Abstract
The mechanistic target of the rapamycin (mTOR) signaling pathway is the central regulator of cell growth and proliferation by integrating growth factor and nutrient availability. Under healthy physiological conditions, this process is tightly coordinated and essential to maintain whole-body homeostasis. Not surprisingly, dysregulated mTOR signaling underpins several diseases with increasing incidence worldwide, including obesity, diabetes, and cancer. Consequently, there is significant clinical interest in developing therapeutic strategies that effectively target this pathway. The transition of mTOR inhibitors from the bench to bedside, however, has largely been marked with challenges and shortcomings, such as the development of therapy resistance and adverse side effects in patients. In this review, we discuss the current status of first-, second-, and third-generation mTOR inhibitors as a cancer therapy in both preclinical and clinical settings, with a particular emphasis on the mechanisms of drug resistance. We focus especially on the emerging role of diet as an important environmental determinant of therapy response, and posit a conceptual framework that links nutrient availability and whole-body metabolic states such as obesity with many of the previously defined processes that drive resistance to mTOR-targeted therapies. Given the role of mTOR as a central integrator of cell metabolism and function, we propose that modulating nutrient inputs through dietary interventions may influence the signaling dynamics of this pathway and compensatory nodes. In doing so, new opportunities for exploiting diet/drug synergies are highlighted that may unlock the therapeutic potential of mTOR inhibitors as a cancer treatment.
Keywords: diet, drug resistance, mTOR, metabolism
Background
A hallmark of tumorigenesis is the remodeling of cellular metabolism to coordinate growth and proliferation with nutrient demand and availability. This is largely achieved by the mechanistic target of rapamycin (mTOR) signaling pathway, which integrates several physiological cues, including levels of growth factors and nutrients (1, 2). mTOR is a serine/threonine protein kinase and the predominant catalytic subunit of the protein complexes mTORC1 and mTORC2. Together, these complexes regulate anabolic metabolism including protein and de novo lipid synthesis, as well as transcriptional and post-translational processes that fuel tumor growth (2). Large-scale genomic and proteomic screens have highlighted the pervasiveness of dysregulated mTOR signaling across human cancers, with one-third of solid tumors and approximately 50% of breast cancers displaying hyperactivation of this pathway (3). Moreover, mTORC1 and mTORC2 have essential roles in various pathologies that are becoming increasingly linked epidemiologically with cancer, including obesity and insulin resistance/type 2 diabetes (T2D) (4-7). Thus, mTOR remains a therapeutic target of significant clinical interest.
The arsenal of mTOR-specific inhibitors has grown significantly over the last 50 years and can broadly be categorized into 3 generations (8-10). Rapamycin and its rapalog derivates such as everolimus and temsirolimus constitute the first generation, and function by binding to FK506-binding protein 12 (FKBP-12) which subsequently interacts with the FKBP-rapamycin-binding (FRB) domain of mTOR to limit substrate access at the catalytic cleft (10, 11). Rapalogs are highly specific allosteric inhibitors of mTORC1, though in some models chronic administration can also inhibit mTORC2 by sequestering mTOR from Rictor (11). In order to improve the robustness of targeting all kinase-dependent functions of mTORC1/2, second-generation adenosine triphosphate (ATP)-competitive inhibitors have been developed including PI-103 and NVP-BEZ-235 (12). Due to the high sequence similarity of the mTOR catalytic domain with phosphatidylinositol 3-kinases (PI3Ks) and PI3K-related kinases (PIKKs), these compounds are prototypical dual PI3K/mTOR inhibitors. Although inhibiting both PI3K and mTOR signaling simultaneously has shown promising antineoplastic properties in preclinical xenograft studies (9, 13-15), concerns arise from toxicity due to the relatively nonspecific inhibition of different PI3K isoforms (16, 17). For this reason, a series of compounds that are up to 1000-fold more specific to mTOR than PI3Ks were developed in the late 2000s (18). Among the best characterized and most commonly used in the laboratory are KU0063794, AZD2014, MLN0128, Torin-1, and Torin-2, which have more than 800-fold selectivity for mTOR over PI3K, though activity toward other PIKKs such as ataxia telangiectasia and Rad3-related protein (ATR), ataxia telangiectasia mutated (ATM), and DNA-dependent protein kinase (DNA-PK) have been reported (19-23).
An exciting advancement in the designment of mTOR-targeted therapies are third-generation compounds such as “RapaLink-1” which directly crosslink rapamycin with ATP-competitive inhibitors to form a bivalent agent (24). This approach leverages the binding specificity of rapamycin with the potency of second-generation inhibitors, and has already shown remarkable potential to overcome drug resistance arising from mutations in either the FRB domain or kinase domain of mTOR in breast cancer models (24). The promising effects of RapaLink-1 have more recently been extended to other tumor types including prostate and glioblastoma where it potently suppresses key metabolic processes such as glutamine and lipid metabolism, while also decreasing primary tumor and metastatic burden (25, 26).
All generations of mTOR inhibitors are at various stages of clinical development (27). Nevertheless, drug resistance remains the biggest obstacle to realizing the potential of these agents as anticancer therapies. Several mechanisms of resistance have been described previously, including incomplete inhibition of mTOR substrates, reactivation of compensatory oncogenic signaling pathways, and mutations arising in either mTOR itself or its interacting partners such as FKBP-12 (8, 11, 28-30). However, these resistance mechanisms have been largely explored in isolation, rather than in the context of the larger nutrient and whole-body metabolic networks that mTOR is at the center of. Specifically, there is renewed interest in investigating the interaction between diet and therapy response, particularly in light of recent evidence suggesting that dietary modifications can remodel the nutrient milieu of the tumor microenvironment, thereby exploiting metabolic vulnerabilities of cancer cells while synergizing with existing therapies (31-38). This review will address the implications of this framework on overcoming drug resistance, and highlight emerging opportunities to leverage these multifaceted interactions at the physiological level to improve the efficacy of mTOR inhibitors.
mTOR Signaling in Healthy Physiology and Disease
In order to understand how diet influences the response to mTOR inhibitors, it is important to consider the role of mTORC1 and mTORC2 in healthy physiological states, and their rewiring in cancer and other epidemiologically linked pathologies like obesity. The intricacies of how upstream signals control mTOR pathway activation have been extensively reviewed previously (1, 2, 39-41). Briefly, 2 main axes are required: (1) endocrine signals encompassing growth factors such as insulin and androgens including testosterone which inhibit the tuberous sclerosis complex (TSC) leading to activation of Rheb, a positive regulator of mTOR (39, 42, 43); and (2) nutrients such as amino acids which facilitate mTOR localization and complete activation at the lysosome through both Rag/Ragulator-dependent and -independent mechanisms (44-48) (Fig. 1). The regulation of lysosomal abundance through Rap1 is also a key regulator of mTORC1 activation in response to amino acid availability (44). Recent studies have further expanded the list of metabolites that can activate mTOR. These include dihydroxyacetone phosphate, which is sensed as a proxy for glucose availability independently of canonical AMP-activated protein kinase (AMPK) signaling (49), lactate which maintains active GTP-bound Rheb by inhibiting its interaction with TSC in mutant KRAS cells (50), and phospholipids such as phosphatidic acid that promote mTOR localization to the lysosome even in the absence of amino acids and growth factors (51).
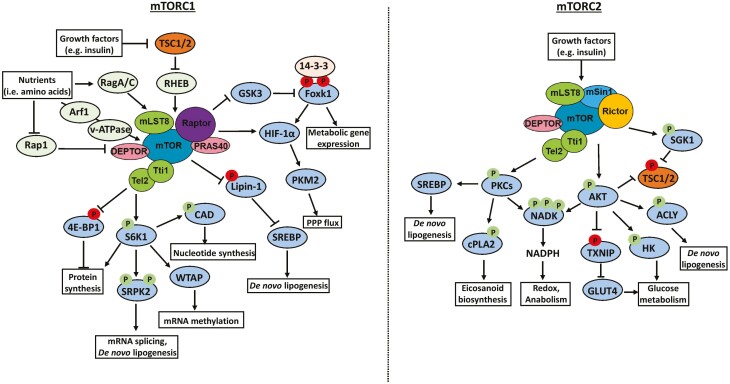
Figure 1.
Regulation of key cellular processes by mTORC1 and mTORC2. The mTOR signaling pathway comprises mTORC1 and mTORC2, which together promote anabolism, growth, and proliferation. mTORC1 is activated by various signals including growth factors and nutrient availability in the form of amino acids and glucose. The downstream processes regulated by mTORC1 include induction of protein synthesis, mRNA processing, and metabolic rewiring toward de novo nucleotide synthesis, lipogenesis, and oxidative PPP. mTORC2 is only activated by growth factors such as insulin, and its best-characterized substrate is AKT, which is a key activator of glucose and lipid metabolism. More recently, essential roles for other kinases such as PKCs and SGKs downstream of mTORC2 have been defined—such as increased bioactive eicosanoid and arachidonic acid metabolism—which provide compensatory signals that drive cell proliferation. Activating and inhibitory phosphorylation events are denoted by an arrowhead or blunt ended lines, respectively. Abbreviations: TSC1/2, tuberous sclerosis complex 1/2; Rag, Ras-related GTPase; RHEB, Ras homolog enriched in brain; Rap1, Ras-related protein 1; HIF, hypoxia inducible factor; PKM2, pyruvate kinase isozyme M2; PPP, pentose phosphate pathway; SREBP, sterol-regulatory element binding protein; CAD, carbamoyl phosphate synthetase 2; WTAP, Wilms’ tumor 1 associated protein; SRPK2, SR protein kinase 2; ACLY, ATP-citrate lyase; HK, hexokinase; SGK1, serum and glucocorticoid-induced protein kinase-1; PKC, protein kinase C; cPLA2, cytosolic phospholipase A2; NADK, NAD kinase.
