Abstract
Objective
The CO2 laser and 532 nm potassium titanyl phosphate (KTP) laser have been applied to treat recurrent respiratory papillomatosis (RRP). This systematic review sought to compare outcome differences between these two methods.
Data Sources
Embase, Web of Science, PubMed, and the Cochrane Library.
Review Methods
CO2 laser and KTP laser studies were obtained by keyword searches of four authoritative medical databases. Articles were screened and retained when conforming to inclusion criteria. The primary outcome was cure rate; the secondary outcomes were recurrence, death, remission, clearance, and human papillomavirus (HPV)‐detected rates, as well as laser effectiveness rates. Postoperative complications rate was the safety outcome measure. All outcomes were summarized within the CO2 and KTP groups, with results statistically compared (p < .05).
Results
Overall, the cure rates were 87.25% (KTP group) and 75.98% (CO2 group; p < .05). Complication rates significantly differed between the KTP (2.32%) and CO2 (17.71%) groups (p < .0001). There was a relatively higher but not significant difference in the recurrence rates between the CO2 (18.6%) and KTP (10.87%) groups (p = .1595). The CO2 group remission rate was considerably lower (38.9%) than the KTP group (88.46%, p < .0001). HPV‐detected and clearance rates were only reported for the CO2 group. The bias risks were 13.1 ± 1.45 (CO2) and 13.6 ± 1.52 (KTP) for the two groups, indicating evidence was of fair quality.
Conclusion
Overall, KTP laser excision showed significantly better postoperative clinical outcomes than the CO2 laser, with a lower failure rate. Available fair‐quality evidence suggests KTP laser excision might be better for treating RRP. Nevertheless, more high‐quality randomized controlled studies are needed to compare these two surgical techniques, particularly in terms of reporting functional data such as vocal outcomes.
Keywords: 532 nm potassium titanyl phosphate laser, CO2 laser, laryngeal papillomatosis, recurrent respiratory papillomatosis, systematic review
This systematic, multilingual review sought to compare outcome differences between the CO2 laser and 532‐nm potassium titanyl phosphate (KTP) laser to treat recurrent respiratory papillomatosis (RRP). KTP laser excision showed significantly better postoperative clinical outcomes than the CO2 laser, with a lower failure rate, and available fair‐quality evidence suggests KTP laser excision might be better for treating RRP. Nevertheless, more high‐quality randomized controlled studies are needed to compare these two surgical techniques, particularly in terms of reporting functional data such as vocal outcomes.
1. INTRODUCTION
Laryngeal papillomatosis (LP) is a benign cell tumor that can afflict the whole respiratory tract and upper digestive tract. 1 It is also termed recurrent respiratory papillomatosis (RRP) due to its high‐frequency, postoperative recurrence rate which requires multiple surgeries overtime. 2 In general, RRP can be divided into juvenile‐ and adult‐onset RRP (AoRRP) based on age of onset. 3 The reported incidence rates are 0.17 and 0.54 per 100,000 people for juvenile‐ and AoRRP, respectively. 4 Like many abnormal exophytic projections, one of RRP's morphological characteristics consists of a center of connective tissue covered by squamous epithelium. 5 , 6 Although benign, RRP is chronic and can have a significant influence on quality of life due to airway obstruction, hoarseness, financial impact from multiple operations, and scarring, as well as the rare possibility of malignant transformation. 4 , 7 , 8
Currently, there is no cure or definitive treatment for RRP available, with clinical management of RRP being primarily dependent on repeated surgical resections with careful preservation of relatively normal tissues based on the surgeon's experience. 9 , 10 Moreover, the clinical treatment for RRP is still frustrating due to unpredictable courses, a high recurrence tendency, and intractable complications. 11 , 12 Effective control of complications is one of the main treatment targets, which is also an important criterion for evaluating cure methods. 2 , 3 , 13 Surgical excision in the operating room under general anesthesia is the traditional management method. 1 , 14 , 15 Powered by advanced technologies, a direct laryngoscopic approach began in the 20th century. 16 , 17 Afterward, with the development of the carbon dioxide (CO2) laser in the 1960s, it quickly became popular in laryngology, but multiple complications (e.g., thermal injuries) have significantly affected its application. 1 , 18 , 19 Subsequently, in 2001, the 585‐nm pulsed dye laser (PDL) was introduced to manage RRP with a fiber delivery system and merit‐absorbable energy. 20 , 21 However, bleeding caused by PDL and its extremely short pulse width prevented the further application of this technology. 2 , 22 , 23 In recent years, adjuvant antiviral drugs have been administrated and studied for potential therapeutic applications, but their therapeutic effects have yet to be confirmed. 24 , 25 , 26
In the past few years, photoangiolytic lasers, such as the 532‐nm potassium titanyl phosphate (KTP) laser, have been widely applied in office‐based laryngeal surgical procedures. 9 , 27 , 28 Many studies have reported successful treatment of multiple vocal diseases, such as papilloma, varix, polyps, Reinke edema, vocal process granuloma, ectasia, and glottal dysplasia. 28 The angiolytic properties of KTP shrink lesions through photothermolysis and the laser energy can be absorbed by hemoglobin. 10 , 29 , 30 Given these advantages, the KTP laser could be the future of RRP treatment. However, the CO2 laser remains the first choice for treating RRP by most hospitals in China due to the lack of comparative studies or consensus on which laser is better for RRP therapy.
Therefore, seeking to address this research gap, this systematic review evaluates and compares the cure, complications, and recurrence rates of CO2 and KTP laser treatments for RRP. We hypothesized that KTP laser yields better outcomes than CO2 laser for RRP treatment, having similar cure but lower complications rates.
2. METHODS
This study followed the PRISMA guidelines 31 (Supporting Information S1).
2.1. Search strategy
For this systematic review, PubMed, EMBASE, Cochrane Library, and Web of Science (WOS) were searched for relevant literature published between January 1, 1900, and December 31, 2020. The search terms used were: (Papilloma* OR Papilloma Virus OR Papillomatosis OR Papillomatoses) AND (Laryngeal* OR throat OR larynx OR throttle) AND (CO2 laser* OR KTP laser* OR potassium titanyl phosphate laser) (see search strategy, Supporting Information S2). Reference lists of previously published reviews and the studies included therein were also assessed in this work to ensure inclusion of as many relevant studies as possible.
2.2. Inclusion criteria
Only clinical studies with original (e.g., reported or published data) were included (review papers were excluded). We considered all research demonstrating the prognosis of RRP after KTP laser or CO2 laser excision. Abstracts and conference proceedings were excluded due to their lack of comprehensiveness. To avoid bias, we placed no restriction on language (beyond having an English abstract) or country of publication.
