Abstract
Background and objectives
We aimed to evaluate the effect of bupivacaine and dexmedetomidine added to bupivacaine used in tranversus abdominis plane (TAP) block on postoperative pain and patient satisfaction in patients undergoing lower abdominal surgery.
Methods
Patients submitted to lower abdominal surgery were enrolled in the study. After anesthesia induction, ultrasound guided TAP block was performed. TAP block was obtained with 21 mL 0.9% saline in Group C (n = 31), 20 mL 0.5% bupivacaine + 1 mL saline in Group B (n = 31), and 20 mL 0.5% bupivacaine + 1 mL dexmedetomidine (100 μg) in Group BD (n = 31).
Results
Visual analog scale scores were lower in Group BD compared to Group C, at all time points (p < 0.05); it was lower in group BD than in group B at 10–24 h. In Group B, it was lower than Group C at 2–8 h (p < 0.05). Total morphine consumption was lower in Group BD compared to other groups and lower in group B than in the controls (p < 0.001). Patient satisfaction was higher in Group BD than in other groups and was higher in both study groups than in the controls (p < 0.001). Nausea-vomiting scores, antiemetic requirement, or additional analgesic administration were not significant among groups (p > 0.05).
Conclusions
The addition of dexmedetomidine to bupivacaine on TAP block decreased postoperative pain scores and morphine consumption; it also increased patient satisfaction in patients undergoing lower abdominal surgery. Dexmedetomidine did not have any effect on nausea and vomiting score and antiemetic requirement.
Keywords: Dexmedetomidine, Bupivacaine, Transversus abdominis plane block, Lower abdominal surgery
Resumo
Justificativa e objetivos
O objetivo do estudo foi avaliar o efeito de bupivacaína e dexmedetomidina adicionada à bupivacaína para bloqueio do plano transverso abdominal (TAP) no controle da dor e satisfação do paciente após cirurgia abdominal inferior.
Métodos
Pacientes submetidos à cirurgia abdominal inferior foram incluídos no estudo. Após a indução da anestesia, o bloqueio TAP guiado por ultrassom foi realizado com 21 mL de solução salina a 0.9% no Grupo C (n = 31), 20 mL de bupivacaína a 0,5% + 1 mL de solução salina no Grupo B (n = 31) e 20 mL de bupivacaína a 0,5% + 1 mL de dexmedetomidina (100 μg) no grupo BD (n = 31).
Resultados
Os escores da escala visual analógica foram menores no Grupo BD comparado ao Grupo C em todos os tempos mensurados (p < 0,05); foi menor no Grupo BD que no Grupo B em 10-24 horas. No Grupo B, os escores VAS foram menores que no Grupo C em 2-8 horas (p < 0,05). O consumo total de morfina foi menor no Grupo BD em comparação com outros grupos e menor no Grupo B que nos controles (p < 0,001). A satisfação do paciente foi maior no Grupo BD que nos outros grupos e maior em ambos os grupos de estudo que nos controles (p < 0,001). Os escores de náusea e vômito, necessidade de antiemético ou de analgésicos adicionais não foram significativos entre os grupos (p > 0,05).
Conclusões
A adição de dexmedetomidina à bupivacaína em bloqueio TAP reduziu os escores de dor e o consumo de morfina no pós-operatório, além de aumentar a satisfação em pacientes submetidos à cirurgia abdominal inferior. Dexmedetomidina não apresentou efeito sobre os escores de náusea e vômito e a necessidade de antiemético.
