Abstract
Tracheal intubation in the critically ill is associated with serious complications, mainly cardiovascular collapse and severe hypoxemia. In this narrative review, we present an update of interventions aiming to decrease these complications. MACOCHA is a simple score that helps to identify patients at risk of difficult intubation in the intensive care unit (ICU). Preoxygenation combining the use of inspiratory support and positive end-expiratory pressure should remain the standard method for preoxygenation of hypoxemic patients. Apneic oxygenation using high-flow nasal oxygen may be supplemented, to prevent further hypoxemia during tracheal intubation. Face mask ventilation after rapid sequence induction may also be used to prevent hypoxemia, in selected patients without high-risk of aspiration. Hemodynamic optimization and management are essential before, during and after the intubation procedure. All these elements can be integrated in a bundle. An airway management algorithm should be adopted in each ICU and adapted to the needs, situation and expertise of each operator. Videolaryngoscopes should be used by experienced operators.
Keywords: Airway, Intubation, Complications, Videolaryngoscope, Videolaryngoscopy
Take-home message
| Preoxygenation differs from apneic oxygenation. While noninvasive ventilation is the preferred method for preoxygenation of critically ill hypoxemic patients, high-flow nasal oxygen may be used for apneic oxygenation to limit the occurrence of desaturation. |
| In patients at high risk of desaturation, without high risk of aspiration, mask ventilation during apnea should be considered. |
| Use of videolaryngoscopy in critically ill patients may help to increase first-attempt intubation success, in the hands of trained operators. |
| Careful hemodynamic management is essential, with the aim to decrease hypotension during the intubation procedure and related cardiac arrest during intubation. |
Introduction
Tracheal intubation is one of the most frequent procedures performed in the intensive care unit (ICU) [1–3]. Tracheal intubation in critically ill patients may be associated with life-threatening complications in up to half of cases [4, 5]. Cardiovascular instability and hypoxemia are the most common complications occurring during intubation of critically ill patients [4, 6]. They are associated with increased 28-day mortality [6] and they may result in cardiac arrest [7, 8], cerebral anoxia, and death [9, 10].
In this narrative review, we summarize the current insights into the measures to be taken to optimize airway management using endotracheal tubes in ICU patients: preoxygenation, apneic oxygenation, appropriate devices, use of an airway management algorithm, hemodynamic optimization, choice of drugs and timing of intubation.
The authors present a narrative review [11], based on the literature, but also on the experience and subjectivity of the authors.
Preoxygenation and apneic oxygenation
Preoxygenation aims to increase the duration of the apnea without desaturation, by an increase of the functional residual capacity and the oxygen reserves, thereby reducing the occurrence of hypoxemia.
Preoxygenation is more effective in the 25° head-up position than in the supine position in patients with severe obesity [12]. Similarly, in patients without obesity, optimal preoxygenation and intubation conditions can be created using a 20° to 30° semi-sitting position, or a reverse Trendelenburg position, avoiding if possible a supine position [13].
Several methods for preoxygenation are available in clinical practice in the spontaneous breathing patient: face mask, high-flow nasal oxygen (HFNO), positive end-expiratory pressure (PEEP) only without any pressure support level, pressure support with PEEP also called noninvasive ventilation (NIV), and the OPTINIV method (NIV combined with HFNO).
Noninvasive ventilation (NIV) for preoxygenation of patients with severe hypoxemic acute respiratory failure is associated with less hypoxemia than preoxygenation with face mask during tracheal intubation [14, 15], even if used in only 11% of cases in the INTUBE study [6]. Combining pressure support with PEEP limits alveolar collapse and atelectasis [16–19].
The face mask is taken off after preoxygenation to allow passage of the endotracheal tube through the mouth. Furthermore, positioning the endotracheal tube into the trachea may take time, from a few seconds to several minutes in case of difficult intubation [5]. Therefore, the use of HFNO provides the advantage of delivering apneic oxygenation during tracheal intubation [20].
Apneic oxygenation is a physiological phenomenon in which the difference between the alveolar rates of oxygen removal and carbon dioxide excretion generates a negative pressure gradient of up to 20 cmH2O. This negative pressure gradient allows the entry of oxygen into the lungs, provided there is airway permeability between the lungs and the atmosphere, open alveoli and high alveolar pressure in oxygen [21]. In 1959, a study reported eight patients scheduled for minor operations who were intubated and paralyzed, while receiving pure oxygen through the endotracheal tube [22]. The patients drastically increased their carbon dioxide tension and developed respiratory acidosis while maintaining 100% oxygen saturation. The interpretation of many studies performed in the field remains difficult because preoxygenation and apneic oxygenation are often evaluated concomitantly. In a randomized controlled study including non-severely hypoxemic patients [23], there was no significant difference between the median lowest SpO2 during intubation in the HFNO group compared with the standard facial mask group. However, there was less severe desaturation < 90% in the HFNO group compared with the standard face mask group. These results confirmed those of the observational study of Miguel Montanes et al. [24], performed in mild hypoxemic patients. However, in severe hypoxemic patients intubated in ICUs, Vourc'h et al. [25] found no difference on the minimal SpO2 values during tracheal intubation between 60 L/min of HFNO and face mask. Similar results were found by Semler et al. [26].
The discrepancies between the results of the studies performed on the field of preoxygenation [24–27] could mainly be explained by differences in the oxygen flow used for the apneic oxygenation, from 15 to 60 L/min, the populations studied, and the severity of hypoxemia. Moreover, if the efficiency of HFNO for preoxygenation and apneic oxygenation is still a matter of debate [28–30], it is mostly because preoxygenation, which is performed before induction of apnea, when the patient is still breathing, is not separated from apneic oxygenation, performed after induction of apnea, when the patient is not breathing anymore. Despite these controversies, a recent clinical practice guideline about the use of HFNO, suggests that HFNO treatment should be continued during intubation for patients who were already receiving HFNO [31]. However, only NIV allows to apply an external PEEP and a pressure support, opening and keeping the alveoli opened [32].
In a randomized controlled trial including 313 patients, NIV was recently compared with HFNO for preoxygenation of critically ill patients with acute hypoxemic respiratory failure [33]. Severe hypoxemia defined by a pulse oximetry < 80% occurred in 33 (23%) of 142 patients after preoxygenation with NIV and 47 (27%) of 171 with HFNO, without significant difference. However, in the subgroup of patients with PaO2/FiO2 lower than 200 mmHg, severe hypoxemia occurred less frequently after preoxygenation with NIV than with HFNO (28 (24%) of 117 patients vs 44 (35%) of 125, adjusted odds ratio 0.56 [0.32–0.99], p = 0.0459). This randomized controlled trial confirmed the results suggested by the meta-analysis performed in 2017 by Zhao et al. [34] and Chaudhuri et al. [35].
Using HFNO combined with NIV may have potential advantages over conventional NIV alone. The OPTINIV method [36], associating preoxygenation with pressure support and PEEP (NIV) and HFNO for both preoxygenation and apneic oxygenation, allowed a significant higher oxygen saturation during the intubation procedure of severe hypoxemic patients, when compared to preoxygenation with NIV alone. It is worth noting that the whole team should be trained to switch from a noninvasive method to invasive ventilation on the ventilator. Similarly, a bag-valve mask connected to oxygen should always be available to switch to manual ventilation if needed.
To summarize, four methods may provide sufficient reserves in oxygen during preoxygenation: facial mask oxygenation, HFNO, NIV, OPTINIV method, the latter permitting higher oxygen saturation during intubation procedure in severe hypoxemic patients.
Though apneic oxygenation may prolong the safe apnea time during endotracheal intubation in the critically ill patients [23], the more efficient way to oxygenate and ventilate patients during the period of apnea remains facial mask ventilation. Conventionally, rapid sequence induction, aimed at limiting gastric insufflation and thus pulmonary aspiration, is performed in the critically ill patients, as they may not be fasted or may have a slower gastric emptying. In the PREVENT study, Casey et al. [37] randomized patients to receive mask ventilation or no ventilation between induction and intubation. Patients receiving mask ventilation experienced a lower incidence of severe hypoxemia compared to controls, without suffering from an increased rate of pulmonary aspiration. Though this study was not powered to look at pulmonary aspiration, it certainly challenges dogma and provides some reassurance for gentle mask ventilation to limit hypoxemia during rapid sequence induction.
