Abstract
Fatigue is one of the most commonly reported symptoms in the context of the post-COVID-19 syndrome. Notably, fatigue is characterised by overlapping physical and psychopathological symptoms, and questions about its trajectory over time and possible predictors remained unanswered. Thus, in the present study we aim to investigate the prevalence, the course over time, and the risk factors of post-COVID fatigue.
We included 495 patients recovered from COVID-19. For all of them we collected one month demographic, clinical and psychopathological characteristics. We evaluated fatigue severity at one, three, six, and twelve-months according to Fatigue Severity Scale (FSS). We explored the potential predictor of long-term post-COVID fatigue (six or twelve months FSS) by implementing 5000 non-parametric bootstraps enhanced elastic net penalised regression.
We found that 22%, 27%, 30%, and 34% of patients self-rated fatigue symptoms in the pathological range at one, three, six, and twelve months respectively. We detected a worsening of fatigue symptomatology over time. From the elastic net regression results, only depressive symptomatology at one month (ZSDS and BDI-13) predicted the presence of post-COVID-19 long-term fatigue. No other clinical or demographic variable was found to predict post-COVID fatigue.
We suggest that, rather independent of COVID-19 severity, depression after COVID-19 is associated with persistent fatigue. Clarifying mechanisms and risk factors of post-COVID fatigue will allow to identify the target population and to tailor specific treatment and rehabilitation interventions to foster recovery.
Keywords: COVID-19, SARS-CoV-2, Fatigue, Depression, Post-COVID syndrome
Contributors
MGM conceived the study. MGM, MP, RDL, PRQ and FB contributed to the inclusion of patients and acquisition of the data. MGM designed the analysis. MGM and GV carried out the analysis and interpreted the data, with contributions from MP, RDL, and FB. MGM and GV wrote the initial draft of the manuscript. All authors contributed to the final version, gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
1. Introduction
In December 2021, the global confirmed case count of Coronavirus Disease (2019) (COVID-19) surpassed 275 million, and the actual case positive rate is estimated to be much higher than the number of diagnosed cases (Havers et al., 2020; Wu et al., 2020a).
More than 30% of individuals affected by COVID-19, who were both symptomatic and asymptomatic during the acute phase of COVID-19, have experienced persistent symptoms after recovering (Huang et al., 2021a; Tenforde et al., 2020). This phenomenon has been termed Post-Acute Sequelae of COVID-19 (PASC) which refers to long-lasting symptoms following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. PASC is now recognized by the National Institute for Health and Care Excellence (NICE) (Venkatesan, 2021), the Centers for Disease Control and Prevention (CDC) (Centers for Disease Control and Prevention, 2022), and the World Health Organization (WHO) (World Health Organization, 2021) even if a clear and shared definition of this condition is still not established. Fatigue, depression, and cognitive impairment, along with other enduring neuropsychiatric and physical manifestations, comprise PACS (Nalbandian et al., 2021).
Fatigue has been consistently reported as one of the most common and debilitating features of the post-COVID-19 syndrome affecting one in three COVID-19 survivors (Ceban et al., 2021; Davis et al., 2021; Marshall, 2020). Post-COVID fatigue was defined as a persistent feeling of physical and mental tiredness characterised by lack of energy, weakness, slowed reactions, and drowsiness. Interestingly, it was not affected by acute COVID severity and persisted over six months after SARS-CoV-2 infection (Ceban et al., 2021). Frequently reported risk factors of post-COVID fatigue included female sex, older age, and pre-existing comorbidities (Ceban et al., 2021). Moreover, the acute COVID-19 severity and the related intensity of care were also associated with increasing risk of post-COVID fatigue respectively from asymptomatic patients, to hospitalized patients, and finally in patients admitted in intensive care unit (ICU) (Al-Aly et al., 2021). Similar incidence rates of fatigue have been reported in consequence of previous coronavirus epidemics, including SARS and MERS (Rogers et al., 2020).
Even depressive symptomatology is now recognized as one of the main enduring symptoms of the PCS. The frequency of depressive symptoms at different follow-ups after COVID-19 ranges from 20 to 40%, with females and patients with a preexisting psychiatric disorder at higher risk of presenting post-COVID fatigue. Post-COVID depression shares psychopathological brain imaging correlates (Benedetti et al., 2021b) and negative thinking styles (Palladini et al., 2021) as core features observed in major depression, and affects fatigue syndrome (Al-Jassas et al., 2022), neurocognitive functioning (Gouraud et al., 2021; Poletti et al., 2021), and quality of life (Babicki et al., 2021; Poletti et al., 2021) in PCS.
Persistent post-COVID symptoms could play a detrimental role on COVID-19 survivors in a self-reinforcing circle of depressive psychopathology, fatigue, and reduction in global functioning. In this context, a bidirectional association between depression and fatigue has been largely demonstrated. Thus, questions about the prevalence and longitudinal course of post-COVID fatigue as well as the possible effects of depression on fatigue and COVID-19 clinical severity on both remained unanswered. In this context, we aim to i) investigate the prevalence of fatigue and its trajectory over time and ii) to predict long-term fatigue symptoms (at six or twelve months) in COVID-19 survivors based on baseline clinical and psychopathological predictors through a machine learning approach.
2. Experimental procedure
2.1. Sample and study design
We included 495 COVID-19 survivors recruited between 6 April 2020 and 28 February 2021 in the ongoing COVID-BioB study at IRCCS San Raffaele Hospital in Milan.
Patients were included if they were hospitalized for clinical and radiological findings suggestive of COVID-19 pneumonia and if SARS-CoV-2 infection was confirmed by positive real-time reverse-transcriptase polymerase chain reaction (RT-PCR). Exclusion criteria were limited to patients under 18 years and difficulty fully understanding self-report questionnaires due to linguistic barrier or intellectual disability.
After inclusion in the study, depressive psychopathology and clinical variables were collected for all patients at the one-month follow-up. Fatigue symptomatology was collected at six and twelve-month follow-up for patients recruited during the first COVID-19 wave (6 April to 9 June 2020); and at one, three, and six-month follow-up for patients recruited during the second COVID-19 wave (1 September 2020 to 28 February 2021). The two COVID-19 waves only differed for the rate of positive psychiatric history (Supplementary eTable 1).