Palavras-chave: Dexmedetomidina, Bupivacaína, Bloqueio do plano transverso abdominal, Cirurgia abdominal inferior
Introduction
Open inguinal hernioplasty and open appendectomy surgery mostly cause mild to severe postoperative pain.1, 2, 3 If not treated, postoperative pain leads to chronic pain and undesirable events ranging from patient discomfort and prolonged immobility to thrombolytic phenomenon and pulmonary complications.4, 5 Regarding chronic pain formation, postoperative pain state, and nerve injury during surgery, as well as insufficient early postoperative pain control, are among the risk factors.4, 6 Expected pain prevalence following hernia repair was determined as 54% and postoperative 2 year cumulative prevalence was found to be 30%.7 Transversus abdominal plane (TAP), one of the peripheral nerve blocks, was reported to reduce postoperative pain following hysterectomy, colorectal surgery, appendectomy, and inguinal hernioplasty.2, 3, 8, 9, 10
TAP is located between the oblique muscles and the transverse abdominis muscles. On TAP iliohypogastric nerve lies and anterolateral abdominal wall afferent T6-L1 nerves is got blocked with blockage of this area.1, 5
Single and continuous TAP block techs have been successfully administered for pain control in the repair of inguinal hernia.11, 12 However, the duration of single-dose administered TAP block is limited to the effect of administered local anesthetics. Addition of adjuvant to local anesthesia may prolong the block's duration.13 Dexmedetomidine is a selective alpha-2 adrenergic agonist with both analgesic and sedative properties.14 When administered as a perineural adjuvant, dexmedetomidine reduces initial blocking time whilst prolonging sensory and motor blockade duration.15
Materials and methods
Local ethics approval for the study was received (2014/37). Then the study was recorded on http://www.clinicaltrials.gov (NCT02064530). After receiving written consent from the patients, 93 ASA I–II patients aged 18–65 years were included in the study and scheduled for open appendectomy repair or inguinal hernia administrations. A placebo-controlled, randomized, prospective and triple-blinded study was carried out, and blinding was applied both to the patients and to the investigators and data collection team. Patients were excluded if they: had a history of allergy to bupivacaine and dexmedetomidine; were or may have been pregnant; had a coagulation disorder, serious cardiac and pulmonary disease; had an administration site infection; or were unable to understand the scoring system. Patients were randomized with sealed envelopes. The control group (Group C) (n = 31), bupivacaine group (Group B) (n = 31) and bupivacaine + dexmedetomidine group (Group BD) (n = 31) were determined. The Groups C, B, and BD were given, respectively, 21 mL 0.9% NaCl, 20 mL 0.5% bupivacaine (without epinephrine) (Bustesin® 5 mg.mL−1, Vem Pharmaceuticals, Ankara, Turkey) + 1 mL 0.9% NaCl solution, and 20 mL 0.5% bupivacaine (without epinephrine) and 100 μg (1 mL) dexmedetomidine (Precedex® 100 μg.mL−1, Meditera, ABD). None of the patients, or the investigators administrating the TAP block and carrying out the postoperative evaluation, or the surgeons performing the operation, were given information on the groups. All patients received standard general anesthesia under standard monitorization, and perioperative mean arterial pressure (MAP) and heart rate (HT) values were recorded. Anesthesia was induced by administration of 5–7 mg.kg−1 thiopental (Pental®, Ulagay, Turkey), 0.6 mg.kg−1 rocuronium bromide (Esmeron®, Schering-Plough, Holland) and 1 μg.kg−1 fentanyl (Talinat®, Vem Pharmaceuticals, Istanbul, Turkey). When muscles were sufficiently relaxed, endotracheal intubation was performed. Patients were monitored and ventilated with an electronic anesthesia device (S/5 Avance, Datex Ohmeda, Finland).
For anesthesia supply, 4–6% desflurane (Suprane® Liquid 100%, Abbott, Norway) was added to 50% oxygen + 50% nitrogen mixture. It was projected to administer 1 μg.kg−1 intravenous fentanyl in any case where MAP and HT values go up to 20% over basal values before induction. A MAP decrease of more than 20% was considered to be hypotension. In such cases, desflurane concentration would be reduced and 5 mg ephedrine would be intravenously administered, if necessary. Slowing down of the heart rate to less than 50 beats/min was considered to be bradycardia, and 0.5 mg atropine was planned to be administered in these cases. The skin antiseptic was provided with 2% chlorhexidine solution following anesthesia induction. A high-frequency (5–10 MHz) ultrasound linear probe (Mindray M7, China) was transversely located on the anterolateral abdominal wall between the iliac crest and the subcostal area, and neurophasia (TAP) between the internal oblique and tranverse abdominis was identified. A 50 mm nerve block needle (Braun Melsungen AG, Melsungen, Germany) was concurrently located on the area and pre-prepared agent was injected after negative aspiration. The injected liquid was observed on ultrasound to be distributed in a dark oval form in TAP. Surgical procedure started after performing TAP block and distance between start and stop point of wound incision was recorded as length of incision.
Anesthesia was discontinued following surgical operation and residual block was reversed using 0.02 mg.kg−1 atropine (Atropin Sülfat® 0.25 mg.mL−1, Biofarma, Istanbul, Turkey) and 0.04 mg.kg−1 neostigmin (Neostigmine® 0.5 mg.mL−1, Adeka, Turkey). When the patient came out of anesthesia and had spontaneously gained sufficient tidal volume and motor function, they were transferred to the Postoperative Care Unit (PCU). One gram of paracetamol (Perfalgan®, Bristol-Myers Squibb, USA) was intravenously administered as a standard postoperative anesthesia regime and attached to a Patient-Controlled Analgesia (PCA), device solution was prepared by adding 100 mg morphine (Morphine® 10 mg.mL−1, Galen, Istanbul, Turkey) to 250 mL 0.9% NaCl. The PCA device was set to have a 7 min lockout time and a 0.5 mg bolus. Cases were transferred to general surgery after 2 h follow-up in PCU during the postoperative period. The severity of pain at rest was assessed using a 10 cm Visual Analog Scale (VAS), scaled from left; 0 = no pain, to right; 10 = worst imaginable pain, and recorded post-operative 0, 2, 6, 8, 10, 12, 18 and 24 h.
