Abstract
Introduction
Advanced hepatic disease may – in addition to the widely recognized hemorrhagic complications – occur with thrombotic events. We describe the case of a cirrhotic patient taking warfarin and whose coagulation management during liver transplantation was guided by thromboelastometry (ROTEM®).
Case report
A 56 year-old male patient diagnosed with alcohol cirrhosis using warfarin (2.5 mg.day−1) for partial portal vein thrombosis with the International Normalized Ratio (INR) of 2.14. At the beginning of surgery, the ROTEM® parameters were all normal. In the anhepatic phase, EXTEM and INTEM remained normal, but FIBTEM showed reduction of amplitude after 10 min and maximum clot firmness. Finally, in the neohepatic phase, there was a slight alteration in the hypocoagulability of most of the parameters of the EXTEM, INTEM and FIBTEM, besides a notable correction of the Coagulation Time (CT) in HEPTEM compared to the CT of the INTEM. Therefore, the patient did not receive any transfusion of blood products during surgery and in the postoperative period, being discharged on the 8th postoperative day.
Discussion
Coagulation deficit resulting from cirrhosis distorts INR as a parameter of anticoagulation adequacy and as a determinant of the need for blood transfusion. Thus, thromboelastometry can provide important information for patient management.
Keywords: Thromboelastometry, Coagulation, Warfarin, Liver transplantation
Resumo
Introdução
A doença hepática avançada pode, além das complicações hemorrágicas amplamente reconhecidas, ocorrer com eventos trombóticos. Descrevemos o caso de um paciente cirrótico em uso de varfarina, cujo manejo da coagulação durante o transplante de fígado foi guiado por tromboelastometria (ROTEM®).
Relato de caso
Paciente do sexo masculino, 56 anos, diagnosticado com cirrose alcoólica, recebendo varfarina (2,5 mg.dia−1) para trombose parcial da veia porta, com razão normalizada internacional (INR) de 2,14. No início da cirurgia, os parâmetros ROTEM® estavam todos normais. Na fase não hepática, EXTEM e INTEM permaneceram normais, mas FIBTEM mostrou redução da amplitude após 10 min e firmeza máxima do coágulo. Por fim, na fase neo-hepática houve uma ligeira alteração da hipocoagulabilidade na maioria dos parâmetros de EXTEM, INTEM e FIBTEM, além de uma correção notável do tempo de coagulação (CT) de HEPTEM em comparação com o CT de INTEM. Portanto, o paciente não recebeu transfusão de hemoderivados durante a cirurgia e no período pós-operatório, obteve alta no oitavo dia de pós-operatório.
Discussão
O déficit de coagulação resultante da cirrose distorce o INR como um parâmetro da adequação da anticoagulação e como um determinante da necessidade de transfusão de sangue. Portanto, a tromboelastometria pode fornecer informações importantes para o manejo do paciente.
Palavras-chave: Tromboelastometria, Coagulação, Varfarina, Transplante hepático
Introduction
In the last two decades, it has become clear that advanced liver disease can – in addition to widely recognized hemorrhagic complications – occur with thrombotic events. This is explained by the fact that the liver synthesizes a large part of pro- and anticoagulant proteins. In healthy individuals, these proteins are present in sufficient amounts so that the hemostasis is balanced and with a safety margin that compensates for possible imbalances in the direction of thrombosis or bleeding. In cirrhosis, however, impairment of hepatocellular function decreases the amount of these proteins. This results in a state of hemostatic lability, characterized by a fragile and easily decompensated equilibrium for hemorrhagic or thrombotic events.1, 2 Hypercoagulability may accelerate the progression of hepatic fibrosis and trigger portal vein thrombosis, deep vein thrombosis, embolism pulmonary and portopulmonary hypertension, and all these complications are associated with increased morbidity and mortality.3, 4
Coumarins are therapeutic options for the treatment of thromboembolic diseases. However, in order to avoid hemorrhagic complications, traditionally, the intensity of the anticoagulation provided by these drugs is titrated to an International Normalized Ratio (INR) in the range 2–3. INR is derived from Prothrombin Time (PT), which, despite being validated to monitor the dose of warfarin, has its limitations in the measurement of hemostatic adequacy.5, 6
By reducing the levels of pro- and anticoagulant factors, cirrhosis per se changes conventional coagulation tests (especially PT and INR), without any correlation with pro- or anticoagulant tendency.2 For this reason, titration of coumarin anticoagulation (or coagulation adequacy) in cirrhotic patients may impose a great challenge on the medical team, especially when the hemostasis-like aggressions typical of the liver transplantation perioperative are added.
