Abstract
Background
In patients with septic shock, the impact of the mean arterial pressure (MAP) target on the course of mottling remains uncertain. In this post hoc analysis of the SEPSISPAM trial, we investigated whether a low-MAP (65 to 70 mmHg) or a high-MAP target (80 to 85 mmHg) would affect the course of mottling and arterial lactate in patients with septic shock.
Methods
The presence of mottling was assessed every 2 h from 2 h after inclusion to catecholamine weaning. We compared mottling and lactate time course between the two MAP target groups. We evaluated the patient’s outcome according to the presence or absence of mottling.
Results
We included 747 patients, 374 were assigned to the low-MAP group and 373 to the high-MAP group. There was no difference in mottling and lactate evolution during the first 24 h between the two MAP groups. After adjustment for MAP and confounding factors, the presence of mottling ≥ 6 h during the first 24 h was associated with a significantly higher risk of death at day 28 and 90. Patients without mottling or with mottling < 6 h and lactate ≥ 2 mmol/L have a higher probability of survival than those with mottling ≥ 6 h and lactate < 2 mmol/L.
Conclusion
Compared with low MAP target, higher MAP target did not alter mottling and lactate course. Mottling lasting for more than 6 h was associated with higher mortality. Compared to arterial lactate, mottling duration appears to be a better marker of mortality.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13613-022-01053-1.
Keywords: Arterial lactate, Lactate clearance, Mean arterial pressure, Microcirculation, Mottling, Septic shock
Background
Septic shock is characterized by macro- and micro-circulatory impairments related to an infection. This condition is associated with a high mortality rate, above 40% according to the SEPSIS-3 criteria [1].
The microcirculatory impairment is a key pathophysiological point and results from several mechanisms (endothelial dysfunction, impaired inter-cell communication, altered glycocalyx, adhesion and rolling of white blood cells as well as platelets, and altered red blood cell deformability) [2]. The persistence of microcirculation dysfunctions during septic shock, assessed by sublingual microvascular damage, is associated with a higher mortality [3].
Both mottling and arterial lactate have been validated as prognostic markers of mortality [1, 4, 5]. Mottling can be easily assessed at the bedside [5, 6]. Its severity as quantified using the mottling score (evaluated 6 h after admission to the intensive care unit), is strongly related to 14-day and 28-day mortality [4, 7, 8]. Moreover, an improvement in mottling score and a shorter duration of mottling are associated with a higher survival at day 28 [4, 8, 9]. Arterial lactate is considered as the mirror of tissue hypoxia and has been validated as a prognostic marker of mortality [10–12].
During septic shock, a dissociation between macrocirculatory hemodynamics and microcirculatory alterations has been reported [13]. Indeed, some patients experience a multiple organ failure with a persistence of microcirculation dysfunctions despite an improvement in systemic hemodynamics [9]. To date, only a few studies involving a small number of patients have assessed the impact of mean arterial pressure (MAP) target on microcirculation alterations, with conflicting results and heterogeneities in the timing of interventions [14–16].
In the SEPSISPAM trial [17], a high-MAP target (80 to 85 mmHg) was compared to a low-MAP target (65 to 70 mmHg), determined at the early phase of septic shock (i.e. within the 6 h following vasopressor introduction). In this post hoc analysis, we investigated whether, in patients with septic shock, different MAP targets may impact the time course of skin mottling, considered as an acceptable surrogate of microcirculation status and/or of arterial lactate normalization, considered as a marker of tissue hypoxia.
Materials and methods
Patient selection
In the randomized SEPSISPAM trial [17], patients were enrolled from 29 centers in France. Randomization was performed with the use of a computer-generated assignment sequence in a centralized, blinded fashion and was stratified according to whether patients had chronic arterial hypertension (i.e., had been receiving antihypertensive treatment or had a history of chronic arterial hypertension). Patients older than 18 years of age were enrolled if they had septic shock (according to SEPSIS-2 definition), if they required vasopressors at a minimum infusion rate of 0.1 µg per kilogram per minute, and if they were evaluated within 6 h after the initiation of vasopressor. After enrollment, patients were assigned to vasopressor treatment that was adjusted to maintain a MAP of 80 to 85 mmHg (high-MAP target group) or 65 to 70 mmHg (low-MAP target group). The MAP target was maintained for a maximum of 5 days or until the patient was weaned from vasopressor support.
For this post hoc analysis of the SEPSISPAM trial [17], we included patients with at least one available data regarding mottling. Data that concerned mottling were considered until the discontinuation of catecholamine or for a maximum of 5 days under vasopressors.
Data collection
In the SEPSISPAM trial, the presence or absence of mottling was assessed by nurses every two hours, from 2 h after the inclusion to the 5 first days or to the discontinuation of catecholamine. In patients weaned from catecholamine for the 5 first days, the presence or absence of mottling was assessed up to 12 h after norepinephrine weaning. Skin mottling was defined as a red-violaceous discoloration of skin on the knee or above it. No information was collected regarding mottling intensity (mottling score). For each patient, we calculated the duration of mottling over the first 24 h after inclusion.