Devices for endotracheal tube positioning and airway management algorithms
Difficult intubation is known to be associated with life-threatening complications [4, 5, 38–41]. Successful first-attempt intubation is an established endpoint in airway management trials [41, 42] and first-attempt failure was reported to be a contributing factor to periprocedural complications and death [43, 44]. First-attempt success in ICU remains around 80% in the INTUBE study [6].
Risk factors for difficult intubation in ICU were assessed in a prospective multicenter observational study [45]. A score used for predicting difficult intubation, the MACOCHA score, was developed and later externally validated. The main predictors of difficult intubation were related to the patient (Mallampati score III or IV, obstructive sleep apnea syndrome (OSAS), reduced mobility of cervical spine, limited mouth opening), the pathology (coma, severe hypoxemia) and the operator (non-anesthesiologist) (Table 1). To rule out a difficult intubation with certainty, a cutoff of 3 was appropriate, allowing optimal negative predictive value and sensitivity.
Table 1.
MACOCHA score calculation worksheet
| Points | |
|---|---|
| Factors related to patient | |
| Mallampati score III or IV | 5 |
| Obstructive sleep apnea syndrome | 2 |
| Reduced mobility of cervical spine | 1 |
| Limited mouth opening < 3 cm | 1 |
| Factors related to pathology | |
| Coma | 1 |
| Severe hypoxemia (< 80%) | 1 |
| Factor related to operator | |
| Non-anesthesiologist | 1 |
| Total | 12 |
M. Mallampati score III or IV
A. Apnea Syndrome (obstructive)
C. Cervical spine limitation
O. Opening mouth < 3 cm
C. Coma
H. Hypoxia
A. Anesthesiologist Non-trained
Coded from 0 to 12
0 = easy
12 = very difficult
In order to improve first-attempt success and reduce the rate of difficult intubation, the device used for intubation is of major importance. Until the coronavirus disease 2019 (COVID-19) pandemic, standard laryngoscopy was the method the most used for intubation, in line with the ICU airway management recommendations [3, 44, 46–50]. Meanwhile, the most widely used technique for tracheal intubation with a standard Macintosh laryngoscope in critically ill patients was tracheal intubation using an endotracheal tube alone [3]. Alternatively, endotracheal tube using an intubating stylet has been proposed to facilitate endotracheal tube insertion, aimed at reducing the complications related to intubation [51]. Some authors suggest that using a preshaped endotracheal tube with a stylet may increase successful first-attempt intubation [51]. However, some traumatic injuries with stylets have been reported in case reports, with a very low incidence, including mucosal bleeding, perforation of the trachea or esophagus, and sore throat [51–53]. To determine the effect of using an intubating stylet on successful first-attempt intubation during endotracheal intubation of critically ill adults, we conducted the STYLET for Orotracheal intubation (STYLETO) trial [54]. We hypothesized that, as compared with endotracheal tube alone, the use of a stylet would significantly increase the successful first-attempt intubation rate. This multicenter randomized controlled trial was conducted in 32 ICUs in 30 university and 2 non-university French hospitals. We found that the use of stylet for tracheal intubation resulted in significantly higher successful first-attempt intubation than the use of endotracheal tube alone [54]. The 11 reported point estimate for successful first-attempt intubation favored endotracheal tube + stylet in every subgroup [54]. The stylet presents some advantages for airway management, being low cost and easy availability worldwide. It has been suggested that the use of a stylet could increase the risk of mucosal bleeding, laryngeal, endotracheal, mediastinal or esophageal injuries [41, 52] during endotracheal intubation. However, our trial reported a similar rate of traumatic injuries both the groups [54]. A recent study compared the use of bougie and stylet among critically ill adults undergoing endotracheal intubation [55]. Among the 1106 patients randomized, use of a bougie did not significantly increase the incidence of successful intubation on the first attempt compared with use of an endotracheal tube with stylet. It is worth noting that this study includes both direct laryngoscopes and videolaryngoscopes, without showing differences in the main result between groups.
Videolaryngoscopes are now recommended to improve airway management in ICU [49]. Three main categories of videolaryngoscopes exist according to the type of blade. First, the Macintosh videolaryngoscopes have Macintosh blades combined with video technology. The glottis can be seen either directly or via a video screen. Second, the anatomically shaped blades, also named hyperangulated blades, giving a view of the glottis without the need to flex or extend the neck, providing only an indirect view of the glottis, with the need to use a preshaped stylet with the endotracheal tube to facilitate tracheal intubation. Third, the anatomically shaped blade with a tube guide, also named channeled videolaryngoscopes, which does not necessitate a preshaped stylet. Despite the better visualization of the glottis, the main challenge when using videolaryngoscopes remains to insert the tube into the trachea. In other terms, achieving a 100% percentage of glottis opening (POGO) view, corresponding to a Cormack–Lehane grade 1 in direct laryngoscopy, during videolaryngoscopy does not guarantee successful intubation, as the tube has to pass a sharp angle to enter the larynx [49].
It has been suggested that videolaryngoscopes could help to reduce the difficult intubation rate [56, 57]. In a before-after study reporting a quality improvement process using a videolaryngoscope in an airway management algorithm [58], the systematic use of a Macintosh videolaryngoscope for intubation significantly reduced the incidence of difficult intubation and/or difficult laryngoscopy [58]. In the multivariate analysis, the “standard laryngoscopy” group was an independent risk factor for difficult intubation and/or difficult laryngoscopy. In addition, in the subgroup of patients with difficult intubation predicted by the MACOCHA score [5], the incidence of difficult intubation was much higher in the “standard laryngoscopy” group (47%) than in the “Macintosh videolaryngoscope” group (0%). These results were confirmed in 2014 by a systematic review and meta-analysis establishing that use of videolaryngoscopes for intubation in ICU could reduce the rate of difficult intubation [50]. Videolaryngoscopy improved difficult intubation, first-attempt success, Cormack 3/4 grades, esophageal intubation, and did not modify severe hypoxemia, severe cardiovascular collapse, airway injury, when compared with direct laryngoscopy. However, in 2016, Lascarrou et al. [1] showed in a large multicenter randomized controlled trial that videolaryngoscopy compared with direct laryngoscopy did not improve first-attempt intubation rate and was associated with higher rates of severe life-threatening complications. Several meta-analyses [59–61] published thereafter, showed conflicting results regarding the superiority of the videolaryngoscopes over direct laryngoscopy for tracheal intubation in critically ill patients. However, there was considerable, heterogeneity among the trials included. Indeed, several factors may influence the effectiveness of videolaryngoscopes compared with direct laryngoscopy, and they should be taken into account when interpreting the results of different studies. A prospective observational study that compares the use of direct laryngoscopy with a conventional Macintosh blade to the C-MAC® videolaryngoscope (Karl–Storz) [62], among operators that had performed, at least, 50 intubations in clinical simulation with the videolaryngoscope, was recently performed. In the videolaryngoscope group, there was a higher first-attempt intubation rate than in the conventional Macintosh blade group.
A recent study [63] showed that using Macintosh-style videolaryngoscope [64] as a first-intention device for tracheal intubation in operating room was associated with a significant increase in the proportion of easy airway, compared to the use of the standard Macintosh laryngoscope. To our knowledge, such a study was not performed in critically ill patients.
It is worth noting that one of the most important point in unchanneled videolaryngoscopes is the use of a stylet to preshape the endotracheal tube. In the study of Lascarrou et al. [1], it was used in less than 20% of cases. Using a preshaped endotracheal tube with a stylet may have potential advantages over conventional endotracheal tube and can help to increase success of intubation using videolaryngoscopy [47, 51, 65]. The type of endotracheal tube is also important, varying in shape and rigidity.
The expertise of operators is also important when assessing the results of published observational and randomized studies. In the study of Lascarrou et al. [1], it is worth noting that more than 80% of the operators were non-experts. More recently, a prospective observational study that compares the use of direct laryngoscopy with a conventional Macintosh blade to the C-MAC® videolaryngoscope (Karl–Storz) [62], among operators that had performed, at least, 50 intubations in clinical simulation with the videolaryngoscope, was performed. In the videolaryngoscope group, there was a higher first-attempt intubation rate than in the conventional Macintosh blade group. The experience required to attain 90% probability of optimal performance with videolaryngoscopes has also been evaluated [66, 67]. At least 75 attempts with hyperangulated videolaryngoscopes were required to achieve that level of proficiency [66, 67]. Similarly, a team recently implemented the McGrath MAC videolaryngoscope (Medtronic) as part of a quality improvement initiative [68]. They positioned the videolaryngoscope as the first-line laryngoscope for every intubation in critically ill patients to reinforce skill training. In the multivariate analysis, the absence of dedicated videolaryngoscopy expertise, junior status, and the presence of coma were independent risk factors of first-attempt failure. They reported for the first time in the critically ill that specific videolaryngoscopy skill training, assessed by the number of previous videolaryngoscopies performed, was an independent factor of first-attempt intubation success. There was an increase of the first-attempt procedure success rate according to the operators’ level of expertise. Having performed more than 15 videolaryngoscopies was associated with a first-attempt success rate of 87%.
