Abstract
Background
Central venous catheters raise the risk of catheter‐related thrombosis (CRT) in patients with cancer, typically affecting the upper extremity. Management of CRT involves catheter removal and anticoagulation. However, robust evidence is lacking on the optimal timing of anticoagulation relative to catheter removal.
Objectives
Our goal is to provide a better understanding of the factors that increase the risk of recurrent venous thromboembolism (VTE) in these patients.
Patients and Methods
We conducted a retrospective chart review of all consecutive patients with cancer in our hospital affected by CRT between January 1, 2015, and December 31, 2017. We measured recurrence of VTE as thrombosis in any vascular bed or pulmonary embolism, for up to 2 years after diagnosis. Logistic and competing risk regression analyses were used to determine the association between different clinical factors and any VTE recurrence in patients with cancer and CRT.
Results
Of the 257 individuals meeting the inclusion criteria, 80.2% had their catheter removed; of these, 50.5% did not receive anticoagulation before the removal. Patients who did not receive anticoagulation before the removal had increased 3‐month and 1‐year risks of recurrent VTE (odds ratio, 5.07 [95% confidence interval [CI], 1.53–23.18]; and hazard ratio, 3.47 [95% CI, 1.34–9.01]), respectively.
Conclusions
Our study supports the use of anticoagulants before catheter removal in patients with CRT. Randomized clinical trials are recommended to establish stronger evidence pertaining to the long‐term risk of VTE recurrence and the effect of catheter reinsertion.
Keywords: cancer, catheter, emergency department, thrombosis, venous thromboembolism
Essentials.
Central venous catheters increase the risk of catheter‐related thrombosis.
Patients with cancer and central venous catheter–associated venous thromboembolism (VTE) were studied.
Catheter removal before anticoagulation increased recurrence of VTE at 3 months.
Our study supports the use of anticoagulation before catheter removal in patients with cancer.
1. INTRODUCTION
Venous thromboembolism (VTE) is a leading cause of morbidity and mortality among patients with cancer. 1 , 2 In addition to malignancy, specific conditions such as the use of prothrombotic therapeutic agents and long‐term indwelling vascular devices increase the risk of VTE. 3 , 4 With the persistent increase in the incidence of cancer and thus more patients undergoing cancer treatment, the long‐term use of central venous vascular access ports, tunneled/nontunneled central venous catheters, or peripherally inserted central catheters are increasing. 5 The use of central venous catheters facilitates the infusion of chemotherapeutic drugs, many of which can be classified as irritants and vesicants. 6 These catheters also allow for prolonged intravenous therapies, such as antibiotics, and parenteral nutrition in those who cannot obtain adequate nutrition via the digestive tract. 7 Central venous catheters have therefore become common in patients with cancer, and their increased use has come with an increase in catheter‐related thrombosis (CRT). 8
Although several clinical practice guidelines provide recommendations for the treatment of cancer‐associated thrombosis, 9 , 10 , 11 , 12 , 13 the evidence behind the treatment recommendations for CRT is at best uncertain and is mostly extrapolated from lower‐extremity VTE data or consensus opinion. 14 , 15 , 16 It is not known whether there is any difference in clinical impact between catheter removal before versus after anticoagulation therapy.
The purpose of our study was to describe a cohort of patients with cancer and CRT of the upper extremity and evaluate the relationship between the timing of catheter removal relative to anticoagulation and recurrence of any VTE, determining if the removal of the catheter before anticoagulation will influence the risk of recurrence.
2. METHODS
2.1. Study cohort and setting
All consecutive patients who visited the University of Texas MD Anderson Cancer Center in Houston, Texas, between January 1, 2015, and December 31, 2017, and who presented with symptomatic upper‐extremity deep vein thrombosis (DVT) associated with a venous catheter were identified by querying billing and radiology databases using International Classification of Diseases (ICD), Ninth Revision and Tenth Revision codes. Exclusion criteria for the identified cohort were (i) no confirmed cancer diagnosis, (ii) age <18 years, (iii) catheter removed >30 days before the CRT event, (iv) DVT not associated with a catheter or associated with apheresis catheters, (v) no acute DVT, (vi) duplicate records, (vii) diagnosis outside MD Anderson Cancer Center, and (viii) treatment with active anticoagulants before presentation. The current study was done in accordance with a clinical research protocol approved by the institutional review board at MD Anderson Cancer Center. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). Waivers of informed consent were granted because this was a retrospective study.
