Abstract
Background/objectives
This study shows the visual and refractive outcomes of cataract surgery in patients with previous radial keratotomy (RK).
Subjects/methods
This is a retrospective case series of 100 eyes (65 patients) with previous RK who had undergone routine cataract surgery with a monofocal intraocular lens implant (IOL) at Moorfields Eye Hospital, London, United Kingdom, between January 2004 and December 2018.
Results
Mean age at the time of surgery was 59.8 years; 39% eyes had ocular copathology. Best-corrected visual acuity (LogMAR; median, interquartile range) improved from 0.30 (0.22, 0.55) to 0.06 (−0.02, 0.21) in eyes without copathology, and from 0.56 (0.30, 1.00) to 0.20 (0.00, 0.20) in eyes with copathology. Haigis formula (19 eyes) resulted in a median prediction error of −0.31 D (−1.07, +0.05), versus −0.55 D (−1.23, +0.22) for Double-K SRK/T (55 eyes) and +0.93 D (0.20, 2.31) for SRK/T (18 eyes). At the final follow-up, 52.6% eyes were within 0.5 D and 68.4% within 1 D of the predicted spherical equivalent for Haigis, versus 32.7% and 52.7% for Double-K SRK/T, and 27.8% and 38.9% for SRK/T. The most frequent complication was RK incision dehiscence (8%).
Conclusions
Although the best-corrected visual acuity outcomes compare with the UK national benchmarks, significantly fewer eyes with previous RK achieved the level of unaided distance visual acuity to allow spectacle independence. Surgeons should be aware of the increased likelihood of wound dehiscence and plan surgery accordingly. Haigis formula tended to have a better predictability of the postoperative spherical equivalent and, since introduced, was the preferred choice for IOL calculation in this group of patients.
Subject terms: Refractive errors, Lens diseases
Introduction
Radial keratotomy (RK) was the most common refractive procedure for myopia correction before the development of excimer laser surgery [1]. Patients who underwent RK in the 1980s and early 1990s are now requiring cataract surgery and this can be challenging for various reasons. Patients with previous refractive surgery tend to require cataract surgery at a younger age and may have an increased intolerance to a refractive surprise [2]. Despite various formulae being used to calculate the intraocular lens (IOL) power in eyes with prior RK, there is little consensus on which is the most predictable [3–8]. Furthermore, owing to the weakening of the cornea, there is an increased risk of intra- or postoperative radial corneal incision dehiscence and a longer duration until refraction stability [9–12]. These eyes often have ocular copathologies associated with myopia, which may limit the visual potential post cataract surgery [13].
Although benchmarks for visual and refractive outcomes following cataract surgery are established [14], there is limited real-world data to allow prediction of outcomes in patients with previous RK.
We report on the visual and refractive outcomes for cataract surgery in patients with previous RK.
Methods
This is a retrospective case series of patients with prior RK who underwent routine cataract surgery at Moorfields Eye Hospital, London, UK, over a period of 15 years (January 2004–December 2018). The study was approved by the Moorfields Eye Hospital Institutional Review Board (Project Number CA-71) and adhered to the tenets of the Declaration of Helsinki.
We included all eyes with previous RK who underwent routine cataract surgery with a monofocal lens implanted in the capsular bag. Eyes with previous refractive surgery, including arcuate keratotomy, photorefractive keratectomy (PRK), and laser-assisted in situ keratomileusis (LASIK), and eyes undergoing combined corneal, glaucoma, or vitreoretinal surgery were excluded.
Past ocular history, pre- and postoperative visual acuity, postoperative refraction (autorefraction or subjective refraction), slit-lamp biomicroscopy, fundus examination and biometry data were collected for all patients. The formula used for IOL selection, IOL model, power, predicted, and postoperative refractive outcomes was also collected. The biometry was performed with an IOLMaster (Carl Zeiss Meditec); where this was not possible, an ultrasound A scan (Humphrey Instruments, Inc.) and handheld keratometer (Nidek Medical Products, Inc.) were used. In two eyes (2%) of the same patient, K values of 37.5 D were used (excluded from PE calculation), and in three eyes, the method of determining K values was not recorded.
Additionally, intra- and postoperative complications were recorded.
We calculated the prediction error (PE) as the difference between the predicted spherical equivalent (SE) and the actual postoperative SE. A negative PE indicates a more myopic outcome than predicted, and a positive PE indicates a more hypermetropic outcome.
The first postoperative visit was considered any visit within six weeks of surgery; further follow-up depended on the surgeons’ practice and any postoperative issues. We considered the last postoperative follow-up the appointment when the patient’s visual acuity was deemed stable and there was no new copathology.
