Abstract
The human pathogen Eikenella corrodens synthesizes type IV pili and exhibits a phase variation involving the irreversible transition from piliated to nonpiliated variants. On solid medium, piliated variants form small (S-phase), corroding colonies whereas nonpiliated variants form large (L-phase), noncorroding colonies. We are studying the molecular basis of this phase variation in the clinical isolate E. corrodens VA1. A genomic fragment encoding the major type IV pilin was cloned from the S-phase variant of strain VA1. Sequence analysis of the fragment revealed four tandemly arranged potential open reading frames (ORFs), designated pilA1, pilA2, pilB, and hagA. Both pilA1 and pilA2 predict a type IV pilin. The protein predicted by pilB shares sequence identity with the Dichelobacter nodosus FimB fimbrial assembly protein. The protein predicted by hagA resembles a hemagglutinin. The region containing these four ORFs was designated the pilA locus. DNA hybridization and sequence analysis showed that the pilA locus of an L-phase variant of strain VA1 was identical to that of the S-phase variant. An abundant pilA1 transcript initiating upstream of pilA1 and terminating at a predicted hairpin structure between pilA1 and pilA2 was detected by several assays, as was a less abundant read-through transcript encompassing pilA1, pilA2, and pilB. Transcription from the pilA locus was nearly indistinguishable between S- and L-phase variants. Electron microscopy and immunochemical analysis showed that S-phase variants synthesize, export, and assemble pilin into pili. In contrast, L-phase variants synthesize pilin but do not export and assemble it into pili. These data suggest that a posttranslational event, possibly involving an alteration in pilin export and assembly, is responsible for phase variation in E. corrodens.
Eikenella corrodens is a gram-negative human pathogen (10) that can cause endocarditis (6, 16), a variety of soft tissue and wound infections (10, 15, 20, 30), and other opportunistic infections. This bacterium has also been associated with periodontal diseases (4, 33, 37), although a causal role has not been clearly established. Despite increasing recognition of its role in disease, as reflected in growing numbers of case studies and clinical reports, little is known about the molecular factors that contribute to E. corrodens pathogenicity and virulence.
Most strains of E. corrodens exhibit an irreversible phase variation that is reflected in colony morphology changes. On solid medium, small (S-phase), so-called corroding, and large (L-phase), so-called noncorroding colonies are observed (11, 23, 28, 46). The L-phase variants arise from S-phase variants at a frequency much greater than mutation rates (24). Colony morphology and phase variation correlates with the presence of pili on S-phase variants and the absence of pili on L-phase variants (22, 23). In terms of piliation, phase variation in E. corrodens resembles the phase variation exhibited by pathogens such as Neisseria gonorrhoeae (7, 36, 50, 51) and Moraxella bovis (18, 34). In the latter species, the phase variation typically involves genomic recombination or mutagenic events that directly affect pilin synthesis or pilus assembly. Given that pili can be determinants of pathogenesis (1, 8, 31, 47), and that modulation of piliation may represent a mechanism to evade host immune response (9, 45, 49), the molecular basis of phase/antigenic variation is of considerable interest.
The pathogens mentioned above, as well as other gram-negative bacterial species, synthesize type IV pili (1, 14, 47). These pili are composed primarily of type IV pilin, a protein which ranges from 150 to 165 amino acids in length. Type IV pilin is synthesized as a precursor form (prepilin) that contains a basic leader sequence of variable length (4 to 25 residues). Following translation, the prepilin is cleaved at an atypical site by a cognate peptidase that simultaneously methylates the resultant amino-terminal amino acid, which is typically a phenylalanine residue. Following processing, the mature pilin is exported to the cell surface by a specific transport mechanism and assembled into pili. Class A type IV pilins from different species share a highly conserved, 30- to 32-residue hydrophobic amino-terminal domain that functions in protein-protein interactions during pilus assembly (47). The remainder of the mature pilin protein is less conserved and contains the major antigenic determinants.
We are examining the molecular basis of the pilus-associated phase variation exhibited by E. corrodens. Recently, we characterized the major pilus protein of clinical isolate strain VA1 and confirmed that the observed pili were type IV (27). Although genes encoding type IV pilin have also been cloned from two other E. corrodens strains (39, 53), their expression and potential role in phase variation have not been defined. In this report, we document the structure and expression of the major type IV pilin gene in S- and L-phase variants of strain VA1. Analyses of pilin gene expression and pilin processing suggest that a posttranslational event, possibly involving an alteration in pilin export and/or assembly, is responsible for phase variation in E. corrodens.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The strains and plasmids used in this study are listed in Table 1. E. corrodens VA1 is a clinical isolate obtained from the Veterans Administration Medical Center, Kansas City, Mo. (12). Strain VA1 forms both S- and L-phase colonies on solid medium. Strains VA1-S1, VA1-S2, and VA1-S3 are independent S-phase isolates of strain VA1 that form S-phase colonies and exhibit typical frequencies of phase variation to L-phase colonies. Strain VA1-L2 is an L-phase isolate of VA1 that forms only large colonies. E. corrodens was cultured aerobically at 35°C on chocolate agar plates purchased from Remel (Lenexa, Kans.).
TABLE 1.
Strains and plasmids used
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| E. corrodens | ||
| VA1 | Clinical isolate | Laboratory collection |
| VA1-S1, -S2, and -S3 | S-phase variants of VA1 | Laboratory collection |
| VA1-L2 | L-phase variant of VA1 | Laboratory collection |
| E. coli | ||
| DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) supE44 λ− thi-1 gyrA96 relA1 | Bethesda Research Laboratories |
| BL21(DE3) | F−ompT hsdSB(rB− mB−) gal dcm (DE3) | Novagen |
| Plasmids | ||
| pGEM3zf(+) | Apr cloning and sequencing vector | Promega |
| pET-22b | Apr expression vector | Novagen |
| pVD203 | Carries pilE1 gene from N. gonorrhoeae | 7 |
| pEC114 | pGEM3zf(+) with 3.9-kbp EcoRI fragment from VA1-S1 containing pilA locus | This study |
| pEC203 | pBluescript SK (+) with 0.6-kbp EcoRI fragment from VA1-S1 containing pilA1 sequences, used to generate pilA1-specific RNA probes | This study |
| pEC205 | pGEM3zf(+) with 0.9-kbp PvuII fragment from VA1-S1 containing mostly pilA2 sequences, used to generate pilA2-specific RNA probes | This study |
| pEC206 | pET-22b with DNA fragment from VA1-S1 encoding residues 26–146 of PilA1 | This study |
Escherichia coli DH5α was used as the host for general cloning vectors. E. coli BL21(D3) was used as the host for pET-22b-based protein expression vectors. Both strains were propagated in liquid or in solid Luria-Bertani medium with antibiotics at standard concentrations (5).
