Abstract
Objective
The primary aim of the study was to investigate the rate of hospitalization and admission diagnoses in severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) positive patients seven months after initial infection. Secondarily, measurement of long-term effects on physical performance, quality of life, and functional outcome was intended.
Design
The study is designed as a controlled follow-up of COVID-19 cases in the district of Constance (FSC19-KN). Setting. A controlled setting is provided due to the recruitment of an equally sized cohort consisting of age- and gender-matched subjects featuring similar cardiovascular risk profiles and negative SARS-CoV-2 antibody titers. Participants. The study examines 206 subjects after polymerase chain reaction (PCR) confirmed SARS-CoV-2 infection seven months after initial infection. Exposure. Infection in the SARS-CoV-2 positive group occurred between March and December 2020. Main Outcome and Measures. The frequency of inpatient admission during the observational period including the related diagnosis was defined as the primary endpoint. Secondary endpoints were health-related quality of life, physical performance, and functional outcome measured by European Quality of Life-5-Dimensions-5-Level (EQ-5D-5L), Short Form Health 36 (SF-36), Six-Minute Walk Test (6MWT), and Post-COVID-19 Functional Status (PCFS).
Results
The study population consisted of mainly nonhospitalized subjects. During the first seven months of observation, frequency of inpatient admission was low and did not differ significantly between both groups (2.4% vs. 2.9% controls: OR 0.8, 95% CI 0.2 to 2.8). Calculation of six-minute walk distance ratios showed no significant difference between both cohorts (0.97 ± 0.17 vs. 0.98 ± 0.16 controls; mean difference −0.01; 95% CI −0.04 to 0.02). However, SARS-CoV-2-positive subjects achieved significantly lower EQ-5D-5L index scores (0.92 ± 0.12 vs. 0.95 ± 0.1 controls; mean difference −0.03, 95% CI −0.05 to −0.01) and SF-36 subscores. Reduced PCFS was reported significantly more often in the SARS-CoV-2 positive cohort (30.6% vs 14.6% controls: OR 2.6, 95% CI 1.6 to 4.2).
Conclusion
The results suggest that mild COVID-19 has no impact on the hospitalization rate during the first seven months after infection. Despite unimpaired performance in cardiopulmonary exercise, SARS-CoV-2-positive subjects reported reduced quality of life and functional sequelae. Underlying psychoneurological mechanisms need further investigation. Trial Registration. This trial is registered with clinicaltrials.gov (identifier: NCT04724434) and German Clinical Trials Register (identifier: DKRS00022409).
1. Background
For over a hundred years, viral pandemics recurrently led to restrictions on public health. The Russian flu—possibly the very first coronavirus pandemic [1]—did not only cost the life of a million people at the end of the 19th century but also involved a prolonged convalescence in the surviving [2]. A more recent example is the secondary development of pulmonary damage in severe acute respiratory syndrome coronavirus (SARS-CoV) patients [3, 4]. Due to a similar taxonomic classification [5], it is possible that the characteristics mentioned above apply to the currently widespread SARS-CoV-2 as well.
The severity of coronavirus infectious disease 2019 (COVID-19) cases varies considerably. Courses are asymptomatic [6] or mild [7], most of the time without the necessity of hospitalization [8], but in some cases characterized by life-threatening acute respiratory distress syndrome (ARDS) [9]. Apart from the acute disease treatment, the clinical management of increasing numbers of post-COVID-19 patients [10] becomes challenging for emergency rooms and general practitioners. Their complaints are often unspecific [11], which makes the identification of secondary diseases a difficult task.
From a socioeconomic perspective, the management of post-COVID-19 patients needs to be planned efficiently on the basis of significant data. Since mild courses represent the majority of COVID-19 cases [12, 13], it is of particular interest to investigate sequelae in nonhospitalized individuals. Despite numerous follow-up studies, there remains a lack of convincing data in this field [14]. Sample sizes, age ranges, and study settings vary considerably, and data were rarely collected in a controlled setting [15]. Hence, the main goal of this single-center prospectivecontrolled follow-up study is to investigate clinical complications in a representative collective of COVID-19 cases.
2. Methods
2.1. Study Design
The prospective single-center cohort study FSC-19-KN was designed as a controlled follow-up of patients after SARS-CoV-2-infection in the local district of Constance (Baden-Wuerttemberg, Germany). Its main objective was to periodically assess sequelae over five years. Approval was given by the ethics committee of Albert Ludwigs University (Freiburg). The study was registered on the German Clinical Trials Register and Clinicaltrials.gov.
