Abstract
Introduction: Brazil has a high number of HTLV-1/2 infections which are unequally distributed in the country. Most prevalence studies have focused on specific populations, such as blood donors and pregnant women. Some areas, for example the state of Bahia, have robust information about HTLV-1/2 infection, however there is no information available about this infection in the general population of Vitória, Espírito Santo, Brazil. Objective: To determine the prevalence of HTLV-1/2 infection in adults from the municipality of Vitoria, ES. Methods: A cross sectional study was performed from September 2010 to December 2011, in individuals of both sexes, aged 18 or older living in Vitória-ES. Venous blood samples were collected and tested for anti-HTLV-1/2 antibodies by chemiluminescent immunoassay (CMIA). Individuals with CMIA reactive results were submitted to a new blood collection for retesting by CMIA, followed by PCR to confirm infection and discriminate the viral type. Results: From 1502 tested samples, eight were reactive in CMIA and all were confirmed by PCR. Therefore, the prevalence of HTLV-1/2 was 0.53% (8/1502, 95% CI: 0.2–1.0%). The infection rate was 0.7% in men (5/711, 95% CI: 0.17–1.51%), and 0.38% in women (3/791, 95% CI: 0–0.81%). Conclusions: The prevalence of HTLV-1/2 infection was 0.53% (8/1502; 95% CI: 0.2–0.9%). Confirmatory test using real-time PCR (qPCR) identified seven individuals positive for HTLV-1 and one for HTLV-2. Considering the risk of infected individuals to develop high morbidity and mortality diseases, it would be important to implement public health policies aimed at stopping transmission of these viruses in this municipality.
Keywords: HTLV-1, HTLV-2, Prevalence, General Population, Vitória, Brazil
Introduction
Human T-cell Lymphotropic Virus type 1 (HTLV-1) is unique as it is both oncogenic1 and capable of triggering HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis (HAM/TSP) and other inflammatory diseases.2 With increased human mobility and migration and limited awareness and implementation of effective prevention strategies, HTLV-1 remains a significant public health threat.3 This virus can be transmitted through unprotected sexual intercourse, by mother-to-child transmission (MTCT), mainly by breastfeeding, and by exposure to infected lymphocytes in blood or tissue, as may occur in needle or syringe sharing among people who inject drugs, blood transfusion, and organ transplantation.4, 5, 6
It is estimated that 10 million people are living with HTLV-1 (PLwHTLV-1)7,8 worldwide. In Brazil an estimated 800,000 people are infected by HTLV-1, with variable prevalence according to geographical regions, being highest in the Northeast and North regions.7 Salvador, the capital city of Bahia state, has been identified as the epicenter of HTLV-1 infection in Brazil as 1.48%8 of its population are infected by this virus, being more prevalent among black/mixed race women, those less educated and, due to the increased likelihood of HTLV acquisition over time, with increasing age.9
A deeper understanding of the epidemiology of HTLV is extremely important as it would guide public policies to prevent virus transmission. Leading HTLV scientists and public health experts called for renewed efforts to eradicate the infection.3 Such call was recently recognized by the World Health Organization (WHO), which published a technical report on HTLV.10 A recent comprehensive epidemiological article addressing HTLV-1 prevalence and distribution in Brazil was published in a bulletin of the Brazilian Ministry of Health7 highlighting the remaining gaps in the knowledge about HTLV-1 in the country. The situation of HTLV-1 infection in some States is unknown where there is no reliable data, including the state of Espírito Santo. Therefore, the present study aimed to estimate the seroprevalence and geographical distribution of HTLV infection in the general population in Vitória, the capital city of Espirito Santo, Brazil.
Methods
Cohort characteristics
This was a population-based cross-sectional study with a random sample of individuals of both sexes, aged 18 years or over, who attended one the 27 health care units (HUs) in the city of Vitória, Espírito Santo. The units are distributed across the six territorial health regions of the city. Companions of patients seeking care in those HUs were randomly invited to participate in the study, from September 2010 to December 2011. After signing the informed consent form, the participant answered a socio-demographic questionnaire and was referred for blood collection.
