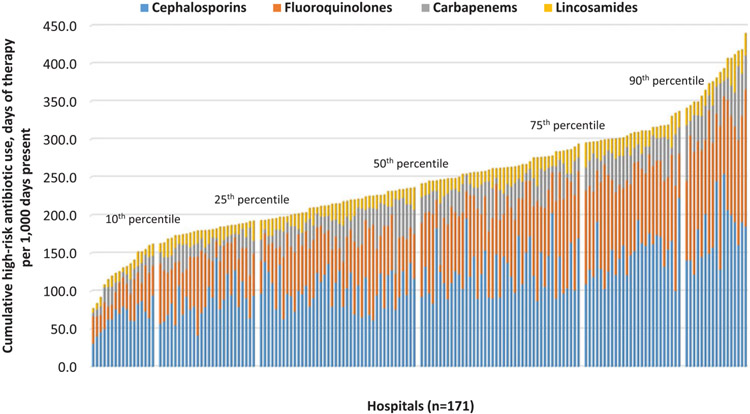
The overall pooled high-risk antibiotic use (DOT per 1,000 DP) across all 171 hospitals was 230.6; the median was 241.2 (interquartile range [IQR, first through third quartiles], 192.6–295.2), which accounted for approximately half of all-risk antibiotic use (pooled mean, 486.6; median, 495.2; IQR, 424.5–565.8) (Table 1). Among high-risk antibiotics, the most frequently used were cephalosporins (47.9%), followed by fluoroquinolones (31.6%), carbapenems (13.0%), and lincosamides (7.6%). Figure 1 shows the cumulative high-risk antibiotic use with the 4 components. For hospitals in the upper quartiles of high-risk antibiotic use, cephalosporins were used more frequently; however, we observed considerable interhospital variations in the relative proportion of each class of drugs.
Table 1.
Overall and Stratified Antibiotic and Other Medication Use
| Variable | Overall Days of Therapy per 1,000 Days Present |
High-Risk antibiotic use quartile (No. of Hospitals), Median (1st–3rd Quartile), Days of Therapy per 1,000 Days Present |
||||
|---|---|---|---|---|---|---|
| Pooled Rate (n=171) |
Median(1st, 3rd quartile)(n=171) |
1st Quartile(n=43) | 2nd Quartile(n=42) | 3rd Quartile(n=43) | 4th Quartile(n=43) | |
| All risk antibiotics | ||||||
| All risk antibiotics, range | N/A | 178.1–835.4 | 178.1–560.9 | 350.5–581.5 | 458.6–648.6 | 461.6–835.4 |
| All risk antibiotics | 486.6 | 495.2 (424.5–565.8) | 397.3 (367.8–440.9) | 462.9 (417.1–521.5) | 508.9 (484.0–556.1) | 596.3 (565.4–651.6) |
| High-risk antibiotics | ||||||
| Overall high-risk antibiotic, range | N/A | 77.2–439.9 | 77.2–192.6 | 192.7–241.2 | 241.3–295.2 | 295.3–439.9 |
| Overall high-risk antibiotics | 230.6 | 241.2 (192.6–295.2) | 173.4 (136.2–182.3) | 215.4 (202.3–225.9) | 261.7 (249.7–276.1) | 317.9 (302–373.6) |
| Cephalosporins, 2nd/3rd/4th generation | 110.5 | 110.7 (86.8–144.9) | 76.1 (62.2–91.5) | 102.2 (79.6–123.4) | 120.2 (97.2–148.8) | 157.4 (129.4–185.1) |
| Fluoroquinolones | 72.8 | 76.6 (55.4–104.2) | 45.2 (35.1–65.2) | 65.1 (50.4–76.6) | 91.7 (76.5–107) | 112.6 (97.5–137.7) |
| Carbapenems | 29.9 | 25.7 (15.7–38.2) | 16.5 (10.9–21.1) | 26.6 (17.2–38.1) | 27.1 (16.8–39) | 35.7 (28.2–45.2) |
| Lincosamides | 17.5 | 17.0 (13.4–21.7) | 13.6 (10.3–18.1) | 16.6 (13.3–20.8) | 17.5 (13.9–22.1) | 20.6 (15.8–26.6) |
| Most frequently used medium- or low-risk antibiotic | ||||||
| Piperacillin/tazobactam | 81.7 | 81.8 (57.2–102.1) | 60.2 (50.0–78.3) | 76.8 (56.6–92.4) | 93.4 (75.6–109.5) | 101.4 (70.0–119.1) |
| Non-antibiotic | ||||||
| Proton pump inhibitor | 326.0 | 334.6 (265–371.9) | 269.5 (212.6–319.8) | 308.5 (236.1–354.4) | 356.1 (320.2–381.1) | 367.5 (331–440.8) |
Note. All data were presented as days of therapy per 1,000 days present. P values for each row were all significant at <.0001.