Patients with a VAS score of more than 4 during assessment were given 50 mg i.v. diclofenac sodium (Dicloron®, Abbott, Norway). The doses of morphine and analgesic consumed were recorded. The satisfaction of patients was recorded in the 24th hour following operation. Patient satisfaction assessment scores were: 1 – poor, 2 – moderate, 3 – good, 4 – perfect.16 Patients were monitored for 24 h in terms of nausea, vomiting, altered mental status, hypotension, hypertension, bradycardia, and tachycardia, all of which might occur as a result of the drugs administered. Nausea and vomiting assessment scores were: 1 – none, 2 – nausea, 3 – retching, 4 – vomiting. All patents having nausea, retching or vomiting were planned to be given an antiemetic.8 In the presence of nausea–vomiting, it was planned to administer 10 mg i.v. metoclopramide HCL (Primperan®, Biofarma, Turkey) first, and then 4 mg i.v. ondansetron (Zofran®, GlaxoSmithKline, Italy) following 1 h follow-up if necessary. The primary outcome of this study was to assess morphine consumption for post-operative purpose. The secondary aim was to meet patient's satisfaction with respect to pain scores assessed with VAS within the postoperative 24 h period, period of hospitalization, nausea, vomiting and antiemetic. To calculate sample size for the study, 10 cases were pre-studied and the averages of postoperative 24 h morphine consumption calculated. When alpha is 0.05, and β (the false negative rate) is 0.20, and the minimum mean difference is 6.3 mg and expected standard.
Statistical analysis
Data was statistically assessed using the Statistical Package for the Social Sciences (SPSS for Windows, Version 21.0, IBM Corp, Armonk, NY). The Shapiro–Wilk normality test was used to determine whether sample distribution was normal or not. Descriptive statistics unit number (n) was given as mean ± standard deviation and median (min–max) values. One way analysis of variance (ANOVA) was used to evaluate whether there was a difference among groups of having normal distribution. To evaluate parameters having abnormal distribution, the Kruskal–Wallis test, one of the non-parametric tests, was used.
The significant ones were compared using pairwise comparisons and it was determined that which group has statistical difference. A value of p < 0.05 was accepted as statistically significant.
Results
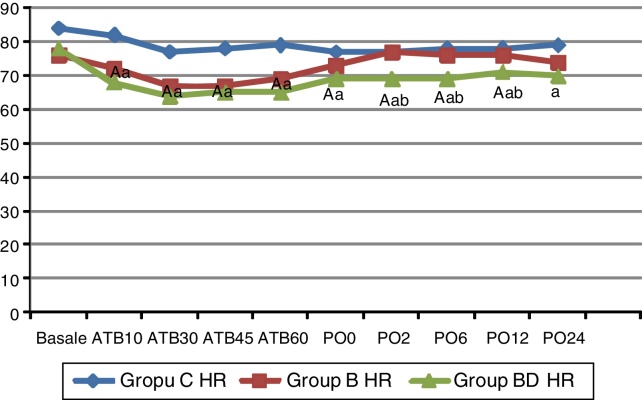
No significant difference was observed in age, weight, length, body mass index, gender, ASA class, surgery type, surgery duration, length of incision, discharge duration, or intraoperative to fentanyl consumption (p > 0.05) (Table 1). When compared to Group C, a significant decrease in heart rate was observed in Groups B and BD in the 10th, 30th, 45th, and 60th minutes of the operation (p < 0.05) (Fig. 1). During the postoperative period, the increase continued in Group BD in 0 and 24th h in comparison with Group C, and in the 120th min, 6th, and 12th h in comparison to both Group C and Group B (p < 0.05) (Fig. 1). In the assessment of heart rate within groups, a fall according to initial heart rates was observed in the 10th, 30th, 45th, and 60th minutes of operation in Group B, and at every measurement time excluding the postoperative 24 h in Group BD (p < 0.05). However, 0.5 mg atropine was needed as two of the patients’ HR went below 50 beats/min. No difference compared to initial values was noticed in Group C (p > 0.05) (Fig. 1).
Table 1.