2.2. Data collection
A chart review of the final patient cohort was done by three trained and monitored investigators using the institution's electronic medical record system. The abstractors used a standard abstraction form with a data dictionary defining the variables of interest to guide data collection and avoid misclassification bias. Patient demographics, cancer and clinical information, and catheter‐related variables were collected. The presence of CRT was confirmed by reviewing the related imaging reports and was defined as the presence of acute upper‐limb DVT in the setting of a venous catheter of the involved limb that was in place at or within 30 days of the CRT event. Anticoagulation was recorded if given at the time of diagnosis or within the following 3 months for treatment of the associated DVT. VTE recurrence was defined as thrombosis in any vascular bed or pulmonary embolism (PE), and it was confirmed by reviewing imaging reports for a follow‐up of 2 years after diagnosis. Local recurrence was defined as a new acute DVT in the same arm diagnosed after 30 days of presentation, indicated by the resolution of the previous original DVT on follow‐up imaging study and/or involvement of new veins. Similarly, the removal of the catheter was recorded if it was removed at any time between the time of diagnosis and 2 years after diagnosis.
2.3. Statistical analysis
Patient and catheter characteristics along with the treatment and outcomes of the patients were summarized using descriptive statistics for the cohort. Categorical variables were analyzed as counts and percentages, while continuous variables were reported as medians and interquartile ranges or means and standard deviations, where appropriate. Significance was appraised using the chi‐square test for categorical variables and the Welch t test or Wilcoxon‐Mann‐Whitney test for continuous variables, where appropriate. We performed univariate logistic regression analysis to determine the association between each clinical variable and venous thrombosis (DVT or PE) recurrence. Significant variables from the univariate analyses and other clinical factors were further analyzed using a multiple logistic regression model reporting the odds ratio (OR) and the 95% confidence interval (CI). For the 1‐year analyses, univariate and multivariable competing risk models were used, with death as a competing event. Cumulative incidence functions, which measure the subdistribution of failure from VTE recurrence, were estimated for each variable reporting the hazard ratio (HR) estimate and its 95% CI. All statistical analyses were performed using R software version 3.6.2 (R Foundation, http://www.r‐project.org).
3. RESULTS
3.1. Demographic and clinical characteristics of patients with cancer and CRT
Of the 288 eligible patients identified, 257 were included in the final analysis after the exclusion criteria were applied. The reasons and number of patients excluded are shown in Figure 1.
FIGURE 1.
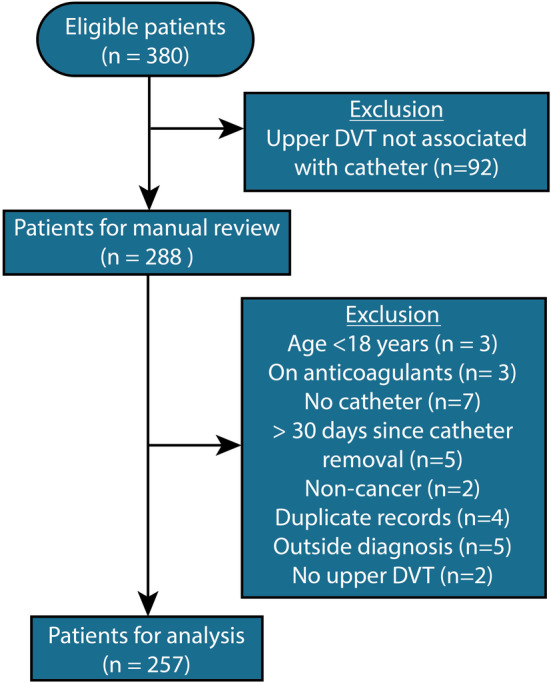
Flow diagram showing the exclusion criteria used to determine the study eligibility for patients with catheter‐related thrombosis. DVT, deep vein thrombosis
The demographic and clinical characteristics of the patients are shown in Table 1. The median age of the patients was 58 years, 55.3% were men, and 69.6% were White or Caucasian. More than half the patients (53.7%) had hematologic malignancies. Other frequent cancer types were gastrointestinal (12.1%), genitourinary (7.0%), and sarcoma (6.2%). Previous history of VTE was confirmed in only 39 (15.2%) patients.
TABLE 1.