Fig. 1.
Cumulative high-risk antibiotic use (sorted by overall high-risk antibiotic use rate).
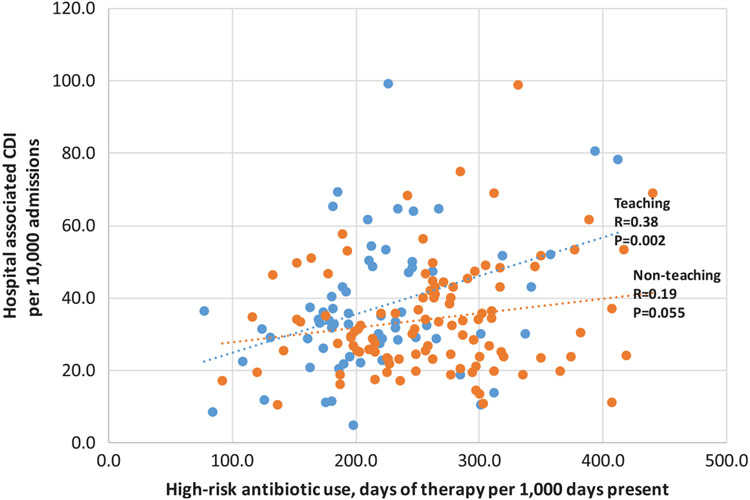
The overall correlation of high-risk antibiotic use and HA CDI was 0.22 (P = .003). Higher correlation was observed in teaching hospitals (R = 0.38; P = .002) versus nonteaching hospitals (R = 0.19; P = .055) (Fig. 2). When HO CDI and CO-HA CDI were evaluated separately in the secondary analysis, the correlation coefficients were 0.18 (P < .05) versus 0.33 (P < .01), respectively (Appendices A and B). When each class of high-risk antibiotics was evaluated, only cephalosporins were significantly correlated with HA CDI (R = 0.23; P < .01). The correlation coefficients for each of the other 3 classes of high-risk antibiotics as well as piperacillin/tazobactam (medium risk) were not statistically significant (all P > .05, data not shown).
Fig. 2.
Correlation of hospital high-risk antibiotic use and hospital-associated Clostridioides difficile infection rates stratified by hospital teaching status. The overall correlation coefficient for all 171 hospitals together was 0.22 (P = .003).
The overall pooled HA CDI rate was 35 per 10,000 admissions (10,305 cases [63% HO; 37% CO-HA] per 2,979,533 admissions). The median was 33 (IQR, 24–43) per 10,000 admissions across 171 hospitals. Univariate analysis on stratified groups showed significant association to HA CDI rates for the following factors: increased high-risk antibiotic use; larger proportion of patients aged >65 years; higher CO-NHA rate; longer average length of stay; and higher PPI use (Table 2). Teaching hospitals had a higher observed CDI rate than nonteaching hospitals (36 vs 33 per 10,000 admissions; P < .0001). Hospitals in the Northeast and Midwest regions had higher CDI rates than the West region (43 vs 35 vs 32 per 10,000 admissions; both P < .05). The CDI rate for the South region was 33 per 10,000 admissions and was not significantly different from the West region (P = .285).