Demographic characteristics, hospital stay and intraoperative fentanyl consumption. Data are given as mean (SD) or number (%).
| Group C (n = 31) Mean ± SD |
Group B (n = 31) Mean ± SD |
Group BD (n = 31) Mean ± SD |
p | |
|---|---|---|---|---|
| Age (years) | 44.2 ± 13.9 | 43.5 ± 15.0 | 43.2 ± 15.0 | 0.962 |
| Weight (kg) | 80.8 ± 14.6 | 76.3 ± 12.0 | 77.1 ± 6.9 | 0.279 |
| Height (cm) | 172.6 ± 7.2 | 171.2 ± 6.5 | 174.1 ± 7.4 | 0.290 |
| BMI | 27.4 ± 5.0 | 26.0 ± 3.7 | 2.0 ± 5.5 | 0.445 |
| Gender (F/M) n (%) |
7 (22.6) 24 (77.4) |
7 (22.6) 24 (77.4) |
6 (19.4) 25 (80.6) |
0.439 |
| ASA (I/II) n (%) |
24 (77.4) 7 (22.6) |
24 (77.4) 7 (22.6) |
28 (90.3) 3 (9.7) |
0.127 |
| Duration of surgery (min) | 90.3 ± 36.3 | 77.1 ± 34.6 | 71.2 ± 21.5 | 0.054 |
| Length of incision (cm) | 6.9 ± 1.5 | 6.8 ± 1.6 | 7.4 ± 1.6 | 0.295 |
| Hospital stay (day/med/min–max) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0.424 |
| I.O. fentanyl (mcg) | 101.6 ± 8.9 | 98.3 ± 8.9 | 98.4 ± 5.4 | 0.451 |
| Surgery type (n) | 0.468 | |||
| Inguinal hernia | 21 | 21 | 21 | |
| Perf. appendectomy | 5 | 4 | 4 | |
| Non-perf. appendectomy | 5 | 6 | 6 |
C, control; B, bupivacaine; BD, bupivacaine + dexmedetomidine; ASA, American Society of Anesthesiologists; BMI, body mass index; I.O., intra operative; Perf, perfore.
Figure 1.
Heart rate (HR) (beats/min). Data are given as median (min–max) or mean (SD). ATB, after TAP block; PO, postoperative; C, control; B, bupivacaine; BD, bupivacaine + dexmedetomidine. (a) Significant difference from Group C; (b) significant difference from Group B; (A) significant difference in the group compared to baseline values.
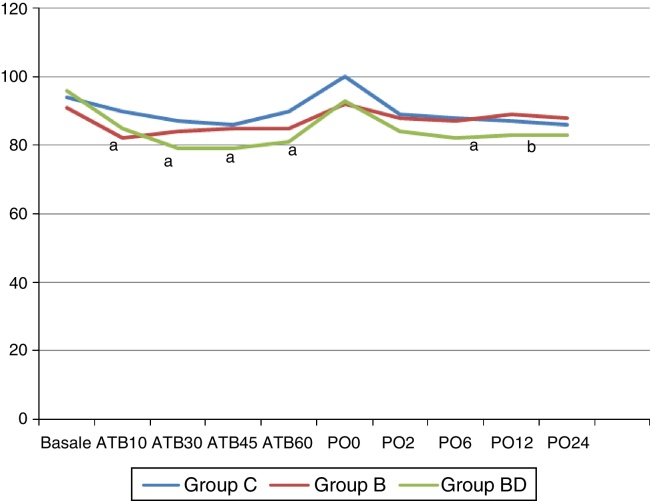
In comparison to Group C, a decrease in the normal clinical level of blood pressure mean was observed in the 10th minute of the operation in Group B; in the 30th, 45th and 60th minutes of the operation, and in the postoperative 6th hour in Group BD; and in the 12th hour comparing with Group B (p > 0.05) (Fig. 2).
Figure 2.
Mean blood pressure (MBP) (mmHg). Data are given as median (min–max) or mean (SD). ATB, after TAP block; PO, postoperative; C, control; B, bupivacaine; BD, bupivacaine + dexmedetomidine. (a) Significant difference from group C; (b) significant difference from group B.
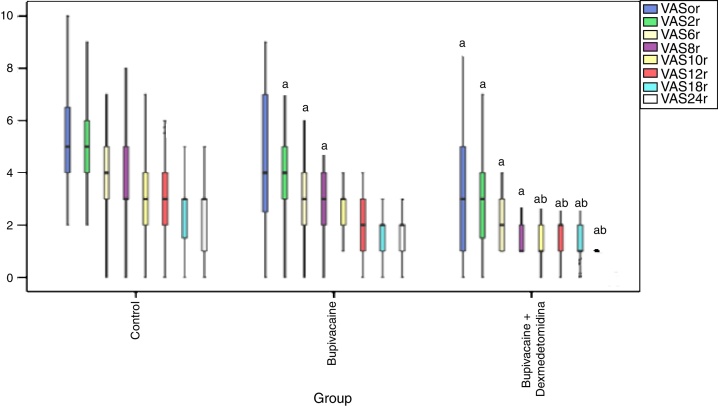
While a statistically significant decrease in VAS score was observed only in Group BD in comparison with Group C in post-operative 0 min, the decrease in both Group B and Group BD in between the postoperative 120th min and the 8th hour was statistically significant (p < 0.05) (Fig. 3). The decrease in Group BD in between the 10th and 24th h postoperatively was statistically significant when compared with Groups C and B (p < 0.05) (Fig. 3).