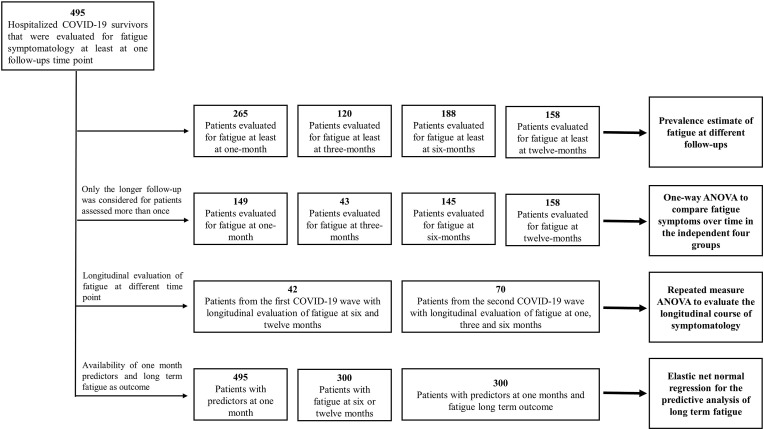
Globally we included 495 hospitalized COVID-19 survivors (males 326, mean age 57.6 ± 11.5) that underwent a psychopathological evaluation at one month and were evaluated for fatigue symptomatology at least at one follow-up time point. In detail, i) 265, 120, 188, and 158 patients were evaluated at one, three, six, and twelve-month (some patients with more than one evaluation of fatigue over time) and were used to estimate the fatigue prevalence; ii) 149, 43, 145, and 158 independent evaluation of fatigue (only the longer follow-up was considered for patients assessed more than one time) were available at different time points and were included in the one-way ANOVA to compare fatigue symptoms in the independent four time point groups; iii) 42 patients from the first COVID-19 wave had a longitudinal evaluation of fatigue at six and twelve months and 70 patients from the second COVID-19 wave were longitudinally evaluated at one, three, and six-month and were included in the repeated measure ANOVA to evaluate the longitudinal course of symptomatology; iv) finally, 300 patients were assessed both for depression (ZSDS and BDI) at one month and fatigue (FSS) at six or twelve months and were included in the elastic net normal regression for the predictive analysis. The detail of patients selection is available in Fig. 1 .
Fig. 1.
Patient selection flowchart.
After a complete description and disclosure of the study to the participants, written informed consent was obtained. The study was approved by the Ethics Committee of San Raffaele Hospital (COVID-BioB protocol NCT04318366) in accordance with the principles in the Declaration of Helsinki.
2.2. Clinical and psychopathological assessment
At one, three, and six-month follow-up, evaluation was performed in an outpatient setting by trained psychiatrists in charge using an unstructured psychiatric interview and validated self-report questionnaires. At twelve-month follow-up, data were collected using encrypted hyperlinks to an online platform.
Fatigue at one, three, six and twelve-month was assessed using the Fatigue Severity Scale (FSS). FSS questionnaire includes nine statements aiming at exploring the severity of fatigue symptoms by asking subjects to choose for each item the number from 1 to 7 which best applied to him/her. FSS was originally conceived to differentiate depression from fatigue, and it proves advantageous to assess fatigue in the aftermath of COVID-19 (Mazza et al., 2021a; Ortelli et al., 2021). We employed the standard cut-off (FSS mean score ≥ 4) to determine clinically significant levels of fatigue (Krupp et al., 1989). Zung Self-Rating Depression Scale (ZSDS) and Beck Depression Inventory-13 (BDI-13) were used to assess depressive psychopathology. The ZSDS consists of a 20-item scale assessing the full spectrum of depressive symptoms) (Zung, 1967). The commonly accepted cut-off to define depression's clinical relevance was used (ZSDS index ≥ 50). The BDI-13 is a self-report questionnaire with 13 items, and each item is rated on a 4-point scale from 0 to 3. BDI-13 was designed to measure the depressive symptoms of an individual during the past two weeks (Beck et al., 1961). The commonly accepted cut-off (BDI sum score ≥ 9) was implemented to identify participants who fell in the pathological range. Both the ZSDS and the BDI-13 have mainly been employed in several studies investigating COVID-19 triggered depression, with the first scale associated with higher prevalence due to its ability to detect even mild depression (Deng et al., 2021).
For all patients, we collected: age, sex, education, ethnicity, body mass index, previous psychiatric history, preexisting treatment with antidepressants, medical comorbidities (e.g. hypertension, coronary artery disease, diabetes mellitus, Chronic obstructive pulmonary disease, renal insufficiency, neoplasm), COVID-19 wave, duration of hospitalization, the need for non-invasive ventilation (NIV), and need of ICU admission, oxygen saturation level (SpO2) at one month, treatment with a cytokine blocking agent.
2.3. Data analysis
Descriptive statistical analyses to compare means and frequencies between patients showing or not post-COVID fatigue (corrected FSS score > 4) at one, three, six or twelve-month follow-up were performed and prevalence of fatigue was calculated at the different time-points. Levels of significance were corrected for multiple comparisons with the method of the adaptive linear step-up procedures that control the FDR, and q-values (FDR-adjusted p-value) were considered.
Then, we investigated the change of fatigue symptoms over time. Considering that, patients evaluated at the different time points partially overlapped, and longitudinal evaluation was available only in small subgroup, we performed two different sets of analyses. First, we investigated whether fatigue symptoms differed between subjects evaluated at one, three, six, and twelve-month follow-up considering different time points as independent groups. Only the longer follow-up was considered in the analysis for patients assessed more than one time. One-way analyses of variance (ANOVA) was performed to compare fatigue symptoms in the independent four groups (one, three, six, and twelve-month follow up). Second, to investigate fatigue changes over time, we performed a repeated measure ANOVA only in the subgroup of patients with a longitudinal evaluation of FSS from the first and second COVID-19 wave. Bonferroni post hoc test has been performed to investigate differences between groups.
Finally, we tested the predictive effect of sociodemographic factors, clinical variable, acute COVID-19 severity, and one-month depressive symptoms (BDI-13 and ZSDS index mean score) in affecting post-COVID long-term fatigue (FSS corrected score). To fully test the predictive role of depression we performed the following sensitivity analyses: i) sensitivity analysis leaving out the item related to fatigue from the ZSDS and BDI-13 (item 10 for ZSDS and item 12 for the BDI-13) to reduce the risk of incorporation bias, ii) sensitivity analysis using binary values of ZSDS and BDI-13 according to conventional cut-off, iii) sensitivity analysis based on to the presence of depression according to at least ZSDS index≥50 or BDI-13 ≥ 9, iv) sensitivity analysis based on to the presence of depression according both to ZSDS index≥50 and BDI-13 ≥ 9. In order to predict long-term post-COVID fatigue, we grouped patients with FSS at six or twelve months; for patients assessed at both time points, only the twelve-month follow-up was considered in the analysis. First, we explored the effect of each predictor in affecting post-COVID long-term fatigue by implementing 5000 non-parametric bootstraps enhanced elastic net penalised normal regression. Before performing the analyses, all the data were normalised (i.e. min-max normalisation). The applied regularisations (L1 and L2 penalties) force a shrinkage of the coefficients, which can also be estimated to zero. This reduces overfitting and eliminates irrelevant or redundant variables, increasing model interpretability and dealing with multicollinearity induced by highly correlated variables. We optimised the model's λ hyperparameter in the inner loop, which defines the regularisation weight to minimise expected deviance. The α hyperparameter was set to 0.5, performing a pure elastic net model. We implemented a non-parametric bootstrap procedure (5000 resamples with replacement) in order to provide estimates of coefficients, related 95% confidence intervals, and variable inclusion probability (VIP), which was the main outcome of the analysis (Bunea et al., 2011). VIP provides a measure of the stability of coefficients as an estimate of the probability of the predictors to be included in the model. We applied a relative conservative threshold of 85% to VIP (Abram et al., 2016) in order to identify predictors that enable to discriminate the post-COVID presence of fatigue. In a second confirmatory step, we modelled the effect of predictors, surviving the VIP threshold, on long-lasting post-COVID-fatigue (FSS correct score at six or twelve months) in the context of the General Linear Model also considering the effect of age and sex as nuisance covariates.