In scenarios of multiplicity of agents and conditions affecting coagulation, viscoelastic coagulation tests may be important tools in characterizing in greater detail the state of coagulation.7 Although EXTEM uses tissue factor for the activation of the extrinsic pathway similar to TP/INR, this viscoelastic test can be within normal limits when the INR is elevated.7, 8
We describe the case of a cirrhotic patient taking warfarin and whose coagulation management during hepatic transplantation was guided by thromboelastometry (ROTEM®).
Case report
A 56 year-old male patient diagnosed with alcohol cirrhosis and Model for End-Stage Liver Disease (MELD) score of 18, using warfarin (2.5 mg.day−1) for partial portal vein thrombosis. He was hemodynamically stable for transplantation and reported having ingested warfarin 6 h prior to transplantation. Preoperative laboratory tests: Hemoglobin (Hb) 13.4 g.dL−1; Hematocrit (Ht) 41.7%; Platelets 117,400/μL; INR 2.14; ratio of activated Partial Thromboplastin Time (aPTT ratio) 1.92; preoperative fibrinogen dosing was not performed.
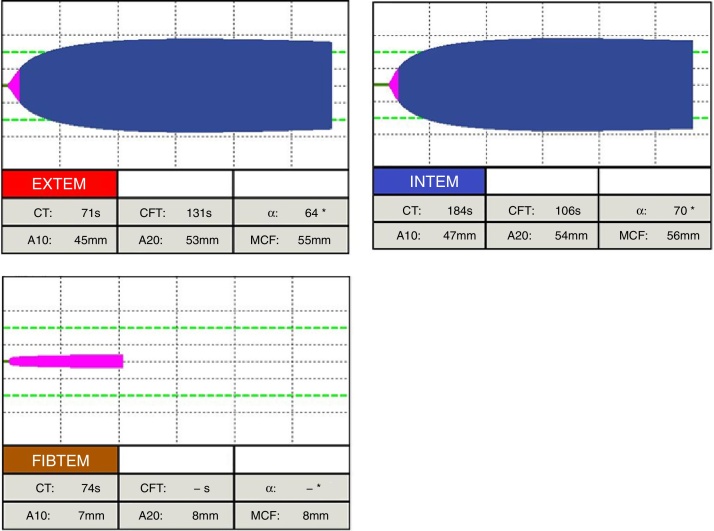
At the beginning of surgery, the ROTEM® parameters were all normal (Fig. 1). In the anhepatic phase (Fig. 2), EXTEM and INTEM remained normal, but FIBTEM presented reduction of A10 and MCF. Finally, in the neohepatic phase (Fig. 3), there was a slight alteration in the hypocoagulability of most of the parameters of EXTEM, INTEM and FIBTEM, besides a notable correction of CT in HEPTEM compared to CT of the INTEM.
Figure 1.
ROTEM® tests at the beginning of surgery.
EXTEM: (activation of extrinsic coagulation) CT (normal range 38–79 s), clotting time; CFT (normal range 34–159 s), clot formation time; α (normal range 63°–83°), alpha angle; A10 (normal range 43–65 mm) amplitude after 10 min; A20 (normal range 50–71 mm), amplitude after 20 min; MCF (normal range 50–72 mm), maximum clot firmness.
INTEM: (Activation of intrinsic coagulation) CT (normal range 100–240 s), clotting time; CFT (normal range 30–110 s), clot formation time; α (normal range 70°–83°), alpha angle; A10 (normal range 44–66 mm) amplitude after 10 min; A20 (normal range 50–71 mm), amplitude after 20 min; MCF (normal range 50–72 mm), maximum clot firmness.
FIBTEM: (Activation of extrinsic coagulation in the presence of cytochalasin D that inhibits platelets): A10 (normal range 7–23 mm) amplitude after 10 min; A20 (normal range 8–24 mm), amplitude after 20 min; MCF (normal range 9–25 mm), maximum clot firmness.
