Abstract
Background
Enhanced recovery after surgery (ERAS) protocols consist of a set of perioperative measures aimed at improving patient recovery and decreasing length of stay and postoperative complications. We assess the implementation and outcomes of an ERAS program for colorectal surgery.
Methods
Single center observational study. Data were collected from consecutive patients undergoing open or laparoscopic colorectal surgery during 2 time periods, 3 years before (Pre-ERAS) and 2 years after (Post-ERAS) the implementation of an ERAS protocol. Baseline characteristics of both groups were compared. The primary outcome was the number of patients with 180 days follow-up with moderate or severe complications; secondary outcomes were postoperative length of stay, and specific complications. Data were extracted from patient records.
Results
There were 360 patients in the Pre-ERAS group and 319 patients in the Post-ERAS Group. 214 (59.8%) patients developed at least one complication in the pre ERAS group, versus 163 patients in the Post-ERAS group (51.10%). More patients in the Pre-ERAS group developed moderate or severe complications (31.9% vs. 22.26%, p = 0.009); and severe complications (15.5% vs. 5.3%; p < 0.0001). The median length of stay was 13 (17) days in Pre-ERAS Group and 11 (10) days in the Post-ERAS Group (p = 0.034). No differences were found on mortality rates (4.7% vs. 2.5%; p = 0.154), or readmission (6.39% vs. 4.39%; p = 0.31). Overall ERAS protocol compliance in the Post-ERAS cohort was 88%.
Conclusions
The implementation of ERAS protocol for colorectal surgery was associated with a significantly reduction of postoperative complications and length of stay.
Keywords: Perioperative, Enhanced recovery after surgery, Postoperative complications
Resumo
Justificativa
O protocolo ERAS — do Inglês Enhanced Recovery After Surgery — consiste em um conjunto de medidas perioperatórias destinadas a melhorar a recuperação do paciente e diminuir o tempo de internação e as complicações pós-operatórias. Avaliamos a implementação e os resultados de um protocolo ERAS para cirurgia colorretal.
Métodos
Estudo observacional em centro único. Os dados foram coletados de pacientes consecutivos submetidos à cirurgia colorretal aberta ou laparoscópica durante dois períodos: três anos antes (pré-ERAS) e dois anos após (pós-ERAS) a implementação de um protocolo ERAS. As características basais de ambos os grupos foram comparadas. O desfecho primário foi o número de pacientes com 180 dias de acompanhamento com complicações moderadas ou graves. Os desfechos secundários foram tempo de internação pós-cirurgia e complicações específicas. Os dados foram extraídos de prontuários dos pacientes.
Resultados
O grupo pré-ERAS foi composto por 360 pacientes e o grupo pós-ERAS por 319. No grupo pré ERAS, 214 pacientes (59,8%) desenvolveram pelo menos uma complicação versus 163 pacientes (51,10%) no grupo pós-ERAS. Um número maior de pacientes do grupo pré-ERAS desenvolveu complicações moderadas ou graves (31,9% vs. 22,26%, p = 0,009); e complicações graves (15,5% vs. 5,3%; p < 0,0001). A mediana do tempo de internação foi de 13 (17) dias no grupo Pré-ERAS e de 11 (10) dias no grupo pós-ERAS (p = 0,034). Não houve diferença nas taxas de mortalidade (4,7% vs. 2,5%; p = 0,1554) ou de reinternação (6,39% vs. 4,39%; p = 0,31). A conformidade geral do protocolo ERAS na coorte pós-ERAS foi de 88%.
Conclusões
A implantação do protocolo ERAS para cirurgia colorretal foi associada a uma redução significativa das complicações pós-operatórias e do tempo de internação.
Palavras-chave: Perioperatório, Aceleração da recuperação pós-operatória, Complicações pós-operatórias
Introduction
Despite all advances in surgical and anesthetic care, morbidity after abdominal surgery it remains high. Colorectal surgery is associated with a high risk of morbidity and mortality in comparison to other general surgical procedures. Overall mortality rates after colorectal surgery range from 1% to 16.4%,1, 2, 3 with morbidity rates as high as 35%.1, 2, 4 The aim of fast-track surgery, also called Enhanced Recovery After Surgery (ERAS) or multimodal surgery involves the use of several perioperative strategies to facilitate better surgical conditions and achieving faster recovery and early discharge from hospital. ERAS protocols have shown repeatedly by reducing the length of stay (LOS)5, 6, 7; without influencing complication or readmission rates.7, 8 Although individual components may vary, most of the ERAS programs include avoidance of fasting, preoperative nutritional optimization, preoperative carbohydrate loading, avoidance of bowel preparation, goal-directed hemodynamic therapy, multimodal analgesia without opiates, avoidance/early removal of tubes (nasogastric tube, Foley catheter, and drains), support of gastrointestinal function, and early convalescence.9, 10
The prediction of success or failure of ERAS has become a matter of interest.11 There are evidences to suggest that increasing overall compliance with the individual elements of an ERAS program improves clinical outcomes.12, 13
This prospective study assessed how implementation of an ERAS program affects postoperative complications in patients undergoing elective colorectal surgery.
Materials and methods
This study is reported according to the STROBE guidelines for the conducting and reporting of observational cohort studies.14
Study design
As part of a quality improvement initiative, the working group was established in 2013 to implement the ERAS protocol for colorectal procedures. The multidisciplinary ERAS group included surgeons, anesthesiologists, nutritionists and a medical librarian to help with literature searches. The group worked with clinical experts, reviewed the literature for best practices in perioperative care and reached consensus on each step of patient care. Drafts were presented to surgeons and anesthesiologists at our institution for reviewing, and an iterative process of review was followed until consensus was reached. An anesthesiologist coordinator managed the project. The main goals of ERAS program were to improve recovery and to decrease postoperative complications through intraoperative Goal Directed Hemodynamic Therapy (GDHT), early nutrition and ambulation, avoidance or prompt removal of drains and tubes. The clinical pathway was based on the ERAS society guidelines.15, 16 The ERAS protocol was implemented in March 2013 after staff training. A 3-month period of implementation allowed all staff to become familiar with the ERAS protocol. The protocol was a hospital-wide, evidence-based quality improvement project and it was considered the standard practice.
