Abstract
Even though macrophages have the potential to harm tissues through excessive release of inflammatory mediators, they play protective roles to maintain tissue integrity. In this study, we hypothesized that lysophosphatidylcholine (LPC), via G2A and A2B receptors, puts brakes on macrophages by the induction of adenosine release which could contribute to termination of inflammation. Mechanistically, LPC-induced PGE2 production followed by the activation of cAMP/protein kinase A (PKA) pathway which results in the activation of LKB1/AMPK signaling pathway leading to increasing Mg2+ influx concomitantly with an increase in mitochondrial membrane potential (MMP, Δψm) and ATP production. Then, ATP is converted to adenosine intracellularly followed by efflux via ENT1. In a parallel pathway, LPC-induced elevation of cytosolic calcium was essential for adenosine release, and Ca2+/calmodulin signaling cooperated with PKA to regulate ENT1 permeation to adenosine. Pharmacological blockade of TRPM7 and antisense treatment suppressed LPC-induced adenosine release and magnesium influx in bone marrow-derived macrophages (BMDMs). Moreover, LPC suppressed LPS-induced phosphorylation of connexin-43, which may counteract TLR4-mediated inflammatory response. Intriguingly, we found LPC increased netrin-1 production from BMDMs. Netrin-1 induces anti-inflammatory signaling via A2B receptor. In the presence of adenosine deaminase which removes adenosine in the medium, the chemotaxis of macrophages toward LPC was significantly increased. Hypoxia and metabolic acidosis are usually developed in a variety of inflammatory situations such as sepsis. We found LPC augmented hypoxia- or acidosis-induced adenosine release from BMDMs. These results provide evidence of LPC-induced brake-like action on macrophages by adenosine release via cellular magnesium signaling.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11302-022-09878-y.
Keywords: Lysophosphatidylcholine, Lipopolysaccharide, Adenosine release, Bone marrow-derived macrophages, TRPM7, Intracellular magnesium
Introduction
Macrophages are effector mononuclear phagocytes, present virtually throughout the body, and they are in a good position to raise alarm when they detect pathogens or tissue damage [1]. This kind of early detection is called innate immune response and forms the foundation of downstream inflammation [1]. They also play a key role in most, if not all, phases of the response to tissue injury or infection through proinflammatory cytokines. On the other hand, anti-inflammatory or immunosuppressive phenotypes of macrophages play a critical role in the resolution of inflammation [2]. As macrophages produce a wide range of biologically active molecules that participate in both beneficial and detrimental outcomes in inflammation, therapeutics targeted to macrophages may open new avenues for controlling inflammatory diseases [3]. Even more than that, they evolve many ways and strategies as protective mechanisms to regulate levels of inflammation including induction of negative feedback regulators such as the production of adenosine [4].
Adenosine is a plurisystem modulator that affects responses in various cell and tissue types and through several widely distributed receptors and cell signaling pathways. It exerts a wide range of immunomodulatory effects, and cells of the mononuclear phagocyte system are among its major targets [5]. Adenosine is known to activate four G protein-coupled receptors A1, A2A, A2B, and A3 [5], whereas activation of macrophages by adenosine occurs mainly through receptors A2A and A2B [6]. Activation of the adenosine pathway is involved in the generation of an effective immune response by regulating the functions of immune and inflammatory cells [7]. Conversely, deletion of adenosine receptors A2B and A2A has been reported to negatively affect immune cells in inflammatory or immune-related diseases such as sepsis [8]. In this context, it is now widely recognized that targeting the adenosinergic system holds enormous potential for the treatment of severe inflammatory diseases. Fine-tuning macrophage responses via the adenosine pathway allow tissues to maintain function [5, 7].
Lysophosphatidylcholine (LPC), an endogenous immunomodulator, is considered a G2A activator [9]. Basically, LPC is mainly derived from the turnover of phosphatidylcholine (PC) in the blood by phospholipase A2 (PLA2) and/or from the transfer of fatty acids to free cholesterol by lecithin-cholesterol acyltransferase (LCAT) [9]. LPC activates several signaling pathways involved in inflammatory responses in different systems or organs. Serum levels of LPC are elevated in a variety of pathophysiological conditions [9], indicating LPC as an important mediator in inflammatory diseases. In addition to proinflammatory effects, LPC can also exert anti-inflammatory effects. These include triggering M2 polarization of macrophages [10], inhibiting neutrophil oxidant production [11], upregulating IL-10 production in dendritic cells [12], and suppressing human Treg function [13], along with the potential to protect against sepsis and organ injury [14, 15]. However, the ability of LPC to modulate innate immune responses via macrophages and modify anti-inflammatory effects is still poorly understood. In the present study, we demonstrated that LPC induces the release of adenosine from bone marrow-derived macrophages (BMDMs), and we analyzed the underlying mechanisms responsible for this. We thought our results provide insight into mechanisms by which LPC controls acute inflammatory response and protects against tissue damage.
Materials and methods
Mice
C57BL/6 J mice wild type (WT) or knockout type (KO, G2A−/− and A2B−/−) (20–25 g, 6–8 weeks old) were used in this study. The experiments were approved by the Experimentation Committee (Hallym University) and managed strictly in accordance with the guide and principles for Use and Care of Laboratory Animals. All the mice were maintained and housed at animal facilities under specific pathogen-free conditions.
Isolation and generation of bone marrow-derived macrophages (BMDMs)
Bone marrow cell cultures were prepared according to the protocol described previously [16] with some modifications. Briefly, mice were sacrificed by cervical dislocation, the skin was removed with sterile scissors and forceps, and the muscles attached to the bones were removed. Then, we took out the tibia and femur, placed them in ice-cold 1 × PBS, and then isolated bone marrow cells from the femur and tibia by rinsing serum-free DMEM with a 25-gauge needle and a 70-μm nylon cell strainer. The filtrate was centrifuged at 1000 rpm for 5 min at 4 °C. The supernatant was carefully discarded, then the pellet was dissociated in complete DMEM heated to 37 °C. We transferred the dissociated cells to a cell culture dish (100 mm) and incubated them for 2–4 h at 37 °C, 5% CO2. We then collected the supernatant and centrifuged it at 1000 rpm for 5 min at 4 °C. The supernatant was discarded, and the pellet was carefully dissociated in complete DMEM with 30% L-929 conditioned medium + 20% FBS (70% complete DMEM + 30% L-929 + 20% FBS), then transferred and seeded in 4–5 Petri dishes for 5 days. The generated macrophages were analyzed for F4/80 expression by flow cytometry, and 80% of isolated cells were positive (Supplementary Fig. S1A, B). Note: we did not use a lysis buffer for erythrocytes because they are rapidly lysed, dead, and do not adhere to cell culture dishes.
LPC preparation
LPC has been shown to be toxic and to promote cytotoxicity in vitro when prepared in a serum-free medium [17]. However, serum albumin effectively neutralizes the toxicity of LPC [18]. Albumin, a major component of FBS, was found to dramatically attenuate LPC-induced cytotoxicity by binding to LPC with multiple binding sites [19]. Therefore, in the current study, LPC was dissolved in DMEM containing 0.3% fatty acid free-BSA in all experiments unless otherwise stated. Stock solutions of LPC were sonicated for 15 s before treatment. We performed LDH assay and MTT assay to further analyze the effect of LPC. We did not detect any cytotoxicity or decreased cell viability (Supplementary Figs. S2 and S3).
Adenosine assay
For measurement of adenosine in the culture supernatant, BMDMs were plated at 3.5 × 105/0.5 ml well in a 24-well flat-bottom tissue culture plate or at 1.4 × 106/2 ml in 6-well flat-bottom tissue culture plate (7 × 105/1 ml) and incubated at 37 °C, 5% CO2 incubator. For extracellular adenosine, supernatants were collected and centrifuged at 10,000 rpm for 5 min at 4 °C to remove insoluble particles. They were then either stored at − 80 °C or assayed directly without dilution. For intracellular adenosine, after washing cells with ice-cold 1 × PBS, collecting and harvesting adherent cells with an appropriate cell scraper using 200 μl or 800 μl ice-cold 1 × PBS/well in a 24-well plate or 6-well plate, respectively, and homogenizing or sonicating the cells on ice, followed by centrifugation at 10,000 rpm for 10 min at 4 °C to remove insoluble particles, supernatants were kept at − 80 °C or assayed immediately using the adenosine assay kit MET-5090 (Cell Biolabs; San Diego, California, USA) according to the manufacturer’s instructions.
ATP assay
Intracellular and extracellular ATP content was measured using the luminescent ATP determination kit (A22066, Molecular Probes; Eugene, Oregon, USA). BMDMs were plated at the number of 3.5 × 105/0.5 ml wells in a 24-well flat-bottomed tissue culture plate. For extracellular ATP, the supernatants were collected and used to quantify ATP. For intracellular ATP, cells (3.5 × 105/0.5 ml/well) were then washed with ice-cold 1 × PBS and lysed in 200 µl Glo-Lysis buffer (Promega; Madison, Wisconsin, USA) on ice. Luciferase activity was measured using a luminometer (Glomax, Promega, Sunnyvale, CA).
Cell viability
BMDMs were cultured in 96-well flat-bottomed plates (1 × 105/200 µl/well) and treated as described above. Cell viability was quantified using the EZ-Cytox Assay kit (EZ-1000, Dogenbio; South Korea) according to the manufacturer’s instructions. Briefly, 10 µl of MTT reagent mixed with 100 µl of culture medium was added to each well and incubated at 37 °C, 5% CO2 for 2 h. Absorbance (optical density OD) was measured at a wavelength of 450 nm using a spectrophotometer (Spectramax M2/e, Molecular Devices). Results were calculated and expressed either as % for each sample directly or normalization with untreated cells (negative control) as 100% and calculated % for each sample.
LDH cytotoxicity assay
LDH activity was measured using the cytotoxicity detection kit (#MK401, Takara; Otsu, Japan) according to the manufacturer’s protocol as follows. Briefly, 50 µl of each sample was added to a 96-well plate and then 50 µl of LDH reagent was added to each well. The plate was incubated for 30–45 min at 25 °C in the dark and then the enzymatic reaction was stopped by adding 50 µl of 1 M HCl. The absorbance was measured at a wavelength of 492 nm. Samples treated with 1% Triton X-100 or samples treated with the culture medium alone were defined as positive controls (100% LDH release) or negative controls (0% LDH release), respectively, and then the relative LDH release (%) for each well was calculated.
Experimental reagents and antibodies
DMEM/high glucose with 4 mM L-glutamine and 4500 mg/l D-glucose (SH30243.01) and RPMI-1640 with 2.05 mM L-glutamine (SH30027.01) were purchased from GE Healthcare Life Sciences. Glucose-free DMEM with 4 mM L-glutamine (11,966–025), high-glucose DMEM (11,965,118), HBSS no calcium no magnesium (14,170,120), Opti-MEM™ I reduced serum medium (31,985,062), and trypsin–EDTA 0.25% (25,200,056) were from Gibco Life Technologies. Fetal bovine serum (FBS) was from Atlas biologicals. Stearoyl LPC (LPC 18:0) was purchased from Avanti Polar Lipids Inc. (Alabaster, AL, USA). Fatty acid-free bovine serum albumin (BSA, A8806) was from Sigma-Aldrich, St. Louis, MO. Lipopolysaccharide (LPS) was from E. coli 055:B5 (L2880, Sigma). Dipyridamole (D9766), S-(4-nitrobenzyl)-6-thioinosine NBTI (N2255), adenosine 5′-(α,β-methylene)diphosphate APCP (M3763), W7 (7 (N- (6-aminohexyl)-5-chloro-1-naphthalene sulfonamide hydrochloride, 681,629), KN-93 (K1385), indomethacin (I7378), diclofenac sodium (D6899), flufenamic acid (F9005), 2-deoxy-D-glucose (D8375), oligomycin A (75,351), NS8593 hydrochloride (N2538), SB203580 (S8307), crystal violet (C0775), and cobalt (II) chloride hexahydrate (C8661) were from Sigma. BAPTA-AM (B4758) was from APExBIO. H-89 dihydrochloride (HY-15979A), MRS 1754 (HY-14121), and ZM241385 (HY-19532) were from MedChemExpress (NJ, USA). PF-04418948 (15,016) and GW-627368 (10,009,162) were obtained from Cayman Chemical, USA. BML-275 (compound C) was obtained from Santa Cruz Biotechnology (sc-200689). Calcium chloride anhydrous (18,235–0301) and magnesium chloride hexahydrate (19,275–0301) were purchased from Junsei Chemical Co. (Tokyo, Japan). Primary antibodies specific for HIF-1α (D2U3T) rabbit mAb, β-actin (D6A8) rabbit mAb, phospho-AMPKα (Thr172), phospho-LKB1 (Ser428) (C67A3) rabbit mAb, and phospho-connexin 43 (Ser368) (D6W8P) rabbit mAb were from Cell Signaling Technology (Beverly, MA, USA). Total connexin 43 (3D8A5) was obtained from Thermo Fisher Scientific, Inc. Rabbit anti-ADK (A304-280A-T) was from Bethyl laboratories, Inc. Montgomery, USA. TRPM7 rabbit polyclonal antibody (NBP1-46,829) was from Novusbio. Rabbit mAb NT5C1A antibody (sc-377244), AMPKα1/2 (sc-74461), and LKB1 (Ley 37D/G6) (sc-32245) were purchased from Santa Cruz Biotechnology (Dallas, 0TX, USA). Secondary antibody goat anti-rabbit IgG, polyclonal antibody, and HRP conjugate (ADI-SAB-300-J) were from Enzo Life sciences. Recombinant human adenosine deaminase protein (rhADA) was obtained from R&D Systems.
Protein assay and preparation of cells for western blot
BMDMs were seeded in 12-well plates at a density of 7 × 105/1 ml/well and kept in the 37 °C, 5% CO2 incubator for more than 20 h to allow cells to reach 80–90% confluence. After treatment, the cells were washed with ice-cold 1 × PBS and then lysed in 300 µl RIPA buffer (Thermo Scientific, 89,900) with protease inhibitor cocktail (Thermo Scientific, 87,786) on ice for 30 min. Cells were collected and centrifuged at 14,000 rpm for 15 min at 4 °C to remove the cellular debris. Protein content was determined using the Bradford assay (Bio-rad, Quick Start Bradford Protein Assay Kit, #500–0202). Cell lysates were heat-denatured in 4 × Laemmli sample buffers for 10 min at 100 °C. Samples were stored at − 80 °C until western blot analysis.