DNA methods.
Restriction endonucleases and modifying enzymes were purchased from Promega (Madison, Wis.). [α-32P]dCTP was purchased from ICN (Costa Mesa, Calif.). [γ-32P]ATP and [35S]dATPαS were purchased from Amersham (Arlington Heights, Ill.). DNA manipulations, including restriction digests, agarose gel electrophoresis, ligations, PCR amplifications, transformation of E. coli, and plasmid minipreparations, were performed by established protocols (5, 43). E. corrodens genomic DNA was prepared by the procedure described for E. coli in reference 43 or with a kit from Qiagen (Chatsworth, Calif.). For DNA hybridization analysis, digested DNA was transferred to a charged nylon membrane (Hybond-N+; Amersham) by the method of Reed and Mann (41). A DNA probe encompassing the pilA locus of strain VA1 (3.9-kbp EcoRI fragment from plasmid pEC114) was generated from the gel-purified fragment by random-primer labeling with a kit from Promega. DNA hybridizations were performed at 58°C as described by Sambrook et al. (43).
Cloning and sequencing of the pilA locus.
Total DNA from strain VA1-S1 was partially digested with Sau3A, and the digestion products were size fractionated by NaCl gradient centrifugation and purified. To generate a subgenomic library, DNA fragments in the range of 15 to 20 kbp were ligated into the BamHI site of phage λGEM11. The phage library was screened by hybridization to a probe for the pilE1 gene (1.4-kbp SmaI fragment from plasmid pVD203) from N. gonorrhoeae MS11 (7), which was kindly provided by M. Koomey. One strongly hybridizing clone was identified, isolated, and designated EP101. Mapping and DNA sequence analyses showed that a terminal 0.6-kbp EcoRI-XhoI fragment from the EP101 genomic insert contained sequences predicting a type IV pilin-like gene. In a genomic DNA hybridization analysis, the 0.6-kbp fragment identified a single 3.9-kbp EcoRI fragment of strain VA1-S1 total DNA. To clone the latter fragment, genomic DNA from strain VA1-S1 was digested with EcoRI and size fractionated on an agarose gel. Fragments in the range of 3.9 kbp were eluted from the gel and ligated into the EcoRI site of vector pGEM-3Zf(−) (Promega), and the ligation products were used to transform E. coli DH5α. The transformants were screened for plasmids containing pilin-like sequences by hybridization against the 0.6-kbp EcoRI-XhoI fragment from EP101, using a colony screening method (5). Plasmid pEC114, which hybridized to the 0.6-kbp probe and contained the predicted 3.9-kbp insert, was chosen for further analysis.
DNA sequence analysis.
Double-stranded DNA sequencing templates were isolated and purified with a kit from Promega. Double-stranded sequencing of the 3.9-kbp EcoRI fragment of pEC114 was performed by the dideoxynucleotide chain termination method (44), using both manual and automated procedures. Manual sequencing was performed with Sequenase version 2.0 modified T7 DNA polymerase purchased from United States Biochemical (Cleveland, Ohio). Automated sequencing was performed on an Applied Biosystems (Foster City, Calif.) model 377 sequencer with AmpliTaq DNA polymerase. Sequencing reactions were primed with M13 universal primers or oligonucleotides synthesized on an Applied Biosystems model 381A oligonucleotide synthesizer. A portion of the pilA locus of strain VA1-L2 was sequenced from a PCR amplification product obtained by using primers 105-R3 (5′-GCCAGCTATTGCAGAATA-3′) and 107-R7 (5′-TGCACCACTTCAAACCG-3′), corresponding to sequences determined from pEC114. DNA and protein sequences were analyzed and compared with sequences in the GenBank database by using MacVector (Oxford Molecular Group, Campbell, Calif.) and BLAST (2) sequence analysis programs.
RNA methods.
Total RNA was isolated from S- and L-phase variants by using an RNeasy kit (Qiagen). Contaminating DNA was digested with RNase-free DNase, and the RNA was further purified by passage through an RNeasy column. For hybridization analysis, RNA samples were denatured, separated by electrophoresis on 1.2% agarose gels (43), and transferred to Hybond-N+ according to the manufacturer’s instructions. The blots were hybridized with radioactive sense or antisense transcripts specific for pilA1 (generated from pEC203) or pilA2 (generated from pEC205), using the Riboprobe system (Promega). RNA hybridizations were performed at 42°C as described by Ausubel et al. (5).
Reverse transcriptase PCR.
Single-stranded cDNA was synthesized from total RNA with an avian myeloblastosis virus reverse transcriptase system (Promega), using oligo(dT) or random primers. A negative control without reverse transcriptase was performed. An aliquot of the reaction volume was amplified by PCR using Taq polymerase (Promega) and the pilA locus-specific primers 105-R1 (5′-TGTTATCGCCATTATCGG-3′), 107-R3B (5′-AAATCCCTCAACGCTTGG-3′), 107-F3 (5′-AGAAGCAACTCGCTTTACCC-3′), RH-2 (5′-GGCAACTTGATGGCAAATATCCTAC-3′), 107-R6 (5′-TGTAAAGGGTTTGGGTGCC-3′), RH-3 (5′-GGCACCCAAACCCTTTACAAG-3′), and 107-R7 (5′-TGGACCACTTCAAACCG-3′). The PCR amplification products were transferred to Hybond-N+ membranes and hybridized to antisense RNA probes for pilA1 and pilA2 as described above.
Primer extension.
cDNA was synthesized with an avian myeloblastosis virus reverse transcriptase primer extension system (Promega) using primer 105-F1 (5′-CAAAATACCGATAATGG-3′; complementary to nucleotides 538 to 555 at the 5′ end of pilA1), which was end labeled with [γ-32P]ATP by using T4 polynucleotide kinase (5). The primer extension products were analyzed against a sequencing ladder on a denaturing polyacrylamide (8% acrylamide) gel.
Cell fractionation.
Cell fractionation was performed essentially as described for E. coli by Studier et al. (48). Cells from plates were suspended in 0.1 ml of buffer (50 mM Tris [pH 8.0]), vigorously mixed in a vortex mixer for 30 s to shear pili, and then pelleted by centrifugation at 12,000 × g. The supernatant (surface fraction) contained proteins, including pilin, that are bound and/or loosely associated with the cell surface. To prepare the soluble and insoluble fractions, the pelleted cells were resuspended in 0.1 ml of buffer, brought to 2 mM EDTA, 0.1 mg of lysozyme per ml, and 0.1% Triton-X100, and incubated for 15 min at 30°C. The suspension was then sonicated (5-s pulse interval) for 1 min on ice and centrifuged at 12,000 × g. The resultant supernatant (soluble protein fraction) and pellet (insoluble protein fraction) were isolated for analysis. All protein fractions were mixed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and stored at −20°C.