After the approval of the Infanta Leonor University Hospital Ethics and Research Committee (approved 2/12/2015), we compared data from consecutive adult patients undergoing elective open or laparoscopic colorectal surgery within the ERAS protocol, with a previous cohort of patients before introduction of the ERAS protocol. Excluding those patients who were operated during the training period, all patients who had an elective colon or rectal resection were included in this analysis. Patients who received conventional perioperative care underwent surgery from November 2010 through January 2013 (Pre-ERAS group). Patients who received perioperative care according to the ERAS protocol underwent surgery from March 2013 through December 2015 (Post-ERAS group). Due to the ERAS program's implementation, was considered as part of the standard patient care, the written consent was not requested to the patients.
Pre-ERAS group management
Before the introduction of the ERAS pathway, there was little standardization of care. Patients were fasted from midnight on the day of the surgery, and all patients received bowel preparation. All other patient managements were at the discretion of the surgical and anesthesia providers. Cardiac output, central venous pressure and invasive blood pressure monitoring were performed at the discretion of the anesthesia providers. Intraoperative fluid administration was usually based on changes in hemodynamic (arterial blood pressure and heart rate) and urine output. Early mobilization and feeding were not undertaken.
Post-ERAS group management
After the implementation of the ERAS pathway, the protocol was standardized using a pathway that was adapted from the evidence described in the ERAS consensus statement16 (Table 1) Patients in the ERAS group were educated in the preoperative surgical clinic about the ERAS pathway. Routine bowel preparation was not performed for colonic procedures, and patients were allowed to drink clear fluids until 2 h preoperatively. Patients also were given 400 mL oral preoperative carbohydrate drink (PreOP®, Nutricia; Numico, Zoetermeer, the Netherlands), which they were told to drink 2 h preoperatively. Preoperative sedatives were not allowed. Epidural anesthesia was not performed in laparoscopic surgery, however, in planed open surgery an epidural catheter was placed at the T8–T10 level In these patients, intraoperative analgesia was provided using a single epidural dose of bupivacaine at induction (25–30 mg), followed by an infusion of bupivacaine (2.5 mg.mL−1 at mL.h−1).
Table 1.
ERAS guidelines recommendations.
| Item | ERAS recommendation |
|---|---|
| Preoperative information, education and counseling | Patients should routinely receive dedicated preoperative counseling |
| Preoperative optimization | Smoking and alcohol consumption (alcohol abusers) should be stopped four weeks before surgery |
| Preoperative bowel preparation | Mechanical bowel preparation should not be used routinely in colonic surgery |
| Preoperative fasting and carbohydrate treatment | Clear fluids should be allowed up to 2 h and solids up to 6 h prior to induction of anesthesia. Preoperative oral carbohydrate treatment should be used routinely |
| Preanesthetic medication | Patients should not routinely receive long- or short- acting sedative medication before surgery because it delays immediate postoperative recover |
| Prophylaxis against thromboembolism | Patients should wear well-fitting compression stockings, have intermittent pneumatic compression, and receive pharmacological prophylaxis |
| Antimicrobial prophylaxis and skin preparation | Routine prophylaxis using intravenous antibiotics should be given 30–60 min before initiating surgery. Additional doses should be given during prolonged operations according to half life of the drug used preparation with chlorhexidine-alcohol should be used |
| Standard anesthetic protocol | A standard anesthetic protocol allowing rapid awakening should be given the anesthetist should control fluid therapy, analgesia and hemodynamic changes to reduce the metabolic stress response |
| Postoperative nausea and vomiting (PONV) | A multimodal approach to PONV prophylaxis should be adopted in all patients with 2 or more risk factors undergoing major colorectal surgery |
| Laparoscopy and modifications of surgical access | Laparoscopic surgery for colonic resections is recommended if the expertise is available |
| Nasogastric intubation | Postoperative nasogastric tubes should not be used routinely. Nasogastric tubes inserted during surgery should be removed before reversal of anesthesia |
| Preventing intraoperative hypothermia | Intraoperative maintenance of normothermia with a suitable warming device and warmed intravenous fluids should be used routinely to keep body temperature |
| Perioperative fluid management | Patients should receive intraoperative fluids (colloids and crystalloids) guided by flow measurements to optimize cardiac output |
| Drainage of peritoneal cavity after colonic anastomosis | Routine drainage is discouraged because it is an unsupported intervention that is likely to impair mobilization. |
| Urinary drainage | Routine transurethral bladder drainage for 1–2 days is recommended |
| Prevention of postoperative ileus | Fluid overload and nasogastric decompression should be avoided |
| Postoperative analgesia | Open surgery: Thoracic epidural anesthesia (TEA) using low-dose local anesthetic and opioids Laparoscopic surgery: No TEA |
| Perioperative nutritional care | Patients should be screened for nutritional status and if at risk of under nutrition given active nutritional support postoperatively patients should be encouraged to take normal food as soon as lucid after surgery |
| Postoperative glucose control | Hyperglycaemia is a risk factor for complications and should therefore be avoided |
| Early mobilization | Prolonged immobilization increases the risk of pneumonia, insulin resistance and muscle weakness. Patients should therefore be mobilized |
IV opioids (fentanyl) were given after induction of anesthesia in all patients undergoing laparoscopic surgery. An infusion of lactated Ringer's solution was started and maintained throughout the procedure using a dedicated infusion pump (set at 3 mL.kg−1.h−1 for laparoscopic colectomy, and 5 mL.kg−1.h−1 for open colectomy, based on lean body weight).