Arterial lactate levels were measured at inclusion, 6 and 12 h after inclusion and once daily thereafter. An arterial lactate level lower than 2 mmol/L was considered as normal.
Outcomes
The primary outcome was the mottling duration for the first 24 h after inclusion. Secondary outcomes were the time to normalization of arterial lactate and mortality at day 28 and day 90.
Ethical concerns
The SEPSISPAM trial (NCT01149278) was approved for all participating centers by the ethic committee at the Angers University Hospital. Written inform consent was obtained from all patients, their next of kin or another surrogate decision-maker, as appropriate. In accordance with ethics policy, if patients were unable to provide informed consent and the next of kin or a designated person was not available, the emergency inclusion procedure was applied and post hoc consent was obtained from patients who survived.
Statistical analysis
Quantitative variables, presented as median [interquartile range] were compared with Mann–Whitney test. Qualitative variables, presented as the absolute value [percentage] were compared with Fisher’s exact test. Survival curves were computed by the Kaplan–Meier method and compared by the log-rank test. First, only available data were analyzed, then, we re-analyzed the dataset of mottling, dealing with missing data, assuming that when mottling state was similar around missing data, the mottling state for the missing data was the same (last observation carried forward: LOCF). For instance, if the patient has mottling at H2 and H6, and the data were missing at H4, then the patient was considered with mottling at H4.
A mixed model was used to explore the evolution of MAP during the 5-day study period according to the MAP target.
A mixed effect logistic regression was used to explore the relation between time course of mottling, MAP target and history of chronic arterial hypertension.
Cox regression models were computed to explore the relation between mottling and mortality at day 90. In the first step, univariate analyses were conducted separately for each inclusion characteristic. In the second step, multivariate Cox regression models were built using variables with p-value < 0.05 in the univariate analyses: age, SAPS II, history of ischemic heart disease and of chronic arterial hypertension, source of infection, MAP, lactate, fluid intake, vasopressor dose and need for mechanical ventilation at inclusion. We add the randomization group in the model. We did not include the pH, SOFA, acute kidney injury or PaO2/FiO2 ratio because of their redundancy with other variables. Results were presented as hazard ratio (HR) with 95% confidence intervals (95% CI).
Statistical analysis was performed using Prism GraphPad Software v3.9.1 (San Diego, California) and R (R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/) v3.6.2. All tests were two-sided, and p-values below 0.05 were considered as statistically significant.
Results
Study population
Thirty-three patients were excluded because of missing data regarding mottling. Seven hundred and forty-seven patients were thus included in the analysis. Among them, 374 and 373 patients were assigned to the low-MAP target group and the high-MAP target group, respectively (Additional file 2: Fig. S1). Baseline characteristics and mortality at day 90 were similar in the low and high-MAP target groups (Additional file 1: Table S1). MAP values measured during the first 5 days were significantly lower in the low-MAP target group than in the high-target MAP group (p < 0.001, Additional file 2: Fig. S2).
Patients in the high-MAP target group received higher doses of norepinephrine during the first 5 days than patients in the low-MAP target group. Fluid intake was similar in both groups (Additional file 1: Table S2).
Among the 747 analyzed patients, 296 (40%) experienced mottling for at least 2 h during the first 24 h after inclusion. The median duration of mottling during the first 24 h after inclusion was 14 [1, 6–22] h. Among patients with mottling, 235 (79.4%) had mottling that lasted more than 6 h.
The whole mottling monitoring (i.e. every 2 h in patients with catecholamines infusion) represented 21,544 timepoints. Data were missing at 610 timepoints (2.8% of the entire dataset), and, after extrapolation with the LOCF procedure, only 40 timepoints data remained missing (0.2% of the dataset).
As presented in Table 1, disease severity was higher in patients with mottling during the first 24 h than in those without mottling (higher Simplified Acute Physiology Score II (SAPS II) [63 (50–77) vs. 53 (43–63), p < 0.0001] and higher Sequential Organ Failure Assessment (SOFA) scores [11 (9–13) vs. 10 (8–12), p < 0.0001]). Patients with mottling had more comorbidities and worse hemodynamic parameters at baseline (MAP, heart rate, arterial lactate). Patients with mottling required higher levels of vasopressor and had higher mortality at day 28 and day 90 (Additional file 1: Table S3).
Table 1.