The periplasmic fraction was prepared as described by Ames et al. (3). Pelleted cells were resuspended in 0.1 ml of a solution containing 30 mM Tris-Cl (pH 8.0), 20% sucrose, and 1 mM EDTA. After the suspension was shaken for 10 min at room temperature, 0.1 ml of ice-cold 5 mM MgSO4 was added and the suspension was shaken for 10 min at 4°C. The periplasmic fraction was isolated as the supernatant following centrifugation of the suspensions at 12,000 × g for 10 min.
Production of PilA1 antisera.
The region of pilA1 encoding residues 26 to 146 of mature PilA1 was amplified from pEC114 by PCR using the following primers to produce flanking NcoI and XhoI sites: RH-4 (5′-GGCTTCCATGGACTACACTGCCCGTGCT-3′) and RH-6 (5′-CTAACTCGAGTTGGCAGCTAGTTGGCAGACGG-3′). The PCR product was digested with NcoI and XhoI and ligated into expression vector pET-22b. The resulting plasmid, designated pEC206, provides for expression of a truncated PilA1 protein containing the added sequence LEH6, at the carboxyl terminus. Plasmid pEC206 was used to transform E. coli BL21(D3), and the His-tagged recombinant PilA1 protein was expressed, isolated, and purified by Ni-nitrilotriacetic acid (Qiagen) chromatography according to the manufacturer’s instructions. A rabbit polyclonal antiserum was raised against the purified PilA1 protein by Atlantic Antibodies (Windham, Maine).
SDS-PAGE and immunoblot analysis.
Protein samples were separated by SDS-PAGE on 20% polyacrylamide gels. Following electrophoresis, proteins were transferred to a nitrocellulose membrane (Nitrobind; Micron Separations Inc., Westborough, Mass.) as described by Ausubel et al. (5). The blots were blocked and incubated with the PilA1 antiserum (1:5,000). Bound antibodies were visualized following incubation of the blots with goat anti-rabbit immunoglobulin G (1:5,000) conjugated to alkaline phosphatase (KLC Laboratories, Gaithersburg, Md.) according to the manufacturer’s instructions.
Electron microscopy.
Negative staining and immunogold electron microscopic examination of whole cells were performed as described (27), using a polyclonal antiserum (1:1,000) prepared against pilin purified from strain VA1-S3.
Nucleotide sequence accession number.
The complete DNA sequence of the pilA locus has been deposited in the GenBank database under accession no. AF079304.
RESULTS
Sequence analysis of the pilA locus.
Plasmid pEC114 contains the 3.9-kbp EcoRI fragment of strain VA1-S1 genomic DNA that was identified by DNA hybridization against the pilE1 gene from N. gonorhoeae MS11. DNA sequence analysis revealed four potential open reading frames (ORFs) arranged in tandem on the pEC114 insert (Fig. 1). The first ORF (nucleotide positions 484 to 943) and second ORF (nucleotide positions 1013 to 1460) predict proteins of 153 and 149 residues, respectively. A BLAST search of the GenBank database showed that both ORFs predict obvious type IV pilins. On the basis of this identity, we designated the first ORF pilA1 and the second ORF pilA2. These and the subsequent gene designations are consistent with recommended bacterial gene nomenclature (17). The predicted PilA1 and PilA2 proteins respectively contain eight- and six-residue amino-terminal leader sequences upstream of the conserved phenylalanine that is presumed to represent the amino terminus of each mature protein. In comparison, the mature PilA1 and PilA2 proteins share 57% overall sequence identity and 87% sequence identity among their 32 amino-terminal residues. The predicted PilA1 amino-terminal sequence matched the amino-terminal sequence determined earlier for pilin of intact pili isolated from cells of strain VA1-S3 (27), demonstrating that PilA1 is the predominant pili component for this strain.
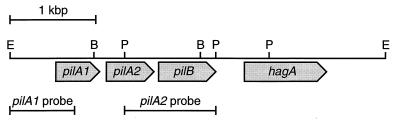
FIG. 1.
Physical map of the pilA locus for E. corrodens VA1-S1. Shaded boxes indicate size and orientation of ORFs as determined by sequence analysis. Flanking and internal restriction sites are shown for enzymes used in cloning and generation of probes. Labeled horizontal bars below map identify regions that correspond to probes used in DNA and RNA hybridization analyses. B, BglII; E, EcoRI; P, PvuII.
The third ORF (nucleotide positions 1550 to 2150) immediately downstream of pilA2 on the pEC114 insert predicts a protein of 200 residues. A BLAST search with the protein predicted by the third ORF identified the Dichelobacter nodosus class I FimB protein, which is hypothesized to function in pilus assembly (25). On the basis of this identity, the third ORF was designated pilB. Overall, the predicted PilB protein shares 21% sequence identity with FimB from D. nodosus.
The fourth ORF (nucleotide positions 2463 to 3324) is located 0.3 kbp downstream of pilB and predicts a protein of 287 residues. A BLAST search revealed that the protein predicted by the fourth ORF shared significant sequence identity with the hemagglutinin encoded by the hae-1 gene of E. corrodens ATCC 23834 (40). Given this identity, the fourth ORF was designated hagA. The predicted HagA protein shares 90% sequence identity with the hae-1 gene product. Given the proximity and structure of the pilA1, pilA2, pilB, and hagA genes, the corresponding genomic region was designated the pilA locus.
pilA locus structure in phase variants.
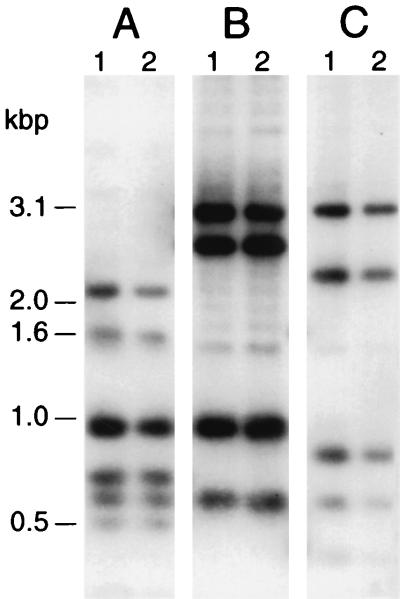
To examine a potential pilA-related genomic basis for their differential piliation, the structure of the pilA locus in E. corrodens S- and L-phase variants was examined by DNA hybridization analysis. Total DNA from strains VA1-S2 and VA1-L2 was digested with PvuII and BglII, PvuII, or PvuII and DraI and hybridized to the 3.9-kbp pEC114 insert (Fig. 2). For each digest, the hybridization profile obtained for strain VA1-S2 was indistinguishable from that obtained for VA1-L2 (Fig. 2; compare lanes 1 and 2). Identical results for S- and L-phase variants were obtained with other restriction enzymes. No hybridizing genomic fragments other than those originating from the pilA1 locus were observed. A similar analysis for other S- and L-phase variants yielded identical results (data not shown). In a related effort, the sequence of a 1.5-kbp region of strain VA1-L2 genomic DNA encompassing pilA and pilB was determined. When compared, the strain VA1-L2 sequence was found to be identical to that for strain VA1-S1 (data not shown).