Demographic and clinical characteristics of the patient cohort
| Characteristic | n (%) (N = 257) |
|---|---|
| Age, years, median (IQR) | 58 (47–66) |
| Sex | |
| Female | 115 (44.7) |
| Male | 142 (55.3) |
| Race | |
| White or Caucasian | 179 (69.6) |
| Black or African American | 29 (11.3) |
| Asian | 18 (7.0) |
| Others or unknown | 31 (12.1) |
| Ethnicity | |
| Not Hispanic or Latino | 181 (70.4) |
| Hispanic or Latino | 43 (16.7) |
| Others or unknown | 33 (12.8) |
| Weight, kg, median (IQR) | 79.1 (65.7–93.2) |
| Cancer type | |
| Hematologic | 138 (53.7) |
| Gastrointestinal | 31 (12.1) |
| Genitourinary | 18 (7.0) |
| Sarcoma | 16 (6.2) |
| Breast | 14 (5.4) |
| Head and neck | 13 (5.1) |
| Lung | 9 (3.5) |
| Gynecology | 6 (2.3) |
| Other | 12 (4.7) |
| Metastatic disease | |
| No | 131 (51.0) |
| Yes | 126 (49.0) |
| Previous history of VTE | |
| No | 218 (84.8) |
| Yes | 39 (15.2) |
| Hypertension | |
| No | 157 (61.1) |
| Yes | 100 (38.9) |
| Diabetes | |
| No | 223 (86.8) |
| Yes | 34 (13.2) |
Abbreviations: IQR, interquartile range; VTE, venous thromboembolism.
3.2. Characteristics, management, and outcomes of CRT cancer‐associated thrombosis
General characteristics of the catheters used in the group of patients with CRT are shown in Table S1. Peripherally inserted central catheter was the most frequent catheter type (77.4%). The median time from catheter insertion to CRT diagnosis was 20 days (interquartile range [IQR], 8–40 days). The most common proximal locations for CRT were the subclavian vein, axillary vein, and jugular vein. Seventeen patients (6.6%) had concurrent PE along with their CRT (Table 2).
TABLE 2.
Characteristics and management of catheter‐related thrombosis
| Variable | n (%) |
|---|---|
| Thrombus location (most proximal) | |
| Subclavian vein | 157 (61.1) |
| Axillary vein | 32 (12.5) |
| Jugular vein | 26 (10.1) |
| Brachial vein | 17 (6.6) |
| Innominate (brachiocephalic) vein | 15 (5.8) |
| Basilic vein | 7 (2.7) |
| SVC | 3 (1.2) |
| Concurrent PE | |
| No | 240 (93.4) |
| Yes | 17 (6.6) |
| Initial anticoagulant | |
| LMWH | 189 (73.5) |
| DOAC | 9 (3.5) |
| UFH | 5 (1.9) |
| Fondaparinux | 1 (0.4) |
| Argatroban | 1 (0.4) |
| None | 52 (20.2) |
| Long‐term anticoagulant | |
| LMWH | 125 (48.6) |
| DOAC | 22 (8.6) |
| VKA | 5 (1.9) |
| Fondaparinux | 2 (0.8) |
| None | 103 (40.1) |
| Duration of long‐term anticoagulants, months, median (IQR) | 3 (3–4) |
| Catheter removed | |
| No | 51 (19.8) |
| Yes | 206 (80.2) |
| Days to catheter removal, median (IQR) a | 4 (1–15) |
| Catheter reinsertion after removal a | |
| No | 73 (35.4) |
| Yes | 133 (64.6) |
| Anticoagulants initiated before catheter removal a | |
| No | 104 (50.5) |
| Yes | 102 (49.5) |
Abbreviations: DOAC, direct oral anticoagulant; IQR, interquartile range; LMWH, low‐molecular‐weight heparin; PE, pulmonary embolism; SVC, superior vena cava; UFH, unfractionated heparin; VKA, vitamin K antagonist.
Only for the cases with catheters removed after presentation.
The catheter was removed in 206 (80.2%) patients, of whom 133 (64.6%) had a catheter reinserted afterward. Of the patients who had their catheter removed, almost half (49.5%) were treated with anticoagulants before the removal (Figure S1). The median time for catheter removal for patients who had their catheter removed after presentation was 4 (IQR, 1‐15) days. Of the remining 104 (50.5%) patients who did not receive anticoagulation before catheter removal, only 22 (21.1%) patients did not receive any type of anticoagulants afterwards, of which 5 (22.7%) had brachial or superficial vein thrombosis. Most patients in our cohort (73.5%) were given low‐molecular‐weight heparin (LMWH) as their initial anticoagulant medication, while 52 patients (20.2%) received no initial anticoagulant. However, only 48.6% of all patients received LMWH as their long‐term anticoagulant treatment, and no long‐term anticoagulants were prescribed in 40.1% of our cohort (Table 2). The median duration of treatment for the patients who had long‐term anticoagulants prescribed was 3 (IQR, 3–4) months. No significant differences were observed in prescribing long‐term anticoagulants between patients who had their catheter removed before anticoagulation compared to the ones who had it removed after (65.7% vs 59.6%, respectively).