Figure 3.
Postoperative VASr scores. (a) Significant difference from group C; (b) significant difference from group B. VASr, Visuel Analog Scale at Rest.
While the postoperative morphine consumption in Group BD was low at all times
When compared to Groups B and C, it was lower in Group B than Group C in the 6th, 12th, 18th and 24th h (p < 0.001) (Table 2).
Table 2.
Postoperative morphine consumption (mg) and additional analgesic need. Data are given as mean (SD) or number.
| Group C (n = 31) | Group B (n = 31) | Group BD (n = 31) | p | |
|---|---|---|---|---|
| Postoperative morphine consumption | ||||
| Postop. 120 min | 5.0 ± 1.8 | 4.6 ± 2.0 | 2.2 ± 1.3a,b | <0.001 |
| Postop. 6 hour | 12.3 ± 4.8 | 9.9 ± 4.1a | 4.3 ± 2.4a,b | <0.001 |
| Postop. 12 hour | 19.1 ± 6.6 | 13.5 ± 4.8a | 6.4 ± 3.5a,b | <0.001 |
| Postop 18 hour | 23.8 ± 4.7 | 15.6 ± 4.7a | 7.2 ± 3.7a,b | <0.001 |
| Postop. 24 hour | 28.8 ± 7.8 | 17.5 ± 4.6a | 8.2 ± 3.9a,b | <0.001 |
| Additional analgesic need (n) | ||||
| 0–2 h | 6 | 5 | 2 | 0.313 |
| 2–6 h | 7 | 3 | 4 | 0.441 |
| 6–12 h | 4 | 1 | 0 | 0.122 |
| 12–24 h | 1 | 0 | 1 | 1.000 |
C, control; B, bupivacaine; BD, bupivacaine + dexmedetomidine.
Significant difference from Group C.
Significant difference from Group B.
There was no significant difference observed among groups in terms of the number of patients having nausea-vomiting scores, antiemetic requirement, or additional analgesic administration (p > 0.05) (Table 2, Table 3).
Table 3.
Patient satisfaction scores, patients with nause and vomitting episodes and patients receiving antiemetics. Data are given as number (%).
| Group C (n = 31) | Group B (n = 31) | Group BD (n = 31) | p | |
|---|---|---|---|---|
| Patient satisfaction scores | ||||
| Very dissatisfied | 0 (0) | 1 (3.2) | 0 (0) | |
| Somewhat satisfied | 12 (39.8) | 4 (12.9)a | 0 (0)a,b | <0.001 |
| Rather satisfied | 19 (61.3) | 18(58.1) | 17 (54.8) | |
| Completely satisfied | 0 (0) | 8 (25.8)a | 14 (45.1)a | |
| Nausea and vomiting | ||||
| None | 17 (54.8) | 22 (71.0) | 21 (65.6) | |
| Nausea | 7 (22.6) | 3 (9.7) | 6 (21.9) | 0.129 |
| Retching | 6 (19.4) | 5 (16.1) | 4 (12.5) | |
| Vomiting | 1 (0.7) | 1 (0.7) | 0 (0) | |
| Patients receiving antiemetics (n) | ||||
| 0–2 h | 7 (22.6) | 5 (16.1) | 5 (16.1) | 0.313 |
| 2–6 h | 5 (16.1) | 4 (12.9) | 4 (12.9) | 0.428 |
| 6–12 h | 3 (9.7) | 2 (6.5) | 1 (3.2) | 0.224 |
| 12–24 h | 0 (0) | 1 (3.2) | 0 (0) | 0.667 |
C, control; B, bupivacaine; BD, bupivacaine + dexmedetomidine.
Significant difference from Group C.
Significant difference from Group B.
There was a significant increase in terms of patient satisfaction scoring in Group B and BD compared to Group C, and also in Group BD when compared to both Group B and C (p < 0.001) (Table 3).
Discussion
It has been stated that direct blockade of abdominal area blocks by ilioinguinal and iliohypogastric nerve blocks with abdominal wall neural afferent nerves, TAP block administration following abdominal surgery, such as inguinal herniorrhaphy and hysterectomy, provide appropriate postoperative pain control.17, 18, 19 In our study, TAP block administration applied after anesthesia induction reduced VAS score when compared to the control group and at the same time reduced postoperative morphine consumption.