2.3. Study selection
After removing duplicate studies obtained from multiple databases, two reviewers (JY and ZX) assessed the titles and abstracts of all articles independently to determine inclusion eligibility. Then they reviewed the full texts of all possibly relevant studies. All the inclusion/exclusion decision differences were resolved paper‐by‐paper through consensus.
2.4. Data extraction
Relevant data were extracted and prepared in a standardized manner. We recorded the demographics and study information of each article, including author, evidence level (EL), population age range, gender, and study type. In addition, lesions, laser settings, clinical outcomes, results, and findings were assessed based on reference standards. Complications, recurrence (e.g., within 1 year), remission (e.g., no recurrence within 2 months), clearance (e.g., no recurrence within 3 years), cure rate (e.g., no recurrence within 5 years), and relative data (when available) were also extracted. The primary outcome was the cure rate. Secondary outcomes were the recurrence, death, remission, clearance, and human papillomavirus (HPV)‐detected rates, as well as the laser effectiveness rate (e.g., tumor remission rate, >50%, or <50%). The postoperative complications rate was the safety outcome measure.
2.5. Risk of bias/quality assessment
Due to the abundance of non‐randomized studies in the included literature, two reviewers (JY and ZX) critically appraised (independently) all eligible studies against the Methodological Index for Nonrandomized Studies (MINORS) to assess their quality. 32 , 33 A senior reviewer (ZL) made the final assessment decision when consensus could not be achieved. The MINORS instrument contains 12 items (Supporting Information S3): eight for noncomparative studies with an additional four for comparative studies. A score of 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate) was given for each item, resulting in an ideal maximum score of 24 for comparative studies and 16 for noncomparative studies. For non‐randomized studies, the methodologic quality was assessed as follows: 0–5, very low quality of evidence; 6–10, low quality; 11–15, fair quality; 16, good quality. The outcomes of the risk of bias and quality assessment provided the confidence level for the conclusions drawn in this review.
2.6. Data synthesis and analysis
In this study, we analyzed the following characteristics: number of patients, study design, EL, study quality, and outcomes used for the evaluation of the surgery/treatment effectiveness. Demographic and clinical outcome data could not be presented as mean ± standard deviation due to the lack of available data in most studies. Thus, we collected and calculated the number of various rates from studies, such as cure rate (e.g., no papilloma for 5 years 34 ), and gained the overall rates of KTP/CO2 laser therapy for RRP to compare their therapeutic effects. The p‐value for a continuous variable was calculated using a t‐test, and Fisher exact test was conducted for a categorical variable. A p‐value < .05 was considered statistically significant in this study. To further assess the robustness of the complete primary outcome results, sample size was calculated based on the cure rate at the final follow‐up. By conducting a two‐tail t‐test of 80% power (1 − b) and a .05 significance level using G power software, an estimated sample size of 58 patients per group was required. When a published work lacked sufficient detail, we attempted to contact the authors of those studies to acquire the necessary information.
3. RESULTS
3.1. Search results
We identified 283 unique English abstracts. After assessing these abstracts for relevance, 51 articles were read in full (written in English, Chinese, Spanish, and German) and 15 passed all inclusion criteria (Figure 1).
FIGURE 1.

PRISMA flow diagram
3.2. Description of studies and study characteristics
The 15 studies passing all inclusion criteria were published between 1982 and 2020 (Table 1). Of these, 12 were published in English, 12 , 29 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 2 were published in Chinese, 10 , 44 and 1 was published in Spanish. 1 Five studies were in the KTP group 10 , 29 , 36 , 37 , 44 and 10 were in the CO2 group. 1 , 12 , 34 , 35 , 38 , 39 , 40 , 41 , 42 , 43 These studies involved a total of 614 RRP patients (all sampled from outpatient departments) receiving 2120 laser surgeries, including 102 KTP cases and 512 CO2 cases. There were eight retrospective studies 1 , 12 , 34 , 35 , 38 , 39 , 40 , 42 with only two prospective works 41 , 43 in the CO2 group, whereas the KTP group contained two retrospective 10 , 44 and three prospective studies. 29 , 36 , 37
TABLE 1.
Listing of included studies with relevant details
| Authors (year) | EL | Characteristics | Study type | Lesions | Laser settings | Outcomes | Results | Key findings |
|---|---|---|---|---|---|---|---|---|
| CO 2 laser studies | ||||||||
| Castillo et al. (2010) | IV |
N/proc: 29/NA Age: 14 months–84 years old Gender: 10F/19M |
Retrospective | Laryngeal papillomatosis |
3–6 W continuous power 100–200 Hz repetition rate |
Complications | n = 3/29 (10.4%) | Papillomatosis is characterized as a pathology with an unpredictable course and low probability of malignancy. CO2 laser surgery has meant a revolution in symptomatic treatment, but there is presently no curative treatment. |
| HPV detected | n = 22/29 (75.8%); Mainly HPV6 and HPV11 | |||||||
| Recurrence | n = 13/29 (44.8%) | |||||||
| Remission (no recurrence occurred within two months) | n = 6/29 (20.7%) | |||||||
| Clearance (no recurrence occurred within 3 years) | n = 10/29(34.5%) | |||||||
| Cure (no recurrence occurred within 5 years) | n = 12/29 (41.3%) | |||||||
| Dedo et al. (1982) | IV |
N/proc: 109/548 Age: NA Gender: 43F/66M |
Retrospective | Laryngeal papillomatosis | NA | Complications: acute upper airway obstruction | n = 2 (1.8%) | Treatment of LP with CO2 laser followed by podophyllum painting represents a clear advance over traditional mechanical methods of papilloma removal when considering voice quality, remission rate, and especially incidence of complications and occurrences of death. |
| Complications: anterior glottic webbing | n = 9 (8.1%) | |||||||
| Remission | n = 45 (41.3%) | |||||||
| Malignant degeneration | n = 3 (2.7%) | |||||||
| Death | n = 0 (0%) | |||||||
| Dedo et al. (2001) | IV |
N/proc: 244/548 Age: NA Gender: 81F/163M |
Retrospective | Respiratory papillomas |
20 W continuous power 0.2 s to continuous exposure time 1–2 mm spot size |
Complications: anterior glottic webbing | n = 68 (27%) | A true cure with elimination of all human papilloma viruses (particularly types 6 and 11) will not be achieved until a uniformly effective vaccine or antiviral and immunomodulating agents are developed. |
| Remission | n = 93 (37.3%) | |||||||