FIG. 2.
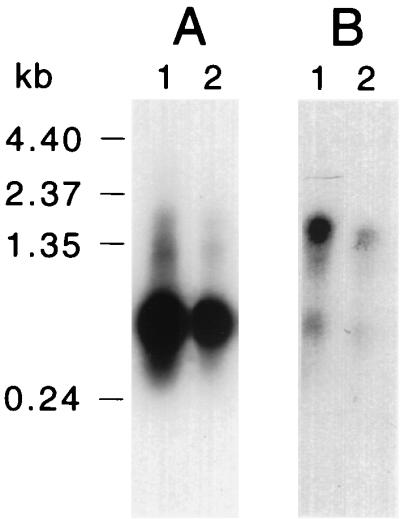
DNA hybridization analysis of pilA locus structure for S- and L-phase variants of E. corrodens VA1. Total DNA (4 μg per lane) was isolated from strain VA1-S1 (lanes 1) or VA1-L2 (lanes 2), digested with PvuII and BglII (A), PvuII (B), or PvuII and DraI (C), and subjected to blot hybridization against a DNA probe for the pilA locus of strain VA1-S1. The positions of DNA molecular size standards are shown at the left.
Pilin gene expression in phase variants.
To characterize differential piliation between S- and L-phase variants at the level of pilin gene expression, transcripts from pilA1 and pilA2 in both variants were examined by RNA hybridization analysis. Total RNA isolated from cells of strains VA1-S1 and VA1-L2 was hybridized to RNA probes specific for pilA1 or pilA2 (includes pilB sequences). Similar hybridization profiles were obtained for both variants: the pilA1 probe detected an abundant 0.6-kb transcript and a less abundant 1.5-kb transcript (Fig. 3A), while the pilA2 probe detected a 1.5-kb transcript (Fig. 3B). Transcripts from pilA1 and pilA2 were also examined in two additional S-phase variants (strains VA1-S2 and VA1-S3) and two additional L-phase variants (strains VA1-L1 and VA1-L3), yielding identical results (data not shown). The 0.6-kb transcript is predicted to include pilA1 sequences, whereas the 1.5-kb transcript is predicted to include pilA1, pilA2, and pilB sequences. For both probes, the relative hybridization signal intensity was lower for strain VA1-L2 (Fig. 3; compare lanes 1 and 2). Replicate experiments showed that the pilA1 and pilA1A2B transcript levels in L-phase variants ranged from 43 to 80% of those in S-phase variants. Despite this difference, these results support transcription of the pilA locus in strain VA1-L2 and, coupled with pilin localization studies (see below), suggest a posttranscriptional basis for the lack of piliation in L-phase variants.
FIG. 3.
Expression of pilA genes in E. corrodens VA1-S1 and VA1-L2. Samples of total RNA (5 μg per lane) from strain VA1-S1 (lanes 1) or VA1-L2 (lanes 2) were subjected to blot hybridization against RNA probes specific for transcripts from pilA1 (A) or pilA2 (B). The positions of RNA molecular size standards are shown at the left.
Transcription of the pilA locus was also examined by reverse transcriptase PCR. The reverse transcriptase reactions were carried out with select primer pairs using total RNA from strains VA1-S1 and VA1-L2 as the template. The cDNAs were amplified by PCR, and the products were analyzed by hybridization against the RNA probes specific for pilA1 or pilA2. PCR products corresponding to transcripts encoding pilA1, pilA2, or pilB were equally detected in both phase variants (data not shown). In addition, PCR products corresponding to polycistronic transcripts encompassing pilA1 and pilA2, as well as pilA1 through pilB, were detected in both variants (data not shown). These data further support equivalent transcription of the pilA locus in S- and L-phase variants.
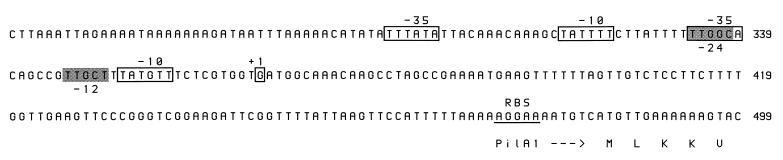
The 5′ end of the pilA1 transcript from strain VA1-S1 was mapped by primer extension. Using a primer complementary to a region in the 5′ end of pilA1 (nucleotides 538 to 555), we detected two products 200 and 130 nucleotides in length (data not shown). The larger product mapped to a guanine located 117 bases upstream (nucleotide 367) of the putative translation initiation codon, whereas the smaller product mapped to a cytosine located 53 bases upstream (nucleotide 431). Identical products were obtained for strain VA1-L2. An analysis of the DNA sequence in the vicinity of these sites suggests that the larger product reflects the native pilA1 transcription initiation site: two potential ς70 consensus promoter sequences and a potential ς54 consensus promoter sequence are located immediately upstream of the identified guanine (Fig. 4). Located further upstream is a 40-bp AT-rich region. A similar analysis of the region between pilA1 and pilA2 did not reveal potential promoter sequences; however, a 25-bp region (nucleotides 968 to 993) predicted to form a near-perfect hairpin was detected.
FIG. 4.
Nucleotide sequence of the E. corrodens VA1-S1 pilA1 promoter region. The putative pilA1 transcription initiation site (+1) determined by primer extension analysis is boxed, as are potential ς70 promoter sequences (−35 and −10). Potential ς54 promoter sequences (−24 and −12) are shaded. A putative ribosome binding site (RBS) is underlined. Numbers to the right correspond to the sequence deposited in the GenBank database (accession no. AF079304).
Pilin localization in phase variants.