As per the National Ophthalmology Database (NOD) Cataract Surgery Report [14], preoperative visual acuity was considered the better of uncorrected distance visual acuity (UCVA) or corrected distance visual acuity (CDVA) within 3 months prior to surgery. Postoperative visual acuity was considered CDVA when present, and the better of UCVA or pinhole VA when CDVA was not recorded.
For the purpose of statistical analysis, the Snellen visual acuities were converted to LogMAR equivalents. Visual acuities of count fingers (CF), hand movements (HM), perception of light (PL), and no perception of light (NPL) were converted to 2.1, 2.4, 2.7 and 3.0 LogMAR equivalents, respectively [14].
Continuous variables are presented as median (interquartile range, IQR) or mean (±1 standard deviation, SD) and categorical variables as proportions. After testing for data distribution, nonparametric tests were performed and two-tailed p-values < 0.05 were considered statistically significant. Statistical analysis was performed using GraphPad Software (San Diego, CA, USA) and SPSS Statistics version 24 (IBM Chicago, IL, USA).
Results
Patients’ characteristics
One-hundred and thirty-six (136) eyes of 90 patients with previous RK had cataract surgery with a monofocal IOL implant over the 15-year period, and 100 eyes of 65 patients were eligible. Demographic and baseline ocular characteristics are shown in Table 1.
Table 1.
Demographics and baseline ocular characteristics.
| Mean (SD) | Median (Range) | |
|---|---|---|
| Age at the time of cataract surgery (n = 65 patients) | 59.8 (12.6) | 62 (33, 81) |
| Age at the time of RK (n = 60 patients) | 37.8 (12.7) | 41 (14, 64) |
| Number of RK incisions (n = 83 eyes) | 8.5 (3.5) | 8 (4, 20) |
| Biometry data (n = 97 eyes) | ||
| Axial length (mm) | 26.84 (2.34) | 26.14 (22.10, 34.92) |
| Anterior chamber depth (mm) | 3.34 (0.45) | 3.35 (2.29, 5.00) |
| Kmin (D) | 36.23 (3.24) | 36.33 (28.87, 45.73) |
| Kmax (D) | 37.96 (3.01) | 38.27 (31.13, 46.04) |
| IOL power (D) (n = 100 eyes) | 21.79 (4.25) | 22 (10, 30) |
| Type of IOL (n = 100 eyes) | ||
| Alcon AcrySof SN60WF | 63 (63%) | |
| Bausch + Lomb Akreos Adapt | 10 (10%) | |
| Alcon AcrySof SN60WS | 9 (9%) | |
| Alcon AcrySof SA60AT | 8 (8%) | |
| Alcon AcrySof MA60AC | 6 (6%) | |
| Alcon AcrySof AU00T0 | 2 (2%) | |
| Alcon AcrySof SN6CWS | 2 (2%) | |
Abbreviations: IOL intraocular lens, RK radial keratotomy, SD standard deviation.
The mean first follow-up visit was 3.0 ± 1.2 weeks (median three weeks, IQR 2.1–3.9 weeks) and the mean final postoperative follow-up was 15.9 ± 9.4 weeks (median 13.6, IQR 8.6–20.9 weeks). Six eyes had no postoperative appointment in the first six weeks and 23 eyes did not have a second follow-up appointment.
Thirty-nine eyes (39%) had ocular copathology: previous retinal detachment surgery (15%), glaucoma or glaucoma surgery (12%), amblyopia (6%), previous vitrectomy for proliferative diabetic retinopathy (2%), myopic degeneration (2%), uveitis (2%), subretinal neovascular membrane (2%), and epiretinal membrane (1%). Four eyes (4%) had more than one ocular copathology.
Intraoperative and postoperative complications
The most frequent complication was RK incision dehiscence occurring in eight out of 100 eyes (8%) requiring suture; of these, six eyes had eight RK incisions, one eye had 16 RK incisions and the number of RK incision was not recorded for one eye. The main incision placement was corneal or limbal, avoiding the RK incisions in 92 eyes (92%) and scleral tunnel in eight eyes (8%); all cases of RK dehiscence occurred when a corneal incision was used. The width of the incision was 2.4 mm in 39 eyes (39%), 2.8 mm in 21 eyes (21%), 3.2 mm in two eyes (2%), and not documented in 38 eyes (38%). In addition to the eight eyes with RK dehiscence, 16/100 eyes (16%) required suturing of the paracentesis or main incision in the absence of RK dehiscence.
Other intraoperative complications included anterior capsular tear (three eyes), intraoperative miosis requiring iris hooks, subsequent zonular dialysis and capsular tension ring insertion (one eye), and a broken haptic requiring wound enlargement and lens replacement (one eye).