Signaling Downstream of mTORC1 and mTORC2 Drives Metabolic Remodeling and Tumorigenesis
The consequences of dysregulated mTORC1 activation range from positive regulation of mRNA processing and protein synthesis to fundamentally shifting cell metabolism toward the anabolism required for tumor growth (2, 52, 53) (Fig. 1). The phosphorylation and consequent inhibition of eukaryotic translation initiation factor 4E-binding protein (4E-BP)—a key negative regulator of mRNA translation—by mTORC1 coupled with the activation of p70 S6 kinases (S6Ks), support protein translation and elongation (53-55). Moreover, S6K activation downstream of mTORC1 regulates mRNA stability through N6-methyladenosine modifications that promote degradation and reduced expression of tumor suppressor proteins such as MAX dimerization protein 2 (MXD2) (56). Finally, mTORC1 can regulate gene expression through spliceosome formation in an S6K1-SRPK2 dependent fashion that promotes the splicing, mRNA stability and translation of key lipogenic genes including FASN, ACLY, and ACSS2 (57).
Rapidly growing and proliferating tumor cells require a constant source of metabolic substrates to fuel anabolic metabolism. mTORC1 facilitates this by eliciting global metabolic rewiring at the transcriptional level by suppressing GSK3-mediated phosphorylation of Foxk1, thereby inducing normoxic hypoxia inducible factor (HIF)-1α expression and genes involved in glycolysis, pentose phosphate pathway (PPP), and 1-carbon metabolism (58). The mTORC1 dependent shift toward aerobic glycolysis also generates NADPH, which is essential for sustaining anabolic processes like de novo nucleotide and lipid synthesis (52, 59-63). There are also several direct mechanisms through which mTORC1 induces lipogenesis including inhibiting lipin-1, a negative regulator of SREBP1 (64), and promoting mRNA splicing and stability of lipogenic genes through SRPK2, as described earlier (57). An additional branch of cancer cell metabolism that is directly regulated by mTORC1 is polyamine synthesis, and this predominantly occurs in prostate tumors driven by loss of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) (65). Mechanistically, mTORC1 increases the post-translational stability of s-adenosyl-L-methionine decarboxylase and contributes to the synthesis of diverse polyamine species that are essential for tumor growth and proliferation (65).
mTORC2 is arguably less well characterized than mTORC1; however, its role as an important complementary and compensatory driver of metabolic remodeling and tumorigenesis is becoming increasingly appreciated (Fig. 1). The best-characterized effector is protein kinase B (AKT), which is directly phosphorylated by mTORC2 on Ser473 and promotes glucose uptake and glycolysis (66). Indeed, inhibition of mTORC2 in mouse livers and the concomitant reduction in AKT activity attenuates de novo lipogenesis and promotes hyperglycemia (67). Moreover, mTORC2 remodels sphingolipid, glycerophospholipid and cardiolipin metabolism in hepatocellular carcinoma independently of mTORC1 (68). Perhaps more importantly is the activation of several AKT-independent compensatory signaling axes downstream of mTORC2 which drive resistance to PI3K/mTOR targeted therapies (69). In this context, mTORC2 and PDK1 phosphorylate and activate serum/glucocorticoid regulated kinase 1 (SGK1), leading to inhibition of TSC2 (69, 70) even when PI3K/AKT signaling is suppressed pharmacologically. members of the protein kinase C (PKC) family of serine/threonine kinases are also important mediators of mTORC2 signaling. Specifically, mTORC2 directly activates PKCα, PKCβII, PKCε, and PKCζ, thus providing an important compensatory node that regulates metabolic phenotypes independently of canonical AKT-mTORC1 signaling (71-75). For instance, arachidonic acid (AA) and the bioactive eicosanoid metabolism is enhanced by a multimodal signaling hub downstream of oncogenic PIK3CA that is specifically centered around the mTORC2-PKCζ–dependent activation of the calcium dependent phospholipase A2 (31) (Fig. 1). PKC and AKT activation downstream of mTORC2 have also been shown to activate nicotinamide adenine dinucleotide kinase, leading to NADPH synthesis and enhanced anabolic metabolism and redox homeostasis (76, 77)
mTOR Signaling Dynamics Are Shaped by Whole-body Metabolic States
Type 2 diabetes, insulin resistance, and mTOR
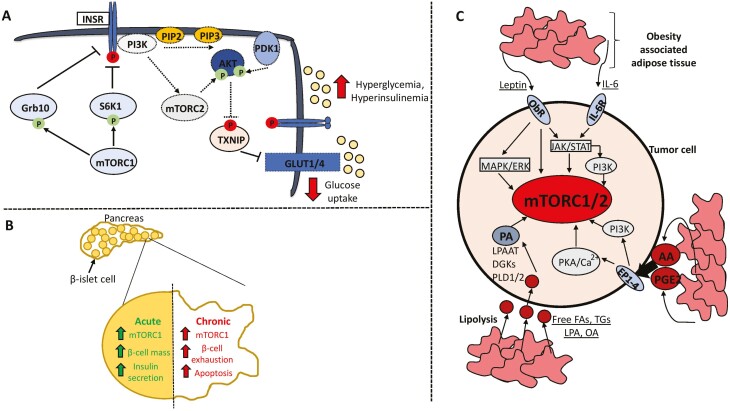
On a broader physiological scale, mTOR signaling regulates glucose homeostasis and adipogenesis, thereby also implicating it in metabolic pathologies such as obesity and diabetes. The incidence of T2D has increased significantly over the last 30 years, with more than 450 million individuals being affected worldwide in 2017 (78). More worryingly is the accumulating epidemiological evidence linking T2D with increased risk of developing certain cancers such as liver, pancreatic, colon, endometrium, and breast (79). Possible mechanisms include hyperinsulinemia, hyperglycemia, and inflammation (79), which are closely associated with the finely tuned balanced between mTORC1 and mTORC2 signaling. Overactivation of mTORC1 in the liver, skeletal muscle, pancreas, and adipose tissue is thought to contribute to insulin resistance through 2 main mechanisms. Firstly, phosphorylation of the insulin receptor substrate 1/2 by S6K1 on several serine residues including 270, 636/639, and 1101 leads to its inhibition and possible degradation, thereby perpetuating a negative feedback loop that attenuates mTORC2-AKT signaling—the predominant node downstream of PI3K that regulates glucose uptake and metabolism (80-84) (Fig. 2A). Indeed, infusing healthy patients with an amino acid mixture to hyperactivate mTORC1 reduces glucose deposition in skeletal muscle and exacerbates hyperinsulinemia (83). An additional regulatory point is the phosphorylation and stabilization of growth factor receptor–bound protein 10 by mTORC1, which inhibits signaling through the insulin receptor and consequent activation of PI3K–mTORC2–AKT (85-87) (Fig. 2A). Independently of its effects on insulin receptor substrate 1/2, mTORC1 also directly impacts pancreatic β-cell functionality in a biphasic manner (2) (Fig. 2B). Constitutive mTORC1 signaling induced by β-cell–specific knockout of TSC1 or TSC2 allows for an initial compensatory response to excessive growth factor and nutrient availability by increasing β-cell size, mass, and insulin production (88, 89). Importantly, these beneficial effects are diminished in older animals due to eventual β-cell exhaustion that closely mimics T2D observed in humans (89), thus highlighting the consequences of perturbing mTOR signaling dynamics outside of physiological thresholds.
Figure 2.
mTOR signaling in the context of type 2 diabetes and obesity. Hyperactivation of mTOR in response to chronic nutrient excess contributes to type 2 diabetes through 2 main pathways. (A) Sustained mTORC1 activity induces the negative-feedback loops converging on activation of the insulin receptor and downstream induction of the mTORC2–AKT axis that promotes glucose uptake. This contributes to hyperglycemia and hyperinsulinemia. (B) Chronic mTORC1 activation leads to exhaustion and apoptosis of pancreatic β-islet cells, culminating in reduced insulin secretion. (C) Obesity-associated adipose tissue profoundly alters the tumor microenvironment and can stimulate mTOR activity through secretion of adipokines such as leptin and IL-6, proinflammatory metabolites including arachidonic acid and prostaglandin E2, and release of fatty acid substrates through lipolysis that can be used to synthesize phosphatidic acid. Activating and inhibitory phosphorylation events are denoted by an arrowhead or blunt ended lines, respectively. Dotted lines or outlines surrounding enzymes denote downregulation/inhibition. Abbreviations: T2D, type 2 diabetes; INSR, insulin receptor; Grb10, growth factor receptor bound protein 10; PIP2, phosphatidylinositol (4,5)-bisphosphate; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; PDK1, phosphoinositide-dependent kinase 1; TXNIP, thioredoxin interacting protein; ObR, leptin receptor; IL-6, interleukin-6; PKA, protein kinase A; AA, arachidonic acid; PGE2, prostaglandin E2; FA, fatty acid; TG, triglycerides; LPA, lysophosphatidic acid; OA, oleic acid; PA, phosphatidic acid; PLD1/2; phospholipase D1/2; DGKs, diacylglycerol kinase; LPAAT, lysophosphatidic acid acyltransferase.