Table 2.
Univariate Analysis
| Variable, Strata Range | Hospital-Associated CDI | |||
|---|---|---|---|---|
| No. of CDI |
No. of Admissions |
CDI per 10,000 Admissions |
P Value | |
| High-risk antibiotic use, day of therapy per 1,000 days present | ||||
| 1st quartile (77–193) | 2,902 | 909,380 | 31.9 | Reference |
| 2nd quartile (194–241) | 2,851 | 837,128 | 34.1 | .0135 |
| 3rd quartile (242–295) | 2,523 | 671,853 | 37.6 | <.0001 |
| 4th quartile (296–440) | 2,029 | 561,172 | 36.2 | <.0001 |
| Proportion of patients age >65, % | ||||
| 1st quartile (0.14–0.33) | 3,080 | 1,044,253 | 29.5 | Reference |
| 2nd quartile (0.34–0.38) | 1,685 | 556,608 | 30.3 | .3899 |
| 3rd quartile (0.39–0.44) | 2,823 | 820,241 | 34.4 | <.0001 |
| 4th quartile (0.45–0.65) | 2,717 | 558,431 | 48.7 | <.0001 |
| Community-onset non-hospital-associated (CO-NHA) CDI per 10,000 admissions | ||||
| 1st quartile (3–35) | 2,180 | 965,859 | 22.6 | Reference |
| 2nd quartile (36–56) | 2,700 | 733,345 | 36.8 | <.0001 |
| 3rd quartile (57–89) | 2,374 | 644,295 | 36.8 | <.0001 |
| 4th quartile (90–273) | 3,051 | 636,034 | 48.0 | <.0001 |
| Average length of stay, days | ||||
| 1st quartile (2.3–3.4) | 1,433 | 524,973 | 27.3 | Reference |
| 2nd quartile (3.5–3.9) | 2,213 | 680,684 | 32.5 | <.0001 |
| 3rd quartile (4.0–4.4) | 2,898 | 814,563 | 35.6 | <.0001 |
| 4th quartile (4.5–6.0) | 3,761 | 959,313 | 39.2 | <.0001 |
| Proton pump inhibitor use, days of therapy per 1,000 days present | ||||
| 1st quartile (107–265) | 2,224 | 756,480 | 29.4 | Reference |
| 2nd quartile (266–335) | 2,610 | 857,357 | 30.4 | .2262 |
| 3rd quartile (336–372) | 2,545 | 641,402 | 39.7 | <.0001 |
| 4th quartile (372–701) | 2,926 | 724,294 | 40.4 | <.0001 |
| Hospital teaching status | ||||
| Nonteaching (n=105) | 4,719 | 1,436,414 | 32.9 | Reference |
| Teaching (n=66) | 5,586 | 1,543,119 | 36.2 | <.0001 |
| Hospital geographic region | ||||
| West (n=19) | 915 | 289,972 | 31.6 | Reference |
| Northeast (n=26) | 1,791 | 418,282 | 42.8 | <.0001 |
| Midwest (n=48) | 2,803 | 808,738 | 34.7 | 0.0135 |
| South (n=78) | 4,796 | 1,462,541 | 32.8 | 0.2846 |
Note. CDI, Clostridioides difficile infection.