| Clearance | n = 15 (6.1%) | |||||||
| Cure | n = 43 (17.2%) | |||||||
| Malignant Transformation | n = 4 (1.6%) | |||||||
| Death | n = 0 (0%) | |||||||
| Holler et al. (2009) | IV |
N/proc: 6/90 Age: 3–17 years old Gender: 6M |
Prospective | Juvenile‐onset recurrent respiratory papillomastosis | NA | Jitter | 4.57% | The data demonstrate a correlation of worsening voice quality with increased exposure to the CO2 laser. |
| Shimmer | 14.66% | |||||||
| NHR | 0.31% | |||||||
| CAPE‐V | 60 | |||||||
| Koji et al. (2019) | IV |
N/proc: 9/14 Age: 30–56 years old Gender: 5 M/4F |
Prospective valid‐action | Recurrent respiratory papillomastosis | 2–3 W continuous or super pulse power | Recurrence | n = 3/9 (33.3%) | CO2 TNFLS is feasible as an in‐office surgery for patients with laryngopharyngeal pathologies. The therapeutic outcome is as expected with the advantages of low patient burden and ease of repetition. |
| Hu et al. (2017) | IV |
N/proc: 6/10 Age: NA Gender: NA |
Retrospective | Recurrent respiratory papillomastosis | 5 W power in super pulse with 0.05 s on and 0.01 s off | Complications | n = 0/10 | With meticulous patient selection, office‐based laryngeal surgery performed using a CO2 laser appears to be a feasible treatment option for various types of vocal lesions. |
| Incomplete surgery | n = 2/10 | |||||||
| Intolerance | n = 1/10 | |||||||
| Preuss et al. (2007) | IV |
N/proc: 64/137 Age: NA Gender: NA |
Retrospective | Recurrent respiratory papillomastosis | 25 W | Complications: glottic webs, scar | n = 4/64 (6%) | Laser microsurgery is the preferential treatment modality due to the low rate of severe scarring and a lower tracheostomy rate as compared with laryngeal microsurgery with cold instruments. |
| Complications: temporary laryngeal edema | n = 2/64 | |||||||
| Complications: airway fire | n = 0/64 | |||||||
| Recurrence, malignant transformation, secondary airway carcinoma | n = 3 (4%) | |||||||
| Robb (1987) | IV |
N/proc: 5/11 Age: 2.5–23 years old Gender: 4F/7M |
Retrospective | Recurrent laryngeal papilloma | 10–30 W, intermittent or pulsed | Complications | n = 0/11 | Compared to other modalities, laser offers the ability to treat frequently the pediatric larynx, with little risk of postoperative edema or bleeding, reduced hospital in‐patient stays, and only mild discomfort. However, using frequent laser treatment, a small number of severely affected children will require tracheotomy for incipient or overt respiratory obstruction. |
| Remission (more than 1 year) | n = 5/11 | |||||||
| Intractable airway obstruction | n = 2/11 | |||||||
| Saleh (1992) | IV |
N/proc: 3/NA Age: 1–7 years old Gender: NA |
Retrospective | Recurrent laryngeal papillomatosis | 8–10 W power | Complications | n = 0/3 | NA |
| Mattot et al. (1990) | IV |
N/proc: 37/595 Age: 1–56 years old Gender: 11F/26M |
Retrospective | Laryngeal papillomatosis | NA | Complications: carcinoma of larynx | n = 1/37 | Number of operations per year does not correlate with eventual remission. |
| Complications: bronchial papillomata | n = 0/37 | |||||||
| Remission | n = 13/37 (35%) | |||||||
| Potassium titanyl phosphate (KTP) laser studies | ||||||||
| Burns et al. (1990) | IV |
N/proc: 37/55 Age: 23–73 years old Gender: 16F/21M |
Prospective uncontrolled | Recurrent laryngeal papillomatosis |
15 ms pulse width 5.25–7.5 J/pulse 2 Hz repetition rate 20–80 J/cm2 fluence |
Complications | n = 0/51 | KTP laser procedure is useful and safe for recurrent papillomatosis. Most patients had >90% of lesion regression at 4–12 weeks postoperation. |
| >90 regression (4–12 weeks) | n = 28/35 | |||||||
| 75%–89% regression | n = 4/35 | |||||||
| 15%–74% regression | n = 3/35 | |||||||
| Hung et al. (2020) | IV |
N/proc: 16/79 Age: 23–73 years old Gender: 6F/10M |
Prospective | Recurrent respiratory papillomatosis |
30–50 ms pulse width 7–8 W 2 Hz repetition rate |
Complications | NA | KTP laser can be an effective tool for managing RRP. Voice quality can be well preserved even after a dozen KTP laser procedures. |
| VHI‐10 | ||||||||
| (1) Before operation | 28.3 | |||||||
| (2) After 1st operation | 12.0 | |||||||
| (3) After 2–5 repeated in‐office or in‐hospital procedures | 10.1 | |||||||
| (4) After 6–10 procedures | 11.0 | |||||||
| CPPs | ||||||||
| (1) Before operation | 6.8 | |||||||
| (2) After 1st operation | 10.5 | |||||||
| (3) After 2–5 repeated in‐office or in‐hospital procedures | 10.9 | |||||||
| (4) After 6–10 procedures | 11.3 | |||||||
| GRB | ||||||||
| (1) Before operation | 5.0 | |||||||
| (2) After 1st operation | 2.4 | |||||||
| (3) After 2–5 repeated in‐office or in‐hospital procedures | 2.4 | |||||||
| (4) After 6–10 procedures | 1.4 | |||||||
| Kaluskar et al. (2009) | IV |
N/proc: 9/NA Age: 39–58 years old Gender: 2F/7M |
Prospective uncontrolled | Inverted papilloma of the nose and paranasal sinuses |
8 W of power in continuous mode At least 80% calibration |
Complications | n = 0/9 | KTP laser is a good option in view of the low rates of recurrence and the minimal postoperative morbidity |
| Recurrence (1 year) | n = 1/9 | |||||||
| Wei et al. (2014) | IV |
N/proc: 18/33 Age: 12–68 years old Gender: F3/M15 |
Retrospective | Recurrence laryngeal papilloma | 6 W of power | Complications | n = 0 | KTP laser is safe and effective in the treatment of recurrent laryngeal papilloma. |
| Cure | n = 11/17 | |||||||
| Effective (tumor remission rate >50%) | n = 3/17 | |||||||
| Ineffective (tumor remission rate <50%) | n = 3/17 | |||||||
| Liu et al. (2005) | IV |
N/proc: 22/NA Age: 3–60 years old Gender: NA |
Retrospective | Laryngeal papilloma | NA | Complications | n = 2/22 | KTP laser treatment is less destructive, with high accuracy and precision, and good hemostatic effect. |
| Cure | n = 19/22 | |||||||
| Recurrence | n = 3/22 | |||||||
Furthermore, the patient populations differed by study, with most studies recruiting patients having recurrent laryngeal/respiratory papillomatosis, whereas others included inverted papilloma, 29 laryngeal papilloma, 1 , 34 , 40 , 44 or juvenile‐onset RRP (Table 1). 41 Note, we included patients with variable locations of papilloma expression (e.g., oral, interpharyngeal) because the pathological states and laser treatments of these patients were similar and thereby sufficiently consistent for inclusion. All studies included both males and females, with a higher percentage of males for both groups (KTP, 65.61%; CO2, 66.25%), but there was no significant difference by sex between the two groups (p > .05; Table 2). However, there was a significant difference between groups for average age before surgery (p < .001; Table 2). In addition, sample sizes per study ranged between just three and 244 (median 39), with sample sizes differing for studies in the CO2 (n = 3–222) and the KTP group (9–39). The ELs of all studies were classified at level IV.