Postoperative complications included intraocular pressure rise requiring tube surgery one week after the cataract surgery (one eye with preexisting advanced glaucoma), and one case of cystoid macular edema 18 weeks postoperatively, which settled by 35 weeks when the visual acuity was 0.00 LogMAR.
Visual acuity outcomes
The mean preoperative CDVA was 0.52 ± 0.45 LogMAR (median 0.49, IQR: 0.24–0.76; n = 100 eyes). The mean CDVA was 0.23 ± 0.33 LogMAR (median 0.20, IQR: 0.04–0.30; n = 94 eyes) at the first postoperative visit and 0.17 ± 0.27 LogMAR (median 0.14, IQR: 0.00–0.30; n = 77 eyes) at the final postoperative visit (p < 0.0001, Friedman test) (Table 2).
Table 2.
Visual acuity for all eyes and by ocular copathology preoperatively (n = 100), first (94 eyes) and final postoperative visit (77 eyes).
| Preoperative CDVA | First postoperative CDVA | Final postoperative CDVA | P value (Friedman test) | |
|---|---|---|---|---|
| Eyes without ocular copathology (n = 61) | ||||
| Mean (SD) | 0.40 (0.34) | 0.17 (0.16) | 0.09 (0.17) | <0.0001 |
| Median (IQR) | 0.30 (0.22, 0.56) | 0.20 (0.02, 0.26) | 0.04 (−0.04, 0.20) | |
| Eyes with ocular copathology (n = 39) | ||||
| Mean (SD) | 0.72 (0.52) | 0.31 (0.48) | 0.28 (0.36) | <0.0001 |
| Median (IQR) | 0.56 (0.30, 1.00) | 0.20 (0.06, 0.30) | 0.22 (0.00, 0.33) | |
| Total (n = 100) | ||||
| Mean (SD) | 0.52 (0.45) | 0.23 (0.33) | 0.17 (0.27) | <0.0001 |
| Median (IQR) | 0.49 (0.24, 0.76) | 0.20 (0.04, 0.30) | 0.14 (0.00, 0.30) | |
Preoperative best-corrected distance visual acuity (CDVA) was considered the better of uncorrected distance visual acuity (UCVA) or CDVA within three months prior to surgery. Postoperative visual acuity was considered CDVA when present, and the better of UCVA or pinhole visual acuity when CDVA was not recorded.
Abbreviations: CDVA best corrected distance visual acuity, UCVA uncorrected distance visual acuity, SD standard deviation, IQR interquartile range.
For 42 eyes with a postoperative refractive aim of emmetropia, we compared the preoperative CDVA with the final postoperative UCVA. The mean preoperative CDVA was 0.55 ± 0.48 LogMAR (median 0.42, IQR: 0.25–0.80) and the mean final postoperative UCVA was 0.43 ± 0.41 LogMAR (median 0.30, IQR: 0.16–0.65) (p = 0.255, Wilcoxon test). While in eyes without ocular copathology, there was no statistically significant difference between preoperative CDVA (mean 0.32 ± 0.26, median 0.25, IQR 0.20–0.50) and the final postoperative UCVA (mean 0.326 ± 0.29, median 0.30, IQR 0.20–0.60) (p = 0.525, Wilcoxon test, n = 23), eyes with ocular copathology achieved a better final postoperative UCVA (mean 0.52 ± 0.52, median 0.50, IQR 0.16–0.80) compared with the preoperative CDVA (mean 0.84 ± 0.54, median 0.64, IQR 0.50–1.00) (p = 0.525, Wilcoxon test, n = 19). The final UCVA was 6/6 or better in 7/42 (16.7%) eyes (7/23, 17.4% without ocular copathology versus 3/19, 15.8% with ocular copathology, p > 0.9999, Fisher’s exact test), 6/9 or better in 15/42 (35.7%) eyes (8/23, 34.8% without ocular copathology versus 7/19, 36.8% with ocular copathology, p > 0.9999, Fisher’s exact test), and 6/12 or better in 22/42 (52.4%) eyes (14/23, 60.9% without ocular copathology versus 8/19, 42.1% with ocular copathology, p = 0.3523, Fisher’s exact test).
Refractive outcomes
Across all eyes, the SE increased from a median of −1.25 D (IQR −1.625, −0.03 D) preoperatively to −0.94 D (IQR −1.63, −0.03 D) postoperatively, but the difference was not statistically significant (p=0.09, Wilcoxon test). There was no significant change in astigmatism between the preoperative (median −1.62 D; IQR −2.50, −1.00 D) and postoperative visit (median −1.63 D; IQR −2.50, −1.00 D) (p = 0.21, Wilcoxon test).