In order to provide effective postoperative analgesia with TAP block, in general it should be administered soon after induction of the block and soon before surgical incision.19, 20 Bharti et al.21 administered TAP block at the end of the operation in a study they carried out, and reported that it did not prolong time to first analgesic request in comparison with the control group; however, they reported reduced total morphine consumption in the postoperative 2nd hour and thereafter. Niraj et al.2 administered unilateral TAP block with 20 mL 0.5% bupivacaine in open appendectomy. In the control group, the mean 24 h morphine consumption was 50 mg, compared to 28 mg in the group in which TAP block was administered. Cho et al.3 reported that TAP block administration with 20 mL 0.5% bupivacaine in open appendectomies reduces intra- operative fentanyl consumption when compared to the control group, and reduces, although not yet statistically significantly, the time to first analgesic administration, and does not change the count of rescue analgesic requirement. Furthermore, they reported that TAP block with 20 mL 0.5% levobupivacaine provided 12 h postoperative analgesia; its effect did not continue up to 24 h postoperatively, and although the time to first analgesic administration was longer in the TAP block group, it was not statistically different when compared to the control group (100.2 min against 40.9 min). However, this result may be due to the limited number of patients.
Erdoğan Arı et al.10 administered TAP block with 20 mL 0.125% bupivacaine in open inguinal herniorrhaphy and administered 0.25% bupivacaine at the end of surgery, and found the same postoperative morphine consumption.
However, they did not compare its effect with 0.5% bupivacaine. Salman et al.19 administered TAP block with 20 mL 0.125% bupivacaine at the end of surgery to patients with inguinal hernia given to spinal anesthesia and found lower postoperative morphine consumption than in the control group. In our study, we administered TAP block with 20 mL 0.5% bupivacaine, and 24 h total morphine consumption was observed to be 60.7% lower in the TAP block group than in the control group.
TAP block duration is limited to the action time of administered local anesthesia. In several studies it has been stated that addition of dexmedetomidine to local anesthesia administered to central neuroaxial and peripheral block prolonged the local anesthetic action time and reduced anesthetic request.8, 21, 22 Agarwal et al.21 indicated in their study that analgesia time was prolonged up to 8 h when they added 100 μg dexmedetomidine to bupivacaine in a supraclavicular block. Almarakbi et al.8 stated that in a study in which they added dexmedetomidine to bupivacaine in TAP block in abdominal hysterectomy, the first time to analgesic administration was significantly longer than in the group that dexmedetomidine (470 min and 280 min, respectively) and total 24 h morphine consumption was significantly lower in this group (19 mg and 29 mg, respectively). In our study, we administered TAP block with 20 mL 0.5% bupivacaine and 100 μg dexmedetomidine + bupivacaine in open appendectomy and open inguinal hernia surgery, and the 24 h morphine consumption in the control group, bupivacaine group and bupivacaine + dexmedetomidine group was 28.8 mg, 17.5 mg, and 8.2 mg, respectively. Morphine consumption in the 2–24 h period in both groups that TAP administered was significantly lower than in the control group, however, lower morphine consumption was observed in the bupivacaine + dexmedetomidine group in all 24 h measuring periods, including the first 2 h, when compared to both control and bupivacaine groups. In this regard, dexmedetomidine is considered to initiate block time earlier and to prolong action time, thereby reducing analgesic consumption. In general, VAS measurement is utilized in postoperative pain studies.
Salman et al.19 found that postoperative 24 h VAS scores in open inguinal hernia operations were lower in the TAP block with 20 mL 0.25% bupivacaine group than in the control group. Cho et al.3 assessed patients administered with TAP block with 20 mL 0.5% levobupivacaine in open appendectomy with verbal numerical rating scale for pain at rest (VNRSr) and on coughing (VNRSc), and stated that VNRSr was 12 h and VNRSc was postoperative 3 h lower than the control group. Niraj et al.2 showed that in open appendectomy, VAS assessment at rest and on coughing in TAP block administration with 20 mL 0.5% bupivacaine was lower in the 30th min and 24 h postoperatively when compared with the control group.
In our VASi scoring studies, there was a significant decrease in Group BD in 0–24 h when compared to the control group and in 10–24 h when compared to Group B. In the BD group, there was a decrease in 2–8 h in comparison with the control group. While in TAP block, only bupivacaine administration provided the decrease in VASi scores for 8 h, addition of dexmedetomidine prolonged this effect up to 24 h. The VASi score was lower in the postoperative 0 min in the group which had dexmedetomidine added, and it was considered that dexmedetomidine induces sensorial block onset earlier, and increases block efficiency.