Sample size
The sample size was calculated based on the prevalence of HTLV infection estimated at 0.6%, with a variation of ± 0.2%, based on the average for Brazil reported in previous studies. The sample size was calculated in order to provide a result with a statistical power of 80% (β error = 0.20) and significance level of 95% (α error = 0.05). Thus, it was planned to enrol 1644 people, which would allow a loss of up to 15%.
Laboratory assays
Blood samples were obtained by venepuncture, using the vacuum collection method or with a disposable syringe, in a collection tube without anticoagulant and with separating gel. From each individual, 5-10 mL of blood were collected in EDTA tube, immediately packed in cool boxes until arrival at the Laboratory of the Infectious Diseases Center in Vitoria. The sera were separated by centrifugation, identified and stored in two cryotube-type plastic tubes at -20° C, until the serological examination was performed.
An aliquot of serum was screened by Chemiluminescent Magnetic Microparticle Immunoassay (CMIA) “ARCHITECT rHTLV-I / II” (ABBOTT–Wiesbaden, Germany) according to manufacturer's instructions, at the Immunology Laboratory of HUCAM, Vitória-ES. This assay provides a qualitative determination of antibodies against HTLV-1 and HTLV-2 in the serum.
Patients with positive results in the screening test (i.e. antibody detected) were recalled, and additional samples were collected in EDTA tube. Those with repeatedly CMIA positive results were subsequently tested using a qualitative molecular test for specific detection of HTLV-1 and HTLV-2 in peripheral blood mononuclear cells (PBMC) using real-time PCR as previously reported.11 This test was carried out by the Hematology and Hemotherapy Center Foundation of Minas Gerais (HEMOMINAS), Belo Horizonte (MG).
Negative results were reported to the HUs where the samples were collected, so volunteers hadd access to their results, as previously agreed. Positive results were delivered directly to the participants, counselling was provided and an appointment at the neurology clinic of the University Hospital Cassiano Antônio de Moraes was scheduled.
Data quality control
Data collection was performed by the first author, including general identification information, socioeconomic, behavioural and health conditions, such as laboratory test results. All data were entered into an electronic Excel spreadsheet (Microsoft). Data accuracy was performed by two research assistants, and subsequently checked by the first and last author.
Statistical analyses
Statistical analysis was performed using the software Statistical Package for the Social Sciences (SPSS) version 17.0. A descriptive analysis was performed, including frequency distribution for qualitative variables and calculation of mean and standard deviation (SD) for quantitative variables. The prevalence of HTLV was estimated by the number of cases diagnosed and confirmed in the tests in relation to the total number of samples tested, and the corresponding 95% confidence interval was calculated. The possible associations between the occurrence of HTLV and risk factors, or demographic variables, were tested using chi-square tests with Yates' correction or Fisher's exact test, when appropriate. Multivariate logistic regression analysis was used to estimate the independent effect of one variable, while controlling the effect of the others, on the probability of occurrence of HTLV infection. Variables with p-value less than or equal to 0.150 in univariate analysis were included in the model, and those with p-value <0.05 were considered significant.
Ethical issues
The study was approved by the ethical Board of the Health Sciences Center of the Federal University of Espírito Santo under no. 089/10 and by the Health Department (SEMUS) of the Municipality of Vitória. Signed informed consent was obtained from all participants prior to study inclusion.
Results
A total of 1502 individuals were included in this study, 91.4% of the invited individuals. The average age of participants was 41.6 years (range: 18-86 years). The average age in men was 42.4 years and in women 40.9 years (p= 0.157). Regarding race/ethnicity, 782 (52.1%) were mixed, 27% white, 19.4% black, 1.1% indigenous, and 0.3% Asian. The majority (936/1502; 65.1%) were married or had a stable marital union; 46.0% reported 9-11 years of schooling and 22.2% between five and eight years. Family monthly income between one to three minimum wages was reported by 46% of participants, while 23.6% had an income of one minimum wage or less.