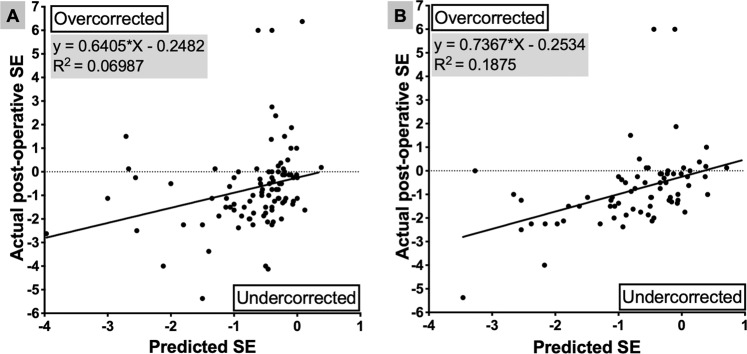
The mean PE for all eyes was +0.001 ± 1.77 D (median PE −0.25 D, IQR: −1.05, +0.48 D). At the final visit, 35 eyes (35%) were within 0.5 D of predicted SE and 52 eyes (53%) were within 1 D of predicted SE; predicted SE or actual postoperative SE was not available for six eyes. The regression analysis plot between the predicted SE and actual postoperative SE is shown in Fig. 1A.
Fig. 1. Comparison between the predicted and achieved refractive outcomes.
Linear regression analysis between the predicted and achieved postoperative spherical equivalent (SE), irrespective of the formula used for intraocular power calculation (n = 96) (A) and if Haigis formula had been used (n = 72) (B).
In 84 eyes (84%), biometry was performed with an IOLMaster (Carl Zeiss Meditec), in 11 eyes (11%) with an ultrasound A scan (Humphrey Instruments, Inc.) and handheld keratometer (Nidek Medical Products, Inc.). There was no significant difference in the PE between eyes using the IOLMaster (+0.03 ± 1.81 D, median −0.28 D, IQR −1.05 to +0.50 D) and manual keratometer (−0.26 ± 1.68 D, median −0.45 D, IQR −1.12 to +0.55 D) (p = 0.75, Mann–Whitney test).
For the eyes with known number of RK incisions, there was no significant difference in the mean PE between 4 cuts (−0.31 ± 0.63 D, median −0.45 D, IQR −0.73 to +0.02 D, n = 10), 8 cuts (−0.06 ± 1.8 D, median −0.27 D, IQR −1.09 to +0.68 D, n = 54), or more than eight cuts (0.94 ± 2.85 D, median 0.23 D, IQR −0.99 to +0.93 D, n = 11) (p = 0.67, Kruskal–Wallis test with Dunn’s multiple comparisons).
The most frequent method for calculating the lens power was the Double-K SRK/T with nomogram adjustment if pre-RK K values were not known (55 eyes), followed by Haigis (19 eyes), SRK/T (18 eyes, for two of which standard Ks were used and were excluded), other (six eyes), and unknown (two eyes). The mean PE was myopic for Haigis (mean −0.36 ± 0.87 D, median −0.31 D, IQR −1.07 to +0.05 D) and Double-K SRK/T (mean −0.38 ± 1.82 D, median −0.55 D, IQR −1.23 to +0.21 D) and hypermetropic for SRK/T (mean +1.32 ± 1.45 D, median +1.10 D, IQR +0.32 to +2.51 D). The PE difference was statistically significant between the Double-K SRK/T and SRK/T, as well as between Haigis and SRK/T, but not between Double-K SRK/T and Haigis or (p = 0.0005, Kruskal–Wallis test with Dunn’s multiple comparisons) (Fig. 2). Eleven out of 19 (57.9%) eyes were within 0.5 D, 14 out of 19 (73.7%) eyes within 1 D, and 19 out of 19 (100%) eyes within 2 D of the predicted SE for Haigis, versus 18 out of 55 (32.7%), 29 out of 55 (52.7%), and 47 out of 55 (85.45%) with Double-K SRK/T, and five out of 16 (31.25%), seven out of 16 (43.75%), and 11 out of 16 (68.75%) for SRK/T.
Fig. 2. Box-and-whisker plot of postoperative prediction error (PE) by the formula used for intraocular lens power calculation.