TABLE 2.
Preoperative study characteristics
| Outcome | KTP laser | Patients | CO2 laser | Patients | p‐Value a |
|---|---|---|---|---|---|
| Age | 49.83 ± 7.05 b | 91 | 34.83 ± 7.36 b | 85 | <.001 |
| Male | 65.61% (292 of 445) | 445 | 66.25% (53 of 80) | 80 | >.05 |
Between group KTP and group CO2. Bold indicates statistically significant.
Values are presented as mean ± SD.
3.3. Surgical techniques
The most important point for surgical techniques was laser setting. We found that 11 studies reported the relevant laser setting information, whereas the other four did not. 34 , 40 , 41 , 44 Additionally, laser settings were inconsistent between studies. In the CO2 group, the laser energy setting ranged widely between 2 and 30 W reported across seven studies, 12 , 34 , 43 , 45 , 46 while the laser frequency ranged between 100 and 300 Hz as reported by two studies. 12 , 34 As for the KTP laser, the energy setting fell within a narrower range of just 6–8 W, which is more accurate compared to the reported CO2 laser energy settings across studies. Moreover, the KTP laser frequency was 2 Hz reported by two studies. 36 , 37 Furthermore, most CO2 laser excision surgeries for RRP were conducted in the operation room under general anesthesia, while the majority of KTP laser excision surgeries were performed using local anesthesia.
3.4. Indications and contradictions
All studies in this review explicitly reported that patients were diagnosed with LP or RRP, which was confirmed by pathological specimens and treated with CO2 laser/KTP laser excision. However, contradictions varied from study to study. Patients treated with adjunctive therapies, such as the HPV vaccine, were excluded from this study. In addition, serious cases with systemic metastases, such as in the lung, were also excluded. Moreover, patients treated not only with CO2 laser/KTP laser but also treated at the same time point with other techniques, such as PDL laser and microblade excision, were not included. Age and gender were not limited in scope.
3.5. Clinical outcomes
Overall, the cure rates were significantly different between the two groups (p = .0127), being higher for the KTP group (87.25%) than the CO2 group (75.98%; Table 3). Although the recurrence rate of the CO2 group (18.63%) was relatively higher than the KTP group (10.87%), the difference was not significant (p = .1595). However, the remission rate of the CO2 group (38.9%) was significantly lower than that of the KTP group (88.46%, p < .0001). Other clinical outcomes, such as death, clearance, and HPV‐detected rates, as well as laser effectiveness rate, could not be compared between the two groups in this study due to these parameters being rarely reported in both groups. Yet, the HPV‐detected rate in the CO2 group was 75.86% while the clearance rate was low (9.88%).
TABLE 3.
Comparison of clinical outcomes. Not Reported (NR) refers to clinical outcomes that were not reported.
| Outcome | KTP laser | Surgery/patients | CO2 laser | Surgery/patients | p‐Value a |
|---|---|---|---|---|---|
| Cure | 87.25% (89 of 102) | 102 | 75.98% (389 of 512) | 512 | .0127 |
| Complications | 2.33% (2 of 86) | 86 | 17.71% (88 of 497) | 497 | <.0001 |
| Recurrence | 10.87% (10 of 92) | 92 | 18.63% (19 of 102) | 93 | .1595 |
| Remission | 88.46% (46 of 52) | 52 | 38.90% (156 of 401) | 401 | <.0001 |
| HPV‐detected | NR | NR | 75.86% (22 of 29) | 29 | |
| Clearance | NR | NR | 9.88% (25 of 253) | 23 |
Between group KTP and group CO2. Bold indicates statistically significant.
3.6. Complications rates
The complications rates were just 2.32% (2/86) in the KTP group, but 17.71% (88 of 497) in the CO2 group (Table 3), being significantly different between the two groups (p < .0001). In the KTP group, only two complications were reported, with webbing formed in front of the vocal fissure, causing hoarseness but not affecting breathing. 44 Yet, for the CO2 laser group, a greater number of complications occurred, including mucosal tears, tooth injuries, laryngeal edemas, scarring, stenosis, and web formation (most were anterior glottic webs), and delayed soft tissue complications (i.e., functionally debilitating scar formation with consecutive voice disorders or airway stenosis). 1 , 12 , 34 , 40 , 42 , 43 These complications depended on the invasiveness of papilloma, the instrumentation used during surgery, and the experience of the surgeon. 12
3.7. Risk of bias assessment
The MINORS scores of the five prospective cases in the KTP group averaged 13.1 (SD, 1.45; range, 11–15), suggesting a fair quality of studies (Supporting Information S3). Similarly, scores of the 10 prospective studies in the CO2 group averaged 13.6 (SD, 1.52; range, 12–15), also suggesting fair quality. No statistical difference existed between the MINORS scores of the two groups (p > .05).
4. DISCUSSION
This systematic review assessed 15 clinical studies involving 614 RRP patients treated with CO2 or KTP laser excision. Of these, 2120 surgeries were performed on 102 KTP cases from 5 studies and 512 CO2 cases from 10 studies, separately. Both CO2 and KTP groups improved postoperatively in clinical outcomes. Nevertheless, compared with the CO2 group, the KTP group showed a significantly better cure rate and lower postoperative complications rate. To the best of our knowledge, this work is the first study that systematically and comprehensively compares the clinical outcomes of CO2 and KTP lasers for RRP. However, it should be noted that we found no study that directly compared CO2 and KTP laser treatment for RRP, so a meta‐analysis approach could not be taken.