Of the 1502 samples tested, eight were positive in the initial CMIA and all of these reactive samples were remained positive on a second sample, and HTLV DNA was detected by PCR. The prevalence of HTLV-1/2 infection was 0.53% (8/1502; 95% CI: 0.2–0.9%). Based on real-time PCR, seven patients were infected with HTLV-1 and one with HTLV-2. The majority were male, married, non-white, and had more than five years of schooling (62.5%). Most of PLwHTLV (75%) reported family income lower than three minimum wages (Table 1). In the final logistic regression model, no variables were independently associated with the diagnosis of HTLV infection (Table 2).
Table 1.
Sociodemographic characteristics of 1502 adult volunteers who were assisted in public health services in the city of Vitória, Espírito Santo, Brazil, 2010.
| Variables | N total (%) | HTLV +N (%) | HTLV –N (%) | p value |
|---|---|---|---|---|
| Age (years) | 0.157 | |||
| >40 | 766 (51.0) | 6 (75.0) | 760 (50.9) | |
| 18 – 39 | 736 (49.0) | 2 (25.0) | 734 (49.1) | |
| Gender | 0.306 | |||
| Male | 711 (47.3) | 5 (62.5) | 706 (47.3) | |
| Female | 791 (52.7) | 3 (37.5) | 788 (52.7) | |
| Scholarity (years) | 0.097 | |||
| 4 | 218 (14.5) | 3 (37.5) | 215 (14.4) | |
| ≥ 5 | 1284 (85.5) | 5 (62.5) | 1279 (85.6) | |
| Marital status | 0.627 | |||
| Single | 343 (22.9) | 1 (12.5) | 342 (22.9) | |
| Married / stable relationship | 936 (62.3) | 5 (62.5) | 931 (62.3) | |
| Separated / Divorced / Widowed | 223 (14.8) | 2 (25.0) | 221 (14.8) | |
| Family income (MW)* | 0.544 | |||
| ≥ 3 | 455 (30.3) | 2 (25.0) | 453 (30.3) | |
| 2 | 1047 (69.7) | 6 (75.0) | 1041 (69.7) | |
| Skin color self-declared | 0.374 | |||
| Other | 1095 (72.9) | 5 (62.5) | 1090 (73.0) | |
| White | 407 (27.1) | 3 (37.5) | 404 (27.0) | |
| Region of health service | 0.125 | |||
| Continental | 477 (31.8) | 2 (25.0) | 475 (31.8) | |
| Maruípe | 290 (19.3) | 1 (12.5) | 289 (19.3) | |
| Central | 133 (8.9) | - | 133 (8.9) | |
| Santo Antônio | 145 (9.7) | - | 145 (9.7) | |
| São Pedro | 130 (8.7) | - | 130 (8.7) | |
| Forte São João | 327 (21.6) | 5 (62.5) | 322 (21.6) |
MW = Minimum wages
Table 2.
Positive cases for HTLV-1/2 in relation to the main behavioral risk factors among 1502 adults in the city of Vitória. Espírito Santo. Brazil. 2010.
| Variables | N total (%) | HTLV +N (%) | HTLV –N (%) | p value |
|---|---|---|---|---|
| Sexual behavior | 0.903 | |||
| Heterossexual | 1483 (98.7) | 8 (100) | 1475 (98.7) | |
| Homo/bissexual | 19 (1.3) | - | 19 (1.3) | |
| Sexual partners in the last year | 0.649 | |||