The recruitment of the SARS-CoV-2-positive group was performed in cooperation with the local health department (see Figure 1). 1200 individuals were randomly sampled from all polymerase chain reaction (PCR)-confirmed cases in the local district of Constance between March 2020 and December 2020 and contacted via mail. During initial visits between January and July 2021, 281 adults who fulfilled the eligibility criteria were enrolled at a mean of 203.5 days after infection. Common eligibility criteria were defined as follows: age ≥18 years, ability to read and sign the consent form, and to grasp the nature of the study.
Figure 1.
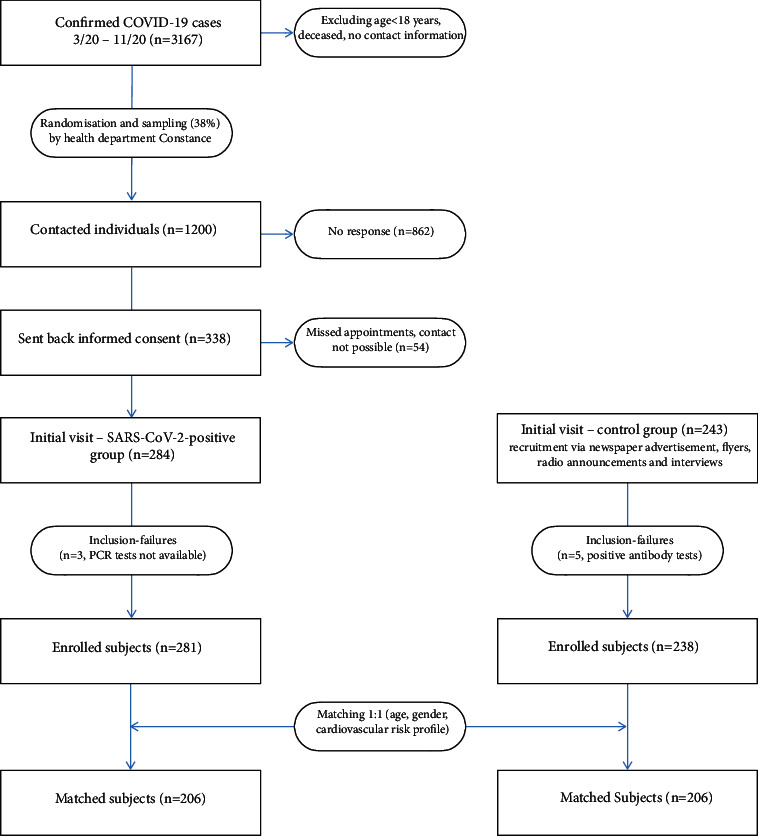
Recruitment and matching. Square boxes contain numbers of recruited subjects; round boxes contain numbers of excluded subjects.
A total of 238 subjects exhibiting similar cardiovascular risk factors and negative SARS-CoV-2 antibody titers (Roche Elecsys Anti-SARS-CoV-2) were recruited as potential matching partners via newspaper advertisements, flyers, radio announcements, and interviews. 206 matching pairs could finally be established. Initially, the matching procedure followed strictly predetermined criteria (age ± 3 years, same gender, same status in terms of arterial hypertension, diabetes, and nicotine abuse). These were punctually loosened for 46 matching pairs in the following manner: age: ±5 years, both smoking and ex-smoking added together.
2.2. Data Collection and Outcome Measurement
All study data were collected in a clinical setting by the medical staff of the Hegau Bodensee academic teaching hospital (District of Constance, Germany). The supervising principal investigators worked as physicians in the field of internal medicine. The data were subsequently managed using a Research Electronic Data Capture (REDCap) platform hosted at redcap.glkn.de [16, 17]. The accuracy of data entries was verified by an external monitor according to guidelines for good clinical practice.
During initial visits, the medical history of the participants was recorded systematically with particular emphasis on COVID-19. This included the presence of symptoms, necessity of hospitalization, monitoring in an intensive care unit and mechanical ventilation, pre-existing medical conditions, and stratification of cardiovascular risk profile. Inpatient admissions within the previous seven months were inquired about to determine clinical events. Relevant medical reports were requested and evaluated. Admission diagnoses were referred to different medical disciplines. Since a greater effect of COVID-19 was expected on cardiopulmonary and neurological events [10, 18, 19], their frequencies were pooled and compared to those of the remaining surgical, gynecological, and orthopedic events.
The six-minute walk test was conducted in a clinical setting under the medical supervision of the principal investigators. Its protocol required documentation of Borg Categorial Ratio (CR) and Rating of Perceived Exertion (RPE) scales before and after physical stress. Ratios of six-minute walk distance were calculated with an age-, gender, and body mass index-dependant formula [20].