This highlights the importance of training and education with the use of videolaryngoscopes, through clinical simulation or practice on cadavers, before implementation of these new techniques in critically ill patients. Table 2 presents ten tips to improve first-attempt intubation success using videolaryngoscopes.
Table 2.
Ten tips to improve first-attempt intubation success using videolaryngoscopes
| 1. Training of the operator in simulation centers |
| 2. Training of the operator with patients: at least 15 intubation performed in patients using the videolaryngoscopes |
| 3. Use of a predefined airway management algorithm |
| 4. Careful assessment of the difficulty of intubation before intubation, using the MACOCHA score for example |
| 5. Choice of a single videolaryngoscopy device |
| 6. Adequate preoxygenation for limiting hypoxemia and rush during the procedure |
| 7. Careful suctioning of secretions |
| 8. Rapid sequence induction with the use of neuromuscular blockers |
| 9. Use of a stylet or bougie for unchanneled videolaryngoscopes |
| 10. To allow easier intubation of the trachea, permit a sub-optimal visualization of the glottis |
The clinical practice guidelines for the management tracheal intubation in the critically ill by the Difficult Airway Society (DAS) [44] recommend the use of videolaryngoscopes in the presence of a difficult airway or as a rescue strategy when the direct laryngoscope has failed, while the All India Difficult Airway Association (AIDAA) guidelines [48] strongly recommend the availability and use of videolaryngoscopes in ICU, especially when a difficult airway is anticipated. Similarly, the expert guidelines on intubation and extubation in intensive care from the Société Francaise d’Anesthésie et de Réanimation (SFAR) and the Société de Réanimation de Langue Francaise (SRLF) published in 2017 [46] have included the videolaryngoscope in the algorithm for the airway management as the first option in the intubation of patients who score ≥ 3 in the MACOCHA score [5], and, as the rescue strategy, when intubation with the direct laryngoscopy fails. In a recent meta-analysis [69], the authors found that the use of videolaryngoscopes reduced the risk of difficult intubation and slightly increased the ratio of successful intubation at the first attempt among adult patients.
The COVID-19 pandemic has further highlighted the place of videolaryngoscopy during intubation in ICU, to limit the contamination of the airway operator. International guidelines for airway management in COVID-19 patients recommend using video laryngoscopy where available to increase the distance between the patient and airway operator, and tracheal intubation to be performed by the most experienced operator [70–73]. If a bougie or a stylet are used, the operator is advised to be careful when removing it so as not to spray secretions on the intubating team [70].
Future trials will better define the role of videolaryngoscopy in ICU, especially with respect to appropriate use of airway adjuncts as stylets. First-attempt intubation success rate alone has demonstrated to be an accurate primary outcome, strongly associated with the occurrence of complications during intubation procedure [43]. The expertise of operator will be a major confounding factor to consider when designing future randomized clinical trials.
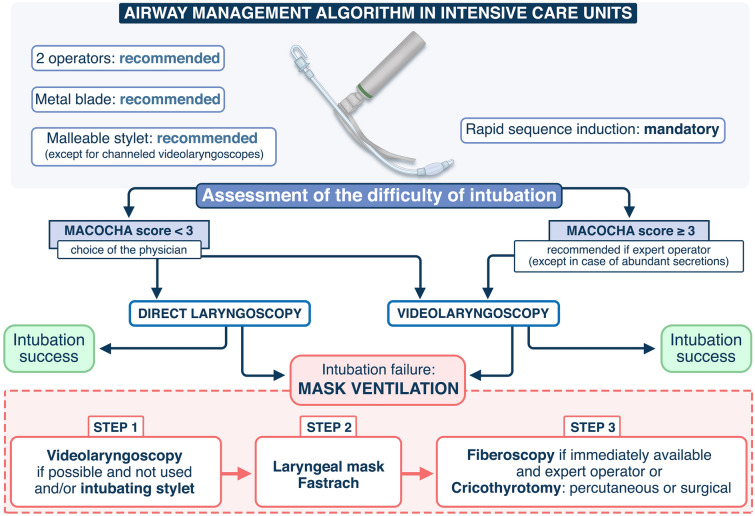
The use of dedicated tracheal intubation algorithms for critically ill patients [38] in case of predicted or unpredicted difficult intubation may also beneficial. We propose an updated airway management algorithm based on recently published trials [54, 55] in Fig. 1. Studies are needed to assess if applying this algorithm in ICU allows reduction of difficult intubation and complications. In each ICU, this airway management algorithm could be adapted according to the needs and situation.
Fig. 1.
Airway management algorithm. The availability of equipment for management of a difficult airway is checked. During the procedure, the patient should be ventilated in case of desaturation < 90%. All the intubation procedures performed in ICU are complicated. To improve first-attempt success, two operators, the use of a metal blade and the use of a malleable stylet (except for channeled videolaryngoscopes) are recommended. A rapid sequence induction is mandatory. In case of predicted difficult intubation (Mallampati score III or IV, OSAS, reduced mobility of cervical spine, limited mouth opening, coma, severe hypoxia, non-anesthesiologist (MACOCHA) score ≥ 3), the use of a videolaryngoscope is recommended if the operator is expert in using it (at least 15 intubations performed using the device), excepted in case of abundant secretions. If the MACOCHA score < 3, the choice of the device is left at the operator discretion (direct laryngoscope or videolaryngoscope). In case of intubation failure, a videolaryngoscope will be used if not used first, and/or an intubating stylet (malleable stylet or long flexible angulated stylet), followed successively using Laryngeal Mask Airway or fastrach, the use of fiberscopy in expert hands and finally the use of rescue percutaneal or surgical airway
Confirmation of tracheal tube position
Unrecognized esophageal intubation may result in profound hypoxemia, brain injury and death [74]. The 4th National Audit Project of the Royal College of Anaesthetists and Difficult Airway Society [75] showed that lack of capnography use contributed to 74% of the airway related deaths in ICU. Hence, clinical examination alone should not be used to exclude esophageal intubation. Following each tracheal intubation, tracheal tube position should be confirmed using continuous sustained waveform capnography of at least 5–7 breaths [48], even in the case of cardiopulmonary resuscitation. The presence of consistent waveforms reinforces tracheal placement of the endotracheal tube, and the effectiveness of cardiopulmonary resuscitation can be monitored by consistently producing EtCO2 values greater than 10–20 mmHg [76]. Inability to detect sustained exhaled carbon dioxide should prompt immediate laryngoscopic or bronchoscopic confirmation of tracheal tube position. Tube removal should be undertaken with ventilation using a facemask or a supraglottic airway, if esophageal placement cannot be excluded.
Hemodynamic optimization and choice of drugs
Hemodynamic failure is one of the most severe complications associated with endotracheal intubation in the critically ill patients [77]. Peri-intubation cardiovascular collapse is associated with an increased risk of both ICU and 28-day mortality [78]. To prevent severe collapse, fluid loading and early introduction of vasopressors together may decrease the occurrence of hemodynamic intubation-related complications [39, 79]. However, the level of evidence remains low. In a pragmatic, multicenter, unblinded, randomized trial [80], 337 critically ill adults patients undergoing tracheal intubation, were randomly assigned to receive either an intravenous bolus of crystalloid solution only or no fluid bolus. Administration of an intravenous fluid bolus alone without systematic administration of vasopressors did not decrease the incidence of cardiovascular collapse during tracheal intubation as compared to no fluid bolus. It is worth noting that the amount of fluid given was very low, which can partially explain the results, and that it was not combined with systematic vasoactive support. Recently, a randomized controlled trial enrolling 1067 critically ill patients undergoing tracheal intubation [81] reported that administration of an intravenous fluid bolus alone without associated to a systematic administration of norepinephrine compared with no fluid bolus did not significantly decrease the incidence of cardiovascular collapse. The FLUVA trial (NCT05318066) is currently underway to assess the effect of fluid loading and introduction of vasopressors before the tracheal intubation to reduce severe cardiovascular collapse.