3. Results
3.1. Prevalence of fatigue
At one, three, six, and twelve-month, respectively, 22% (59 out of 265 patients), 27% (32 out of 120 patients), 30% (56 out of 188 patients), and 34% (54 out of 158 patients) of patients self-rated fatigue symptoms in the pathological range (Table 1 ).
Table 1.
Complete description of the sample baseline characteristics according to the presence of post-COVID fatigue at one, three, six, and twelve follow-up months. Levels of significance of the observed differences (Student's t-test and Chi-square) are reported both as p and q-value (false discovery rate corrected p-value). Patients self-rated their symptoms on the Beck's Depression Inventory (BDI) and Zung Self-rating Depression Scale (ZSDS).
| One month follow-up (n = 265) |
Three months follow-up (n = 120) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FSS<4 (n = 206) | FSS≥4 (n = 59) | t or χ2 | p-value | q-value | FSS<4 (n = 88) | FSS≥4 (n = 32) | t or χ2 | p-value | q-value | |
| Age | 56.786 ± 10.981 | 56.881 ± 9.624 | −0.06 | 0.952 | 0.952 | 57.432 ± 10.067 | 58.031 ± 9.823 | −0.29 | 0.772 | 0.966 |
| Duration of hospitalization | 11.762 ± 5.753 | 13.559 ± 7.101 | −2.003 | 0.046 | 0.087 | 12.705 ± 6.754 | 14.156 ± 7.505 | −1.011 | 0.314 | 0.524 |
| Oxygen saturation level at one month follow-up | 97.534 ± 1.103 | 97.661 ± 0.921 | −0.808 | 0.420 | 0.700 | 97.602 ± 0.953 | 97.594 ± 1.012 | 0.043 | 0.966 | 0.966 |
| Body mass index | 27.915 ± 4.916 | 27.785 ± 5.748 | 0.172 | 0.864 | 0.928 | 28.078 ± 4.813 | 27.245 ± 5.853 | 0.791 | 0.431 | 0.728 |
| Year of education | 12.893 ± 4.027 | 12.678 ± 3.739 | 0.368 | 0.713 | 0.823 | 12.534 ± 4.065 | 12.375 ± 3.499 | 0.196 | 0.845 | 0.966 |
| BDI-13 at one month follow-up | 2.524 ± 3.606 | 6.542 ± 5.627 | −6.577 | <0.001 | <0.001 | 3.102 ± 3.595 | 6.188 ± 5.251 | −3.65 | <0.001 | 0.002 |
| ZSDS index at one month follow-up | 40.051 ± 10.873 | 50.216 ± 11.103 | −6.302 | <0.001 | <0.001 | 41.307 ± 10.387 | 51.523 ± 9.779 | −4.837 | <0.001 | <0.001 |
| Males (Females) | 139 (67) | 30 (29) | 5,489 | 0.019 | 0.041 | 69 (19) | 15 (17) | 11,112 | 0.001 | 0.003 |
| Treated with cytokine blocking agents Yes (No) | 42 (164) | 10 (49) | 0.344 | 0.558 | 0.760 | 20 (68) | 3 (29) | 2,700 | 0.100 | 0.188 |
| Positive psychiatric history Yes (No) | 19 (187) | 18 (41) | 17,298 | <0.001 | <0.001 | 11 (77) | 8 (24) | 2,751 | 0.097 | 0.188 |
| Under conventional antidepressant treatment Yes (No) | 12 (194) | 18 (41) | 27,834 | <0.001 | <0.001 | 5 (83) | 7 (25) | 6,837 | 0.008 | 0.020 |
| Intensive care unit for acute COVID-19 Yes (No) | 16 (190) | 3 (56) | 0.496 | 0.481 | 0.722 | 12 (76) | 4 (28) | 0.026 | 0.871 | 0.966 |
| Non invasive ventilation for acute COVID-19 Yes (No) | 44 (162) | 13 (46) | 0.012 | 0.911 | 0.952 | 26 (62) | 8 (24) | 0.239 | 0.625 | 0.938 |
| Presence of organic comorbidity Yes (No) | 90 (116) | 28 (31) | 0.264 | 0.608 | 0.760 | 43 (45) | 16 (16) | 0.012 | 0.912 | 0.966 |
| BDI-13 ≥ 9 Yes (No) | 11 (195) | 18 (41) | 29,812 | <0.001 | <0.001 | 6 (82) | 8 (24) | 7,528 | 0.006 | 0.018 |
| ZSDS index ≥ 50 Yes (No) | 38 (168) | 28 (31) | 20,639 | <0.001 | <0.001 | 16 (72) | 19 (13) | 19,274 | <0.001 | <0.001 |
| Six months follow-up (n = 188) |
Twelve months follow-up (n = 158) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FSS<4 (n = 132) | FSS≥4 (n = 56) | t or χ2 | p-value | q-value | FSS<4 (n = 104) | FSS≥4 (n = 54) | t or χ2 | p-value | q-value | |