Baseline characteristics of study patients according to the presence or absence of mottling at baseline or during the first 24 h
| Characteristic | No mottling n = 451 |
Mottling n = 296 |
p-value |
|---|---|---|---|
| Age—years | 65 [54–75] | 69 [58–78] | 0.002 |
| Male sex | 302 [67] | 201 [67] | 0.81 |
| SAPS II | 53 [43–63] | 63 [50–77] | < 0.0001 |
| SOFA | 10 [8–12] | 11 [9–13] | < 0.0001 |
| Pre-existing condition—no (%) | |||
| Ischemic heart disease | 35 [8] | 43 [14] | 0.003 |
| Chronic heart failure | 55 [12] | 53 [18] | 0.03 |
| COPD | 63 [14] | 39 [13] | 0.83 |
| Chronic kidney disease | 49 [11] | 42 [14] | 0.21 |
| Cirrhosis | 29 [6] | 23 [8] | 0.56 |
| Chronic arterial hypertension | 208 [46] | 166 [57] | 0.008 |
| Cancer or autoimmune disease | 167 [37] | 97 [33] | 0.24 |
| Source of infection—no (%) | |||
| Lung | 252 [56] | 136 [46] | 0.009 |
| Abdomen | 60 [13] | 66 [22] | 0.001 |
| Urinary tract | 59 [13] | 26 [9] | 0.08 |
| Others | 80 [18] | 68 [23] | 0.09 |
| Community-acquired infection—no (%) | 296 [66] | 199 [67] | 0.69 |
| Hemodynamic and biochemical variable | |||
| Mean arterial pressure -mmHg | 74 [65–83] | 71 [61–81] | 0.01 |
| Heart rate—beats/min | 100 [81–117] | 107 [88–126] | < 0.0001 |
| Arterial pH | 7.34 [7.28–7.39] | 7 .25 [7.15–7.35] | < 0.0001 |
| Serum lactate level—mmol/L | 2 [1.3–3.1] | 3.2 [1.8–5.4] | < 0.0001 |
| Fluid therapy before inclusion—mL | 2500 [2000–3250] | 3000 [2500–4000] | < 0.0001 |
| Vasoactive drug infusions at randomization—no (%) | |||
| Norepinephrine | 434 [96] | 274 [93] | 0.04 |
| Dobutamine | 21 [5] | 20 [7] | 0.25 |
| Epinephrine | 18 [4] | 36 [12] | 0.0004 |
| Median vasopressor dose at randomization—µg/kg/min | |||
| Norepinephrine | 0.32 [0.2–0.52] | 0.44 [0.22–0.84] | 0.0004 |
| Epinephrine | 0.34 [0.16–0.57] | 0.21 [0.14–0.5] | 0.35 |
| Mechanical ventilation—no (%) | 317 [70] | 253 [85] | < 0.0001 |
| PaO2/FiO2 ratio -mmHg | 178 [122–267] | 158 [90–245] | 0.0098 |
| Acute kidney injury—no (%) | 175 [39] | 169 [57] | < 0.0001 |
Patients in the “no mottling” group had no mottling during the first 24 h of septic shock. Patients in the mottling group had mottling for at least 2 h during the first 24 h of septic shock
COPD: chronic obstructive pulmonary disease
Values are represented as median [interquartile range]. The target mean arterial pressure was 80 to 85 mmHg in the high-target group and 65 to 70 mmHg in the low-target group
The Simplified Acute Physiology Score (SAPS) II is based on 17 variables and scores range from 0 to 163, with a higher score indicating a more severe disease
The score on the Sequential Organ Failure Assessment (SOFA) includes sub-scores ranging from 0 to 4 for each of five components (circulation, lungs, liver, kidneys and coagulation). Aggregated scores range from 0 to 20, with higher scores indicating more severe organ failure
Others sources of infection included blood, soft tissue, skin, central venous system, bones and joints, cardiac system, reproductive organs and unknown sources
Acute kidney injury was defined as a renal SOFA score of 2 or more (plasma creatinine level > 1.9 mg/dL (168 µmol/L) or urinary output, < 500 mL per day)
Patients with mottling had higher arterial lactate levels at inclusion than patients without mottling [3.2 (1.8–5.4) vs. 2.0 (1.3–3.1), p < 0.0001], without difference between MAP-target groups (Additional file 2: Fig. S3). The duration of mottling during the first 24 h was higher in patients with an arterial lactate level ≥ 2 mmol/L at inclusion than in patients with an arterial lactate level < 2 mmol/L [[0.0 (0.0–14.0) h] vs 0.0 (0.0–2.0) h, p < 0.0001]. The time required to normalize arterial lactate was shorter in patients without mottling than in those with mottling (p < 0.0001, Additional file 2: Fig. S4).
Impact of MAP target on mottling and arterial lactate
There was no difference in mottling duration during the first 24 h between low and high-MAP target groups (Fig. 1), nor in the proportion of patients with mottling while under vasopressors according to MAP target (Fig. 2, Additional file 1: Table S4). Among the 747 patients, 519 were weaned from catecholamines during the first 5 days. There was no difference in mottling duration after weaning of norepinephrine between low [0 (0–0) h) and High-MAP target group [0 (0–0) h, p value = 0.68] (Additional file 1: Table S4) and mottling during period of weaning was observed only in 21 patients in the low-MAP target group and in 16 patients in the high-MAP target group (p value = 0.74). In both groups of patients with catecholamines and weaned from catecholamines, there was no difference in the proportion of patients with or without mottling at H24, H48 and H72 of the inclusion according to the MAP target (Additional file 1: Table S4).