Figure 2.
ROTEM® tests in the anhepatic phase.
EXTEM: (activation of extrinsic coagulation) CT (normal range 38–79 s), clotting time; CFT (normal range 34–159 s), clot formation time; α (normal range 63°–83°), alpha angle; A10 (normal range 43–65 mm) amplitude after 10 min; A20 (normal range 50–71 mm), amplitude after 20 min; MCF (normal range 50–72 mm), maximum clot firmness.
INTEM: (Activation of intrinsic coagulation) CT (normal range 100–240 s), clotting time; CFT (normal range 30–110 s), clot formation time; α (normal range 70°–83°), alpha angle; A10 (normal range 44–66 mm) amplitude after 10 min; A20 (normal range 50–71 mm), amplitude after 20 min; MCF (normal range 50–72 mm), maximum clot firmness.
FIBTEM: (Activation of extrinsic coagulation in the presence of cytochalasin D that inhibits platelets): A10 (normal range 7–23 mm) amplitude after 10 min; A20 (normal range 8–24 mm), amplitude after 20 min; MCF (normal range 9–25 mm), maximum clot firmness.
Figure 3.
ROTEM® tests in the neohepatic phase.
EXTEM: (activation of extrinsic coagulation) CT (normal range 38–79 s), clotting time; CFT (normal range 34–159 s), clot formation time; α (normal range 63°–83°), alpha angle; A10 (normal range 43–65 mm) amplitude after 10 min; A20 (normal range 50–71 mm), amplitude after 20 min; MCF (normal range 50–72 mm), maximum clot firmness.
INTEM: (Activation of intrinsic coagulation) CT (normal range 100–240 s), clotting time; CFT (normal range 30–110 s), clot formation time; α (normal range 70°–83°), alpha angle; A10 (normal range 44–66 mm) amplitude after 10 min; A20 (normal range 50–71 mm), amplitude after 20 min; MCF (normal range 50–72 mm), maximum clot firmness.
FIBTEM: (Activation of extrinsic coagulation in the presence of cytochalasin D that inhibits platelets): A10 (normal range 7–23 mm) amplitude after 10 min; A20 (normal range 8–24 mm), amplitude after 20 min; MCF (normal range 9–25 mm), maximum clot firmness.
In surgery, the patient received 5000 mL of a lactated ringer's solution with 2% albumin and received no any transfusion of blood products. No hyperfibrinolysis was detected by ROTEM®, and therefore no antifibrinolytics were used. Laboratory tests at the end of transplantation: Hb 7.8 mg.dL−1; Ht 24.1%; Platelets 57810 μL; INR 2.48; aPTT ratio 1.85. At the end of the surgery, the patient was referred to the ICU with infusion of noradrenaline (0.05 mcg.kg−1.min−1). Hospital discharge occurred on the 8th postoperative day without receiving any blood transfusion.
Discussion
Current guidelines recommend that patients taking coumarins who require emergency surgery should have their anticoagulation reversed in order to reduce the risk of bleeding during surgery. This can be done with prothrombin complex concentrates or fresh frozen plasma where it is not available. The laboratory target varies from an INR of less than 2.0 or 1.5.6 However, in the case described the patient presented cirrhosis as a factor of distortion of INR as a reference for the state of coagulation. For this reason, the authors chose to conduct the management of hemostasis guided by ROTEM®.
At the beginning of surgery, despite a high initial INR, the parameters of ROTEM® were normal and the surgical field did not present excessive bleeding, so that the patient received no blood transfusion. Until the end of the transplantation, hemostasis evolved with a progressive tendency to hypocoagulation (as routinely observed even in patients who do not use coumarin), but without the occurrence of clinical coagulopathy or blood transfusion necessity. An outcome similar was described by Durila et al.9 Where they reported that warfarin-induced coagulopathy can be diagnosed by ROTEM® and in case it is normal, surgical procedures can be performed safely and without the need for PT-INR correction.
Hepner et al.10 observed in a prospective study that Thromboelastography (TEG®) was normal in patients taking warfarin, when the mean INR was 1.48 ± 0.3. Haas et al.11 also concluded that there is little correlation between conventional laboratory tests (PT and activated Partial Thromboplastin Time (aPTT) and ROTEM® tests (CT-EXTEM and CT-INTEM)) respectively, and the accepted limits for PT and aPTT can overestimate the intervention in coagulation.