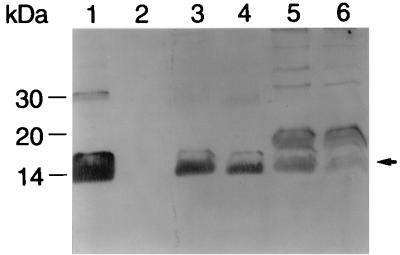
The experiments described above indicated that both S- and L-phase variants express intact pilA1 transcripts. To define a cellular basis for the lack of piliation among L-phase variants, we examined the synthesis and localization of PilA1 in both S- and L-phase variants. Surface, soluble, and insoluble protein fractions were isolated from strains VA1-S1 and VA1-L2 and analyzed for PilA1 content by using a PilA1-specific antiserum. The strain VA1-S1 surface fraction, which should include pilin from pili and pilin loosely associated with the cell surface, contained a considerable amount of mature PilA1 (Fig. 5, lane 1). In contrast, no PilA1 was detected in the surface fraction of strain VA1-L2 (lane 2). Comparable amounts of mature PilA1 were detected in the soluble fraction of both strains (compare lanes 3 and 4). Similarly, the insoluble fraction of both strains contained comparable amounts of mature PilA1 (compare lanes 5 and 6). In addition to mature pilin, the insoluble fraction of both strains contained a unidentified 19-kDa immunoreactive protein (lanes 5 and 6). Control assays with different pilA1 and pilA2 mutants indicated that the 19-kDa immunoreactive species is ubiquitous to the insoluble fraction and is not a form of pilin. No PilA1 was detected in the periplasmic fraction of either strain VA1-S1 or strain VA1-L2 (data not shown). Collectively, these data suggest that the lack of piliation in L-phase variants is due to a posttranslational event, possibly involving PilA1 export and/or pilus assembly.
FIG. 5.
Localization of PilA1 in S- and L-phase variants of E. corrodens VA1. Surface (lanes 1 and 2), soluble (lanes 3 and 4), and insoluble (lanes 5 and 6) protein fractions from strains VA1-S1 (lanes 1, 3, and 5) and VA1-L2 (lanes 2, 4, and 6) were subjected to immunoblot analysis with a polyclonal antiserum specific for PilA1. The arrow marks the position of mature pilin. The positions of protein molecular weight standards are shown at the left.
Piliation of phase variants.
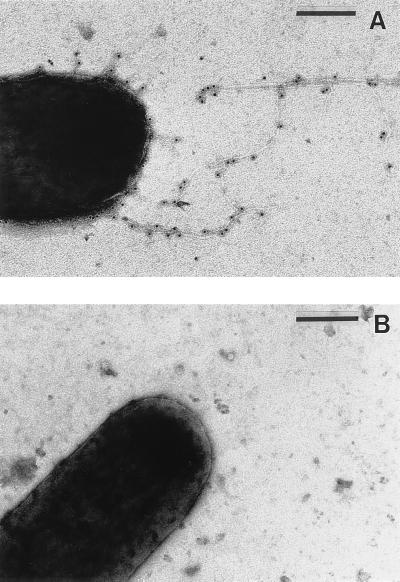
Previous reports have described differential piliation among S- and L-phase variants of E. corrodens (22, 23). However, this phenomenon was not well documented, and subsequent studies have questioned the presence of pili on E. corrodens (32, 38). To document their differential piliation, cells of strains VA1-S3 and VA1-L2 were examined by immunogold electron microscopy using a polyclonal antiserum raised against pilin purified from strain VA1-S1 (27). In an analysis of over 200 strain VA1 cells, we detected one or more immunoreactive pili on 47% of the strain VA1-S3 cells but on none of the strain VA1-L2 cells. Furthermore, no immunoreactive material was observed on the cell surface of either strain. A representative electron micrograph for strains VA1-S3 and VA1-S2 is presented in Fig. 6. In a similar analysis of over 200 E. corrodens ATCC 23834 cells, immunoreactive pili were detected on all of the S-phase cells and none of the L-phase cells.
FIG. 6.
Differential piliation of S- and L-phase variants of E. corrodens VA1. (A) Immunogold electron micrograph of a strain VA1-S3 cell; (B) equivalent micrograph of a strain VA1-L2 cell. Bars = 200 nm.
DISCUSSION
The gram-negative pathogen E. corrodens elaborates type IV pili and exhibits a phase variation involving an irreversible transition from piliated to nonpiliated cells. To initiate an investigation into the molecular basis of this transition event, we have cloned and characterized the pilA locus from E. corrodens VA1. The pilA locus includes four putative genes arranged in tandem. The first two genes, designated pilA1 and pilA2, each encode a type IV pilin. The third gene, designated pilB, encodes a potential pilus assembly protein, whereas the fourth gene, designated hagA, encodes a putative hemagglutinin. In terms of the pilin and hemagglutinin genes, the strain VA1 pilA locus structurally resembles the similar locus described for E. corrodens ATCC 23834 (39) (see below). However, the strain VA1 pilA locus differs from the strain ATCC 23834 locus by the presence of pilB. Given that DNA hybridization analyses of genomic DNA using heterologous and homologous probes failed to detect additional hybridizing fragments, pilA1 and pilA2 most likely represent the only type IV pilin genes present in strain VA1.
This study showed that S- and L-phase variants of E. corrodens are identical with respect to pilA1 and pilA2 structure and sequence. This differs from what is most commonly observed for the best-characterized bacterial pathogens exhibiting a similar phase and/or antigenic variation that involves expression of type IV pilin. Among these are N. gonorrhoeae and N. meningitidis, which possess one or two pilin (pilE) genes and a variable number of silent partial pilin (pilS) genes. For these strains, phase variation can be achieved by spontaneous mutations in pilE, which results in nonpiliated cells (7, 50, 51). In addition, irreversible intragenic recombination events involving the pilE and pilS genes result in the synthesis of structurally altered pilins, giving rise to antigenic variation (19, 36, 45, 52). Another example is M. bovis, in which two different pilin genes (fimL and fimQ) are alternatively expressed by means of a DNA inversion that links one or the other gene to a single common promoter (18, 34). Thus, the most common phase and/or antigenic variation exhibited by N. gonorrhoeae, N. meningitidis, and M. bovis results from a genomic mutation or recombination events directly involving type IV pilin genes. In comparison, the phase variation exhibited by E. corrodens is unique in that it does not involve a pilin gene-associated mutation or genomic recombination event.