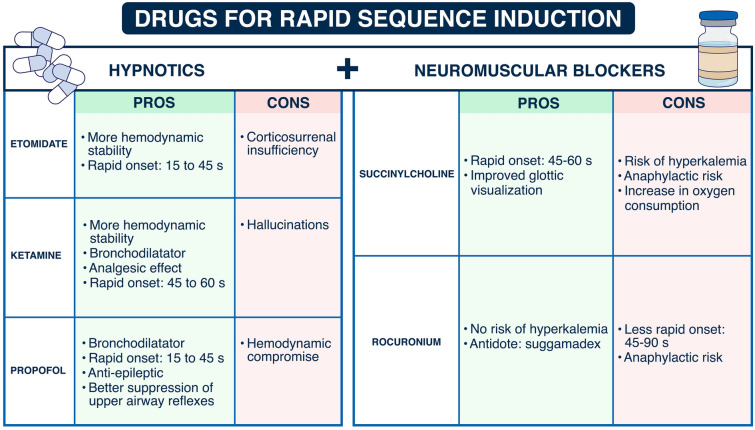
The drugs used for intubation [82] are especially important when dealing with hemodynamic complications. After a period of maximal activation of the sympathomimetic system all anesthetic drugs will rapidly lead to hemodynamic instability after induction. Vasopressors should be largely used in a preventive way. The respective advantages and benefits of drugs used for intubation are presented in Fig. 2. Russoto et al. [6] recently warned us about the risks of hemodynamic complications using propofol in a post hoc analysis [78] of the INTUBE study. Importantly, these hemodynamic complications were associated with an increased risk of death. Surprisingly, rapid sequence induction, combining the use of a neuromuscular blocker and a rapid-onset hypnotic, was used in only 75% of cases [78]. Among patients undergoing endotracheal intubation in an out-of-hospital emergency setting, rocuronium, compared with succinylcholine, failed to demonstrate noninferiority with regard to first-attempt intubation success rate [42]. However, the differences between the two drugs were clinically not significant, suggesting that both drugs can be used safely.
Fig. 2.
Drugs used for the intubation procedure: pros and cons
Intubation bundle to limit complications related to intubation procedure (update of the Montpellier-ICU intubation algorithm)
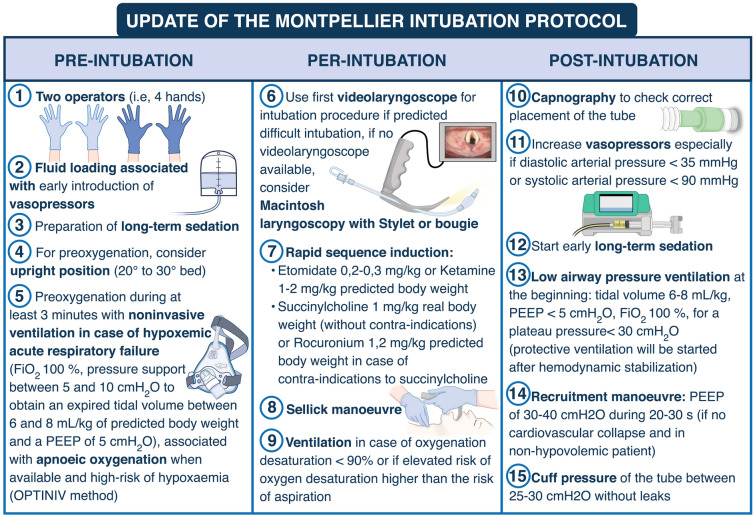
Jaber et al. [39] developed an intubation protocol designed to provide a practical tool that help with planning and optimizing the procedure of intubation. The updated version of the Montpellier intubation protocol [39], presented in Fig. 3, comprises of a list of items required, things to be done, or points to be considered during each of the phases of tracheal intubation: pre-intubation, per-intubation and post-intubation [83]. Application of this bundle has demonstrated improved safety during tracheal intubation [39]. In this study, Jaber et al. [39] demonstrated that the use of the Montpellier intubation protocol in the intervention phase was associated with significant diminution in life-threatening complications (21% vs. 34%, p = 0.03) and other complications (9 vs. 21%, p = 0.01) compared to the control phase. An external validation of the Montpellier intubation protocol, using a modified version of the protocol, was then performed and published in 2018 by Corl et al. [84]. They found that a modified Montpellier protocol was associated with a significant 16.2% [95% CI 5.1–30.0%] increase in first-attempt intubation success and a 12.6% [95% CI 1.2–23.6%] reduction in all intubation-related complications. Similar to these two studies, in the recent STYLETO trial performed by Jaber et al. [54], only 25% of patients had a severe complication, which is lower than the rate reported by Russoto et al. in their large observational international study [6].One explanation could be that in the STYLETO study [54], applying the Montpellier intubation protocol was highly recommended, which may explain the relatively low rate of complications observed in both groups (endotracheal tube + stylet and endotracheal tube alone), compared to the rate observed in the INTUBE study [6]. For example, in the INTUBE study [6], capnography was used in only 25% of cases.
Fig. 3.
Update of the Montpellier intubation protocol. Briefly, pre-intubation period interventions consist in fluid loading associated with early introduction of vasopressors, preoxygenation with NIV in the case of acute respiratory failure, preparation of sedation by the nursing team and the presence of two operators. NIV is applied during the 3-min preoxygenation phase with an ICU ventilator and a standard face mask. The PSV level is set between 5 and 10 cmH2O, adjusted to obtain an expired tidal volume of 6 to 8 ml/kg of ideal body weight. The FiO2 is set at 100% and the PEEP level of 5 cmH2O. During the intubation period, recommended induction is rapid sequence induction using short acting, well-tolerated hypnotics (etomidate or ketamine), and a rapid-onset muscle relaxant (succinylcholine or rocuronium), with application of cricoid pressure (Sellick maneuver). The Sellick maneuver is performed to prevent gastric contents from leaking into the pharynx, by external obstruction of the esophagus, and associated inhalation of substances into the lungs, as well as vomiting into an unprotected airway. Just after the intubation (post-intubation period), we recommend verification of the tube’s position by capnography (a technique which allows to confirm the endotracheal position of the tube and to verify the absence of esophageal placement), initiation of long-term sedation as soon as possible (to avoid agitation) and use of “protective” mechanical ventilation settings, as defined by the ARDS network
The combination of a limited physiologic reserve in the critically ill patients and the potential for difficult mask ventilation and intubation [45] mandates careful planning and justifies the use of an algorithmic approach to tracheal intubation, though the benefits of implementation need to be further evaluated.
A randomized controlled trial failed to show superiority of a verbal checklist prior to intubation in reducing lower arterial pressure or saturation during the intubation procedure [85]. It is worth noting that this checklist lacked interventions aimed at improving physiological parameters, such as adequate preoxygenation, fluid load, and vasopressors. Moreover, with the prior significant expertise in airway management in the participating centers and the use of checklists for other ICU procedures, a high penetrance of checklist items may have been present in the control group. Nevertheless, a pre-intubation checklist may be effective with less experienced teams and where the checklist includes interventions to optimize physiology [86].
