Abstract
Background and objectives
A transversus abdominis plane block is a peripheral block method that has been used successfully for pain relief after total abdominal hysterectomy. However, the effects of the combination of the transversus abdominis plane block and general anesthesia on analgesic and anesthetic requirements remain unclear. This randomized placebo-controlled study is aimed to evaluate the effects of transversus abdominis plane block on analgesic and anesthetic consumption during total abdominal hysterectomy under general anesthesia.
Methods
Sixty-six women undergoing total abdominal hysterectomy were randomized into two groups to receive general anesthesia alone (control group) or with transversus abdominis plane block using 20 mL of 0.25% bupivacaine (transversus abdominis plane group). Intraoperative remifentanil and sevoflurane consumption were recorded. We also evaluated the postoperative pain, nausea, quality of recovery scores and rescue analgesic requirement during postoperative 24 hours.
Results
The total remifentanil and sevoflurane consumption is significantly lower in transversus abdominis plane group; respectively mean (SD) 0.130 (0.25) vs. 0.094 (0.02) mcg.kg−1.min−1; p < 0.01 and 0.295 (0.05) vs. 0.243 (0.06) mL.min−1; p < 0.01. In the postoperative period, pain scores were significantly reduced in transversus abdominis plane group soon after surgery; median (range) 6 (2–10) vs. 3 (0–5); p < 0.001, at 2 h (5 [3–9] vs. 2.5 [0–6]; p < 0.001), at 6 h (4 [2–7] vs. 3[0–6], p < 0.001), at 12 h (3.5 [1–6] vs. 2 [1–5]; p = 0.003). The patients in the transversus abdominis plane group had significantly higher QoR-40 scores 190.5 (175–197) vs. 176.5 (141–187); p < 0.001).
Conclusion
Combining transversus abdominis plane block with general anesthesia can provide reduced opioid and anesthetic consumption and can improve postoperative pain and quality of recovery scores in patients undergoing total abdominal hysterectomy.
Keywords: Anesthesia, general; Anesthesia, regional; Transversus abdominis plane block; Hysterectomy
Resumo
Justificativa e objetivos
O bloqueio do plano transverso abdominal é um método de bloqueio periférico que tem sido usado com sucesso para alívio da dor após histerectomia abdominal total. No entanto, os efeitos da combinação do bloqueio do plano transverso abdominal e da anestesia geral sobre a necessidade de analgésico e anestésico ainda não estão claros. Este estudo randômico e controlado com placebo tem como objetivo avaliar os efeitos do bloqueio do plano transverso abdominal sobre o consumo de analgésico e anestésico durante histerectomia abdominal total sob anestesia geral.
Métodos
Foram randomizadas em dois grupos 66 mulheres submetidas à histerectomia abdominal total para receber apenas anestesia geral (grupo controle) ou associada a bloqueio do plano transverso abdominal usando 20 mL de bupivacaína a 0,25% (grupo plano transverso abdominal). O consumo de remifentanil e sevoflurano no período intraoperatório foi registrado. Também avaliamos a dor pós-cirurgia, náusea, qualidade dos escores de recuperação e necessidade de analgésico de resgate durante as 24 horas de pós-operatório.
Resultados
O consumo total de remifentanil e sevoflurano foi significativamente menor no grupo plano transverso abdominal, respectivamente, média (DP): 0,130 (0,25) vs. 0,094 (0,02) mcg.kg−1.min−1; p < 0,01 e 0,295 (0,05) vs. 0,243 (0,06) mL.min−1; p < 0,01. No pós-operatório, os escores de dor foram significativamente reduzidos no grupo plano transverso abdominal logo após a cirurgia; mediana (intervalo): 6 (2-10) vs. 3 (0-5); p < 0,001, em 2 h (5 [3-9] vs. 2,5 [0-6]; p < 0,001), em 6 h (4 [2-7] vs. 3 [0-6], p < 0,001), em 12 h (3,5 [1-6] vs. 2 [1-5]; p = 0,003). As pacientes do grupo plano transverso abdominal apresentaram escores QoR-40 significativamente maiores: 190,5 (175-197) vs. 176,5 (141-187); p < 0,001).
Conclusão
A combinação de bloqueio do plano transverso abdominal e anestesia geral pode proporcionar um consumo reduzido de opioides e anestésicos e melhorar a dor pós-cirúrgica e a qualidade dos escores de recuperação em pacientes submetidas à histerectomia abdominal total.