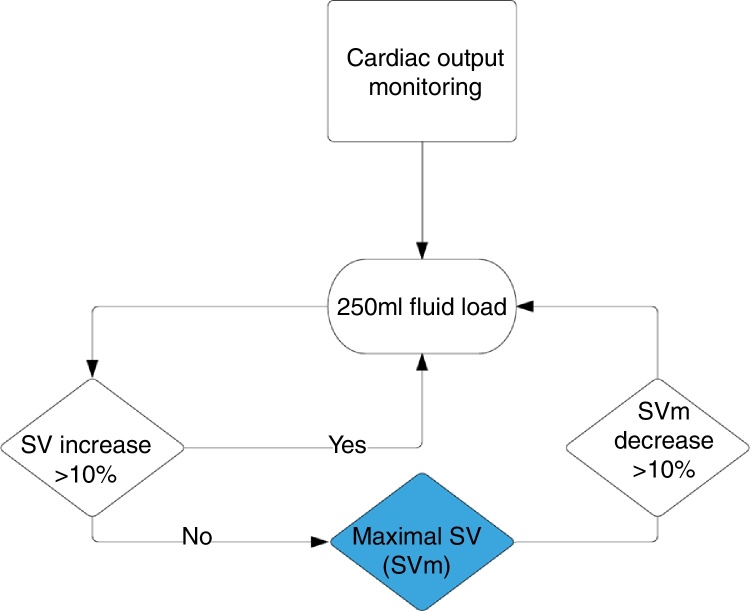
All patients received intraoperative Goal-Directed Hemodynamic Therapy (GDHT) with a minimally invasive Cardiac Output (CO) monitor. Oesophageal Doppler (EDM™ Deltex Medical, Inc., Irving, TX) or Vigileo/Flotrac (Edwards, Irvine, USA) were used according to the need for invasive monitoring of blood pressure, which was not protocolised, and was based on the preferences of the anesthesiologist. Bolus of IV colloid (hydroxyethyl starch 130/04, Voluven, Fresenius Kabi, Germany) was given to optimize stroke volume (SV) using a 10% algorithm (Fig. 1). Hypotension was preferably treated with a vasopressor agent (ephedrine 5 mg or phenylephrine 0.1 mg) instead of intravenous fluid bolus in order to maintain a neutral fluid balance. Ondansetron and dexamethasone were administered according individual Apfel score17 to prevent postoperative nausea and vomiting. Nasogastric tubes and surgical drains were not routinely used.
Figure 1.
Goal Directed Fluid Therapy algorithm (SV, stroke volume; SVm, maximal stroke volume).
Postoperatively patient's urinary catheter was removed on the day after surgery. Postoperative analgesia was provided by using acetaminophen and no steroidal anti-inflammatory drugs. Opioid were avoided when possible. Patients were encouraged to drink liquids immediately after surgery. IV fluid administration was discontinued once adequate oral intake was achieved, usually on the first morning after surgery. Patients were encouraged to get up on a chair 6 h after surgery, and to walk after the first 12 postoperative hours.
Interventions unchanged between groups
All patients underwent basic anesthetic monitoring with five-lead-electrocardiogram, pulse oximetry and blood pressure cuff; at least two peripheral intravenous lines were established. Antibiotic prophylaxis (cefazolin 2 g and metronidazole 500 mg intravenously) was given 30 min before surgery incision. All patients received general anesthesia with an oral endotracheal tube. All patients received balanced or total intravenous anesthesia, intravenous anesthetic induction, and neuromuscular relaxants; for pragmatic reasons, their administration was made at the discretion of the anesthesiologist. Bispectral Index monitoring system (BIS, Medtronic, Dublin, Ireland) was used to monitor the depth of the anesthesia. Sevoflurane or propofol was used for anesthesia maintenance; with the target range of BIS values between 40–60. All patients received standard measures to maintain optimal oxygenation (oxygen saturation by pulse oximetry ≥94%), core temperature (37 °C) and heart rate (<100 min). Ventilation with inspired oxygen fraction of 60% was mechanically controlled to maintain PaCO2 between 35 and 45 mmHg, with a positive end-expiratory pressure of 4–6 mmHg and tidal volume of 6–8 mL.kg−1. Normothermia was maintained using convective air warming system (Bair Hugger; 3M-Switzerland, Rüschlikon, Switzerland) and Hotline® fluid warmer (Smith Medical International Ltd., Ashford, Kent, United Kingdom).
At the end the intervention all patients were followed in intensive care for at least 24 h. Postoperative administration of fluids was not protocolized.
Outcomes
The aim of our study was the prospective evaluation of an ERAS protocol that includes the whole application of all ERAS principles in adult patients undergoing elective colorectal surgery. The primary endpoint was the percentage of patients who developed pre-defined moderate-severe postoperative complications within 180 days after surgery, including complications that occurred before or after hospital discharge and required ambulatory or in-hospital care.
Postoperative complications were defined according to standard definitions and using the outcome measures for clinical effectiveness research in perioperative medicine (EPCO).18 All complications were classified in mild, moderate and severe.
Secondary endpoints were: LOS (defined as the number of days spent in the hospital: from the day of surgery to hospital discharge or death), re-admission, all-cause mortality within 180 days after surgery, and ERAS protocol compliance (Table 2). Overall ERAS protocol compliance was calculated in the ERAS cohort as the average of all pre- and intraoperative ERAS elements, as specified in the ERAS Guidelines of Colon and Rectal surgery. Hospital readmission for any postoperative complication occurring within 180 days after discharge was also recorded.
Table 2.