Western blotting
Thirty to 50 µg of protein of the same concentration of each sample was loaded on 10%, 6%, or 12.5% SDS–PAGE (sodium dodecyl sulfate–polyacrylamide gel) and separated by electrophoresis and then transferred to PVDF (polyvinylidene fluoride) membranes. The membranes were blocked for 1 h at room temperature (RT) in 5% non-fat dry milk /1 × TBST (Tris-buffered saline, 0.1% Tween-20). After washing 4–5 times, membranes were incubated overnight at 4 °C with primary antibodies. Primary antibodies against phospho-cx43, total cx43, HIF-1α, β-actin, TRPM7, phospho-AMPK, phospho-LKB1, and ADK were prepared as a ratio of 1:1000. Primary antibodies against CN1A, AMPK, and LKB1 were prepared as a ratio of 1:500. After washing 3 to 4 times, the membranes were probed with secondary antibodies for 1–2 h at RT. Membranes were then washed 3 to 4 times and ECL reagent (Western HRP Substrate (WBLUF0100, Millipore)) was added. Imaging and chemiluminescent signals were quantified. Densitometric data were analyzed using ImageJ 1.52a software (National Institutes of Health, USA).
Acidosis challenge
To temporarily expose BMDMs to acidic conditions similar to those in vivo, we followed a previous report [20] to adjust the extracellular pH of the medium. Extracellular acidosis (pH 6.5) was introduced dropwise into the medium (10% DMEM or BSA with free-fatty acid in serum-free DMEM) with 1 M HCl, then the pH was standardized and adjusted to 6.5 using the SevenEasy™ pH meter. Prior to treatment, all acidic media were filter-sterilized (0.22 mm). BMDMs were incubated at (3.5 × 105/0.5 ml well in a 24-well plate for eADO, iADO, eATP, iATP) or (1 × 105/0.2 ml well in a 96-well plate for LDH assay, MTT assay) in 10% acidic DMEM for 3.5 h followed by treatment with LPC (0, 10, 30 µM) or LPS (0, 0.1, 1 µg/ml) in acidic 0.3% BSA DMEM for 30 min in parallel with normal pH 7.4 of 10% DMEM and then 0.3% BSA DMEM with LPC, LPS with the same concentrations, and treatment duration. We examined cytotoxicity (LDH assay) and cell viability (MTT assay) and observed a minimum level of cellular toxicity (Supplementary Figs. S2 and S3).
Hypoxia induction and HIF-1α quantification
Stock solutions of CoCl2 (a mimetic agent/chemical inducer) were prepared by direct dissolution in autoclaved DDW (100 mM), filter-sterilized (0.22 mm), and stored at − 20 °C. The final concentration was 100 µM. To determine extracellular adenosine, extracellular ATP, and intracellular ATP, BMDMs were seeded at 3.5 × 105/0.5 ml well in a 24-well plate at 37 °C, 5% CO2 incubator for less than 1 day, allowing the cells to reach adequate confluence. After washing cells with serum-free DMEM, CoCl2 diluted in 10% DMEM (final concentration 100 µM) and LPS (stock solution in autoclaved DDW at 1 mg/ml) was also diluted in 10% DMEM (final concentration 100 ng/ml) and then treated for 24 h followed by washing with serum-free DMEM. Thereafter, CoCl2 and LPS (which were subsequently prepared in 0.3% fatty acid-free BSA at a final dilution of 100 µM and 100 ng/ml respectively) were treated either alone, together, or with the addition of LPC (10, 30 µM) as triplicate treatment (final medium 0.3% BSA with CoCl2 + LPS + LPC at the indicated concentrations) for 30 min. All wells including the vehicles contained the same concentration of DDW (0.1%). After 24 h of treatment, cytosolic and nuclear extracts were isolated using the NE-PER nuclear and cytoplasmic extraction reagents kit (78,833, Thermo Fisher Scientific, Inc.) followed by western blotting to quantify the efficiency of hypoxia by measuring HIF-1α protein expression. We measured cytotoxicity (LDH assay) and cell viability (MTT assay) and observed a minimum level of cellular toxicity (Supplementary Figs. S2 and S3).
Knockdown of ENT1 in BMDMs
Cells were seeded at 3.5 × 105/0.5 ml well in a 24-well plate and 6 × 105 cells/ml in 100-mm dishes at 37 °C, 5% CO2 incubator. Cells were then transiently transfected for 72 h with Trilencer-27 siRNA kit (Origene, Rockville, USA) using TransIT-TKO® siRNA transfection reagent (MIRUS, MIR 2150) according to the manufacturer’s protocol. The siRNA specific for ENT1 was designed and chemically synthesized as follows: Slc29a1 (mouse)—3 unique 27 mer siRNA duplexes (SR426873, final concentration 10 nM). Scrambled negative control siRNA duplex (SR30004, final concentration 10 nM). The efficiency of siRNA transfection was checked 72 h after the transfection by flow cytometry. Cells were successfully transfected with improved efficacy and minimal cytotoxicity as confirmed by LDH assay and MTT assay (Supplementary Figs. S2 and S3).
FACS analysis for ENT1 expression in transfected BMDMs
Cells were plated at 6 × 105 cells/ml in 100-mm dishes at 37 °C, 5% CO2 incubator 1 day before transfection to allow sufficient confluency. At 72 h after transfection, cells were washed and collected from the plate surface using a cell scraper and then centrifuged at 4 °C, 1000 rpm for 5 min. The cell pellet was then resuspended in a fixation buffer (2% paraformaldehyde) for 15 min at 4 °C. Then, cells were centrifuged at 4 °C, 1800 rpm for 4 min, resuspended in 1 × PBS, counted, transferred to polypropylene FACS tubes (0.5 × 106 cells/ml), and centrifuged at 4 °C, 1800 rpm for 4 min. For surface staining, cells were incubated with fluorescence-conjugated monoclonal antibodies against ENT1-PE (1:100 dilution) (sc-377283, Santa Cruz Biotechnology) and F4/80-FITC (1:200) (Abcam, ab60343) on ice for 30 min in the dark. Cells were then re-pelleted by centrifugation and resuspended in FACS staining buffer (2% FBS) (554,656, BD Biosciences). Cells were filtered in new FACS tubes and immediately probed using 300 µl FACS staining buffer. Flow cytometry was analyzed by FACSCalibur (BD Bioscience, San Diego, CA, USA) Cell Quest Pro 6.0 (BD Bioscience, San Diego, CA, USA). At least 10,000 cells within the gated region were analyzed.
TRPM7 gene silencing in BMDMs
Cells were plated 1 day before transfection at 3.5 × 105/0.5 ml well in a 24-well plate, 1 × 105/0.2 ml well in a 96-well plate, and 7 × 105 cells/ml in a 12-well plate. To silence TRPM7, macrophages were transfected for 72 h with two different siRNA duplexes (25 nM final concentration each) targeting mouse TRPM7. TransIT-TKO® siRNA transfection reagent (Mirus, MIR 2150) was used for this purpose according to the manufacturer’s protocol. siRNA duplexes were chemically synthesized, pre-engineered, and purchased from Bioneer, South Korea. A non-specific siRNA duplex was used as a negative control (SN-1012, Bioneer). The efficiency of siRNA transfection was checked by western blots 72 h after transfection. Cells were successfully transfected with improved efficacy and minimal cytotoxicity as determined by LDH assay and MTT assay (Supplementary Figs. S2 and S3).
Measurement of mitochondrial membrane potential (MMP, ΔΨm) using fluorescence microplate reader
Cells were plated at 1 × 105 cells/0.2 ml in a 96-well plate at 37 °C, 5% CO2 incubator 1 day before treatment. After washing cells with 1 × PBS, cells were then treated with LPC (30 µM) or vehicle (0.3% BSA DMEM) for 30 min. Cells were pretreated with PKA inhibitor (H89 10 µM), AMPK inhibitor (compound C, 10 µM), TRPM7 inhibitor (NS8593, 50 µM), or EP4 blocker (GW-627368, 1 µM) for 30 min followed by co-treatment with LPC (30 µM) for 30 min. Stock solutions of these drugs were prepared in DMSO; all subsequent dilutions were prepared in 10% DMEM for the first 30 min or prepared in 0.3% BSA-DMEM for the second 30 min. All cells were treated with the same concentration of DMSO (0.1%). CCCP (carbonyl cyanide m-chlorophenyl hydrazone) (20 µM) was used as a depolarizing agent. Following that, MMP was estimated using Cell Meter™ Mitochondrion Membrane Potential Assay Kit-Red Fluorescence Optimized for Microplate Reader (AAT Bioquest, CA, USA) according to the manufacturer’s protocol. Results were normalized to vehicle or control (untreated wells).
Fluorescence measurement of [Ca.2+]i
Intracellular calcium concentration was measured using the fluorescent Ca2+ indicator Fluo-3 (Thermo Fisher Scientific, F1241). Briefly, BMDMs were plated in a 96-well plate at 1 × 105/0.2 ml well 1 day before the experiment. Cells were loaded with Fluo-3 AM (4 µM) in serum-free DMEM for 30 min at 37 °C, 5% CO2 incubator. After washing twice with HBSS without calcium and magnesium, LPC (30 µM) or pre-treatment for 15 min then co-treatment of indicated drugs with LPC as described above were added to cells following 5 min pre-read. Traces of intracellular calcium in Fluo-3 AM-loaded macrophages were measured with 490 nm/526 nm using Spectramax M2/e fluorescence microplate reader (Molecular Devices). Fluorescent emission readings were recorded every 10 s for 20 min then average was calculated after the run time (20 min) finished. Raw fluorescence was subtracted with average fluorescence 5 min before the treatment. The changes of [Ca2+]i were presented as background-subtracted normalized fluorescence (F/F0).
Fluorescence measurement of [Mg.2+]i
Intracellular magnesium concentration was labeled by Mag-Fluo-4 AM (Thermo Fisher Scientific, M14206). Briefly, BMDMs were plated in a 96-well plate at 1 × 105/0.2 ml well 1 day before the experiment. Cells were loaded with Mag-Fluo-4 AM (10 µM) in serum-free DMEM for 30 min at 37 °C, 5% CO2 incubator. After washing twice with HBSS without calcium and magnesium, LPC (30 µM) or pre-treatment for 15 min then co-treatment of indicated drugs with LPC as described above were added to cells following 5 min pre-read. Traces of intracellular calcium in Mag-Fluo-4 AM-loaded macrophages were measured with 490 nm/526 nm using Spectramax M2/e fluorescence microplate reader (Molecular Devices). Fluorescent emission readings were recorded every 10 s for 20 min then average was calculated after the run time (20 min) finished. Raw fluorescence was subtracted with average fluorescence 5 min before the treatment. The changes of [Mg2+]i were presented as background-subtracted normalized fluorescence (F/F0).
Measurement of PGE2, IL-10, and netrin-1
BMDMs were seeded at 7 × 105/1 ml in a 12-well plate then incubated at 37 °C, 5% CO2 incubator overnight for appropriate confluency. After washing, cells were stimulated with LPC alone or co-treated with other drugs as shown for 12 h (LPC and all drugs were prepared in 5% FBS-DMEM) then supernatants were collected, centrifuged, and kept at − 80 °C or directly assayed. PGE2 was measured using prostaglandin E2 ELISA kit-monoclonal (Cayman Chemical Company, Ann Arbor, MI, USA). IL-10 was quantified using EliKine™ mouse IL-10 ELISA Kit (Abbkine, Inc., China). Netrin-1 concentration was determined using a RayBio® mouse Netrin-1 ELISA kit (RayBiotech, Norcross, GA, USA). The standard curve for each one was constructed in parallel with the analysis of the samples.
RNA purification and real-time RT- PCR
BMDMs were seeded at 1.4 × 106/2 ml in a 6-well plate and treated with LPC or vehicle (5% DMEM) for 3 h. Then, total RNA was extracted and purified using Direct-zol™ RNA Miniprep Plus (Zymo Research, Irvine, CA, USA). cDNA was subsequently synthesized using Maxime RT PreMix kit (Cat. No. 25081, Intron Biotechnology, South Korea) following the manufacturer’s instructions. Analysis was done by real-time PCR using RealMOD™ Probe M2 2 × qPCR mix (with UDG) (Cat. No. 25360.100, Intron Biotechnology, South Korea). GAPDH was used as endogenous control. Primers sequences for mouse IL-10: (forward) 5′-CATACTGCTAACCGACTCC-3′, (reverse) 5′-CAAATGCTCCTTGATTTCTGG-3′, mouse GAPDH: (forward) 5′-GAGTCTACTGGTGTCTTCAC-3′, (reverse) 5′-CATATTTCTCGTGGTTCACAC-3′. All primers were obtained from Bioneer, South Korea. PCR products were analyzed in a comparative manner relative to endogenous control GAPDH (ΔCT).
Transmigration assay for chemotaxis
BMDMs (7 × 105/mL in serum-free DMEM) were added into the upper chambers of a 24-well transwell plate with 8-μm pore size polycarbonate membrane filters (catalog no. 35224, SPL). The plate was equilibrated at 37 °C, 5% CO2 cell culture incubator for 30 min prior to assay. LPC was prepared and diluted in serum-free DMEM to a final concentration of 30 μM. We added LPC 30 μM or LPC (30 μM) + rhADA (1 μg/ml) and vehicle (serum-free DMEM only) into the lower chambers of the transwells. After 3 h at 37 °C in a 5% CO2 cell culture incubator, cells were washed twice in 1 × PBS then fixed with 4% paraformaldehyde at RT for 2 min. After washing twice with 1 × PBS, cells were permeabilized by 100% methanol at RT for 20 min. After that, BMDMs were stained with crystal violet (0.1%) at RT for 15 min. The cells remaining on the top of the membrane (inner side of the insert) were gently removed with a cotton swab. The number of migrated cells was counted under a microscope. For each sample, cells in 5 randomly picked fields under 200 × magnification were counted.
Statistical analysis
All data are presented as means ± standard error of the mean (SEM) and representative of at least 3 independent experiments (n = 3). p values less than 0.05 were considered to indicate statistical significance, determined by either one-way ANOVA followed by Bonferroni test or Student’s t test. GraphPad Prism Version 8.0.1 (GraphPad Software, San Diego, CA, USA) was used.