The RNA hybridization analysis indicated that transcripts from pilA1, but not pilA2, were abundant in both S- and L-phase variants of strain VA1, consistent with previous work showing that PilA1 was the major pilus protein for this strain (27). The 5′-end mapping and sequence analyses suggest that the abundant pilA1 transcript originates from a ς70-type promoter located 200 bp upstream of the putative PilA1 translation initiation codon. If correct, the 0.6-kb pilA1 transcript terminates at the pilA1-pilA2 intergenic region, the sequence of which predicts a potential hairpin structure. A low-abundance 1.5-kbp transcript that hybridized to probes for both pilA1 and pilA2 was also detected in the S- and L-phase variants. This larger transcript is most likely the product of transcription from the pilA1 promoter through the pilA1-pilA2 intergenic region, giving rise to a pilA1A2B polycistronic message. Presumably, the predicted hairpin structure between pilA1 and pilA2 represents the terminator component of a transcription attenuation mechanism. Such an attenuation mechanism would provide for controlled expression of pilA1, pilA2, and pilB as required for pilus formation and resembles a similar mechanism that has been proposed for D. nodosus (25).
The detection of transcripts from pilA1 and pilA2 in the L-phase variant of strain VA1 was somewhat unexpected. In the absence of a detectable pilin gene-associated recombination or mutagenesis event, it was hypothesized that the phase variation exhibited by strain VA1 might be achieved through differential expression of pilA1 or pilA2. The abundance of both the 0.6-kb pilA1 transcript and 1.5-kb pilA1A2B transcript was consistently lower in L-phase variants; however, this difference was deemed insufficient to account for their lack of piliation. We suspect that the decreased level of the pilA1 and pilA1A2B transcripts is related to factors associated with extraction of RNA from the morphologically distinct L-phase variants, as opposed to factors associated with transcription. Collectively, the pilA1 and pilA2 structure and transcription data support a posttranscriptional basis for the nonpiliated phenotype of the L-phase variants.
The pilin localization studies revealed that L-phase variants are not compromised in pilin biosynthesis but that they differ from S-phase variants with respect to the fate of synthesized pilin. For both S- and L-phase variants, similar levels of mature PilA1 were detected in the insoluble protein fraction. Because this fraction includes the cytoplasmic membrane and associated components, the presence of mature PilA1 is consistent with studies of Pseudomonas aeruginosa and other species showing that initial processing of type IV pilins (leader sequence cleavage and amino-terminal methylation) is accomplished on the cytoplasmic surface of the cytoplasmic membrane by membrane-associated cognate prepilin peptidases (47). Moreover, the similarity in pilin composition of the insoluble protein fractions suggests that initial processing of PilA1 is not significantly altered in the L-phase variants. The S- and L-phase variants were also indistinguishable in the PilA1 composition of their soluble protein fractions, as similar levels of mature PilA1 were detected for both. In contrast to these results is the PilA1 composition of the surface protein fraction: whereas mature PilA1 was readily detected in the S-phase variant surface protein fraction, none was detected in the corresponding fraction from the L-phase variant. This lack of pilin in the L-phase variant surface fraction is consistent with the immunogold electron microscopic analysis showing that only S-phase variants possessed intact pili. On the basis of these data, we conclude that an altered pilin posttranslational event, possibly involving one or more steps in pilin export and/or assembly, is responsible for the lack of piliation associated with the L-phase variants of E. corrodens VA1.
The specific stage or event in export or assembly of pili that might be affected in the L-phase variants is not known. In general, the pathway for processing, export, and assembly of type IV pilins is not well defined. Perhaps the best-characterized type IV pilus biosynthetic pathway is that of P. aeruginosa, for which more than 22 genes involved in pilin expression and/or pilus assembly and function have been identified by transposon tagging (35). More than half of these genes appear to be directly involved in pilus assembly and function and are thought to represent a subset of a general system for the formation of surface-associated protein complexes (26). Of particular interest is the observation that mutations in the individual genes in this subset result in the lack of piliation. Homologs to many of the putative pilus assembly genes have been identified in other type IV piliated bacteria, supporting a common mechanism for processing, export, and assembly of type IV pili. Presumably, E. corrodens shares this mechanism; if this is so, events that affect one or more of the corresponding genes might be involved in the transition from S- to L-phase variants. Experiments to examine this possibility are in progress.
A precedence for phase variation mediated by a type IV pilin-associated posttranslational event has been established for N. gonorrhoeae MS11. This strain, like most examined N. gonorrhoeae strains, contains two unlinked copies of the pilC gene (not a P. aeruginosa pilC homolog), designated pilC1 and pilC2, which encode a protein involved in pilus assembly (29). Only pilC2 is expressed in piliated MS11 cells due to a translational frameshift in pilC1. Spontaneous phase variation of MS11 cells is achieved by frameshift mutations in a run of G residues within the region of pilC2 encoding the signal peptide of PilC, abolishing translation of the protein. Pilin synthesis is maintained in the absence of PilC, but no pili are assembled. In this regard, the strain MS11 pilC2 mutants and the L-phase variants of E. corrodens VA1 are phenotypically indistinguishable. Whether E. corrodens VA1 possesses a homolog to pilC from N. gonorrhoeae remains to be examined; however, we note that thus far, PilC appears to be unique to N. gonorrhoeae (47). A significant difference between the pilC2-based phase variation in N. gonorrhoeae MS11 and the phase variation in E. corrodens VA1 is that the former is reversible. Piliated revertants to strain MS11 pilC2 mutants are readily obtainable; the reversion involves mutations in pilC1 or pilC2 that result in translation of PilC. In contrast, no L- to S-phase revertants of E. corrodens VA1 have been observed. Given these data, we predict that the putative posttranslational basis of phase variation in E. corrodens VA1 differs mechanistically from pilC2-based phase variation in N. gonorrhoeae.
Genes encoding type IV pilins have been cloned from two other E. corrodens strains, ATCC 23834 (39) and ATCC 31745 (53). A feature common to these two strains and strain VA1 is the presence of two tandemly arranged type IV pilin genes separated by 70 to 80 bp. The strain ATCC 23834 pilin genes are designated ecpA and ecpB (39), whereas the strain 31745 pilin genes are designated ecpC and ecpD (53). For the strain VA1 pilin genes pilA1 and pilA2, we adopted the designations of the P. aeruginosa pilin gene system (1), which is consistent with recommendations for bacterial gene nomenclature (17). The six predicted pilins share 80 to 97% sequence identity at their amino termini (first 32 residues of the mature protein), which is typical for all examined type IV pilins (47). Overall, the six predicted pilins share 35 to 43% sequence identity, which is consistent with the reported decreased sequence conservation within the carboxyl regions of type IV pilins (47). In general, the two pilins from a given strain share greater overall sequence identity with each other than with pilins from another strain, suggesting that for each strain, the pilin gene pairs arose by a duplication event. The G+C contents of pilA1 and pilA2 (47.9 and 40.1%, respectively) significantly differ from the genomic G+C contents (53 to 58%) reported for several different E. corrodens strains (13, 28, 42), suggesting that the pilin gene(s) may have been acquired by horizontal transfer. Since these are characteristics of a variety of virulence genes acquired by intra- and interspecies gene transfer in bacterial pathogens (21, 54), the pilA locus may represent an acquired region encoding pathogenicity-associated proteins.