Palavras-chave: Anestesia, geral; Anestesia, regional; Bloqueio do plano transverso abdominal; Histerectomia
Introduction
Improving the quality of perioperative care in surgical patients is an important aim of the anesthesiologist. Balancing anesthesia with anesthetic and analgesic drugs is the preferred technique in maintaining stability during surgical procedures. The commonly used combination is inhalation anesthetics with opioid drugs that are accepted as beneficial agents that prevent the response of noxious stimuli. Although there are some undesired effects of these agents like prolonged recovery from anesthesia, nausea and vomiting, bowel dysfunction, respiratory depression, increased postoperative pain, the rise in environmental pollution and health cost.1, 2, 3, 4, 5 Therefore minimizing the intraoperative anesthetic and analgesic consumption is prominent focus of the perioperative physicians.
With the increasing evidence concerning postoperative complications related to neuroaxial analgesia, and the safety of peripheral regional anesthesia under ultrasound guidance, multimodal analgesia with peripheral nerve blocks have become more popular.6 A Transversus Abdominis Plane (TAP) block is a peripheral block method that anesthetizes the somatic nerves underlying the abdominal wall, which is an important component of the pain during abdominal incisions.7 TAP block has been used successfully for pain relief after Total Abdominal Hysterectomy (TAH).8 However, the effects of the combination of the TAP block and general anesthesia on analgesic and anesthetic requirements remain unclear.
To the best of our knowledge, this randomized, controlled trial is the first to focus on the effects of the TAP block on analgesic and anesthetic consumption during TAH procedures. We hypothesized that the TAP block would decrease the remifentanil and sevoflurane requirements in patients undergoing TAH. The secondary aim of this study was to assess the quality of recovery, postoperative pain, nausea, and vomiting scores.
Materials and methods
This study was performed as a prospective, randomized, single-blind, parallel-group, placebo-controlled clinical trial in patients undergoing TAH surgery. This study was approved by the Gaziosmanpasa University Clinical Research Ethics Board (14-KAEK-155, 22/07/2014) and registered at www.clinicaltrials.gov (NCT02296619).
After obtaining the patients’ written informed consents, we enrolled the patients (18–65 years) with American Society of Anesthesiologist (ASA) physical Status I and II who were scheduled for elective TAH at the Gaziosmanpasa University School of Medicine Hospital. The eligibility for participation was evaluated and determined by an anesthesiologist not involved in the study. The patients who had allergies to local anesthetics, drug abuse or addiction, and bleeding tendencies were excluded from the study. The patients were randomized into two groups (TAP or Control group) on a 1:1 ratio using a computer-generated random table. The patient's assigned group information was elicited from a sealed opaque envelope by the anesthesiologist who performed the TAP bloc and was not involved in further data collection or patient care. The patients and both the surgical and anesthesia teams, who were responsible for patient care, were blinded from the group assignments.
In this study, no premedication was given to the patients before the operation. All patients were monitored using electrocardiography, pulse-oximetry, capnography, thermometer, noninvasive blood pressure, and Bispectral Index (BIS) in the operating room and received a standard anesthesia induction with intravenous propofol (1 mg.kg−1) and fentanyl (1 mcg.kg−1). After muscle relaxation with rocuronium (0.5 mg.kg−1), tracheal intubation was performed and mechanical ventilation was applied while maintaining the end-tidal CO2 between 30 and 35 mmHg.
After the intubation, the bilateral TAP block with ultrasound (MyLab Gold, Easote, Genova) guidance was performed in the patients who were assigned to the TAP block group, as described by Hebbard et al.9 The external oblique abdominis, the internal oblique abdominis, and the transversus abdominis muscles were visualized with a linear 6–18 MHz ultrasound transducer and 20 mL of 0.25% bupivacaine was injected into the area between the internal oblique and transversus abdominis muscles on each side via a 90 mm echogenic block needle (Pajunk, Geisingen, Germany). The bilateral transversus abdominis plane visualization with an ultrasound transducer without drug injection was also performed in the patients assigned to the control group.