Results
LPC induced adenosine release from BMDMs through G2A and A2B receptors
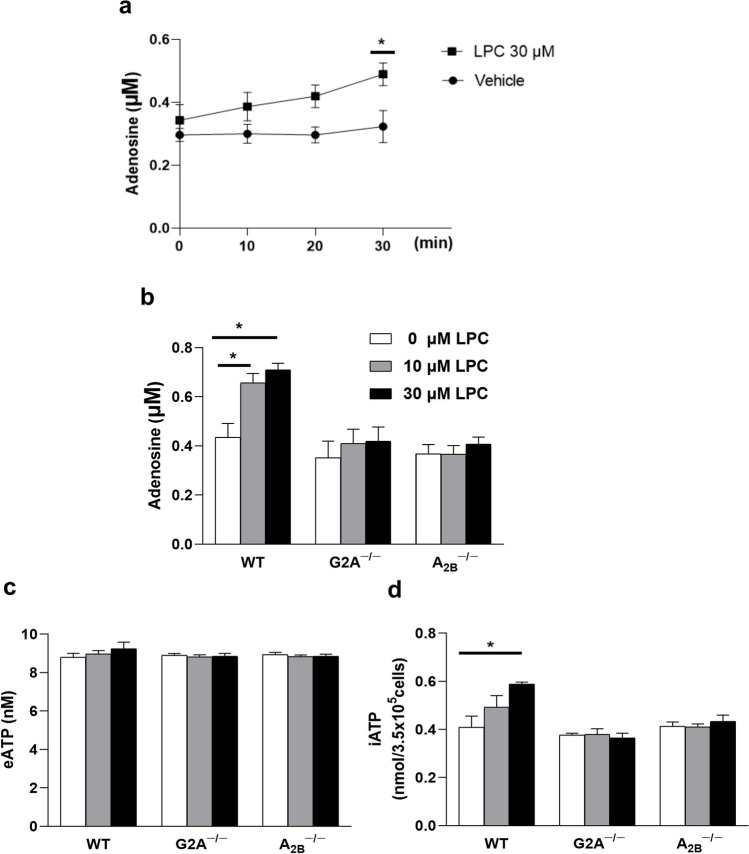
A direct physical interaction was observed between G2A and A2B receptors [15]. LPC has a protective effect against cecal ligation and puncture (CLP)-induced lethality, that is, dependent on macrophages, G2A, and A2B receptors [15]. Moreover, LPC increases plasma adenosine levels [15]. Overall, these results prompted us to measure adenosine release from macrophages. We tested two different concentrations of LPC to examine dose-dependent effects. We also assayed LPC effect at different time points (0, 10, 20, and 30 min) and found that LPC (30 µM) induced adenosine release in a significant way after 30-min incubation (Fig. 1a). In our initial experiments, we treated wild-type (WT), G2A knockout (KO), and A2B KO bone marrow-derived macrophages (BMDM) with LPC for 30 min. Our results showed that treatment with LPC caused a significant increase in extracellular adenosine release in a G2A- and A2B-dependent manner (Fig. 1b). Because dephosphorylation of ATP is the major pathway for adenosine formation, we sought to measure ATP content in extracellular and intracellular compartments. Surprisingly, we found no change in extracellular ATP of BMDMs treated with LPC (Fig. 1c), but LPC (30 µM) significantly increased intracellular ATP via G2A and A2B receptors (Fig. 1d).
Fig. 1.
LPC induced adenosine release from BMDMs through G2A and A2B receptors. a Bone marrow-derived macrophages (BMDMs) were treated with LPC 30 µM or vehicle (0.3% free-fatty acid BSA) for 0, 10, 20, and 30 min. BMDMs from wild type and knockout (KO) mice were treated with LPC (0, 10, 30 µM) prepared in 0.3% free-fatty acid BSA DMEM for 30 min, then b extracellular adenosine, c extracellular ATP were measured in the supernatant, and d intracellular ATP level was estimated as nmol/number of cells each well (3.5× 105/0.5 ml) at 24-well plate. Values are mean ± SEM (standard error of mean) of an average of 3 independent experiments (n = 3), *p < 0.05
LPC inhibited lipopolysaccharide (LPS)-induced phosphorylation of connexin 43 and did not augment LPS-induced adenosine release or intracellular ATP increase
To determine the precise mechanisms of LPC-induced adenosine release, we treated cells with LPC in conjunction with LPS (a known ligand that stimulates adenosine signaling (21)). As shown in Fig. 2a, LPC (10, 30 µM) or LPS (100 ng/ml) induced the release of adenosine from BMDMs, whereas LPC did not induce any additional increase when treated simultaneously with LPS, without changing extracellular ATP (Fig. 2b). LPC (10, 30 µM) and LPS (100 ng/ml) increased intracellular ATP in BMDMs (Fig. 2c), but no additional effect on intracellular ATP was observed in the co-treatment of LPC with LPS. No significant change was observed in intracellular adenosine after LPC or LPS treatment (Supplementary Fig. 4e).
Fig. 2.
LPC inhibited lipopolysaccharide (LPS)-induced phosphorylation of connexin 43 and did not augment LPS-induced adenosine release or intracellular ATP increase. BMDMs were treated either with LPC or LPS alone or both as indicated for 30 min; supernatants were collected and centrifuged to measure a extracellular adenosine, b extracellular ATP, and c intracellular ATP. The expression of connexin-43 (phosphorylated and total form) was measured at the protein level by western blotting, and d β-actin was used as an endogenous control. LPC or LPS were prepared in 0.3% BSA DMEM and all wells contained 0.1% DDW. Results are representative of mean ± SEM of 3 independent experiments (n = 3), *p < 0.05 and **p < 0.01
Connexin 43 (Cx43) belongs to gap junctions consisting of two hemichannels and plays an important role in extracellular ATP release [2]. Pharmacological blockade of Cx43 decreases LPS-stimulated ATP release in peritoneal macrophages [23]. TLR4-mediated ATP release from Cx43 in inflammatory cells occurs via activation of the ERK pathway [24]. LPC has been shown to reduce LPS-stimulated ERK phosphorylation in macrophages [25]. In the next step, we examined the effect of LPC and LPS, and their co-treatment for 30 min on Cx43 using western blot analysis. As shown in Fig. 2d, LPS (100 ng/ml) significantly increased the phosphorylation of Cx-43. Interestingly, LPC (30 µM) reduced the LPS-induced phosphorylation of Cx-43 in BMDMs (Fig. 2d). All these data suggest that LPC inhibits TLR4-mediated Cx43 phosphorylation by blocking the ERK pathway.
Glucose-free medium blocked LPS-, but not LPC-, induced adenosine release and intracellular ATP increase
It is becoming increasingly clear that immune cells switch their metabolism from oxidative phosphorylation to glycolysis in response to proinflammatory stimuli including LPS [3]. To test whether the LPC- or LPS-triggered adenosine release and intracellular ATP increase are dependent on glycolysis, we treated BMDMs with LPC or LPS in glucose-free DMEM for 30 min. As shown in Fig. 3a, b, LPC (10, 30 µM) still significantly increased adenosine release and intracellular ATP, but LPS had no effect in the glucose-free medium. Intracellular adenosine and extracellular ATP after LPC or LPS treatment were not significantly altered (Supplementary Fig. 1e). These data suggest that the effects of LPS are dependent on glycolysis, on the other hand, glucose uptake is not required for LPC-induced adenosine release and intracellular ATP increase.
Fig. 3.
Glucose-free medium blocked LPS-, but not LPC-, induced adenosine release and intracellular ATP increase. BMDMs were incubated in the absence or presence of different concentrations of LPC or LPS for 30 min in glucose-free DMEM (containing 0.3% BSA). a Extracellular adenosine was measured in the culture supernatant and b iATP was measured from cell lysates. Data values represent mean ± SEM of 3 independent experiments (n = 3), *p < 0.05 and **p < 0.01
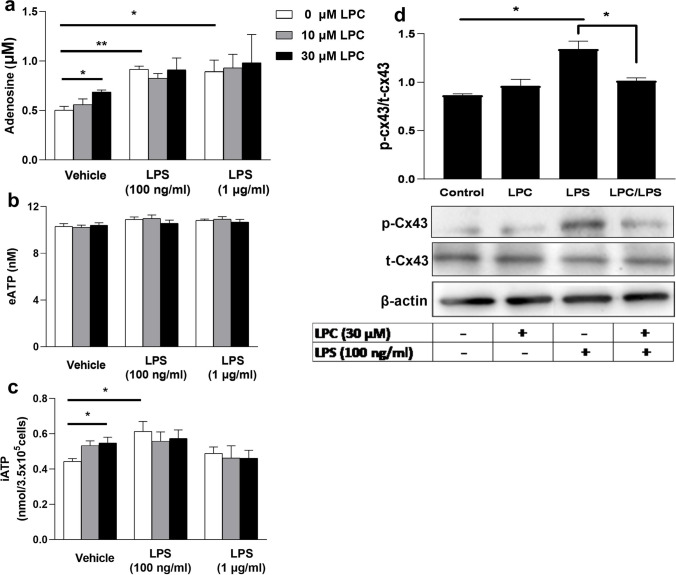
LPC, but not LPS, induced an increase in adenosine release from BMDMs via ENT1
There are several pathways to produce extracellular amounts of adenosine. ATP is delivered to the extracellular environment and then converted to adenosine by the enzymatic activity of ectonucleotidases CD39 and CD73 [5]. Otherwise, intracellular ATP is sequentially dephosphorylated by cytosolic nucleotidases to generate adenosine, which then flows across cell membranes via nucleoside transporters [5]. This highlights the importance of nucleoside transporters or ectoenzyme CD73 as a master regulator of extracellular adenosine levels. In the extracellular compartment, phospho-hydrolysis of AMP to adenosine occurs through CD73, which is the pacemaker enzyme for extracellular adenosine production [4]. ENT1 (SLC29A1) is expressed ubiquitously in mammalian tissues and has a dominant role in purines homeostasis [26]. To determine which pathway LPC stimulates to induce the release of adenosine, we analyzed the effect of the specific inhibitors for CD73 (APCP) and ENT1 (NBTI) on extracellular adenosine levels induced by LPC or LPS, and LPC + LPS. Although NBTI (5 µM) suppressed the increased adenosine level induced by LPC (30 µM), APCP (100 µM) had no effect, unlike the case of LPS (100 ng/ml) (Fig. 4a). Moreover, LPC, but not LPS, significantly increased intracellular adenosine in the presence of NBTI (Fig. 4b). We also observed that the LPC-induced adenosine release disappeared with dipyridamole (20 µM), another ENT1 inhibitor (data not shown). In addition, dipyridamole increased intracellular ATP in BMDMs (Supplementary Fig. 1f). Collectively, these findings suggest that LPC stimulates intracellular dephosphorylation of ATP to adenosine, which is then exported extracellularly via ENT1. Next, we tested whether ENT1 is required for the LPC effect by using siRNA to downregulate ENT1 in BMDMs. Macrophages were transfected with scrambled RNA (as a negative control) or with siRNAs against ENT1 (siENT1). Seventy-two hours later, cells were treated with or without LPC (30 µM) for 30 min. We found that LPC-induced adenosine release was blocked by siENT1 (Fig. 4c). We did not observe any change in eATP (Supplementary Fig. 4b). The efficacy of transfection at the ENT1 protein level was analyzed with flow cytometry (Fig. 4d).
Fig. 4.
LPC, but not LPS, induced an increase in adenosine release from BMDMs via ENT1. BMDMs (3.5 × 105/0.5 ml well in a 24-well plate) were pretreated with NBTI or APCP for 30 min then co-treated with LPC or LPS for 30 min as shown. a Extracellular adenosine was measured in collected media. NBTI was prepared in DMSO, and APCP was prepared in 10% DMEM or 0.3% BSA-DMEM; all subsequent dilutions were prepared in 10% DMEM for the first 30 min or in 0.3% BSA-DMEM for the second 30 min. b Intracellular adenosine was quantified from BMDMs (1.4 × 10.6/2 ml well in a 6-well plate) that were treated either by LPC or LPS alone or cotreated with NBTI for 30 min. The maximal DMSO and DDW concentration applied to cells in culture. a, b Did not exceed 0.1% at all wells including vehicles. c siRNA knockdown (KD) of ENT1 in BMDMs as described then treatment with or without LPC for 30 min. d Quantitation of efficacy of knockdown transfection protocol at the protein level by flow cytometry. Values are shown as mean ± SEM of 3 independent experiments (n = 3), *p < 0.05 and **p < 0.01
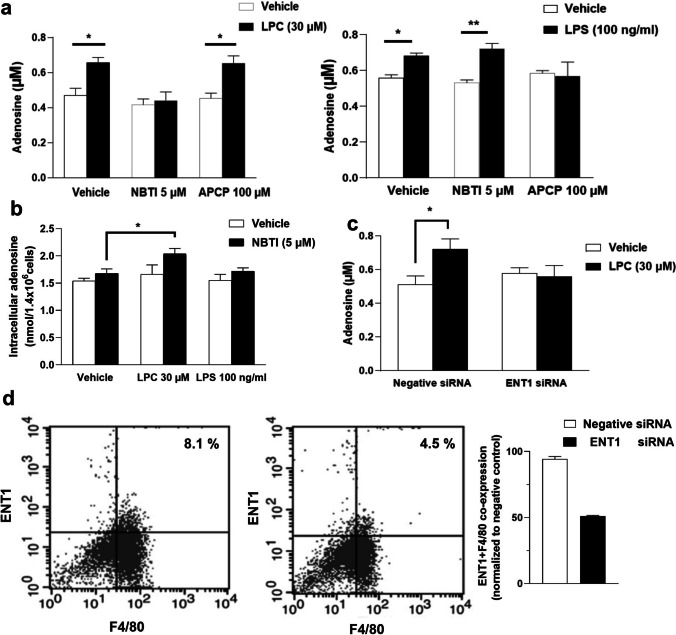
LPC increased adenosine release through Ca2+ signaling (except CaMKII), cAMP-dependent PKA signaling, AMPK, and PGE2 (via EP4)
To better understand the underlying mechanisms of the LPC-triggered adenosine release, we performed a screening analysis of regulatory signal transduction pathways reported to be activated by LPC. Calcium influx across the plasma membrane via various Ca2+ channels is an important signaling pathway for immune cell activation [27]. LPC increases Ca2+ influx via transient receptor potential ankyrin 1 (TRPA1) or TRPM2 channels in macrophages [28, 29]. In addition, Ca2+-dependent calmodulin-binding has been reported to regulate the flux of nucleosides (including adenosine) via interaction with ENT1 [30]. Calcium/calmodulin-dependent kinase kinase 2 (CaMKII) plays a key role in regulating inflammatory responses in macrophages [31]. To investigate the involvement of the Ca2+ pathway, we treated macrophages with BAPTA-AM (a calcium chelator), W-7 (a calmodulin inhibitor), and KN-93 (a CaMKII inhibitor) at concentrations 20, 25, and 10 µM, respectively, for 30 min, and then treated them with LPC (30 µM) for 30 min. The results showed that LPC-induced adenosine release was drastically blocked by BAPTA-AM and also inhibited by W-7 but not by KN-93 (Fig. 5a). Basal adenosine levels sharply declined upon chelation of intracellular Ca2+ by BAPTA-AM in BMDMs (Fig. 5a). In addition, BAPTA-AM induced a significant reduction in eATP (Supplementary Fig. 4c). As shown in Fig. 5e, f, LPC (30 µM) promoted the increase of [Ca2+]i in BMDMs, but the LPC-induced increase in [Ca2+]i was not affected by the drugs shown.