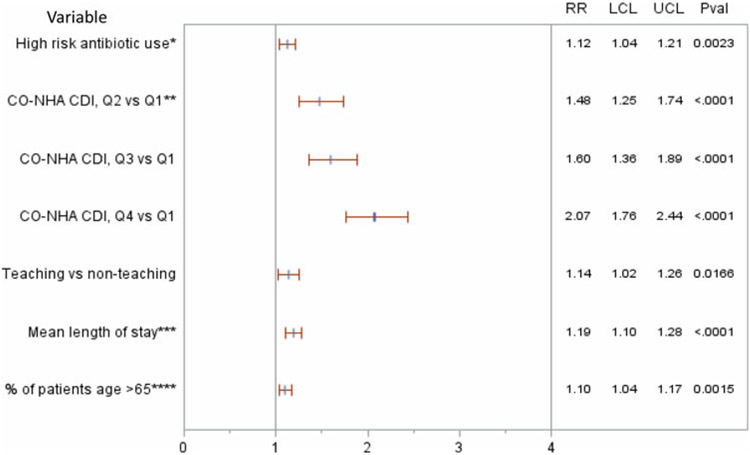
Adjusting for significant confounders, high-risk antibiotic use was independently associated with significant risk for HA CDI. For every 100-day increase of DOT per 1,000 DP in high-risk antibiotic use, there was a 12% increase in HA CDI (RR, 1.12; 95% CI, 1.04–1.21; P = .002) (Fig. 3). This increase translates to 4 additional HA CDI cases with every 100 DOT increase per 1,000 DP. Other significant independent predictors for CDI included higher CO-NHA CDI rate, longer patient average length of stay, higher percent of admissions of older patients, and hospital teaching status. PPI use and geographic regions were not independent predictors of CDI; hence, both were dropped from the final model.
Fig. 3.
Multivariable Poisson model results. Note: *Per 1,000 days present, per increase of 100 days of therapy. **CO-NHA CDI: community-onset non–hospital-associated Clostridioides difficile infection. ***Per 1 day increase of mean length of stay. ****Per 10% increase of patient’s age >65 years. Note. Q1, first quartile; Q2, second quartile; Q3, third quartile; Q4, fourth quartile; RR, relative risk; LCL, lower confidence limit; UCL, upper confidence limit; Pval, P value.
Discussion
Using a large and current dataset, we found an independent impact of hospital-level high-risk antibiotic use on HA CDI even after adjusting for confounding factors such as community CDI pressure, proportion of patients aged 65 years or older, average length of stay, and hospital teaching status. This investigation demonstrates a robust relationship between hospital-level high-risk antibiotic use and CDI risk, building upon a recent multicenter study using claims data.16 Our recent data sheds additional light on high-risk antimicrobial use since the CMS and the Joint Commission support for antimicrobial stewardship programs (ASPs), which increased the proportion of hospitals reporting incorporation of all 7 elements of CDC core elements in their facility ASPs (from 41% in 2014 to 76% in 2017).24,25
Our finding that facility-level high-risk antibiotic use was independently associated with HA CDI differs from a VA study in which facility-level antibiotic use was independently correlated with facility-onset CDI only in long-term care facilities but not in the acute-care facilities.5 This difference may be explained, in part, by our finding of a wide range of high-risk antibiotic use (a 110% increase from 10th to the 90th percentile), whereas the VA study found a narrow range of antibiotic use across 131 VA acute-care facilities (only a 14% increase from the 10th to 90th percentile). Such relative homogeneity in antibiotic use across the 131 VA acute care facilities may partially explain the lack of correlation between the acute-care facility-level antibiotic use and the facility-onset CDI rate.5
Our findings, along with the recent multicenter study,16 support the clinical relevance of hospital-level tracking of high-risk antimicrobials for CDI. High-risk antibiotics accounted for approximately half of all-risk antibiotics analyzed and may represent a more practical focus for CDI reduction programs in antimicrobial stewardship that may be short on resources.1,26 Targeted stewardship has been successfully implemented: the United Kingdom reported a reduction in CDI after implementation of cephalosporin- and fluoroquinolone-sparing programs.27
Regarding specific antibiotic use at the facility level, Kazakova et al16 reported when assessed individually, cephalosporins (third and fourth generations) and carbapenems were significantly associated with HO CDI. Our study largely confirms these findings, although we included second generation in our evaluation of cephalosporins and we did not find carbapenem use alone to be associated with CDI. This finding may be due to less frequent carbapenem use (13% of overall high-risk antibiotic use; median 25.7 per 1,000 DP) in this more recent analysis, which was conducted >2 years after ASP guidelines were introduced.24
The lack of association of fluoroquinolones and CDI in our study may reflect changes in antibiotic use in a post-ASP environment.28 With ASP in place for multiple years and 2016 additions to black box warnings for quinolone use,29 quinolones’ current contribution to CDI seems reduced, perhaps partially due to its reduced use. Meanwhile, cephalosporin use appeared in the highest volume and is corroborated here as a significant CDI risk. The “preferred” class of high-risk CDI antibiotic use may continue to change in the future. Thus, tracking high-risk antibiotic use as a group with several components is likely needed in time- and volume-based assessments, to better inform stewardship in the mitigation of CDI risk. This continuous monitoring is now feasible because the NHSN’s standardized antimicrobial administration ratio (SAAR) metric30 enables tracking clinical subclasses of drugs at the facility level. The SAAR provides an infrastructure to support more timely updating of ASP practices and CDI risk based on current epidemiology and practice patterns.