Health-related quality of life was evaluated via German versions of European Quality of Life 5 Dimension 5 Level (EQ-5D-5L) and Short-Form Health 36 (SF-36). EQ-5D-5L index score was calculated using the German crosswalk value set according to the authors' algorithm [21] and the Visual Analogue Scale (VAS) score was directly read out. SF-36 subscores were calculated according to the authors' instructions [22]. Due to overlapping content, a translation algorithm from both questionnaires into the recently validated Post-COVID-Functional Status (PCFS) scale [23, 24] was developed and applied to generate further evidence for its clinical practicability (see Figure 2).
Figure 2.
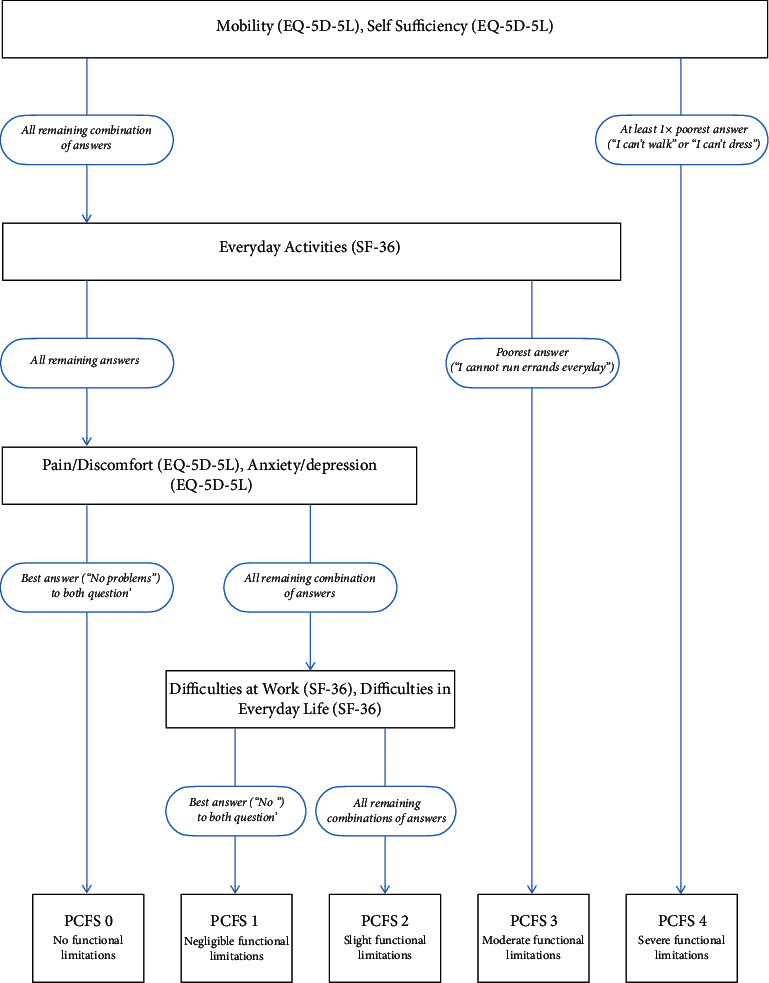
Translation algorithm from EQ-5D-5L and SF-36 into PCFS. Square boxes contain items from EQ-5D-5L/SF-36 and PCFS states; round boxes contain required response options.
2.3. Statistical Analysis
Descriptive statistics were used for a comparative presentation of sociodemographic data, cardiovascular risk profiles, pre-existing medical conditions, and COVID-19-specific data. Results are presented only for matched data (n = 206, respectively). Discrete variables were presented as the absolute frequency with proportional value in brackets. Continuous variables were indicated as mean ± standard deviation. Missing values were recorded accurately, but not included during data analysis.
For Borg CR and RPE scales, six-minute walk distance ratios, EQ-5D-5L-index scores, SF-36 component, and subscores data were given as mean ± standard deviation. The difference in means (MD) was indicated and the respective 95%-confidence interval (CI) was estimated via t-test for independent samples with pooled variances. Results of PCFS were summarised into a fourfold table to calculate odds ratios (OR). Respective confidence intervals were determined by the logarithmic odds ratio function.
3. Results
3.1. Study Population
SARS-CoV-2-positive subjects were enrolled at a mean of 203.5 days after initial PCR testing. The majority had suffered from at least one symptom during the initial infection (95.1%). Rates of hospitalisation (2.4%), ICU-monitoring (0.7%), and mechanical ventilation (0.4%) were low.