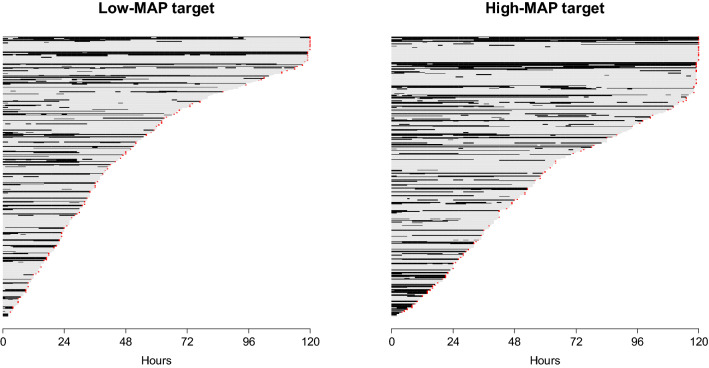
Fig. 1.
Course of mottling in patients with septic shock, according to the mean arterial pressure (MAP) target. Low-MAP target group: 65–70 mmHg, high-MAP target group: 80–85 mmHg. Each horizontal line represents a patient follow-up. The length of the line represents the length of observation time of the patient. Black line corresponds to a period of time with mottling; and grey line corresponds to a period of time without mottling for the patient. There was no difference in mottling time course according to the MAP target. A red asterisk at the end of a line represents the death of the patient
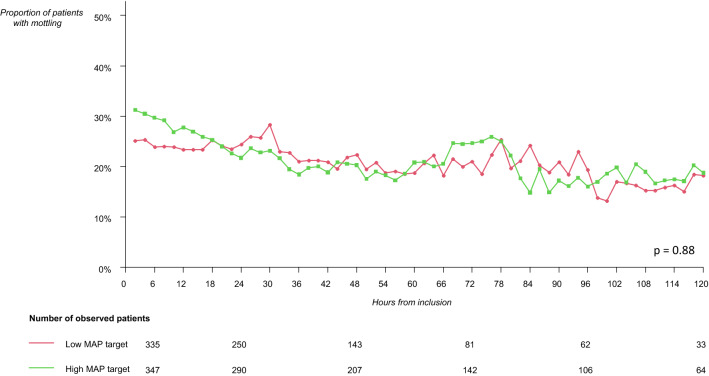
Fig. 2.
Evolution of the proportion of patients with mottling according to the mean arterial pressure (MAP) target during septic shock. Low-MAP target group: 65–70 mmHg, high-MAP target group: 80–85 mmHg. This figure represents the proportion of patients with mottling (number of patients with mottling out of the number of patients observed) at each time. There was no difference in the evolution of proportion of patients with mottling during septic shock according to the MAP target (Wilcoxon test: p = 0.88)
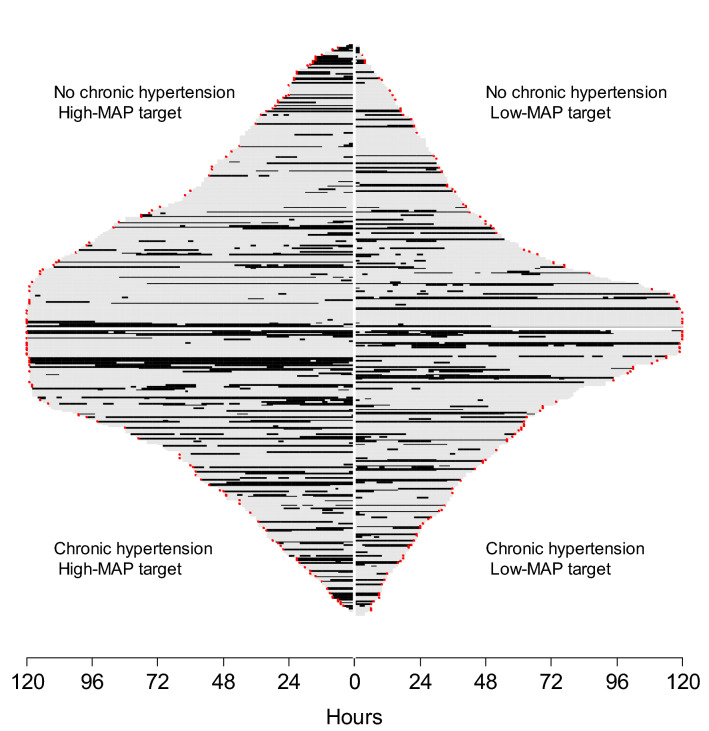
Furthermore, no difference was observed in the duration of mottling according to the history of chronic arterial hypertension and MAP target during the first 24 h (Fig. 3). Indeed there was no significant association between the probability of observing mottling according to the MAP target (p = 0.369) and to the history of chronic arterial hypertension (p = 0.144). Our results were similar when considering only patients who met the criteria of the SEPSIS-3 definition of septic shock (Additional file 2: Fig. S5) and when considering mottling with the LOCF procedure (Additional file 2: Figs. S6 and S7).