Declarations
Conflicts of interest
SJ Jaber reports receiving consulting fees from Drager, Medtronic, Mindray, Fresenius, Baxter, and Fisher & Paykel. ADJ reports receiving remuneration for presentations from Medtronic, Drager and Fisher & Paykel. OR reports receiving research grant from Hamilton Medical AG, speaker fees from Hamilton Medical AG, Fisher & Paykel, Aerogen Ltd and Ambu, and non-financial research support from Timpel. No potential conflict of interest relevant to this article was reported for the other authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lascarrou JB, Boisrame-Helms J, Bailly A, Le Thuaut A, Kamel T, Mercier E, Ricard JD, Lemiale V, Colin G, Mira JP, Meziani F, Messika J, Dequin PF, Boulain T, Azoulay E, Champigneulle B, Reignier J. Video laryngoscopy vs direct laryngoscopy on successful first-pass orotracheal intubation among ICU patients: a randomized clinical trial. JAMA. 2017;317:483–493. doi: 10.1001/jama.2016.20603. [DOI] [PubMed] [Google Scholar]
- 2.Roux D, Reignier J, Thiery G, Boyer A, Hayon J, Souweine B, Papazian L, Mercat A, Bernardin G, Combes A, Chiche JD, Diehl JL, du Cheyron D, L'Her E, Perrotin D, Schneider F, Thuong M, Wolff M, Zeni F, Dreyfuss D, Ricard JD. Acquiring procedural skills in ICUs: a prospective multicenter study*. Crit Care Med. 2014;42:886–895. doi: 10.1097/CCM.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 3.Martin M, Decamps P, Seguin A, Garret C, Crosby L, Zambon O, Miailhe AF, Canet E, Reignier J, Lascarrou JB. Nationwide survey on training and device utilization during tracheal intubation in French intensive care units. Ann Intensive Care. 2020;10:2. doi: 10.1186/s13613-019-0621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaber S, Amraoui J, Lefrant J-Y, Arich C, Cohendy R, Landreau L, Calvet Y, Capdevila X, Mahamat A, Eledjam J-J. Clinical practice and risk factors for immediate complications of endotracheal intubation in the intensive care unit: a prospective, multiple-center study. Crit Care Med. 2006;34:2355–2361. doi: 10.1097/01.CCM.0000233879.58720.87. [DOI] [PubMed] [Google Scholar]
- 5.De Jong A, Molinari N, Terzi N, Mongardon N, Arnal JM, Guitton C, Allaouchiche B, Paugam-Burtz C, Constantin JM, Lefrant JY, Leone M, Papazian L, Asehnoune K, Maziers N, Azoulay E, Pradel G, Jung B, Jaber S. Early identification of patients at risk for difficult intubation in the intensive care unit: development and validation of the MACOCHA score in a multicenter cohort study. Am J Respir Crit Care Med. 2013;187:832–839. doi: 10.1164/rccm.201210-1851OC. [DOI] [PubMed] [Google Scholar]
- 6.Russotto V, Myatra SN, Laffey JG, Tassistro E, Antolini L, Bauer P, Lascarrou JB, Szuldrzynski K, Camporota L, Pelosi P, Sorbello M, Higgs A, Greif R, Putensen C, Agvald-Öhman C, Chalkias A, Bokums K, Brewster D, Rossi E, Fumagalli R, Pesenti A, Foti G, Bellani G. Intubation practices and adverse peri-intubation events in critically ill patients from 29 countries. JAMA. 2021;325:1164–1172. doi: 10.1001/jama.2021.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mort TC. The incidence and risk factors for cardiac arrest during emergency tracheal intubation: a justification for incorporating the ASA Guidelines in the remote location. J Clin Anesth. 2004;16:508–516. doi: 10.1016/j.jclinane.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 8.De Jong A, Rolle A, Molinari N, Paugam-Burtz C, Constantin JM, Lefrant JY, Asehnoune K, Jung B, Futier E, Chanques G, Azoulay E, Jaber S. Cardiac arrest and mortality related to intubation procedure in critically ill adult patients: a multicenter cohort study. Crit Care Med. 2018;46:532–539. doi: 10.1097/CCM.0000000000002925. [DOI] [PubMed] [Google Scholar]
- 9.Cook TM, Scott S, Mihai R. Litigation related to airway and respiratory complications of anaesthesia: an analysis of claims against the NHS in England 1995–2007. Anaesthesia. 2010;65:556–563. doi: 10.1111/j.1365-2044.2010.06331.x. [DOI] [PubMed] [Google Scholar]
- 10.Monet C, De Jong A, Jaber S (2021) Intubation in the ICU. In: Editor (ed)^(eds) Book Intubation in the ICU. City, pp. 100916 [DOI] [PubMed]
- 11.Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J. 2009;26:91–108. doi: 10.1111/j.1471-1842.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- 12.Dixon BJ, Dixon JB, Carden JR, Burn AJ, Schachter LM, Playfair JM, Laurie CP, O'Brien PE. Preoxygenation is more effective in the 25 degrees head-up position than in the supine position in severely obese patients: a randomized controlled study. Anesthesiology. 2005;102:1110–1115. doi: 10.1097/00000542-200506000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Mosier JM, Hypes CD, Sakles JC. Understanding preoxygenation and apneic oxygenation during intubation in the critically ill. Intensive Care Med. 2017;43:226–228. doi: 10.1007/s00134-016-4426-0. [DOI] [PubMed] [Google Scholar]
- 14.Baillard C, Fosse JP, Sebbane M, Chanques G, Vincent F, Courouble P, Cohen Y, Eledjam JJ, Adnet F, Jaber S. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am J Respir Crit Care Med. 2006;174:171–177. doi: 10.1164/rccm.200509-1507OC. [DOI] [PubMed] [Google Scholar]
- 15.Baillard C, Prat G, Jung B, Futier E, Lefrant JY, Vincent F, Hamdi A, Vicaut E, Jaber S. Effect of preoxygenation using non-invasive ventilation before intubation on subsequent organ failures in hypoxaemic patients: a randomised clinical trial. Br J Anaesth. 2018;120:361–367. doi: 10.1016/j.bja.2017.11.067. [DOI] [PubMed] [Google Scholar]
- 16.Pepin JL, Timsit JF, Tamisier R, Borel JC, Levy P, Jaber S. Prevention and care of respiratory failure in obese patients. Lancet Respir Med. 2016;4:407–418. doi: 10.1016/S2213-2600(16)00054-0. [DOI] [PubMed] [Google Scholar]
- 17.Hemmes SN, Gama de Abreu M, Pelosi P, Schultz MJ. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet. 2014;384:495–503. doi: 10.1016/S0140-6736(14)62030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delay JM, Sebbane M, Jung B, Nocca D, Verzilli D, Pouzeratte Y, Kamel ME, Fabre JM, Eledjam JJ, Jaber S. The effectiveness of noninvasive positive pressure ventilation to enhance preoxygenation in morbidly obese patients: a randomized controlled study. Anesth Analg. 2008;107:1707–1713. doi: 10.1213/ane.0b013e318183909b. [DOI] [PubMed] [Google Scholar]
- 19.Futier E, Constantin JM, Pelosi P, Chanques G, Massone A, Petit A, Kwiatkowski F, Bazin JE, Jaber S. Noninvasive ventilation and alveolar recruitment maneuver improve respiratory function during and after intubation of morbidly obese patients: a randomized controlled study. Anesthesiology. 2011;114:1354–1363. doi: 10.1097/ALN.0b013e31821811ba. [DOI] [PubMed] [Google Scholar]
- 20.Papazian L, Corley A, Hess D, Fraser JF, Frat JP, Guitton C, Jaber S, Maggiore SM, Nava S, Rello J, Ricard JD, Stephan F, Trisolini R, Azoulay E. Use of high-flow nasal cannula oxygenation in ICU adults: a narrative review. Intensive Care Med. 2016;42:1336–1349. doi: 10.1007/s00134-016-4277-8. [DOI] [PubMed] [Google Scholar]
- 21.De Jong A, Rollé A, Ducros L, Aarab Y, Monet C, Jaber S. Clinical applications of high-flow nasal cannula in the operating RoomHigh flow nasal cannula. Cham: Springer; 2021. pp. 101–108. [Google Scholar]
- 22.Frumin MJ, Epstein RM, Cohen G. Apneic oxygenation in man. Anesthesiology. 1959;20:789–798. doi: 10.1097/00000542-195911000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Guitton C, Ehrmann S, Volteau C, Colin G, Maamar A, Jean-Michel V, Mahe PJ, Landais M, Brule N, Bretonnière C, Zambon O, Vourc'h M. Nasal high-flow preoxygenation for endotracheal intubation in the critically ill patient: a randomized clinical trial. Intensive Care Med. 2019;45:447–458. doi: 10.1007/s00134-019-05529-w. [DOI] [PubMed] [Google Scholar]