At 15 min after the TAP block or control procedure, the anesthesia team was changed with another team blinded to the patient's allocation and allowed the skin incision. The anesthesia was maintained with sevoflurane in 50% oxygen-air mixture at 3 L.min−1 flow rate. The sevoflurane was started at 2% concentration and adjusted to obtain an adequate level of anesthesia by titrating the concentration according to the BIS monitoring (BIS XP, A-2000, Version 3.31, Aspect Medical Systems, Newton, Mass, USA) to keep the BIS value between 40 and 60 during surgery. The sevoflurane concentration was changed by 0.2 units when the BIS value was >60 or <40 for more than 1 min. Remifentanil infusion was started at 0.1 mcg.kg−1.min−1 and the dose was adjusted every 5 min to maintain the Mean Arterial blood Pressure (MAP) value within 20% of baseline, but not below 60 mmHg, and the heart rate within 20% of baseline, but not below 50 beats.min−1. The remifentanil dose was changed in 0.02 mcg.kg−1.min−1 increments, if necessary. If hypotension, hypertension, bradycardia, or tachycardia persisted longer than 2 min despite remifentanil adjustment, we intravenously administered ephedrine (5 mg) for hypotension, atropine (0.5 mg) for bradycardia, glycerol trinitrate (0.1 mg) for hypertension, and metoprolol (1 mg) for tachycardia.
Approximately 30 min before ending the operation, all patients received paracetamol (1 g) intravenously.
At the end of the surgery, sevoflurane and remifentanil were discontinued and the muscle relaxant was antagonized with neostigmine (0.04 mg.kg−1) and atropine (0.01 mg.kg−1).
All patients were admitted to the Post-Anesthesia Care Unit (PACU) at the end of surgery and were monitored by a nurse who was blinded to the patient's group. The patient's pain was evaluated using a 10 cm Visual Analog Scale (VAS) and morphine was administered in 2 mg increments up to a total dose 15 mg when the VAS score was higher than 4.
Postoperative Nausea and Vomiting (PONV) were treated with ondansetron (4 mg) up to a total dose of 16 mg, as needed. The patients were transferred to their rooms according to the institutional PACU discharge criteria and the anesthesia team evaluated the patients during the first postoperative 24 h. All patients received a standard analgesic protocol consisting of 1 g of metamizol sodium every 8 h and morphine (0.05 mg.kg−1) as a rescue analgesic when the VAS > 4.
The primary outcome was the total intraoperative remifentanil consumption and recorded during surgery. The other primary outcome was the total sevoflurane consumption and it was calculated as mL.min−1 using the recorded fresh gas flows and applied concentration of the sevoflurane, including the duration during surgery according to this formula previously described by Biro10:Fluid Volatil Agent (mL) = [Fresh Gas Flow (mL.dk−1) × Volatil Agent Concentration (Vol %) × Anesthesia Duration (min)]/[Saturated Gas Volume (mL.mL−1) × 100]
Intraoperative hemodynamic variables were evaluated from the noninvasive arterial blood pressure and heart rates during anesthesia. The secondary outcomes were postoperative pain and PONV at 0 (15 min after extubation), 2, 6, 12, and 24 h, which were recorded by the anesthesia team unaware of the patient's group. Pain was evaluated with the VAS and PONV was measured with a 5 point Likert scale (1:always to 5:never). The other secondary outcome concerning the quality of recovery was assessed by the Quality of Recovery-40 questionnaire (QoR-40) at 24 h after surgery. The QoR-40 was developed by Myles et al. and recently validated and translated into the Turkish language.11, 12 The QoR-40 is a self-rated, five-point Likert scale (1:none of the time to 5:all of the time) questionnaire comprised of 40 items with five subscales including emotional state (9 items), physical comfort (12 items), patient support (7 items), physical independence (5 items), and pain (7 items). The total score can vary from 40 to 200.
Based on a previous study, we estimated the total intraoperative remifentanil consumption and Standard Deviation (SD).13 We then hypothesized that the intraoperative total remifentanil consumption could be reduced 30% in patients with TAP blocks. We calculated that a minimum of 26 patients would be required per group assuming a two-sided type 1 error of 0.05 (α = 0.05) and a power of 0.80 (β = 0.02). We recruited 66 patients to achieve an adequate power in case of patient drop outs.