| Age | 58.659 ± 10.943 | 59.75 ± 11.743 | −0.612 | 0.542 | 0.542 | 58.856 ± 12.263 | 57.352 ± 12.706 | 0.722 | 0.471 | 0.544 |
| Duration of hospitalization | 16.644 ± 12.303 | 14.482 ± 9.169 | 1.182 | 0.239 | 0.358 | 13.269 ± 12.425 | 10.426 ± 10.177 | 1.448 | 0.150 | 0.250 |
| Oxygen saturation level at one month follow-up | 97.75 ± 1.115 | 97.536 ± 0.953 | 1.257 | 0.210 | 0.351 | 97.798 ± 1.056 | 97.833 ± 1.194 | −0.19 | 0.849 | 0.849 |
| Body mass index | 27.831 ± 4.252 | 28.848 ± 5.803 | −1.339 | 0.182 | 0.297 | 27.42 ± 4.403 | 28.438 ± 5.518 | −1.261 | 0.209 | 0.301 |
| Year of education | 12.652 ± 3.404 | 12.107 ± 3.944 | 0.956 | 0.341 | 0.393 | 12.904 ± 4.018 | 13.481 ± 4.264 | −0.839 | 0.403 | 0.544 |
| BDI-13 at one month follow-up | 2.659 ± 3.243 | 5.196 ± 4.27 | −4.447 | <0.001 | 0.023 | 2.404 ± 2.858 | 4.593 ± 5.214 | −3.412 | 0.001 | 0.012 |
| ZSDS index at one month follow-up | 41.723 ± 9.471 | 49.549 ± 12.075 | −4.76 | <0.001 | 0.004 | 42.163 ± 9.285 | 46.653 ± 14.524 | −2.36 | 0.019 | 0.058 |
| Males (Females) | 99 (33) | 28 (28) | 11,211 | 0.008 | 0.023 | 79 (25) | 29 (25) | 8,141 | 0.004 | 0.019 |
| Treated with cytokine blocking agents Yes (No) | 35 (97) | 11 (45) | 1,005 | 0.316 | 0.393 | 24 (80) | 4 (49) | 4,529 | 0.033 | 0.069 |
| Positive psychiatric history Yes (No) | 24 (108) | 16 (40) | 2,534 | 0.111 | 0.238 | 23 (81) | 21 (33) | 4,977 | 0.026 | 0.065 |
| Under conventional antidepressant treatment Yes (No) | 12 (120) | 13 (43) | 6,803 | 0.009 | 0.023 | 7 (97) | 13 (41) | 9,670 | 0.002 | 0.015 |
| Intensive care unit for acute COVID-19 Yes (No) | 15 (117) | 4 (52) | 0.771 | 0.379 | 0.406 | 4 (100) | 4 (50) | 0.938 | 0.333 | 0.500 |
| Non invasive ventilation for acute COVID-19 Yes (No) | 45 (87) | 15 (41) | 0.966 | 0.326 | 0.393 | 25 (79) | 11 (43) | 0.272 | 0.602 | 0.645 |
| Presence of organic comorbidity Yes (No) | 55 (77) | 30 (26) | 2,249 | 0.134 | 0.251 | 47 (57) | 21 (33) | 0.576 | 0.448 | 0.544 |
| BDI-13 ≥ 9 Yes (No) | 9 (123) | 11 (45) | 6,803 | 0.009 | <0.001 | 7 (97) | 12 (42) | 8,063 | 0.005 | 0.019 |
| ZSDS index ≥ 50 Yes (No) | 27 (105) | 25 (31) | 11,497 | 0.001 | <0.001 | 24 (80) | 21 (33) | 4,363 | 0.037 | 0.069 |
Depressive symptoms (ZSDS and BDI-13), female sex, and preexisting antidepressant treatment were consistently associated with the presence of fatigue at different time points after the correction for multiple comparisons (Table 1). The severity of acute COVID-19 (duration of hospitalization, need of NIV, and ICU admission) was not associated with the presence of fatigue as well as the presence of organic comorbidity. No other clinical and demographical variable was consistently associated with the presence of fatigue. The full description of the sample at different time points, according to the presence of fatigue, is summarised in Table 1.
3.2. Change of fatigue symptoms over time
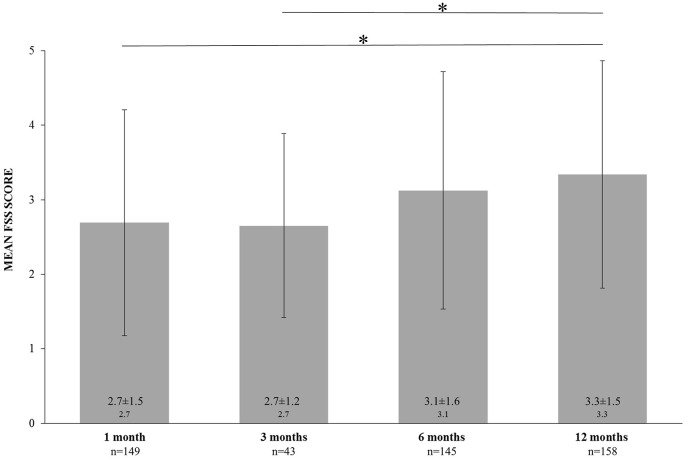
The one-way ANOVA comparing FSS mean scores between different follow-ups found increased fatigue severity over time (F = 5.741, p < 0.001). Bonferroni post-hoc analysis revealed a significant statistical difference between one-month FSS vs twelve-month FSS (p = 0.001), and three-month FSS vs twelve-month FSS (p = 0.041) (Fig. 2 ).
Fig. 2.
Differences in Fatigue Severity Scale (FSS) mean score at one, three, six, and twelve-month follow-up considering different time points as independent groups. One-way analyses of variance and Bonferroni post hoc test revealed significant statistical differences between groups. * indicates p < 0.05.
