Abstract
Purpose
Sphenopalatine ganglion block is widely accepted in chronic pain; however it has been underestimated in post dural puncture headache treatment. The ganglion block does not restore normal cerebrospinal fluid dynamics but effectively reduces symptoms associated with resultant hypotension. When correctly applied it may avoid performance of epidural blood patch. The transnasal approach is a simple and minimally invasive technique. In the cases presented, we attempted to perform and report the ganglion block effectiveness and duration, using ropivacaine.
Clinical features
We present four obstetrics patients with post dural puncture headache, after epidural or combined techniques, with Tuohy needle 18G that underwent a safe and successful sphenopalatine ganglion block. We performed the block 24–48 h after dural puncture, with 4 mL of ropivacaine 0.75% in each nostril. In three cases pain recurred within 12–48 h, although less intense. In one patient a second block was performed with complete relief and without further recurrence. In the other two patients a blood patch was performed without success. All patients were asymptomatic within 7 days.
Conclusion
The average duration of analgesic effect of the block remains poorly defined. In the cases reported, blocking with ropivacaine was a simple, safe and effective technique, with immediate and sustained pain relief for at least 12–24 h.
Keywords: Postdural puncture headache, Sphenopalatine ganglion block, Postpartum care
Resumo
Justificativa e objetivo
O bloqueio do gânglio esfenopalatino é amplamente aceito em dor crônica; porém, esse bloqueio tem sido subestimado no tratamento de cefaleia pós-punção dural. O bloqueio do gânglio não restaura a dinâmica normal do líquido cefalorraquidiano, mas reduz de modo eficaz os sintomas associados à hipotensão resultante. Quando aplicado corretamente, pode evitar a realização de tampão sanguíneo epidural. A abordagem transnasal é uma técnica simples e minimamente invasiva. Nos casos apresentados, tentamos realizar o bloqueio do gânglio e relatar sua eficácia e duração usando ropivacaína.
Características clínicas
Apresentamos quatro pacientes de obstetrícia com cefaleia pós-punção dural, após técnica epidural ou técnicas combinadas, com agulha Tuohy (18 G), que foram submetidas ao bloqueio do gânglio esfenopalatino de forma segura e bem-sucedida. Realizamos o bloqueio após 24 a 48 horas da punção dural, com 4 mL de ropivacaína a 0,75% em cada narina. Em três casos, a dor voltou em 1–48 horas, embora menos intensa. Em uma paciente, um segundo bloqueio foi realizado com alívio completo e sem recorrência. Nas outras duas pacientes, um tampão sanguíneo foi realizado sem sucesso. Todas as pacientes estavam assintomáticas dentro de sete dias.
Conclusão
A duração média do efeito analgésico do bloqueio continua mal definida. Nos casos relatados, o bloqueio com ropivacaína foi uma técnica simples, segura e eficaz, com alívio imediato e prolongado da dor durante pelo menos 12–24 horas.