Fig. 3.
Course of mottling in patients with septic shock, according to the mean arterial pressure (MAP) target and chronical arterial hypertension. Low-MAP target group: 65–70 mmHg, High-MAP target group: 80–85 mmHg. Each horizontal line represents a patient follow-up. Solid line corresponds to a period of time with mottling; and hatched line corresponds to a period of time without mottling for the patient. A red asterisk at the end of a line represents the death of the patient. There was no difference in mottling time course according to the MAP target and chronic arterial hypertension
In addition, in patients with elevated arterial lactate at inclusion (according to the SEPSIS-3 definition [1]), there was no difference in the time course changes of arterial lactate concentrations between the two MAP-target groups (Additional file 2: Fig. S8).
Association between mottling and mortality
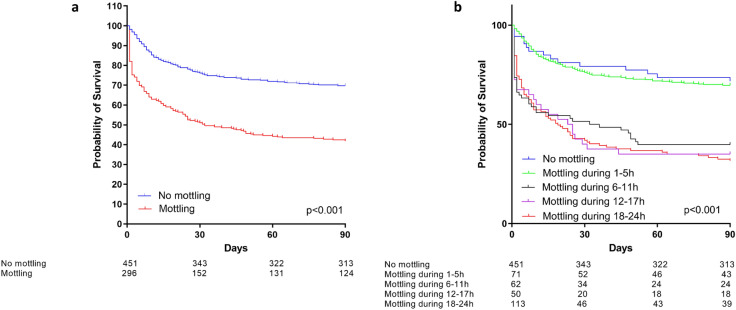
The presence of mottling within the first 24 h after inclusion was associated with a significant increase in the risk of death at day 90 (log-rank test p < 0.001) (Fig. 4a). Similar results were found when considering only patients who met the criteria of the SEPSIS-3 definition of septic shock [1].
Fig. 4.
Survival according to the presence (mottling) or absence of mottling (no mottling) during the first 24 h after inclusion (a) and according to the duration of mottling (b). a p-value refers to the difference between patients with mottling and those without. Log rank test: p < 0.0001. b p-value refers to the difference between each subgroup of mottling duration
When compared to patients with mottling lasting for more than 6 h, patients without mottling or with mottling lasting for less than 6 h had a lower risk of death at day 90 (Fig. 4b and Table 2). In Table 2, the adjustment of the multivariate analysis was performed on the MAP target and on differences between patients at baseline (age, SAPS II, history of chronic arterial hypertension or of myocardial infarction, source of infection and MAP, fluid intake, vasopressor dose and need for mechanical ventilation at inclusion).
Table 2.
Univariate and multivariate analysis evaluating the impact of the course of mottling on day 90 mortality in septic shock
| Duration of mottling within the first 24 h | Univariate analysis n = 747 |
Multivariate analysis† n = 656 |
||
|---|---|---|---|---|
| HR | p-value | HR | p-value | |
| No mottling (reference) | 1 | – | 1 | – |
| 1–5 h | 1.24 [0.79–1.92] | 0.35 | 1.03 [0.62–1.72] | 0.91 |
| 6–24 h | 3.32 [2.64–4.18] | < 0.001 | 2.14 [1.63–2.81] | < 0.001 |
| 1–5 h (reference) | 1 | – | 1 | – |
| 6–24 h | 2.69 [1.73–4.17] | < 0.001 | 2.08 [1.25–3.46] | 0.005 |
†Multivariate analysis based on the randomization group (low-MAP or high-MAP target), lactate at inclusion, age, SAPS II, history of chronic arterial hypertension, history of ischemic heart disease, and MAP, fluid intake, vasopressor dose and need for mechanical ventilation at inclusion. We did not include the pH, SOFA, acute kidney injury or PaO2/FiO2 ratio for competing with other variables. Ninety-one patients were excluded because of missing data on at least 1 adjustment variable
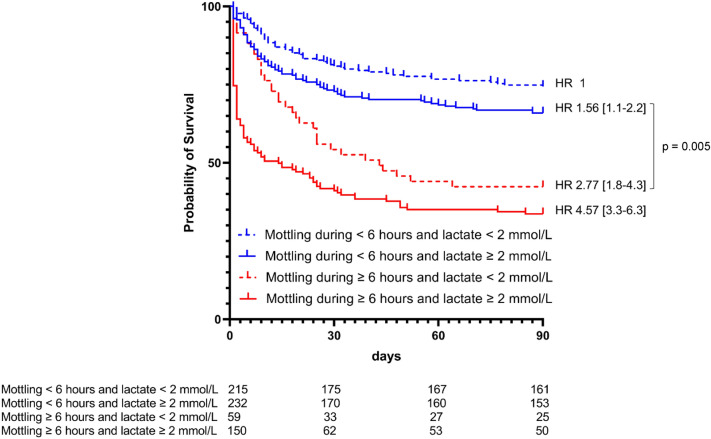
Figure 5 illustrates the respective weight of mottling duration and arterial lactate level on mortality: patients without mottling or mottling lasting for less than 6 h and with arterial lactate ≥ 2 mmol/L have a higher probability of survival as compared with patients with mottling lasting for more than 6 h and arterial lactate < 2 mmol/L (p = 0.005). Indeed, 153 of the 232 patients without mottling or mottling lasting for less than 6 h and with arterial lactate ≥ 2 mmol/L were alive at day 90 as compared to 25 of the 59 patients with mottling lasting during for more than 6 h and arterial lactate < 2 mmol/L.