The analysis of demographic and basic biometric data proved a similar composition of both study groups attributable to successful matching (Table 1). The mean age was 47 years with a moderately higher female participation rate of 58.2%, and body mass indices were at the threshold of mild obesity. The stratification of cardiovascular risk profiles showed no difference between both cohorts: 36% of participants were smokers, 13.1% suffered from arterial hypertension, and 1% from diabetes mellitus. Comorbidities were similarly prevalent in both cohorts with regard to chronic obstructive pulmonary disease, pulmonary embolism, deep venous thrombosis, myocardial infarction, cerebral ischemia, and coronary/peripheral artery disease. Merely bronchial asthma was found significantly more often in the SARS-CoV-2-positive cohort (8.9% vs. 3.0% controls: OR 2.5, 95% CI 1.1 to 6.0).
Table 1.
Study population.
| SARS-CoV-2, n = 206 | Control, n = 206 | Missing values, SARS-CoV-2/control | |
|---|---|---|---|
| Demographics/biometrics | |||
| Age (years) | 47.0 ± 15.2 | 47.0 ± 15.0 | 0/0 |
| 18–39 (no. (%)) | 69 (33.5) | 63 (30.6) | 0/0 |
| 40 (no. (%)) | 93 (45.1) | 97 (47.1) | 0/0 |
| 60–79 (no. (%)) | 43 (20.9) | 46 (22.3) | 0/0 |
| ≥80 (no. (%)) | 1 (0.01) | 0 (0) | 0/0 |
|
| |||
| Gender | |||
| Male (no. (%)) | 86 (41.8) | 86 (41.8) | 0/0 |
| Female (no. (%)) | 120 (58.2) | 120 (58.2) | 0/0 |
|
| |||
| Body mass index (kg/m2) | 25.3 ± 4.0 | 24.8 ± 4.3 | 4/0 |
|
| |||
| Cardiovascular risk factors | |||
| Diabetes mellitus (no. (%)) | 1 (0.5) | 1 (0.5) | 0/0 |
| Arterial hypertension (no. (%)) | 27 (13.1) | 27 (13.1) | 0/0 |
| Hypercholesterolemia (no. (%)) | 24 (11.6) | 15 (7.3) | 2/0 |
| Smoking (no. (%)) | 79 (36.9) | 79 (36.9) | 0/0 |
| Family history of coronary artery disease (no. (%)) | 37 (18.9) | 36 (17.5) | 3/2 |
|
| |||
| Clinical history | |||
| Chronic obstructive pulmonary disease (no. (%)) | 3 (1.5) | 2 (1.0) | 10/6 |
| Interstitial lung disease (no. (%)) | 0 (0.0) | 0 (0.0) | 9/7 |
| Pulmonary embolism (no. (%)) | 1 (0.5) | 1 (0.5) | 8/7 |
| Deep vein thrombosis (no. (%)) | 2 (1.0) | 4 (1.9) | 9/7 |
| Asthma (no. (%)) | 18 (8.9) | 8 (3.9) | 22/12 |
| Myocardial infarction (no. (%)) | 3 (1.5) | 3 (1.5) | 0/0 |
| Transient ischemic attack/stroke (no. (%)) | 2 (1.0) | 2 (1.0) | 0/0 |
| Coronary artery disease (no. (%)) | 4 (1.9) | 4 (1.9) | 0/0 |
| Peripheral artery disease (no. (%)) | 1 (0.5) | 1 (0.5) | 1/0 |
Data are given as absolute value (percentage)/mean ± standard deviation.
3.2. Primary Endpoints
Only clinical events leading to hospitalization were recorded (Table 2). They were distributed evenly among both study groups (2.4% vs. 2.9% controls: OR 0.8, 95% CI 0.2 to 2.8). Admission diagnoses were cardiopulmonary, neurological, orthopedic, gynecological, or surgical. Combined frequencies of cardiopulmonary and neurological events did not differ significantly between both groups (1.4% vs. 0.5% controls: OR 3.1, 95% CI 0.3 to 31.0).
Table 2.
Clinical events leading to hospitalization and admission diagnoses.
| Total | SARS-CoV-2, n = 206 5 (2.4) |
Control, n = 206 6 (2.9) |
||
|---|---|---|---|---|
| Classification | Number | Admission diagnoses | Number | Admission diagnoses |
| Cardiopulmonal/neurological | 3 (1.4) | Exclusion of coronary artery disease, atrial fibrillation, vestibular neuritis | 1 (0.5) | Obstructive sleep apnoea |
|
| ||||
| Orthopedic/surgical/gynecological | 2 (0.9) | Tibial head fracture, meniscal lesion | 5 (2.4) | Birth arrest, acute appendicitis, acute pancreatitis, meniscal lesion, spinal canal stenosis |
Data are given as absolute value (percentage).