Obesity-associated Endocrine Signals Remodel mTOR Pathway Activity
Obesity rates, much like T2D, have increased dramatically since the 1970s with an estimated ~42% of the adult population in the United States being obese, and almost 18% of adolescents between 5 and 18 years old globally (5, 90). This is concerning given the multifactorial interactions between obesity and T2D-induced physiological changes that may influence the incidence of certain tumor types such as gastrointestinal and breast cancers. It is now widely accepted that altered lipid metabolism is a bone fide metabolic hallmark of cancer cells, with mTOR functioning as a key driver of this phenotype and general adipogenesis (91). Adipocytes present in obese and nonobese individuals are functionally distinct, with the former characterized by hypoxia, fibrosis and inflammation arising from the accumulation of lipid droplets (5, 92-94). Consequently, obesity-associated adipose tissue is a potent endocrine system that mediates metabolic cross-talk between adipocytes and tumor cells, and activates pro-oncogenic signaling pathways including mTOR (95-98) (Fig. 2C). Increased fat mass is also associated with the release of bioactive adipokines such as leptin, which normally regulates satiety but is aberrantly secreted in obese individuals (99). Importantly, serum levels of leptin are positively correlated with breast cancer risk, grade, and worse prognosis, and this is attributable at least in part to the activation of JAK-STAT, PI3K-mTOR, and MAPK signaling downstream of the leptin receptor (ObR) (100-103). A specific functional association between leptin levels and mTOR activity was first described in the hypothalamus, where leptin specifically induced mTORC1 and phosphorylation of p70S6K and S6 ribosomal protein (104, 105). Ultimately, this axis is essential for the mTORC1-dependent sensing of nutrient sufficiency and can be perturbed with rapamycin leading to increased food intake in already satiated rats (104). The relevance of leptin-induced mTOR signaling has also been demonstrated in colorectal and breast cancers, with mTORC1 being a critical mediator of lipid droplet accumulation, COX-2 expression, and cell proliferation (106-108).
In conjunction with leptin secretion, adipose tissue in obese patients mirrors a state of chronic inflammation characterized by a secretome comprising interleukin (IL)-6, IL-8, and tumor necrosis factor alpha, which potently activate mTOR signaling (109) (Fig. 2C). Prostaglandins, an important class of bioactive lipids produced by adipocytes, also shift the tumor microenvironment toward a chronic inflammatory, immunosuppressive, and proproliferative state through autocrine and paracrine mechanisms (109, 110). These effects are largely mediated by G-protein–coupled prostanoid receptors such as EP1-4 and DP1, and PGF, PGI, and TX receptors, which link elevated prostaglandin metabolism with enhanced PI3K-mTOR signaling (111-116) (Fig. 2C).
Obesity-associated Adipose Tissue Alters the Metabolic Milieu of the Tumor Microenvironment
Adipocytes fundamentally alter the nutrient composition of the tumor microenvironment by actively secreting lipids in the form of free fatty acids (FAs) such as oleate, triglycerides, and diglycerides following chronic lipolysis of stored lipid droplets (95, 117). In this setting, cancer cells shift their metabolism toward exogenous FA uptake and β-oxidation to produce ATP and reducing power in the form NADPH to sustain anabolic metabolism (118-122). Indeed, coculture systems have highlighted the role of adipocytes in promoting both proliferation and metastasis by effectively providing cancer cells with a consistent source of free FAs (95). These models provide at least 1 explanation for why tumors having high dependencies on lipid metabolism, such as breast, prostate, and ovarian cancers, preferentially metastasize to the visceral omentum in patients (123).
At the signaling level, mTOR is activated by phosphatidic acid (PA), which can be produced in cancer cells using the lipid substrates secreted by adipocytes (124-127) (Fig. 2C). For example, oleic acid and lysophosphatidic acid are metabolized by lysophosphatidic acid acyltransferase-β and acyl-CoA synthetase long chain 5 to PA (128), while di- and triglycerides feed into PA synthesis through the activity of diacylglycerol kinases (129). Additionally, a major contributor to the cellular PA pool is phospholipase D1 and D2 (PLD1/2) which are both overexpressed and hyperactive in breast, gastric and colorectal cancers (130-133). In the context of mTOR, supplementing breast and pancreatic cancer cells with PA containing at least 1 monounsaturated FA tail activates mTORC1 and mTORC2 in a dose- and time-dependent manner (128). Mechanistic insights for this association include the direct binding of PA to the FRB domain of mTOR and stabilizing the interaction with its functional partners Raptor or Rictor, thereby facilitating substrate recognition (124, 134). More recently, PA has been shown to directly promote the lysosomal localization of mTOR independently of amino acids, growth factors, and the Ragulator complex, though these inputs are still necessary for complete activation (51). This implies that PA is an obligate priming event for driving mTORC1 signaling. Adding to the intricacies of this regulatory network is the observation that PLD2 contains a TOR signaling motifs (TOS)-like motif and physically associates with mTOR and Raptor (135), suggesting that the localized synthesis of PA is an important modulator of mTOR activity. Intriguingly, elevated PLD expression and activity is correlated with rapamycin resistance at least in vitro, and this could be attributable to PA outcompeting rapamycin for binding to the FRB domain (136, 137). Surprisingly, these findings have largely been overlooked in studies using rapalogs as a cancer therapy, thereby highlighting exciting opportunities to investigate the role of other lipid classes, such as dietary essential FAs, as potential nutrient inputs into mTOR.
Giving mTOR inhibitors a Boost: Harnessing Diet to Overcome Resistance
A Conceptual Framework for How Diet Could Influence mTOR Therapy Resistance
Based on the evidence described thus far, different metabolic states can perpetuate a “snowball effect” that reshapes the mTOR signaling landscape in cancer cells and the wider microenvironment. With this in mind, what evidence is there to support that modifying dietary habits can alter sensitivity to mTOR inhibitors in tumors? Within the last 5 years, several studies have begun to delineate the biological mechanisms by which consumption of proteins, carbohydrates and fats impact tumor growth and therapy response (138). These include the ketogenic diet enhancing the efficacy of PI3K inhibitors by suppressing insulin feedback (38), and fat-limiting diets synergizing with anti-inflammatory inhibitors to block arachidonic acid and eicosanoid metabolism in PIK3CA mutant breast cancers (31). Moreover, high-fat diet–induced obesity can rewire the metabolism of tumor cells toward FA uptake and oxidation, thereby limiting nutrient resources for infiltrating cytotoxic CD8+ T-cells (139). In terms of dietary protein intake, restricting specific amino acids, such as histidine (37), methionine (36), and serine/glycine (140), has been shown to improve both the efficacy and tolerability of existing chemotherapeutic regimes, while limiting asparagine availability alone is sufficient to inhibit metastatic potential (33). Finally, caloric restriction whereby 30% to 40% of calorie intake is reduced by limiting carbohydrate consumption, and plant-based fasting-mimicking diets that have low-protein/carbohydrate content, can profoundly alter both nutrient availability in the tumor microenvironment (TME) and systemic endocrine signals (32, 35). In the case of fasting-mimicking diets, this amounts to lower circulating leptin, insulin, and insulin-like growth factor 1, which inhibit PI3K–AKT–mTOR signaling in estrogen receptor–positive breast cancers without causing feedback hyperinsulinemia, thereby potentiating hormone-based therapies such as tamoxifen and fulvestrant (35).
Dietary interventions could also conceivably alter the access and utilization of nutrients that directly modulate mTOR activity in cancer cells. The most direct evidence supporting this notion comes from protein restriction and intermittent fasting. Indeed, reducing dietary protein content from a standard ~21% of calorie intake to ~7% reduced estrogen receptor–positive breast xenograft growth in vivo and inhibited the 40S ribosomal protein S6, but not AKT, phosphorylation (141). Notably, intermittent fasting with a 21% calorie from protein diet could attenuate both mTORC1 and mTORC2 signaling in tumor xenografts, highlighting nuanced differences between nutrient content and meal timing (141). The inhibitory effects of protein restriction on mTOR signaling can also be recapitulated by only removing dietary branched-chain amino acid (BCAAs) (142). In studies of hepatocellular carcinoma, downregulation of BCAA catabolic enzymes such as branched chain keto acid dehydrogenase E1 and acyl-CoA dehydrogenases (ACADS and ACADSB) chronically activate mTORC1 through accumulation of BCAAs, and indeed loss of BCAA catabolic capacity is associated with tumor progression and worse overall survival (143). This is also consistent in BRCA1 haplo-insufficient breast cancers displaying elevated levels of BCAAs compared with wild-type cells and mTORC1 hyperactivation (144, 145). Although this is not necessarily surprising given the well-defined mechanisms for BCAA sensing by mTOR (46, 146, 147), these findings nevertheless raise the interesting prospect of investigating the extent to which protein restriction could simultaneously modulate both the rapalog-sensitive and -resistant signaling nodes downstream of mTORC1. In terms of other dietary nutrients, limiting carbohydrate intake by adopting a ketogenic diet can inhibit mTOR by lowering circulating insulin levels and associated feedback signaling described earlier in this review (Figs. 2 and 3A) (38). The metabolic changes induced by the ketogenic diet also converge on activation of AMPK—an established negative regulator of mTOR signaling (148).
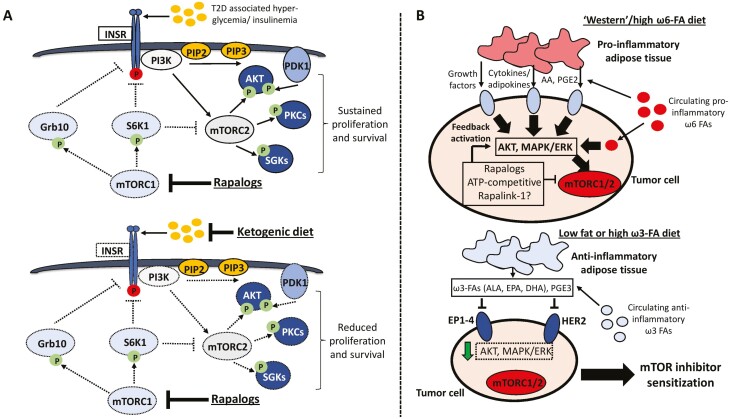
Figure 3.