Fig. 5.
Survival according to mottling duration during the first 24 h and arterial lactate at inclusion. Kaplan–Meier curves represent the survival according to the duration of mottling and the arterial lactate level. Hazard ratios (HR) were calculated using a Cox model. We defined the group of patients with mottling duration < 6 h during the first 24 h of septic shock and lactate < 2 mmol/L at inclusion as the reference. HR were adjusted on randomization group, age, SAPS II, past medical history (chronic arterial hypertension, myocardial infarction,) and MAP, lactate, fluid intake, vasopressor dose at inclusion and need for mechanical ventilation at inclusion. Patients without mottling or mottling lasting for less than 6 h and with arterial lactate ≥ 2 mmol/L had a higher probability of survival than patients with mottling lasting for ≥ 6 h and arterial lactate < 2 mmol/L (p = 0.005)
Discussion
In this post hoc analysis of the SEPSISPAM trial, the course of mottling was not found to be associated with the MAP target level (low-MAP versus high-MAP target) or with the time course of arterial lactate levels. In addition, we confirmed the strong relationship between mottling duration ≥ 6 h and mortality. Moreover, the multivariate analysis showed that the presence of mottling during the first 24 h was more strongly associated with mortality than the arterial lactate level.
In patients with septic shock, increasing MAP target in patients with chronic arterial hypertension may reduce the need for renal replacement therapy [17]. However, the increase in doses of norepinephrine to achieve a higher MAP target may also increase the risk of tissue ischemia [18]. Our results show that increasing MAP in patients with septic shock is safe regarding microcirculation.
Microcirculation impairment and its dissociation from macrocirculatory hemodynamics is one of the cornerstones of the pathophysiology of septic shock [3]. Results of previous studies regarding the effect of MAP level on microcirculation are conflicting. Small sample size studies reported an improvement of the sublingual or cutaneous microcirculation with an increase in MAP target [14, 19, 20], while others did not identify any difference between two levels of MAP [16]. Dubin et al. reported a large variability in microvascular responses with different levels of MAP [16]. Our study provides new information on the dissociation between micro- and macro-circulation during septic shock [3], as we found no difference in mottling course and arterial lactate normalization between the two MAP target groups in a large number of patients, with a homogeneous definition of septic shock (according to the SEPSIS-2 definition) and a strong external validity (mortality rate) [21].
Of note, the design of the SEPSISPAM trial and our post hoc analysis did not allow to explain the pathophysiological impairment of microcirculatory blood flow. Whether mottling is more than a microcirculation surrogate and may convey a broader array of underlying mechanisms (adrenergia, endothelial dysfunction, microthrombosis, microcirculatory low flow, etc.) remains unanswered.
Beyond its interest as an acceptable marker of microcirculation impairment, mottling has already been reported to be a marker of mortality [4, 7]. Ait-oufella et al. described the association between mottling and its intensity (through a semi-quantitative approach, namely the mottling score) and worse outcomes in 60 patients [4]. In addition, mottling duration (more than 6 h despite adequate resuscitation) was reported to be predictive of death at day 14 [4, 9] and day 28 [7, 8] in heterogeneous populations. Similar results were found in sepsis [9, 22].
Coudroy et al. [8] also reported a higher mortality of ICU patients with mottling lasting more than 6 h. In this study, only 65 out of 791 patients had, however, been referred to ICU for septic shock [8]. Our results confirm the poor outcomes of patients with mottling in a well-defined population of septic shock according to SEPSIS-2 [23] and SEPSIS-3 definitions [1]. Furthermore, we confirm on a larger scale that patients with mottling lasting less than 6 h have the same outcomes than those without mottling.