Significant differences in cancer type and platelet count were observed among patients who received initial anticoagulant compared to the ones who did not (Table S2). The majority (75%) of the patients who did not receive initial anticoagulants had hematologic malignancies, and the median platelet count for this group was significantly lower when compared to patients who received initial anticoagulants (28 K/μl [IQR, 14‐74] vs 171 K/μl [IQR, 103‐250]). For the 52 (20.2%) patients who did not receive initial anticoagulants, 36 (69.2%) had thrombocytopenia, and 11 (21.2%) had active or recent bleeding as the main reason for not prescribing any initial anticoagulants.
Venous thromboembolism recurrences within 3 months and within 1 year occurred in 18 (7%) and 26 (10.1%) patients, respectively. Table S3 reports the incidence of VTE recurrences stratified by different demographic and clinical risk factors. Most VTE recurrences were DVT in the contralateral upper arm, with some patients having more than one type of VTE recurrence at the same time (Table S4). The mortality rates were 13.2% and 30.7% for the first 3 months and 1 year after presentation, respectively. The median overall survival for the whole cohort was 55.3 months, with substantially lower median overall survival for patients who had VTE recurrence within 1 year of presentation and for patients who did not receive long‐term anticoagulation (Table S5).
3.3. Predictors of venous thromboembolic event recurrence in patients with cancer and CRT
Univariate and multivariable analyses were used to determine the association between different clinical factors and any VTE recurrence in patients with cancer and CRT (Tables 3, 4, 5 and 6). The timing of the catheter removal and whether a catheter had been reinserted (either ipsilateral or contralateral) were the main predictors for 3‐month, 6‐month, and 1‐year recurrence. Patients who had their catheter removed before anticoagulant initiation had significantly higher risk of any VTE recurrence compared to those who had anticoagulants started before removal of the catheter (3‐month recurrence: univariate OR, 5.13 [95% CI, 1.61–22.79]; multivariable OR, 5.07 [95% CI, 1.53–23.18]; and 1‐year recurrence: univariate HR, 3.38 [95% CI, 1.36–8.40]; multivariable HR, 3.47 [95% CI, 1.34–9.01]) (Tables 3 and 5). Patients who had a catheter reinserted had significantly higher risk of VTE recurrence within 3 months (multivariable OR, 9.96 [95% CI, 1.82–186.75]) and 1 year (multivariable sub‐distribution HR, 3.07 [95% CI, 1.09–8.67]) (Tables 4 and 6)Similar results were observed in a subanalysis after excluding the patients with brachial and superficial vein thrombosis (Table S6).
TABLE 3.
Univariate analysis of the association between clinical factors and venous thromboembolic recurrence in patients with cancer and catheter‐related thrombosis
| Variable | VTE recurrence | |||
|---|---|---|---|---|
| 3 months | 6 months | |||
| OR (95% CI) | p | OR (95% CI) | p | |
| Age, years | 0.99 (0.96–1.02) | 0.57 | 1.00 (0.97–1.03) | 0.99 |
| Sex | ||||
| Female | Reference | |||
| Male | 2.22 (0.81‐7.09) | 0.14 | 1.96 (0.80–5.26) | 0.15 |
| Weight, kg | 0.98 (0.95–1.00) | 0.09 | 0.99 (0.96–1.01) | 0.20 |
| Cancer type | ||||
| Hematologic | Reference | |||
| Solid | 0.42 (0.13–1.16) | 0.11 | 0.48 (0.18–1.16) | 0.12 |
| Concurrent PE | 0.82 (0.04–4.41) | 0.85 | 1.39 (0.21–5.39) | 0.68 |
| Prior history of VTE | ||||
| No | Reference | |||
| Yes | 0.68 (0.11–2.54) | 0.62 | 1.20 (0.33–3.42) | 0.76 |
| Timing of catheter removal | ||||
| Anticoagulants started before catheter removed | Reference | |||
| Catheter was not removed | 0.66 (0.03–5.30) | 0.72 | 0.32 (0.02–1.94) | 0.30 |
| Catheter removed before anticoagulants started | 5.13 (1.61–22.79) | 0.01 | 2.91 (1.14–8.41) | 0.03 |
| Anticoagulants prescribed | ||||
| No | Reference | |||
| Yes | 0.64 (0.23–2.06) | 0.41 | 0.54 (0.22–1.49) | 0.21 |
| Catheter reinserted after removal | ||||
| No | Reference | |||
| Yes | 7.28 (2.01–46.70) | 0.009 | 4.09 (1.47–14.48) | 0.01 |
Note: Age and weight are continuous variables with odds ratio associated with a one‐unit increment (per each 1‐year or 1‐kg increase, respectively) for each variable. Boldface indicates statistical significance.
Abbreviations: CI, confidence interval; OR, odds ratio; PE, pulmonary embolism; VTE, venous thromboembolism.
TABLE 4.