Although RRP is a benign disease, it is frequently associated with substantial morbidity and mortality. 4 Therefore, balancing treatment goals (e.g., voice preservation and disease regression and/or restoration) with the morbidity and cost of treatment is necessary and should be seriously considered before treatment. 7 Recent studies have found that HPV (a DNA virus) is the cause of RRP. Additional evidence suggests that more than 100 genotypes of HPV infection exist. 47 , 48 , 49 For instance, HPV‐6 and HPV‐11 result in the low‐risk and most common RRP infections, whereas HPV‐16 and HPV‐18 infections are high‐risk but rarely occur. 50 According to the clinical cases reviewed, there were two onset age categories. Juvenile onset RRP (JoRRP) represents patients whose RRP onset began before 12 years old, while AoRRP refers to patients whose RRP onset occurred after 12 years old. 5 , 8 Most JoRRP is transmitted vertically during pregnancy or acquired from an infected mother at birth. As for AoRRP, it is often sexually transmitted, especially via oral sex. 51
Data indicate a trimodal distribution with peak RRP onset ages at seven, 35, and 64 years old. 52 Despite this, researchers often find a bimodal distribution. 5 , 51 , 53 Consequently, a series of anti‐viral drugs have been investigated for preventing and/or treating RRP, including the HPV vaccine, Interferon, Cidofovir, Bevacizumab, and Celecoxib, among others. 5 , 54 For instance, the meta‐analysis of Rosenberg et al. 55 found that the number of surgeries/month significantly declined after long‐term HPV vaccination compared with no vaccination. These adjuvant treatments may benefit patients with RRP treated with surgical excision and more studies are needed to assess the effects of combining KTP laser surgery with adjuvant therapies.
In our review, we found that both KTP and CO2 laser groups demonstrated satisfactory outcomes for RRP in terms of cure rate. Nevertheless, the cure rate for the KTP laser (87.25%) was significantly higher than for the CO2 laser (75.98%), demonstrating that the main therapeutic effect of KTP laser was superior. In addition, though not significant, there was a relatively higher recurrence rate in the CO2 group than the KTP group (18.6% vs. 10.87%, p = .1595). It may appear that the overall recurrence rate was unexpectedly low, but this is due to the definition adopted for this parameter being re‐occurrence within 1 year (see Section 2.4). Another evaluation indicator was remission rate, of which the CO2 group was considerably lower than the KTP group (38.9% vs. 88.46%, p < .0001), while the HPV‐detected and clearance rates were only reported in the CO2 group. Moreover, the safety outcome‐complication rate of the KTP group (2.32%) was considerably lower than the CO2 group (17.71%; p < .0001). These findings indicate that the KTP laser can yield comparatively better outcomes than the CO2 laser for RRP treatment.
According to the literature, several reasons may explain why the KTP laser is superior to the CO2 laser for treating RRP. The CO2 laser—quickly gained popularity for treating RRP since the 1960s. 14 , 18 Many therapeutic options have also been advocated for RRP, such as microblade and PDL laser, but surgical removal using CO2 laser remains the most important single treatment choice available. 4 , 56 , 57 Although the 10,600 nm wavelength of the CO2 laser is well absorbed by water in biological tissue and is suitable for fine surgical cutting, its application to remove laryngeal lesions is not without risk. 38 , 58 Thermal injury, excessive resection, and repeated surgeries may result in a loss of pliable vocal fold tissue, fibrosis, and scar formation, which can significantly affect the voice and quality of life. 36 , 59
In contrast, KTP laser treatment has both the cutting function of the CO2 laser and the hemostatic effect of the PDL laser, so its application in laryngeal microsurgery has many advantages: (1) The operation is significantly less destructive than the traditional approach, without laryngeal laceration and with less tracheotomy ratio. In addition, KTP laser is more likely to preserve postoperative laryngeal function, and the postoperative hospital stays are shorter (meaning less cost), requiring only 2 or 3 days. 44 (2) The accuracy and precision of surgery are improved by using a KTP laser. The tumor boundary can be clearly seen under the microscope, and the level of incision can be distinguished so that the lesion can be completely removed while minimizing collateral damage. 60 (3) The fiber‐based delivery of the KTP laser with the technical advancement of distal‐tip endoscopy enables surgical procedures to be performed in office settings under local anesthesia, meaning that considerable time and medical expenses can be saved. 30 , 37 Nevertheless, it should be noted that CO2 laser treatment has also been reported in a recent study to have been performed in an office setting. 43 Thus, further studies should be conducted that specifically compare differences when the two lasers are used in an office setting to treat RRP. (4) The KTP laser has a good hemostatic effect and allows the surgery to be performed in a bloodless manner. Especially in children with laryngeal papilloma invading the supraglottis, tumors have rich blood supplies. Thus, under these conditions, the KTP laser can be applied to great advantage, resulting in a clearer field and a well‐defined incision, reducing collateral damage. 5 , 55 Nevertheless, despite the abovementioned advantages of KTP, there is still a major limitation on its adoption and widespread use, namely that KTP laser equipment is relatively more expensive than CO2 lasers.
Our review has several limitations that should be mentioned. First, the available studies and data about KTP laser used for RRP were very limited (102 patients). The case number only achieved the minimal sample number after conducting a sample size estimation (58 in each group). Second, much data reported by different studies were not consistent between the KTP and CO2 groups. For example, the HPV positive and clearance rates for RRP were always presented in the CO2 group but rarely appeared in the KTP group, 1 , 42 making it impossible to compare those parameters and potentially influencing the results of this study. We, therefore, recommend that future studies report these data for patients with RRP whenever possible. In addition, the age difference (before surgery) between the CO2 group and KTP group should be noted. That being said, we believe that due to the way most studies in the CO2 group did not report the detailed ages of patients (419/510 patients), the age data from the two groups were not entirely representative, so the difference is not likely to be a serious problem. Furthermore, most studies did not clearly report their follow‐up times, which may result in unavoidable heterogeneity in parameters such as cure rate. Third, high‐quality comparative evidence is noticeably insufficient as most studies meeting our inclusion criteria were at level IV. Nevertheless, the MINORS scores of these studies averaged 13.1 and 13.6 in the CO2 and KTP groups, respectively, demonstrating the evidence was of fair quality. Future research is necessary in the form of standard‐evaluation prospective multicenter randomized controlled studies. Fourth, key functional parameters such as vocal outcomes were not reported in sufficient detail in the available studies, limiting the safety outcome only to complications. Future studies should take care to clearly report these data whenever possible.