Akın et al.22 administered epidural analgesia with bupivacaine, and bupivacaine + dexmedetomidine, to patients undergoing abdominal surgery. Patient satisfaction scores in the group to which dexmedetomidine was administered were significantly higher than in the control group. Kaur et al.23 found no statistically significant difference in patient satisfaction scores in a study in which they added levobupivacaine dexmedetomidine in supraclavicular brachial plexus block. In our study, there was a significant increase in satisfaction scores in Group B when compared to the control group and in Group BD when compared to both the control group and the bupivacaine group. In all groups, rathet satisfied rate in patient satisfaction scores are similar, but in group B and group BD somewhat satisfied rate was lower and completely satisfied rate was higher. So, in group BD patient satisfaction rate was higher.
In this study, a significant decrease was observed in HR and MAP values recorded after block, in comparison with pre-block values in Group B and BD; however the effect was longer in Group BD.
The decrease observed in hemodynamic data was long-lasting, and it was considered that it might be its role in blocking response to stress following relief of postoperative pain with dexmedetomidine effect. Patients did not require treatment with vasoactive drugs.
Only two patients in Group BD had intra-operative atropine request due to bradycardia.
When nausea-vomiting scores were assessed in this study, there was no statistically significant difference among groups. Administration of bupivacaine and dexmedetomidine for TAP block did not elevate nausea-vomiting scores. Almarakbi et al.8 indicated that 50 of sample size included in the study and first degree nausea was observed in 3 samples from the group in which dexmedetomidine was added to bupivacaine in TAP block, and 11 samples from the group given bupivacaine, and there was no statistically significant difference among samples in terms of nausea-vomiting and antiemetic treatment.
As a result, addition of bupivacaine and bupivacaine dexmedetomidine to TAP block following lower abdomen surgery leads to a decrease in HR and MAP normal clinical levels of the cases. Addition of bupivacaine dexmedetomidine reduced morphine requests and VAS scores in the 24 h postoperative period. Even though addition of only bupivacaine reduced 24 h morphine consumption in comparison with the control group, it reduced VAS scores effectively for hours.
It has been found that postoperative patient satisfaction scores in the group in which bupivacaine dexmedetomidine was added was higher than in the other two groups.
Funding
This research was supported by Erciyes University BAP Unit.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgment
This work was supported by Erciyes University BAP Funding.
References
- 1.Petersen P.L., Mathiesen O., Stjernholm P., et al. The effect of transversus abdominis plane block or local anaesthetic infiltration in inguinal hernia repair: a randomised clinical trial. Eur J Anaesthesiol. 2013;30:415–421. doi: 10.1097/EJA.0b013e32835fc86f. [DOI] [PubMed] [Google Scholar]
- 2.Niraj G., Searle A., Mathews M., et al. Analgesic efficacy of ultrasound-guided transversus abdominis plane block in patients undergoing open appendicectomy. Br J Anaesth. 2009;103:601–605. doi: 10.1093/bja/aep175. [DOI] [PubMed] [Google Scholar]
- 3.Cho S., Kim Y.J., Kim D.Y., et al. Postoperative analgesic effects of ultrasound-guided transversus abdominis plane block for open appendectomy. J Korean Surg Soc. 2013;85:128–133. doi: 10.4174/jkss.2013.85.3.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alfieri S., Amid P.K., Campanelli G., et al. International guidelines for prevention and management of post-operative chronic pain following inguinal hernia surgery. Hernia. 2011;15:239–249. doi: 10.1007/s10029-011-0798-9. [DOI] [PubMed] [Google Scholar]
- 5.Ganai S., Lee K.F., Merrill A., et al. Adverse outcomes of geriatric patients undergoing abdominal surgery who are at high risk for delirium. Arch Surg. 2007;142:1072–1078. doi: 10.1001/archsurg.142.11.1072. [DOI] [PubMed] [Google Scholar]
- 6.Bay-Nielsen M., Perkins F.M., Kehlet H. Danish Hernia Database. Pain and functional impairment 1 year after inguinal herniorrhaphy: a nationwide questionnaire study. Ann Surg. 2001;233:1–7. doi: 10.1097/00000658-200101000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elahi F., Reddy C., Ho D. Ultrasound guided peripheral nerve stimulation implant for management of intractable pain after inguinal herniorrhaphy. Pain Physician. 2015;18:E31–E38. [PubMed] [Google Scholar]
