Abstract
We have reported the rare case of an intermittent endoleak via an aneurysm–venous fistula (AVF). An 89-year-old woman had experienced postoperative sac expansion 6 years after she had undergone endovascular aneurysm repair. During aneurysmorrhaphy, we detected a small AVF, which was the source of the endoleak responsible for the aneurysmal sac expansion. This AVF had a check valve-like mechanism that allowed the inflow of blood from the iliac vein to the sac when the venous pressure exceeded the endotension. Our case has demonstrated the occurrence of an AVF after endovascular aneurysm repair that had resulted in an endoleak that was invisible on imaging studies and the presence of endotension.
Keywords: Aortocaval fistula, Caval pressure, Check valve, Endotension, Sac expansion
An endoleak (EL) after endovascular aneurysm repair (EVAR) has been considered one of the main mechanisms responsible for the increase in the pressure inside the aneurysm.1 Depending on wall compliance, the aneurysmal sac can expand to equilibrate with the retroperitoneal pressure. Current research has focused on arterial ELs, including type II ELs.2 However, few studies have reported the occurrence of an EL from a venous source as a cause of postoperative sac expansion. A few studies have reported the creation of a sac–caval fistula that mitigated the increase in sac pressure and postoperative expansion.3 In the present report, we have described a rare type of intermittent EL via an aneurysm–right common iliac venous fistula, which could not be detected by any preoperative imaging modalities. The patient provided written informed consent for the report of her case details and imaging studies.
Case report
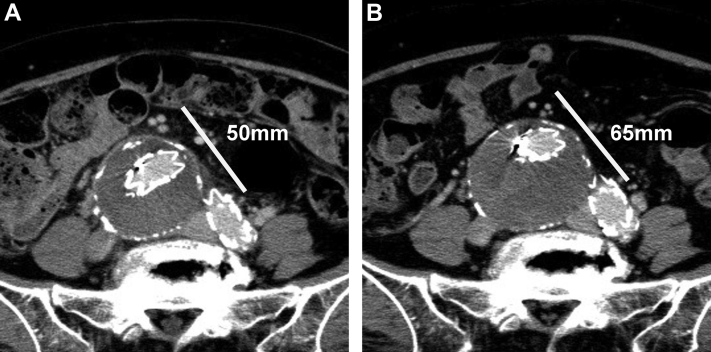
An 83-year-old woman had presented with a 50-mm solitary aneurysm of the right common iliac artery. Despite her relatively advanced age at the index procedure, her preoperative evaluation revealed that she was otherwise healthy. The patient preferred endovascular treatment over an open procedure for the aneurysm and underwent EVAR using the Endurant II stent graft system (Medtronic Endovascular, Santa Rosa, CA). The right hypogastric artery was embolized to obtain a sufficient distal sealing zone in the right external iliac artery. Neither an EL nor aneurysm–venous fistula (AVF) was detected during pre- and periprocedural angiography. Postoperative sac expansion was observed, although contrast-enhanced computed tomography (CT) in the early and delayed phases (Fig 1) and duplex ultrasound imaging after EVAR could not detect the EL. Six years after the EVAR, when the maximal diameter of the sac had exceeded 80 mm, aneurysmorrhaphy was indicated to prevent subsequent rupture. Before open treatment, arterial angiography was performed; however, no form of EL could be identified.
Fig 1.
Delayed phase of contrast-enhanced computed tomography (CT). White line indicates maximal small diameter at 1 week (A) and 3 years (B) after endovascular aneurysm repair (EVAR). No endoleak (EL) was found.