ACKNOWLEDGMENTS
We thank D. Mason for help in the initial cloning of the E. corrodens pilA locus, D. Law and staff for assistance with electron microscopy, and W. Thomas and staff for assistance with DNA sequencing.
This research was partially supported by Public Health Service grant DE10439 (R.L.H.) from the National Institutes of Health.
REFERENCES
- 1.Alm R A, Mattick J S. Genes involved in the biogenesis and function of type-4 fimbriae in Pseudomonas aeruginosa. Gene. 1997;192:89–98. doi: 10.1016/s0378-1119(96)00805-0. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Ames G F, Prody C, Kustu S. Simple, rapid, and quantitative release of periplasmic proteins by chloroform. J Bacteriol. 1984;160:1181–1183. doi: 10.1128/jb.160.3.1181-1183.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashimoto A, Chen C, Bakler I, Slots J. Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontal lesions. Oral Microbiol Immunol. 1998;11:226–273. doi: 10.1111/j.1399-302x.1996.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 5.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1989. [Google Scholar]
- 6.Berbari E F, Cockerill F R I, Steckelberg J M. Infective endocarditis due to unusual or fastidious microorganisms. Mayo Clin Proc. 1998;72:532–542. doi: 10.4065/72.6.532. [DOI] [PubMed] [Google Scholar]
- 7.Bergström S, Robbins K, Koomey J M, Swanson J. Piliation control mechanisms in Neisseria gonorrhoeae. Proc Natl Acad Sci USA. 1986;83:3890–3894. doi: 10.1073/pnas.83.11.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bieber D, Ramer S W, Wu C Y, Murray W J, Tobe T, Fernandez R, Schoolnik G K. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science. 1998;280:2114–2118. doi: 10.1126/science.280.5372.2114. [DOI] [PubMed] [Google Scholar]
- 9.Brunham R C, Plummer F A, Stephens R S. Bacterial antigenic variation, host immune response, and pathogen-host coevolution. Infect Immun. 1993;61:2273–2276. doi: 10.1128/iai.61.6.2273-2276.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C K, Wilson M E. Eikenella corrodens in human oral and non-oral infections: a review. J Periodontol. 1992;63:941–953. doi: 10.1902/jop.1992.63.12.941. [DOI] [PubMed] [Google Scholar]
- 11.Chen C K, Wilson M E. Outer membrane protein and lipopolysaccharide heterogeneity among Eikenella corrodens isolates. J Infect Dis. 1990;162:664–671. doi: 10.1093/infdis/162.3.664. [DOI] [PubMed] [Google Scholar]
- 12.Cobb C M, Helber J T, Hirschberg R. Scanning electron microscopy of Eikenella corrodens colony morphology variants. J Periodontal Res. 1994;29:410–417. doi: 10.1111/j.1600-0765.1994.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 13.Coykendall A L, Kaczmarek F S. DNA homologies among Eikenella corrodens strains. J Periodontal Res. 1980;15:615–620. doi: 10.1111/j.1600-0765.1980.tb00320.x. [DOI] [PubMed] [Google Scholar]
- 14.Dalrymple B, Mattick J S. An analysis of the organization and evolution of type 4 fimbrial (MePhe) subunit proteins. J Mol Evol. 1987;25:261–269. doi: 10.1007/BF02100020. [DOI] [PubMed] [Google Scholar]
- 15.Decker M D. Eikenella corrodens. Infect Control. 1986;7:36–41. doi: 10.1017/s0195941700063797. [DOI] [PubMed] [Google Scholar]
- 16.Decker M D, Graham B S, Hunter E B, Liebowitz S M. Endocarditis and infections of intravascular devices due to Eikenella corrodens. Am J Med Sci. 1986;292:209–212. doi: 10.1097/00000441-198610000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Demerec M, Adelberg E A, Clark A J, Hartman P E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966;54:61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fulks K A, Marrs C F, Stevens S P, Green M R. Sequence analysis of the inversion region containing the pilin genes of Moraxella bovis. J Bacteriol. 1990;172:310–316. doi: 10.1128/jb.172.1.310-316.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fussenegger M, Rudel T, Barten R, Ryll R, Meyer T F. Transformation competence and type-4 pilus biogenesis in Neisseria gonorrhoeae—a review. Gene. 1997;192:125–134. doi: 10.1016/s0378-1119(97)00038-3. [DOI] [PubMed] [Google Scholar]
- 20.Griego R D, Rosen T, Orengo I F, Wolf J E. Dog, cat, and human bites: a review. J Am Acad Dermatol. 1995;33:1019–1029. doi: 10.1016/0190-9622(95)90296-1. [DOI] [PubMed] [Google Scholar]
- 21.Hacker J, Blum-Oehler G, Muhldorfer I, Tschape H. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol Microbiol. 1997;23:1089–1097. doi: 10.1046/j.1365-2958.1997.3101672.x. [DOI] [PubMed] [Google Scholar]
- 22.Henrichsen J. The occurrence of twitching motility among gram-negative bacteria. Acta Pathol Microbiol Scand Sect B. 1975;83:171–178. doi: 10.1111/j.1699-0463.1975.tb00089.x. [DOI] [PubMed] [Google Scholar]
- 23.Henrichsen J, Blom J. Examination of fimbriation of some gram-negative rods with and without twitching and gliding motility. Acta Pathol Microbiol Scand Sect B. 1975;83:161–170. doi: 10.1111/j.1699-0463.1975.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 24.Hirschberg, R. L. Unpublished data.