5. CONCLUSION
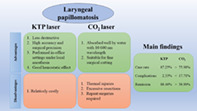
This systematic review demonstrated that the clinical outcomes of KTP laser and CO2 laser were good for treating RRP. However, overall, our findings indicate that KTP laser may be a good treatment option with superior outcome and lower postoperative complication rates than CO2 laser treatment (Figure 2). In the future, more high‐quality randomized controlled studies on the long‐term outcomes of these two techniques are needed to further evaluate them, especially study designs that directly compare the two laser treatment approaches. Studies must also provide all relevant pre‐ and postoperative functional parameters.
FIGURE 2.

Comparison of KTP and CO2 laser for treating laryngeal papillomatosis/ recurrent respiratory papillomatosis
FUNDING INFORMATION
This study did not receive any specific funding from agencies in the public, private, or not‐for‐profit sectors.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Data S1 PRISMA checklist.
Data S2 Search strategy.
Data S3 MINORS score.
ACKNOWLEDGMENT
The authors would like to thank Dr. Zhiwen Luo for providing critical support and expertise in preparing this manuscript.
Yang J, Xie Z, Seyler BC. Comparing KTP and CO2 laser excision for recurrent respiratory papillomatosis: A systematic review. Laryngoscope Investigative Otolaryngology. 2022;7(4):970‐981. doi: 10.1002/lio2.871
Jimin Yang and Zhongcheng Xie contributed equally to this study.
DATA AVAILABILITY STATEMENT
All data that support the findings of this work are available upon reasonable request to the corresponding author.
REFERENCES
- 1. Gutiérrez Castillo C, Monerris García E, Duran MD, Sancho Mestre M, Gras JR. Papilomas y papilomatosis laríngea. Tratamiento con láser CO2. Nuestra experiencia en 15 años. Acta Otorrinolaringol Esp. 2010;61(6):422‐427. doi: 10.1016/j.otorri.2010.07.006 [DOI] [PubMed] [Google Scholar]
- 2. Kim HT, Baizhumanova AS. Is recurrent respiratory papillomatosis a manageable or curable disease? Laryngoscope. 2016;126(6):1359‐1364. doi: 10.1002/lary.25795 [DOI] [PubMed] [Google Scholar]
- 3. Maturo SC, Hartnick CJ, Maturo SC, Hartnick CJ. Juvenile‐onset recurrent respiratory papillomatosis. Pediatr Airw Surg. 2012;73:105‐108. doi: 10.1159/000334457 [DOI] [PubMed] [Google Scholar]
- 4. Valerie A, Vasiliki KE. Recurrent respiratory papillomatosis: an extensive review. Int J Hematol Stem Cell Res. 2012;6(3):19‐28. http://ijhoscr.tums.ac.ir/index.php/ijhoscr/article/view/312/325 [Google Scholar]
- 5. Ivancic R, Iqbal H, deSilva B , Pan Q, Matrka L. Current and future management of recurrent respiratory papillomatosis. Laryngoscope Investig Otolaryngol. 2018;3(1):22‐34. doi: 10.1002/lio2.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kurita T, Chitose S‐I, Sato K, et al. Pathological mechanisms of laryngeal papillomatosis based on laryngeal epithelial characteristics. Laryngoscope Investig Otolaryngol. 2019;4(1):89‐94. doi: 10.1002/lio2.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim D, Siegel J, Chouake RJ, et al. Implication and management of incidental oropharyngeal papillomas—a retrospective case series review. Ear Nose Throat J. 2019;100(4):145561319871228. 10.1177/0145561319871228 [DOI] [PubMed] [Google Scholar]
- 8. Wiatrak BJ. Overview of recurrent respiratory papillomatosis. Curr Opin Otolaryngol Head Neck Surg. 2003;11(6):433‐441. doi: 10.1097/00020840-200312000-00005 [DOI] [PubMed] [Google Scholar]
- 9. Lechien JR, Burns JA, Akst LM. The use of 532‐nanometer‐pulsed potassium‐titanyl‐phosphate (ktp) laser in laryngology: a systematic review of current indications, safety, and voice outcomes. Ear Nose Throat J. 2020;100(1):4S‐13S. doi: 10.1177/0145561319899183 Published online 2020. [DOI] [PubMed] [Google Scholar]
- 10. Wei C, Zhang P, Peijie He KX. KTP laser treatment for recurrent laryngeal papilloma. 13th Natl Acad Conf Otolaryngol Neck Surg Chinese Med Assoc. 2014:202–203.