| 0–1 | 1273 (84.8) | 7 (87.5) | 1266 (84.7) | |
| 2 or more | 229 (15.2) | 1 (12.5) | 228 (15.3) | |
| Condom use* | 0.483 | |||
| Uses regularly | 305 (20.3) | 1 (12.5) | 304 (20.3) | |
| Rarely or never | 994 (66.2) | 6 (75.0) | 988 (66.2) | |
| History of Sexually Transmitted Diseases (STD) | 0.418 | |||
| No | 1241 (82.6) | 6 (75.0) | 1235 (82.7) | |
| Yes | 261 (17.4) | 2 (25.0) | 258 (17.3) | |
| Have already used injecting drugs | 0.979 | |||
| No | 1498 (99.7) | 8 (100) | 1490 (99.7) | |
| Yes | 4 (0.3) | - | 4 (0.3) | |
| Have shared syringes | 0.989 | |||
| No | 1500 (99.9) | 8 (100) | 1492 (99.9) | |
| Yes | 2 (0.1) | - | 2 (0.1) | |
| Having been breastfed | 0.655 | |||
| No | 75 (5.0) | - | 75 (5.0) | |
| Yes | 1359 (90.5) | 8 (100) | 1351 (90.4) | |
| Do not know / do not remember | 68 (4.5) | - | 68 (4.6) | |
| Breastfeeding time (days) | 0.236 | |||
| 180 | 840 (55.9) | 6 (75.0) | 834 (55.8) | |
| ≥181 | 662 (44.1) | 2 (25.0) | 660 (44.2) | |
| History of blood transfusion | 0.218 | |||
| No | 1336 (88.9) | 6 (75.0) | 1330 (89.0) | |
| Yes | 166 (11.1) | 2 (25.0) | 164 (11.0) | |
| Tattoo use | 0.228 | |||
| No | 1249 (83.2) | 8 (100) | 1241 (83.1) | |
| Yes | 253 (16.8) | - | 253 (16.9) | |
| Use of alcohol (last month) | 0.196 | |||
| 4 times | 1347 (89.7) | 6 (75.0) | 1341 (89.8) | |
| ≥ 5 times | 155 (10.3) | 2 (25.0) | 153 (10.2) | |
| Cocaine use | 0.138 | |||
| No | 1377 (91.7) | 6 (75.0) | 1371 (91.8) | |
| Yes | 125 (8.3) | 2 (25.0) | 123 (8.2) | |
| Metilenodioximetanfetamine (MDME) use | 0.062 | |||
| No | 1490 (99.2) | 7 (87.5) | 1483 (99.3) | |
| Yes | 12 (0.8) | 1 (12.5) | 11 (0.7) | |
A total of 203 volunteers have no sexual activity in the last year.
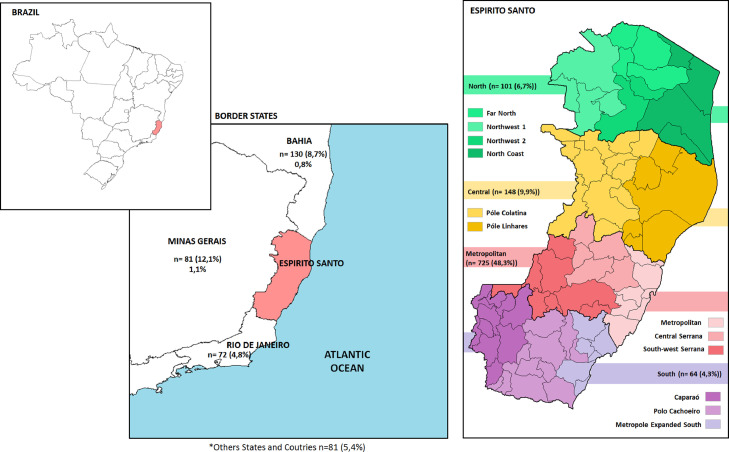
Fig. 1 shows the distribution of participants in the regions of Espirito Santo and in the border states. The prevalence of HTLV-1/2 varied between 0 to 1.5% according to the health regions of the city of Vitória. No case of HTLV infection was detected in Central, Santo Antonio, and São Pedro regions. In Maruípe, the prevalence was 0.3% (1/290, 95% CI 0-1%), while in Continental region was 0.4% (2/477, 95% CI 0-1%), and 1.5% (5/327, 95% CI 0.2–2.8%) in Forte de São João. Considering the place of birth, 2% (2/101) of individuals born in the North region and 0.4% (3/725) in the Metropolitan region were infected by HTLV. Interestingly, 0.8% (1/130) of patients born in Bahia and 1.1% (2/181) of those born in Minas Gerais were diagnosed with HTLV infection (Fig. 1).
Fig. 1.
Distribution of origin of volunteers in Espirito Santo and border stares.