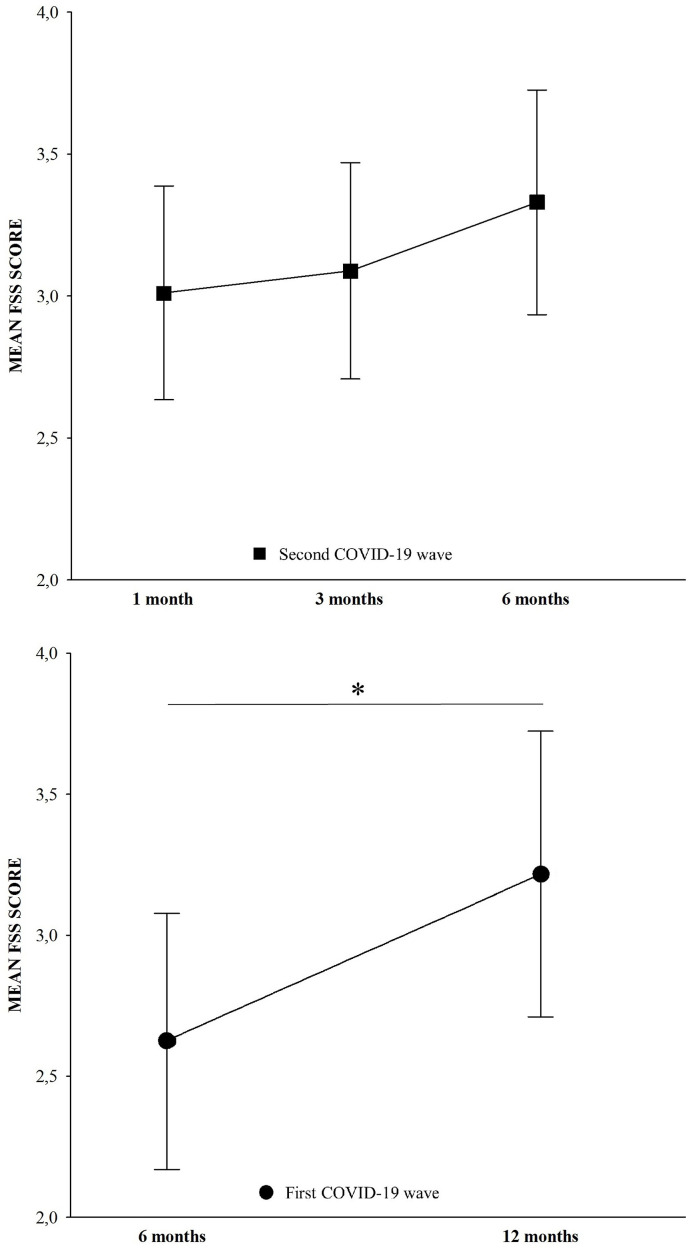
Considering the two longitudinal cohorts, we consistently found a significant increasing trend in fatigue symptoms. Repeated measure ANOVA showed a significant increase in FSS means over time in the subgroup of 42 patients from the first COVID-19 wave with a longitudinal evaluation at six and twelve months (six-month FSS 2.624 ± 1.461, twelve-months FSS 3.216 ± 1.625, F = 11.425, p = 0.001). As well, a statistical trend suggesting FSS increase over time was found in the subgroup of 70 patients from the second COVID-19 wave longitudinally evaluated at one, three, and six-month (one-month FSS 3.011 ± 1.578, three-month FSS 3.089 ± 1.599, six-month FSS 3.330 ± 1.659, F = 2.399, p = 0.094). Bonferroni post-hoc test revealed no statistical difference between groups (Fig. 3 ).
Fig. 3.
Longitudinal changes of Fatigue Severity Scale (FSS) mean score over time at one, three, and six months during the second wave (upper), and at six and twelve during the first wave (bottom). The repeated measure analyses of variance (ANOVA) showed significant increasing FSS score from six to twelve months and a trend of significant increasing FSS score over one, three, and six months. * indicates p < 0.05.
3.3. Prediction of long-lasting fatigue symptoms
When entering demographical, clinical, and psychopathological predictors in the elastic net penalised normal regression, we found that only depressive symptomatology at one month (according to ZSDS and BDI-13 mean score) significantly predicted the presence of post-COVID-19 long-term fatigue (Table 2 ). No other clinical or demographic variable was found to predict post-COVID fatigue. The sensitivities analyses consistently confirmed the predictive role of depressive psychopathology at one month in predicting long-term fatigue (Supplementary eTable 2). Second-step GLM regression analyses confirmed these findings showing a central role of depressive psychopathology in affecting post-COVID fatigue higher BDI-13 scores (b = 0.222, F = 24.755, p < 0.001) and higher ZSDS index scores (b = 0.288, F = 22.101, p < 0.001) predicted increased long-term fatigue severity.
Table 2.
Description of the sample used for the predictive analysis and coefficients estimate for elastic net regression predicting the presence of fatigue at 6 or 12 months follow-up (Fatigue Severity Scale corrected mean score). Levels of significance of the observed differences (Student's t-test and Chi-square) is reported both as p and q-value (false discovery rate corrected p-value). Patients self-rated their symptoms on the Beck's Depression Inventory (BDI); Zung Self-rating Depression Scale (ZSDS). Bold represents q < 0.05 and variable inclusion probability >85%.
| Predictors | Descriptive statistics |
Elastic net coefficients |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 300) | Absence of long-term fatigue (n = 199) | Presence of long-term fatigue (n = 101) | t or χ2 | p-value | q-value | Coefficient | Standard deviation | 95% Lower CI | 95% Upper CI | Variable inclusion probability | |
| Intercept | 0.2748 | 0.0782 | 0.1215 | 0.4281 | 100.0 | ||||||
| BDI-13 mean score at one month follow-up | 3.267 ± 3.813 | 2.533 ± 3.078 | 4.713 ± 4.64 | −4.854 | <0.001 | <0.001 | 0.2010 | 0.1175 | −0.0292 | 0.4312 | 94.1 |
| ZSDS index mean score at one month follow-up | 43.959 ± 11.154 | 41.866 ± 9.467 | 48.084 ± 12.985 | −4.723 | <0.001 | <0.001 | 0.1659 | 0.1065 | −0.0430 | 0.3747 | 92.0 |
| Males Yes (%) | 201 (67%) | 148 (74%) | 53 (52%) | 14.528 | 0.001 | 0.002 | −0.0340 | 0.0296 | −0.0921 | 0.0240 | 81.1 |
| Under conventional antidepressant treatment Yes (%) | 37 (12%) | 15 (8%) | 22 (22%) | 12.573 | <0.001 | 0.002 | 0.0488 | 0.0439 | −0.0371 | 0.1348 | 80.3 |
| Positive psychiatric history Yes (%) | 71 (24%) | 38 (19%) | 33 (33%) | 6.836 | 0.009 | 0.017 | 0.0307 | 0.0310 | −0.0300 | 0.0914 | 73.1 |
| Body mass index | 27.953 ± 4.918 | 27.604 ± 4.401 | 28.641 ± 5.767 | −1.731 | 0.084 | 0.156 | 0.0929 | 0.0947 | −0.0927 | 0.2786 | 70.9 |
| Presence of organic comorbidity Yes (%) | 136 (45%) | 90 (45%) | 46 (46%) | 0.002 | 0.958 | 0.958 | 0.0506 | 0.0571 | −0.0614 | 0.1626 | 67.8 |
| Treated with cytokine blocking agents Yes (%) | 59 (20%) | 45 (23%) | 14 (14%) | 3.247 | 0.072 | 0.123 | −0.0211 | 0.0276 | −0.0753 | 0.0331 | 61.8 |
| Duration of hospitalization | 13.523 ± 11.23 | 14.271 ± 12.074 | 12.05 ± 9.225 | 1.624 | 0.105 | 0.164 | −0.0405 | 0.0614 | −0.1609 | 0.0798 | 52.6 |
| Oxygen saturation level at one month follow-up | 97.697 ± 1.093 | 97.704 ± 1.095 | 97.683 ± 1.095 | 0.152 | 0.879 | 0.958 | −0.0280 | 0.0545 | −0.1348 | 0.0788 | 49.9 |
| COVID-19 first wave Yes (%) | 93 (31%) | 57 (29%) | 36 (36%) | 1.534 | 0.251 | 0.354 | 0.0070 | 0.0195 | −0.0313 | 0.0453 | 45.8 |
| Hospitalized for acute COVID-19 Yes (%) | 266 (89%) | 177 (89%) | 89 (88%) | 0.45 | 0.831 | 0.95 | −0.0130 | 0.0312 | −0.0743 | 0.0482 | 45.3 |
| Caucasian Yes (%) | 280 (93%) | 189 (95%) | 91 (90%) | 2.559 | 0.109 | 0.164 | −0.0031 | 0.0356 | −0.0729 | 0.0667 | 42.3 |
| Intensive care unit for acute COVID-19 Yes (%) | 24 (8%) | 18 (9%) | 6 (6%) | 0.877 | 0.349 | 0.465 | −0.0075 | 0.0318 | −0.0698 | 0.0549 | 42.0 |
| Year of education | 12.77 ± 3.881 | 12.839 ± 3.706 | 12.634 ± 4.223 | 0.433 | 0.665 | 0.798 | 0.0016 | 0.0387 | −0.0742 | 0.0773 | 40.7 |
| Non invasive ventilation for acute COVID-19 Yes (%) | 76 (25%) | 52 (26%) | 24 (24%) | 0.198 | 0.656 | 0.798 | −0.0020 | 0.0204 | −0.0419 | 0.0380 | 38.1 |