- 11. Dodhia S, Baxter PC, Ye F, et al. Investigation of the presence of HPV on KTP laser fibers following KTP laser treatment of papilloma. Laryngoscope. 2018;128(4):926‐928. doi: 10.1002/lary.27018 [DOI] [PubMed] [Google Scholar]
- 12. Florian Preuss S, Peter Klussmann J, Jungehulsing M, Edmund Eckel H, Guntinas‐Lichius O, Damm M. Long‐term results of surgical treatment for recurrent respiratory papillomatosis. Acta Otolaryngol. 2007;127(11):1196‐1201. doi: 10.1080/00016480701200350 [DOI] [PubMed] [Google Scholar]
- 13. Toma S, Hudson K, Harriess M. Abstracts of the 14th British Academic Conference in Otolaryngology Posters—laryngology. Clin Otolaryngol. 2012;37(suppl 1):118‐129. doi: 10.1111/j.1749-4486.2012.02513.x [DOI] [PubMed] [Google Scholar]
- 14. Motta G, Villari G, Pucci V, De Clemente M. CO2 laser in laryngeal microsurgery. Int Surg. 1987;72(3):175‐178. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed3&NEWS=N&AN=18673423 [PubMed] [Google Scholar]
- 15. Pratt LW. The CO2 laser in otolaryngology. J Maine Med Assoc. 1980;71(2):39‐45. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed3&NEWS=N&AN=10530249 [PubMed] [Google Scholar]
- 16. Damm M, Eckel HE, Schneider D, Arnold G. CO2 laser surgery for verrucous carcinoma of the larynx. Lasers Surg Med. 1997;21(2):117‐123. doi: [DOI] [PubMed] [Google Scholar]
- 17. Keane WM, Atkins JP. CO2 laser surgery of the upper airway. Surg Clin North Am. 1984;64(1):955‐971. doi: 10.1016/S0039-6109(16)43439-0 [DOI] [PubMed] [Google Scholar]
- 18. Wellenstein DJ, Honings J, Schimberg AS, et al. Office‐based CO2 laser surgery for benign and premalignant laryngeal lesions. Laryngoscope. 2020;130(6):1503‐1507. doi: 10.1002/lary.28278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Strong MS, Vaughan CW, Cooperband SR, Healy GB, Clemente MA. Recurrent respiratory papillomatosis. Management with the CO2 laser. Ann Otol Rhinol Laryngol. 1976;85(4I):508‐516. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed2&NEWS=N&AN=7146817 [DOI] [PubMed] [Google Scholar]
- 20. McMillan K, Shapshay SM, McGilligan JA, Wang Z, Rebeiz EE. Preliminary clinical results of pulsed dye laser therapy for recurrent respiratory papillomatosis. In: Anderson RR, Bartels KE, Bass LS, et al., eds. Lasers in Surgery: Advanced Characterization, Therapeutics, and Systems VIII, Proceedings of Vol 3245: Proceedings of the Society of Photo‐Optical Instrumentation Engineers. SPIE; 1998:182‐190. doi: 10.1117/12.312286 [DOI] [Google Scholar]
- 21. Valdez TA, McMillan K, Shapshay SM. A new laser treatment for vocal cord papilloma—585‐nm pulsed dye. Otolaryngol Head Neck Surg. 2001;124(4):421‐425. doi: 10.1067/mhn.2001.113944 [DOI] [PubMed] [Google Scholar]
- 22. McMillan K, Shapshay SM, McGilligan JA, et al. A 585‐nanometer pulsed dye laser treatment of laryngeal papillomas: preliminary report. Laryngoscope. 1998;108(7):968‐972. doi: 10.1097/00005537-199807000-00003 [DOI] [PubMed] [Google Scholar]
- 23. Abitol J, Abitol P. Pulse dye laser: laryngeal microsurgical procedure by non‐invasive laser surgery on outpatients about 57 cases. Lasers Surg Med. 2013;45(suppl 25):67‐68. doi: 10.1002/lsm.22127 23440713 [DOI] [Google Scholar]
- 24. Snoeck R, Wellens W, Desloovere C, et al. Treatment of severe laryngeal papillomatosis with intralesional injections of cidofovir [(S)‐1‐(3‐hydroxy‐2‐phosphonylmethoxypropyl)cytosine]. J Med Virol. 1998;54(3):219‐225. doi: [DOI] [PubMed] [Google Scholar]
- 25. Coulombeau B, Nusa Naiman A, Ceruse P, Froehlich P. Anti‐viral injectable treatment (cidofovir) in laryngeal papillomatosis. Rev Laryngol Otol Rhinol (Bord). 2002;123(5):315‐320. [PubMed] [Google Scholar]
- 26. Chhetri DK, Blumin JH, Shapiro NL, Berke GS. Office‐based treatment of laryngeal papillomatosis with percutaneous injection of cidofovir. Otolaryngol Head Neck Surg. 2002;126(6):642‐648. doi: 10.1067/mhn.2002.125604 [DOI] [PubMed] [Google Scholar]
- 27. Koufman J. Office‐based 532‐nm pulsed KTP laser treatment of glottal papillomatosis and dysplasia. Ann Otol Rhinol Laryngol. 2007;116(4):317. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed10&NEWS=N&AN=46821157 [PubMed] [Google Scholar]
- 28. Xie X, Young J, Kost K, McGregor M. KTP 532‐nm laser for laryngeal lesions. A systematic review. J Voice. 2013;27(2):245‐249. doi: 10.1016/j.jvoice.2012.11.006 [DOI] [PubMed] [Google Scholar]
- 29. Kaluskar SK, Mehta R, Farnan TB, Basha SI. Endoscopic 532‐nm KTP laser excision of inverted papilloma of the nose and paranasal sinuses: a series of 9 patients. Ear Nose Throat J. 2009;88(4):880‐887. [PubMed] [Google Scholar]
- 30. Hess M, Fleischer S. Photoangiolytic lasers in laryngology. Laryngorhinootologie. 2020;99(9):185‐191. doi: 10.1055/a-1071-0410 [DOI] [PubMed] [Google Scholar]
- 31. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group T. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Rev Esp Nutr Humana Diet. 2014;18(3):172‐181. doi: 10.14306/renhyd.18.3.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luo Z, Lin J, Sun Y, Zhu K, Wang C, Chen J. Outcome comparison of latissimus dorsi transfer and pectoralis major transfer for irreparable subscapularis tendon tear: a systematic review. Am J Sports Med. 2021;50(7):2032‐2041. doi: 10.1177/03635465211018216 [DOI] [PubMed] [Google Scholar]
- 33. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non‐randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712‐716. doi: 10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- 34. Dedo HH, Jackler RK. Laryngeal papilloma: results of treatment with the CO2 laser and podophyllum. Ann Otol Rhinol Laryngol. 1982;91(4 I):425‐430. doi: 10.1177/000348948209100421 [DOI] [PubMed] [Google Scholar]
- 35. Hu H‐C, Lin S‐Y, Hung Y‐T, Chang S‐Y. Feasibility and associated limitations of office‐based laryngeal surgery using carbon dioxide lasers. JAMA Otolaryngol Neck Surg. 2017;143(5):485‐491. doi: 10.1001/jamaoto.2016.4129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hung WC, Lo WC, Fang KM, Cheng PW, Wang CT. Longitudinal voice outcomes following serial potassium titanyl phosphate laser procedures for recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol. 2020;130(4):363‐369. doi: 10.1177/0003489420950374 [DOI] [PubMed] [Google Scholar]