Discussion
Espírito Santo is one of the 27 federative units in Brazil with Vitória as the capital city, but Serra is the most populous municipality. Located in the Southeast region, the state borders the Atlantic Ocean to the east, Bahia to the north, Minas Gerais to the west and the state of Rio de Janeiro to the south. In 2010, the population of the state of Espírito Santo reached a total of 3,514,952 inhabitants.12
Most studies about HTLV-1/2 seroprevalence in Brazil focus on specific populations, such as blood donors, pregnant women, and people who inject drugs. Although valuable, these studies cannot be extrapolated to the general population. This is the first population-based study in Espírito Santo designed to assess the prevalence of HTLV-1/2 infection. In this context, the results showed a prevalence of 0.53% of HTLV. Although considered intermediate, the rate is higher than that observed in the South and Midwest regions, lower than that observed in the North and Northeast regions, and similar to that observed in the other states of the Southeast region.9,13, 14, 15, 16
Although HTLV-1 infection occurs in different parts of the world, its prevalence varies according to geographic location, ethnic and racial factors, and with social behaviour, that can lead to a greater exposure, as occurs with some vulnerable groups, such as people who inject drugs and sex workers.17 Worldwide, 5 to 10 million are estimated to be HTLV-1 infected. This meta-analysis was based on data covering nearly 1.5 billion individuals in regions with reliable epidemiological data17,18 but may considerably underestimate the actual prevalence given the paucity of data for more than 2/3 of the global population.
HTLV-1 is endemic in South America, affecting 13 countries and prevailing in several ethnic groups.19, 20, 21 In Brazil, HTLV-1 is found in immigrants from endemic areas such as Africa and Japan,8,21, 22, 23 their descendants and in the descendants of Amerindians who have inhabited South America for thousands of years.24, 25, 26 Several studies performed in Brazil on large populations of blood donors have found an heterogeneous HTLV-1 seroprevalence ranging from 0.04 to 1% depending on the geographical location.17
The results of this study reveal that the distribution of cases was unequal even between different regions of the city, which may be related to the origin of the residents. Among the five positive cases in the Forte São João Region, three originated in other states (Bahia and Minas Gerais), regions where the prevalence of the HTLV-1 is higher than in other Brazilian states.7,27
The prevalence found in this study was higher when compared with data obtained in the blood bank of the State of Espírito Santo (HEMOES) in 2011 (0.07%, in 45,898 donors without confirmatory testing; personal communication). However, it was lower than that reported in a study with 447 low-income pregnant women and parturients in Vitória, which had a prevalence of 1.3% (8/1502 – 6/447).28 However, the previous study did not include confirmatory test and was conducted in an HIV specialized center, which may bias the participants towards a high-risk population for sexually transmitted diseases, and with a history of surgery or blood transfusion.28
The distribution in relation to sex showed a predominance among men. This is notable as female sex has commonly been associated with higher rates. However, the sex distribution of HTLV-1/2 infection in Brazil, based on data from population samples, has shown variable results: predominance of women in the states of Bahia, Pará, Goiás and Mato Grosso do Sul,14,15,29, 30, 31 and higher predominance in men in specific populations such as co-infected patients and men who have sex with men.23,32 In the current study the average age of the women was 40.9 years suggesting that they may not yet have reached the age when susceptibility to HTLV-1 infection increases (i.e. post-menopause).
The selected sample was proportional to the number of inhabitants of each region, but a limitation was the inclusion of only individuals who attended public healthcare services in the city of Vitória, which may limit extrapolations to the entire population. Importantly, they were not themselves attending for care. Furthermore, we believe that the adequate sample size and the high acceptance rate (only 8.6% refusal) are important elements to favour representativeness of the situation of infection by HTLV-1/2 in the studied population.
Most prevalence studies show that on average the educational level of PLwHTLV-1/2 is 10 years.5,23,33, 34, 35 In the present study, a high degree of education in PLwHTLV-1/2 was observed, which could be explained by a sample population including some health professionals of these units.
In view of the findings of this study and considering the risk of infected individuals to develop high morbidity and mortality diseases, it would be important to implement public health policies aimed at stopping the transmission of these viruses in this municipality. It would be important to recommend preventive measures such as the inclusion of serological screening for these viruses during antenatal period, and the counselling of infected pregnant women in relation to the interruption of breastfeeding, since the possibility of transmission through this route is high and prophylactic measures are simple and effective. Additionally, it is necessary to test and advise the sexual partners of infected individuals, in order to mitigate the spread of HTLV-1/2 viruses.