| Age | 58.69 ± 11.748 | 58.653 ± 11.503 | 58.762 ± 12.274 | −0.076 | 0.94 | 0.958 | 0.0127 | 0.0484 | −0.0821 | 0.1075 | 37.7 |
4. Discussion
Post-COVID fatigue has been reported to be one of the major complaints during the acute COVID-19 disease as well as in the post-COVID syndrome. The present findings i) confirm a high prevalence of fatigue at short, medium, and long-term follow-up after SARS-CoV-2 infection, ii) highlight a potential worsening of fatigue symptomatology over time, iii) support a predictive role of acute depressive symptomatology in inducing long term post-COVID fatigue.
A recent meta-analysis including 68 original studies revealed that 32% of the patients (n = 25,268) experienced fatigue twelve or more weeks following recovery from SARS-CoV-2 infection (Ceban et al., 2021). Interestingly, the meta-analysis also showed that fatigue, in contrast to other persistent symptoms which may be self-limiting (e.g., anosmia, ageusia, sleep disorders), appeared to persist and to potentially worsen over time, as evidenced by subgroup analysis reporting similar proportions of affected individuals at <6 and ≥ 6 months follow-up (Ceban et al., 2021). Sparse single studies investigating the longitudinal course of post-COVID fatigue reported inconsistent results. Huang et al. found a decreasing trend from six to twelve-month follow-up (Huang et al., 2021b). On the contrary, Augustin et al. reported an increasing prevalence of fatigue from four to seven months after COVID-19 (Augustin et al., 2021), and Abdelrahman et al. reported persistent fatigue in 37% of patients during a ten-month longitudinal follow-up (Abdelrahman et al., 2021). Consistently with this background, we found persistent fatigue symptoms over twelve months evaluation with prevalence ranging from 22% at one month to 34% at twelve months. Interestingly, both the statistical model used to test the trajectory of fatigue revealed a significant worsening of severity over time. Considering that chronic fatigue negatively impacts daily and on return to work despite being medically deemed recovered from their primary illness (Townsend et al., 2020), the reported worsening fatigue symptom over time highlights the importance of following all patients diagnosed with COVID-19.
When exploring the effect of potential baseline risk factors in affecting long-term post-COVID fatigue in a sample of 300 patients, we found that, among several predictors, only depressive psychopathology significantly predicted the presence of fatigue six or twelve months after infection. Accordingly, Al-Jassas et al. showed that in the acute phase of COVID-19, the appearance of depressive symptoms and physio-somatic fatigue symptoms is strongly interrelated, as happens in major depression and ME/CFS (Al-Jassas et al., 2022). Moreover, Townsend et al. found a significant association between preexisting diagnosis of depression and the use of antidepressant medications and the presence of post-COVID fatigue (Townsend et al., 2020). Finally, Grover et al. showed a strong association between anxiety and depressive symptoms and post-COVID-19 fatigue (Grover et al., 2021). Our findings, based on elastic net penalised regressions, corroborate this piece of literature. The implemented statistical framework allows managing a large set of highly correlated predictors, exploring the marginal contribution of each variable. Moreover, thanks to L1 penalty, the elastic net performs an embedded feature selection directly optimised on the predictive estimated model by a data-driven rule. Interesting, and consistent with other previous studies (Al-Jassas et al., 2022; Townsend et al., 2020), there was no association between age, organic comorbidity, COVID-19 severity (duration of hospitalization, intensive care unit, non-invasive ventilation, oxygen saturation) and post-COVID fatigue. We suggest that, rather independent of COVID-19 severity, depression after COVID-19 is associated with persistent fatigue, thus worsening the load of a non-communicable condition potentially triggered by infection-related systemic inflammation (Benedetti et al., 2021a; De Lorenzo et al., 2020; Mazza et al., 2020, 2021b) but then persisting on its own. It seems that post-COVID fatigue and post-COVID depression potentially share the same pathophysiological mechanisms possibly related to indirect immune-inflammatory mediated damage and neuroinflammation (Troyer et al., 2020; Wu et al., 2020b). The causal relationship between systemic inflammation and post-COVID depression as well as post-COVID fatigue has been hypothesized and confirmed by several findings (Benedetti et al., 2021b; Ceban et al., 2021; Mazza et al., 2020, 2021b). Considering that, apart from fatigue, depressive symptoms have also been associated with neurocognitive functioning (Gouraud et al., 2021; Mattioli et al., 2021; Mazza et al., 2021b; Poletti et al., 2021) and quality of life (Babicki et al., 2021; Poletti et al., 2021) in COVID-19 survivors, the reported evidence confirm the central role of depression, which seems to be one of the most relevant predictors of the post-COVID syndrome. From a clinical perspective, considering the alarming prevalence of post-COVID-19 depression ranging from 21% (Khraisat et al., 2021) up to 45% (Deng et al., 2021), according to current literature (Nalbandian et al., 2021) follow-up services for COVID-19 survivors should be implemented irrespectively of acute COVID-19 severity in order to monitor mental health and provide early treatment thus reducing the associated high disease burden.