- 37. Burns JA, Zeitels SM, Akst LM, Broadhurst MS, Hillman RE, Anderson R. 532 nm pulsed potassium‐titanyl‐phosphate laser treatment of laryngeal papillomatosis under general anesthesia. Laryngoscope. 2007;117(8):1500‐1504. doi: 10.1097/MLG.0b013e318064e869 [DOI] [PubMed] [Google Scholar]
- 38. Saleh EM. Complications of treatment of recurrent laryngeal papillomatosis with the carbon dioxide laser in children. J Laryngol Otol. 1992;106(8):715‐718. doi: 10.1017/S0022215100120663 [DOI] [PubMed] [Google Scholar]
- 39. Robb PJ. The CO2 laser and management of recurrent laryngeal papilloma: the Guy's experience by. J Laryngol Otol. 1987;101(4):369‐375. doi: 10.1017/S0022215100101811 [DOI] [Google Scholar]
- 40. Mattot M, Ninane J, Hamoir M, et al. Combined CO2‐laser and alfa recombinant interferon treatment in five children with juvenile laryngeal papillomatosis. Acta Clin Belg. 1990;45(3):158‐163. doi: 10.1080/17843286.1990.11718082 [DOI] [PubMed] [Google Scholar]
- 41. Holler T, Allegro J, Chadha NK, et al. Voice outcomes following repeated surgical resection of laryngeal papillomata in children. Otolaryngol Neck Surg. 2009;141(4):522‐526. doi: 10.1016/j.otohns.2009.06.080 [DOI] [PubMed] [Google Scholar]
- 42. Dedo HH, Yu KCY. CO2 laser treatment in 244 patients with respiratory papillomas. Laryngoscope. 2001;111(9):1639‐1644. doi: 10.1097/00005537-200109000-00028 [DOI] [PubMed] [Google Scholar]
- 43. Araki K, Tomifuji M, Uno K, et al. Feasibility of transnasal flexible carbon dioxide laser surgery for laryngopharyngeal lesions. Auris Nasus Larynx. 2019;46(5):772‐778. doi: 10.1016/j.anl.2019.01.008 [DOI] [PubMed] [Google Scholar]
- 44. Liu F, Shi L, Ji WY. 22 cases of laryngeal papilloma treated with potassium titanium phosphate laser. J Clin Otorhinolaryngol Head Neck Surg. 2005;01(18):1001‐1781. [Google Scholar]
- 45. Gutierrez Castillo C, Monerris Garcia E, Duran MD, et al. Papillomas & laryngeal papillomatosis. Treatment with CO2 laser surgery. Our experience over 15 years. Acta Otorrinolaringol Esp. 2010;61(6):422‐427. doi: 10.1016/j.otorri.2010.07.006 [DOI] [PubMed] [Google Scholar]
- 46. Hu H, Zhang Q, Sun G, et al. Treatment of recurrent laryngeal papilloma by submucosal resection and the effect on prognosis. Lin chuang er bi yan hou tou jing wai ke za zhi = J Clin Otorhinolaryngol Head Neck Surg. 2015;29(21):1873‐1877. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed16&NEWS=N&AN=611139790 [PubMed] [Google Scholar]
- 47. Best SR, Esquivel D, Mellinger‐Pilgrim R, et al. Infectivity of murine papillomavirus in the surgical byproducts of treated tail warts. Laryngoscope. 2020;130(3):712‐717. doi: 10.1002/lary.28026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Best SR, Esquivel D, Mellinger‐Pilgrim R, RBS R, Pitman MJ. Nasopharyngeal papillomas treated with CO2 laser and human papillomavirus vaccination. Laryngoscope. 2017;127(10):2279‐2281. doi: 10.1002/lary.26580 [DOI] [PubMed] [Google Scholar]
- 49. Ilmarinen T, Auvinen E, Hiltunen‐Back E, Ranki A, Aaltonen LM, Pitkäranta A. Transmission of human papillomavirus DNA from patient to surgical masks, gloves and oral mucosa of medical personnel during treatment of laryngeal papillomas and genital warts. Eur Arch Oto‐Rhino‐Laryngol. 2012;269(11):2367‐2371. doi: 10.1007/s00405-012-2049-9 [DOI] [PubMed] [Google Scholar]
- 50. Snoeck R, Wellens W, Desloovere C, et al. Laryngeal papillomatosis: clinical, histopathologic and molecular studies. Clin Otolaryngol Off. 2015;11(3):221‐224. doi: 10.2147/TCRM.S81825 [DOI] [Google Scholar]
- 51. Fortes HR, von Ranke FM, Escuissato DL, et al. Recurrent respiratory papillomatosis: a state‐of‐the‐art review. Respir Med. 2017;126:116‐121. doi: 10.1016/j.rmed.2017.03.030 [DOI] [PubMed] [Google Scholar]
- 52. San Giorgi MRM, van den Heuvel ER, Tjon Pian Gi REA, et al. Age of onset of recurrent respiratory papillomatosis: a distribution analysis. Clin Otolaryngol. 2016;41(5):448‐453. doi: 10.1111/coa.12565 [DOI] [PubMed] [Google Scholar]
- 53. Maïga S, Ndiaye C, Diouf MS, et al. Laryngeal papillomatosis in Senegal: a ten‐year experience. Eur Ann Otorhinolaryngol Neck Dis. 2018;135(3):221‐224. doi: 10.1016/j.anorl.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 54. Kin Cho Goon P, Scholtz LU, Sudhoff H. Recurrent respiratory papillomatosis (RRP)‐time for a reckoning? Laryngoscope Investig Otolaryngol. 2017;2(4):184‐186. doi: 10.1002/lio2.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tu Y‐P, Jennings R, Hart B, et al. Therapeutic use of the HPV vaccine on recurrent respiratory papillomatosis: a systematic review and meta‐analysis Rosenberg. J Gerontol Ser A Biol Sci Med Sci. 2018;0813(April):1‐11. [Google Scholar]
- 56. Scatolini ML, Labedz G, Cocciaglia A, et al. Laryngeal sequelae secondary to surgical treatment for recurrent respiratory papillomatosis in children. Int J Pediatr Otorhinolaryngol. 2020;130:109815. doi: 10.1016/j.ijporl.2019.109815 [DOI] [PubMed] [Google Scholar]
- 57. Nielsen NR, Rasmussen N. Laryngeal papillomatosis treated with CO2 laser. Ugeskr Laeger. 2010;172(4):284‐289. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed11&NEWS=N&AN=358573061 [PubMed] [Google Scholar]
- 58. Halum SL, Moberly AC. Patient tolerance of the flexible CO2 laser for office‐based laryngeal surgery. J Voice. 2010;24(6):750‐754. doi: 10.1016/j.jvoice.2009.04.005 [DOI] [PubMed] [Google Scholar]
- 59. Hirano M. Clinical examination of voice by Minoru Hirano. J Acoust Soc Am. 1986;80(4):1273. [Google Scholar]
- 60. Koufman J, Zeitels SM, Akst LM, et al. Office‐based 532 nm pulsed KTP laser treatment of glottal papillomatosis and dysplasia. Ann Otol Rhinol Laryngol. 2006;115(9I):679‐685. doi: 10.1177/000348940611500905 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 PRISMA checklist.
Data S2 Search strategy.
Data S3 MINORS score.
Data Availability Statement
All data that support the findings of this work are available upon reasonable request to the corresponding author.