- 8.Almarakbi W.A., Kaki A.M. Addition of dexmedetomidine to bupivacaine in transversus abdominis plane block potentiates post-operative pain relief among abdominal hysterectomy patients: a prospective randomized controlled trial. Saudi J Anaesth. 2014;8:161–166. doi: 10.4103/1658-354X.130683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niraj G., Kelkar A., Hart E., et al. Comparison of analgesic efficacy of four-quadrant transversus abdominis plane (TAP) block and continuous posterior TAP analgesia with epidural analgesia in patients undergoing laparoscopic colorectal surgery: an open-label, randomised, non-inferiority trial. Anaesthesia. 2014;69:348–355. doi: 10.1111/anae.12546. [DOI] [PubMed] [Google Scholar]
- 10.Erdoğan Arı D., Yıldırım Ar A., Karadoğan F., et al. Ultrasound-guided transversus abdominis plane block in patients undergoing open inguinal hernia repair: 0.125% bupivacaine provides similar analgesic effect compared to 0.25% bupivacaine. J Clin Anesth. 2016;28:41–46. doi: 10.1016/j.jclinane.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Milone M., Di Minno M.N., Musella M., et al. Outpatient inguinal hernia repair under local anaesthesia: feasibility and efficacy of ultrasound-guided transversus abdominis plane block. Hernia. 2013;17:749–755. doi: 10.1007/s10029-012-1022-2. [DOI] [PubMed] [Google Scholar]
- 12.Heil J.W., Ilfeld B.M., Loland V.J., et al. Ultrasound-guided transversus abdominis plane catheters and ambulatory perineural infusions for outpatient inguinal hernia repair. Reg Anesth Pain Med. 2010;35:556–558. doi: 10.1097/AAP.0b013e3181fa69e9. [DOI] [PubMed] [Google Scholar]
- 13.Ammar A.S., Mahmoud K.M. Effect of adding dexamethasone to bupivacaine on transversus abdominis plane block for abdominal hysterectomy: a prospective randomized controlled trial. Saudi J Anaesth. 2012;6:229–233. doi: 10.4103/1658-354X.101213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coursin D.B., Coursin D.B., Maccioli G.A. Dexmedetomidine. Curr Opin Crit Care. 2001;7:221–226. doi: 10.1097/00075198-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Brummett C.M., Norat M.A., Palmisano J.M., et al. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology. 2008;109:502–511. doi: 10.1097/ALN.0b013e318182c26b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Comez M., Celik M., Dostbil A., et al. The effect of pre-emptive intravenous dexketoprofen + thoracal epidural analgesia on the chronic post-thoracotomy pain. Int J Clin Exp Med. 2015;8:8101–8107. [PMC free article] [PubMed] [Google Scholar]
- 17.Khedkar S.M., Bhalerao P.M., Yemul-Golhar S.R., et al. Ultrasound-guided ilioinguinal and iliohypogastric nerve block, a comparison with the conventional technique: an observational study. Saudi J Anaesth. 2015;9:293–297. doi: 10.4103/1658-354X.154730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carney J., McDonnell J.G., Ochana A., et al. The transversus abdominis plane block provides effective postoperative analgesia in patients undergoing total abdominal hysterectomy. Anesth Analg. 2008;107:2056–2060. doi: 10.1213/ane.0b013e3181871313. [DOI] [PubMed] [Google Scholar]
- 19.Salman A.E., Yetişir F., Yürekli B., et al. The efficacy of the semi-blind approach of transversus abdominis plane block on postoperative analgesia in patients undergoing inguinal hernia repair: a prospective randomized double-blind study. Local Reg Anesth. 2013;6:1–7. doi: 10.2147/LRA.S38359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonnell J.G., O’Donnell B., Curley G., et al. The analgesic efficacy of transversus abdominis plane block after abdominal surgery: a prospective randomized controlled trial. Anesth Analg. 2007;104:193–197. doi: 10.1213/01.ane.0000250223.49963.0f. [DOI] [PubMed] [Google Scholar]
- 21.Bharti N., Kumar P., Bala I., et al. The efficacy of a novel approach to transversus abdominis plane block for postoperative analgesia after colorectal surgery. Anesth Analg. 2011;112:1504–1508. doi: 10.1213/ANE.0b013e3182159bf8. [DOI] [PubMed] [Google Scholar]
- 22.Akin S., Aribogan A., Arslan G. Dexmedetomidine as an adjunct to epidural analgesia after abdominal surgery in elderly intensive care patients: a prospective, double-blind, clinical trial. Curr Ther Res. 2008;69:16–28. doi: 10.1016/j.curtheres.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaur H., Singh G., Rani S., et al. Effect of dexmedetomidine as an adjuvant to levobupivacaine in supraclavicular brachial plexus block: a randomized double-blind prospective study. J Anaesth Clin Pharmacol. 2015;31:333–338. doi: 10.4103/0970-9185.161668. [DOI] [PMC free article] [PubMed] [Google Scholar]