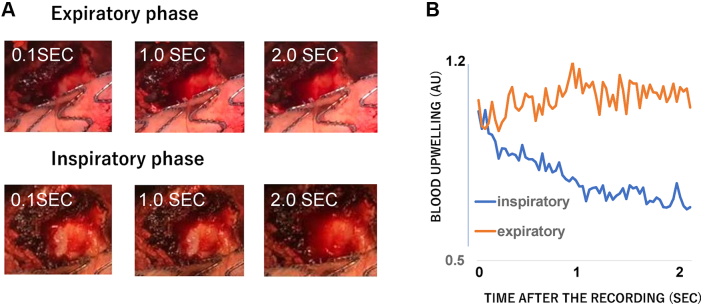
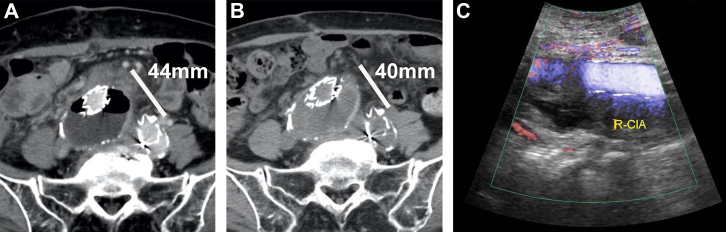
With the patient under general anesthesia, aneurysmorrhaphy was performed using a midline approach. The sac was filled with fresh thrombus and unclotted blood. Exploration revealed a dime-size wall defect in the right inferoposterior aspect of the aneurysm. The anterior wall of the right common iliac vein (CIV) was partially visualized. Venous blood upwelled under the mound around the ulcer-like lesion, where a small AVF was observed. Blood egress was observed during the inspiratory phase (Fig 2). The low pressure on the mound over the AVF was sufficient for control. Because adhesion of the aneurysm wall was too severe for complete exposure of the right CIV, the fistula was closed with 4-0 Prolene suture from inside the aneurysm. The endograft was completely enfolded using the remnant sac and the peritoneum to prevent direct contact with the intestine. The postoperative course was uneventful. Two-phase contrast-enhanced CT on the fifth postoperative day did not detect any EL. At 3 months after aneurysmorrhaphy, noncontrast-enhanced CT did not detect postoperative sac re-expansion. Duplex ultrasound could not detect an EL during any respiratory phases (Fig 3).
Fig 2.
Difference in temporal profiles of blood upwelling speed between inspiratory and expiratory phases. A, Blood was stored inside the dime-size defect in the inspiratory phase. B, The amount of accumulated blood was quantified and expressed as the change in the average gray scale in the observed area compared with that at the beginning of the recording. The region of interest was set at the adventitial defect of the aneurysmal sac. The ordinate indicates the average gray scale change in the region of interest, and the abscissa, the time after the video recording. The decrease in the averaged gray scale indicates the increase in the amount of bleeding. Open-source software (Image J; National Institutes of Health, Bethesda, MD) was used for the analysis.
Fig 3.
A, Delayed phase of contrast-enhanced computed tomography (CT) on fifth postoperative day. Noncontrast-enhanced CT (B) and duplex ultrasound imaging (C) were performed 3 months after aneurysmorrhaphy. No endoleak (EL) was detected in any respiratory phase. The aneurysm showed no evidence of re-expansion. R-CIA, Right common iliac artery.
Discussion
EL is a risk factor for postoperative sac expansion.1 Several studies have reported type II ELs from the inferior mesenteric and lumbar arteries.4 In the present patient, no arterial ELs were detected before or during the procedure. A rare form of EL via an AVF, with a check valve-like mechanism, had served as a source of blood accumulation within the aneurysmal sac.
Aneurysms can erode the walls of the contacting tissues and organs, resulting in enteric or caval fistulas.5 Depending on the amount of shunt flow, caval fistulas can be recognized as symptomatic right heart failure, complicated bleeding during open surgery, or early phase caval or iliac venous contrast filling on aortography during EVAR.6 The AVF in our patient was not observed during the initial EVAR. Before the secondary procedure, a postoperative ultrasound study was performed to identify the common arterial sources of ELs but not to investigate posture-dependent ELs.7
The AVF demonstrated a check valve-like mechanism directing the venous blood into the sac. The peak CIV pressure occurred during the inspiratory phase of mechanical ventilation.8 Because bleeding was observed in the inspiratory phase, the valve threshold (ie, the difference between the intrasaccular pressure and the pressure of the inferior vena cava [IVC]) was lower than the difference between the peak pressure of the IVC and the barometric pressure. Under mechanical ventilation, assuming that the IVC pressure has been equilibrated to the peritoneal pressure, the peak pressure of the IVC will be approximately equal to the peak peritoneal pressure of ∼10 mm Hg.9 Given that the sac was open to barometric pressure, the pressure threshold of the valve would have been <10 mm Hg. If the AVF had been open continuously, it might have resulted in the formation of thromboembolism after dislodgment of the aneurysmal sac debris. No evidence of pulmonary embolism was identified in our patient. This finding also suggested the existence of a check valve-like mechanism.