3.3. Secondary Endpoints
Normal six-minute walk distance ratios were measured in both study groups (Table 3; means 0.97 ± 0.17 vs. 0.98 ± 0.16 controls) without a significant difference (MD −0.01, 95% CI −0.04 to 0.02). However, means were significantly higher in SARS-CoV-2-positive subjects' self-evaluation scores. This applied for the Borg CR scale at rest (0.05 ± 0.24 vs. 0.01 ± 0.08 controls; MD 0.04, 95% CI 0.01 to 0.09), after stress (0.8 ± 1.23vs. 0.41 ± 0.91 controls; MD 0.39, 95% CI 0.19 to 0.59), and RPE scale (9.6 ± 2.9 vs. 8.7 ± 2.6 controls; MD 0.8, 95% CI 0.3 to 1.4).
Table 3.
Physical performance, health-related quality of life, and functional outcome.
| SARS-CoV-2 n = 206 | Control n = 206 | Difference in means/odds ratio | Missing values, SARS-CoV-2/control | |
|---|---|---|---|---|
| 6-minute walk-test results | ||||
| Walk distance (meters) | 590.8 ± 77.7 | 600.8 ± 92.4 | −10.4 [ −26.6; 6.5] | 0/3 |
| Walk distance ratio (0-1) | 0.97 ± 0.17 | 0.98 ± 0.16 | −0.01 [ −0.04; 0.02] | 4/3 |
|
| ||||
| Borg scales | ||||
| Borg CR scale (0-10) at rest | 0.05 ± 0.24 | 0.01 ± 0.08 | 0.04 [0.01; 0.09] | 0/1 |
| Borg CR scale (0-10) after stress | 0.8 ± 1.23 | 0.41 ± 0.91 | 0.39 [0.19; 0.59] | 0/1 |
| Borg rating of perceived exertion (6-20) | 9.6 ± 2.9 | 8.7 ± 2.6 | 0.8 [0.3; 1.4] | 0/1 |
|
| ||||
| EQ-5D-5L (European quality of life 5-dimension-5-level) | ||||
| VAS-index-score (%) | 83.6 ± 15.2 | 88.6 ± 12.4 | −4.9 [ − 7.6; −2.3] | 0/0 |
| Calculated index-score (0-1) | 0.92 ± 0.12 | 0.95 ± 0.1 | −0.03 [ − 0.05; −0.01] | 0/1 |
|
| ||||
| SF-36 (short form 36) scores | ||||
| Physical functioning score (0–100) | 88.0 ± 15.3 | 93.6 ± 10.9 | −5.6 [ − 8.2; −2.9] | 11/2 |
| Role functioning/physical score (0–100) | 76.0 ± 33.9 | 92.0 ± 22.7 | −16.0 [ − 21.6; −10.4] | 1/0 |
| Role functioning/emotional score (0–100) | 73.6 ± 37.8 | 88.0 ± 27.0 | −14.4 [ − 20.8; −7.9] | 10/3 |
| Energy/fatigue score (0–100) | 56.8 ± 23.04 | 67.9 ± 21.5 | −11.0 [ − 15.4; −6.7] | 1/0 |
| Emotional well-being score (0–100) | 72.8 ± 17.6 | 81.1 ± 15.2 | −8.2 [ − 11.4; −5.0] | 1/0 |
| Social functioning score (0–100) | 83.7 ± 22.5 | 90.7 ± 17.6 | −7.0 [ − 11.0; −3.1] | 8/2 |
| Pain score (0–100) | 84.9 ± 22.1 | 90.7 ± 17.2 | −5.9 [ − 9.7; −2.0] | 9/2 |
| General health score (0–100) | 72.8 ± 21.0 | 80.1 ± 16.9 | −7.3 [ − 11.0; −3.6] | 0/0 |
|
| ||||
| PCFS (post-COVID-19 functional status) | ||||
| PCFS 2/3 (no. (%) | 63 (30.6) | 30 (14.5) | OR 2.6 [1.6; 4.2] | 0/0 |
Data are given as mean ± standard deviation, and in square brackets, 95% confidence interval is given; significant differences in means/odds ratios are written in bold type.