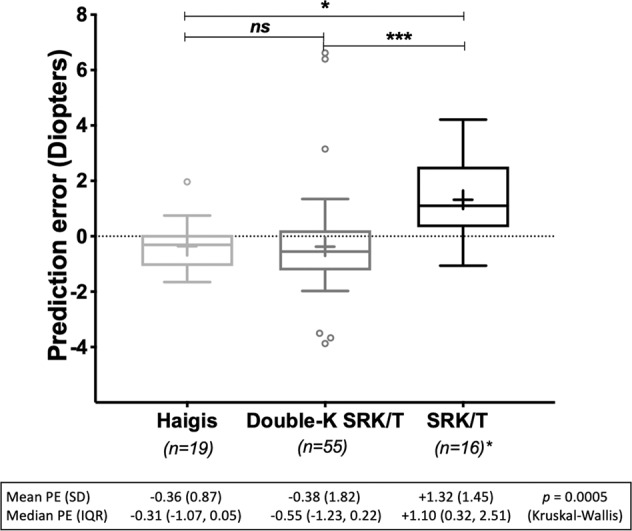
The upper and lower limits of the boxes are the 25th and 75th percentiles, the median is represented by the horizontal line inside the box and the mean as a cross. Outliers are shown as small circles outside the boxes. *The two eyes where standard Ks have been used were excluded from PE calculation. Abbreviations: IQR interquartile range, SD standard deviation.
Four eyes (4%) of three patients underwent further refractive procedure after the cataract surgery: one eye with hypermetropic surprise (+6.375 D) required IOL exchange, one eye required cross-linking and subsequent secondary piggy-back IOL implant due to diurnal fluctuations in vision, and both eyes of one patient underwent laser-assisted subepithelial keratomileusis.
Nine eyes (seven patients) had a prediction error larger than 2.5 D. Seven eyes (five patients) achieved a hypermetropic outcome (PE +2.72 to +6.40 D), – 5/7 had an AL > 30 mm and high corneal astigmatism (−2.0D); double-K SRK/T was used for 3/7, SRKT for 3/7, and online calculator for 1/7. Two eyes achieved a myopic outcome (PE −3.665 and −3.875)—one had AL > 30 mm, both had <2.0 D of astigmatism, and Double-K SRK/T was used for both eyes.
Analysis of predicted error if Haigis formula had been used
As the analysis of refractive outcomes suggests that Haigis formula had a better refraction predictability, we compared the predicted spherical equivalent (SE) and the actual post-op SE as if Haigis had been used instead to select the actual implanted intraocular lens (IOL). In addition to the 19 eyes where Haigis was actually used, a further 53 eyes had complete preoperative biometry to allow calculation of the predicted SE of the same IOL power for Haigis formula, using an online calculator (http://www.eyecalcs.com). When comparing the Haigis-predicted SE to the actual postoperative SE for these 72 eyes, Haigis formula would have resulted in 34 out of 72 (47.2%) eyes within 0.5 D and 51 out of 72 eyes (70.8%) within 1 D and 68 out of 72 (94.4%) within 2 D of predicted refraction. The mean (SD) PE would have been −0.07 D (1.47) and median (IQR) −0.15 D (−0.95, +0.22) if standard Haigis formula had been used. The regression analysis plot between the predicted SE if Haigis had been used and the achieved postoperative SE is shown in Fig. 1B.
Discussion
In this study, we report the outcomes of routine cataract surgery with monofocal IOL in a large series of patients with previous RK in a UK tertiary referral centre experienced in performing cataract surgery in eyes with previous refractive surgery.
Our study shows that cataract surgery in eyes with previous RK results in similar best-corrected visual acuity compared with the UK national benchmarks [14]. In our series the median LogMAR CDVA improved from 0.49 preoperatively to 0.20 at the final postoperative visit in the entire group; when we excluded the eyes with ocular copathology, the median LogMAR CDVA improved from 0.30 preoperatively to 0.06 at the final postoperative visit. The preoperative CDVA in our patients is similar and the postoperative CDVA is one Snellen line equivalent worse than those reported by the Royal College of Ophthalmologists’ National Ophthalmology Database study of cataract surgery (RCOphth NOD) (0.50 preoperatively and 0.10 logMAR postoperatively) [14]. At the final postoperative visit, 36% of eyes achieved a CDVA of 0.00 LogMAR or better and 90% achieved a CDVA of 0.30 LogMAR or better. The percentages of eyes achieving a logMAR CDVA of 0.30 or better compare with those reported by the RCOphth NOD study for both the whole group and eyes without ocular pathology (90% and 96.7% in our study versus 89% and 94% in the RCOphth NOD). However, in our study, fewer eyes achieved a final logMAR CDVA of 0.00 or better compared with the RCOphth NOD study (36% versus 44% for the whole group and 39.3% versus 50% for the eyes without ocular copathology).