ERAS protocol compliance in the ERAS group.
| % | |
|---|---|
| Preoperative information and counseling | 98.9 |
| Preoperative optimization | 98.9 |
| Preoperative bowel preparation | 92.4 |
| Preoperative fasting and carbohydrate treatment | 67.5 |
| Pre anesthesic medication | 96.2 |
| Prophylaxis against thromboembolism | 100 |
| Antimicrobial prophylaxis | 100 |
| Standard anesthesic protocol | 100 |
| PONV | 71.6 |
| Laparoscopy | 79.2 |
| Nasogastric intubation | 87.9 |
| Preventing intraoperative hypothermia | 99.6 |
| Goal Directed Hemodynamic therapy | 100 |
| Drainage of peritoneal cavity | 70.9 |
| Urinary drainage | 99.6 |
| Fluid balance < 1500 mL/24 h | 85.5 |
| Postoperative analgesia | 100 |
| Perioperative nutricional care | 68.5 |
| Postoperative glucose control | 100 |
| Early mobilization | 50.2 |
| Overall compliance | 88.4 |
PONV, postoperative nausea and vomiting.
Data collection
Three researchers who did not participate in the perioperative management of patients performed data collection. All data were retrieved from the patients’ database and clinical records, which in our center is fully computerized. The following data were extracted: sex, age, comorbidity (diabetes mellitus, hypertension, ischemic heart disease, respiratory disease), American Society of Anesthesiologists.
Physical Status (ASA), surgical approach (open, laparoscopic), type of anesthetic procedure, operation time and complications.
Statistical analysis
Descriptive statistics of the pre-ERAS and post-ERAS groups were compared for all relevant patient characteristics, perioperative and postoperative data. The discrete variables were described as n (%) and the continuous variables as mean (standard deviation) and median (interquartile range). Statistical comparisons between pre-ERAS and post-ERAS groups were performed with the Mann–Whitney U tests, and Fisher's exact test as appropriate. The statistical significance level for all comparisons was set at a two-tailed α = 0.05.
The data set was analyzed using the percentage of patients with postoperative complications. The influence of the following factors was assessed: sex, age, ASA status, surgical approach (open, laparoscopic), type of anesthetic procedure, duration of surgery, intraoperative fluid administration and first 24 h fluid balance; and ERAS protocol.
All analyses were performed using JMP version 12.1.0 (SAS Institute, Cary, Carolina del Norte, USA).
Results
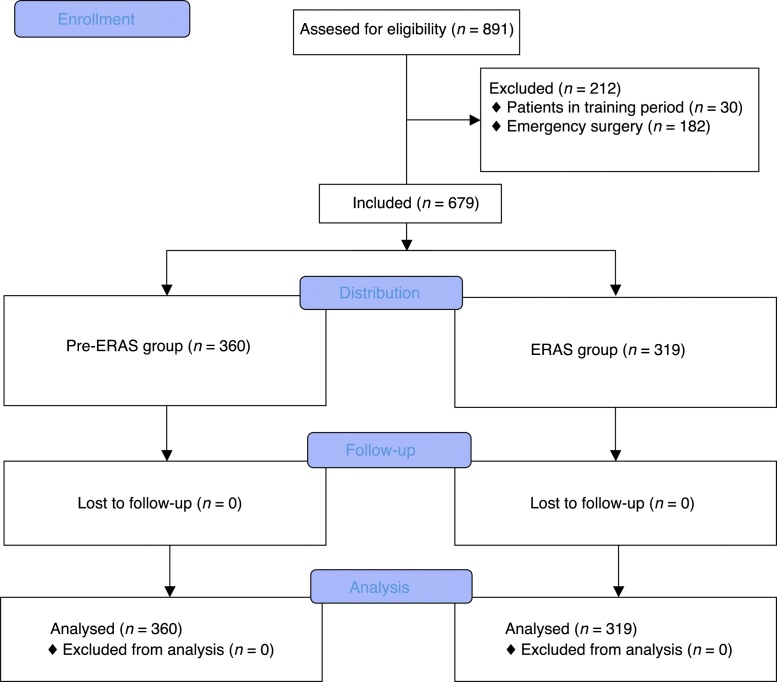
There were 360 procedures on 360 individual patients in the Pre-ERAS group and 319 procedures on 319 individual patients in the Post-ERAS Group. There were no patients or procedures excluded or deleted from either cohort of consecutive cases enrolled in the analysis. There were no losses of follow up (Fig. 2).
Figure 2.
CONSORT flow chart.
Patient characteristics are reported in Table 1. There was no statistically significant difference in age, body mass index, or ASA rating. However, there were more women in the pre-ERAS group. Chronic health conditions such as diabetes and chronic cardiac conditions were equally represented in both groups. More patients in the Pre-ERAS group underwent surgery with open surgical approach, and more patients received combined anesthesia (general epidural). Significantly less intravenous fluid were administered during the day of surgery and in the first 24 h after surgery in patients receiving ERAS perioperative care (Table 1).
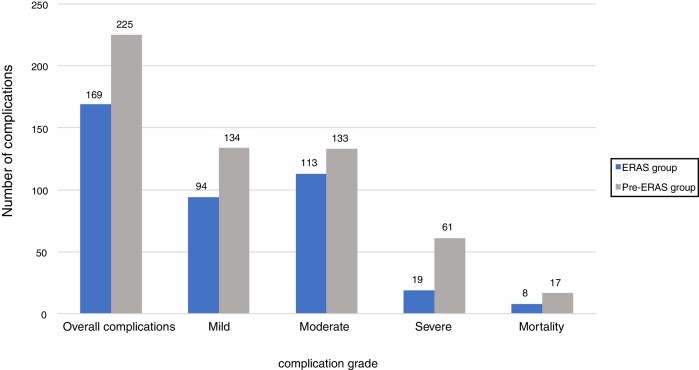
A total of 214 (59.8%) patients developed at least one complication in the Pre-ERAS group, versus 163 patients in the ERAS group (51.1%). More patients in the Pre-ERAS group developed moderate or severe complications (31.9% vs. 22.2%, p = 0.009); and severe complications (15.5% vs. 5.3%; p < 0.001). No differences were found on mortality rates (4.7% vs. 2.5%; p = 0.154) (Fig. 3) nor readmission (6.39% vs. 4.39%; p = 0.31). The rate of specific complications is presented in Table 3. Postoperative ileus was the most frequent complications in both groups, however there were significant fewer patients in the Post-ERAS group (33.6% vs. 19.2%, p < 0.001). There were also significant fewer patients with deep and organ-space surgical site infection in the Post-ERAS group. More patients developed anastomotic breakdown in the Pre-ERAS group (10% vs. 4.7%; p = 0.012). No significant differences in other analyzed complications were observed. There was a significant difference in LOS between both groups. The median postoperative hospital stay was 13 (17) days for patients receiving conventional care and 11 (10) days for patients that had followed the ERAS protocol (p = 0.034). Patient characteristics, operative factors, and ERAS component that influenced the development of complications are summarized in Table 4.