Regarding the EQ-5D-5L, means of both VAS (83.6 ± 15.2% vs. 88.6 ± 12.4% controls; MD −4.9, 95% CI −7.6 to −2.3) and calculated index scores (0.92 ± 0.12 vs. 0.95 ± 0.1 controls; MD −0.03, 95% CI −0.05 to −0.01) were significantly lower in the SARS-CoV-2-positive group.
Equally, SARS-CoV-2-positive subjects achieved significantly lower results in each of the eight SF-36 subscores (Table 3).
The majority of subjects altogether achieved lower PCFS states <2 (77.4%). However, reduced functional outcomes (PCFS ≥2) were detected significantly more often in the SARS-CoV-2-positive cohort (30.6% vs. 14.5% controls: OR 2.6, 95% CI 1.6 to 4.2).
4. Discussion
4.1. Interpretation of Results
Rates of hospitalization, monitoring in an intensive care unit, and mechanical ventilation were relatively low in the SARS-CoV-2-positive cohort. Most of the subjects were symptomatic but presented with a mild course of the disease.
No effect of SARS-CoV-2 infection could be detected on the frequency of clinical events during the first seven months after the initial infection.
Similarly, no significant effect of SARS-CoV-2 infection on physical performance during six-minute walk tests was observed. This harshly contrasts with poorer results in PCFS and Borg scales as well as EQ-5D-5L and SF-36 scores. Thus, the results suggest a negative impact of COVID-19 on functional outcomes and self-perceived quality of life.
4.2. Contextual Evidence
A huge number of recovered COVID-19 patients still suffer from symptoms six to eight months after mild infection [25, 26]. These symptoms are often unspecific and manifest in various organ systems [27], which makes it difficult to prove causality between prior SARS-CoV-2 infection and patients' complaints. According to a follow-up study carried out by the University of Ulm, there is a discrepancy between functional complaints and barely measurable organ damage in post-COVID-19 patients [28]. This resembles the contrast between poor self-evaluation and normal physical performance tests in the SARS-CoV-2-positive cohort at hand.
A large controlled follow-up study of COVID-19 patients in the United States investigated hospitalization rates six months after infection and provided evidence for higher incidences of pulmonary embolisms after COVID-19 [29]. Another equally scaled observational study from the same geographic region provided contradicting evidence stating that rates of thromboembolic events return to baseline pre-COVID-19 levels six to seven weeks after infection [30]. The SARS-CoV-2-positive cohort at hand showed no increase in thromboembolic events during the observational period.
The reduction of quality of life and functional outcome was reproduced by several other COVID-19 follow-up studies regardless of initial disease severity and cohort size [31–34]. Evidence has been published for postinfectious damage of neurons in the peripheral and central nervous system [35], which might contribute to the development of this very pattern. From a psychological perspective, mechanisms of social stigmatization [36] and the sheer knowledge of previous SARS-CoV-2-infection [37] should be additionally taken into account as harmful factors. A large multidisciplinary COVID-19 follow-up study initiated by University Mainz deals with this very subject by differentiating between knowingly and unknowingly infected subjects [37].
4.3. Limitations
First, the low hospitalization rate of the SARS-CoV-2-positive cohort seems to limitate the applicability of the findings. Since the severity of COVID-19 is mild in most cases [12, 13, 38], the study results are relevant for the majority of patients.
Second, the observational period has only been seven months so far. Long-term effects might not have been fully developed during initial visits yet. However, yearly follow-ups have been planned over the next five years to re-evaluate the results. Additional effects of SARS-CoV-2 vaccination and SARS-CoV-2 infection within the control cohort will have to be considered by then.
Third, there is growing evidence that postinfectious syndromes resemble psychiatric conditions such as fatigue syndrome and depression [39]. Mental symptoms have only been recorded to some extent via SF-36 subscores. However, a substudy has emerged from the study at hand dealing with this topic specifically.
Lastly, subclinical events requiring outpatient diagnostic and therapeutic procedures were not inquired about during initial visits. Considering the low frequency of clinical events so far, inclusion of these subclinical data is intended during future annual follow-ups.
5. Conclusion
In this study, SARS-CoV-2-positive subjects were mainly nonhospitalized and went through mild clinical courses. The initial data analysis suggests that there is no objective difference in terms of inpatient admission and physical performance between both study groups within the first seven months after infection. However, there is a pattern of negative self-assessment in the SARS-CoV-2-positive cohort with regards to health-related quality of life and functional status. Underlying psychoneurological mechanisms need to be investigated. During annual follow-up over five years, the dynamics of these effects will be monitored.
Data Availability
The data used to support the findings of this study are available in https://redcap.glkn.de/surveys/?__report = 9AKLDFRDCAER7AWF.