- 24.Miguel-Montanes R, Hajage D, Messika J, Bertrand F, Gaudry S, Rafat C, Labbe V, Dufour N, Jean-Baptiste S, Bedet A, Dreyfuss D, Ricard JD. Use of high-flow nasal cannula oxygen therapy to prevent desaturation during tracheal intubation of intensive care patients with mild-to-moderate hypoxemia. Crit Care Med. 2015;43:574–583. doi: 10.1097/CCM.0000000000000743. [DOI] [PubMed] [Google Scholar]
- 25.Vourc'h M, Asfar P, Volteau C, Bachoumas K, Clavieras N, Egreteau PY, Asehnoune K, Mercat A, Reignier J, Jaber S, Prat G, Roquilly A, Brule N, Villers D, Bretonniere C, Guitton C. High-flow nasal cannula oxygen during endotracheal intubation in hypoxemic patients: a randomized controlled clinical trial. Intensive Care Med. 2015;41:1538–1548. doi: 10.1007/s00134-015-3796-z. [DOI] [PubMed] [Google Scholar]
- 26.Semler MW, Janz DR, Lentz RJ, Matthews DT, Norman BC, Assad TR, Keriwala RD, Ferrell BA, Noto MJ, McKown AC, Kocurek EG, Warren MA, Huerta LE, Rice TW. Randomized trial of apneic oxygenation during endotracheal intubation of the critically ill. Am J Respir Crit Care Med. 2016;193:273–280. doi: 10.1164/rccm.201507-1294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakles JC, Mosier JM, Patanwala AE, Dicken JM. Apneic oxygenation is associated with a reduction in the incidence of hypoxemia during the RSI of patients with intracranial hemorrhage in the emergency department. Intern Emerg Med. 2016;11:983–992. doi: 10.1007/s11739-016-1396-8. [DOI] [PubMed] [Google Scholar]
- 28.Chanques G, Jaber S. Nasal high-flow preoxygenation for endotracheal intubation in the critically ill patient? Maybe. Intensive Care Med. 2019;45:532–534. doi: 10.1007/s00134-019-05598-x. [DOI] [PubMed] [Google Scholar]
- 29.Ricard JD, Gregoretti C. Nasal high-flow preoxygenation for endotracheal intubation in the critically ill patient? Pro. Intensive Care Med. 2019;45:529–531. doi: 10.1007/s00134-019-05588-z. [DOI] [PubMed] [Google Scholar]
- 30.Hanouz JL, Gerard JL, Fischer MO. Nasal high-flow preoxygenation for endotracheal intubation in the critically ill patient? Con. Intensive Care Med. 2019;45:526–528. doi: 10.1007/s00134-019-05563-8. [DOI] [PubMed] [Google Scholar]
- 31.Rochwerg B, Einav S, Chaudhuri D, Mancebo J, Mauri T, Helviz Y, Goligher EC, Jaber S, Ricard JD, Rittayamai N, Roca O, Antonelli M, Maggiore SM, Demoule A, Hodgson CL, Mercat A, Wilcox ME, Granton D, Wang D, Azoulay E, Ouanes-Besbes L, Cinnella G, Rauseo M, Carvalho C, Dessap-Mekontso A, Fraser J, Frat JP, Gomersall C, Grasselli G, Hernandez G, Jog S, Pesenti A, Riviello ED, Slutsky AS, Stapleton RD, Talmor D, Thille AW, Brochard L, Burns KEA. The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline. Intensive Care Med. 2020;46:2226–2237. doi: 10.1007/s00134-020-06312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Jong A, Futier E, Millot A, Coisel Y, Jung B, Chanques G, Baillard C, Jaber S (2014) How to preoxygenate in operative room: healthy subjects and situations “at risk”. In: Editor (ed)^(eds) Book How to preoxygenate in operative room: healthy subjects and situations “at risk”. Elsevier, Masson, pp. 457–461 [DOI] [PubMed]
- 33.Frat JP, Ricard JD, Quenot JP, Pichon N, Demoule A, Forel JM, Mira JP, Coudroy R, Berquier G, Voisin B, Colin G, Pons B, Danin PE, Devaquet J, Prat G, Clere-Jehl R, Petitpas F, Vivier E, Razazi K, Nay MA, Souday V, Dellamonica J, Argaud L, Ehrmann S, Gibelin A, Girault C, Andreu P, Vignon P, Dangers L, Ragot S, Thille AW. Non-invasive ventilation versus high-flow nasal cannula oxygen therapy with apnoeic oxygenation for preoxygenation before intubation of patients with acute hypoxaemic respiratory failure: a randomised, multicentre, open-label trial. Lancet Respir Med. 2019;7:303–312. doi: 10.1016/S2213-2600(19)30048-7. [DOI] [PubMed] [Google Scholar]
- 34.Zhao H, Wang H, Sun F, Lyu S, An Y. High-flow nasal cannula oxygen therapy is superior to conventional oxygen therapy but not to noninvasive mechanical ventilation on intubation rate: a systematic review and meta-analysis. Crit Care. 2017;21:184. doi: 10.1186/s13054-017-1760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaudhuri D, Granton D, Wang DX, Einav S, Helviz Y, Mauri T, Ricard JD, Mancebo J, Frat JP, Jog S, Hernandez G, Maggiore SM, Hodgson C, Jaber S, Brochard L, Burns KEA, Rochwerg B. Moderate certainty evidence suggests the use of high-flow nasal cannula does not decrease hypoxia when compared with conventional oxygen therapy in the peri-intubation period: results of a systematic review and meta-analysis. Crit Care Med. 2020;48:571–578. doi: 10.1097/CCM.0000000000004217. [DOI] [PubMed] [Google Scholar]
- 36.Jaber S, Monnin M, Girard M, Conseil M, Cisse M, Carr J, Mahul M, Delay JM, Belafia F, Chanques G, Molinari N, De Jong A. Apnoeic oxygenation via high-flow nasal cannula oxygen combined with non-invasive ventilation preoxygenation for intubation in hypoxaemic patients in the intensive care unit: the single-centre, blinded, randomised controlled OPTINIV trial. Intensive Care Med. 2016;42:1877–1887. doi: 10.1007/s00134-016-4588-9. [DOI] [PubMed] [Google Scholar]
- 37.Casey JD, Janz DR, Russell DW, Vonderhaar DJ, Joffe AM, Dischert KM, Brown RM, Zouk AN, Gulati S, Heideman BE, Lester MG, Toporek AH, Bentov I, Self WH, Rice TW, Semler MW. Bag-mask ventilation during tracheal intubation of critically ill adults. N Engl J Med. 2019;380:811–821. doi: 10.1056/NEJMoa1812405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Jong A, Jung B, Jaber S. Intubation in the ICU: we could improve our practice. Crit Care. 2014;18:209. doi: 10.1186/cc13776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaber S, Jung B, Corne P, Sebbane M, Muller L, Chanques G, Verzilli D, Jonquet O, Eledjam J-J, Lefrant J-Y. An intervention to decrease complications related to endotracheal intubation in the intensive care unit: a prospective, multiple-center study. Intensive Care Med. 2010;36:248–255. doi: 10.1007/s00134-009-1717-8. [DOI] [PubMed] [Google Scholar]
- 40.Martin LD, Mhyre JM, Shanks AM, Tremper KK, Kheterpal S. 3,423 emergency tracheal intubations at a university hospital: airway outcomes and complications. Anesthesiology. 2011;114:42–48. doi: 10.1097/ALN.0b013e318201c415. [DOI] [PubMed] [Google Scholar]
- 41.Driver BE, Prekker ME, Klein LR, Reardon RF, Miner JR, Fagerstrom ET, Cleghorn MR, McGill JW, Cole JB. Effect of use of a Bougie vs endotracheal tube and stylet on first-attempt intubation success among patients with difficult airways undergoing emergency intubation: a randomized clinical trial. JAMA. 2018;319:2179–2189. doi: 10.1001/jama.2018.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guihard B, Chollet-Xémard C, Lakhnati P, Vivien B, Broche C, Savary D, Ricard-Hibon A, Marianne Dit Cassou PJ, Adnet F, Wiel E, Deutsch J, Tissier C, Loeb T, Bounes V, Rousseau E, Jabre P, Huiart L, Ferdynus C, Combes X. Effect of rocuronium vs succinylcholine on endotracheal intubation success rate among patients undergoing out-of-hospital rapid sequence intubation: a randomized clinical trial. JAMA. 2019;322:2303–2312. doi: 10.1001/jama.2019.18254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Jong A, Rolle A, Pensier J, Capdevila M, Jaber S. First-attempt success is associated with fewer complications related to intubation in the intensive care unit. Intensive Care Med. 2020;46:1278–1280. doi: 10.1007/s00134-020-06041-2. [DOI] [PubMed] [Google Scholar]