From a statistical perspective, a lack of statistical significance for the association of a specific class of antibiotics to CDI does not necessarily mean “no risk.” Volume of use, the range of variations, sample size, study design, and other factors may affect statistical test results. For this reason, aggregating subclasses of antibiotics based on prior documented risk for CDI may be a practical and more robust way to detect signals and to identify drivers of CDI.
Although we corroborate some findings in the study by Kazakova et al,16 there are methodologic differences. First, the Kazakova study used International Classification of Disease, Ninth Revision (ICD-9) secondary diagnosis of CDI in conjunction with oral anti-CDI antibiotics to identify cases, whereas we used positive nonduplicate microbiological test results to define cases, consistent with the current CDC NHSN AU module.23 Second, the Kazakova study used pharmacy billing records to calculate the antibiotic use (DOT),31 whereas we used hospital pharmacy prescription start and stop orders to calculate the DOT. Third, our CDI categories are broader, including both HO CDI and same-hospital–associated, CO CDI, which potentially enabled assessment of a more representative burden of HA CDI. Because of the inclusion of the latter, we used admissions as the denominator to calculate the HA CDI rate rather than patient days to calculate the HO CDI rates. Despite these methodological and data-source differences, both studies found convergent independent association of acute-care facility-level antibiotic use and increased risk for CDI.
Our definition of HA CDI was previously used in a study that included both index HO and delayed outpatient-onset CDI as the outcome.7 The linked assessment of index HO (ie, HO) and delayed postdischarge (ie, CO-HA) CDI may better capture the full impact of hospital-level antibiotic use. If a patient had a prior, same-hospital stay within a short period, it may be reasonable to assume a potential delayed effect of hospital-level antibiotic use in the development of CDI. Our secondary analysis on HO- and CO-HA separately demonstrated that in-hospital antibiotic use increase CDI risk not only during hospital stay but also after hospital discharge. Nationally, the standardized infection ratio, a risk-adjusted metric of HO-CDI used by the NHSN, appears to be slowly decreasing whereas community-associated CDI (a subset of community-onset CDI), as reported by the CDC Emerging Infection Program, appears to be increasing or unchanged.32 With more HA CDI manifesting in the outpatient setting, spread of CDI spores may become an increasingly important issue.21 CDI prevention initiatives focused on reducing C. difficile transmission and unnecessary antimicrobial use in both inpatient and outpatient settings may therefore be necessary to address the changing epidemiology of CDI.
The finding of moderate correlation between the hospital-level high-risk antibiotic use and HA CDI rates reflects considerable interhospital variation and may be partially due to unmeasured factors such as facility-level infection prevention and stewardship efforts that may modify the impact of antibiotic use on CDI. Another unmeasured variable is potential testing practice change, which may affect observed CDI rate. This aspect may be better addressed with longitudinal data. Furthermore, community differences in prescribing antimicrobials in the outpatient and other non-acute healthcare facilities could also be contributing factors. Although we included the CO-NHA CDI rate as a covariate in the model, it is not a direct assessment of variation of the outpatient antibiotic use.