Our results should be taken in the context of some limitations. First, the monocentric nature of our study limits the generalizability of the findings, raising the possibility of population stratification. Second, the small sample size of patients assessed longitudinally limits our findings' interpretation and power. Third, during the first wave, we did not evaluate fatigue since the pandemic beginning; thus, evaluation was limited at six and twelve months, making it impossible to compare the fatigue rate between the two waves and to have a complete longitudinal assessment over the twelve months. Fourth, the lack of a comparison group of subjects not affected by COVID-19 but experiencing the same psychological stressful situation (lockdown, fear, doubt, stigma, and social isolation) did not allow to disentangle the effect of COVID-19 infection from the psychological stressors. Fifth, limited health care resources forced us to assess psychopathology at 12 months using only self-rated questionnaires instead of a direct clinical interview. Finally, we included only hospitalized patients showing clinical and radiological findings suggestive of COVID-19 pneumonia thus selection bias could possibly limit the generalization of current results, especially considering that post-COVID fatigue also occurs after mild acute infection that don't require hospitalization.
Further larger multicentric longitudinal studies also exploring the effect of heterogeneous predictors are needed to overcome these limitations.
These weaknesses, however, do not bias our findings confirming that post-COVID-19 fatigue, mainly predicted by depressive psychopathology, persists in the months following hospital discharge with potential worsening over time. This finding has major preventive and clinical implications. The global burden of COVID-19 infection and the highly prevalent post-COVID sequelae will require integrated multi-disciplinary management strategies in the following months and years. Moreover, prioritisation of follow-up care should be considered for patients at high risk for post-acute COVID-19, including those who had severe illness during acute COVID-19 and patients showing depressive symptomatology. Post-COVID syndrome will affect COVID-19 survivors' daily functioning and place an additional burden on the healthcare system. Clarifying the mechanisms and risk factors underlying such long-term symptomatology is essential to identify target population and to tailor specific treatment and rehabilitation interventions to foster recovery.
Role of funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations of competing interest
None.
Aknowledgments
MP salary: Italian Ministry of University, XXXVII PhD cycle, FSE REACT-EU 2021 PON projects, Action IV.5.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpsychires.2022.08.008.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abdelrahman M.M., Abd-Elrahman N.M., Bakheet T.M. Persistence of symptoms after improvement of acute COVID19 infection, a longitudinal study. J. Med. Virol. 2021;93:5942–5946. doi: 10.1002/jmv.27156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abram S.V., Helwig N.E., Moodie C.A., DeYoung C.G., MacDonald A.W., Waller N.G. Bootstrap enhanced penalized regression for variable selection with neuroimaging data. Front. Neurosci.-Switz. 2016;10 doi: 10.3389/fnins.2016.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Aly Z., Xie Y., Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- Al-Jassas H.K., Al-Hakeim H.K., Maes M. Intersections between pneumonia, lowered oxygen saturation percentage and immune activation mediate depression, anxiety, and chronic fatigue syndrome-like symptoms due to COVID-19: a nomothetic network approach. J. Affect. Disord. 2022;297:233–245. doi: 10.1016/j.jad.2021.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin M., Schommers P., Stecher M., Dewald F., Gieselmann L., Gruell H., Horn C., Vanshylla K., Cristanziano V.D., Osebold L., Roventa M., Riaz T., Tschernoster N., Altmueller J., Rose L., Salomon S., Priesner V., Luers J.C., Albus C., Rosenkranz S., Gathof B., Fatkenheuer G., Hallek M., Klein F., Suarez I., Lehmann C. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg. Health. Eur. 2021;6 doi: 10.1016/j.lanepe.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babicki M., Bogudzinska B., Kowalski K., Mastalerz-Migas A. Anxiety and depressive disorders and quality of life assessment of Poles-A study covering two waves of the COVID-19 pandemic. Front. Psychiatr. 2021;12 doi: 10.3389/fpsyt.2021.704248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatr. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Benedetti F., Mazza M., Cavalli G., Ciceri F., Dagna L., Rovere-Querini P. Can cytokine blocking prevent depression in COVID-19 survivors? J. Neuroimmune Pharmacol. 2021;16:1–3. doi: 10.1007/s11481-020-09966-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F., Palladini M., Paolini M., Melloni E., Vai B., De Lorenzo R., Furlan R., Rovere-Querini P., Falini A., Mazza M.G. Brain correlates of depression, post-traumatic distress, and inflammatory biomarkers in COVID-19 survivors: a multimodal magnetic resonance imaging study. Brain Behav. Immun. Health. 2021;18 doi: 10.1016/j.bbih.2021.100387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunea F., She Y., Ombao H., Gongvatana A., Devlin K., Cohen R. Penalized least squares regression methods and applications to neuroimaging. Neuroimage. 2011;55:1519–1527. doi: 10.1016/j.neuroimage.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceban F., Ling S., Lui L.M.W., Lee Y., Gill H., Teopiz K.M., Rodrigues N.B., Subramaniapillai M., Di Vincenzo J.D., Cao B., Lin K., Mansur R.B., Ho R.C., Rosenblat J.D., Miskowiak K.W., Vinberg M., Maletic V., McIntyre R.S. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: a systematic review and meta-analysis. Brain Behav. Immun. 2021;101:93–135. doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention C. 2022. Long COVID or Post-COVID Conditions. [Google Scholar]