Funding
The TA was funded by FAPESP 2016/03025-2; CNPq (Scholarship to JC), FFM (support to JC).
Authors contribution
MPSO data collected, laboratorial tests and writing; TA statistical analyses and text revision; GDS laboratorial tests, MLM laboratorial tests, CR revision, JC revision; GT revision and English, JBFF concept and laboratorial tests, FELP concept, AEM concept and revision.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
We would like to thank all volunteers for their participation.
Contributor Information
Tatiane Assone, Email: tatiane.assone@aids.gov.br.
Angélica Espinosa Miranda, Email: angelica.miranda@aids.gov.br.
References
- 1.Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bangham CRM. Human T Cell Leukemia Virus Type 1: Persistence and Pathogenesis. Annu Rev Immunol. 2018;36:43–71. doi: 10.1146/annurev-immunol-042617-053222. [DOI] [PubMed] [Google Scholar]
- 3.Martin F, Tagaya Y, Gallo R. Time to eradicate HTLV-1: an open letter to WHO. Lancet Lond Engl. 2018;391(10133):1893–1894. doi: 10.1016/S0140-6736(18)30974-7. [DOI] [PubMed] [Google Scholar]
- 4.Rosadas C, Taylor GP. Mother-to-Child HTLV-1 transmission: unmet research needs. Front Microbiol. 2019;10:999. doi: 10.3389/fmicb.2019.00999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paiva A, Smid J, Haziot MEJ, et al. High risk of heterosexual transmission of human T-cell lymphotropic virus type 1 infection in Brazil. J Med Virol. 2017;89(7):1287–1294. doi: 10.1002/jmv.24745. [DOI] [PubMed] [Google Scholar]
- 6.Araujo AC de, Casseb JS, Neitzert E, et al. HTLV-I and HTLV-II infections among HIV-1 seropositive patients in Sao Paulo, Brazil. Eur J Epidemiol. 1994;10(2):165–171. doi: 10.1007/BF01730366. [DOI] [PubMed] [Google Scholar]
- 7.Coordenação-Geral de Vigilância das Infecções Sexualmente Transmissíveis (CGIST/DCCI/SVS): Carolina Rosadas, Angélica Espinosa Miranda,, Denise Utsch Gonçalves**, Adele Caterino-de-Araujo**, Tatiane Assone**, Ricardo Ishak** Prevalência da infecção por HTLV-1/2 no Brasil–Boletim Epidemiológico. Secretaria de Vigilância em Saúde | Ministério da Saúde. 2020;51(48) https://www.gov.br/saude/pt-br/media/pdf/2020/dezembro/11/boletim_epidemiologico_svs_48.pdf Available. [Google Scholar]
- 8.Nunes D, Boa-Sorte N, Grassi MFR, et al. HTLV-1 is predominantly sexually transmitted in Salvador, the city with the highest HTLV-1 prevalence in Brazil. PloS One. 2017;12(2) doi: 10.1371/journal.pone.0171303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereira FM, Almeida M da CC, Santos FLN de, Carreiro RP, Galvão-Castro B, Grassi MFR. Distribution of Human T-Lymphotropic Virus (HTLV) and Hepatitis C Co-infection in Bahia, Brazil. PloS One. 2020;15(7) doi: 10.1371/journal.pone.0223087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.New WHO report on Human T-lymphotropic virus type 1 highlights strategies for its prevention and control [Internet]. citado 20 de abril de 2021. Available: https://www.who.int/news/item/03-03-2021-new-who-report-on-human-t-lymphotropic-virus-type-1-indicates-the-future-path-for-its-prevention-and-control
- 11.Andrade RG, Ribeiro MA, Namen-Lopes MSS, et al. Análise do uso da PCR em tempo real para HTLV-1 e 2 como teste confirmatório na triagem de doadores de sangue. Rev Soc Bras Med Trop. 2010;43(2):111–115. [Google Scholar]
- 12.IBGE Censo [Internet] 2010 https://censo2010.ibge.gov.br/sinopse/index.php?uf=32&dados=26 [citado 23 de julho de 2021]. Disponível em. [Google Scholar]
