To the Editor: The alarming increase in melanoma incidence demands the evaluation of biases in screening and histologic diagnosis.1 Absent disease outcome data, no gold standard exists for the accuracy of histopathology.2 We thus examined intraobserver reproducibility among board-certified or fellowship-trained dermatopathologists, the study design we believed a priori would capture the highest experimental reproducibility rates for melanocytic lesion diagnosis. We focused on the conceptual “common” pathway of melanomagenesis, namely nevus, dysplastic nevus, melanoma in situ (MIS), and invasive melanoma, reflecting low cumulative solar damage and representing the most frequently biopsied (∼80%) melanocytic lesions.3 We evaluated this subset from a previous study to focus results in order to be used as a reference guide.4
Dermatopathologists interpreted sets of 48 glass slides on 2 occasions separated by ≥8 months. They were not informed that phase 2 cases were identical to phase 1. Details on the study design are described elsewhere (Supplementary Appendix, available via Mendeley at https://data.mendeley.com/datasets/ssrfvm5pgg/1).4 After informed consent, participants used a form with >50 diagnostic options grouped into 5 categories.5 To maximize relevance to routine practice, we selected interpretation pairs with phase 1 diagnoses within the common pathway (Supplementary Table I, available via Mendeley at https://data.mendeley.com/datasets/ssrfvm5pgg/1). Diagnoses outside this pathway were classified as “Other” (Supplementary Table II, available via Mendeley at https://data.mendeley.com/datasets/ssrfvm5pgg/1). Analysis units were interpretation pairs of the same case by the same dermatopathologist in both phases. The outcome was the proportion of phase 1 interpretations receiving phase 2 diagnoses in the same category. An “Other” category diagnosis in phase 2 was considered discordant. Confidence intervals used logit transformation (SAS 9.4, SAS Institute Inc).
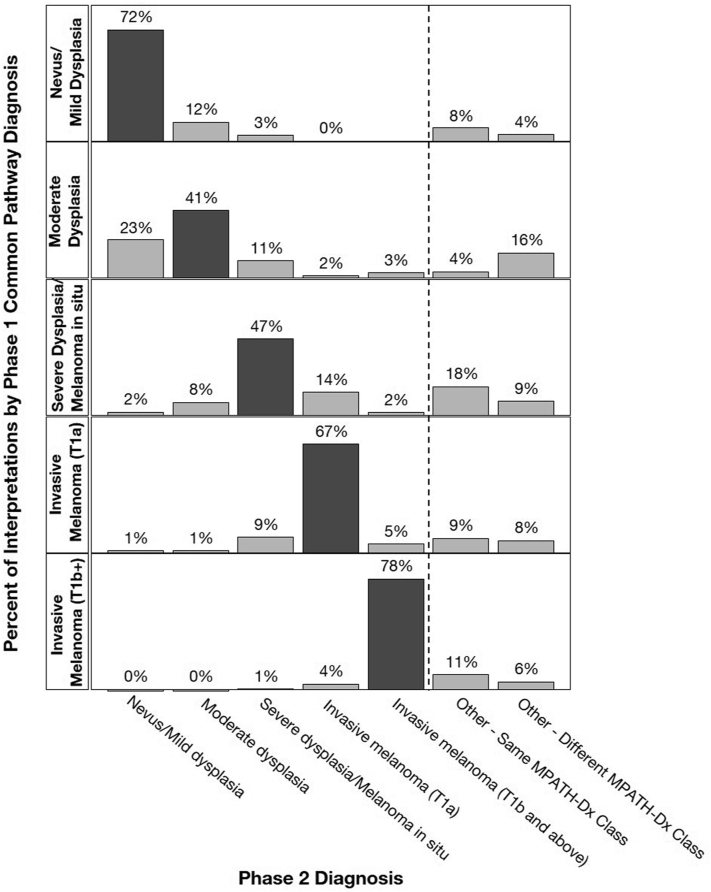
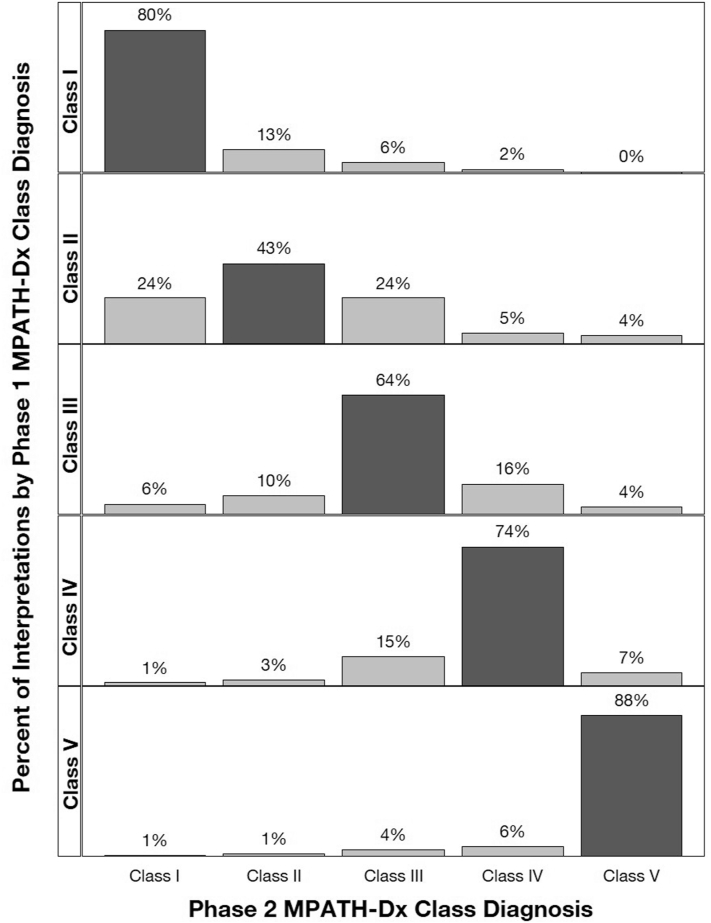
Forty-nine dermatopathologists completed both phases (Supplementary Table III, available via Mendeley at https://data.mendeley.com/datasets/ssrfvm5pgg/1). There were 1396 phase 1 common pathway diagnoses: nevus/mild dysplasia, 293 (21%); moderate dysplasia, 193 (14%); severe dysplasia/MIS, 266 (19%); invasive melanoma (pT1a), 383 (27%); and invasive melanoma pT1b and above, 261 (19%). Fig 1 displays intraobserver reproducibility between phases: nevus/mild dysplasia: 72% (95% CI, 67%-76%), moderate dysplasia: 41% (95% CI, 34%-50%), severe dysplasia/MIS: 47% (95% CI, 40%-54%), pT1a invasive melanoma: 67% (95% CI, 60%-73%), and ≥pT1b melanoma: 78% (95% CI, 71%-83%) (Supplementary Table IV, available via Mendeley at https://data.mendeley.com/datasets/ssrfvm5pgg/1). Reproducibility improves when using Melanocytic Pathology Assessment Tool and Hierarchy for Diagnosis, a reporting schema which stratifies lesions by pathologists’ assessment of risk and suggested management (Fig 2; Supplementary Table V, available via Mendeley at https://data.mendeley.com/datasets/ssrfvm5pgg/1).5
Fig 1.
Intraobserver reproducibility using the common melanoma pathway. Initial phase 1 common diagnosis categories are displayed as row panels and the distribution of the paired phase 2 diagnosis category is shown as vertical bars. Interpretation pairs are limited to those in which the phase 1 diagnosis was within the common melanoma pathway categories (N = 1396).