All statistical analyses were performed with SPSS software version 20.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were presented as mean (±SD) for continuous data, as median (range) for ordinal data, or as frequencies for categorical data. Normality was tested using a one-sample Kolmogorov–Smirnov test. Continuous variables were compared with independent samples t-tests if normally distributed, otherwise with Mann–Whitney U tests. Categorical variables were analyzed using Pearson's Chi-square or Fischer's exact tests. Repeated measures were evaluated by repeated measures analysis of variance. p-Values of <0.05 were considered to be statistically significant.
Results
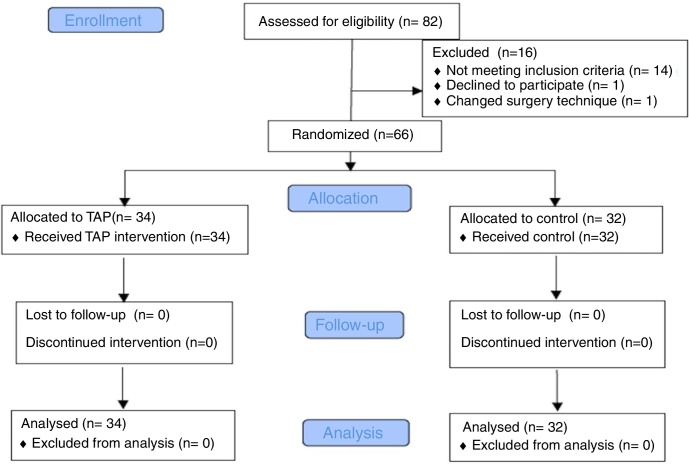
Seventy patients scheduled for TAH between November 2014 and February 2016 were assessed for eligibility to participate and 66 were enrolled in the study. The consort flow diagram for the study is shown in Fig. 1.
Figure 1.
Study flow.
Patients and perioperative demographic characteristics were comparable in the two groups (Table 1). In all patients, the layers of the abdominal wall were easily visualized and the TAP block was performed after one attempt without complications in the TAP group.
Table 1.
Demographic and operative characteristics.
| Parameter | Control (n = 32) |
TAP (n = 34) |
p-Value |
|---|---|---|---|
| Age (year) | 48.9 ± 4.7 | 46.6 ± 4.6 | 0.12 |
| ASA classification (I/II) | 16/16 | 20/14 | 0.47 |
| Height (cm) | 161.5 ± 4.1 | 162.2 ± 5.9 | 0.59 |
| Weight (kg) | 77.0 ± 11.9 | 75.2 ± 12.7 | 0.56 |
| Body Mass Index (kg.m−2) | 29.5 ± 4.4 | 28.5 ± 4.7 | 0.39 |
| Duration of surgery (min) | 125.00 ± 32.5 | 136.62 ± 35.7 | 0.38 |
| Duration of anesthesia (min) | 134.2 ± 33.6 | 148.8 ± 35.9 | 0.18 |
Data are presented as mean ± SD or number.
The total intraoperative remifentanil dose is presented as mcg.kg−1.min−1 and remifentanil consumption was significantly higher in the control group (0.13 vs. 0.094 mcg.kg−1.min−1; p < 0.01) (Table 2). The total sevoflurane dose was also significantly higher in the control group (0.295 vs. 0.243 mL.min−1; p < 0.01) (Table 2). There were no statistically significant differences between the two groups’ hemodynamic variables (both heart rate and MAP) at any time (Two way repeated measures ANOVA; p = 0.207 for heart rate and p = 0.466 for MAP).
Table 2.
Intraoperative cumulative analgesic and anesthetic consumption.
| Control | TAP | p-Value | |
|---|---|---|---|
| Remifentanil (mcg.kg−1.min−1) | 0.130 ± 0.25 | 0.094 ± 0.02 | 0.00a |
| Sevoflurane (mL.min−1) | 0.295 ± 0.05 | 0.243 ± 0.06 | 0.00a |
Data are presented as mean ± SD.
Significantly difference (Independent sample t-test).
VAS scores in the TAP group were significantly lower at 0, 2, 6 and 12 h, but not at 24 h (Table 3). The cumulative morphine consumption in the PACU was 0 (0–6) mg in TAP group and 4 (0–12) mg in Control group (p < 0.001). The rescue analgesic requirement level was significantly higher in the control group (2 [0–4] in Control group vs. 0 [0–2] in Tap group; p < 0.001). There was no significant difference with respect to PONV scores between groups (3.4 [2–5] vs. 3.4 [1–5]; p = 0.9).