Multivariable analysis of the association between clinical factors and venous thromboembolic recurrence within 3 months in patients with cancer and catheter‐related thrombosis
| Variable | VTE recurrence | |
|---|---|---|
| OR (95% CI) | p | |
| Age, years | 1.00 (0.97–1.03) | 0.84 |
| Sex | ||
| Female | Reference | |
| Male | 3.57 (1.15–13.65) | 0.04 |
| Cancer type | ||
| Hematologic | Reference | |
| Solid | 0.86 (0.24–2.73) | 0.80 |
| Catheter removed before anticoagulants | ||
| No | Reference | |
| Yes | 5.07 (1.53–23.18) | 0.02 |
| Anticoagulants prescribed | ||
| No | Reference | |
| Yes | 0.85 (0.26–3.05) | 0.79 |
| Catheter reinserted after removal | ||
| No | Reference | |
| Yes | 9.96 (1.82–186.75) | 0.03 |
Note: Boldface indicates statistical significance.
Abbreviations: CI, confidence interval; OR, odds ratio; VTE, venous thromboembolism.
TABLE 5.
Univariate competing risk regression analysis of clinical factors and 1‐year venous thromboembolic recurrence in cancer patients with catheter‐related thrombosis
| Variable | HR (95% CI) | p |
|---|---|---|
| Age, years | 1.00 (0.97–1.02) | 0.73 |
| Sex | ||
| Female | ||
| Male | 1.55 (0.72–3.33) | 0.26 |
| Weight, kg | 0.99 (0.97–1.00) | 0.10 |
| Cancer type | ||
| Hematologic | ||
| Solid | 0.46 (0.20–1.03) | 0.06 |
| Concurrent PE | 1.09 (0.26–4.61) | 0.91 |
| Prior history of VTE | ||
| No | ||
| Yes | 1.23 (0.48–3.17) | 0.67 |
| Timing of catheter removal | ||
| Anticoagulants started before catheter removed | ||
| Catheter was not removed | 0.65 (0.13–3.20) | 0.60 |
| Catheter removed before anticoagulants started | 3.38 (1.36–8.40) | 0.009 |
| Anticoagulants prescribed | ||
| No | ||
| Yes | 0.59 (0.26–1.34) | 0.21 |
| Catheter reinserted after removal | ||
| No | ||
| Yes | 3.26 (1.33–7.98) | 0.01 |
Note: Boldface indicates statistical significance.
Abbreviations: CI, confidence interval; HR, hazard ratio; PE, pulmonary embolism; VTE, venous thromboembolism.
TABLE 6.
Multivariable competing risk regression analysis of clinical factors and 1‐year venous thromboembolic recurrence in patients with cancer and catheter‐related thrombosis
| Variable | HR (95% CI) | p |
|---|---|---|
| Age, years | 1.00 (0.98–1.02) | 0.97 |
| Sex | ||
| Female | ||
| Male | 1.92 (0.84–4.38) | 0.12 |
| Cancer type | ||
| Hematologic | ||
| Solid | 0.87 (0.38–1.95) | 0.73 |
| Catheter removed before anticoagulants | ||
| No | ||
| Yes | 3.47 (1.34–9.01) | 0.01 |
| Anticoagulants prescribed | ||
| No | ||
| Yes | 0.89 (0.36–2.19) | 0.80 |
| Catheter reinserted after removal | ||
| No | ||
| Yes | 3.07 (1.09–8.67) | 0.03 |
Note: Boldface indicates statistical significance.
Abbreviations: CI, confidence interval; HR, hazard ratio.
4. DISCUSSION
Symptomatic and asymptomatic VTEs are common complications in patients with cancer owing to the multiple clinical and cancer‐related risk factors found in these patients including chemotherapy, multiple surgeries, immobility, and the malignancy itself. 17 , 18 , 19 , 20 , 21 , 22 In addition, endothelial damage and vessel wall injury caused by the insertion of venous catheters tends to contribute significantly to the development of CRT. 19 In critical care settings, including cancer care, venous catheters are commonly used to establish stable venous access for different indications, including chemotherapy administration, fluid resuscitation, and antibiotic administration. 23 , 24 , 25 Despite their great benefit, venous catheters increase the long‐term risk of thrombosis, 26 , 27 , 28 with reported rates ranging from <1% to 28% owing to the widely different study settings and inclusion criteria, including various cancer types. 5 , 27 , 29 , 30 , 31 , 32 , 33
It is known that cancer‐related VTE often recurs, despite optimal anticoagulation, 34 and the rate of VTE recurrence for patients with CRT has been reported to be around 7% to 10%. 35 In this study, we examined the presentation and outcomes of patients with cancer and upper‐extremity CRT and found an association between the timing of anticoagulant initiation and catheter removal and the recurrence of VTE.