Fig 2.
Intraobserver reproducibility using MPATH-Dx Classes. The MPATH-Dx classes of phase 1 interpretations are shown as row panels and the distribution of the paired phase 2 MPATH-Dx classes are shown as vertical bars. All interpretation pairs regardless of inclusion in the common melanoma pathway categories are shown (N = 2064). MPATH-Dx, Melanocytic Pathology Assessment Tool and Hierarchy for Diagnosis.
Although it has long been known that melanocytic lesion histology is variable to a level impacting clinical management,4,5 this report presents detailed new results on reproducibility within the common melanoma pathway. Diagnoses from moderately dysplastic nevus to MIS were not reproducible (intraobserver reproducibility <50%); reproducibility of pT1a invasive melanoma was modestly better. The extremes of nevus/mild dysplasia and invasive melanoma (≥pT1b) were the most reproducible.
We restricted the study to board-certified/fellowship-trained dermatopathologists, whose reproducibility is higher than general pathologists (Supplementary Tables VI and VII and Supplementary Figs 1 and 2, available via Mendeley at https://data.mendeley.com/datasets/ssrfvm5pgg/1). We employed a testing environment without access to additional clinical information or special testing, possibly limiting generalizability.
Cognizance of the limitations of histopathology is needed to avoid overdiagnosis.1 Poor diagnostic reliability encompasses MIS to pT1a invasive melanoma, which together are more prevalent than all other stages of melanoma collectively.3,4 For providers who drive utilization of dermatopathology services, we offer a concrete reference guide to counsel patients on the limits of histopathology in melanocytic lesion diagnosis.
Conflicts of interest
Dr Elmore serves as Editor-in-Chief of Primary Care (Adult) topics at UpToDate.
Footnotes
Funding sources: Supported by the National Cancer Institute (R01 CA151306, R01 CA201376). The funding agency had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
IRB approval status: Reviewed and approved by the University of Washington IRB (#44309).
References
- 1.Welch H.G., Mazer B.L., Adamson A.S. The rapid rise in cutaneous melanoma diagnoses. N Engl J Med. 2021;384(1):72–79. doi: 10.1056/NEJMsb2019760. [DOI] [PubMed] [Google Scholar]
- 2.Elston D.M., Luo Y., Echols K., Rensch G., Metcalf J. Changes in melanoma diagnosis after presurgical tertiary care center review. J Am Acad Dermatol. 2021;85(2):480–481. doi: 10.1016/j.jaad.2018.04.052. [DOI] [PubMed] [Google Scholar]
- 3.Lott J.P., Boudreau D.M., Barnhill R.L., et al. Population-based analysis of histologically confirmed melanocytic proliferations using natural language processing. JAMA Dermatol. 2018;154(1):24–29. doi: 10.1001/jamadermatol.2017.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elmore J.G., Barnhill R.L., Elder D.E., et al. Pathologists' diagnosis of invasive melanoma and melanocytic proliferations: observer accuracy and reproducibility study. BMJ. 2017;357:j2813. doi: 10.1136/bmj.j2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piepkorn M.W., Barnhill R.L., Elder D.E., et al. The MPATH-Dx reporting schema for melanocytic proliferations and melanoma. J Am Acad Dermatol. 2014;70(1):131–141. doi: 10.1016/j.jaad.2013.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]