Fig. 5.
LPC increased adenosine release through Ca2+ signaling (except CaMKII), cAMP-dependent PKA signaling, AMPK, and PGE2 (via EP4). a, b, c, d Adenosine measurement in supernatants of BMDMs (3.5 × 105/0.5 ml well in a 24-well plate) pretreated with shown drugs for 30 min (in 10% DMEM) then co-treated with LPC (0.3% BSA-DMEM) for 30 min. Stock solutions of all drugs were prepared in DMSO; all subsequent dilutions were prepared in 10% DMEM for the first 30 min or prepared in 0.3% BSA-DMEM for the second 30 min. Parallel cultures were treated with 0.1% DMSO-containing media to rule out the possibility that the observed effects (adenosine release) were due to solvent. e, f LPC-induced increase in [Ca2+]i and [Mg2+]i was measured in the presence of indicated drugs that were pretreated for 15 min then co-treated with LPC in BMDMs (1× 105/0.2 ml well in a 96-well plate). Statistical analysis (mean ± SEM) was done from 3 independent experiments (n = 3), *p < 0.05, **p < 0.01
LPC is known to activate mitogen-activated protein kinases (MAPKs) signaling such as JNK and p38 but not ERK in macrophages [25]. LPC elevates cAMP levels, thereby activating the intracellular cAMP-dependent PKA signaling pathway in macrophages [32]. We examined whether inhibition of p38 MAPK by SB203580 (10 µM) or PKA inhibition by H89 (10 µM) affects the LPC effect. Interestingly, SB203580 did not inhibit the adenosine level increased by LPC, whereas H89 suppressed the LPC effect (Fig. 5b), and H89 itself significantly reduced the basal adenosine level. These results demonstrate that both Ca2+ signaling machinery and the PKA pathway are required as early signaling events for the LPC effect.
ATP has been shown to be the central pathway of adenosine production, and the major source of ATP relies on glycolysis and mitochondrial metabolic programs in mammalian cells [3–5]. To better assess the role of mitochondria, we investigated the effects of 2-D-glucose (2-DG, which inhibits glycolysis and oxidative phosphorylation) and oligomycin (an inhibitor of ATP synthase). 2-DG and oligomycin dramatically decrease mitochondrial ATP synthesis [33]. We found that the LPC effect disappeared in the presence of 2-DG (10 mM) or oligomycin (2.5 µM) (Fig. 5b). Interestingly, 2-DG significantly reduced eATP (Supplementary Fig. 4c). These results strongly suggest that LPC increases oxidative phosphorylation and thus ATP production, and subsequently converts it to adenosine.
Nonsteroidal anti-inflammatory drugs (NSAIDs), the most widely used analgesic and anti-inflammatory agents, also inhibit oxidative phosphorylation, ATP synthesis, and mitochondrial membrane potential [34–36]. They also inhibit the uptake of adenosine by blocking the nucleoside transporters ENT1 and ENT2 [37]. Therefore, in the present study, we aimed to test whether NSAIDs have an effect on LPC-induced adenosine release from BMDMs. We used indomethacin, diclofenac, and flufenamic acid (FFA), which represent different classes of NSAIDs. Figure 5c shows that indomethacin (10 µM), diclofenac (15 µM), and FFA (100 µM) suppressed LPC-induced increase in adenosine release. In addition, indomethacin significantly decreased basal adenosine release in BMDMs. These data compelled us to examine whether cyclooxygenase (COX) activity and prostaglandin synthesis are involved in the LPC-induced increase in adenosine levels in BMDMs. LPC has been shown to increase prostaglandin E2 (PGE2) secretion in macrophages [10]. PGE2 stimulates the production of IL-10 and triggers the M2 phenotype in macrophages [38, 39]. Among the four PGE2 receptors (EP)1–4, EP2, and EP4 are specifically most important for stimulation of intracellular c-AMP-dependent PKA signaling in macrophages [38, 39]. To elucidate the role of PGE2, we investigated the effect of pharmacological inhibition of EP2 and EP4 signaling on LPC action. A selective EP4 antagonist (GW-627368, 1 µM) markedly blocked the LPC-induced increase in adenosine levels (Fig. 5d), but a specific EP2 blocker (PF-04418948, 10 µM) had no effect.
AMPK is a master regulator of metabolism and can be activated by metabolic stress resulting from a suppression of ATP production or an increase in ATP consumption [40, 41]. Notably, LPC induces AMPK activation in macrophages in a time- and dose-dependent manner and suppresses LPS-mediated extracellular release of HMGB1 in macrophages through AMPK activation [42, 43]. Therefore, we considered the possibility that the LPC-induced adenosine increase is AMPK-dependent. To investigate whether AMPK mediates LPC-stimulated adenosine release, we used compound C 10 µM (an AMPK inhibitor) (Fig. 5d) and found that the LPC-induced increase in adenosine level was abolished by compound C. Compound C (10 µM) has been shown to suppress LPC-activated AMPK phosphorylation previously [43].
LPC increases [Mg.2+]i in BMDMs
Magnesium ion (Mg2+) is required for ATP synthesis [44] and proper mitochondrial function in macrophages [45]. Therefore, we investigated whether LPC increases [Mg2+]i in macrophages. Fluorescence-based detection of intracellular Mg2+ with Mag-fluo-4 AM has been shown previously [46]. The data show that LPC (30 µM) increases [Mg2+]i in BMDMs (Fig. 5e, f). Interestingly, the LPC-induced increase in [Mg2+]i was markedly inhibited by H89 (10 µM), compound C (10 µM), indomethacin (10 µM), diclofenac (15 µM), and GW-627368 (1 µM). In contrast, the same drugs had no effect on intracellular Ca2+ increased by LPC. These results suggest that PKA, AMPK, and PGE2 via EP4 are critical for the increased [Mg2+]i by LPC.
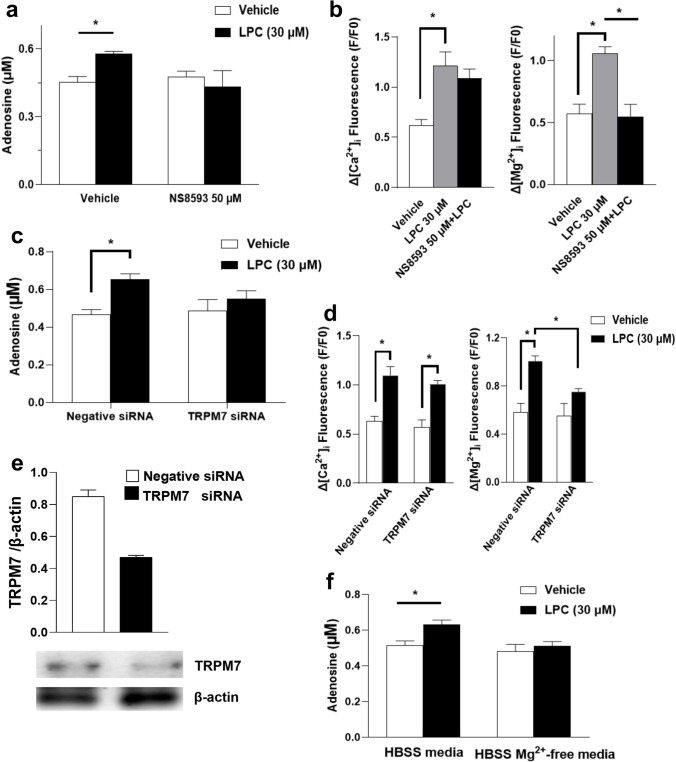
Functional TRPM7 channel was essential for LPC-induced adenosine release in BMDMs
Transient receptor potential melastatin7 (TRPM7) channels are divalent cation-selective ion channels permeable to Ca2+ and Mg2+ [47]. New insights have been gained into the key roles of TRPM7 in modulating macrophage signaling pathways linked to Mg2+ flux [48, 49]. To assess the functional role of the TRPM7 channel in the LPC actions, we tested NS8593, a potent inhibitor of TRPM7 [50]. Figure 6a and b show that NS8593 (50 µM) blocked both LPC-induced adenosine release and LPC-induced [Mg2+]i increase, but NS8593 had no effect on the LPC-induced increase in [Ca2+]i. To rule out the possibility that NS8593 caused off-target effects, we selectively suppressed the expression of TRPM7 by siRNA-mediated knockdown. Figure 6c shows the downregulation of TRPM7 suppresses LPC-induced adenosine release from BMDMs. Interestingly, TRPM7 siRNA significantly inhibited the increased [Mg2+]i caused by LPC but had no effect on the increased [Ca2+]i caused by LPC (Fig. 6d). We did not observe any change in eATP (Supplementary Fig. 4b). The efficacy of transfection at the protein level was assessed by western blot analysis of TRPM7 protein (Fig. 6e). To further confirm the prominent role of Mg2+ in increasing adenosine levels by LPC, we incubated BMDMs in Mg2+-free HBSS medium with or without LPC (30 µM) for 30 min. Figure 6f shows that the LPC effect disappeared in the absence of Mg2+. We observed no change in eATP (Supplemental Fig. 4A), and no cytotoxicity (Supplementary Figs. S2 and S3).
Fig. 6.
Functional TRPM7 channel was essential for LPC-induced adenosine release in BMDMs. a Macrophages (3.5 × 105/0.5 ml well in a 24-well plate) were pretreated by NS8593 (50 µM) for 30 min then co-treated with LPC (30 µM) for 30 min as indicated to measure extracellular adenosine or b pretreated by NS8593 at 96-well plate (1 × 105/0.2 ml well) for 15 min before co-treatment with LPC to measure [Ca2+]i and [Mg2+]i increase. NS8593 was dissolved in DMSO, and subsequent preparations were in 10% DMEM or 0.3% BSA-DMEM. All vehicle wells contained 0.1% DMSO. We silenced TRPM7 via siRNA-mediated knockdown in BMDMs; cells (3.5 × 105/0.5 ml well in a 24-well plate or 1 × 105/0.2 ml well in a 96-well plate) were transfected for 72 h followed by LPC treatment for 30 min then measurement of c extracellular adenosine (eADO) or d [Ca2+]i and [Mg2+]i increase. e We assessed the efficacy of knockdown of TRPM7 post-transfection by western blotting of TRPM7 protein in BMDMs (7 × 105/1 ml well in a 12-well plate). f BMDMs (3.5 × 105/0.5 ml well in a 24-well plate) were incubated in HBSS media or HBSS containing Mg2+-free media containing 0.3% BSA with or without LPC for 30 min, then eADO was measured. At first, we had HBSS media with no Ca2+ no Mg2+ then we put CaCl2 (as a source of Ca2+ at the same concentration to normal HBSS media) in order to have HBSS media without Mg2+ or we put CaCl2 and MgCl2 (as a source of Mg2+ at the same concentration to normal HBSS media) in order to have HBSS full media then, we added 0.3% BSA-free fatty acid before treatment. Values represent mean ± SEM of 3 independent experiments (n=3), *p < 0.05; relative western blot values (TRPM7/β-actin) are representative of 3 separate experiments
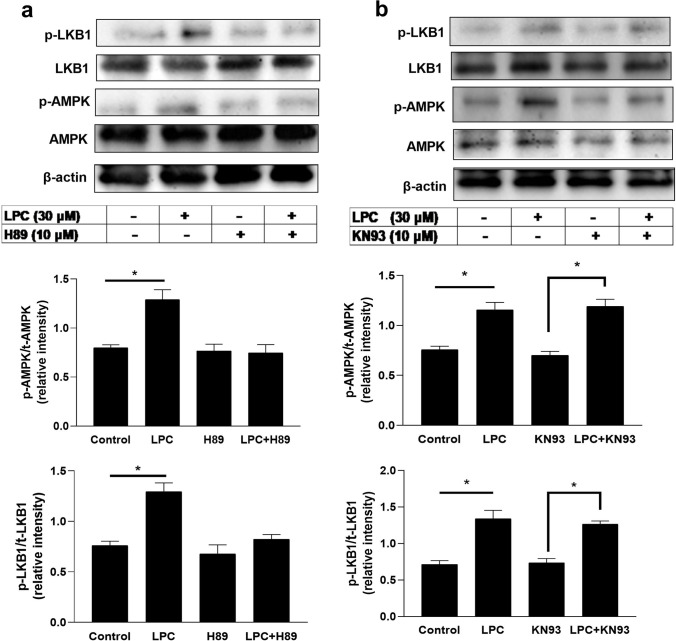
LPC activated the LKB1-AMPK axis pathway in a PKA-, but not CaMKII-, dependent manner in BMDMs
AMPK can be directly phosphorylated on Thr172 by the calcium-sensitive kinase CaMKK2 (also known as CaMKKβ) or by liver kinase B1 (also known as STK11) [40]. In our study, we found LPC-induced adenosine release is dependent on AMPK (Fig. 5d). Therefore, we investigated the possible pathways by which LPC increased AMPK phosphorylation. Figure 7a shows that LPC (30 µM) remarkably increased the phosphorylation of AMPK. In the presence of PKA inhibitor H89 (10 µM), the LPC effect disappeared. Similarly, LPC (30 µM) significantly increased the phosphorylation of LKB1, and H89 (10 µM) blocked this effect. Figure 7a and b confirm that LPC (30 µM) induced the phosphorylation of AMPK and LKB1. However, CaMKII inhibitor KN93 (10 µM) did not block LPC-mediated phosphorylation of AMPK and LKB1 (Fig. 7b). These findings suggest that LPC-mediated AMPK activation via its upstream LKB1, and PKA plays a central regulatory role in stimulating this pathway.
Fig. 7.