Most patients in our cohort (80.2%) had their catheters removed despite guidance from the ISTH to keep the catheter in place if not infected and still functioning. It is likely that the practice at our center differed from this guidance due to the limited evidence, based on a single prospective study that included 74 patients with cancer without a control group. Additionally, there is usually a delay from the time a guideline is published until effective implementation in clinical practice, and our study period was shortly after guidance release. 36 Finally, it is possible that a case‐by‐case approach was used due to the lack of strong evidence for the guidance. Of the patients who had their catheter removed, half (50.5%) were not treated with anticoagulants before the removal. Those who had anticoagulants initiated had a median of 4 days between the initiation of the anticoagulants and catheter removal. Interestingly, we found the removal of the catheter before anticoagulant initiation to be associated with increased risk of VTE recurrence. The ISTH recommends anticoagulation for 3 to 5 days before catheter removal, 12 while the National Comprehensive Cancer Network and the Spanish Society of Medical Oncology recommend 5 to 7 days of anticoagulation before catheter removal. 13 However, these recommendations to overlap therapeutic anticoagulation with catheter removal are not based on strong evidence, and the variation in recommended days can be confusing for clinicians, thus leading to the lack of uniformity in treatment approaches. Anticoagulation for several days before removal is intended to minimize the risk of embolization; however, a recent study by Houghton et al 37 showed that early catheter removal (<48 hours after anticoagulant initiation) in patients with hematologic malignancies and CRT was not associated with increased risk of pulmonary embolism within 7 days. This finding aligns with our study, as none of the patients developed PE within 7 days from presentation.
About half of our patients (53.7%) had hematologic malignancies. While the common recommendation for such patients is to leave the catheter in place if it is functioning properly and still needed for further care, patients with hematologic malignancies often have prolonged and severe thrombocytopenia, which prevents the use of anticoagulants, and catheter removal is often indicated. 37 , 38 , 39 A study by Vu et al 40 in patients with leukemia reported a VTE recurrence rate of nearly 20%, which was attributed to improper anticoagulation in the setting of thrombocytopenia. However, that study did not examine the relationship between catheter removal and VTE recurrence. Furthermore, while initial anticoagulants were given in 79.8% of our cases, with LMWH being the most common initial anticoagulant, 40.1% of the patients were not prescribed any long‐term anticoagulants. Delluc and colleagues described a recurrence rate of 7% at 1 year after CRT for patients who discontinued anticoagulants while still having active cancer. 41 It is possible that the lack of continued anticoagulation in our cohort contributed to the recurrence rate.
The data presented here should be interpreted in the context of the following limitations. First, this was a retrospective study, which has its own inherent limitations and biases, including potential indication bias for catheter removal. We tried to minimize selection bias by including all consecutive patients with cancer in the study period and carefully reviewing the electronic medical records and abstracting data in a systematic manner. Second, this was a single‐center study in a cancer‐specific hospital, which may not make the results generalizable to other institutions. However, to our knowledge, this is the only study evaluating the relationship between timing of catheter removal and anticoagulation to VTE recurrence. Our data showed an association between anticoagulation and timing of catheter removal with VTE recurrence, but a future prospective study will be needed to confirm this observation and determine if anticoagulation before catheter removal can decrease the risk of VTE recurrence at 3 months. Finally, only a few variables and covariates related to VTE were used in the final multivariable analysis to fulfill the accepted rule criteria that require a specific number of outcome events for each predictor to be included in the model. 42 Although some important variables like previous history of VTE and weight (or body mass index), which are predictors of VTE, 43 , 44 were not significant in the univariate analysis, future larger study is needed to confirm their association with VTE recurrence in patients presenting with CRT.
5. CONCLUSION
In summary, removal of a catheter before anticoagulant administration was associated with an increased risk of VTE recurrence. In instances when immediate removal is not needed, delaying the catheter removal until the patient can be properly anticoagulated may be beneficial.
AUTHOR CONTRIBUTIONS
TWR and DNL conceived and designed the study. AQ and DNL analyzed and interpreted the data. All authors contributed critical writing and revising the intellectual content as well as final approval of the version to be published.
Funding information
None.
RELATIONSHIP DISCLOSURE
DNL is employed by IQVIA Biotech. The rest of the authors declare no conflicts of interest.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
The authors thank Bryan Tutt, scientific editor at the MD Anderson Cancer Center's medical research library, for his editorial support.
Lipe DN, Qdaisat A, Rajha E, et al. Characteristics and predictors of venous thrombosis recurrence in patients with cancer and catheter‐related thrombosis. Res Pract Thromb Haemost. 2022;6:e12761. doi: 10.1002/rth2.12761
Handling Editor: Dr Lana Castellucci
Contributor Information
Demis N. Lipe, Email: demis.ros@gmail.com, @DemisLipe.