Proposed model of how dietary factors could modulate resistance to mTOR inhibitors. (A) One of the most pervasive mechanisms of resistance to rapalogs is the hyperactivation of mTORC2 signaling and downstream AKT, PKC and SGK signaling following mTORC1-S6K inhibition. Furthermore, in the context of hyperglycemia and hyperinsulinemia observed in patients with type-2 diabetes and even following chronic rapalog treatment, feedback activation of the insulin receptor may occur which overrides the therapeutic benefit of these inhibitors. In this scenario, a very low carbohydrate diet such as the ketogenic diet could lower the metabolic feedback signals that hyperactivate resistance pathways downstream of the insulin receptor. (B) Schematic of how altering dietary fat content in the form of pro-inflammatory ω-6 or anti-inflammatory ω-3 FA ratios could impact the physiology of adipose tissue that shapes autocrine/paracrine signaling networks in the tumor microenvironment. In this context, “Western” diets containing an excess of ω-6 FAs such as linoleic and arachidonic acid may cooperate with the feedback hyperactivation of AKT and MAPK/ERK signaling observed following first- and second-generation mTOR inhibitor treatment to drive therapy resistance. Conversely, a diet rich in ω-3 FAs such as linolenic acid, EPA, and DHA may suppress the reactivation of these oncogenic pathways. Dotted lines or outlines surrounding enzymes denote downregulation/inhibition. Abbreviations: T2D, type 2 diabetes; INSR, insulin receptor; Grb10, growth factor receptor bound protein 10; PIP2, phosphatidylinositol (4,5)-bisphosphate; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; PDK1, phosphoinositide-dependent kinase 1; SGK1, serum and glucocorticoid-induced protein kinase 1; PKC, protein kinase C; AA, arachidonic acid; PGE2, prostaglandin E2; ALA, alpha-linolenic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; PGE3, prostaglandin E3; EP1-4, Prostaglandin E2 receptor 1-4; HER2, receptor tyrosine-protein kinase erbB-2.
There are currently more than 200 clinical trials investigating the role of mTOR inhibitors as either a monotherapy in cancer or in combination with existing targeted and chemotherapeutic agents including taxol, cisplatin, tamoxifen, and aromatase inhibitors (9, 149, 150). Notably, there is a paucity of studies exploring potential synergy between mTOR-targeted therapies and dietary interventions, highlighting heretofore unexplored opportunities to evaluate nutrition as a key determinant of clinical responsiveness. These concepts are explored in more detail below.
Attenuating Insulin-dependent Mechanisms of Resistance Through Low Carbohydrate Diets
Perhaps one of the most relevant molecular events driving adaptive resistance to both first and second-generation mTOR inhibitors is the rewiring of oncogenic signaling networks and reactivation of compensatory nodes that override the antitumorigenic effects of these compounds (11). In the case of rapamycin and rapalogs, inhibiting mTORC1-S6K alleviates the negative regulation of insulin receptor signaling thereby hyperactivating mTORC2 and AKT (9, 11, 151). In terms of targeting this node through dietary avenues, the ketogenic diet has the strongest evidence for effectively lowering blood glucose and circulating insulin levels that ultimately suppress the feedback activation of PI3K-AKT signaling (38). Indeed, recent studies have provided preclinical evidence that the ketogenic diet synergizes with rapamycin to inhibit breast cancer growth and metastasis to the lung (152). In light of several observations demonstrating that chronic rapamycin treatment worsens hyperglycemia and insulin resistance (153), the therapeutic benefit of combining rapalogs with carbohydrate-restricted diets could be explained at least in part by attenuating the reactivation of insulin receptor signaling (Fig. 3A). This idea could also be extended to melanoma models that develop resistance to catalytic mTORC1/2 inhibitors such as Torin1 through mTOR-independent reactivation of AKT (154). In this context, the PI3K-dependent induction of integrin α2/focal adhesion kinase signaling converges on integrin-linked kinase activation downstream of insulin-like growth factor receptor (IGFR) and the insulin receptor, which phosphorylates AKT and drives xenograft growth even with Torin1 treatment (154). Since dual administration of catalytic mTORC1/2 and IGFR inhibitors is sufficient to overcome resistance (154), it would be interesting to investigate if similar synergistic effects can also be achieved with low carbohydrate diets.
Modulating Compensatory Autocrine and Paracrine Signals by Altering Dietary Fat Intake
The consequences of abrogating the negative feedback loops following mTORC1 inhibition are not just limited to PI3K-AKT, but also extend to other prosurvival pathways such as ERK-MAPK signaling (151). Strikingly, reactivation of the MAPK cascade is observed in breast and prostate cancer biopsy specimens following treatment with everolimus at weekly high doses (50-70 mg), and to a lesser extent in patients administered daily low (10 mg) doses (155). Mechanistically, the activation of ERK downstream of mTORC1 inhibition requires an S6K1-PI3K feedback loop that is independent of mTORC2 and AKT (155). Similar observations have also been corroborated in pancreatic cancer; however, in this context ERK signaling could be reactivated by second-generation ATP-competitive inhibitors such as KU63794, but not rapamycin, in a PI3K-independent fashion (156). Together, these findings provide a strong rationale for combining mTOR and MAPK inhibitors to overcome feedback activation of ERK, however concerns arise relating to long-term tolerability in patients and timing of dosing regimens (157, 158). Alternatively, could dietary interventions be exploited to perturb the compensatory induction of ERK signaling following treatment with first and second-generation mTOR inhibitors? Epidemiological case–control studies in thousands of colon and rectal cancer patients suggest that dietary intake of polyunsaturated fatty acids are associated with particular single nucleotide polymorphisms in MAPK1 (ERK2) and MAPK3 (ERK1) that increased disease risk (159). While the mechanisms behind these interactions remain to be defined, this finding is particularly intriguing because it suggests that dietary factors may act as a selection pressure that influences tumor-intrinsic ERK signaling dynamics. Furthermore, treating gastric carcinoma cell lines with the essential ω-6 FA linoleic acid promotes invasiveness phenotypes in vitro, and metastatic dissemination to the omentum and liver in mice fed a very high linoleic acid diet with 12% of total fat content derived from ω-6 FAs (160). These findings were attributable to the COX-1–dependent metabolism of linoleic acid to arachidonic acid and prostaglandins that activated ERK1/2 through autocrine and paracrine activation of G protein coupled receptors (GPCRs) (160).
Essential polyunsaturated fatty acids such as ω-6 (linoleic acid) and ω-3 (linolenic acid) FAs, cannot be synthesized endogenously in humans and must be obtained from the diet. Physiologically, ω-6 FAs are predominantly associated with the synthesis of prostaglandins that mediate the inflammatory response, while ω-3 FAs have anti-inflammatory properties (161, 162). Thus, these nutrients are implicated in a wide range of disorders including obesity and autoimmune diseases (163, 164), and may also contribute to the pathogenesis of certain tumor types such as breast and colorectal cancer (165-170). Metabolic derivatives of ω-6 FAs including AA and prostaglandins can stimulate cell proliferation while also fostering an immunosuppressive microenvironment (31, 91, 110, 171). Conversely, in addition to being anti-inflammatory by directly competing with AA for COX-2 binding, ω-3 FAs have been shown to be anti-estrogenic and inhibit signaling through HER2 (172, 173). From a nutritional standpoint, the recommended dietary ratio of ω-6:3 is 1:1; however, ratios exceeding 20:1 are common in Western diets (174). Since adipose tissue is an active mediator of autocrine/paracrine signaling through lipolysis and secretion of free FAs described earlier, it is conceivable that modifying dietary fat intake could reshape the availability of FA nutrients that stimulate ERK signaling. In doing so, combining first- and second-generation mTOR inhibitors with diets containing either a balanced ratio of ω-6:ω-3 FAs, or an excess of the latter, could provide a strategy for alleviating the feedback activation of compensatory ERK and proinflammatory signaling (Figs. 2C and 3B).
Third-generation mTOR inhibitors such as Rapalink-1 have shown great promise in targeting mutations in the FRB or kinase domains of mTOR that otherwise confer resistance to rapalogs and ATP competitive inhibitors, respectively (24). Moreover, Rapalink-1 displays greater potency than rapalogs as demonstrated by inhibiting the phosphorylation of rapamycin-insensitive substrates such as 4E-BP1 (24, 175). Nevertheless, preclinical studies in glioblastoma suggest that despite a period of significant tumor shrinkage following Rapalink-1 monotherapy regrowth invariably occurs, albeit at a slower rate than rapalog treatment (26). The basis for recurrence in this context could be driven by the same signaling feedback loops that impact the efficacy of first and second-generation mTOR inhibitors. These should be carefully evaluated in preclinical and future long-term clinical trials using Rapalink-1 as a cancer therapy, along with identifying combinations—including dietary interventions—that block the molecular events driving recurrence.