Table 3.
Patients postoperative VAS scores.
| 0 | 2 h | 6 h | 12 h | 24 h | |
|---|---|---|---|---|---|
| Control | 6 (2–10) | 5 (3–9) | 4 (2–7) | 3.5 (1–6) | 3 (0–4) |
| TAP | 3 (0–5) | 2.5 (0–6) | 3 (0–6) | 2 (1–5) | 2 (1–4) |
| p | <0.001a | <0.001a | <0.001a | 0.003a | 0.25 |
Data are presented as median (range). 0, 15 min after extubation; 2 h, 2 h after surgery; 6 h, 6 h after surgery; 12 h, 12 h after surgery; 24 h, 24 h after surgery.
Significantly difference (Mann Whitney-U test).
The patients in the TAP group had significantly higher median QoR-40 global, physical comfort, physical independence, pain, emotional status, and support scores at 24 hours after TAH compared with the controls (Table 4).
Table 4.
QoR-40 dimensions and global scores.
| Control | TAP | p-Value | |
|---|---|---|---|
| Physical independence | 21.5 (15–25) | 24 (20–25) | <0.001a |
| Physical comfort | 50.5 (37–56) | 55.5 (44–58) | <0.001a |
| Pain | 29 (23–34) | 33.5 (27–35) | <0.001a |
| Support | 33 (24–35) | 34 (33–35) | <0.001a |
| Emotional status | 42 (34–45) | 44 (41–45) | <0.001a |
| Global QoR-40 score | 176.5 (141–187) | 190.5 (175–197) | <0.001a |
Data are presented as median (range).
Statistically difference (Mann Whitney-U test).
Discussions
This randomized controlled study demonstrated that a TAP block combined with general anesthesia maintained reduced intraoperative opioid and anesthetic consumption in patients undergoing TAH. The TAP block also improved the quality of recovery at 24 h after surgery and the postoperative pain scores during the first 6 h with a decreased requirement for rescue analgesics.
A hysterectomy is one of the most commonly performed gynecologic surgical procedures and can cause considerable postoperative pain, which arises from the compounded effects of multiple factors. The parietal component of the pain after a trans abdominal intervention originates from the surgical incision of the abdominal wall and the sensory afferents that lie in the transversus abdominis plane that can be blocked with the TAP block method.8 Several studies have demonstrated the analgesic efficacy of the TAP block for postoperative pain in patients undergoing hysterectomy.8, 14, 15 However, the reduction in intraoperative opioid consumption with the TAP block during abdominal surgery has been investigated in the few studies.16, 17, 18 Bhattacharjee et al.18 evaluated the intraoperative fentanyl requirement as a secondary outcome of their study and showed that preemptively performed TAP block reduced the intraoperative opioid (fentanyl) consumption compared with placebo, similar to our results. In this study, differing from us, the anesthetic consumption was not evaluated by Bhattacharjee et al.18 In another study, it was shown that the fentanyl requirement was significant decrease during abdominal hysterectomy in the pre-incisional TAP block group.19 However, this study did not give any information about the anesthetic gas consumption.19 According to our best knowledge, there is no randomized clinical study that present the decreased sevoflurane requirement with a preemptive TAP block during TAH. On the other hand, Kokulu et al.20 showed that the TAP block could modulate the anesthetic consumption during laparoscopic cholecystectomy. During surgery, to ensure hemodynamic stability, adequate analgesia is mandatory. This can be provided by a satisfactory anesthesia with balanced anesthetic and analgesic drugs. Moreover, it is known that preemptive regional anesthesia could modulate the anesthetic and analgesic requirements during surgery.18, 19 Furthermore, Tsuchiya et al.21 applied a TAP block in combination with general anesthesia in high-risk abdominal surgery and found that this provided better intraoperative hemodynamic control than general anesthesia alone with reduced sevoflurane consumption. Recently, Abu Elyazed et al.22 concluded that TAP block could be attenuation factor for the neuroendocrine stress response induced by surgery. And also, by several studies it was shown that the local anesthetic drugs could reduce the MAC of anesthetic agents.23, 24 So, in our study, we suggest that the reduction of sevoflurane consumption may arise from two reasons; TAP block's blocking effect on the transmission of sensorial impulses and neuroendocrine stress response induced by surgery. The second is the local anesthetic's reduction effect on the MAC value of the anesthetic agent.