Palavras-chave: Cefaleia pós-punção dural, Bloqueio do gânglio esfenopalatino, Cuidados pós-parto
Introduction
The sphenopalatine ganglion (SPG) is a cranial ganglion and a complex neuronal centre. This structure lies in the Pterygopalatine fossa bilaterally, and receives multiple sensitive and autonomic afferents, sending efferent connections to the nasopharingeal cavity, meningeal structures, and probably displaying an important role in neural modulation.1, 2
The shenopalatine ganglion block (SPGB), is known for its efficacy in severe migraine and trigeminal neuralgia management, as in other cranial pain syndromes.1, 2, 3, 4 There are also some reports regarding the success of this block in post dural puncture headache (PDPH) secondary to diagnostic lumbar puncture,5 however reports regarding application of this procedure in post partum PDPH due to neuraxial techniques in labour are scarce.6, 7, 8, 9
The aim of SPGB in PDPH is symptomatic relief. The block does not change cerebral fluid production or circulation. Its analgesic effect is due to the trigeminal and parasympathetic block, therefore changing the meningeal vessel tone and nociception transmission.2, 5
It is important to note that even though the resolution of the dural tear is a spontaneous process that usually takes less than seven days, this analgesic procedure allows an asymptomatic recovery until then.7, 8, 9, 10
The PDPH in obstetric population has an incidence of 1%, and is associated with a low therapeutic efficacy.10, 11, 12, 13 Epidural blood patch (EBP) is presently considered the therapeutic gold standard, although it's an invasive technique, its associated with major complications and only with a 30% complete success rate (defined by Numeric Scale Rate [NSR 0–10] of 0 after management).10, 11, 12, 13
Although more studies are required, SPGB could be discussed as a first line option because it is effective and it may prevent the need for EBP, a more invasive technique, up to 69%.6, 7, 11
Several block techniques were described, but the transnasal approach is the simplest and least invasive.1, 2, 4 The only local anesthetic known to have been used in this procedure is lidocaine,5, 6, 7, 8, 9 however, there aren’t any reports regarding the duration of the analgesic effect.14
In the reported cases, we attempted to perform the ganglion block using ropivacaine. We present a small case series of transnasal SPGB in obstetric patients, in order to highlight our drug regiment, ropivacaine 0.75%, 2 mL in each nostril plus 2 mL (after 10 min), duration of the block analgesic effect, its safety and effectiveness in the postpartum period.
Consent for publication
The patients reviewed the case report and gave written permission to the authors to publish the report. All the authors described in the case report participated in the care of the patients.
Case description
Case 1
27 years old woman with obesity and gestational diabetes, who had an epidural catheter inserted for pain relief during labour. The technique was performed with an 18G Tuohy needle without immediate complications. Twenty four hour postpartum she complained about occipital headache that was worse when standing and with head motion and that irradiated to the neck. Despite that, there was no dural tear noticed during the technique. The symptoms suggested a PDPH, and according to the International Classification of Headache Disorders criteria this was the best accounted diagnosis. Conservative treatment was started, oral caffeine, hydration and ketorolac, without improvement. Twenty four hours later, as she rated her pain with similar intensity, the SPGB was performed with immediate relief of pain to a NSR 0 in one hour span. Hospital discharge was allowed. When contacted by phone, seven days later, she remained asymptomatic.
Case 2
During epidural catheter insertion, for labour analgesia in a healthy 27 year old woman, a positive aspiration test is noticed (18G Tuohy needle). Twenty four hours after puncture the patient complained about headache, compatible with PDPH, NRS 6 when lying and NRS 8 supine. The SPGB was performed with total pain relief NRS 0. Twelve hours after the blockage, pain recurrence, NSR 4, when in supine position, motivated a second SPGB that completely relieved symptoms with no pain recurrence. She was discharged the next day. In the follow up contact, seven days later, she reported no symptoms.
Case 3
A healthy 26 year old woman that underwent a combined anesthesia for an urgent caesarean section (demanded by a failure to progress in labour). The combined anesthesia was technically difficult, with multiple punctures with an 18G Tuohy needle and 25G Whitacre. Twenty four hours later, she reported head and neck pain, NRS 4 when lying and 6 when standing. As there were technical difficulties and multiple punctures, and the pain pattern was suggestive of PDPH we performed a SPGB with immediate regression of symptoms to NSR 0. The patient maintained asymptomatic throughout the first 48 h after the blockage but later reported headache NSR 6 when standing. Rather than repeating SPGB, the anesthesiologist attempted to relieve the pain with EBP with 20 mL of autologous blood, ineffectively. Patient refused a second blood patch. Despite a mild pain score, NRS 3, the patients requested home discharge the same day.