- 44.Higgs A, McGrath BA, Goddard C, Rangasami J, Suntharalingam G, Gale R, Cook TM. Guidelines for the management of tracheal intubation in critically ill adults. Br J Anaesth. 2018;120:323–352. doi: 10.1016/j.bja.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 45.De Jong A, Molinari N, Terzi N, Mongardon N, Arnal J-M, Guitton C, Allaouchiche B, Paugam-Burtz C, Constantin J-M, Lefrant J-Y, Leone M, Papazian L, Asehnoune K, Maziers N, Azoulay E, Pradel G, Jung B, Jaber S. Early identification of patients at risk for difficult intubation in ICU: development and validation of the MACOCHA score in a multicenter cohort study. Am J Respir Crit Care Med. 2013;187:832–839. doi: 10.1164/rccm.201210-1851OC. [DOI] [PubMed] [Google Scholar]
- 46.Quintard H, l’Her E, Pottecher J, Adnet F, Constantin JM, De Jong A, Diemunsch P, Fesseau R, Freynet A, Girault C, Guitton C, Hamonic Y, Maury E, Mekontso-Dessap A, Michel F, Nolent P, Perbet S, Prat G, Roquilly A, Tazarourte K, Terzi N, Thille AW, Alves M, Gayat E, Donetti L. Intubation and extubation of the ICU patient. Anaesthesia Crit Care Pain Med. 2017;36:327–341. doi: 10.1016/j.accpm.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Apfelbaum JL, Hagberg CA, Caplan RA, Blitt CD, Connis RT, Nickinovich DG, Benumof JL, Berry FA, Bode RH, Cheney FW, Guidry OF, Ovassapian A. Practice guidelines for management of the difficult airway: an updated report by the American society of anesthesiologists task force on management of the difficult airway. Anesthesiology. 2013;118:251–270. doi: 10.1097/ALN.0b013e31828604c6. [DOI] [PubMed] [Google Scholar]
- 48.Myatra SN, Ahmed SM, Kundra P, Garg R, Ramkumar V, Patwa A, Shah A, Raveendra US, Shetty SR, Doctor JR, Pawar DK, Ramesh S, Das S, Divatia JV. Republication: all India difficult airway association 2016 guidelines for tracheal intubation in the intensive care unit. Indian J Crit Care Med. 2017;21:146–153. doi: 10.4103/ijccm.IJCCM_57_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaber S, De Jong A, Pelosi P, Cabrini L, Reignier J, Lascarrou JB. Videolaryngoscopy in critically ill patients. Crit Care. 2019;23:221. doi: 10.1186/s13054-019-2487-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Jong A, Molinari N, Conseil M, Coisel Y, Pouzeratte Y, Belafia F, Jung B, Chanques G, Jaber S. Video laryngoscopy versus direct laryngoscopy for orotracheal intubation in the intensive care unit: a systematic review and meta-analysis. Intensive Care Med. 2014;40:629–639. doi: 10.1007/s00134-014-3236-5. [DOI] [PubMed] [Google Scholar]
- 51.Sorbello M, Hodzovic I. Tracheal tube introducers (Bougies), stylets and airway exchange catheters. In: Kristensen MS, Cook T, editors. Core topics in airway management. Cambridge: Cambridge University Press; 2020. pp. 130–139. [Google Scholar]
- 52.Kotoda M, Oguchi T, Mitsui K, Hishiyama S, Ueda K, Kawakami A, Matsukawa T. Removal methods of rigid stylets to minimise adverse force and tracheal tube movement: a mathematical and in-vitro analysis in manikins. Anaesthesia. 2019;74:1041–1046. doi: 10.1111/anae.14699. [DOI] [PubMed] [Google Scholar]
- 53.Brodsky MB, Akst LM, Jedlanek E, Pandian V, Blackford B, Price C, Cole G, Mendez-Tellez PA, Hillel AT, Best SR, Levy MJ. Laryngeal injury and upper airway symptoms after endotracheal intubation during surgery: a systematic review and meta-analysis. Anesth Analg. 2020;134:1023–1032. doi: 10.1213/ANE.0000000000005276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jaber S, Rollé A, Godet T, Terzi N, Riu B, Asfar P, Bourenne J, Ramin S, Lemiale V, Quenot JP, Guitton C, Prudhomme E, Quemeneur C, Blondonnet R, Biais M, Muller L, Ouattara A, Ferrandiere M, Saint-Léger P, Rimmelé T, Pottecher J, Chanques G, Belafia F, Chauveton C, Huguet H, Asehnoune K, Futier E, Azoulay E, Molinari N, De Jong A. Effect of the use of an endotracheal tube and stylet versus an endotracheal tube alone on first-attempt intubation success: a multicentre, randomised clinical trial in 999 patients. Intensive Care Med. 2021;47:653–664. doi: 10.1007/s00134-021-06417-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Driver BE, Semler MW, Self WH, Ginde AA, Trent SA, Gandotra S, Smith LM, Page DB, Vonderhaar DJ, West JR, Joffe AM, Mitchell SH, Doerschug KC, Hughes CG, High K, Landsperger JS, Jackson KE, Howell MP, Robison SW, Gaillard JP, Whitson MR, Barnes CM, Latimer AJ, Koppurapu VS, Alvis BD, Russell DW, Gibbs KW, Wang L, Lindsell CJ, Janz DR, Rice TW, Prekker ME, Casey JD. Effect of use of a Bougie vs endotracheal tube with stylet on successful intubation on the first attempt among critically ill patients undergoing tracheal intubation: a randomized clinical trial. JAMA. 2021;326:2488–2497. doi: 10.1001/jama.2021.22002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kory P, Guevarra K, Mathew JP, Hegde A, Mayo PH. The impact of video laryngoscopy use during urgent endotracheal intubation in the critically ill. Anesth Analg. 2013;117:144–149. doi: 10.1213/ANE.0b013e3182917f2a. [DOI] [PubMed] [Google Scholar]
- 57.Lakticova V, Koenig SJ, Narasimhan M, Mayo PH. Video laryngoscopy is associated with increased first pass success and decreased rate of esophageal intubations during urgent endotracheal intubation in a medical intensive care unit when compared to direct laryngoscopy. J Intensive Care Med. 2013;30:44–48. doi: 10.1177/0885066613492641. [DOI] [PubMed] [Google Scholar]
- 58.De Jong A, Clavieras N, Conseil M, Coisel Y, Moury PH, Pouzeratte Y, Cisse M, Belafia F, Jung B, Chanques G, Molinari N, Jaber S. Implementation of a combo videolaryngoscope for intubation in critically ill patients: a before-after comparative study. Intensive Care Med. 2013;39:2144–2152. doi: 10.1007/s00134-013-3099-1. [DOI] [PubMed] [Google Scholar]
- 59.Arulkumaran N, Lowe J, Ions R, Mendoza M, Bennett V, Dunser MW. Videolaryngoscopy versus direct laryngoscopy for emergency orotracheal intubation outside the operating room: a systematic review and meta-analysis. Br J Anaesth. 2018;120:712–724. doi: 10.1016/j.bja.2017.12.041. [DOI] [PubMed] [Google Scholar]
- 60.Zhao BC, Huang TY, Liu KX. Video laryngoscopy for ICU intubation: a meta-analysis of randomised trials. Intensive Care Med. 2017;43:947–948. doi: 10.1007/s00134-017-4741-0. [DOI] [PubMed] [Google Scholar]
- 61.Huang HB, Peng JM, Xu B, Liu GY, Du B. Video laryngoscopy for endotracheal intubation of critically ill adults: a systemic review and meta-analysis. Chest. 2017;152:510–517. doi: 10.1016/j.chest.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 62.Dey S, Pradhan D, Saikia P, Bhattacharyya P, Khandelwal H, Adarsha KN. Intubation in the Intensive Care Unit: C-MAC video laryngoscope versus Macintosh laryngoscope. Med Intensiva. 2020;44:135–141. doi: 10.1016/j.medin.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 63.De Jong A, Sfara T, Pouzeratte Y, Pensier J, Rolle A, Chanques G, Jaber S. A videolaryngoscope as a first-intention device for tracheal intubation in unselected patients in the operating room. Br J Anaesth. 2022 doi: 10.1016/j.bja.2022.05.030. [DOI] [PubMed] [Google Scholar]
- 64.De Jong A, Pouzeratte Y, Laplace A, Normanno M, Rollé A, Verzilli D, Perrigault P-F, Colson P, Capdevila X, Molinari N. Macintosh videolaryngoscope for intubation in the operating room: a comparative quality improvement project. Anesth Analg. 2021;132:524–535. doi: 10.1213/ANE.0000000000005031. [DOI] [PubMed] [Google Scholar]