Aiham Qdaisat, @AQdaisat.
Eva Rajha, @RajhaEva.
Patrick Chaftari, @pchaftari.
Terry W. Rice, @CancerEDdoc.
REFERENCES
- 1. Verso M, Agnelli G. Venous thromboembolism associated with long‐term use of central venous catheters in cancer patients. J Clin Oncol. 2003;21(19):3665‐3675. [DOI] [PubMed] [Google Scholar]
- 2. Ay C, Pabinger I, Cohen AT. Cancer‐associated venous thromboembolism: burden, mechanisms, and management. Thromb Haemost. 2017;117(2):219‐230. [DOI] [PubMed] [Google Scholar]
- 3. Baskin JL, Pui CH, Reiss U, et al. Management of occlusion and thrombosis associated with long‐term indwelling central venous catheters. Lancet. 2009;374(9684):159‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sheth RA, Niekamp A, Quencer KB, et al. Thrombosis in cancer patients: etiology, incidence, and management. Cardiovasc Diagn Ther. 2017;7(Suppl 3):S178‐S185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Decousus H, Bourmaud A, Fournel P, et al. Cancer‐associated thrombosis in patients with implanted ports: a prospective multicenter French cohort study (ONCOCIP). Blood. 2018;132(7):707‐716. [DOI] [PubMed] [Google Scholar]
- 6. Lingegowda D, Gehani A, Sen S, Mukhopadhyay S, Ghosh P. Centrally inserted tunnelled peripherally inserted central catheter: off‐label use for venous access in oncology patients. J Vasc Access. 2020;21(5):773‐777. [DOI] [PubMed] [Google Scholar]
- 7. Geerts W. Central venous catheter‐related thrombosis. Hematology Am Soc Hematol Educ Program. 2014;2014(1):306‐311. [DOI] [PubMed] [Google Scholar]
- 8. Citla Sridhar D, Abou‐Ismail MY, Ahuja SP. Central venous catheter‐related thrombosis in children and adults. Thromb Res. 2020;187:103‐112. [DOI] [PubMed] [Google Scholar]
- 9. Farge D, Frere C, Connors JM, et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20(10):e566‐e581. [DOI] [PubMed] [Google Scholar]
- 10. Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2020;38(5):496‐520. [DOI] [PubMed] [Google Scholar]
- 11. Lyman GH, Carrier M, Ay C, et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021;5(4):927‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zwicker JI, Connolly G, Carrier M, Kamphuisen PW, Lee AY. Catheter‐associated deep vein thrombosis of the upper extremity in cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2014;12(5):796‐800. [DOI] [PubMed] [Google Scholar]
- 13. Munoz Martin AJ, Gallardo Diaz E, Garcia Escobar I, et al. SEOM clinical guideline of venous thromboembolism (VTE) and cancer (2019). Clin Transl Oncol. 2020;22(2):171‐186. [DOI] [PubMed] [Google Scholar]
- 14. Rajasekhar A, Streiff MB. Etiology and management of upper‐extremity catheter‐related thrombosis in cancer patients. Cancer Treat Res. 2019;179:117‐137. [DOI] [PubMed] [Google Scholar]
- 15. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S‐e496S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Debourdeau P, Farge D, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of thrombosis associated with central venous catheters in patients with cancer. J Thromb Haemost. 2013;11(1):71‐80. [DOI] [PubMed] [Google Scholar]
- 17. Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation. 2003;107(23 Suppl 1):I17‐I21. [DOI] [PubMed] [Google Scholar]
- 18. Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293(6):715‐722. [DOI] [PubMed] [Google Scholar]
- 19. Qdaisat A, Yeung SJ, Variyam DE, et al. Evaluation of cancer patients with suspected pulmonary embolism: performance of the American College of Physicians Guideline. J Am Coll Radiol. 2020;17(1 Pt A):22‐30. [DOI] [PubMed] [Google Scholar]
- 20. Blom JW, Vanderschoot JP, Oostindier MJ, Osanto S, van der Meer FJ, Rosendaal FR. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost. 2006;4(3):529‐535. [DOI] [PubMed] [Google Scholar]
- 21. Qdaisat A, Kamal M, Al‐Breiki A, et al. Clinical characteristics, management, and outcome of incidental pulmonary embolism in cancer patients. Blood Adv. 2020;4(8):1606‐1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prandoni P, Falanga A, Piccioli A. Cancer and venous thromboembolism. Lancet Oncol. 2005;6(6):401‐410. [DOI] [PubMed] [Google Scholar]
- 23. Teichgraber UK, Pfitzmann R, Hofmann HA. Central venous port systems as an integral part of chemotherapy. Dtsch Arztebl Int. 2011;108(9):147‐153; quiz 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schiffer CA, Mangu PB, Wade JC, et al. Central venous catheter care for the patient with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31(10):1357‐1370. [DOI] [PubMed] [Google Scholar]