Figure 3.
Overall complications.
Table 3.
Demographic and perioperative characteristics of included patients.
| Pre-ERAS group | ERAS group | |
|---|---|---|
| Age (years) mean (SD) | 68.5 (13.6) | 70.5 (12.4) |
| Gender, n (%) | ||
| Female | 120 (33.3) | 137 (42.9) |
| Male | 240 (66.7) | 182 (57) |
| Body mass index (kg.m−2) Mean (SD) | 27.8 (4.2) | 27.8 (4.7) |
| ASA grade, n (%) | ||
| I | 37 (10.2) | 23 (7.2) |
| II | 204 (56.6) | 173 (54.2) |
| III | 117 (32.5) | 116 (36.3) |
| IV | 2 (0.5) | 7 (2.2) |
| Hypertension, n (%) | 201 (55.8) | 199 (62.4) |
| Diabetes, n (%) | 91 (25.3) | 76 (23.8) |
| COPD, n (%) | 57 (15.8) | 47 (14.7) |
| Chronic renal disease, n (%) | 35 (9.7) | 22 (6.9) |
| Ischemic heart disease, n (%) | 40 (11.1) | 29 (9.1) |
| Preoperative Hb (g.dL−1) Mean (SD) | 12.7 (1.8) | 12.7 (2.0) |
| Preoperative Albumin (g.dL−1) Mean (SD) | 3.8 (0.6) | 3.7 (0.7) |
| Surgery, n (%) | ||
| Open approach | 167 (46.4) | 116 (36.4) |
| Laparoscopic approach | 193 (53.6) | 203 (63.6) |
| Conversion to open, n (%) | 16 (4.4) | 15 (4.7) |
| Epidural, n (%) | 76 (21.2) | 25 (7.8) |
| Length of surgery (min) Mean (SD) | 147.2 (54.1) | 121.4 (49.2) |
| Intraoperative fluids (milliliters) Mean (SD) | 2466.9 (1033.5) | 1731.9 (702.8) |
| 24 h fluids (milliliters) Mean (SD) | 5463.7 (1687.2) | 4356.4 (1236.6) |
| 24 h balance (milliliters) Mean (SD) | 2717.1 (1714.1) | 2056.3 (1375.3) |
ASA, American Society of Anesthesiologists physical status classification; COPD, chronic obstructive pulmonary disease; h, hours; BMI, body mass index.
Table 4.
Postoperative complications after colorectal surgery.
| Pre-ERAS group | ERAS group | p-value | |
|---|---|---|---|
| AKI, n (%) | 34 (9.4) | 29 (9.1) | 0.895 |
| ARDS, n (%) | 13 (3.6) | 4 (1.2) | 0.082 |
| Acute myocardial infarction, n (%) | 1 (0.3) | 0 (0) | 1.000 |
| MINS, n (%) | 5 (1.4) | 1 (0.3) | 0.221 |
| Arritmia, n (%) | 34 (9.4) | 33 (10.3) | 0.701 |
| Cardiac arrest, n (%) | 5 (1.4) | 4 (1.2) | 1.000 |
| Cardiogenic pulmonar edema, n (%) | 11 (3.1) | 6 (1.8) | 0.462 |
| DVT, n (%) | 0 (0) | 1 (0.3) | 0.469 |
| PE, n (%) | 2 (0.5) | 1 (0.3) | 1.000 |
| Stroke, n (%) | 3 (0.8) | 0 (0) | 0.252 |
| Infection, source uncertain, n (%) | 35 (9.7) | 25 (7.8) | 0.418 |
| Infection, laboratory confirmed, n (%) | 47 (13.1) | 15 (4.7) | 0.000 |
| Surgical site infection (superficial), n (%) | 46 (12.8) | 26 (8.1) | 0.060 |
| Surgical site infection (deep), n (%) | 42 (11.6) | 11 (3.4) | 0.000 |
| Surgical site infection (organ/space), n (%) | 39 (10.8) | 15 (4.7) | 0.004 |
| Anastomotic breakdown, n (%) | 36 (10) | 15 (4.7) | 0.012 |
| Urinary tract infection, n (%) | 13 (3.6) | 7 (2.2) | 0.364 |
| Pneumonia, n (%) | 13 (3.6) | 10 (3.1) | 0.833 |
| Gastrointestinal bleed, n (%) | 11 (3.1) | 18 (5.6) | 0.127 |
| Postoperative Haemorrage, n (%) | 84 (23.3) | 80 (25.1) | 0.653 |
| Paralytic ileus, n (%) | 121 (33.6) | 61 (19.1) | 0.000 |
| Delirium, n (%) | 39 (10.8) | 18 (5.6) | 0.018 |
| LOS Mean (SD) | 13 (17) | 11 (10) | 0.034 |
AKI, acute kidney injury; ARDS, acute respiratory distress syndrome; MINS, myocardial injury after non-cardiac surgery; DVT, deep vein thrombosis; PE, pulmonary embolism; LOS, length of stay.
The average percentage of completion was 88.4% in the Post-ERAS patient cohort (Table 5). All patients complied with the items on antithrombotic and antimicrobial prophylaxis, anesthetic protocol, fluid therapy, postoperative analgesia and glycaemic control. The lowest compliance item was early mobilization (50.2%), followed by fasting and preoperative carbohydrate loading (67.5%), perioperative nutritional care (68.5%) and non-placement of surgical drains (70.9%).