- 65.Jaber S, Rolle A, Jung B, Chanques G, Bertet H, Galeazzi D, Chauveton C, Molinari N, De Jong A. Effect of endotracheal tube plus stylet versus endotracheal tube alone on successful first-attempt tracheal intubation among critically ill patients: the multicentre randomised STYLETO study protocol. BMJ Open. 2020;10:e036718. doi: 10.1136/bmjopen-2019-036718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aziz MF, Abrons RO, Cattano D, Bayman EO, Swanson DE, Hagberg CA, Todd MM, Brambrink AM. First-attempt intubation success of video laryngoscopy in patients with anticipated difficult direct laryngoscopy: a multicenter randomized controlled trial comparing the C-MAC D-blade versus the glidescope in a mixed provider and diverse patient population. Anesth Analg. 2016;122:740–750. doi: 10.1213/ANE.0000000000001084. [DOI] [PubMed] [Google Scholar]
- 67.Cortellazzi P, Caldiroli D, Byrne A, Sommariva A, Orena EF, Tramacere I. Defining and developing expertise in tracheal intubation using a GlideScope((R)) for anaesthetists with expertise in Macintosh direct laryngoscopy: an in-vivo longitudinal study. Anaesthesia. 2015;70:290–295. doi: 10.1111/anae.12878. [DOI] [PubMed] [Google Scholar]
- 68.Amalric M, Larcher R, Brunot V, Garnier F, De Jong A, Moulaire Rigollet V, Corne P, Klouche K, Jung B. Impact of videolaryngoscopy expertise on first-attempt intubation success in critically ill patients. Crit Care Med. 2020;48:e889–e896. doi: 10.1097/CCM.0000000000004497. [DOI] [PubMed] [Google Scholar]
- 69.Vargas M, Servillo G, Buonanno P, Iacovazzo C, Marra A, Putensen-Himmer G, Ehrentraut S, Ball L, Patroniti N, Pelosi P, Putensen C. Video vs. direct laryngoscopy for adult surgical and intensive care unit patients requiring tracheal intubation: a systematic review and meta-analysis of randomized controlled trials. Eur Rev Med Pharmacol Sci. 2021;25:7734–7749. doi: 10.26355/eurrev_202112_27620. [DOI] [PubMed] [Google Scholar]
- 70.Cook TM, El-Boghdadly K, McGuire B, McNarry AF, Patel A, Higgs A. Consensus guidelines for managing the airway in patients with COVID-19: Guidelines from the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia. 2020;75:785–799. doi: 10.1111/anae.15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Patwa A, Shah A, Garg R, Divatia JV, Kundra P, Doctor JR, Shetty SR, Ahmed SM, Das S, Myatra SN. All India difficult airway association (AIDAA) consensus guidelines for airway management in the operating room during the COVID-19 pandemic. Indian J Anaesth. 2020;64:S107–s115. doi: 10.4103/ija.IJA_498_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Jong A, Khanna AK. Airway management in the critically ill patient with COVID-19. Curr Opin Anaesthesiol. 2022;35:137–143. doi: 10.1097/ACO.0000000000001101. [DOI] [PubMed] [Google Scholar]
- 73.Velly L, Gayat E, Quintard H, Weiss E, De Jong A, Cuvillon P, Audibert G, Amour J, Beaussier M, Biais M, Bloc S, Bonnet MP, Bouzat P, Brezac G, Dahyot-Fizelier C, Dahmani S, de Queiroz M, Di Maria S, Ecoffey C, Futier E, Geeraerts T, Jaber H, Heyer L, Hoteit R, Joannes-Boyau O, Kern D, Langeron O, Lasocki S, Launey Y, le Saché F, Lukaszewicz AC, Maurice-Szamburski A, Mayeur N, Michel F, Minville V, Mirek S, Montravers P, Morau E, Muller L, Muret J, Nouette-Gaulain K, Orban JC, Orliaguet G, Perrigault PF, Plantet F, Pottecher J, Quesnel C, Reubrecht V, Rozec B, Tavernier B, Veber B, Veyckmans F, Charbonneau H, Constant I, Frasca D, Fischer MO, Huraux C, Blet A, Garnier M. Guidelines: Anaesthesia in the context of COVID-19 pandemic. Anaesthesia, critical care & pain medicine. 2020;39:395–415. doi: 10.1016/j.accpm.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holland R, Webb RK, Runciman WB. The Australian Incident Monitoring Study. Oesophageal intubation: an analysis of 2000 incident reports. Anaesth Intensive Care. 1993;21:608–610. doi: 10.1177/0310057X9302100519. [DOI] [PubMed] [Google Scholar]
- 75.Cook TM, Woodall N, Harper J, Benger J. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 2: intensive care and emergency departments. Br J Anaesth. 2011;106:632–642. doi: 10.1093/bja/aer059. [DOI] [PubMed] [Google Scholar]
- 76.Kodali BS, Urman RD. Capnography during cardiopulmonary resuscitation: current evidence and future directions. J Emerg Trauma Shock. 2014;7:332–340. doi: 10.4103/0974-2700.142778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Russotto V, Myatra SN, Laffey JG. What's new in airway management of the critically ill. Intensive Care Med. 2019;45:1615–1618. doi: 10.1007/s00134-019-05757-0. [DOI] [PubMed] [Google Scholar]
- 78.Russotto V, Tassistro E, Myatra SN, Parotto M, Antolini L, Bauer P, Lascarrou JB, Szułdrzyński K, Camporota L, Putensen C, Pelosi P, Sorbello M, Higgs A, Greif R, Pesenti A, Valsecchi MG, Fumagalli R, Foti G, Bellani G, Laffey JG. Peri-intubation cardiovascular collapse in critically ill patients: insights from the INTUBE study. Am J Respir Crit Care Med. 2022 doi: 10.1164/rccm.202111-2575OC. [DOI] [PubMed] [Google Scholar]
- 79.Perbet S, De Jong A, Delmas J, Futier E, Pereira B, Jaber S, Constantin JM. Incidence of and risk factors for severe cardiovascular collapse after endotracheal intubation in the ICU: a multicenter observational study. Crit Care. 2015;19:257. doi: 10.1186/s13054-015-0975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Janz DR, Casey JD, Semler MW, Russell DW, Dargin J, Vonderhaar DJ, Dischert KM, West JR, Stempek S, Wozniak J, Caputo N, Heideman BE, Zouk AN, Gulati S, Stigler WS, Bentov I, Joffe AM, Rice TW. Effect of a fluid bolus on cardiovascular collapse among critically ill adults undergoing tracheal intubation (PrePARE): a randomised controlled trial. Lancet Respir Med. 2019;7:1039–1047. doi: 10.1016/S2213-2600(19)30246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Russell DW, Casey JD, Gibbs KW, Ghamande S, Dargin JM, Vonderhaar DJ, Joffe AM, Khan A, Prekker ME, Brewer JM, Dutta S, Landsperger JS, White HD, Robison SW, Wozniak JM, Stempek S, Barnes CR, Krol OF, Arroliga AC, Lat T, Gandotra S, Gulati S, Bentov I, Walters AM, Dischert KM, Nonas S, Driver BE, Wang L, Lindsell CJ, Self WH, Rice TW, Janz DR, Semler MW. Effect of fluid bolus administration on cardiovascular collapse among critically ill patients undergoing tracheal intubation: a randomized clinical trial. Jama. 2022;328(3):270–279. doi: 10.1001/jama.2022.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jung B, Clavieras N, Nougaret S, Molinari N, Roquilly A, Cisse M, Carr J, Chanques G, Asehnoune K, Jaber S. Effects of etomidate on complications related to intubation and on mortality in septic shock patients treated with hydrocortisone: a propensity score analysis. Crit Care. 2012;16:R224. doi: 10.1186/cc11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Constantin J-M, Futier E, Cherprenet A-L, Chanques G, Guerin R, Cayot-Constantin S, Jabaudon M, Perbet S, Chartier C, Jung B, Guelon D, Jaber S, Bazin J-E. A recruitment maneuver increases oxygenation after intubation of hypoxemic intensive care unit patients: a randomized controlled study. Critical Care (London, England) 2010;14(2):R76. doi: 10.1186/cc8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Corl KA, Dado C, Agarwal A, Azab N, Amass T, Marks SJ, Levy MM, Merchant RC, Aliotta J. A modified Montpellier protocol for intubating intensive care unit patients is associated with an increase in first-pass intubation success and fewer complications. J Crit Care. 2018;44:191–195. doi: 10.1016/j.jcrc.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Janz DR, Semler MW, Joffe AM, Casey JD, Lentz RJ, de Boisblanc BP, Khan YA, Santanilla JI, Bentov I, Rice TW. A multicenter, randomized trial of a checklist for endotracheal intubation of critically ill adults. CHEST J. 2017;153(4):816–824. doi: 10.1016/j.chest.2017.08.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De Jong A, Jaber S. Do not throw the intubation checklist out with the bath water! Chest. 2018;153:771–773. doi: 10.1016/j.chest.2017.08.1170. [DOI] [PubMed] [Google Scholar]