Feasibility of Modifying Dietary Fat Content in Patients
Recent studies have provided fundamental preclinical evidence demonstrating the role of dietary fat intake as a modulator of cancer risk, disease progression, and therapy response (31, 176-178). However, several important considerations must be accounted for when extrapolating preclinical dietary studies to patients. Firstly, the composition of systemic adipose tissue varies significantly between obese and nonobese patients, with the former characterized by enrichment in proinflammatory ω-6 FAs, leptin, and IL-6 (5, 179). Given that sustained lipolysis could provide a chronic source of FAs that activate mTOR and compensatory signaling nodes, an important question emerges: How long do patients need to be on a balanced or ω-3-rich diet to reverse this proinflammatory adipose composition, thereby shifting systemic metabolism to favor synergy with mTOR inhibitors? Secondly, essential dietary ω-6 and ω-3 FAs serve fundamental roles in normal human physiology, therefore altering the intake of these nutrients needs to be carefully optimized on a patient-by-patient basis. Recent human trials have begun to shed light on these questions and suggest that administering eicosapentaenoic acid and docosahexaenoic acid daily to patients with advanced inoperable non-small cell lung cancer (NSCLC) is well tolerated and significantly ameliorates systemic inflammatory and oxidative stress during chemotherapy (180). Although there is limited evidence that ω-3 FA supplementation could be an effective monotherapy for breast cancer, there are exciting findings suggesting that these nutrients may synergize with existing therapies and limit toxicity. Most notably, a treatment regime combining docosahexaenoic acid and conventional anthracycline-based chemotherapies almost doubled survival in patients with aggressive metastatic breast cancer (181). There is also an ongoing clinical trial to evaluate the combination of ω-3 FAs and the aromatase inhibitor letrozole to reduce estrogen levels in obese patients diagnosed with breast cancer (NCT02538484).
Practical Considerations and Future Perspectives
Developing resistance to small molecule inhibitors remains a significant clinical obstacle to the successful treatment of cancer. The recent boom in research efforts aimed at characterizing the role of diet as a modifier of drug responses highlights exciting opportunities to explore nutritional interventions as an alternative synergistic avenue to improve the efficacy of existing clinically approved therapies (138). These lessons could also be applied to mTOR inhibitors, especially given the role of mTOR as an essential integrator of whole-body metabolism that dynamically interacts with nutrient availability. Indeed, the ketogenic, protein or fat-limiting, and fast-mimicking diets directly modulate mTOR activity and the compensatory oncogenic signaling nodes that contribute to drug resistance (35, 38, 141). To further interrogate the effects of these dietary regimens at the cellular level, future work should focus on comparing how they impact the rapamycin-insensitive substrates downstream of mTORC1 such as 4E-BP1, as well as mTORC2-dependent signaling axes mediated by SGK and PKC that drive tumor recurrence.
Combining mTOR inhibitors with dietary interventions could also provide opportunities to modify dosing regimens such that lower doses are administered intermittently rather than chronically, which may extend the therapeutic window for long-term treatment (182, 183). Evidence supporting this notion has focused on combining low doses of drugs that target common nodes of an oncogenic signaling pathway, most notably in EGFR inhibitor resistant NSCLC (184). As these treatment strategies gain more traction in the clinic, an exciting opportunity emerges to assess whether nutritional interventions can potentiate their effects on abrogating the development of drug resistance.
Despite the increasingly important role diet appears to play in shaping cancer outcomes and therapy response, this evidence is largely preclinical. Consequently, several unanswered questions remain including the extent to which dietary factors alone are either preventative or protumorigenic, the appropriate timing of introducing specific dietary modifications during treatment, and the long-term tolerability of altering nutrient intake in cancer patients. An added layer of complexity also emerges when considering how the nutrient requirements of a primary tumor change with disease progression and may not necessarily be preserved in secondary macrometastases (185-187). Nevertheless, there is reason for optimism despite this complexity: recent studies have provided valuable proof-of-concept that dietary interventions are physiologically actionable and do in fact impact the availability of specific nutrients to tumors in vivo (34, 138). The most exciting prospect for this area of research is the emphasis on molecular, genetic, and nutritional stratification, which could fundamentally change the way cancer is treated by matching patients with tailored diet/drug combinations that are more efficacious and tolerable.
Acknowledgments
We thank the Blenis lab members Drs. Michal Nagiec, Bethany Schaffer, Long He, and Sungyun Cho for helpful discussions and comments on this manuscript.
Glossary
Abbreviations
- AA
arachidonic acid
- AKT
protein kinase B
- AMPK
AMP-activated protein kinase
- ATP
adenosine triphosphate
- ATR
ataxia telangiectasia and Rad3-related protein
- ATM
ataxia telangiectasia mutated
- BCAA
branched-chain amino acid
- DNA-PK
DNA-dependent protein kinase
- ER
estrogen receptor
- FA
fatty acid
- FRB
FKBP-rapamycin-binding
- FKBP
FK506-binding protein
- GPCR
G protein coupled receptor
- HIF
hypoxia inducible factor
- IGF
insulin like growth factor
- IGFR
insulin-like growth factor receptor
- IL
interleukin
- mTOR
mechanistic target of the rapamycin
- PA
phosphatidic acid
- PLD1/2
phospholipase D1/2
- PI3K
phosphatidylinositol 3-kinase
- PIKK
PI3K-related kinase
- PKC
protein kinase C
- PLD
phospholipase
- PPP
pentose phosphate pathway
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
- S6K
S6 kinase
- SGK
serum/glucocorticoid regulated kinase
- T2D
type 2 diabetes
- TOS
TOR signaling motifs
- TSC
tuberous sclerosis complex
- TME
tumor microenvironment
- 4E-BP
eukaryotic translation initiation factor 4E-binding protein
Funding Support
The work in the Blenis lab is supported by National Institutes of Health Research Project Grants R01 CA046595 and R01 GM051405.
Disclosure Summary
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Kim SG, Buel GR, Blenis J. Nutrient regulation of the mTOR complex 1 signaling pathway. Mol Cells. 2013;35(6):463-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saxton RA, Sabatini DM. mTOR signaling in growth. Metab Dis Cell. 2017;169(2):361-371. [DOI] [PubMed] [Google Scholar]
- 3. Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, et al. . An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68(15):6084-6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brännmark C, Nyman E, Fagerholm S, et al. . Insulin signaling in type 2 diabetes: experimental and modeling analyses reveal mechanisms of insulin resistance in human adipocytes. J Biol Chem. 2013;288(14):9867-9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown KA. Metabolic pathways in obesity-related breast cancer. Nat Rev Endocrinol. 2021;17(6):350-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146(3):1473-1481. [DOI] [PubMed] [Google Scholar]
- 7. Malley CO, Pidgeon GP. The mTOR pathway in obesity driven gastrointestinal cancers: potential targets and clinical trials. BBA Clin. 2016;5:29-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Formisano L, Napolitano F, Rosa R, et al. . Mechanisms of resistance to mTOR inhibitors. Crit Rev Oncol Hematol. 2020;147:102886. [DOI] [PubMed] [Google Scholar]
- 9. Hua H, Kong Q, Zhang H, Wang J, Luo T, Jiang Y. Targeting mTOR for cancer therapy. J Hematol Oncol. 2019;12(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zheng Y, Jiang Y. mTOR Inhibitors at a Glance. Mol Cell Pharmacol. 2015;7(2):15-20. [PMC free article] [PubMed] [Google Scholar]
- 11. Li J, Kim SG, Blenis J. Rapamycin: one drug, many effects. Cell Metab. 2014;19(3):373-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schenone S, Brullo C, Musumeci F, Radi M, Botta M. ATP-competitive inhibitors of mTOR: an update. Curr Med Chem. 2011;18(20):2995-3014. [DOI] [PubMed] [Google Scholar]
- 13. Chen D, Lin X, Zhang C, et al. . Dual PI3K/mTOR inhibitor BEZ235 as a promising therapeutic strategy against paclitaxel-resistant gastric cancer via targeting PI3K/Akt/mTOR pathway. Cell Death Dis. 2018;9(2):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu Z, Xie G, Zhou G, et al. . NVP-BEZ235, a novel dual PI3K-mTOR inhibitor displays anti-glioma activity and reduces chemoresistance to temozolomide in human glioma cells. Cancer Lett. 2015;367(1):58-68. [DOI] [PubMed] [Google Scholar]
- 15. Maira SM, Stauffer F, Brueggen J, et al. . Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7(7):1851-1863. [DOI] [PubMed] [Google Scholar]
- 16. Tarantelli C, Lupia A, Stathis A, Bertoni F. Is there a role for dual PI3K/mTOR inhibitors for patients affected with lymphoma? Int J Mol Sci . 2020;21(3):1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hillmann P, Fabbro D. PI3K/mTOR pathway inhibition: opportunities in oncology and rare genetic diseases. Int J Mol Sci . 2019;20(22):5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu Q, Kang SA, Thoreen CC, et al. . Development of ATP-competitive mTOR inhibitors. Methods Mol Biol. 2012;821:447-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Francipane MG, Lagasse E. Selective targeting of human colon cancer stem-like cells by the mTOR inhibitor Torin-1. Oncotarget. 2013;4(11):1948-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Slotkin EK, Patwardhan PP, Vasudeva SD, et al. . 0128, an ATP-competitive mTOR kinase inhibitor with potent in vitro and in vivo antitumor activity, as potential therapy for bone and soft-tissue sarcoma. Mol Cancer Ther. 2015;14(2):395-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gökmen-Polar Y, Liu Y, Toroni RA, et al. . Investigational drug MLN0128, a novel TORC1/2 inhibitor, demonstrates potent oral antitumor activity in human breast cancer xenograft models. Breast Cancer Res Treat. 2012;136(3):673-682. [DOI] [PubMed] [Google Scholar]