- 25. Sousa B, Furlanetto J, Hutka M, et al. Central venous access in oncology: ESMO clinical practice guidelines. Ann Oncol. 2015;26(Suppl 5):v152‐v168. [DOI] [PubMed] [Google Scholar]
- 26. Jones D, Wismayer K, Bozas G, Palmer J, Elliott M, Maraveyas A. The risk of venous thromboembolism associated with peripherally inserted central catheters in ambulant cancer patients. Thromb J. 2017;15:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kang J, Chen W, Sun W, et al. Peripherally inserted central catheter‐related complications in cancer patients: a prospective study of over 50,000 catheter days. J Vasc Access. 2017;18(2):153‐157. [DOI] [PubMed] [Google Scholar]
- 28. Mansour A, Saadeh SS, Abdel‐Razeq N, Khozouz O, Abunasser M, Taqash A. Clinical course and complications of catheter and non‐catheter‐related upper extremity deep vein thrombosis in patients with cancer. Clin Appl Thromb Hemost. 2018;24(8):1234‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Raaf JH. Results from use of 826 vascular access devices in cancer patients. Cancer. 1985;55(6):1312‐1321. [DOI] [PubMed] [Google Scholar]
- 30. Lokich JJ, Becker B. Subclavian vein thrombosis in patients treated with infusion chemotherapy for advanced malignancy. Cancer. 1983;52(9):1586‐1589. [DOI] [PubMed] [Google Scholar]
- 31. Debourdeau P, Espie M, Chevret S, et al. Incidence, risk factors, and outcomes of central venous catheter‐related thromboembolism in breast cancer patients: the CAVECCAS study. Cancer Med. 2017;6(11):2732‐2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dridi M, Mejri N, Labidi S, et al. Implantable port thrombosis in cancer patients: a monocentric experience. Cancer Biol Med. 2016;13(3):384‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee AY, Kamphuisen PW. Epidemiology and prevention of catheter‐related thrombosis in patients with cancer. J Thromb Haemost. 2012;10(8):1491‐1499. [DOI] [PubMed] [Google Scholar]
- 34. Lee AY, Levine MN, Baker RI, et al. Low‐molecular‐weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146‐153. [DOI] [PubMed] [Google Scholar]
- 35. Baumann Kreuziger L, Onwuemene O, Kolesar E, Crowther M, Lim W. Systematic review of anticoagulant treatment of catheter‐related thrombosis. Thromb Res. 2015;136(6):1103‐1109. [DOI] [PubMed] [Google Scholar]
- 36. Kovacs MJ, Kahn SR, Rodger M, et al. A pilot study of central venous catheter survival in cancer patients using low‐molecular‐weight heparin (dalteparin) and warfarin without catheter removal for the treatment of upper extremity deep vein thrombosis (the catheter study). J Thromb Haemost. 2007;5(8):1650‐1653. [DOI] [PubMed] [Google Scholar]
- 37. Houghton DE, Billett HH, Gaddh M, et al. Risk of pulmonary emboli after removal of an upper extremity central catheter associated with a deep vein thrombosis. Blood Adv. 2021;5(14):2807‐2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Samuelson Bannow BR, Lee AYY, Khorana AA, et al. Management of anticoagulation for cancer‐associated thrombosis in patients with thrombocytopenia: a systematic review. Res Pract Thromb Haemost. 2018;2(4):664‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Houghton DE, Key NS, Zakai NA, Laux JP, Shea TC, Moll S. Analysis of anticoagulation strategies for venous thromboembolism during severe thrombocytopenia in patients with hematologic malignancies: a retrospective cohort. Leuk Lymphoma. 2017;58(11):2573‐2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vu K, Luong NV, Hubbard J, et al. A retrospective study of venous thromboembolism in acute leukemia patients treated at the University of Texas MD Anderson Cancer Center. Cancer Med. 2015;4(1):27‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Delluc A, Le Gal G, Scarvelis D, Carrier M. Outcome of central venous catheter associated upper extremity deep vein thrombosis in cancer patients. Thromb Res. 2015;135(2):298‐302. [DOI] [PubMed] [Google Scholar]
- 42. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373‐1379. [DOI] [PubMed] [Google Scholar]
- 43. Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110(10):2339‐2346. [DOI] [PubMed] [Google Scholar]
- 44. Qdaisat A, Wu W, Lin JZ, et al. Clinical and cancer‐related predictors for venous thromboembolism in cancer patients presenting to the emergency department. J Emerg Med. 2020;58(6):932‐941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