Additional Points
Question: does a follow-up investigation of COVID-19 cases in a controlled setting show an increased hospitalization rate during the first seven months after infection? Findings: in this controlled follow-up study, the seven-month observation of 206 mildly affected SARS-CoV-2 positive subjects showed no significant increase in clinical events. Despite good performance in cardiopulmonary exercise, reduced quality of life and functional sequelae were reported. Meaning: long-term impact of mild COVID-19 does not manifest in binary endpoints such as hospitalization, but self-perceived health status.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Elisabeth Haberland and Jonas Haberland contributed equally to this work.
References
- 1.Brüssow H., Brüssow L. Clinical evidence that the pandemic from 1889 to 1891 commonly called the russian flu might have been an earlier coronavirus pandemic. Microbial Biotechnology . 3 July 2021;14(5):1860–1870. doi: 10.1111/1751-7915.13889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Honigsbaum M. The great dread: cultural and psychological impacts and responses to the “russian” influenza in the United Kingdom, 1889–1893. Social History of Medicine . 10 June 2010;23(2):299–319. doi: 10.1093/shm/hkq011. [DOI] [Google Scholar]
- 3.Ngai J. C., Ko F. W., Ng S. S., To K. W., Tong M., Hui D. S. The long‐term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology . 2010;15(3):543–550. doi: 10.1111/j.1440-1843.2010.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang P., Li J., Liu H., et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Resorption . 2020;8(1) doi: 10.1038/s41413-020-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiology . 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sah P., Fitzpatrick M. C., Zimmer C. F., et al. Asymptomatic SARS-CoV-2 infection: a systematic review and meta-analysis. Proceedings of the National Academy of Sciences of the United States of America . 2021;118(34) doi: 10.1073/pnas.2109229118.e2109229118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandhi R. T., Lynch J. B., Del Rio C. Mild or moderate covid-19. New England Journal of Medicine . 2020;383(18):1757–1766. doi: 10.1056/nejmcp2009249. [DOI] [PubMed] [Google Scholar]
- 8.Blair J. E., Gotimukul A., Wang F., et al. Mild to moderate COVID-19 illness in adult outpatients. Medicine . 2021;100(24) doi: 10.1097/md.0000000000026371.e26371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzotzos S. J., Fischer B., Fischer H., Zeitlinger M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Critical Care . 2020;24(1) doi: 10.1186/s13054-020-03240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nature Medicine . 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matta J., Wiernik E., Robineau O., et al. Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among French adults during the COVID-19 pandemic. JAMA Internal Medicine . 2022;182(1):19–25. doi: 10.1001/jamainternmed.2021.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia L., Chen J., Friedemann T., et al. The course of mild and moderate COVID-19 infections—the unexpected long lasting challenge. Open Forum Infectious Diseases . 2020;7(9) doi: 10.1093/ofid/ofaa286.ofaa286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boban M. Novel coronavirus disease (COVID‐19) update on epidemiology, pathogenicity, clinical course and treatments. International Journal of Clinical Practice . 2020;75(4) doi: 10.1111/ijcp.13868.e13868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koczulla A., Ankermann T., Behrends U., Berlit P. S1-Leitlinie Post-COVID/Long-COVID—AWMF online. 2021. https://www.awmf.org/uploads/tx_szleitlinien/020-027l_S1_Post_COVID_Long_COVID_2021-07.pdf .
- 15.Amin-Chowdhury Z., Ladhani S. N. Causation or confounding: why controls are critical for characterizing long COVID. Nature Medicine . 2021;27(7):1129–1130. doi: 10.1038/s41591-021-01402-w. [DOI] [PubMed] [Google Scholar]
- 16.Harris P., Taylor R., Minor B., Elliott V. The REDCap consortium: building an international community of software partners. Journal of Biomedical Informatics . 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris P. A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J. G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics . 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cilazi M., Duffy E., Thakkar A., Michos E. Intermediate and long-term impact of COVID-19 on cardiovascular disease. 2021. https://www.acc.org/latest-in-cardiology/articles/2021/04/21/13/08/intermediate-and-long-term-impact-of-covid-19-on-cardiovascular-disease .
- 19.Pilotto A., Cristillo V., Cotti Piccinelli S., et al. Long-term neurological manifestations of COVID-19: prevalence and predictive factors. Neurological Sciences . 2021;42(12):4903–4907. doi: 10.1007/s10072-021-05586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enright P., Sherrill D. Reference equations for the six-minute walk in healthy adults. American Journal of Respiratory and Critical Care Medicine . 1998;158(5):1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 21.Euroquol Research Foundation. Euroquol instruments—EQ-5D-5L | valuation | crosswalk index value calculator. 2019. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/valuation-standard-value-sets/crosswalk-index-value-calculator/
- 22.RAND Corporation. 36-item short form survey (SF-36) scoring instructions. 2021. https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form/scoring.html .