Table 5.
Multivariable analysis of complications development after colorectal surgery in all included patients (pre-ERAS and ERAS groups).
| Correlation | Lower end 95% CI | Upper end 95% CI | p-value | |
|---|---|---|---|---|
| Patients with complications (all severity) | ||||
| ERAS | −0.08 | −0.15 | −0.01 | 0.030 |
| Age | 0.15 | 0.07 | 0.22 | <0.001 |
| ASA | 0.21 | 0.13 | 0.27 | <0.001 |
| Laparoscopy | −0.26 | −0.32 | −0.18 | <0.001 |
| Female | 0.09 | 0.01 | 0.16 | 0.020 |
| Epidural | 0.08 | 0.01 | 0.15 | 0.035 |
| Duration | 0.18 | 0.11 | 0.26 | <0.001 |
| Intraoperative fluids | 0.21 | 0.14 | 0.28 | <0.001 |
| 24 h fluids | 0.25 | 0.18 | 0.32 | <0.001 |
| 24 h fluid balance | 0.26 | 0.19 | 0.33 | <0.001 |
| Patients with complications (moderate or severe) | ||||
| ERAS | −0.10 | −0.17 | −0.02 | 0.008 |
| Age | 0.11 | 0.04 | 0.19 | 0.002 |
| ASA | 0.17 | 0.10 | 0.25 | <0.001 |
| Laparoscopy | −0.23 | −0.30 | −0.15 | <0.001 |
| Female | 0.16 | 0.09 | 0.24 | <0.001 |
| Epidural | 0.08 | 0.01 | 0.16 | 0.021 |
| Duration | 0.19 | 0.12 | 0.26 | <0.001 |
| Intraoperative fluids | 0.23 | 0.16 | 0.30 | <0.001 |
| 24 h fluids | 0.22 | 0.15 | 0.30 | <0.001 |
| 24 h fluid balance | 0.20 | 0.13 | 0.27 | <0.001 |
| Patients with complications (severe) | ||||
| ERAS | −0.16 | −0.23 | −0.09 | <0.001 |
| Age | 0.09 | 0.01 | 0.16 | 0.017 |
| ASA | 0.12 | 0.04 | 0.19 | 0.001 |
| Laparoscopy | −0.15 | −0.22 | −0.07 | <0.001 |
| Female | 0.14 | 0.07 | 0.21 | <0.001 |
| Epidural | 0.04 | −0.03 | 0.11 | 0.283 |
| Duration | 0.18 | 0.11 | 0.25 | <0.001 |
| Intraoperative fluids | 0.16 | 0.08 | 0.23 | <0.001 |
| 24 h fluid balance | 0.18 | 0.10 | 0.25 | <0.001 |
ERAS, Enhanced Recovery After Surgery; ASA, American Society of Anesthesiologists physical status classification; CI, confidence interval; ERAS, Enhanced Recovery After Surgery.
Discussion
When implementing an ERAS protocol, it is imperative to examine the Pre- and Post-ERAS implementation data to ensure that any unintended consequences are appropriately detected, and the hypothesized improvements actually occurred in patient outcomes. The results of this study suggest that the ERAS program was superior to conventional postoperative care for patients undergoing elective colonic or rectal resection. Patients treated according to the ERAS program developed significantly fewer complications and had shorter hospital stay. However, no differences in mortality were found.
Postoperative complications occur in up to one-third of patients undergoing colorectal surgery.1 The most common complications reported are infectious, wound infection or organ space infection, and Gastrointestinal (GI) motility complications, including ileus and bowel obstruction.19 In a recent meta-analysis, Geco et al. showed that ERAS pathway was associated to a reduction of overall morbidity (Relative Ratio [RR] = 0.60; 95% CI 0.46–0.76), particularly with respect to no surgical complications; and the ERAS pathway shortened two-day LOS, without increasing readmission rates.20
In our study, we found similar results that were previously reported, fewer patients presented infectious complications, ileus, and anastomotic breakdown. In the Post-ERAS group, patients received better perioperative management of fluids, both intraoperatively, and in the immediate postoperative period. Volume overload was associated with postoperative complications21, 22 and mortality23; while GDHT had shown that it could reduce the incidence of postoperative complications; its role within the ERAS program was recently questioned.24, 25 Nonetheless, Gustafsson et al. identified restriction of intravenous fluid and use of a preoperative carbohydrate drink as major independent predictors of success.12 Similarly, a restrictive perioperative fluid therapy, was the only individual ERAS item associated with a reduction of postoperative complications in the metacentre ERAS registry data, after analysing more than 2000 patients undergoing colorectal surgery.26 Our study data provide further evidence that perioperative fluid management -in particular to prevent volume overload, is an important predictor of outcome, which underscores the need to keep this item on clinical pathways,27 despite particular controversies,28 and contradictory results of recent meta-analysis.24
In our study, we found that restrictive fluid administration was associated with a reduction of complications; however, due to the 100% compliance in GDHT, we cannot draw conclusions regarding its usefulness.
The relationship between the onset of postoperative complications or LOS, and the rate of compliance to the ERAS protocol is subject to discussion. A high ERAS adherence has been shown to be associated with a significant reduction in LOS,29 Nevertheless, its association with a reduction of complications is less evident.29 Meaningfully, LOS is an irrelevant resource of measure, and has often been used as a surrogate measure for postoperative complications.30 It is not necessarily directly related to postoperative morbidity. Even though, in our study, despite not found a decrease in mortality, we found a significant decrease in moderate and severe complications.