As patients who had previous refractive surgery may have higher expectations of achieving spectacle independence, we analyzed the final postoperative UCVA in those eyes who had a postoperative refractive aim of emmetropia. Our results show that significantly fewer eyes without ocular copathology will achieve a UDVA of 0.00 and 0.30 LogMAR compared with the RCOphth NOD Audit (18% versus 27% and 65% versus 81%, respectively). The postoperative refractive error, refraction instability, and irregular astigmatism associated with RK may partially account for the lower number of eyes achieving good unaided visual outcomes.
As RKs induce changes in both anterior and posterior corneal curvature, the main challenges in calculating the IOL power are overestimating the corneal power and accurately predicting the effective lens position (ELP), resulting in postoperative hypermetropia. Numerous methods have been described to overcome these challenges and improve the accuracy of IOL power calculation in eyes with previous RK, with various results [3–6, 8, 15]. While Aramberri double-K method uses the pre-RK corneal power to estimate the ELP and post-RK power to calculate the IOL power [3], Haigis formula allows ELP prediction without taking into account the corneal power [16]. In our study, the most frequent method used for IOL power calculation was the Double-K SRK/T [3] (with nomogram adjustment [17]), followed by standard Haigis and standard SRK/T formulae. Compared with the Double-K SRK/T and Haigis, standard SRK/T resulted in a more hypermetropic outcome than predicted. The mean PE was similar between Haigis and Double-K SRK/T eyes (−0.36 D versus −0.38 D). The Haigis formula in post-RK eyes was adopted in our institution in July 2017, following published evidence that using IOLMaster K readings with standard Haigis formula and aiming for −1.0 D resulted in postoperative mean refraction of −0.66 D, with 69% eyes within 0.50 D of target [15]. Although only used for 19 eyes, its superiority is reinforced by the recalculation of postoperative refractive outcomes in another 53 eyes. Although originally not aimed for eyes with previous refractive surgery, the main advantage of Haigis over other formulae is that it eliminates the need to estimate the corneal power in order to predict the effective lens position, making it more reliable in eyes with flattened corneas post radial keratotomies. Our results are in agreement with a recently published study on 52 eyes with prior RK, showing that Haigis formula aiming for emmetropia achieved similar results with Barrett True-K (No History) in eyes with no previous refractive history (mean predicted error −0.006 versus 0.269, 69.2% eyes within 0.5 D of predicted outcome) [8]. In another study on 44 eyes with previous RK, Haigis formula resulted in a mean error of +0.27 D, with 54.5% of eyes within 0.5 D of the prediction [7].
As RKs induce changes in both the anterior and posterior corneal curvature, including the posterior corneal curvature in the total corneal power and applying it to IOL power calculation formulae has the potential advantage of reducing the keratometric index error. Total keratometric power has been traditionally measured using Scheimpflug technology and more recently with swept-source OCT biometer (IOLMaster 700, Carl Zeiss Meditec AG) with integrated total keratometry. While incorporating total keratometry in the Barrett or Haigis formulae was found to achieve higher prediction accuracy in eyes with no previous refractive surgery [18] or myopic laser-refractive surgery [19], using total keratometry in Haigis formula in eyes with previous RK resulted in worse prediction error compared with standard Haigis or Barrett True K (+0.41 D versus +0.27 D versus +0.33 D) [7].
In order to overcome the challenges derived from the altered anterior:posterior curvature ratio, some authors suggested using central keratometry values measured with a Pentacam rotating Scheimpflug camera [5]. Despite the theoretical advantage, using Pentacam central keratometry for IOL power calculation has not been proven superior to using IOLMaster keratometry, with 40–42% of eyes within 0.50 D of target, and 69–76% within 1.00 D [5, 20].
A recent study on 47 eyes with previous RK showed that intraoperative aberrometry is comparable with Barrett True K (No History) in predicting the refractive outcome in eyes with previous RK and both methods were more reliable in eyes with less than eight cuts compared with eyes with eight or more cuts [21].
Our results indicate that standard Haigis formula aiming for emmetropia achieves better IOL power estimation compared with the traditional Double-K SRK/T, with 73.7% of eyes within 1.0 D in eyes with prior RK and it is a reliable and simple method when refractive history is not available. Although eyes with less than eight cuts show reasonable refraction accuracy and stability after cataract surgery (with only 1/28 eyes outside of ±1.00 D), during the first year after cataract surgery, in eyes with eight or more cuts, stability decreases and the refraction errors are often large (−3.125 to + 4.0 D) [21]. As the hypermetropic shift may continue for years after the cataract surgery, aiming for a slightly myopic outcome (−0.50 D) should be considered, particularly in younger patients.
