Abstract
Objectives
Children with type I laryngeal clefts and sialorrhea can have posterior drooling, aspiration of oral secretions, and respiratory complications. Laryngeal cleft injection laryngoplasty (LCIL) and salivary botulinum injections (Sal‐Bot) have been used separately for short‐term treatment of type I laryngeal clefts and sialorrhea. Our goal was to evaluate combined LCIL and Sal‐Bot and create recommendations for further treatment based on response to initial treatment.
Methods
Retrospective chart review of nine patients who underwent direct laryngoscopy and bronchoscopy with combined LCIL and Sal‐Bot from 2012 to 2019. Charts were reviewed for patient characteristics, response to treatment, and pre and post‐op hospitalizations. Subsequent procedures were performed depending on efficacy of initial treatments.
Results
Nine patients were identified. All had pre‐existing neurologic conditions, gastrostomy tubes, and a history of coughing and choking on secretions. Only one patient was able to feed orally (purees). 1 U/kg of botulinum toxin per gland was injected into each parotid and submandibular gland. The average units of botulinum toxin injected was 67 U. The mean laryngeal cleft injection volume was 0.35 cc. Subsequent treatment was based on timing of symptomatic improvement and individual patient factors. Five patients had respiratory‐related hospitalizations in year preceding the procedures (median 1.5, range 1–10). Three (60%) had a reduction in respiratory‐related hospitalization 1 year post procedure (median 1, range 1–3). One patient died during the follow up period due to continued chronic respiratory failure.
Conclusions
This is the first study of combined laryngeal cleft injection laryngoplasty and botulinum toxin injections for patients with posterior laryngeal penetration and aspiration of oropharyngeal secretions. We highlight strategies for choosing subsequent procedures based on response to initial treatment.
Level of Evidence
4.
Keywords: aspiration, botulinum injection, laryngeal cleft, laryngeal cleft injection laryngoplasty, sialorrhea
We evaluated combined laryngeal cleft injection laryngoplasty (LCIL) and salivary botulinum injections (Sal‐Bot) for posterior drooling, aspiration of oral secretions, and respiratory complications in nine patients with type I laryngeal clefts and sialorrhea and proposed a treatment paradigm for similar patients.
1. INTRODUCTION
Laryngeal clefts in children can present with respiratory symptoms such as choking, feeding issues, recurrent respiratory infections, pneumonia, stridor, and cyanosis. 1 A laryngeal cleft is an abnormal posterior communication between the larynx and the pharynx/esophagus. 2 Based on the Benjamin and Inglis classification scheme, type I laryngeal clefts do not extend below the level of the vocal cords. 3 In children evaluated for aspiration or other respiratory diseases, the incidence of type 1 laryngeal clefts was found to be 4.4%. 4
The overarching goal of type 1 laryngeal cleft management is the prevention of pulmonary complications. Laryngeal cleft injection laryngoplasty (LCIL) is an accepted method of diagnosing and treating type 1 laryngeal clefts. LCIL involves injecting a biologic material into the submucosa of the interarytenoid space. 5 LCIL has seen favorable results in reduction of symptoms such as dysphagia and aspiration in pediatric patients. There are no permanent injectable materials, but LCIL can been useful in identifying patients that might benefit from further surgical intervention. 6 , 7 , 8
Children with neurological disorders may have difficulty managing their own secretions and thus present with sialorrhea and/or aspiration of secretions, which may result in aspiration pneumonia. 9 There are two type of sialorrhea: anterior drooling, saliva excretion through the front of the mouth, and posterior drooling, saliva spilling posteriorly into the oropharynx. 10 Posterior drooling has been associated with aspiration, which is a major cause of morbidity and mortality in children with cerebral palsy. 11
Salivary gland botulinum toxin injection (Sal‐Bot) with or without ultrasound guidance/sedation is often used for sialorrhea management. 9 , 12 In this procedure, botulinum toxin is injected into the submandibular and/or parotid glands. The toxin blocks the release of acetylcholine and causes decreased saliva production. Salivary gland botulinum injections have been shown to decrease drooling frequency and severity, lessening the impact of drooling on patients and their families. 13
Decreased saliva production after Sal‐Bot with Botox‐A in the short term occurs in >80% of children with sialorrhea. 14 In a small number of patients there is no change in secretions. For those with a response, the decrease in saliva production lasts 2–5 months, with the peak occurring around 2–4 weeks. 14 A randomized controlled trial showed that Sal‐Bot had a 26.9% efficacy in reducing symptoms by over 50% based on survey after 32 weeks. 15 In contrast, the effect of LCIL is almost immediate. 16 Per the manufacturer carboxymethylcellulose is resorbed in 3–6 months. When observed in a canine model, the carboxymethylcellulose was completely resorbed around 6–8 weeks. 2 Anecdotally, it is our experience that LCIL are resorbed in 1–3 months. Therefore, in patients with laryngeal clefts, recurrence of symptoms could be seen a few months after LCIL. 17
There is a population of pediatric patients with both type I laryngeal clefts and sialorrhea. Although LCIL and Sal‐Bot have been used separately as therapies for type I laryngeal clefts and sialorrhea, there is little information on combined LCIL and Sal‐Bot.
The purpose of this study is to review our experience with concurrent LCIC and Sal‐Bot injections.
2. METHODS
With approval from the Baylor College of Medicine Institutional Review Board (H‐47290), a retrospective chart review was performed on patients who underwent concurrent LCIL and Sal‐Bot injections from January 2012 to January 2020. Patients gave informed consent prior to participation in the study. Only patients who had Type 1 laryngeal clefts were included in the study. Data collected included demographics, past medical history, feeding modality, number of bibs used per day, number of hospitalizations for respiratory diagnoses including acute respiratory distress, acute on chronic respiratory failure and pneumonia 1 year before and 1 year after LCIL and Sal‐Bot Injections. Neurology notes were reviewed to estimate achieve developmental milestones.