Additionally, in the current study, we confirmed the beneficial effect of a TAP block on postoperative pain during the first 6 h after the operation. Postoperative opioid requirements were also higher in the control group, and PONV was not different between groups, confirming previous reports.8, 18 Intraoperative opioid and anesthetic consumptions are important risk factors for PONV, unless there are other determining properties that may include patient and surgical-specific factors.2 Therefore, the anticipated effect on PONV may not appear in the current study. Despite the TAP block's useless effect on PONV, the improved postoperative quality of recovery scores at 24 h after surgery were observed in our study. Although the relationship of PONV with patient dissatisfaction and discomfort is notable, De Oliveira et al.15 presented an inverse linear correlation between opioid consumption and the quality of recovery with a similar antiemetic consumption after laparoscopic hysterectomies. These findings support the importance of pain management in a patient's quality of recovery.
There are several limitations in this study. First, the operations were performed by different two surgeons, but the surgeons used the same technique. Second, the preoperative risk factors of the patients for POVN were not evaluated. Third, to avoid from bias, we did not evaluate the loss of sensation in the abdominal wall after TAP block at the end of the surgery that could led to awareness of patient's allocated group.
Conclusion
In patients undergoing TAH, the combination of an ultrasound guided TAP block and general anesthesia can provide reduced opioid and anesthetic consumption, and can improve postoperative pain and quality of recovery scores.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The departmental funding only. All authors declare that have no conflict of interest. The abstract of this study was presented in ARUD 2017 Balkan States Anesthesia Days – IV, 2017, Sarajevo, Bosnia and Herzegovina.
References
- 1.Guignard B., Bossard A.E., Coste C., et al. Acute opioid tolerance: intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology. 2000;93:409–417. doi: 10.1097/00000542-200008000-00019. [DOI] [PubMed] [Google Scholar]
- 2.Gan T.J., Meyer T., Apfel C.C., et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg. 2003;97:62–71. doi: 10.1213/01.ane.0000068580.00245.95. [DOI] [PubMed] [Google Scholar]
- 3.Kurz A., Sessler D.I. Opioid-induced bowel dysfunction: pathophysiology and potential new therapies. Drugs. 2003;63:649–671. doi: 10.2165/00003495-200363070-00003. [DOI] [PubMed] [Google Scholar]
- 4.Lee L.A., Caplan R.A., Stephens L.S., et al. Postoperative opioid induced respiratory depression: a closed claims analysis. Anesthesiology. 2015;122:659–665. doi: 10.1097/ALN.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 5.Epstein R.H., Dexter F., Maguire D.P., et al. Economic and environmental considerations during low fresh gas flow volatile agent administration after change to a nonreactive carbon dioxide absorbent. Anesth Analg. 2016;122:996–1006. doi: 10.1213/ANE.0000000000001124. [DOI] [PubMed] [Google Scholar]
- 6.Belavy D., Janda M., Baker J., et al. Epidural analgesia is associated with an increased incidence of postoperative complications in patients requiring an abdominal hysterectomy for early stage endometrial cancer. Gynecol Oncol. 2013;131:423–429. doi: 10.1016/j.ygyno.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 7.Rafi A.N. Abdominal field block: a new approach via the lumbar triangle. Anaesthesia. 2001;56:1024–1026. doi: 10.1046/j.1365-2044.2001.02279-40.x. [DOI] [PubMed] [Google Scholar]
- 8.Carney J., McDonnell J.G., Ochana A., et al. The transversus abdominis plane block provides effective postoperative analgesia in patients undergoing total abdominal hysterectomy. Anesth Analg. 2008;107:2056–2060. doi: 10.1213/ane.0b013e3181871313. [DOI] [PubMed] [Google Scholar]
- 9.Hebbard P., Fujiwara Y., Shibata Y., et al. Ultrasound-guided transversus abdominis plane (TAP) block. Anaesth Intensive Care. 2007;35:616–617. [PubMed] [Google Scholar]