The observation that teaching hospitals had higher HA CDI risk might be a result of detecting admissions of more severely ill patients. Prior studies have shown higher risk for CDI in the elderly, different ethnicities, and multiple antibiotic class exposure.5,7,21 Teaching hospitals may use multiple antimicrobial classes due to sicker patients, or as a byproduct of a hierarchical training environment.
CO-NHA CDI appears to contribute to the HO-CDI risk in admitting hospitals; hence, adjusting HO rates by a community-onset CDI variable appears to be appropriate for hospital performance reporting.33,34 The role of CDI community pressure on hospital CDI risk is likely multifactorial, including skin contamination and environmental shedding of C. difficile spores, which is particularly high among patients recently treated for CDI.11,12 The overall wide range of CO-NHA CDI rates observed in this study suggests a large variation in community CDI pressure, and as such, awareness of local CO-NHA CDI pressure can help inform stewardship and infection prevention strategies.
Our study focused on high-risk antibiotics at a facility-level in the new era of ASP. It may be helpful to further delineate the relationship of facility thresholds of total and tiered (high/medium/low CDI risk) antimicrobial use to help inform targets for antimicrobial stewardship programs. In this paradigm, the concept of lower-risk antibiotics becomes of clinical value because a replacement agent will often be needed to treat the infecting organism.35 New in 2019, a specific SAAR has been developed to measure use of antibiotics that pose a high-risk for CDI; this metric includes most of the same antibiotic classes included in our study.23 From a stewardship perspective, a comprehensive CDI reduction program would therefore ideally involve assessment of specific patient demographics, risk profiles, and community pressure in addition to high-risk antimicrobials.
This study has several limitations. First, despite the study including a large number of community hospitals of diverse characteristics, it does not necessarily represent the characteristics of all US hospitals. Second, as an ecologic study, it depicts associations and not causal relations. Nevertheless, ecological studies are important for estimating likely degree of impact of a population-level intervention, especially through convergent insights involving diverse data sources, patient population, and methodologies. Although the complex physiologic interactions between the intestinal microbiome and antibiotics are not completely understood, prescribing practices comprise a risk factor that is malleable and can change with antimicrobial stewardship and benchmarking.1,4-7,16,34 Future studies would ideally combine both facility- and patient-level measures of high-risk and total antibiotic use to further inform CDI mitigation strategies.
Supplementary Material
Acknowledgments.
The authors thank John Murray and Kennedy Shurke for their efforts to create the dataset to support this study. The findings and conclusions in this report are those of the authors and do not necessarily represent the official positions of the Centers for Disease Control and Prevention.
Financial support.
The funding for this study was provided to the BD Insights Research Team of Becton, Dickinson and Co. (Franklin Lakes, NJ) by Nabriva Therapeutics (King of Prussia, PA).
Footnotes
PREVIOUS PRESENTATION. The preliminary results of this study were presented in part at the European Society of Clinical Microbiology and Infectious Diseases Conference on April 21, 2018, in Madrid, Spain, and at the American Society for Microbiology conference on June 9, 2018, in Atlanta, Georgia.
Conflicts of interest. Y.P.T., K.C.Y., S.G.K., V.G. are full-time employees of Becton, Dickinson and Co., a medical technology company, whose life science business segment develops, manufactures, and sells diagnostics for infectious diseases, including CDI, as part of its business. S.G. is a full-time employee of Nabriva. P.J.S. was a full-time employee of Nabriva at the time of this study. A.S. and L.C.M. do not have any conflicts related to this article.