In addition to variable visual and refractive outcomes, cataract surgery in eyes with previous RK has a higher risk of intraoperative or postoperative complications. Radial corneal incision dehiscence has been previously reported [4, 9–12] and different approaches to wound construction have been proposed in order to reduce the risk [22, 23]. In our study, dehiscence of the RK incision occurred in 8% of the eyes, and in all cases, the main cataract surgery incision was corneal. The width and the location of the corneal incision were determined by the operating surgeon for each case and we presume precautions were taken to minimize the risk of RK wound dehiscence.
The main limitations of our study are its retrospective nature and variability of follow-up. While some patients were followed up for a longer period and had hospital-subjective refraction due to unstable vision, patients who achieved stable vision and had no other ocular copathologies were discharged after a minimum of 14 days postoperatively, reflecting the standard of care in the UK national health system. However, our institution is a national referral centere for complex cataract surgery and patients would have been rereferred if further management was required. Due to the retrospective nature of the study, it was not practical to collect patient-reported outcomes or dependence on spectacles or contact lenses after cataract surgery.
In summary, this study provides real-world visual and refractive outcomes of cataract surgery in eyes with prior RK. Although best-corrected visual acuity compares with standard cataract surgery, significantly fewer eyes with previous RK achieve the level of unaided visual acuity that may allow spectacle independence. Our study also highlights that Haigis formula aiming for emmetropia is reliable for IOL power calculation, and with the advantage of simplicity and universal availability, should be considered in eyes with no refractive history. Although cataract surgery in eyes with previous RK is safe, surgeons must be aware of the increased likelihood of wound dehiscence. These findings are important for preoperative counseling on patient expectations and surgical planning.
Summary
What was known before:
Although cataract surgery is the most common surgery performed in the National Health Service (NHS), there are no real-world data to allow prediction of surgical outcomes in eyes with previous radial keratotomy (RK). Various formulae are being used to calculate the intraocular lens power in eyes with previous RK, but there is no consensus on which is the best to avoid postoperative refractive surprises.
What this study adds:
Cataract surgery in eyes with previous RK results in similar best-corrected visual acuity compared with the UK national benchmarks, but significantly fewer eyes with previous RK achieve the level of unaided visual acuity to allow spectacle independence. Among formulae used for intraocular lens power calculation, standard Haigis aiming for emmetropia has a better predictability of the spherical equivalent and should be considered for IOL calculation in eyes with previous RK.
Acknowledgements
We would like to thank Elyn Fernandez from the Audit Department at Moorfields Eye Hospital for the support in providing the case notes. Presented in part at the The Oxford Ophthalmological Congress, Oxford, UK, July 2019, the European Society of Cataract and Refractive Surgeons (ESCRS), Paris, France, September 2019, and United Kingdom and Ireland Society of Cataract and Refractive Surgeons (UKICRS), Hinckley, UK, October 2019.
Author contributions
All authors contributed to the conception and design of the study and reviewed, critiqued and approved the final version of the paper. CS and DP undertook data collection, analysis and interpretation and drafted the paper. AI reviewed the paper and supervised the execution of the study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang SC, Chen HC. Overview of laser refractive surgery. Chang Gung Med J. 2008;31:237–52. [PubMed] [Google Scholar]
- 2.Moshirfar M, Ostler EM, Smedley JG, Mamalis N, Muthappan V. Age of cataract extraction in post-refractive surgery patients. J Cataract Refract Surg. 2014;40:841–2. doi: 10.1016/j.jcrs.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Aramberri J. Intraocular lens power calculation after corneal refractive surgery: double-K method. J Cataract Refract Surg. 2003;29:2063–8. doi: 10.1016/S0886-3350(03)00957-X. [DOI] [PubMed] [Google Scholar]