- 23.Machado F. V. C., Meys R., Delbressine J. M., et al. Construct validity of the post-COVID-19 functional status scale in adult subjects with COVID-19. Health and Quality of Life Outcomes . 2021;19(1) doi: 10.1186/s12955-021-01691-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klok F. A., Boon G. J., Barco S., et al. The Post-COVID-19 Functional Status scale: a tool to measure functional status over time after COVID-19. European Respiratory Journal . 2020;56(1) doi: 10.1183/13993003.01494-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menges D., Ballouz T., Anagnostopoulos A., et al. Burden of post-COVID-19 syndrome and implications for healthcare service planning: a population-based cohort study. PLoS One . 2021;16(7) doi: 10.1371/journal.pone.0254523.e0254523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kashif A., Chaudhry M., Fayyaz T., et al. Follow-up of COVID-19 recovered patients with mild disease. Scientific Reports . 2021;11(1) doi: 10.1038/s41598-021-92717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis H. E., Assaf G. S., McCorkell L., et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine . 2021;38 doi: 10.1016/j.eclinm.2021.101019.101019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kersten J., Baumhardt M., Hartveg P., et al. Long COVID: distinction between organ damage and deconditioning. Journal of Clinical Medicine . 2021;10(17):p. 3782. doi: 10.3390/jcm10173782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chevinsky J. R., Tao G., Lavery A. M., et al. Late conditions diagnosed 1–4 Months following an initial coronavirus disease 2019 (COVID-19) encounter: a matched-cohort study using inpatient and outpatient administrative data—United States, 1 march–30 june 2020. Clinical Infectious Diseases . 2021;73:S5–S16. doi: 10.1093/cid/ciab338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasha A. K., McBane R. D., Chaudhary R., et al. Timing of venous thromboembolism diagnosis in hospitalized and non-hospitalized patients with COVID-19. Thrombosis Research . 2021;207:150–157. doi: 10.1016/j.thromres.2021.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaes A., Goërtz Y., Van Herck M., Machado F. Recovery from COVID-19: a sprint or marathon? 6-month follow-up data from online long COVID-19 support group members. ERJ Open Research . 2021;7 doi: 10.1183/23120541.00141-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Betschart M., Rezek S., Unger I., et al. One year follow-up of physical performance and quality of life in patients surviving COVID-19: a prospective cohort study. Swiss Medical Weekly . 2021;151 doi: 10.4414/smw.2021.w30072. [DOI] [PubMed] [Google Scholar]
- 33.Pizarro-Pennarolli C., Sánchez-Rojas C., Torres-Castro R., et al. Assessment of activities of daily living in patients post COVID-19: a systematic review. PeerJ . 2021;9 doi: 10.7717/peerj.11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qu G., Zhen Q., Wang W., et al. Health-related quality of life of COVID-19 patients after discharge: a multicenter follow-up study. Journal of Clinical Nursing . 2021;30:1742–1750. doi: 10.1111/jocn.15733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang F., Kream R. M., Stefano G. B. Long-term respiratory and neurological sequelae of COVID-19. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research . 2020;26 doi: 10.12659/msm.928996.e928996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhanot D., Singh T., Verma S. K., Sharad S. Stigma and discrimination during COVID-19 pandemic. Frontiers in Public Health . 2021;8 doi: 10.3389/fpubh.2020.577018.577018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wild P. Spätfolgen einer SARS-CoV-2-Infektion in der Bevölkerung und Konzept der Gutenberg Long COVID Studie . 2021. https://www.unimedizin-mainz.de/GCS/dashboard/#/app/pages/AktuelleErgebnisse/ergebnisselc . [Google Scholar]
- 38.Schilling J., Tolksdorf K., Marquis A., Faber M. Die verschiedenen Phasen der COVID-19-Pandemie in Deutschland: Eine deskriptive Analyse von Januar 2020 bis Februar 2021. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz . 2021;64:1093–1106. doi: 10.1007/s00103-021-03394-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taquet M., Geddes J. R., Husain M., Luciano S., Harrison P. J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry . 2021;8(5):416–427. doi: 10.1016/s2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available in https://redcap.glkn.de/surveys/?__report = 9AKLDFRDCAER7AWF.