A single-center prospective cohort study showed a significant decrease in postoperative complications (OR = 0.73; 95% CI 0.55–0.98). Following an overall increase in preoperative and perioperative adherence to the ERAS protocol from 43.3% to 70.6%.12 Similarly, The United Kingdom Enhanced Recovery Partnership Program (ERPP) found that a ≥80% ERAS compliance was associated with a shorter median LOS by two days,31 and recently, the Francophone Group for Enhanced Recovery after Surgery (“Groupe francophone de réhabilitation améliorée après chirurgie” [GRACE]) found a statistically significant relationship between protocol compliance and LOS for colorectal surgery, in a large multicentre prospective study including 490 patients; the threshold value of 68% of the elements applied was associated with a statistically significant effect on LOS.32 A high compliance to the items of the protocol, has been evoked to be essential to obtain the best outcomes.33 Our results are similar to those previously published.
A limitation of the present study is that the analysis was retrospective and the results should have been interpreted as such. Despite this, database entry was done prospectively, which minimized data loss. Another limitation of this study included a relatively small sample size, and low risk patients. Moreover, both groups were not balanced in terms of population size, gender and open surgical vs. laparoscopic approach. Especially the greater number of patients with a laparoscopic approach may have been influenced to obtain better outcome, however, the routine application of this approach constitutes a measure of the ERAS protocol itself. The use of Esophageal Doppler and Vigileo/Flotrac was not protocolized. Studies have shown that there is a limited concordance between the two devices to measure cardiac output and preload sensitive parameters and their response to clinical interventions.34 This difference could be a source of misleading intraoperative management. However, due to the pragmatic features of this study, this limitation may be considered unimportant.
Conclusions
ERAS program is feasible, and its results are superior to conventional postoperative care for patients undergoing elective colonic or rectal resection. Patients treated according to an ERAS program develop significant fewer complications and have shorter hospital stay. However, no difference in mortality has been found.
Funding
This study has no funding. Grupo Español de Rehabilitación Multimodal (GERM), and Evidence Anesthesia Review (EAR) group supports this study.
JRM received travel funding from Deltex Medical and honoraria for lectures from Fresenius Kabi, Edwards Lifesciences, Deltex Medical and Merck Sharp & Dohme. He is currently the Chief of Fluid Management section of Grupo Español de Rehabilitación Multimodal (GERM/ERAS Spain Chapter). RCF received honoraria and travel funding for lectures from Merck Sharp & Dohme and Deltex Medical. AAGR received honoraria and travel funding for lectures from Merck Sharp &Dohme. JMCV received travel funding from Deltex Medical and honoraria for lectures from Fresenius Kabi, Edwards Lifesciences, Deltex Medical and Merck Sharp & Dohme. He is currently the vice chief of Grupo Español de Rehabilitación Multimodal (GERM/ERAS Spain Chapter).
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
Ane Abad-Motos, Norma Aracil-Escoda, Ana Tirado-Errazquin and Kateri Chao Novo, Infanta Leonor University Hospital anesthesiologists. Patricia Ortega, Alicia Ruíz de la Hermosa, Daniel Serralta, Carlos Johanson, Infanta Leonor University Hospital surgeons; Belen Silveira, Infanta Leonor University Hospital nutritionist. Teresa de la Torre Aragonés and Rocío Gálvez, Infanta Leonor University Hospital Professional librarian.
References
- 1.Alves A., Panis Y., Mathieu P., et al. Postoperative mortality and morbidity in French patients undergoing colorectal surgery: results of a prospective multicenter study. Arch Surg. 2005;140:278–283. doi: 10.1001/archsurg.140.3.278. [DOI] [PubMed] [Google Scholar]
- 2.Longo W.E., Virgo K.S., Johnson F.E., et al. Risk factors for morbidity and mortality after colectomy for colon cancer. Dis Colon Rectum. 2000;43:83–91. doi: 10.1007/BF02237249. [DOI] [PubMed] [Google Scholar]
- 3.Tevis S.E., Carchman E.H., Foley E.F., et al. Postoperative ileus-more than just prolonged length of stay? J Gastrointest Surg. 2015;19:1684–1690. doi: 10.1007/s11605-015-2877-1. [DOI] [PubMed] [Google Scholar]
- 4.de Silva S., Ma C., Proulx M.C., et al. Postoperative complications and mortality following colectomy for ulcerative colitis. Clin Gastroenterol Hepatol. 2011;9:972–980. doi: 10.1016/j.cgh.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 5.King P.M., Blazeby J.M., Ewings P., et al. Randomized clinical trial comparing laparoscopic and open surgery for colorectal cancer within an enhanced recovery programme. Br J Surg. 2006;93:300–308. doi: 10.1002/bjs.5216. [DOI] [PubMed] [Google Scholar]
- 6.Lovely J.K., Maxson P.M., Jacob A.K., et al. Case-matched series of enhanced versus standard recovery pathway in minimally invasive colorectal surgery. Br J Surg. 2012;99:120–126. doi: 10.1002/bjs.7692. [DOI] [PubMed] [Google Scholar]
- 7.Keane C., Savage S., McFarlane K., et al. Enhanced recovery after surgery versus conventional care in colonic and rectal surgery. ANZ J Surg. 2012;82:697–703. doi: 10.1111/j.1445-2197.2012.06139.x. [DOI] [PubMed] [Google Scholar]
- 8.Wind J., Vlug M.S., Hollmann M.W., et al. Perioperative strategy in colonic surgery; laparoscopy and/or FAst track multimodal management versus standard care (LAFA trial) BMC Surg. 2006;6:16. doi: 10.1186/1471-2482-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott M.J., Baldini G., Fearon K.C.H., et al. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 1: pathophysiological considerations. Acta Anesthesiol Scand. 2015;59:1212–1231. doi: 10.1111/aas.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casans Frances R., Ripolles Melchor J., Abad-Gurumeta A., et al. The role of the anesthesiologist in enhanced recovery programs. Rev Esp Anestesiol Reanim. 2016;63:273–288. doi: 10.1016/j.redar.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Ripolles Melchor J., Casans Frances R., Abad-Gurumeta A., et al. Spanish survey on enhanced recovery after surgery. Rev Esp Anestesiol Reanim. 2016;63:376–383. doi: 10.1016/j.redar.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Gustafsson U.O., Hausel J., Thorell A., et al. Adherence to the enhanced recovery after surgery protocol and outcomes after colorectal cancer surgery. Arch Surg. 2011;146:571–577. doi: 10.1001/archsurg.2010.309. [DOI] [PubMed] [Google Scholar]