Our study shows a higher mortality in patients with mottling lasting for more than 6 h without hyperlactatemia as compared to patients without mottling and with arterial lactate ≥ 2 mmol/L at inclusion. These results are consistent with the analysis of Ait-oufella et al. [4] where the mottling score was a stronger predictor of mortality than arterial lactate. These results were confirmed by Dumas et al. [9]. The better ability of mottling to predict death could be explained by the fact that arterial lactate is the result of a balance between peripheral tissue production and several pathogenic mechanisms (stress-related adrenergic-induced glycolysis, impaired hepatic lactate clearance) [24, 25], whereas skin mottling is probably a more direct assessment of microcirculatory impairment. However, skin mottling is not the only marker of microcirculation impairment. Brunauer et al. reported that mottling intensity was less correlated to visceral organ pulsatility indices than capillary refill time [26].
Our study has several limitations. First of all, the post hoc design is well known for its biases. Second, the data regarding mottling collected in the SEPSISPAM trial consisted only of the presence or absence of mottling, without information on the intensity and extent of mottling. Such information, namely the mottling score [4], could have been of interest in our study, to better assess whether MAP target may have an impact on mottling score intensity. However, discriminating patients with mottling from those without decreases the risk of interobserver variability and increases the robustness of mottling assessment. Third, as in the SEPSISPAM trial, achieved MAP was higher than targeted MAP, although there was still a significant difference in MAP between the low-target and high-target MAP groups. Finally, we acknowledge that a delayed impact of higher vasopressor dose could have been missed due to the end of the monitoring of mottling. There was no difference between duration of mottling after weaning of norepinephrine in both groups as mottling was assessed up to 12 h after norepinephrine weaning. As the half-life of norepinephrine is short, a persistent late effect seems unlikely.
Conclusion
This study shows that in patients with septic shock, a MAP target between 80 and 85 mmHg, achieved through increased vasopressor doses, did not alter the course of mottling nor lactate normalization. This study confirms that the presence of mottling lasting for ≥ 6 h was associated with a higher mortality at day 28 and 90 in these patients. In addition, compared to the arterial lactate level at inclusion, mottling duration appears to be a stronger marker of mortality risk. Our results suggest that a deeper pathophysiological understanding of mottling is still pending.
Supplementary Information
Abbreviations
- LOCF
Last observation carried forward
- MAP
Mean arterial pressure
- HR
Hazard ratio
- CI
Confidence intervals
- SAPS II
Simplified Acute Physiology Score II
- SOFA
Sequential Organ Failure Assessment
Author contributions
NF, JD and PA designed the study. NF collected data in hospitalization reports. NF and VS performed statistical analysis. NF, JD, VS, PR and PA drafted the article. HM, FG, BM, NA, JPM, PFQ, SG, NW, FL, YLT, MC, RC, FG, CG, FT, JMT, PG, TVDL, AVB, EM, GP, OL, JDR, FH, DDC, CG, JLT and PA included patients in the SEPSISPAM trial and helped to grant access to hospitalization reports. All authors read and approved the final manuscript.
Funding
The SEPSISPAM trial was supported by the French Ministry of Health.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The SEPSISPAM trial (NCT01149278) was approved for all participating centers by the ethic committee at the Angers University Hospital. Written informed consent was obtained from all patients, their next of kin or another surrogate decision-maker, as appropriate. In accordance with ethics policy, if patients were unable to provide informed consent and the next of kin or a designated person was not available, the emergency inclusion procedure was applied and post hoc consent was obtained from patients who survived.
Consent for publication
Not applicable.
Competing interests
Dr. Weiss received consultant fees from Med-Day Pharmaceuticals beyond the scope of this article. Dr. Dequin's institution received funding from Angers University Hospital, the French Ministry of Health, Abionic, Atox Bio, Sphingotec GMBH, Adrenomed, Medspace, Aridis, Merck, Combioxin, GSK, Med-Immune, Genentech INH, Rev-Immune, Faron, Kenta and Tigenix. He also received support for article research from the French Ministry of Health. Dr. Gonzalez disclosed work for hire. Dr. Teboul received funding from Getinge/Pulsion. Dr. Radermacher's institution received funding from Deutsche Forschungsgemeinschaft and the German Ministry of Defence. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Backer D, Donadello K, Taccone FS, et al. Microcirculatory alterations: potential mechanisms and implications for therapy. Ann Intensive Care. 2011;1:27. doi: 10.1186/2110-5820-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakr Y, Dubois M-J, De Backer D, et al. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32:1825–1831. doi: 10.1097/01.CCM.0000138558.16257.3F. [DOI] [PubMed] [Google Scholar]