LPC activated the LKB1-AMPK axis pathway in a PKA-, but not CaMKII-, dependent manner in bone marrow-derived macrophages (BMDMs). BMDMs (7 × 105/1 ml well in a 12-well plate) were pretreated with PKA inhibitor (H89, 10 µM) or CaMKII inhibitor (KN93, 10 µM) for 30 min then treatment or co-treatment with LPC (30 µM). Both drugs (H89, KN93) were prepared in DMSO as a stock solution then all subsequent dilutions in 10% DMEM or 0.3% BSA-DMEM. 10% DMEM for the first 30 min or in 0.3% BSA-DMEM for the second 30 min. All wells including vehicles contained 0.1% DMSO. a, b Representative western blots using antibodies specific for phosphorylated AMPK (Thr172), phosphorylated LKB1 (Ser428), total AMPK, total LKB1, and actin, and also show the densitometric ratio of phosphorylated AMPKThr172 to total AMPK or phosphorylated LKB1.Ser428 to total LKB1 calculated using data from 3 independent experiments. Each bar represents the mean ± SEM. *p < 0.05 compared to the control
LPC increased mitochondrial membrane potential (MMP, Δψm) in BMDMs
The mitochondrion regulates the oxidative state required for optimal immune cell activation and plays a critical role in a variety of macrophage functions [51]. Conversely, mitochondrial dysfunction impairs immune cell function, worsens the progression of inflammation, and induces sepsis [51, 52]. There are many methods to assess mitochondrial function or its ability to produce ATP. Measurement of mitochondrial membrane potential (MMP) provides accurate information about mitochondrial ATP synthesis [53]. One of the existing methods for precise measurement of MMP where changes of Δψm can be monitored is using of the fluorescence-based microplate reader [54, 55]. To examine the effect of LPC on MMP, we treated cells with LPC (30 µM) for 30 min, and Fig. 8 shows that LPC significantly increased MMP compared with vehicles (0.3% BSA). One of the important functions of AMPK has been shown to promote mitochondrial health [40, 41]. Mg2+ influx via TRPM7 into macrophages is necessary for intracellular ATP increase [45]. Importantly, inhibition of EP4 receptor expression is associated with MMP collapse and a significant decrease in mitochondrial ATP [56]. Consistent with these studies, Fig. 8 shows PKA inhibitor (H89 10 µM), TRPM7 antagonist (NS8593 50 µM), EP4 antagonist (GW-627368 1 µM), and AMPK inhibitor (compound C 10 µM) completely blocked the action of LPC. These data provide useful insights into the mechanism by which LPC induces an increase in intracellular ATP content.
Fig. 8.
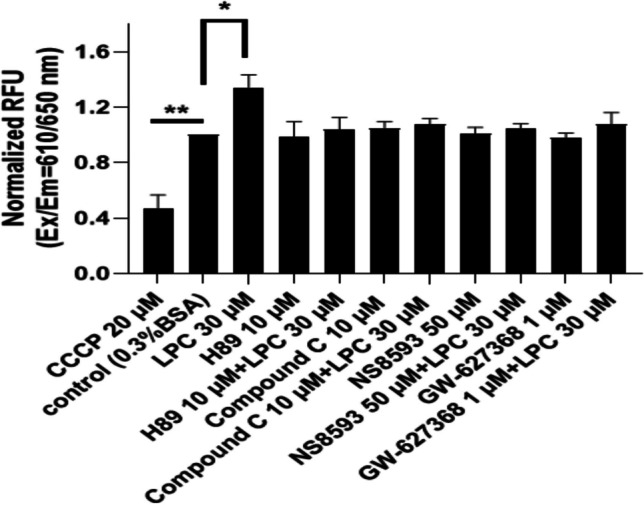
LPC increased mitochondrial membrane potential (MMP, Δψm) in BMDMs. MMP was measured with a fluorescence microplate reader after treatment of LPC 30 µM or LPC + different drugs as indicated. CCCP (20 µM) is a potent uncoupler of mitochondrial oxidative phosphorylation. Each bar represents the mean ± SEM of 3 different independent experiments. *p < 0.05 compared to the control
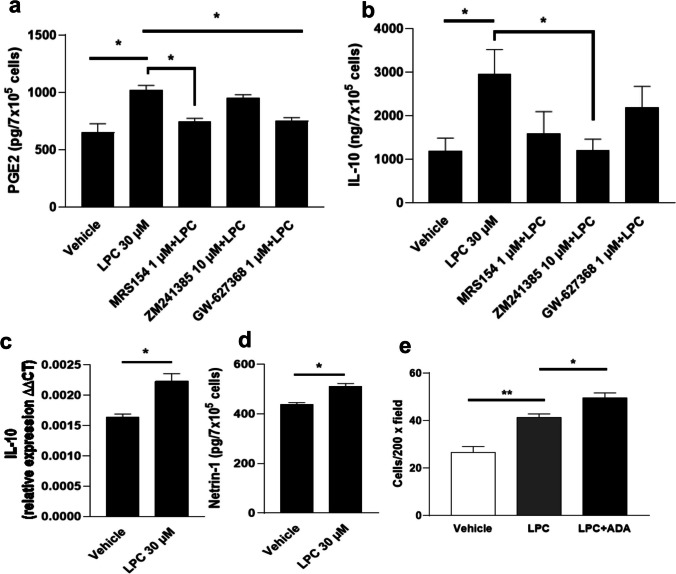
The effects of LPC on the production of PGE2, IL-10, netrin-1, and macrophages chemotaxis
LPC (30 µM) significantly induced PGE2 release in the supernatant of BMDMs (Fig. 9a). However, in the presence of the A2B receptor antagonist (MRS154, 1 µM), but not the A2A receptor antagonist (ZM241385, 10 µM), the LPC effect was abolished, suggesting that A2B receptor mediates LPC-induced PGE2 production. In the presence of GW-627368 1 µM (a specific EP4 blocker), the effect of LPC also disappeared after 12 h of treatment, suggesting that EP4 receptor is involved in the LPC-induced PGE2 production. The anti-inflammatory effects of adenosine such as the upregulation of IL-10 were mediated through A2A, A2B receptors in macrophages [2, 4]. We analyzed the effect of LPC on IL-10 upregulation at the protein and mRNA levels in macrophages. Figure 9b shows LPC (30 µM) significantly increased the release of IL-10 in the supernatant of BMDMs, and ZM241385 significantly blocked the LPC effect. However, in the presence of MRS154 or GW-627368, the reduction in the LPC effect did not reach statistical significance. In addition, no change in eATP was observed (Supplementary Fig. 4g). Figure 9c shows that LPC (30 µM) significantly increased the mRNA level of IL-10.
Fig. 9.
The effects of LPC on the production of PGE2, IL-10, netrin-1, and macrophages chemotaxis. Quantification of a PGE2, b IL-10, and d Netrin-1 in the supernatant of bone marrow-derived macrophages (BMDMs) (7 × 105/1 ml well in a 12-well plate) 12 h after stimulation with LPC 30 µM or LPC + different drugs as shown using ELISA. c Three hours after incubation, cells with LPC 30 µM or LPC + different drugs are shown, total RNA was extracted, and IL-10 mRNA levels were determined by qRT-PCR. Results were normalized against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. LPC was prepared in 5% DMEM and all drugs were subsequently diluted in 5% DMEM. All cells were treated with the same concentration of DMSO (0.1%). e Transwell migration assay was used to examine the effect of LPC (30 µM) or LPC (30 µM) + adenosine deaminase (rhADA 1 μg/mL) (serum-free DMEM was used to resuspend cells and prepare LPC) on BMDMs chemotaxis (cells were seeded at 3.5× 105/0.5 ml well in a 24-well plate) after treatment for 3 h. Each bar represents 3 different independent experiments (n = 3). Results represent the mean ± SEM. *p < 0.05, **p < 0.01
Netrin-1 has anti-inflammatory actions on immune cells and protective effects against organ damage by targeting the adenosine A2B receptor [57–59]. These common actions between LPC and netrin led us to investigate whether LPC induces netrin-1 release from BMDMs. We incubated the cells with LPC (30 µM) and after 12 h, supernatants were collected for netrin-1 quantification using ELISA assay. Remarkably, LPC (30 µM) increased netrin-1 production in the supernatant of macrophages (Fig. 9d). To the best of our knowledge, this is the first report on the release of netrin-1 in the cell culture supernatant.
Next, we analyzed whether adenosine release by LPC from macrophages contributes to the regulation of a different aspect of macrophage—not releasing effect—but behavioral aspects like chemotaxis. LPC induces macrophage chemotaxis via G2A [60]. In this context, we aimed to investigate whether the chemotaxis of BMDMs toward LPC is affected by adenosine induction by LPC. We treated cells for 3 h with LPC (30 µM) in the presence of adenosine deaminase (rhADA 1 μg/mL) (to rapidly remove adenosine from the medium) or in the absence of ADA. Figure 9e shows that the number of migrated macrophages increased significantly toward LPC. Interestingly, co-incubation of LPC with ADA significantly increased the number of migrated macrophages compared with LPC alone. Thus, these data support our hypothesis that adenosine release (induced by LPC) puts brakes on macrophage chemotaxis toward LPC. All these data in Fig. 9 strongly suggest that LPC-induced adenosine release activates autocrine anti-inflammatory signaling pathways.
LPC augmented hypoxia-elicited adenosine release but decreased hypoxia-induced extracellular and intracellular ATP
Hypoxia and inflammation often coexist and may reinforce each other [61]. The transcription factor hypoxia-inducible factor-1α (HIF-1α) plays a key role in the acute response to hypoxia [61]. HIF-1α-driven adenosine signaling attenuates harmful inflammatory immune responses, promotes tissue integrity, and minimizes organ damage [62, 63]. To investigate the role of LPC during hypoxia, we attempted to mimic hypoxic conditions in vitro using cobalt chloride (CoCl2), which induces HIF-1α stabilization [64], in addition to LPS treatment as a mimicry for inflammatory hypoxia. Figure 10a clearly shows that hypoxic conditions (CoCl2, 100 µM) or LPS (100 ng/ml) markedly increased adenosine release, and CoCl2 plus LPS further significantly enhanced adenosine release in BMDMs. Importantly, LPC (30 µM) significantly increased hypoxia-induced adenosine in BMDMs (Fig. 10a), but no change in adenosine level was observed when LPC was added to CoCl2 plus LPS for 30 min. We also determined extracellular and intracellular ATP, and found that hypoxia (CoCl2, 100 µM) or LPS (100 ng/ml) increased extracellular and intracellular ATP in BMDMs (Fig. 10b, c). However, in contrast to our observation regarding adenosine, LPC (30 µM) blocked the increase in extracellular and intracellular ATP induced by hypoxia (CoCl2, 100 µM) (Fig. 10b, c). Hypoxic cells shift their metabolism to glycolytic ATP production via the HIF-1α-mediated pathway [62]. Confirming mimetic hypoxic condition, CoCl2 (100 µM) dramatically activated HIF-1α, LPS (100 ng/ml) did likewise, and CoCl2 plus LPS synergistically induced an upregulation of HIF-1α expression (more significantly than either agent) (Fig. 10d). The results suggest that CoCl2 (100 µM), LPS (100 ng/ml), and CoCl2 plus LPS markedly increased the release of adenosine from BMDMs in the context of the HIF-1α-dependent pathway. Additionally, we examined intracellular adenosine, but no significant effect was observed (Supplementary Fig. 4f).
Fig. 10.
LPC augmented hypoxia-elicited adenosine release but decreased extracellular and intracellular ATP produced by hypoxia. To mimic hypoxic conditions, BMDMs were cultured and treated as described before. Supernatants were collected to measure a extracellular adenosine, b eATP, and c iATP. Normoxic or hypoxic cell lysates (cytosolic and nuclear fraction) were harvested and analyzed by western blotting for d β-actin, HIF-1α expression, or e phospho-Cx43, total Cx43 proteins quantification, and results were expressed relative to that in resting normoxic state cells or β-actin content of the sample. Results are derived from 3 different experiments (mean ± SEM, n = 3). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
The efflux of ATP through hemichannels leads to the accumulation of a degradation product, adenosine, which has a protective effect in hypoxia and other pathological conditions [65, 66]. Cx43 plays a key role in cellular resistance to severe injury such as hypoxia, and phosphorylation of Cx43 protein is sensitive to hypoxia [66, 67]. Therefore, to determine the effect of hypoxia on Cx43 expression in BMDMs, we performed a western blotting analysis of Cx-43 after induction of hypoxic and inflammatory hypoxic conditions. Increased phosphorylation of Cx43 was observed after treatment with CoCl2 (100 µM) or LPS (100 ng/ml) (Fig. 10e). However, co-treatment of CoCl2 (100 µM) and LPS (100 ng/ml) reversed Cx43 phosphorylation to the control value. Although CoCl2 (100 µM) did not increase the total amount of Cx43, it significantly inhibited the LPS-induced increase in total Cx43. A previous study [67] has shown that phospho-Cx43 is regulated by cellular ATP such that a decrease in intracellular ATP is accompanied by significant dephosphorylation of Cx43, which is consistent with our data showing that CoCl2 remarkably inhibited the LPS-induced intracellular ATP increase, and also inhibited the LPS-induced increase in phosphorylation of Cx43 in BMDMs (Fig. 10c, e).
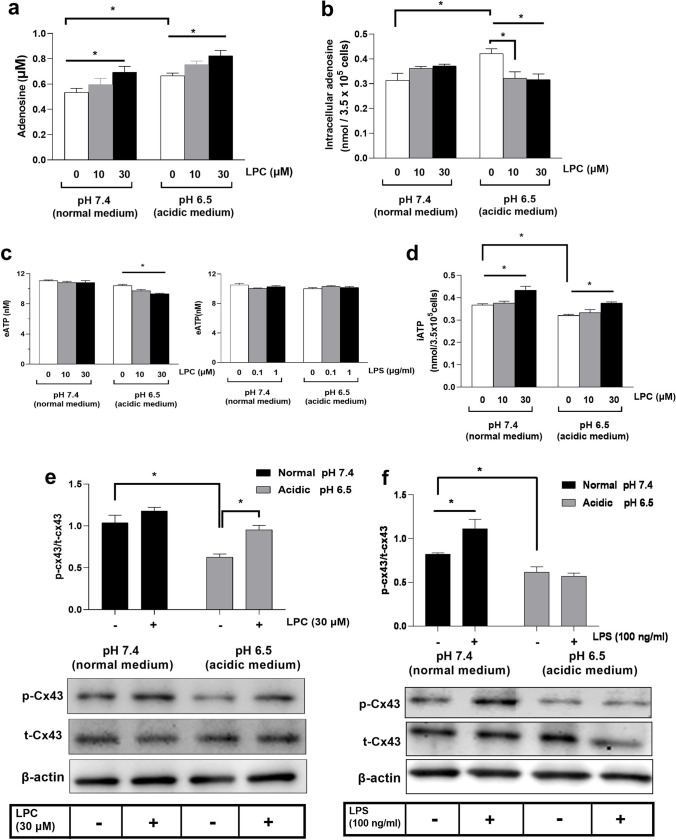
Impact of LPC on acidosis-induced signaling
Extracellular acidosis stimulates endocytosis and IL-10 secretion in BMDMs [20]. We developed an acidic environment by transiently exposing BMDMs to low extracellular pH (pH 6.5) compared with neutral media (pH 7.4). Acidosis (pH 6.5) per se significantly increased adenosine release and intracellular adenosine production in BMDMs (Fig. 11a, b). LPC (30 µM) significantly induced extracellular adenosine release at a normal pH of 7.4 and enhanced the adenosine increase triggered by acidosis (Fig. 11a). Surprisingly, LPC (10, 30 µM) markedly inhibited the intracellular adenosine increase triggered by acidosis (Fig. 11b). We observed no change in extracellular ATP between normal medium pH 7.4 and acidic medium pH 6.5 in BMDMs (Fig. 11c). Although LPC (30 µM) induced a significant reduction in extracellular ATP only in acidic medium pH 6.5, not in normal medium, LPS had no effect either in normal medium pH 7.4 or acidic medium pH 6.5 (Fig. 11c). Figure 11d shows that acidosis (pH 6.5) significantly reduced intracellular ATP levels in BMDMs, and LPC (30 µM) significantly increased intracellular ATP production in a normal medium (pH 7.4). Moreover, LPC (30 µM) significantly reversed the acidosis-induced decrease in intracellular ATP level. It remains to be elucidated how acidosis decreased intracellular ATP.