- 13.Boulind C.E., Yeo M., Burkill C., et al. Factors predicting deviation from an enhanced recovery programme and delayed discharge after laparoscopic colorectal surgery. Colorectal Dis. 2012;14:e103–e110. doi: 10.1111/j.1463-1318.2011.02799.x. [DOI] [PubMed] [Google Scholar]
- 14.von Elm E., Altman D.G., Egger M., et al. STROBE initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Nygren J., Thacker J., Carli F., et al. Enhanced Recovery After Surgery Society. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. Clin Nutr. 2012;31:801–816. doi: 10.1016/j.clnu.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Gustafsson U.O., Scott M.J., Schwenk W., et al. Enhanced Recovery After Surgery Society. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. Clin Nutr. 2012;31:783–800. doi: 10.1016/j.clnu.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Apfel C.C., Greim C.A., Haubitz I., et al. A risk score to predict the probability of postoperative vomiting in adults. Acta Anesthesiol Scand. 1998;42:495–501. doi: 10.1111/j.1399-6576.1998.tb05157.x. [DOI] [PubMed] [Google Scholar]
- 18.Jammer I., Wickboldt N., Sander M., et al. European Society of Anesthesiology (ESA) and the European Society of Intensive Care Medicine (ESICM); European Society of Anesthesiology; European Society of Intensive Care Medicine. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anesthesiol. 2015;32:88–105. doi: 10.1097/EJA.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 19.Tevis S.E., Kennedy G.D. Postoperative complications: looking forward to a safer future. Clin Colon Rectal Surg. 2016;29:246–252. doi: 10.1055/s-0036-1584501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greco M., Capretti G., Beretta L., et al. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg. 2014;38:1531–1541. doi: 10.1007/s00268-013-2416-8. [DOI] [PubMed] [Google Scholar]
- 21.Ripolles Melchor J., Casans-Frances R., Espinosa A., et al. Goal directed hemodymanic therapy based in esophageal doppler flow parameters: a systematic review, meta-analysis and trial sequential analysis. Rev Esp Anestesiol Reanim. 2016;63:384–405. doi: 10.1016/j.redar.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Brandstrup B., Tønnesen H., Beier-Holgersen R., et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238:641–648. doi: 10.1097/01.sla.0000094387.50865.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva J.M.J., de Oliveira A.M., Nogueira F.A., et al. The effect of excess fluid balance on the mortality rate of surgical patients: a multicenter prospective study. Crit Care. 2013;17:R288. doi: 10.1186/cc13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rollins K.E., Lobo D.N. Intraoperative goal-directed fluid therapy in elective major abdominal surgery: a meta-analysis of randomized controlled trials. Ann Surg. 2016;263:465–476. doi: 10.1097/SLA.0000000000001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ripolles Melchor J., Chappell D., Aya H.D., et al. Fluid therapy recommendations for major abdominal surgery. Via RICA recommendations revisited. Part II: Goal directed hemodynamic therapy. Rationale for optimising intravascular volume. Rev Esp Anestesiol Reanim. 2017;64:339–347. doi: 10.1016/j.redar.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 26.ERAS Compliance Group The impact of enhanced recovery protocol compliance on elective colorectal cancer resection: results from an international registry. Ann Surg. 2015;261:1153–1159. doi: 10.1097/SLA.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 27.Navarro L.H.C., Kramer G.C. Goal directed hemodynamic therapy: the time to implement is now. Rev Esp Anestesiol Reanim. 2016;63:373–375. doi: 10.1016/j.redar.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Ripolles Melchor J., Espinosa A. Goal directed fluid therapy controversies in non-cardiac surgery. Rev Esp Anestesiol Reanim. 2014;61:477–480. doi: 10.1016/j.redar.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Messenger D.E., Curtis N.J., Jones A., et al. Factors predicting outcome from enhanced recovery programmes in laparoscopic colorectal surgery: a systematic review. Surg Endosc. 2017;31:2050–2071. doi: 10.1007/s00464-016-5205-2. [DOI] [PubMed] [Google Scholar]
- 30.Moonesinghe S.R., Mythen M.G., Grocott M.P.W. High-risk surgery: epidemiology and outcomes. Anesth Analg. 2011;112:891–901. doi: 10.1213/ANE.0b013e3181e1655b. [DOI] [PubMed] [Google Scholar]
- 31.Simpson J.C., Moonesinghe S.R., Grocott M.P.W., et al. National Enhanced Recovery Partnership Advisory Board. Enhanced recovery from surgery in the UK: an audit of the enhanced recovery partnership programme 2009–2012. Br J Anesth. 2015;115:560–568. doi: 10.1093/bja/aev105. [DOI] [PubMed] [Google Scholar]
- 32.Veziant J., Raspado O., Entremont A., et al. Large-scale implementation of enhanced recovery programs after surgery. A francophone experience. J Visc Surg. 2017;154:159–166. doi: 10.1016/j.jviscsurg.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Maessen J., Dejong C.H., Hausel J., et al. A protocol is not enough to implement an enhanced recovery programme for colorectal resection. Br J Surg. 2007;94:224–231. doi: 10.1002/bjs.5468. [DOI] [PubMed] [Google Scholar]
- 34.Phan T.D., Kluger R., Wan C. Minimally invasive cardiac output monitoring: agreement of oesophageal Doppler, LiDCOrapid and Vigileo FloTrac monitors in non-cardiac surgery. Anesth Intensive Care. 2016;44:382–390. doi: 10.1177/0310057X1604400313. [DOI] [PubMed] [Google Scholar]