- 4.Ait-Oufella H, Lemoinne S, Boelle PY, et al. Mottling score predicts survival in septic shock. Intensive Care Med. 2011;37:801–807. doi: 10.1007/s00134-011-2163-y. [DOI] [PubMed] [Google Scholar]
- 5.Lima A, Bakker J. Noninvasive monitoring of peripheral perfusion. Intensive Care Med. 2005;31:1316–1326. doi: 10.1007/s00134-005-2790-2. [DOI] [PubMed] [Google Scholar]
- 6.Ait-Oufella H, Bakker J. Understanding clinical signs of poor tissue perfusion during septic shock. Intensive Care Med. 2016;42:2070–2072. doi: 10.1007/s00134-016-4250-6. [DOI] [PubMed] [Google Scholar]
- 7.de Moura EB, Amorim FF, da Cruz Santana AN, et al. Skin mottling score as a predictor of 28-day mortality in patients with septic shock. Intensive Care Med. 2016;42:479–480. doi: 10.1007/s00134-015-4184-4. [DOI] [PubMed] [Google Scholar]
- 8.Coudroy R, Jamet A, Frat J-P, et al. Incidence and impact of skin mottling over the knee and its duration on outcome in critically ill patients. Intensive Care Med. 2015;41:452–459. doi: 10.1007/s00134-014-3600-5. [DOI] [PubMed] [Google Scholar]
- 9.Dumas G, Lavillegrand J-R, Joffre J, et al. Mottling score is a strong predictor of 14-day mortality in septic patients whatever vasopressor doses and other tissue perfusion parameters. Crit Care. 2019;23:211. doi: 10.1186/s13054-019-2496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vincent J-L, De Backer D. Circulatory shock. N Engl J Med. 2013;369:1726–1734. doi: 10.1056/NEJMra1208943. [DOI] [PubMed] [Google Scholar]
- 11.Vincent J-L, Quintairos e Silva A, Couto L, et al. The value of blood lactate kinetics in critically ill patients: a systematic review. Crit Care. 2016;20:257. doi: 10.1186/s13054-016-1403-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gotmaker R, Peake SL, Forbes A, et al. Mortality is greater in septic patients with hyperlactatemia than with refractory hypotension. Shock Augusta Ga. 2017;48:294–300. doi: 10.1097/SHK.0000000000000861. [DOI] [PubMed] [Google Scholar]
- 13.De Backer D, Creteur J, Preiser J-C, et al. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002;166:98–104. doi: 10.1164/rccm.200109-016OC. [DOI] [PubMed] [Google Scholar]
- 14.Xu J-Y, Ma S-Q, Pan C, et al. A high mean arterial pressure target is associated with improved microcirculation in septic shock patients with previous hypertension: a prospective open label study. Crit Care. 2015;19:130. doi: 10.1186/s13054-015-0866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thooft A, Favory R, Salgado DR, et al. Effects of changes in arterial pressure on organ perfusion during septic shock. Crit Care. 2011;15:R222. doi: 10.1186/cc10462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubin A, Pozo MO, Casabella CA, et al. Increasing arterial blood pressure with norepinephrine does not improve microcirculatory blood flow: a prospective study. Crit Care. 2009;13:R92. doi: 10.1186/cc7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asfar P, Meziani F, Hamel J-F, et al. High versus low blood-pressure target in patients with septic shock. N Engl J Med. 2014;370:1583–1593. doi: 10.1056/NEJMoa1312173. [DOI] [PubMed] [Google Scholar]
- 18.Friesenecker BE, Tsai AG, Martini J, et al. Arteriolar vasoconstrictive response: comparing the effects of arginine vasopressin and norepinephrine. Crit Care Lond Engl. 2006;10:R75. doi: 10.1186/cc4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jhanji S, Stirling S, Patel N, et al. The effect of increasing doses of norepinephrine on tissue oxygenation and microvascular flow in patients with septic shock. Crit Care Med. 2009;37:1961–1966. doi: 10.1097/CCM.0b013e3181a00a1c. [DOI] [PubMed] [Google Scholar]
- 20.Jozwiak M, Chambaz M, Sentenac P, et al. Assessment of tissue oxygenation to personalize mean arterial pressure target in patients with septic shock. Microvasc Res. 2020;132:104068. doi: 10.1016/j.mvr.2020.104068. [DOI] [PubMed] [Google Scholar]
- 21.Cecconi M, Evans L, Levy M, et al. Sepsis and septic shock. The Lancet. 2018;392:75–87. doi: 10.1016/S0140-6736(18)30696-2. [DOI] [PubMed] [Google Scholar]
- 22.Preda G, Bourcier S, Joffre J, et al. Mottling score is associated with 28-day mortality in critically ill patients with sepsis. Minerva Anestesiol. 2017;83:664–666. doi: 10.23736/S0375-9393.17.11816-X. [DOI] [PubMed] [Google Scholar]
- 23.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez G, Bellomo R, Bakker J. The ten pitfalls of lactate clearance in sepsis. Intensive Care Med. 2019;45:82–85. doi: 10.1007/s00134-018-5213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vincent J-L, Bakker J. Blood lactate levels in sepsis: in 8 questions. Curr Opin Crit Care. 2021;27:298–302. doi: 10.1097/MCC.0000000000000824. [DOI] [PubMed] [Google Scholar]
- 26.Brunauer A, Koköfer A, Bataar O, et al. Changes in peripheral perfusion relate to visceral organ perfusion in early septic shock: A pilot study. J Crit Care. 2016;35:105–109. doi: 10.1016/j.jcrc.2016.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.