- 10.Biro P. Calculation of volatile anaesthetics consumption from agent concentration and fresh gas flow. Acta Anaesthesiol Scand. 2014;58:968–972. doi: 10.1111/aas.12374. [DOI] [PubMed] [Google Scholar]
- 11.Myles P.S., Weitkamp B., Jones K., et al. Validity and reliability of a postoperative quality of recovery score: the QoR-40. Br J Anaesth. 2000;84:11–15. doi: 10.1093/oxfordjournals.bja.a013366. [DOI] [PubMed] [Google Scholar]
- 12.Karaman S., Arici S., Dogru S., et al. Validation of the Turkish version of the quality of recovery-40 questionnaire. Health Qual Life Outcomes. 2014;12:8. doi: 10.1186/1477-7525-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jokela R., Ahonen J., Tallgren M., et al. Premedication with pregabalin 75 or 150 mg with ibuprofen to control pain after day-case gynaecological laparoscopic surgery. Br J Anaesth. 2008;100:834–840. doi: 10.1093/bja/aen098. [DOI] [PubMed] [Google Scholar]
- 14.Gasanova I., Grant E., Way M., et al. Ultrasound-guided transversus abdominal plane block with multimodal analgesia for pain management after total abdominal hysterectomy. Arch Gynecol Obstet. 2013;288:105–111. doi: 10.1007/s00404-012-2698-3. [DOI] [PubMed] [Google Scholar]
- 15.De Oliveira G.S., Jr., Milad M.P., Fitzgerald P., et al. Transversus abdominis plane infiltration and quality of recovery after laparoscopic hysterectomy: a randomized controlled trial. Obstet Gynecol. 2011;118:1230–1237. doi: 10.1097/AOG.0b013e318236f67f. [DOI] [PubMed] [Google Scholar]
- 16.El-Dawlatly A.A., Turkistani A., Kettner S.C., et al. Ultrasound-guided transversus abdominis plane block: description of a new technique and comparison with conventional systemic analgesia during laparoscopic cholecystectomy. Br J Anaesth. 2009;102:763–767. doi: 10.1093/bja/aep067. [DOI] [PubMed] [Google Scholar]
- 17.Mukhtar K., Khattak I. Transversus abdominis plane block for renal transplant recipients. Br J Anaesth. 2010;104:663–664. doi: 10.1093/bja/aeq077. [DOI] [PubMed] [Google Scholar]
- 18.Bhattacharjee S., Ray M., Ghose T., et al. Analgesic efficacy of transversus abdominis plane block in providing effective perioperative analgesia in patients undergoing total abdominal hysterectomy: a randomized controlled trial. J Anaesthesiol Clin Pharmacol. 2014;30:391–396. doi: 10.4103/0970-9185.137274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amr Y.M., Amin S.M. Comparative study between effect of pre-versus post-incisional transversus abdominis plane block on acute and chronic post-abdominal hysterectomy pain. Anesth Essays Res. 2011;5:77–82. doi: 10.4103/0259-1162.84199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kokulu S., Bakı E.D., Kaçar E., et al. Effect of transversus abdominis plane block on cost of laparoscopic cholecystectomy anesthesia. Med Sci Monit. 2014;20:2783–2787. doi: 10.12659/MSM.892055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuchiya M., Takahashi R., Furukawa A., et al. Transversus abdominis plane block in combination with general anesthesia provides better intraoperative hemodynamic control and quicker recovery than general anesthesia alone in high-risk abdominal surgery patients. Minerva Anestesiol. 2012;78:1241–1247. [PubMed] [Google Scholar]
- 22.Abu Elyazed M.M., Mostafa S.F., Abdullah M.A., et al. The effect of ultrasound-guided transversus abdominis plane (TAP) block on postoperative analgesia and neuroendocrine stress response in pediatric patients undergoing elective open inguinal hernia repair. Paediatr Anaesth. 2016;26:1165–1171. doi: 10.1111/pan.12999. [DOI] [PubMed] [Google Scholar]
- 23.Granier M., Dadure C., Bringuier S., et al. Intranasal lidocaine plus naphazoline nitrate improves surgical conditions and perioperative analgesia in septorhinoplasty surgery. Can J Anaesth. 2009;56:102–108. doi: 10.1007/s12630-008-9020-7. [DOI] [PubMed] [Google Scholar]
- 24.Goktas U., Isik D., Kati I., et al. Effects of lidocaine infiltration on cost of rhinoplasty made under general anesthesia. J Craniofac Surg. 2011;22:2176–2178. doi: 10.1097/SCS.0b013e318232414c. [DOI] [PubMed] [Google Scholar]