Supplementary material. For supplementary materials referred to in this article, please visit https://doi.org/10.1017/ice.2019.236
References
- 1.McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018;66(7):e1–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014;370:1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015;372:825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med 2015;373(3):287–288. [DOI] [PubMed] [Google Scholar]
- 5.Brown KA, Daneman N, Jones M, et al. The drivers of acute and long-term care Clostridium difficile infection rates: a retrospective multilevel cohort study of 251 facilities. Clin Infect Dis 2017;65:1282–1288. [DOI] [PubMed] [Google Scholar]
- 6.Slimings C, Riley TV. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother 2014;69:881–891. [DOI] [PubMed] [Google Scholar]
- 7.Tartof SY, Rieg GK, Wei R, Tseng HF, Jacobsen SJ, Yu KC. A comprehensive assessment across the healthcare continuum: risk of hospital-associated Clostridium difficile infection due to outpatient and inpatient antibiotic exposure. Infect Control Hosp Epidemiol 2015;36:1409–1416. [DOI] [PubMed] [Google Scholar]
- 8.Gross AE, Johannes RS, Gupta V, Tabak YP, Srinivasan A, Bleasdale SC. The effect of a piperacillin/tazobactam shortage on antimicrobial prescribing and Clostridium difficile risk in 88 US medical centers. Clin Infect Dis 2017;15:613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996–2003. Emerg Infect Dis 2006;12:409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antibiotic use in the United States, 2017: progress and opportunities. Centers for Disease Control and Prevention; website. https://www.cdc.gov/antibiotic-use/stewardship-report/pdf/stewardship-report.pdf. Published 2017. Accessed September 21, 2018. [Google Scholar]
- 11.Bobulsky GS, Al-Nassir WN, Riggs MM, Sethi AK, Donskey CJ. Clostridium difficile skin contamination in patients with C. difficile-associated disease. Clin Infect Dis 2008;46:447–450. [DOI] [PubMed] [Google Scholar]
- 12.Sethi AK, Al-Nassir WN, Nerandzic MM, Bobulsky GS, Donskey CJ. Persistence of skin contamination and environmental shedding of Clostridium difficile during and after treatment of C. difficile infection. Infect Control Hosp Epidemiol 2010;31:21–27. [DOI] [PubMed] [Google Scholar]
- 13.Brown K, Valenta K, Fisman D, Simor A, Daneman N. Hospital ward antibiotic prescribing and the risks of Clostridium difficile infection. JAMA Intern Med 2015;175:626–633. [DOI] [PubMed] [Google Scholar]
- 14.Freedberg DE, Salmasian H, Cohen B, Abrams JA, Larson EL. Receipt of antibiotics in hospitalized patients and risk for Clostridium difficile infection in subsequent patients who occupy the same bed. JAMA Intern Med 2016;176:1801–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Get smart: know when antibiotics work. Centers for Disease Control and Prevention; website. https://www.cdc.gov/getsmart/community/materials-references/print-materials/everyone/index.html. Accessed September 21, 2018. [Google Scholar]
- 16.Kazakova SV, Baggs J, McDonald LC, et al. Association between antibiotic use and hospital-onset Clostridioides difficile infection in US acute care hospitals, 2006–2012: an ecologic analysis. Clin Infect Dis 2019. doi: 10.1093/cid/ciz169. [DOI] [PubMed] [Google Scholar]
- 17.Villafuerte-Galvez JA, Kelly CP. Proton pump inhibitors and risk of Clostridium difficile infection: association or causation? Curr Opin Gastroenterol 2018;34:11–18. [DOI] [PubMed] [Google Scholar]
- 18.Cao F, Chen CX, Wang M, et al. Updated meta-analysis of controlled observational studies: proton-pump inhibitors and risk of Clostridium difficile infection. J Hosp Infect 2018;98:4–13. [DOI] [PubMed] [Google Scholar]
- 19.Barletta JF, Sclar DA. Proton pump inhibitors increase the risk for hospital-acquired Clostridium difficile infection in critically ill patients. Crit Care 2014;18:714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faleck DM, Salmasian H, Furuya EY, Larson EL, Abrams JA, Freedberg DE. Proton pump inhibitors do not increase risk for Clostridium difficile infection in the intensive care unit. Am J Gastroenterol 2016;111:1641–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zilberberg MD, Tabak YP, Sievert DM, et al. Using electronic health information to risk-stratify rates of Clostridium difficile infection in US hospitals. Infect Control Hosp Epidemiol 2011;32:649–655. [DOI] [PubMed] [Google Scholar]