Fig. 11.
Impact of LPC on acidosis-induced signaling. BMDMs were grown in a 24-well plate (3.5× 105/0.5 ml well) and acidic environment induction for 3.5 h, pH 6.5, then treated by LPC (30 µM) or LPS (100 ng/ml) for 30 min, then a eADO in supernatants and b iADO in cellular lysates were measured. c Extracellular ATP or d intracellular ATP were estimated. Representative western blots for acidic medium-induced dephosphorylation of Cx-43 (that was prevented by e LPC, not by f LPS) using β-actin as a loading control, and relative protein levels (p-Cx43/total Cx43) were shown. Statistical analysis (mean ± SEM) was done from 3 different experiments (n = 3). *p < 0.05
Because the state of Cx43 phosphorylation correlates with cellular ATP [68], we next examined the effect of LPC (30 µM) or LPS (100 ng/ml) on Cx43 phosphorylation. Acidosis (pH 6.5) resulted in dephosphorylation of Cx43 (Fig. 11e, f), which LPC (30 µM) effectively returned to normal (pH 7.4). Although LPS (100 ng/ml) showed no effect under the acidic condition, it induced phosphorylation of Cx43 in the normal medium (pH 7.4).
Discussion
Excessive inflammatory immune responses triggered by hyperactive macrophages are considered as a major cause of increased mortality in sepsis [21, 23]. LPC is a candidate therapeutic for sepsis, as evidenced by increased bacterial clearance, decreased lethality in the CLP model, and suppression of the inflammatory immune response [14, 15]. In the current study, we demonstrated that LPC exerts a restraining effect on macrophages by inducing adenosine release. One of the most promising targets for improving sepsis outcomes is adenosine release [7, 68]. Adenosine controls the innate immune response, limits inflammation and tissue damage, and therefore plays an important anti-inflammatory role [5, 6].
We found that LPC remarkably induced the release of adenosine and the increase of intracellular ATP production (Fig. 1b, d) in a manner dependent on G2A and A2B receptors. To better understand the underlying mechanisms of LPC action, we used LPS as a comparison to LPC. It has been previously established that LPS treatment activates the adenosine signaling pathway [4, 21]. Similar to the effect of LPC, LPS increased adenosine release and intracellular ATP production (Fig. 2a, c). However, whereas LPS increases extracellular ATP (eATP) release from peritoneal macrophages after 30 min incubation [23], we observed no change in eATP levels after 30 min LPC (Fig. 1c), LPS, or LPS + LPC treatment in BMDMs (Fig. 2b). Cohen et al. [21] showed ATP release after 3-h stimulation with LPS in RAW264.7 cells; however, no detectable level of eATP up to 8-h stimulation with LPS in BMDMs in opposite to RAW264.7 cells. They nicely showed that the expression of CD39, the major ATP degrading enzyme is ten times higher in BMDMs than in RAW264.7 cells [21]. Their results exactly match with our one that we could not detect eATP level in BMDMs after 30-min treatment of LPS (Fig. 2b). The reason why we could not detect any change in eATP levels (in our manuscript and Cohen et al. [21]) is BMDMs express higher number of ectonucleotide CD39 that rapidly hydrolyzes eATP.
ATP release from macrophages is an active process that occurs in response to the agonists for TLR-2 and TLR-4 in a CX43-dependent manner [23, 24]. Cx43 has been shown to be phosphorylated in different cell types through multiple pathways [24]. In inflammatory cells, LPS stimulates phosphorylation of Cx43 mediated by the ERK1/2-MAPK pathway [24]. LPC blocks LPS-induced ERK1/2 activation, whereas LPC alone does not induce ERK activation in macrophages [25]. Moreover, LPC reduces fMLP-stimulated phosphorylation of ERK in neutrophils [11]. Interestingly, LPC counteracted TLR4-mediated phosphorylation of Cx43 (Fig. 2d), presumably via inhibition of ERK1/2 activation. Thus, LPC may play an important role in the immune regulation of macrophage responses via Cx43 during the progression of an inflammatory disease.
Macrophages play a critical role in the pathogenesis of sepsis via the upregulation of glycolysis [69]. Intriguingly, downregulation of macrophage glycolysis shows greater resistance to sepsis induced by CLP as well as by LPS, as evidenced by prolonged survival, attenuated lung injury, and lower levels of pro-inflammatory cytokines [69]. In the glucose-free medium, LPC still effectively increased adenosine release and intracellular ATP production, whereas the effect of LPS was completely abolished (Fig. 3a, b). These data suggest that glucose uptake is indispensable for the LPS effect, but not for the LPC effect. We further analyzed the role of mitochondria as the major source of cellular ATP, and found that LPC-induced adenosine release was abolished in the presence of 2-DG (that inhibits glycolysis and OXPHOS) or oligomycin (an OXPHOS inhibitor) (Fig. 5b). Mitochondrial dysfunction is a common metabolic feature of sepsis and organ damage [51]. In the same line, lower mitochondrial membrane potential (MMP, Δψm) correlates with higher levels of proinflammatory cytokines and increased risk for many diseases [70]. In this sense, the stabilization of MMP could help to protect against severe inflammation. Interestingly, LPC increased MMP [Fig. 8]. One of the most important consequences of the elevation in MMP is the increase in ATP production [53–55]. Figure 1b and d showed extracellular adenosine and intracellular ATP significantly increased by LPC. These data show a plausible link between the improvement of mitochondrial function (by increasing MMP) and the release of adenosine, suggesting that mitochondria are the target for the action of LPC.
In fact, intracellular ATP is involved in many cellular processes including activity of numerous enzymes that require ATP. Thus, the increase in intracellular ATP by OXPHOS in response to LPC could have many other cellular effects, apart from generation of adenosine.
Leishmania-infected macrophages produce extracellular adenosine either by dephosphorylation of eATP via ectoenzymes (CD39 and CD73) or by sequential degradation of iATP via cytosolic nucleotidase enzymes that generate iADO which passes the cell membrane through equilibrative nucleoside transporters (ENTs) [71, 72]. Adenosine kinase (ADK) is an important regulator of adenosine homeostasis [73]. AMP-selective 5′-nucleotidase (CN1A) acts on AMP to produce iADO [74]. We did not detect any change in the total amount of ADK and CN1A proteins after 30 min of LPC or LPS treatment (Supplementary Fig. 1c). ENT1 plays a critical regulatory role in the efflux of intracellular adenosine across cell membranes in macrophages [26]. mENT1 plays a central role in regulating plasma adenosine levels [26]. In our study, we aimed to analyze the pathway of adenosine release after treatment with LPC in parallel with treatment with LPS. The effect of LPC persisted in the presence of concomitant treatment with a CD73 inhibitor (APCP), whereas in the presence of a specific ENT1 blocker (NBTI), the effect of LPC disappeared (Fig. 4a, left panel). LPC alone had a slight tendency to increase intracellular adenosine (Supplementary Fig. 4c). In the presence of NBTI, LPC significantly increased intracellular adenosine levels (Fig. 4b). Moreover, siRNA-mediated silencing of ENT1 abolished LPC-induced adenosine release (Fig. 4c). In contrast to LPC, adenosine release induced by LPS treatment was not affected by the presence of a specific ENT1 blocker (NBTI), although the specific CD73 inhibitor (APCP) completely blocked the LPS effect (Fig. 4a, right panel). This result is in agreement with previous reports showing that CD39 and CD73 elicit an effective immunoregulatory response after TLR4 activation by LPS in macrophages [4, 21]. Thus, our results suggest that LPC and LPS differ in the way they activate the adenosine signaling pathway.
Inhibition of Ca2+ signaling impairs many effector functions of various immune cell types [27]. LPC induces an increase in cytoplasmic Ca2+ influx via the TRPM2 channel or the TRPA1 channel in macrophages [28, 29]. In our study, we demonstrated that BAPTA-AM (an intracellular Ca2+ chelator) abolished LPC-induced adenosine release and W7 (a specific calmodulin antagonist) also blocked the LPC effect (Fig. 5a). However, the effect of LPC was unchanged in the presence of KN-93 (a CaMKII inhibitor) (Fig. 5a). It is to be noted that CaMKII depletion has no effect on mitochondrial Ca2+ uptake and mitochondrial functions such as ATP production [75].
The channel kinase TRPM7 plays a dominant role in the regulation of immune responses [47, 48]. In addition to Ca2+ flux, it is essentially involved in cellular and systemic Mg2+ homeostasis [76]. We believe that dual assessment of Δ[Mg2+]i together with Δ[Ca2+]i provides a complete picture of TRPM7 functionality in LPC treatment. Both the addition of a potent TRPM7 blocker (NS8593) and experimental knockdown of TRPM7 blocked the release of adenosine (Fig. 6a, c) and significantly inhibited the increase in [Mg2+]i caused by LPC (Fig. 6b, d). In the Mg2+-free medium, the effect of LPC was blocked (Fig. 6f). Moreover, NS8593 (a specific TRPM7 blocker) negated the mitochondrial membrane potential increased by LPC (Fig. 8). All these results identify TRPM7 as an important cellular element essential for adenosine release by LPC. Ca2+ influx via TRPM7 is critical for LPS-induced activation of murine macrophages [77]. On the contrary, the increase in [Ca2+]i by LPC was not affected by pharmacological inhibition (with NS8593), and by siRNA-mediated downregulation of TRPM7 (Fig. 6b, d), suggesting that TRPM7 is not required for LPC-induced [Ca2+]i increase.
LPC induces an elevation of intracellular cAMP level, thus stimulating the cAMP-PKA pathway in macrophages [32]. We investigated the role of the PKA pathway in the effects of LPC using H89 (a selective PKA inhibitor). The data showed that PKA inhibition blocked LPC-induced increase in adenosine release (Fig. 5b), [Mg2+]i (Fig. 5e), and MMP (Fig. 8).
AMPK is considered a potential target for the treatment of diseases associated with metabolic disorders, and functions as a central mediator of the cellular response to energetic stress and mitochondrial insults [40, 41]. LPC-induced AMPK activation suppresses HMGB1 translocation, increases bacterial phagocytosis in macrophages, and compound C (an AMPK inhibitor) reverses LPC effects [42, 43]. Our results showed that compound C blocked LPC-induced increase in adenosine release (Fig. 5d), and [Mg2+]i (Fig. 5e) in BMDMs. These data suggest a novel link between AMPK and TRPM7, i.e., TRPM7 may be a downstream target of AMPK activation.
LKB1 and CaMKII have been shown to be upstream kinases for AMPK phosphorylation [40, 41]. However, studies of cells devoid of LKB1 indicate that LKB1 mediates nearly all of the activation of AMPK in response to mitochondrial insults [40]. Moreover, LKB1 inhibits pro-inflammatory signaling in response to LPS in macrophages [78]. Stimulation of the LKB1/AMPK pathway improves survival in sepsis and protects against endotoxemia [79]. In the present study, we demonstrated that H89 (a PKA inhibitor) significantly reduced LPC-induced AMPK phosphorylation and LKB1 phosphorylation (Fig. 7a), whereas the CaMKII inhibitor (KN-93) had no effect (Fig. 7b). Recently, PKA and AMPK were shown to cooperate in the regulation of mitochondrial functions [80]. We demonstrated that inhibition of AMPK by compound C blocked the LPC-induced increase in MMP (Fig. 8). LPC activates MAPK signaling molecules such as JNK and p38 in macrophages [25]. Therefore, we tested a selective p38 MAPK inhibitor (SB203580) for the induction of adenosine release by LPC. We found that SB203580 did not affect the effect of LPC (Fig. 5b), suggesting that the p38 MAPK pathway is not required for LPC-mediated enhancement of adenosine release. Consistent with our data, SB203580 did not reduce LPC-induced phosphorylation of AMPK [43].
Recently, blocked PGs synthesis was shown to negatively affect adenosine flux across cell membranes [37]. Co-incubation of LPC with three different NSAIDs (indomethacin, diclofenac, and FFA) resulted in the blockade of LPC-induced adenosine release in BMDMs (Fig. 5c). These three NSAIDs have been shown to inhibit mitochondrial ATP synthesis and decrease MMP (Δψm) in various cell types [34–36]. In addition, FFA has been reported to trigger the mitochondrial permeability transition, thereby decreasing MMP, mitochondrial function, and oxidative phosphorylation [36] which agrees with our findings (Supplementary Fig. 1d) that FFA reliably decreased intracellular ATP, and LPC (30 µM) reversed this event. Moreover, FFA slightly decreased eATP (Supplementary Fig. 4d). Indomethacin and diclofenac blocked the LPC-induced increase in [Mg2+]i, but not [Ca2+]i (Fig. 5f) in BMDMs. These data highlight the involvement of PGs in the action of LPC. It is to be noted that LPC increases PGE2 secretion in macrophages [10] which is consistent with our data (Fig. 9a). PGE2 is required for the stimulation of the adenosine pathway in immune cells [81]. Moreover, PGE2 induces a variety of anti-inflammatory effects in macrophages [38, 39]. We tested blockers for EP2 (PF-04418948) and EP4 (GW-627368) on LPC-induced adenosine release. The data showed that EP4 receptor was required for LPC-induced adenosine release, whereas the EP2 antagonist had no effect (Fig. 5d). In the next experiment, to further investigate the role of PGE2 via EP4, we measured [Mg2+]i and MMP. The results showed that specific antagonism of EP4 significantly inhibited the LPC-induced increase in MMP (Fig. 8) and in [Mg2+]i (Fig. 5f). Consistently, reduction in EP4 receptor expression led to a collapse in MMP and consequent inhibition of mitochondrial ATP production [56]. Interestingly, EP4 in macrophages was found to promote the resolution of inflammation and limit liver damage [82]. Adenosine (acting mainly on A2A and A2B receptors) stimulates anti-inflammatory signaling pathways including IL-10 production in macrophages [6]. Western blotting and qRT-PCR results showed that LPC increased the production of IL-10 (Fig. 9b, c). The blockade of A2A abolished LPC-induced IL-10 release (Fig. 9b). Taken together, the release of PGE2 is essential for the autocrine anti-inflammatory effects of LPC via adenosine release.