- 25.Hobbs M, Dalrymple B P, Cox P T, Livingstone S P, Delaney S F, Mattick J S. Organization of the fimbrial gene region of Bacteroides nodosus: class I and class II strains. Mol Microbiol. 1991;5:543–560. doi: 10.1111/j.1365-2958.1991.tb00726.x. [DOI] [PubMed] [Google Scholar]
- 26.Hobbs M, Mattick J S. Common components in the assembly of type 4 fimbriae, DNA transfer systems, filamentous phage and protein-secretion apparatus: a general system for the formation of surface-associated protein complexes. Mol Microbiol. 1993;10:233–243. doi: 10.1111/j.1365-2958.1993.tb01949.x. [DOI] [PubMed] [Google Scholar]
- 27.Hood B L, Hirschberg R. Purification and characterization of Eikenella corrodens type IV pilin. Infect Immun. 1995;63:3693–3696. doi: 10.1128/iai.63.9.3693-3696.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson F L, Goodman Y. Eikenella. In: Krieg N R, editor. Bergey’s manual of systematic bacteriology. 9th ed. Vol. 1. Baltimore, Md: Williams & Wilkins; 1984. pp. 591–597. [Google Scholar]
- 29.Jonsson A B, Nyberg G, Normark S. Phase variation of gonococcal pili by frameshift mutation in pilC, a novel gene for pilus assembly. EMBO J. 1991;10:477–488. doi: 10.1002/j.1460-2075.1991.tb07970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kentos A, De Vuyst P, Stuelens M J, Jacobs F, de Francquen P, Delaere B, Demaeyer P, Thys J P. Lung abscess due to Eikenella corrodens: three cases and review. Eur J Clin Microbiol Infect Dis. 1995;14:146–148. doi: 10.1007/BF02111877. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 31.Krogfelt K A. Bacterial adhesion: genetics, biogenesis, and role in pathogenesis of fimbrial adhesins of Escherichia coli. Rev Infect Dis. 1991;13:721–735. doi: 10.1093/clinids/13.4.721. [DOI] [PubMed] [Google Scholar]
- 32.Lai C H, Listgarten M A, Tanner A C R, Socransky S S. Ultrastructures of Bacteroides gracilis, Wolinella recta, and Eikenella corrodens all from humans with periodontal disease. Int J Syst Bacteriol. 1981;31:465–475. [Google Scholar]
- 33.Lowenguth R A, Chin I, Caton J G, Cobb C M, Drisko C L, Killoy W J, Michalowicz B S, Pihlstrom B L, Goodson J M. Evaluation of periodontal treatments using controlled-release tetracycline fibers: microbiological response. J Periodontol. 1995;66:700–707. doi: 10.1902/jop.1995.66.8.700. [DOI] [PubMed] [Google Scholar]
- 34.Marrs C F, Ruehl W W, Schoolnik G K, Falkow S. Pilin-gene phase variation of Moraxella bovis is caused by an inversion of the pilin genes. J Bacteriol. 1988;170:3032–3039. doi: 10.1128/jb.170.7.3032-3039.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattick J S, Whitchurch C B, Alm R A. The molecular genetics of type-4 fimbriae in Pseudomonas aeruginosa—a review. Gene. 1996;179:147–155. doi: 10.1016/s0378-1119(96)00441-6. [DOI] [PubMed] [Google Scholar]
- 36.Meyer T F, Gibbs C P, Haas R. Variation and control of protein expression in Neisseria. Annu Rev Microbiol. 1990;44:451–477. doi: 10.1146/annurev.mi.44.100190.002315. [DOI] [PubMed] [Google Scholar]
- 37.Page R C. Current understanding of the aetiology and progression of periodontal disease. Int Dent J. 1986;36:153–161. [PubMed] [Google Scholar]
- 38.Progulske A, Holt S C. Transmission-scanning electron microscopic observations of selected Eikenella corrodens strains. J Bacteriol. 1980;143:1003–1018. doi: 10.1128/jb.143.2.1003-1018.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao V K, Progulske-Fox A. Cloning and sequencing of two type 4 (N-methylphenylalanine) pilin genes from Eikenella corrodens. J Gen Microbiol. 1993;139:651–660. doi: 10.1099/00221287-139-3-651. [DOI] [PubMed] [Google Scholar]
- 40.Rao V K, Whitlock J A, Proguske-Fox A. Cloning, characterization and sequencing of two haemagglutinin genes from Eikenella corrodens. J Gen Microbiol. 1993;139:639–650. doi: 10.1099/00221287-139-3-639. [DOI] [PubMed] [Google Scholar]
- 41.Reed K C, Mann D A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985;13:7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossau R, Vandenbussche G, Thielemans S, Segers P, Grosch H, Göthe E, Mannheim W, Ley J D. Ribosomal nucleic acid cistron similarities and deoxyribonucleic acid homologies of Neisseria, Kingella, Eikenella, Simonsiella, Alysiella, and Centers for Disease Control Groups EF-4 and M-5 in the emended family of Neisseriaceae. Int J Syst Bacteriol. 1989;39:185–198. [Google Scholar]
- 43.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 44.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seifert H S. Questions about gonococcal pilus phase- and antigenic variation. Mol Microbiol. 1996;21:433–440. doi: 10.1111/j.1365-2958.1996.tb02552.x. [DOI] [PubMed] [Google Scholar]
- 46.Shiozu I, Shiozu J, Takazoe I, Okuda K. Corroding characteristics of Eikenella corrodens. Bull Tokyo Dent Coll. 1992;33:1–6. [PubMed] [Google Scholar]
- 47.Strom M S, Lory S. Structure-function and biogenesis of the type IV pili. Annu Rev Microbiol. 1993;47:565–596. doi: 10.1146/annurev.mi.47.100193.003025. [DOI] [PubMed] [Google Scholar]
- 48.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 49.Swanson J, Belland R J, Hill S A. Neisserial surface variation: how and why? Curr Opin Genet Dev. 1992;2:805–811. doi: 10.1016/s0959-437x(05)80143-1. [DOI] [PubMed] [Google Scholar]
- 50.Swanson J, Bergström S, Barrera O, Robbins K, Corwin D. Pilus- gonococcal variants. Evidence for multiple forms of piliation control. J Exp Med. 1985;162:729–744. doi: 10.1084/jem.162.2.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swanson J, Bergström S, Robbins K, Barrera O, Corwin D, Koomey J M. Gene conversion involving the pilin structural gene correlates with pilus+ in equilibrium with pilus− changes in Neisseria gonorrhoeae. Cell. 1986;47:267–276. doi: 10.1016/0092-8674(86)90449-6. [DOI] [PubMed] [Google Scholar]
- 52.Tonjum T, Koomey M. The pilus colonization factor of pathogenic neisserial species: organelle biogenesis and structure/function relationships-a review. Gene. 1997;192:155–163. doi: 10.1016/s0378-1119(97)00018-8. [DOI] [PubMed] [Google Scholar]
- 53.Tonjum T, Weir S, Bovre K, Progulske-Fox A, Marrs C F. Sequence divergence in two tandemly located pilin genes of Eikenella corrodens. Infect Immun. 1993;61:1909–1016. doi: 10.1128/iai.61.5.1909-1916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolff K, Stern A. Identification and characterization of specific sequences encoding pathogenicity associated proteins in the genome of commensal Neisseria species. FEMS Microbiol Lett. 1995;125:255–263. doi: 10.1111/j.1574-6968.1995.tb07366.x. [DOI] [PubMed] [Google Scholar]