Case 4
A healthy 21 year old woman, who received combined analgesia for pain relief during labour (18G Tuohy needle and 27G Whitacre). A positive aspiration test is noticed when introducing the catheter. Twenty-four hours after eutocic labour, she mentioned headache, NSR 3 that was worse when standing, NRS 7. A SPGB was performed with immediate regression of pain to NSR 0. The patient was pain free for 48 h until she reported a progressive increase in headache intensity up to NRS 8 when standing. Rather than repeating SPGB, the anesthesiologist attempted to relieve the pain with EBP with 20 mL of autologous blood, ineffectively (NRS 4–6). The patient refused a second blood patch. She became asymptomatic the next day and was discharged. In the follow up contact, 7 days later, she reported no symptoms.
Discussion
The SPG locates approximately 3 mm from mucosal surface of posterior wall of the nasal cavity, at the middle turbinate level. To perform the SPGB the patient should be in supine position, with a slight cervical extension. Two cotton tipped applicators should be soaked in 2 mL of anesthetic drug and introduced smoothly and rapidly up to the posterior wall of both nostrils. After 10 min, 2 mL of anesthetic drug should be instilled over the applicator cable, thereafter rotating the applicators in order to confirm the correct location, where they are well tolerated by the patient. The applicator must remain in the nasal cavity for 15–20 min (Fig. 1). In case of headache recurrence, this technique can be repeated.6
Figure 1.
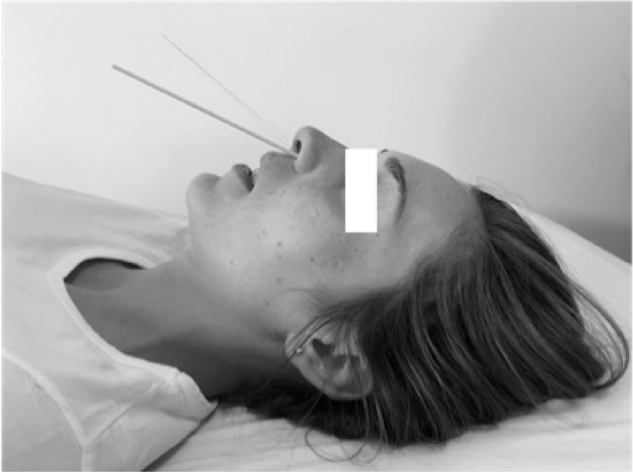
Cotton tipped applicators in correct position at middle turbinate level at posterior nostril wall during performance of sphenopalatine gangion block.
Due to its successful, yet unpredictable analgesic effect duration, this procedure can be taught to the patient, allowing repeated self-ganglion block in an ambulatory setting and earlier discharge home.1, 2, 3, 4, 5, 6
No major complications associated with this technique have been described. Nevertheless, minor bleeding due to traumatic applicator introduction, paresthesia and initial discomfort of the nasopharynx, related to the spread of anesthetic, have been reported.1, 2, 3, 4, 5, 15
The four cases presented obstetric patients with symptoms compatible with PDPH who underwent a safe and successful SPGB, within 24–48 h after puncture. In all cases the SPGB led to a complete pain relief, NRS 0, in one hour span. In Case 1 there was no pain recurrence. In Case 2, the pain recurred after 12 h, although less intense, and a second block also provided complete pain relief. In Case 3 and 4, SPGB had a prolonged efficacy of 48 h. Both patients 3 and 4 performed an EBP after 48 h with only partial symptomatic relief. As expected, and according to the natural disease course, all patients were asymptomatic within 7 days of dural puncture.
The pain relief of 12–48 h was the expected for the pharmacokinetic characteristics of ropivacaine.
Heterogeneity of treatment, due to patient management by different professionals, limited SPGB repetition and education in all patients.
The average duration of analgesic effect of SPGB remains poorly defined and is currently the main technical limitation as the anesthetic spread in the nasal cavity is unpredictable and dependent on anatomy. Which factors are associated with technical success or failure; how different anesthetic drugs act in this context; how effective are block repetitions; how conservative therapy or EBP are affected by SPGB, these are some of the questions that need to be addressed in further studies in order to produce high evidence recommendations.