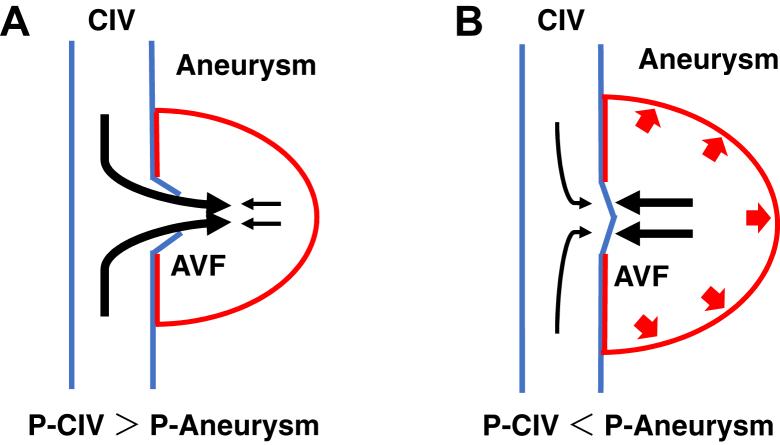
Our patient’s EL had caused sac expansion. The average sac pressure in the absence of an arterial EL will be 20 to 30 mm Hg,10 greater than the CIV pressure in the recumbent position.11 Under these circumstances, this rare form of EL could not have been detected because the valve was closed. However, in a standing position, the hydrostatic CIV pressure will increase to ∼31 mm Hg.12 The CIV pressure might further increase due to certain physiologic behavior. The Valsalva maneuver can increase the CIV pressure to ∼74 mm Hg.11 A previous study showed that the sac pressure without an EL present will correlate positively with the systemic pressure.13 At normal systemic pressure (100-150 mm Hg), the sac pressure will be 20 to 30 mm Hg; thus, the pressure conduction in the sac will not be >20% of the systemic pressure. If the Valsalva maneuver increases the systemic pressure ≤200 mm Hg, the sac pressure can be approximately less than 40 mm Hg. Therefore, the pressure difference between the CIV and the sac must have transiently exceeded this threshold. An acute increase in CIV pressure resulted in an EL from the vein and increased intrasaccular pressure. After valve closure, the sac expanded until the pressure had reached equilibrium with its surroundings (Fig 4). As explained, an AVF might serve, not only as a depressor, but also as a pressor.3
Fig 4.
Scheme of intermittent endoleak (EL). A, Increased common iliac vein (CIV) pressure opened the valve, leading to an increase in intrasaccular pressure. B, When the aneurysm–venous fistula (AVF) closed, the sac expanded until the intrasaccular pressure was in equilibrium with that in its surroundings. P-Aneurysm, Pressure of aneurysm; P-CIV, pressure of common iliac vein.
Even retrospectively, the EL of our patient was difficult to visualize. First, the delicate pressure balance between the aneurysm and CIV must be reproduced. In addition, the imaging modality must be able to detect the small volume of blood inflow from the CIV to the aneurysm. Contrast-enhanced duplex ultrasound or cavography might be a reasonable option for visualization. Ultrasound has the advantage of modulating the pressure balance; however, a small amount of EL might be challenging to detect, even with contrast material. Cavography has the advantage of detecting a small EL if the contrast agent has been injected from inside the CIV and close to the AVF. However, difficulties can be encountered when reproducing the pressure balance owing to positional limitations. If the EL could be detected preoperatively, liquid embolization of the aneurysm, using n-butyl cyanoacrylate (Histoacryl; B. Braun, AG, Melsungen, Germany) or an ethylene–vinyl alcohol copolymer liquid embolic system (Onyx; Covidien, Irvine, CA) might be able to close the valve permanently. The deployment of a covered stent inside the CIV could be another endovascular option. However, liquid embolization requires invasive and complicated procedures. In addition, the iliac–venous deployment of stent grafts appears to have a non-negligible risk of obstruction and migration.