Netrin-1 is involved in axon guidance during fetal development and leukocyte migration in response to various chemotactic stimuli [83]. We noted several similarities between LPC and netrin-1 as follows: the A2B receptor is required for the protective effects of both against acute systemic injury [15, 57–59], plasma levels of LPC significantly decrease in sepsis [84], netrin-1 remarkably decreases in the lungs of septic rats [85], and macrophages are functional targets for both [15, 59]. As shown in Fig. 9d, LPC significantly increased netrin-1 secretion in BMDMs. Another aspect to be highlighted is the role that LPC-induced adenosine release plays in regulating macrophage chemotaxis toward LPC. We demonstrated that the removal of adenosine from the medium (using ADA) significantly increased the migration of macrophages toward LPC (Fig. 9e). These results support our hypothesis that LPC puts brakes on macrophages via adenosine release.
Inflamed tissues are characterized by hypoxia and acidic foci [20, 61]. Macrophages not only sense injury and infection, but also other types of detrimental conditions such as hypoxia and metabolic stress such as acidosis. Pharmacological strategies to enhance adenosine release triggered by stress or to promote adenosine signaling through adenosine receptors contribute to limiting inflammatory processes [7, 62]. Thus, we simulated hypoxic and acidic conditions in vitro. Figures 10a and 11a show that LPC enhanced the hypoxia-evoked/acidosis-induced adenosine production in BMDMs.
Conclusion
We delineated in this study the mechanisms induced by LPC to stimulate adenosine release from the macrophages (Fig. 12). It is the first-ever report that links cellular Mg2+ status to adenosine release. Released adenosine can limit inflammatory responses by activating an autocrine anti-inflammatory negative feedback loop. Thus, LPC may put a brakes-like system on hyperactive macrophages during a severe inflammatory state.
Fig. 12.
A schematic diagram for LPC action. The increased release of PGE2 (which possibly resulted from phospholipase A2 (PLA2) activation mediated by LPC-induced calcium influx) activates EP4 receptor which results in potentiation of activation of cAMP-PKA pathway. Stimulated PKA signaling leads to activation of LKB1/AMPK axis followed by increasing Mg.2+ influx concomitantly with an increase in mitochondrial ATP. Then, ATP is converted to adenosine intracellularly followed by efflux via ENT1. Released adenosine can act on A2A and A2B receptors to stimulate anti-inflammatory effects. The broken arrows indicate a signaling pathway from literature [28, 29, 89] but not determined in this study
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Shin-Hae Kang for helping AMY with the experiments.
Abbreviations
- AMPK
AMP-activated protein kinase
- APCP
Adenosine 5′-(α,β-methylene)diphosphate
- BMDMs
Bone marrow-derived macrophages
- BAPTA-AM
1,2-Bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- CaMKII
Calcium/calmodulin-dependent kinase kinase 2
- ENT1
Equilibrative nucleoside transporter
- HIF-1α
Hypoxia-inducible factor 1α
- LPC
Lysophosphatidylcholine
- LPS
Lipopolysaccharide
- LKB1
Liver kinase B1
- MMP
Mitochondrial membrane potential
- NBTI
S-(4-nitrobenzyl)-6-thioinosine
- TRPM7
Transient receptor potential melastatin member 7
Author contribution
DKS conceived, DKS and AMY designed this study. AMY did the experiments. AMY and DKS analyzed the data and wrote the manuscript.
Funding
This research was supported by the basic research program of the National Research Foundation of Korea (NRF), funded by the Ministry of Science ICT (NRF-2020R1F1A1067708).
Data availability
Not applicable.
Declarations
Ethics approval
We hereby confirm that we have complied with ethical standards.
Informed consent
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s11302-023-09962-x
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/12/2023
This article has been retracted. Please see the Retraction Notice for more detail: 10.1007/s11302-023-09962-x
References
- 1.Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:281–286. doi: 10.2174/1568010054022024. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe S, Alexander M, Misharin AV, Budinger GRS. The role of macrophages in the resolution of inflammation. J Clin Invest. 2019;129:2619–2628. doi: 10.1172/JCI124615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly B, O’Neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25:771–784. doi: 10.1038/cr.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cekic C, Linden J. Purinergic regulation of the immune system. Nat Rev Immunol. 2016;16:177–192. doi: 10.1038/nri.2016.4. [DOI] [PubMed] [Google Scholar]
- 5.Leitão-Rocha A, Sousa J, Diniz C (2012) Adenosinergic system in the mesenteric vessels, the cardiovascular system - Physiology, Diagnostics and Clinical Implications, Dr. David Gaze, ed. IntechOpen, London, UK. p. 109–134.
- 6.Csoka B, Selmeczy Z, Koscso B, Nemeth ZH, Pacher P, Murray PJ, Kepka-Lenhart D, Morris SM, Jr, Gause WC, Leibovich SJ, Hasko G. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J. 2012;26:376–386. doi: 10.1096/fj.11-190934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Effendi WI, Nagano T, Kobayashi K, Nishimura Y. Focusing on adenosine receptors as a potential targeted therapy in human diseases. Cells. 2020;9:785. doi: 10.3390/cells9030785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryzhov S, Zaynagetdinov R, Goldstein AE, Novitskiy SV, Blackburn MR, Biaggioni I, Feoktistov I. Effect of A2B adenosine receptor gene ablation on adenosine-dependent regulation of proinflammatory cytokines. J Pharmacol Exp Ther. 2008;324:694–700. doi: 10.1124/jpet.107.131540. [DOI] [PubMed] [Google Scholar]
- 9.Law SH, Chan ML, Marathe GK, Parveen F, Chen CH, Ke LY. An updated review of lysophosphatidylcholine metabolism in human diseases. Int J Mol Sci. 2019;20:1149. doi: 10.3390/ijms20051149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Assunção LS, Magalhães KG, Carneiro AB, Molinaro R, Almeida PE, Atella GC, Castro-Faria-Neto HC, Bozza PT. Schistosomal-derived lysophosphatidylcholine triggers M2 polarization of macrophages through PPARγ dependent mechanisms. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862:246–254. doi: 10.1016/j.bbalip.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Lin P, Welch EJ, Gao XP, Malik AB, Ye RD. Lysophosphatidylcholine modulates neutrophil oxidant production through elevation of cyclic AMP. J Immunol. 2005;174:2981–2989. doi: 10.4049/jimmunol.174.5.2981. [DOI] [PubMed] [Google Scholar]
- 12.Tounsi N, Meghari S, Moser M, Djerdjouri B. Lysophosphatidylcholine exacerbates Leishmania major-dendritic cell infection through interleukin-10 and a burst in arginase1 and indoleamine 2,3-dioxygenase activities. Int Immunopharmacol. 2015;25:1–9. doi: 10.1016/j.intimp.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa H, Lei J, Matsumoto T, Onishi S, Suemori K, Yasukawa M. Lysophosphatidylcholine enhances the suppressive function of human naturally occurring regulatory T cells through TGF-β production. Biochem Biophys Res Commun. 2011;415:526–531. doi: 10.1016/j.bbrc.2011.10.119. [DOI] [PubMed] [Google Scholar]
- 14.Yan JJ, Jung JS, Lee JE, Lee J, Huh SO, Kim HS, Jung KC, Cho JY, Nam JS, Suh HW, Kim YH, Song DK. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat Med. 2004;10:161–167. doi: 10.1038/nm989. [DOI] [PubMed] [Google Scholar]
- 15.Li HM, Jang JH, Jung JS, Shin J, Park CO, Kim YJ, Ahn WG, Nam JS, Hong CW, Lee J, Jung YJ, Chen JF, Ravid K, Lee HT, Huh WK, Kabarowski JH, Song DK. G2A protects mice against sepsis by modulating Kupffer cell activation: cooperativity with adenosine receptor 2b. J Immunol. 2019;202:527–538. doi: 10.4049/jimmunol.1700783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trouplin V, Boucherit N, Gorvel L, Conti F, Mottola G, Ghigo E (2013) Bone marrow-derived macrophage production. J Vis Exp: e50966. 10.3791/50966 [DOI] [PMC free article] [PubMed]
- 17.Corrêa R, Silva LFF, Ribeiro DJS, Almeida RDN, Santos IO, Corrêa LH, de Sant’Ana LP, Assunção LS, Bozza PT, Magalhães KG. Lysophosphatidylcholine induces NLRP3 inflammasome-mediated foam cell formation and pyroptosis in human monocytes and endothelial cells. Front Immunol. 2019;10:2927. doi: 10.3389/fimmu.2019.02927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mochizuki M, Zigler JS, Jr, Russell P, Gery I. Serum proteins neutralize the toxic effect of lysophosphatidylcholine. Curr Eye Res. 1982;2:621–624. doi: 10.3109/02713688208996363. [DOI] [PubMed] [Google Scholar]
- 19.Kim YL, Im YJ, Ha NC, Im DS. Albumin inhibits cytotoxic activity of lysophosphatidylcholine by direct binding. Prostaglandins Other Lipid Mediat. 2007;83:130–138. doi: 10.1016/j.prostaglandins.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Kong X, Tang X, Du W, Tong J, Yan Y, Zheng F, Fang M, Gong F, Tan Z. Extracellular acidosis modulates the endocytosis and maturation of macrophages. Cell Immunol. 2013;281:44–50. doi: 10.1016/j.cellimm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Cohen HB, Briggs KT, Marino JP, Ravid K, Robson SC, Mosser DM. TLR stimulation initiates a CD39-based autoregulatory mechanism that limits macrophage inflammatory responses. Blood. 2013;122:1935–1945. doi: 10.1182/blood-2013-04-496216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dosch M, Zindel J, Jebbawi F, Melin N, Sanchez-Taltavull D, Stroka D, Candinas D, Beldi G. Connexin-43-dependent ATP release mediates macrophage activation during sepsis. Elife. 2019;8:42670. doi: 10.7554/eLife.42670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dosch M, Gerber J, Jebbawi F, Beldi G. Mechanisms of ATP release by inflammatory cells. Int J Mol Sci. 2018;19:1222. doi: 10.3390/ijms19041222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carneiro AB, Iaciura BM, Nohara LL, Lopes CD, Veas EM, Mariano VS, Bozza PT, Lopes UG, Atella GC, Almeida IC, Silva-Neto MA. Lysophosphatidylcholine triggers TLR2- and TLR4-mediated signaling pathways but counteracts LPS-induced NO synthesis in peritoneal macrophages by inhibiting NF-κB translocation and MAPK/ERK phosphorylation. PLoS One. 2013;8:e76233. doi: 10.1371/journal.pone.0076233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boswell-Casteel RC, Hays FA. Equilibrative nucleoside transporters-a review. Nucleosides Nucleotides Nucleic Acids. 2017;36:7–30. doi: 10.1080/15257770.2016.1210805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaeth M, Zee I, Concepcion AR, Maus M, Shaw P, Portal-Celhay C, Zahra A, Kozhaya L, Weidinger C, Philips J, Unutmaz D, Feske S. Ca2+ signaling but not store-operated Ca2+ entry is required for the function of macrophages and dendritic cells. J Immunol. 2015;195:1202–1217. doi: 10.4049/jimmunol.1403013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian C, Huang R, Tang F, Lin Z, Cheng N, Han X, Li S, Zhou P, Deng S, Huang H, Zhao H, Xu J, Li Z. Transient receptor potential Ankyrin 1 contributes to lysophosphatidylcholine-induced intracellular calcium regulation and THP-1-derived macrophage activation. J Membr Biol. 2020;253:43–55. doi: 10.1007/s00232-019-00104-2. [DOI] [PubMed] [Google Scholar]
- 29.Jeong H, Kim YH, Lee Y, Jung SJ, Oh SB. TRPM2 contributes to LPC-induced intracellular Ca(2+) influx and microglial activation. Biochem Biophys Res Commun. 2017;485:301–306. doi: 10.1016/j.bbrc.2017.02.087. [DOI] [PubMed] [Google Scholar]
- 30.Bicket A, Mehrabi P, Naydenova Z, Wong V, Donaldson L, Stagljar I, Coe IR. Novel regulation of equlibrative nucleoside transporter 1 (ENT1) by receptor-stimulated Ca2+-dependent calmodulin binding. Am J Physiol Cell Physiol. 2016;310:C808–C820. doi: 10.1152/ajpcell.00243.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Racioppi L, Noeldner PK, Lin F, Arvai S, Means AR. Calcium/calmodulin-dependent protein kinase kinase 2 regulates macrophage-mediated inflammatory responses. J Biol Chem. 2012;287:11579–11591. doi: 10.1074/jbc.M111.336032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee HJ, Ko HJ, Song DK, Jung YJ. Lysophosphatidylcholine promotes phagosome maturation and regulates inflammatory mediator production through the protein kinase A-phosphatidylinositol 3 kinase-p38 mitogen-activated protein kinase signaling pathway during mycobacterium tuberculosis infection in mouse macrophages. Front Immunol. 2018;9:920. doi: 10.3389/fimmu.2018.00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Depaoli MR, Karsten F, Madreiter-Sokolowski CT, Klec C, Gottschalk B, Bischof H, Eroglu E, Waldeck-Weiermair M, Simmen T, Graier WF, Malli R. Real-time imaging of mitochondrial ATP dynamics reveals the metabolic setting of single cells. Cell Rep. 2018;25:501–12.e3. doi: 10.1016/j.celrep.2018.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Syed M, Skonberg C, Hansen SH. Mitochondrial toxicity of diclofenac and its metabolites via inhibition of oxidative phosphorylation (ATP synthesis) in rat liver mitochondria: possible role in drug induced liver injury (DILI) Toxicol In Vitro. 2016;31:93–102. doi: 10.1016/j.tiv.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 35.Matsui H, Shimokawa O, Kaneko T, Nagano Y, Rai K, Hyodo I. The pathophysiology of non-steroidal anti-inflammatory drug (NSAID)-induced mucosal injuries in stomach and small intestine. J Clin Biochem Nutr. 2011;48:107–111. doi: 10.3164/jcbn.10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jordani MC, Santos AC, Prado IM, Uyemura SA, Curti C. Flufenamic acid as an inducer of mitochondrial permeability transition. Mol Cell Biochem. 2000;210:153–158. doi: 10.1023/a:1007185825101. [DOI] [PubMed] [Google Scholar]