On the other hand, Schmidt et al.8 demonstrated in one study the possibility of the ROTEM® test using the activated tissue factor, to identify an INR elevation with a high positive and negative predictive value. It is important not to exclude anticoagulated patients with warfarin from the analysis with viscoelastic tests. Blasi et al.12 reported in a prospective study with 54 patients in treatment with acenocoumarol after valve replacement heart surgery that ROTEM® is a viable method of diagnosing the insufficient reversal of anticoagulant therapy, by demonstrating that the EXTEM CT ≥ 84 s, predicts an INR > 1.5 in 93% of the cases, while the EXTEM CT < 84 s, predicts an INR < 1.5 in 100% of cases.
The INR remains the only validated laboratory tool for the titration of the anticoagulation level with coumarins. Coagulation deficit resulting from cirrhosis distorts INR as a parameter of anticoagulation adequacy and as a determinant of the need for transfusion of blood products. In scenarios like this, especially in the context of emergency, thromboelastometry can provide important information for patient management.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Tripodi A., Primignani M., Chantarangkul V., et al. An imbalance of pro- vs anti coagulation factors in plasma from patients with cirrhosis. Gastroenterology. 2009;137:2105–2111. doi: 10.1053/j.gastro.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 2.Lisman T., Porte R.J. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood. 2010;116:878–885. doi: 10.1182/blood-2010-02-261891. [DOI] [PubMed] [Google Scholar]
- 3.Northup P.G. Hypercoagulation in liver disease. Clin Liver Dis. 2009;13:109–116. doi: 10.1016/j.cld.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Wanless I.R., Wong F., Blendis L.M., et al. Hepatic and portal vein thrombosis in cirrhosis: possible role in development of parenchymal extinction and portal hypertension. Hepatology. 1995;21:1238–1247. [PubMed] [Google Scholar]
- 5.Dargaud Y., Hoffman M., Lefrapper L., et al. Bleeding risk in warfarinized patients with a therapeutic international normalized ratio: the effect of low factor IX levels. J Thromb Haemost. 2013;11:1043–1052. doi: 10.1111/jth.12244. [DOI] [PubMed] [Google Scholar]
- 6.Leissinger C.A., Blatt P.M., Hoots W.K., et al. Role of prothrombin complex concentrates in reversing warfarin anticoagulation: a review of the literature. Am J Hematol. 2008;83:137–143. doi: 10.1002/ajh.21046. [DOI] [PubMed] [Google Scholar]
- 7.Whiting D., Dinardo J.A. TEG and ROTEM: technology and clinical applications. Am J Hematol. 2014;89:228–232. doi: 10.1002/ajh.23599. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt D.E., Holmström M., Majeed A., et al. Detection of elevated INR by thromboelastometry and thromboelastography in warfarin treated patients and healthy controls. Thromb Res. 2015;135:1007–1011. doi: 10.1016/j.thromres.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 9.Durila M., Schützner J., Vymazal T. The role of rotational thromboelastometry (ROTEM) in the perioperative period in a warfarinized patient (case report) Rozhl Chir. 2016;95:329–332. [PubMed] [Google Scholar]
- 10.Hepner D.L., Concepcion M., Bhavani-Shankar K. Coagulation status using thromboelastography in patients receiving warfarin prophylaxis and epidural analgesia. J Clin Anesth. 2002;14:405–410. doi: 10.1016/s0952-8180(02)00373-2. [DOI] [PubMed] [Google Scholar]
- 11.Haas T., Spielmann N., Mauch J., et al. Comparison of thromboelastometry (ROTEM®) with standard plasmatic coagulation testing in paediatric surgery. Br J Anaesth. 2012;108:36–41. doi: 10.1093/bja/aer342. [DOI] [PubMed] [Google Scholar]
- 12.Blasi A., Muñoz G., De Soto I., et al. Reliability of thromboelastometry for detecting the safe coagulation threshold in patients taking acenocoumarol after elective heart valve replacement. Thromb Res. 2015;136:669–672. doi: 10.1016/j.thromres.2015.07.003. [DOI] [PubMed] [Google Scholar]