- 4.Awwad ST, Dwarakanathan S, Bowman RW, Cavanagh HD, Verity SM, Mootha VV, et al. Intraocular lens power calculation after radial keratotomy: estimating the refractive corneal power. J Cataract Refract Surg. 2007;33:1045–50. doi: 10.1016/j.jcrs.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Potvin R, Hill W. New algorithm for post-radial keratotomy intraocular lens power calculations based on rotating Scheimpflug camera data. J Cataract Refract Surg. 2013;39:358–65. doi: 10.1016/j.jcrs.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Ma JX, Tang M, Wang L, Weikert MP, Huang D, Koch DD. Comparison of newer IOL power calculation methods for eyes with previous radial keratotomy. Invest Ophthalmol Vis Sci. 2016;57:OCT162–8. doi: 10.1167/iovs.15-18948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Spektor T, de Souza RG, Koch DD. Evaluation of total keratometry and its accuracy for intraocular lens power calculation in eyes after corneal refractive surgery. J Cataract Refract Surg. 2019;45:1416–21. doi: 10.1016/j.jcrs.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 8.Turnbull AMJ, Crawford GJ, Barrett GD. Methods for intraocular lens power calculation in cataract surgery after radial keratotomy. Ophthalmology. 2020;127:45–51. doi: 10.1016/j.ophtha.2019.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Budak K, Friedman NJ, Koch DD. Dehiscence of a radial keratotomy incision during clear corneal cataract surgery. J Cataract Refract Surg. 1998;24:278–80. doi: 10.1016/S0886-3350(98)80211-3. [DOI] [PubMed] [Google Scholar]
- 10.Behl S, Kothari K. Rupture of a radial keratotomy incision after 11 years during clear corneal phacoemulsification. J Cataract Refract Surg. 2001;27:1132–4. doi: 10.1016/S0886-3350(01)00763-5. [DOI] [PubMed] [Google Scholar]
- 11.Day A, Seward H. Delayed radial keratotomy dehiscence following uneventful phacoemulsification cataract surgery. Eye. 2007;21:886–7. doi: 10.1038/sj.eye.6702762. [DOI] [PubMed] [Google Scholar]
- 12.Zhang JS, Liu X, Wang JD, Xiong Y, Li J, Li XX, et al. Outcomes of phacoemulsification using different size of clear corneal incision in eyes with previous radial keratotomy. PLoS ONE. 2016;11:e0165474. doi: 10.1371/journal.pone.0165474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai CY, Chang TJ, Kuo LL, Chou P, Woung LC. Visual outcomes and associated risk factors of cataract surgeries in highly myopic Taiwanese. Ophthalmologica. 2008;222:130–5. doi: 10.1159/000112631. [DOI] [PubMed] [Google Scholar]
- 14.Day AC, Donachie PH, Sparrow JM, Johnston RL, Royal College of Ophthalmologists’ National Ophthalmology Database. The Royal College of Ophthalmologists’ National Ophthalmology Database study of cataract surgery: report 1, visual outcomes and complications. Eye. 2015;29:552–60. doi: 10.1038/eye.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geggel HS. Intraocular lens power selection after radial keratotomy: topography, manual, and IOLMaster keratometry results using haigis formulas. Ophthalmology. 2015;122:897–902. doi: 10.1016/j.ophtha.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Haigis W, Lege B, Miller N, Schneider B. Comparison of immersion ultrasound biometry and partial coherence interferometry for intraocular lens calculation according to Haigis. Graefes Arch Clin Exp Ophthalmol. 2000;238:765–73. doi: 10.1007/s004170000188. [DOI] [PubMed] [Google Scholar]
- 17.Koch DD, Wang L. Calculating IOL power in eyes that have had refractive surgery. J Cataract Refract Surg. 2003;29:2039–42. doi: 10.1016/j.jcrs.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Fabian E, Wehner W. Prediction accuracy of total keratometry compared to standard keratometry using different intraocular lens power formulas. J Refract Surg. 2019;35:362–8. doi: 10.3928/1081597X-20190422-02. [DOI] [PubMed] [Google Scholar]
- 19.Yeo TK, Heng WJ, Pek D, Wong J, Fam HB. Accuracy of intraocular lens formulas using total keratometry in eyes with previous myopic laser refractive surgery. Eye. 2020;35:1705–11. [DOI] [PMC free article] [PubMed]
- 20.Turnbull AMJ, Crawford GJ, Barrett GD. Methods for intraocular lens power calculation in cataract surgery after radial keratotomy. Ophthalmology. 2020;127:45–51. [DOI] [PubMed]
- 21.Dawson VJ, Patnaik JL, Ifantides C, Miller DC, Lynch AM, Christopher KL. Comparison of refractive prediction for intraoperative aberrometry and Barrett True K no history formula in cataract surgery patients with prior radial keratotomy. Acta Ophthalmol. 2020. 10.1111/aos.14688. [DOI] [PubMed]
- 22.Jin H, Zhang Q, Zhao P. Modification of the wound construction to prevent dehiscence of radial keratotomy incision in cataract surgery: Wave-shaped scleral incision. J Cataract Refract Surg. 2017;43:449–55. doi: 10.1016/j.jcrs.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Meduri A, Urso M, Signorino GA, Rechichi M, Mazzotta C, Kaufman S. Cataract surgery on post radial keratotomy patients. Int J Ophthalmol. 2017;10:1168–70. doi: 10.18240/ijo.2017.07.23. [DOI] [PMC free article] [PubMed] [Google Scholar]