- 37.Li RW, Seto SW, Au AL, Kwan YW, Chan SW, Lee SM, Tse CM, Leung GP. Inhibitory effect of nonsteroidal anti-inflammatory drugs on adenosine transport in vascular smooth muscle cells. Eur J Pharmacol. 2009;612:15–20. doi: 10.1016/j.ejphar.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 38.MacKenzie KF, Clark K, Naqvi S, McGuire VA, Nöehren G, Kristariyanto Y, van den Bosch M, Mudaliar M, McCarthy PC, Pattison MJ, Pedrioli PG, Barton GJ, Toth R, Prescott A, Arthur JS. PGE(2) induces macrophage IL-10 production and a regulatory-like phenotype via a protein kinase A-SIK-CRTC3 pathway. J Immunol. 2013;190:565–577. doi: 10.4049/jimmunol.1202462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Na YR, Jung D, Yoon BR, Lee WW, Seok SH. Endogenous prostaglandin E2 potentiates anti-inflammatory phenotype of macrophage through the CREB-C/EBP-β cascade. Eur J Immunol. 2015;45:2661–2671. doi: 10.1002/eji.201545471. [DOI] [PubMed] [Google Scholar]
- 40.Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19:121–135. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hardie DG. The AMP-activated protein kinase pathway—new players upstream and downstream. J Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 42.Kim JM, Han HJ, Hur YH, Quan H, Kwak SH, Choi JI, Bae HB. Stearoyl lysophosphatidylcholine prevents lipopolysaccharide-induced extracellular release of high mobility group box-1 through AMP-activated protein kinase activation. Int Immunopharmacol. 2015;28:540–545. doi: 10.1016/j.intimp.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 43.Quan H, Hur YH, Xin C, Kim JM, Choi JI, Kim MY, Bae HB. Stearoyl lysophosphatidylcholine enhances the phagocytic ability of macrophages through the AMP-activated protein kinase/p38 mitogen activated protein kinase pathway. Int Immunopharmacol. 2016;39:328–334. doi: 10.1016/j.intimp.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 44.Rubin H. Central role for magnesium in coordinate control of metabolism and growth in animal cells. Proc Natl Acad Sci U S A. 1975;72:3551–3555. doi: 10.1073/pnas.72.9.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiao W, Wong KHM, Shen J, Wang W, Wu J, Li J, Lin Z, Chen Z, Matinlinna JP, Zheng Y, Wu S, Liu X, Lai KP, Chen Z, Lam YW, Cheung KMC, Yeung KWK. TRPM7 kinase-mediated immunomodulation in macrophage plays a central role in magnesium ion-induced bone regeneration. Nat Commun. 2021;12:2885. doi: 10.1038/s41467-021-23005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawahara K, Sato R, Iwabuchi S, Matsuyama D. Rhythmic fluctuations in the concentration of intracellular Mg2+ in association with spontaneous rhythmic contraction in cultured cardiac myocytes. Chronobiol Int. 2008;25:868–881. doi: 10.1080/07420520802536387. [DOI] [PubMed] [Google Scholar]
- 47.Nadolni W, Zierler S. The channel-kinase TRPM7 as novel regulator of immune system homeostasis. Cells. 2018;7:109. doi: 10.3390/cells7080109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schilling T, Miralles F, Eder C. TRPM7 regulates proliferation and polarisation of macrophages. J Cell Sci. 2014;127:4561–4566. doi: 10.1242/jcs.151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chubanov V, Gudermann T. Mapping TRPM7 function by NS8593. Int J Mol Sci. 2020;21:7017. doi: 10.3390/ijms21197017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Angajala A, Lim S, Phillips JB, Kim JH, Yates C, You Z, Tan M. Diverse roles of mitochondria in immune responses: novel insights into immuno-metabolism. Front Immunol. 2018;9:1605. doi: 10.3389/fimmu.2018.01605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mantzarlis K, Tsolaki V, Zakynthinos E. Role of oxidative stress and mitochondrial dysfunction in sepsis and potential therapies. Oxid Med Cell Longev. 2017;2017:5985209. doi: 10.1155/2017/5985209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arts RJ, Gresnigt MS, Joosten LA, Netea MG. Cellular metabolism of myeloid cells in sepsis. J Leukoc Biol. 2017;101:151–164. doi: 10.1189/jlb.4MR0216-066R. [DOI] [PubMed] [Google Scholar]
- 53.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques. 2011;50:98–115. doi: 10.2144/000113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ding C, Han F, Xiang H, Wang Y, Dou M, Xia X, Li Y, Zheng J, Ding X, Xue W, Tian P. Role of prostaglandin E2 receptor 4 in the modulation of apoptosis and mitophagy during ischemia/reperfusion injury in the kidney. Mol Med Rep. 2019;20:3337–3346. doi: 10.3892/mmr.2019.10576. [DOI] [PubMed] [Google Scholar]
- 56.Mirakaj V, Gatidou D, Pötzsch C, König K, Rosenberger P. Netrin-1 signaling dampens inflammatory peritonitis. J Immunol. 2011;186:549–555. doi: 10.4049/jimmunol.1002671. [DOI] [PubMed] [Google Scholar]
- 57.Duan L, Woolbright BL, Jaeschke H, Ramachandran A. Late protective effect of Netrin-1 in the murine acetaminophen hepatotoxicity model. Toxicol Sci. 2020;175:168–181. doi: 10.1093/toxsci/kfaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aherne CM, Collins CB, Masterson JC, Tizzano M, Boyle TA, Westrich JA, Parnes JA, Furuta GT, Rivera-Nieves J, Eltzschig HK. Neuronal guidance molecule netrin-1 attenuates inflammatory cell trafficking during acute experimental colitis. Gut. 2012;61:695–705. doi: 10.1136/gutjnl-2011-300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang LV, Radu CG, Wang L, Riedinger M, Witte ON. Gi-independent macrophage chemotaxis to lysophosphatidylcholine via the immunoregulatory GPCR G2A. Blood. 2005;105:1127–1134. doi: 10.1182/blood-2004-05-1916. [DOI] [PubMed] [Google Scholar]
- 60.Watts ER, Walmsley SR. Inflammation and hypoxia: HIF and PHD isoform selectivity. Trends Mol Med. 2019;25:33–46. doi: 10.1016/j.molmed.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 61.Bowser JL, Lee JW, Yuan X, Eltzschig HK. The hypoxia-adenosine link during inflammation. J Appl Physiol. 2017;123:1303–1320. doi: 10.1152/japplphysiol.00101.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grenz A, Homann D, Eltzschig HK. Extracellular adenosine: a safety signal that dampens hypoxia-induced inflammation during ischemia. Antioxid Redox Signal. 2011;15:2221–2234. doi: 10.1089/ars.2010.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shweta MKP, Chanda S, Singh SB, Ganju L. A comparative immunological analysis of CoCl2 treated cells with in vitro hypoxic exposure. Biometals. 2015;28:175–185. doi: 10.1007/s10534-014-9813-9. [DOI] [PubMed] [Google Scholar]
- 64.Dou L, Chen YF, Cowan PJ, Chen XP. Extracellular ATP signaling and clinical relevance. Clin Immunol. 2018;188:67–73. doi: 10.1016/j.clim.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 65.Lin JH, Lou N, Kang N, Takano T, Hu F, Han X, Xu Q, Lovatt D, Torres A, Willecke K, Yang J, Kang J, Nedergaard M. A central role of connexin 43 in hypoxic preconditioning. J Neurosci. 2008;28:681–695. doi: 10.1523/JNEUROSCI.3827-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Turner MS, Haywood GA, Andreka P, You L, Martin PE, Evans WH, Webster KA, Bishopric NH. Reversible connexin 43 dephosphorylation during hypoxia and reoxygenation is linked to cellular ATP levels. Circ Res. 2004;95:726–733. doi: 10.1161/01.RES.0000144805.11519.1e. [DOI] [PubMed] [Google Scholar]
- 67.Sivak KV, Vasin AV, Egorov VV, Tsevtkov VB, Kuzmich NN, Savina VA, Kiselev OI. Adenosine A2A receptor as a drug target for treatment of sepsis. Mol Biol (Mosk) 2016;50:231–245. doi: 10.7868/S0026898416020233. [DOI] [PubMed] [Google Scholar]
- 68.Wang Z, Kong L, Tan S, Zhang Y, Song X, Wang T, Lin Q, Wu Z, Xiang P, Li C, Gao L, Liang X, Ma C. Zhx2 accelerates sepsis by promoting macrophage glycolysis via Pfkfb3. J Immunol. 2020;204:2232–2241. doi: 10.4049/jimmunol.1901246. [DOI] [PubMed] [Google Scholar]
- 69.Zorova LD, Popkov VA, Plotnikov EY, Silachev DN, Pevzner IB, Jankauskas SS, Babenko VA, Zorov SD, Balakireva AV, Juhaszova M, Sollott SJ, Zorov DB. Mitochondrial membrane potential. Anal Biochem. 2018;552:50–59. doi: 10.1016/j.ab.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Basu M, Gupta P, Dutta A, Jana K, and Ukil A (2020) Increased host ATP efflux and its conversion to extracellular adenosine is crucial for establishing Leishmania infection. J Cell Sci 133. 10.1242/jcs.239939 [DOI] [PubMed]
- 71.Silva D, Moreira D, Cordeiro-da-Silva A, Quintas C, Gonçalves J, Fresco P. Intracellular adenosine released from THP-1 differentiated human macrophages is involved in an autocrine control of Leishmania parasitic burden, mediated by adenosine A(2A) and A(2B) receptors. Eur J Pharmacol. 2020;885:173504. doi: 10.1016/j.ejphar.2020.173504. [DOI] [PubMed] [Google Scholar]
- 72.Boison D, Jarvis MF. Adenosine kinase: A key regulator of purinergic physiology. Biochem Pharmacol. 2021;187:114321. doi: 10.1016/j.bcp.2020.114321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Borowiec A, Lechward K, Tkacz-Stachowska K, Składanowski AC. Adenosine as a metabolic regulator of tissue function: production of adenosine by cytoplasmic 5′-nucleotidases. Acta Biochim Pol. 2006;53:269–278. doi: 10.18388/abp.2006_3339. [DOI] [PubMed] [Google Scholar]
- 74.Nickel AG, Kohlhaas M, Bertero E, Wilhelm D, Wagner M, Sequeira V, Kreusser MM, Dewenter M, Kappl R, Hoth M, Dudek J, Backs J, Maack C. CaMKII does not control mitochondrial Ca(2+) uptake in cardiac myocytes. J Physiol. 2020;598:1361–1376. doi: 10.1113/JP276766. [DOI] [PubMed] [Google Scholar]
- 75.Ryazanova LV, Rondon LJ, Zierler S, Hu Z, Galli J, Yamaguchi TP, Mazur A, Fleig A, Ryazanov AG. TRPM7 is essential for Mg(2+) homeostasis in mammals. Nat Commun. 2010;1:109. doi: 10.1038/ncomms1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schappe MS, Szteyn K, Stremska ME, Mendu SK, Downs TK, Seegren PV, Mahoney MA, Dixit S, Krupa JK, Stipes EJ, Rogers JS, Adamson SE, Leitinger N, Desai BN. Chanzyme TRPM7 mediates the Ca(2+) influx essential for lipopolysaccharide-induced toll-like receptor 4 endocytosis and macrophage activation. Immunity. 2018;48:59–74.e5. doi: 10.1016/j.immuni.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Z, Zhang W, Zhang M, Zhu H, Moriasi C, Zou MH. Liver kinase B1 suppresses lipopolysaccharide-induced nuclear factor κB (NF-κB) activation in macrophages. J Biol Chem. 2015;290:2312–2320. doi: 10.1074/jbc.M114.616441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang Y, Dong R, Hu D, Chen Z, Fu M, Wang DW, Xu X, Tu L. Liver kinase B1/AMP-activated protein kinase pathway activation attenuated the progression of endotoxemia in the diabetic mice. Cell Physiol Biochem. 2017;42:761–779. doi: 10.1159/000478068. [DOI] [PubMed] [Google Scholar]
- 79.Zhang J, Wang Y, Liu X, Dagda RK, Zhang Y. How AMPK and PKA interplay to regulate mitochondrial function and survival in models of ischemia and diabetes. Oxid Med Cell Longev. 2017;2017:4353510. doi: 10.1155/2017/4353510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Al-Taei S, Salimu J, Spary LK, Clayton A, Lester JF, Tabi Z. Prostaglandin E(2)-mediated adenosinergic effects on CD14(+) cells: self-amplifying immunosuppression in cancer. Oncoimmunology. 2017;6:e1268308. doi: 10.1080/2162402X.2016.1268308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang J, Liu Y, Ding H, Shi X, Ren H. Mesenchymal stem cell-secreted prostaglandin E(2) ameliorates acute liver failure via attenuation of cell death and regulation of macrophage polarization. Stem Cell Res Ther. 2021;12:15. doi: 10.1186/s13287-020-02070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Claro V, Ferro A. Netrin-1: focus on its role in cardiovascular physiology and atherosclerosis. JRSM Cardiovasc Dis. 2020;9:2048004020959574. doi: 10.1177/2048004020959574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Amunugama K, Pike DP, Ford DA. The lipid biology of sepsis. J Lipid Res. 2021;62:100090. doi: 10.1016/j.jlr.2021.100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu J, Du J, Cheng X, Zhang X, Li Y, Fu X, Chen X. Effect of Netrin-1 anti-inflammatory factor on acute lung injury in sepsis rats. Med Sci Monit. 2019;25:7928–35. doi: 10.12659/MSM.917279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leslie CC. Cytosolic phospholipase A2: physiological function and role in disease. J Lipid Res. 2015;8:1386–402. doi: 10.1194/jlr.R057588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.