- 22.Tabak YP, Zilberberg MD, Johannes RS, Sun X, McDonald LC. Attributable burden of hospital-onset Clostridium difficile infection: a propensity score matching study. Infect Control Hosp Epidemiol 2013;34:588–596. [DOI] [PubMed] [Google Scholar]
- 23.Antimicrobial use and resistance (AUR) module. Centers for Disease Control and Prevention; website. https://www.cdc.gov/nhsn/PDFs/pscManual/11pscAURcurrent.pdf. Published 2019. Accessed April 3, 2019. [Google Scholar]
- 24.Core elements of hospital antibiotic stewardship programs. Centers for Disease Control and Prevention; website. https://www.cdc.gov/antibiotic-use/healthcare/pdfs/core-elements.pdf. Published 2014. Accessed July 8, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reported uptake of CDC’s core elements of hospital antibiotic stewardship programs in US acute care hospitals, 2017. Centers for Disease Control and Prevention; website. https://gis.cdc.gov/grasp/PSA/STMapView.html. Published 2017. Accessed July 26, 2019. [Google Scholar]
- 26.Yu K, Rho J, Morcos M, et al. Evaluation of dedicated infectious diseases pharmacists on antimicrobial stewardship teams. Am J Health Syst Pharm 2014;71:1019–1028. [DOI] [PubMed] [Google Scholar]
- 27.Dingle KE, Didelot X, Quan TP, et al. Effects of control interventions on Clostridium difficile infection in England: an observational study. Lancet Infect Dis 2017;17:411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National action plan to prevent health care-associated infections: road map to elimination. Phase four: coordination among Federal partners to leverage HAI prevention and antibiotic. Office of Disease Prevention and Health Promotion; website. https://health.gov/hcq/pdfs/National_Action_Plan_to_Prevent_HAIs_Phase_IV_2018.pdf. Published 2018. Accessed July 8, 2019. [Google Scholar]
- 29.FDA updates warnings for fluoroquinolone antibiotics. US Food and Drug Administration; website. https://www.fda.gov/news-events/press-announcements/fda-updates-warnings-fluoroquinolone-antibiotics. Published 2014. Accessed July 8, 2019. [Google Scholar]
- 30.van Santen KL, Edwards JR, Webb AK, et al. The standardized antimicrobial administration ratio: a new metric for measuring and comparing antibiotic use. Clin Infect Dis 2018;67:179–185. [DOI] [PubMed] [Google Scholar]
- 31.Kazakova S, Baggs J, McDonald L, et al. Association of hospital-onset Clostridium difficile infection rates and antibiotic use in US acute care hospitals, 2006–2012: an ecologic analysis. Oral abstract session: CDI Prevention. October 5, 2017, IDWEEK, San Diego, CA. Infectious Diseases Society of America; website. https://idsa.confex.com/idsa/2017/webprogram/Paper64581.html. Published 2017. Accessed March 22, 2019. [Google Scholar]
- 32.Data summary of HAIs in the United States: assessing progress 2006–2016. Centers for Disease Control and Prevention; website. https://www.cdc.gov/hai/data/archive/data-summary-assessing-progress.html. Published 2018. Accessed December 14, 2018. [Google Scholar]
- 33.The NHSN standardized infection ratio (SIR): a guide to the SIR. Centers for Disease Control and Prevention; website. https://www.cdc.gov/nhsn/pdfs/ps-analysis-resources/nhsn-sir-guide.pdf. Published 2017. Accessed September 19, 2018. [Google Scholar]
- 34.Multidrug-resistant organism and Clostridioides difficile infection (MDRO/CDI) module. Centers for Disease Control and Prevention; website. https://www.cdc.gov/nhsn/pdfs/pscmanual/12pscmdro_cdadcurrent.pdf. Accessed December 14, 2018. [Google Scholar]
- 35.Talpaert MJ, Gopal Rao G, Cooper BS, Wade P. Impact of guidelines and enhanced antibiotic stewardship on reducing broad-spectrum antibiotic usage and its effect on incidence of Clostridium difficile infection. J Antimicrob Chemother 2011;66:2168–2174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.