- 22. Liu Q, Wang J, Kang SA, et al. . Discovery of 9-(6-aminopyridin-3-yl)-1-(3-(trifluoromethyl)phenyl)benzo[h][1,6]naphthyridin-2(1H)-one (Torin2) as a potent, selective, and orally available mammalian target of rapamycin (mTOR) inhibitor for treatment of cancer. J Med Chem. 2011;54(5):1473-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu Q, Xu C, Kirubakaran S, et al. . Characterization of Torin2, an ATP-competitive inhibitor of mTOR, ATM, and ATR. Cancer Res. 2013;73(8):2574-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rodrik-Outmezguine VS, Okaniwa M, Yao Z, et al. . Overcoming mTOR resistance mutations with a new-generation mTOR inhibitor. Nature. 2016;534(7606):272-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. La Manna F, De Menna M, Patel N, et al. . Dual-mTOR inhibitor rapalink-1 reduces prostate cancer patient-derived xenograft growth and alters tumor heterogeneity. Front Oncol. 2020;10:1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fan Q, Aksoy O, Wong RA, et al. . A kinase inhibitor targeted to mTORC1 drives regression in glioblastoma. Cancer Cell. 2017;31(3):424-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zou Z, Tao T, Li H, Zhu X. mTOR signaling pathway and mTOR inhibitors in cancer: progress and challenges. Cell Biosci. 2020;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grabiner BC, Nardi V, Birsoy K, et al. . A diverse array of cancer-associated MTOR mutations are hyperactivating and can predict rapamycin sensitivity. Cancer Discov. 2014;4(5):554-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lorenz MC, Heitman J. TOR mutations confer rapamycin resistance by preventing interaction with FKBP12-rapamycin. J Biol Chem. 1995;270(46):27531-27537. [DOI] [PubMed] [Google Scholar]
- 30. Kurmasheva RT, Huang S, Houghton PJ. Predicted mechanisms of resistance to mTOR inhibitors. Br J Cancer. 2006;95(8):955-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koundouros N, Karali E, Tripp A, et al. . Metabolic fingerprinting links oncogenic PIK3CA with enhanced arachidonic acid-derived eicosanoids. Cell. 2020;181(7):1596-1611.e1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lien EC, Westermark AM, Zhang Y, et al. . Low glycaemic diets alter lipid metabolism to influence tumour growth. Nature. 2021;599(7884):302-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Knott SRV, Wagenblast E, Khan S, et al. . Asparagine bioavailability governs metastasis in a model of breast cancer. Nature. 2018;554(7692):378-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vernieri C, Fuca G, Ligorio F, et al. . Fasting-mimicking diet is safe and reshapes metabolism and antitumor immunity in cancer patients. Cancer Discov. 2022;12(1):90-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Caffa I, Spagnolo V, Vernieri C, et al. . Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature. 2020;583(7817):620-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gao X, Sanderson SM, Dai Z, et al. . Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature. 2019;572(7769):397-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kanarek N, Keys HR, Cantor JR, et al. . Histidine catabolism is a major determinant of methotrexate sensitivity. Nature. 2018;559(7715):632-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hopkins BD, Pauli C, Du X, et al. . Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature. 2018;560(7719):499-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412(2):179-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Melick CH, Jewell JL. Regulation of mTORC1 by upstream stimuli. Genes (Basel). 2020;11(9):989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang K, Fingar DC. Growing knowledge of the mTOR signaling network. Semin Cell Dev Biol. 2014;36:79-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Menon S, Dibble CC, Talbott G, et al. . Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell. 2014;156(4):771-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. White JP, Gao S, Puppa MJ, Sato S, Welle SL, Carson JA. Testosterone regulation of Akt/mTORC1/FoxO3a signaling in skeletal muscle. Mol Cell Endocrinol. 2013;365(2):174-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mutvei AP, Nagiec MJ, Hamann JC, Kim SG, Vincent CT, Blenis J. Rap1-GTPases control mTORC1 activity by coordinating lysosome organization with amino acid availability. Nat Commun. 2020;11(1):1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141(2):290-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Saxton RA, Knockenhauer KE, Wolfson RL, et al. . Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Science. 2016;351(6268):53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jewell JL, Kim YC, Russell RC, et al. . Metabolism. Differential regulation of mTORC1 by leucine and glutamine. Science. 2015;347(6218):194-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Meng D, Yang Q, Wang H, et al. . Glutamine and asparagine activate mTORC1 independently of Rag GTPases. J Biol Chem. 2020;295(10):2890-2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Orozco JM, Krawczyk PA, Scaria SM, et al. . Dihydroxyacetone phosphate signals glucose availability to mTORC1. Nat Metab. 2020;2(9):893-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Byun JK, Park M, Yun JW, et al. . Oncogenic KRAS signaling activates mTORC1 through COUP-TFII-mediated lactate production. EMBO Rep. 2019;20(6):e47451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Frias MA, Mukhopadhyay S, Lehman E, et al. . Phosphatidic acid drives mTORC1 lysosomal translocation in the absence of amino acids. J Biol Chem. 2020;295(1):263-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ben-Sahra I, Manning BD. mTORC1 signaling and the metabolic control of cell growth. Curr Opin Cell Biol. 2017;45:72-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10(5):307-318. [DOI] [PubMed] [Google Scholar]
- 54. Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA. 1998;95(4):1432-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science. 2006;314(5798):467-471. [DOI] [PubMed] [Google Scholar]
- 56. Cho S, Lee G, Pickering BF, et al. . mTORC1 promotes cell growth via m. Mol Cell. 2021;81(10):2064-2075.e2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee G, Zheng Y, Cho S, et al. . Post-transcriptional regulation of de novo lipogenesis by mTORC1-S6K1-SRPK2 signaling. Cell. 2017;171(7):1545-1558.e1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. He L, Gomes AP, Wang X, et al. . mTORC1 promotes metabolic reprogramming by the suppression of GSK3-dependent Foxk1 phosphorylation. Mol Cell. 2018;70(5):949-960.e944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ben-Sahra I, Hoxhaj G, Ricoult SJH, Asara JM, Manning BD. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science. 2016;351(6274):728-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ben-Sahra I, Howell JJ, Asara JM, Manning BD. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science. 2013;339(6125):1323-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Robitaille AM, Christen S, Shimobayashi M, et al. . Quantitative phosphoproteomics reveal mTORC1 activates de novo pyrimidine synthesis. Science. 2013;339(6125):1320-1323. [DOI] [PubMed] [Google Scholar]
- 62. Düvel K, Yecies JL, Menon S, et al. . Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39(2):171-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sun Q, Chen X, Ma J, et al. . Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc Natl Acad Sci USA. 2011;108(10):4129-4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Peterson TR, Sengupta SS, Harris TE, et al. . mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146(3):408-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zabala-Letona A, Arruabarrena-Aristorena A, Martín-Martín N, et al. . mTORC1-dependent AMD1 regulation sustains polyamine metabolism in prostate cancer. Nature. 2017;547(7661):109-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122(Pt 20):3589-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hagiwara A, Cornu M, Cybulski N, et al. . Hepatic mTORC2 activates glycolysis and lipogenesis through Akt, glucokinase, and SREBP1c. Cell Metab. 2012;15(5):725-738. [DOI] [PubMed] [Google Scholar]
- 68. Guri Y, Colombi M, Dazert E, et al. . mTORC2 promotes tumorigenesis via lipid synthesis. Cancer Cell. 2017;32(6):807-823.e812. [DOI] [PubMed] [Google Scholar]
- 69. Castel P, Ellis H, Bago R, et al. . PDK1-SGK1 signaling sustains AKT-independent mTORC1 activation and confers resistance to PI3Kα inhibition. Cancer Cell. 2016;30(2):229-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. García-Martínez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem J. 2008;416(3):375-385. [DOI] [PubMed] [Google Scholar]
- 71. Garg R, Benedetti LG, Abera MB, Wang H, Abba M, Kazanietz MG. Protein kinase C and cancer: what we know and what we do not. Oncogene. 2014;33(45):5225-5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cozzoli DK, Courson J, Rostock C, et al. . Protein kinase C epsilon activity in the nucleus accumbens and central nucleus of the amygdala mediates binge alcohol consumption. Biol Psychiatry. 2016;79(6):443-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Das F, Ghosh-Choudhury N, Mariappan MM, Kasinath BS, Choudhury GG. Hydrophobic motif site-phosphorylated protein kinase CβII between mTORC2 and Akt regulates high glucose-induced mesangial cell hypertrophy. Am J Physiol Cell Physiol. 2016;310(7):C583-C596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 2008;27(14):1919-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Li X, Gao T. mTORC2 phosphorylates protein kinase Cζ to regulate its stability and activity. EMBO Rep. 2014;15(2):191-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hoxhaj G, Ben-Sahra I, Lockwood SE, et al. . Direct stimulation of NADP. Science. 2019;363(6431):1088-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schild T, McReynolds MR, Shea C, et al. . NADK is activated by oncogenic signaling to sustain pancreatic ductal adenocarcinoma. Cell Rep. 2021;35(11):109238. [DOI] [PubMed] [Google Scholar]
- 78. Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes - global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10(1):107-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Giovannucci E, Harlan DM, Archer MC, et al. . Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhang J, Gao Z, Yin J, Quon MJ, Ye J. S6K directly phosphorylates IRS-1 on Ser-270 to promote insulin resistance in response to TNF-(alpha) signaling through IKK2. J Biol Chem. 2008;283(51):35375-35382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Um SH, D’Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006;3(6):393-402. [DOI] [PubMed] [Google Scholar]