- 13.Guimarães de Souza V, Lobato MM, Carneiro-Proietti AB de F, et al. High prevalence of HTLV-1 and 2 viruses in pregnant women in São Luis, state of Maranhão, Brazil. Rev Soc Bras Med Trop. 2012;45(2):159–162. doi: 10.1590/s0037-86822012000200004. [DOI] [PubMed] [Google Scholar]
- 14.Rebouças KAAF, Narici FM, Santos Junior MN, Neres NS, de M, Oliveira MV, Souza CL. Seroprevalence of transfusion-transmissible infectious diseases at a hemotherapy service located in southwest Bahia, Brazil. Hematol Transfus Cell Ther. 2019;41(4):324–328. doi: 10.1016/j.htct.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morimoto HK, Caterino-De-Araujo A, Morimoto AA, et al. Seroprevalence and risk factors for human T cell lymphotropic virus type 1 and 2 infection in human immunodeficiency virus-infected patients attending AIDS referral center health units in Londrina and other communities in Paraná, Brazil. AIDS Res Hum Retroviruses. 2005;21(4):256–262. doi: 10.1089/aid.2005.21.256. [DOI] [PubMed] [Google Scholar]
- 16.Alves FA, Campos KR, Lemos MF, Moreira RC, Caterino de-Araujo A. Hepatitis C viral load in HCV-monoinfected and HCV/HIV-1-, HCV/HTLV-1/-2-, and HCV/HIV/HTLV-1/-2-co-infected patients from São Paulo, Brazil. Braz J Infect Dis Off Publ Braz Soc Infect Dis. 2018;22(2):123–128. doi: 10.1016/j.bjid.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gessain A, Cassar O. Epidemiological Aspects and World Distribution of HTLV-1 Infection. Front Microbiol. 2012;3:388. doi: 10.3389/fmicb.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eusebio-Ponce E, Anguita E, Paulino-Ramirez R, Candel FJ. HTLV-1 infection: an emerging risk. Pathogenesis, epidemiology, diagnosis and associated diseases. Rev Esp Quimioter. 2019;32(6):485–496. [PMC free article] [PubMed] [Google Scholar]
- 19.Gotuzzo E, Moody J, Verdonck K, et al. Frequent HTLV-1 infection in the offspring of Peruvian women with HTLV-1-associated myelopathy/tropical spastic paraparesis or strongyloidiasis. Rev Panam Salud Publica Pan Am J Public Health. 2007;22(4):223–230. doi: 10.1590/s1020-49892007000900001. [DOI] [PubMed] [Google Scholar]
- 20.Ramassamy J-L, Tortevoye P, Ntab B, et al. Adult T-cell leukemia/lymphoma incidence rate in French Guiana: a prospective cohort of women infected with HTLV-1. Blood Adv. 2020;4(9):2044–2048. doi: 10.1182/bloodadvances.2020001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carneiro-Proietti ABF, Catalan-Soares BC, Castro-Costa CM, et al. HTLV in the Americas: challenges and perspectives. Rev Panam Salud Publica Pan Am J Public Health. 2006;19(1):44–53. doi: 10.1590/s1020-49892006000100007. [DOI] [PubMed] [Google Scholar]
- 22.Bandeira LM, Puga MAM, Weis-Torres SMS, et al. Human T-cell leukemia virus type 1 infection among Japanese immigrants and their descendants living in Southeast Brazil: A call for preventive and control responses. PLoS Negl Trop Dis. 2021;15(2) doi: 10.1371/journal.pntd.0009066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castro LS, Rezende GR, Fernandes FRP de, et al. Human T cell lymphotropic virus type 1 infection among men who have sex with men in Central Brazil. Braz J Infect Dis Off Publ Braz Soc Infect Dis. 2018;22(6):472–476. doi: 10.1016/j.bjid.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talarmin A, Vion B, Ureta-Vidal A, Du Fou G, Marty C, Kazanji M. First seroepidemiological study and phylogenetic characterization of human T-cell lymphotropic virus type I and II infection among Amerindians in French Guiana. J Gen Virol. 1999;80(Pt 12):3083–3088. doi: 10.1099/0022-1317-80-12-3083. [DOI] [PubMed] [Google Scholar]