These cases highlight the effectiveness and safety of SPGB with ropivacaine on immediate and sustained pain relief for at least 12–24 h in obstetric context.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Khonsan S., Villablanca P., Emerson J., et al. Clinical functional anatomy of the pterygopalatine ganglion, cephalgia and related dysautonomias: a review. Surg Neurol Int. 2013;4:422–428. doi: 10.4103/2152-7806.121628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gharaei H., Nabi N. Sphenopalatine ganglion block a jack of all trades block. J Anesth Crit Care Open Access. 2015;3:00091. [Google Scholar]
- 3.Piagkou M., Demestisha T., Troupis T. The pterygopalatine ganglion and its role in various pain syndromes: from anatomy to clinical practice. Pain Pract. 2012;12:399–412. doi: 10.1111/j.1533-2500.2011.00507.x. [DOI] [PubMed] [Google Scholar]
- 4.Spencer R. Technique for sphenopalatine ganglion block. Reg Anesth. 1997;22:483–489. doi: 10.1016/s1098-7339(97)80040-5. [DOI] [PubMed] [Google Scholar]
- 5.Kent S., Mehaffey G. Transnasal sphenopalatine ganglion block for the treatment of postdural puncture headache in the ED. Am J Emerg Med. 2015;3:00091. doi: 10.1016/j.ajem.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 6.Cohen S., Sakr A., Katyal S., et al. Sphenopalatine ganglion block for postdural puncture headache. Anaesthesia. 2009;64:574–575. doi: 10.1111/j.1365-2044.2009.05925.x. [DOI] [PubMed] [Google Scholar]
- 7.Cohen S., Ramos D., Grubb W. Sphenopalatine ganglion block a safer alternative to epidural blood patch for postdural puncture headache. Reg Anesth Pain Med. 2014;39:563. doi: 10.1097/AAP.0000000000000172. [DOI] [PubMed] [Google Scholar]
- 8.Cohen S., Trnovski S., Zada Y. A new interest in an old remedy for headache and backache for our obstetric patients: a sphenopalatine ganglion block. Anaesthesia. 2000;56:585–610. [PubMed] [Google Scholar]
- 9.Sakr A., Rah K., Cohen S. Paper presented at American Society of Regional Anesthesia and Pain Medicine 12th Annual Pain Medicine Meeting, A358. 2013. Can we offer sphenopalatine ganglion block for our obstetric patients following accidental dural puncture? [Google Scholar]
- 10.Turnbull D., Shepherd D. Post-dural puncture headache: pathogenesis, prevention and treatment. Br J Anaesth. 2003;91:718–729. doi: 10.1093/bja/aeg231. [DOI] [PubMed] [Google Scholar]
- 11.Darvish B., Gupta A., Alahuhta S., et al. Management of accidental dural puncture and post-dural puncture headache after labour: a Nordic survey. Acta Anaesthesiol Scand. 2011;55:46–53. doi: 10.1111/j.1399-6576.2010.02335.x. [DOI] [PubMed] [Google Scholar]
- 12.D’Angelo J., Booth L., Pan H., et al. A retrospective review of an epidural blood patch database: the incidence of epidural blood patch associated with obstetric neuraxial anesthetic techniques and the effect of blood volume on efficacy. Int J Obstet Anesth. 2017;29:10–17. doi: 10.1016/j.ijoa.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Schroede K. ARSA News, Publication of American Society of Regional Anesthesia and Pain Medicine; 2014. Postdural puncture headaches: what works, what doesn’t, and what is new? [Google Scholar]
- 14.Berde C., Strichartz G. In: Anesthesia. 7th ed. Miller R.D., editor. Churchill Livingstone Inc; New York: 2016. Local anesthetics; pp. 913–939. [Google Scholar]
- 15.Candido K., Massey S. A novel revision to the classical transnasal topical sphenopalatine ganglion block for the treatment of headache and facial pain. Pain Physician. 2013;16:769–778. [PubMed] [Google Scholar]