- Davis H.E., Assaf G.S., McCorkell L., Wei H., Low R.J., Re'em Y., Redfield S., Austin J.P., Akrami A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinical. Med. 2021;38 doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorenzo R., Conte C., Lanzani C., Benedetti F., Roveri L., Mazza M.G., Brioni E., Giacalone G., Canti V., Sofia V., D'Amico M., Di Napoli D., Ambrosio A., Scarpellini P., Castagna A., Landoni G., Zangrillo A., Bosi E., Tresoldi M., Ciceri F., Rovere-Querini P. Residual clinical damage after COVID-19: a retrospective and prospective observational cohort study. PLoS One. 2020;15 doi: 10.1371/journal.pone.0239570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J., Zhou F., Hou W., Silver Z., Wong C.Y., Chang O., Huang E., Zuo Q.K. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: a meta-analysis. Ann. N. Y. Acad. Sci. 2021;1486:90–111. doi: 10.1111/nyas.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouraud C., Bottemanne H., Lahlou-Laforet K., Blanchard A., Gunther S., Batti S.E., Auclin E., Limosin F., Hulot J.S., Lebeaux D., Lemogne C. Association between psychological distress, cognitive complaints, and neuropsychological status after a severe COVID-19 episode: a cross-sectional study. Front. Psychiatr. 2021;12 doi: 10.3389/fpsyt.2021.725861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover S., Sahoo S., Mishra E., Gill K.S., Mehra A., Nehra R., Suman A., Bhalla A., Puri G.D. Fatigue, perceived stigma, self-reported cognitive deficits and psychological morbidity in patients recovered from COVID-19 infection. Asian J. Psychiatr. 2021;64 doi: 10.1016/j.ajp.2021.102815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havers F.P., Reed C., Lim T., Montgomery J.M., Klena J.D., Hall A.J., Fry A.M., Cannon D.L., Chiang C.F., Gibbons A., Krapiunaya I., Morales-Betoulle M., Roguski K., Rasheed M.A.U., Freeman B., Lester S., Mills L., Carroll D.S., Owen S.M., Johnson J.A., Semenova V., Blackmore C., Blog D., Chai S.J., Dunn A., Hand J., Jain S., Lindquist S., Lynfield R., Pritchard S., Sokol T., Sosa L., Turabelidze G., Watkins S.M., Wiesman J., Williams R.W., Yendell S., Schiffer J., Thornburg N.J. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, march 23-may 12, 2020. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.4130. [DOI] [PubMed] [Google Scholar]
- Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., Luo J., Huang Z., Tu S., Zhao Y., Chen L., Xu D., Li Y., Li C., Peng L., Li Y., Xie W., Cui D., Shang L., Fan G., Xu J., Wang G., Wang Y., Zhong J., Wang C., Wang J., Zhang D., Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Yao Q., Gu X., Wang Q., Ren L., Wang Y., Hu P., Guo L., Liu M., Xu J., Zhang X., Qu Y., Fan Y., Li X., Li C., Yu T., Xia J., Wei M., Chen L., Li Y., Xiao F., Liu D., Wang J., Wang X., Cao B. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398:747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khraisat B., Toubasi A., AlZoubi L., Al-Sayegh T., Mansour A. Meta-analysis of prevalence: the psychological sequelae among COVID-19 survivors. Int. J. Psychiatr. Clin. Pract. 2021:1–10. doi: 10.1080/13651501.2021.1993924. [DOI] [PubMed] [Google Scholar]
- Krupp L.B., LaRocca N.G., Muir-Nash J., Steinberg A.D. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- Marshall M. The lasting misery of coronavirus long-haulers. Nature. 2020;585:339–341. doi: 10.1038/d41586-020-02598-6. [DOI] [PubMed] [Google Scholar]
- Mattioli F., Stampatori C., Righetti F., Sala E., Tomasi C., De Palma G. Neurological and cognitive sequelae of Covid-19: a four month follow-up. J. Neurol. 2021;268:4422–4428. doi: 10.1007/s00415-021-10579-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., De Lorenzo R., Conte C., Poletti S., Vai B., Bollettini I., Melloni E.M.T., Furlan R., Ciceri F., Rovere-Querini P., Benedetti F. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav. Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., Palladini M., De Lorenzo R., Bravi B., Poletti S., Furlan R., Ciceri F., group C.-B.O.C.S., Rovere-Querini P., Benedetti F. One-year mental health outcomes in a cohort of COVID-19 survivors. J. Psychiatr. Res. 2021;145:118–124. doi: 10.1016/j.jpsychires.2021.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., Palladini M., De Lorenzo R., Magnaghi C., Poletti S., Furlan R., Ciceri F., Rovere-Querini P., Benedetti F. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021;94:138–147. doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., Ahluwalia N., Bikdeli B., Dietz D., Der-Nigoghossian C., Liyanage-Don N., Rosner G.F., Bernstein E.J., Mohan S., Beckley A.A., Seres D.S., Choueiri T.K., Uriel N., Ausiello J.C., Accili D., Freedberg D.E., Baldwin M., Schwartz A., Brodie D., Garcia C.K., Elkind M.S.V., Connors J.M., Bilezikian J.P., Landry D.W., Wan E.Y. Post-acute COVID-19 syndrome. Nat. Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortelli P., Ferrazzoli D., Sebastianelli L., Engl M., Romanello R., Nardone R., Bonini I., Koch G., Saltuari L., Quartarone A. Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: insights into a challenging symptom. J. neurol. sci. 2021;420 doi: 10.1016/j.jns.2020.117271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladini M., Mazza M., Rovere-Querini P., Benedetti F. P. 0691 Mood-congruent cognitive distortion and processing bias in depressed covid-19 survivors: a comparison with major depressive disorder. Eur. Neuropsychopharmacol: J. Europ. Coll. Neuropsych. 2021;53:S505–S506. [Google Scholar]
- Poletti S., Palladini M., Mazza M.G., De Lorenzo R., group C.-B.O.C.S., Furlan R., Ciceri F., Rovere-Querini P., Benedetti F. Long-term consequences of COVID-19 on cognitive functioning up to 6 months after discharge: role of depression and impact on quality of life. Eur. Arch. Psychiatr. Clin. Neurosci. 2021 doi: 10.1007/s00406-021-01346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J.P., Chesney E., Oliver D., Pollak T.A., McGuire P., Fusar-Poli P., Zandi M.S., Lewis G., David A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatr. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenforde M.W., Kim S.S., Lindsell C.J., Billig Rose E., Shapiro N.I., Files D.C., Gibbs K.W., Erickson H.L., Steingrub J.S., Smithline H.A., Gong M.N., Aboodi M.S., Exline M.C., Henning D.J., Wilson J.G., Khan A., Qadir N., Brown S.M., Peltan I.D., Rice T.W., Hager D.N., Ginde A.A., Stubblefield W.B., Patel M.M., Self W.H., Feldstein L.R., Investigators I.V.Y.N., Team C.C.-R., Investigators I.V.Y.N. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network - United States, march-june 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend L., Dyer A.H., Jones K., Dunne J., Mooney A., Gaffney F., O'Connor L., Leavy D., O'Brien K., Dowds J., Sugrue J.A., Hopkins D., Martin-Loeches I., Ni Cheallaigh C., Nadarajan P., McLaughlin A.M., Bourke N.M., Bergin C., O'Farrelly C., Bannan C., Conlon N. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15 doi: 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer E.A., Kohn J.N., Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav. Immun. 2020;87:34–39. doi: 10.1016/j.bbi.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan P. NICE guideline on long COVID. Lancet Respir. Med. 2021;9:129. doi: 10.1016/S2213-2600(21)00031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2021. Coronavirus Disease (COVID-19): Post COVID-19 Condition. [Google Scholar]
- Wu S.L., Mertens A.N., Crider Y.S., Nguyen A., Pokpongkiat N.N., Djajadi S., Seth A., Hsiang M.S., Colford J.M., Jr., Reingold A., Arnold B.F., Hubbard A., Benjamin-Chung J. Substantial underestimation of SARS-CoV-2 infection in the United States. Nat. Commun. 2020;11:4507. doi: 10.1038/s41467-020-18272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zung W.W. Factors influencing the self-rating depression scale. Arch. Gen. Psychiatr. 1967;16:543–547. doi: 10.1001/archpsyc.1967.01730230027003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.