Conclusions
We have described a post-EVAR AVF that served as an intermittent EL. Such an AVF can increase the endotension via a check valve-like mechanism. In addition, an intermittent posture-dependent EL can cause postoperative sac expansion. Although difficult to detect using preoperative imaging studies, postoperative AVF formation can serve as a source of occult EL and endotension.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Jones J.E., Atkins M.D., Brewster D.C., Chung T.K., Kwolek C.J., LaMuraglia G.M., et al. Persistent type 2 endoleak after endovascular repair of abdominal aortic aneurysm is associated with adverse late outcomes. J Vasc Surg. 2007;46:1–8. doi: 10.1016/j.jvs.2007.02.073. [DOI] [PubMed] [Google Scholar]
- 2.Ward T.J., Cohen S., Patel R.S., Kim E., Fischman A.M., Nowakowski F.S., et al. Anatomic risk factors for type-2 endoleak following EVAR: a retrospective review of preoperative CT angiography in 326 patients. Cardiovasc Intervent Radiol. 2014;37:324–328. doi: 10.1007/s00270-013-0646-7. [DOI] [PubMed] [Google Scholar]
- 3.van de Luijtgaarden K.M., Bastos Gonçalves F., Rouwet E.V., Hendriks J.M., Ten Raa S., Verhagen H.J. Conservative management of persistent aortocaval fistula after endovascular aortic repair. J Vasc Surg. 2013;58:1080–1083. doi: 10.1016/j.jvs.2012.10.138. [DOI] [PubMed] [Google Scholar]
- 4.Lalys F., Durrmann V., Duménil A., Göksu C., Cardon A., Clochard E., et al. Systematic review and meta-analysis of preoperative risk factors of type II endoleaks after endovascular aneurysm repair. Ann Vasc Surg. 2017;41:284–293. doi: 10.1016/j.avsg.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Chenu C., Marcheix B., Barcelo C., Rousseau H. Aorto-enteric fistula after endovascular abdominal aortic aneurysm repair: case report and review. Eur J Vasc Endovasc Surg. 2009;37:401–406. doi: 10.1016/j.ejvs.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 6.Cinara I.S., Davidovic L.B., Kostic D.M., Cvetkovic S.D., Jakoyljevic N.S., Koncar I.B. Aorto-caval fistulas: a review of eighteen years experience. Acta Chir Belg. 2005;105:616–620. doi: 10.1080/00015458.2005.11679788. [DOI] [PubMed] [Google Scholar]
- 7.May J., Harris J.P. Intermittent, posture-dependent, and late endoleaks after endovascular aortic aneurysm repair. Semin Vasc Surg. 2012;25:167–173. doi: 10.1053/j.semvascsurg.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Michard F. Changes in arterial pressure during mechanical ventilation. Anesthesiology. 2005;103:419–428. doi: 10.1097/00000542-200508000-00026. [DOI] [PubMed] [Google Scholar]
- 9.Langer T., Santini A., Bottino N., Crotti S., Batchinsky A.I., Pesenti A., et al. “Awake” extracorporeal membrane oxygenation (ECMO): pathophysiology, technical considerations, and clinical pioneering. Crit Care. 2016;20:150. doi: 10.1186/s13054-016-1329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baum R.A., Carpenter J.P., Cope C., Golden M.A., Velazquez O.M., Neschis D.G., et al. Aneurysm sac pressure measurements after endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2001;33:32–41. doi: 10.1067/mva.2001.111807. [DOI] [PubMed] [Google Scholar]
- 11.Laborda A., Sierre S., Malvé M., De Blas I., Ioakeim I., Kuo W.T., et al. Influence of breathing movements and Valsalva maneuver on vena caval dynamics. World J Radiol. 2014;6:833–839. doi: 10.4329/wjr.v6.i10.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meissner M.H., Moneta G., Burnand K., Gloviczki P., Lohr J.M., Lurie F., et al. The hemodynamics and diagnosis of venous disease. J Vasc Surg. 2007;46(Suppl S):4S–24S. doi: 10.1016/j.jvs.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 13.Gawenda M., Knez P., Winter S., Jaschke G., Wassmer G., Schmitz-Rixen T., et al. Endotension is influenced by wall compliance in a latex aneurysm model. Eur J Vasc Endovasc Surg. 2004;27:45–50. doi: 10.1016/j.ejvs.2003.10.013. [DOI] [PubMed] [Google Scholar]