- 25.Ishak R, Guimarães de O, Ishak M, Vallinoto ACR. The challenge of describing the epidemiology of HTLV in the Amazon region of Brazil. Retrovirology. 2020;17(1):4. doi: 10.1186/s12977-020-0512-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paiva A, Casseb J. Origin and prevalence of human T-lymphotropic virus type 1 (HTLV-1) and type 2 (HTLV-2) among indigenous populations in the Americas. Rev Inst Med Trop Sao Paulo. 2015;57(1):1–13. doi: 10.1590/S0036-46652015000100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Catalan-Soares B, Carneiro-Proietti AB de F, Proietti FA, Interdisciplinary HTLV Research Group Heterogeneous geographic distribution of human T-cell lymphotropic viruses I and II (HTLV-I/II): serological screening prevalence rates in blood donors from large urban areas in Brazil. Cad Saude Publica. 2005;21(3):926–931. doi: 10.1590/s0102-311x2005000300027. [DOI] [PubMed] [Google Scholar]
- 28.Lima LHM de, Viana MC. Prevalence and risk factors for HIV, syphilis, hepatitis B, hepatitis C, and HTLV-I/II infection in low-income postpartum and pregnant women in Greater Metropolitan Vitória, Espírito Santo State, Brazil. Cad Saude Publica. 2009;25(3):668–676. doi: 10.1590/s0102-311x2009000300021. [DOI] [PubMed] [Google Scholar]
- 29.Kozlowski AG, Matos MAD de, Carneiro MADS, et al. Seroprevalence of HTLV in a population of HIV1-infected patients in midwestern Brazil. Rev Inst Med Trop Sao Paulo. 2016;58:80. doi: 10.1590/S1678-9946201658080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galetto LR, Lunge VR, Béria JU, Tietzmann DC, Stein AT, Simon D. Short communication: Prevalence and risk factors for human T cell lymphotropic virus infection in Southern Brazilian HIV-positive patients. AIDS Res Hum Retroviruses. 2014;30(9):907–911. doi: 10.1089/AID.2013.0210. [DOI] [PubMed] [Google Scholar]
- 31.Ml M, Jc G, Jg R, Lc R, Ab de FC-P. Long-term follow-up of HTLV-1 proviral load in asymptomatic carriers and in incident cases of HAM/TSP: what is its relevance as a prognostic marker for neurologic disease? [Internet] J Neurovirol. 2017;23 doi: 10.1007/s13365-016-0484-x. https://pubmed.ncbi.nlm.nih.gov/27678093/ [citado 2 de dezembro de 2020]. Disponível em. [DOI] [PubMed] [Google Scholar]
- 32.Caterino-de-Araujo A, Campos KR, Petrucci TVB, Silva RX, da, Lemos MF, Moreira RC. Surveillance of human retroviruses in blood samples from patients with hepatitis B and C in São Paulo, Brazil. Rev Soc Bras Med Trop. 2020;53 doi: 10.1590/0037-8682-0378-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paiva AM, Assone T, Haziot MEJ, et al. Risk factors associated with HTLV-1 vertical transmission in Brazil: longer breastfeeding, higher maternal proviral load and previous HTLV-1-infected offspring. Sci Rep. 2018;8(1):7742. doi: 10.1038/s41598-018-25939-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Lima WMG, Esteves FAM, Torres M do CMR, Pires ESF. Prevalence of human T-cell lymphotropic virus types 1 and 2 in blood donors of the Caruaru Blood Center (Hemope) Rev Bras Hematol E Hemoter. 2013;35(4):268–271. doi: 10.5581/1516-8484.20130061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caterino-de-Araujo A, Sacchi CT, Gonçalves MG, et al. Short communication: current prevalence and risk factors associated with human T Lymphotropic virus Type 1 and Human T Lymphotropic virus Type 2 infections among HIV/AIDS patients in São Paulo, Brazil. AIDS Res Hum Retroviruses. 2015;31(5):543–549. doi: 10.1089/aid.2014.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]