- 82. Tremblay F, Brûlé S, Hee Um S, et al. . Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci USA. 2007;104(35):14056-14061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tremblay F, Krebs M, Dombrowski L, et al. . Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes. 2005;54(9):2674-2684. [DOI] [PubMed] [Google Scholar]
- 84. Tremblay F, Marette A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J Biol Chem. 2001;276(41):38052-38060. [DOI] [PubMed] [Google Scholar]
- 85. Yu Y, Yoon SO, Poulogiannis G, et al. . Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science. 2011;332(6035):1322-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Edick AM, Auclair O, Burgos SA. Role of Grb10 in mTORC1-dependent regulation of insulin signaling and action in human skeletal muscle cells. Am J Physiol Endocrinol Metab. 2020;318(2):E173-E183. [DOI] [PubMed] [Google Scholar]
- 87. Hsu PP, Kang SA, Rameseder J, et al. . The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 2011;332(6035):1317-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mori H, Inoki K, Opland D, et al. . Critical roles for the TSC-mTOR pathway in β-cell function. Am J Physiol Endocrinol Metab. 2009;297(5):E1013-E1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Shigeyama Y, Kobayashi T, Kido Y, et al. . Biphasic response of pancreatic beta-cell mass to ablation of tuberous sclerosis complex 2 in mice. Mol Cell Biol. 2008;28(9):2971-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 2020(360):1-8. [PubMed] [Google Scholar]
- 91. Koundouros N, Poulogiannis G. Reprogramming of fatty acid metabolism in cancer. Br J Cancer. 2020;122(1):4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Corvera S, Gealekman O. Adipose tissue angiogenesis: impact on obesity and type-2 diabetes. Biochim Biophys Acta. 2014;1842(3):463-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Engin A. The pathogenesis of obesity-associated adipose tissue inflammation. Adv Exp Med Biol. 2017;960:221-245. [DOI] [PubMed] [Google Scholar]
- 94. Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 2013;1831(10):1533-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Nieman KM, Kenny HA, Penicka CV, et al. . Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17(11):1498-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Olzmann JA, Carvalho P. Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol. 2019;20(3):137-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wu Q, Li B, Li Z, Li J, Sun S. Cancer-associated adipocytes: key players in breast cancer progression. J Hematol Oncol. 2019;12(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wang YY, Attané C, Milhas D, et al. . Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight. 2017;2(4):e87489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Friedman JM. Leptin and the endocrine control of energy balance. Nat Metab. 2019;1(8):754-764. [DOI] [PubMed] [Google Scholar]
- 100. Gui Y, Pan Q, Chen X, Xu S, Luo X, Chen L. The association between obesity related adipokines and risk of breast cancer: a meta-analysis. Oncotarget. 2017;8(43):75389-75399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Frankenberry KA, Skinner H, Somasundar P, McFadden DW, Vona-Davis LC. Leptin receptor expression and cell signaling in breast cancer. Int J Oncol. 2006;28(4):985-993. [PubMed] [Google Scholar]
- 102. Garofalo C, Koda M, Cascio S, et al. . Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res. 2006;12(5):1447-1453. [DOI] [PubMed] [Google Scholar]
- 103. Khabaz MN, Abdelrahman A, Butt N, et al. . Immunohistochemical staining of leptin is associated with grade, stage, lymph node involvement, recurrence, and hormone receptor phenotypes in breast cancer. BMC Womens Health. 2017;17(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cota D, Proulx K, Smith KA, et al. . Hypothalamic mTOR signaling regulates food intake. Science. 2006;312(5775):927-930. [DOI] [PubMed] [Google Scholar]
- 105. Villanueva EC, Münzberg H, Cota D, et al. . Complex regulation of mammalian target of rapamycin complex 1 in the basomedial hypothalamus by leptin and nutritional status. Endocrinology. 2009;150(10):4541-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Maya-Monteiro CM, Bozza PT. Leptin and mTOR: partners in metabolism and inflammation. Cell Cycle. 2008;7(12):1713-1717. [DOI] [PubMed] [Google Scholar]
- 107. Fazolini NP, Cruz AL, Werneck MB, Viola JP, Maya-Monteiro CM, Bozza PT. Leptin activation of mTOR pathway in intestinal epithelial cell triggers lipid droplet formation, cytokine production and increased cell proliferation. Cell Cycle. 2015;14(16):2667-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wang D, Chen J, Chen H, et al. . Leptin regulates proliferation and apoptosis of colorectal carcinoma through PI3K/Akt/mTOR signalling pathway. J Biosci. 2012;37(1):91-101. [DOI] [PubMed] [Google Scholar]
- 109. Johnson AR, Milner JJ, Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev. 2012;249(1):218-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10(3):181-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Fujino H, West KA, Regan JW. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. J Biol Chem. 2002;277(4):2614-2619. [DOI] [PubMed] [Google Scholar]
- 112. Fujino H, Xu W, Regan JW. Prostaglandin E2 induced functional expression of early growth response factor-1 by EP4, but not EP2, prostanoid receptors via the phosphatidylinositol 3-kinase and extracellular signal-regulated kinases. J Biol Chem. 2003;278(14):12151-12156. [DOI] [PubMed] [Google Scholar]
- 113. Greenhough A, Smartt HJ, Moore AE, et al. . The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30(3):377-386. [DOI] [PubMed] [Google Scholar]
- 114. Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med. 2002;8(3):289-293. [DOI] [PubMed] [Google Scholar]
- 115. Wen ZH, Su YC, Lai PL, et al. . Critical role of arachidonic acid-activated mTOR signaling in breast carcinogenesis and angiogenesis. Oncogene. 2013;32(2):160-170. [DOI] [PubMed] [Google Scholar]
- 116. Chang HH, Young SH, Sinnett-Smith J, et al. . Prostaglandin E2 activates the mTORC1 pathway through an EP4/cAMP/PKA- and EP1/Ca2+-mediated mechanism in the human pancreatic carcinoma cell line PANC-1. Am J Physiol Cell Physiol. 2015;309(10):C639-C649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ladanyi A, Mukherjee A, Kenny HA, et al. . Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene. 2018;37(17):2285-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer. 2013;13(4):227-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pascual G, Avgustinova A, Mejetta S, et al. . Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541(7635):41-45. [DOI] [PubMed] [Google Scholar]
- 120. Samovski D, Sun J, Pietka T, et al. . Regulation of AMPK activation by CD36 links fatty acid uptake to β-oxidation. Diabetes. 2015;64(2):353-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wu Q, Li J, Li Z, et al. . Exosomes from the tumour-adipocyte interplay stimulate beige/brown differentiation and reprogram metabolism in stromal adipocytes to promote tumour progression. J Exp Clin Cancer Res. 2019;38(1):223. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 122. Balaban S, Shearer RF, Lee LS, et al. . Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. 2017;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lengyel E, Makowski L, DiGiovanni J, Kolonin MG. Cancer as a matter of fat: the crosstalk between adipose tissue and tumors. Trends Cancer. 2018;4(5):374-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294(5548):1942-1945. [DOI] [PubMed] [Google Scholar]
- 125. Foster DA. Regulation of mTOR by phosphatidic acid? Cancer Res. 2007;67(1):1-4. [DOI] [PubMed] [Google Scholar]
- 126. Foster DA. Phosphatidic acid signaling to mTOR: signals for the survival of human cancer cells. Biochim Biophys Acta. 2009;1791(9):949-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Foster DA. Phosphatidic acid and lipid-sensing by mTOR. Trends Endocrinol Metab. 2013;24(6):272-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Menon D, Salloum D, Bernfeld E, et al. . Lipid sensing by mTOR complexes via. J Biol Chem. 2017;292(15):6303-6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. You JS, Lincoln HC, Kim CR, et al. . The role of diacylglycerol kinase ζ and phosphatidic acid in the mechanical activation of mammalian target of rapamycin (mTOR) signaling and skeletal muscle hypertrophy. J Biol Chem. 2014;289(3):1551-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hornberger TA, Chu WK, Mak YW, Hsiung JW, Huang SA, Chien S. The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. Proc Natl Acad Sci USA. 2006;103(12):4741-4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Bernfeld E, Menon D, Vaghela V, et al. . Phospholipase D-dependent mTOR complex 1 (mTORC1) activation by glutamine. J Biol Chem. 2018;293(42):16390-16401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Brown HA, Thomas PG, Lindsley CW. Targeting phospholipase D in cancer, infection and neurodegenerative disorders. Nat Rev Drug Discov. 2017;16(5):351-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Foster DA, Salloum D, Menon D, Frias MA. Phospholipase D and the maintenance of phosphatidic acid levels for regulation of mammalian target of rapamycin (mTOR). J Biol Chem. 2014;289(33):22583-22588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Toschi A, Lee E, Xu L, Garcia A, Gadir N, Foster DA. Regulation of mTORC1 and mTORC2 complex assembly by phosphatidic acid: competition with rapamycin. Mol Cell Biol. 2009;29(6):1411-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ha SH, Kim DH, Kim IS, et al. . PLD2 forms a functional complex with mTOR/raptor to transduce mitogenic signals. Cell Signal. 2006;18(12):2283-2291. [DOI] [PubMed] [Google Scholar]
- 136. Chen Y, Zheng Y, Foster DA. Phospholipase D confers rapamycin resistance in human breast cancer cells. Oncogene. 2003;22(25):3937-3942. [DOI] [PubMed] [Google Scholar]
- 137. Yoon MS, Sun Y, Arauz E, Jiang Y, Chen J. Phosphatidic acid activates mammalian target of rapamycin complex 1 (mTORC1) kinase by displacing FK506 binding protein 38 (FKBP38) and exerting an allosteric effect. J Biol Chem. 2011;286(34):29568-29574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lien EC, Vander Heiden MG. A framework for examining how diet impacts tumour metabolism. Nat Rev Cancer. 2019;19(11):651-661. [DOI] [PubMed] [Google Scholar]
- 139. Ringel AE, Drijvers JM, Baker GJ, et al. . Obesity shapes metabolism in the tumor microenvironment to suppress anti-tumor immunity. Cell. 2020;183(7):1848-1866.e1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Maddocks ODK, Athineos D, Cheung EC, et al. . Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature. 2017;544(7650):372-376. [DOI] [PubMed] [Google Scholar]
- 141. Lamming DW, Cummings NE, Rastelli AL, et al. . Restriction of dietary protein decreases mTORC1 in tumors and somatic tissues of a tumor-bearing mouse xenograft model. Oncotarget. 2015;6(31):31233-31240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Green CL, Lamming DW, Fontana L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat Rev Mol Cell Biol. 2022;23(1):56-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ericksen RE, Lim SL, McDonnell E, et al. . Loss of BCAA catabolism during carcinogenesis enhances mTORC1 activity and promotes tumor development and progression. Cell Metab. 2019;29(5):1151-1165.e1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Cuyàs E, Fernández-Arroyo S, Alarcón T, Lupu R, Joven J, Menendez JA. Germline BRCA1 mutation reprograms breast epithelial cell metabolism towards mitochondrial-dependent biosynthesis: evidence for metformin-based “starvation” strategies in BRCA1 carriers. Oncotarget. 2016;7(33):52974-52992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Ananieva EA, Wilkinson AC. Branched-chain amino acid metabolism in cancer. Curr Opin Clin Nutr Metab Care. 2018;21(1):64-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Solon-Biet SM, McMahon AC, Ballard JWO, et al. . The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2020;31(3):654. [DOI] [PubMed] [Google Scholar]
- 147. Wolfson RL, Chantranupong L, Saxton RA, et al. . Sestrin2 is a leucine sensor for the mTORC1 pathway. Science. 2016;351(6268):43-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Miller VJ, LaFountain RA, Barnhart E, et al. . A ketogenic diet combined with exercise alters mitochondrial function in human skeletal muscle while improving metabolic health. Am J Physiol Endocrinol Metab. 2020;319(6):E995-E1007. [DOI] [PubMed] [Google Scholar]
- 149. Leisching GR, Loos B, Botha MH, Engelbrecht AM. The role of mTOR during cisplatin treatment in an in vitro and ex vivo model of cervical cancer. Toxicology. 2015;335:72-78. [DOI] [PubMed] [Google Scholar]
- 150. deGraffenried LA, Friedrichs WE, Russell DH, et al. . Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt activity. Clin Cancer Res. 2004;10(23):8059-8067. [DOI] [PubMed] [Google Scholar]
- 151. Rozengurt E, Soares HP, Sinnet-Smith J. Suppression of feedback loops mediated by PI3K/mTOR induces multiple overactivation of compensatory pathways: an unintended consequence leading to drug resistance. Mol Cancer Ther. 2014;13(11):2477-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Zou Y, Fineberg S, Pearlman A, Feinman RD, Fine EJ. The effect of a ketogenic diet and synergy with rapamycin in a mouse model of breast cancer. PLoS One. 2020;15(12):e0233662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Houde VP, Brûlé S, Festuccia WT, et al. . Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes. 2010;59(6):1338-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Yoon SO, Shin S, Karreth FA, et al. . Focal adhesion- and IGF1R-dependent survival and migratory pathways mediate tumor resistance to mTORC1/2 inhibition. Mol Cell. 2017;67(3):512-527.e514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Carracedo A, Ma L, Teruya-Feldstein J, et al. . Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118(9):3065-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Soares HP, Ni Y, Kisfalvi K, Sinnett-Smith J, Rozengurt E. Different patterns of Akt and ERK feedback activation in response to rapamycin, active-site mTOR inhibitors and metformin in pancreatic cancer cells. PLoS One. 2013;8(2):e57289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Grilley-Olson JE, Bedard PL, Fasolo A, et al. . A phase Ib dose-escalation study of the MEK inhibitor trametinib in combination with the PI3K/mTOR inhibitor GSK2126458 in patients with advanced solid tumors. Invest New Drugs. 2016;34(6):740-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Infante JR, Cohen RB, Kim KB, et al. . A phase I dose-escalation study of Selumetinib in combination with Erlotinib or Temsirolimus in patients with advanced solid tumors. Invest New Drugs. 2017;35(5):576-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Slattery ML, Lundgreen A, Wolff RK. Dietary influence on MAPK-signaling pathways and risk of colon and rectal cancer. Nutr Cancer. 2013;65(5):729-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Matsuoka T, Adair JE, Lih FB, et al. . Elevated dietary linoleic acid increases gastric carcinoma cell invasion and metastasis in mice. Br J Cancer. 2010;103(8):1182-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood). 2008;233(6):674-688. [DOI] [PubMed] [Google Scholar]
- 162. Simopoulos AP. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients. 2016;8(3):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21(6):495-505. [DOI] [PubMed] [Google Scholar]
- 164. Bates MA, Brandenberger C, Langohr II, et al. . Silica-triggered autoimmunity in lupus-prone mice blocked by docosahexaenoic acid consumption. PLoS One. 2016;11(8):e0160622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Hall MN, Chavarro JE, Lee IM, Willett WC, Ma J. A 22-year prospective study of fish, n-3 fatty acid intake, and colorectal cancer risk in men. Cancer Epidemiol Biomarkers Prev. 2008;17(5):1136-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Song M, Zhang X, Meyerhardt JA, et al. . Marine ω-3 polyunsaturated fatty acid intake and survival after colorectal cancer diagnosis. Gut. 2017;66(10):1790-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Klein V, Chajès V, Germain E, et al. . Low alpha-linolenic acid content of adipose breast tissue is associated with an increased risk of breast cancer. Eur J Cancer. 2000;36(3):335-340. [DOI] [PubMed] [Google Scholar]
- 168. Shannon J, King IB, Moshofsky R, et al. . Erythrocyte fatty acids and breast cancer risk: a case-control study in Shanghai, China. Am J Clin Nutr. 2007;85(4):1090-1097. [DOI] [PubMed] [Google Scholar]
- 169. Kuriki K, Hirose K, Wakai K, et al. . Breast cancer risk and erythrocyte compositions of n-3 highly unsaturated fatty acids in Japanese. Int J Cancer. 2007;121(2):377-385. [DOI] [PubMed] [Google Scholar]
- 170. Zheng JS, Hu XJ, Zhao YM, Yang J, Li D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: meta-analysis of data from 21 independent prospective cohort studies. BMJ. 2013;346:f3706. [DOI] [PubMed] [Google Scholar]
- 171. Zelenay S, van der Veen AG, Böttcher JP, et al. . Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell. 2015;162(6):1257-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Nagata C, Iwasa S, Shiraki M, Sahashi Y, Shimizu H. Association of maternal fat and alcohol intake with maternal and umbilical hormone levels and birth weight. Cancer Sci. 2007;98(6):869-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Menéndez JA, Vázquez-Martín A, Ropero S, Colomer R, Lupu R. HER2 (erbB-2)-targeted effects of the omega-3 polyunsaturated fatty acid, alpha-linolenic acid (ALA; 18:3n-3), in breast cancer cells: the “fat features” of the “Mediterranean diet” as an “anti-HER2 cocktail”. Clin Transl Oncol. 2006;8(11):812-820. [DOI] [PubMed] [Google Scholar]
- 174. Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56(8):365-379. [DOI] [PubMed] [Google Scholar]
- 175. Kuroshima K, Yoshino H, Okamura S, et al. . Potential new therapy of Rapalink-1, a new generation mammalian target of rapamycin inhibitor, against sunitinib-resistant renal cell carcinoma. Cancer Sci. 2020;111(5):1607-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Zhu Y, Aupperlee MD, Haslam SZ, Schwartz RC. Pubertally initiated high-fat diet promotes mammary tumorigenesis in obesity-prone FVB mice similarly to obesity-resistant BALB/c mice. Transl Oncol. 2017;10(6):928-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. La Merrill M, Baston DS, Denison MS, Birnbaum LS, Pomp D, Threadgill DW. Mouse breast cancer model-dependent changes in metabolic syndrome-associated phenotypes caused by maternal dioxin exposure and dietary fat. Am J Physiol Endocrinol Metab. 2009;296(1):E203-E210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Khalid S, Hwang D, Babichev Y, et al. . Evidence for a tumor promoting effect of high-fat diet independent of insulin resistance in HER2/Neu mammary carcinogenesis. Breast Cancer Res Treat. 2010;122(3):647-659. [DOI] [PubMed] [Google Scholar]
- 179. Fuster JJ, Ouchi N, Gokce N, Walsh K. Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ Res. 2016;118(11):1786-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Finocchiaro C, Segre O, Fadda M, et al. . Effect of n-3 fatty acids on patients with advanced lung cancer: a double-blind, placebo-controlled study. Br J Nutr. 2012;108(2):327-333. [DOI] [PubMed] [Google Scholar]
- 181. Bougnoux P, Hajjaji N, Ferrasson MN, Giraudeau B, Couet C, Le Floch O. Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: a phase II trial. Br J Cancer. 2009;101(12):1978-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Busaidy NL, Farooki A, Dowlati A, et al. . Management of metabolic effects associated with anticancer agents targeting the PI3K-Akt-mTOR pathway. J Clin Oncol. 2012;30(23):2919-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Pallet N, Legendre C. Adverse events associated with mTOR inhibitors. Expert Opin Drug Saf. 2013;12(2):177-186. [DOI] [PubMed] [Google Scholar]
- 184. Fernandes Neto JM, Nadal E, Bosdriesz E, et al. . Multiple low dose therapy as an effective strategy to treat EGFR inhibitor-resistant NSCLC tumours. Nat Commun. 2020;11(1):3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Schild T, Low V, Blenis J, Gomes AP. Unique metabolic adaptations dictate distal organ-specific metastatic colonization. Cancer Cell. 2018;33(3):347-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Dupuy F, Tabariès S, Andrzejewski S, et al. . PDK1-dependent metabolic reprogramming dictates metastatic potential in breast cancer. Cell Metab. 2015;22(4):577-589. [DOI] [PubMed] [Google Scholar]
- 187. Pollari S, Käkönen SM, Edgren H, et al. . Enhanced serine production by bone metastatic breast cancer cells stimulates osteoclastogenesis. Breast Cancer Res Treat. 2011;125(2):421-430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.