All patients underwent direct laryngoscopy and bronchoscopy. After suspension laryngoscopy, the interarytenoid region was palpated to confirm the presence of a type 1 laryngeal cleft. Palpation for the presence of a laryngeal cleft was performed in cases with signs or symptoms of aspiration including coughing and choking on secretions. A laryngeal cleft was then injected with carboxymethycellulose (Prolaryn voice gel, Raleigh, NC) only in patients who has a confirmed laryngeal cleft at the discretion of the performing surgeon. In addition, Onabotulinum Toxin A (Botox, Allergan, Dublin, Ireland) was injected at 1 U/kg per gland into the parotid and submandibular glands with ultrasound guidance (max dose 100 units total). Patients were followed for response to treatment, and subsequent procedures were performed depending on efficacy of initial treatments.
3. RESULTS
Nine patients were identified who had undergone concurrent LCIL and Sal‐Bot injections. All patients in this study had neurologic conditions such as cerebral palsy (CP) or hypoxic ischemic encephalopathy (HIE) (Table 1). The average age was 4.18 years (1.12–10.89 years). All patients had feeding difficulties that required placement of gastronomy tube. One patient (patient 1) was able to tolerate pureed feeds by mouth. One patient (patient 8) had a pre‐existing tracheostomy tube.
TABLE 1.
Patient demographics and medical history.
| Pt | Age (years) | Gender | Developmental notes | Medical comorbidities | Gastronomy | Units of botulinum toxin injected | Volume of prolaryn gel injected (cc) |
|---|---|---|---|---|---|---|---|
| (Y/N) | |||||||
| 1 | 1.89 | M | 6‐month‐old developmental age | Dandy Walker malformation, seizure, polymicrogyria | Y | 100, 100, 30 | 0.50 |
| 2 | 4.44 | M | Responsive to painful stimuli, unable to follow commands and nonverbal | Myoclonic epilepsy, static encephalopathy, dystonic CP | Y | 80 | 0.70 |
| 3 | 8.26 | M | Non‐verbal, poor head control, does not sit up | Seizure disorder, CP, dystonia, seizure, hydrancephaly | Y | 56 | 0.50 |
| 4 | 1.13 | M | Non‐verbal, poor head control, sits with support | Moderate to severe HIE, epilepsy | Y | 40 | 0.25 |
| 5 | 2.96 | M | Globally delayed, but no specific assessments due to frequent hospitalizations | CP, spastic, severe HIE | Y | 72 | 0.30 |
| 6 | 2.38 | M | 9‐month‐old developmental age | 24‐week gestation, Dandy Walker malformation, ataxic CP | Y | 40 | 0.15 |
| 7 | 1.51 | F | 4‐month‐old developmental age | Severe HIE, hypotonia, seizures | Y | 50 | Not recorded |
| 8 | 10.89 | F | Non‐verbal, full dependence for activities of daily living | HIE, CP quadriplegic, anoxic brain injury, tracheostomy | Y | 100, 100 | 0.20 |
| 9 | 1.64 | M | 4‐month‐old developmental age | Spastic CP, HIE | Y | 40 | 0.20 |
Abbreviations: HIE, hypoxic–ischemic encephalopathy; CP, cerebral palsy.
Between 10 and 25 U of botulinum were injected into each parotid and submandibular gland. The total amount of botulinum injected over four glands ranged from 40 to 100 U. Patient 1 was injected with botulinum in three separate sessions with the first two using 100 U each and the last using 30 U. Patient 8 received two sessions of botulinum injections with 100 U used in each. All other patients received a single session of botulinum injections. The average units of botulinum toxin injected was 67 U (30–100 U). The average volume of the laryngeal cleft injections was 0.35 cc (0.15–0.7 cc).
Five patients had at least one respiratory‐related hospitalization in the year prior to the procedures (Table 2). The median number of pre‐injection respiratory hospitalizations in this group was 1.5 (range 1–10). Among those patients, the median number of 1‐year respiratory hospitalizations post‐injection was 1 (range 1–3). Patient 5 died during the 1 year follow up period due to continued chronic respiratory failure related to their underlying disease process and was excluded from pre and post procedure hospitalization statistics.
TABLE 2.
Patient symptoms, hospitalizations, and subsequent procedures.
| Pt | Presenting symptoms | Respiratory hospitalizations 1 year before LCIL and Sal‐Bot injection | Respiratory hospitalizations 1 year after LCIL and Sal‐Bot injection | 30‐day adverse events from LCIL and Sal‐Bot injection | Subsequent procedure | 30‐day adverse events from subsequent procedure |
|---|---|---|---|---|---|---|
| 1 | Coughing and choking on secretions, 6 bibs/day | 0 | 0 | None | Botulinum toxin injections and eventual submandibular gland excision | None |
| 2 | Coughing, choking on secretions, aspiration, recurrent respiratory infections | 5 | 2 | None | Planned botulinum toxin and laryngeal cleft injection, but patient had respiratory failure requiring tracheostomy | N/A |
| 3 | Choking on secretions, 1–2 bibs/day | 0 | 0 | Transient increase in apneic events 1 week post procedure | No subsequent procedures needed | N/A |
| 4 | Choking on secretions, 3–4 bibs/day, recurrent respiratory symptoms | 3 | 2 | None | Laryngeal cleft repair & repeat botulinum injection | None |
| 5 | Choking on secretions, constant drooling, recurrent respiratory symptoms | 8 | 4 | Bronchospasm, ICU admission | Hospice care, deceased | N/A |
| 6 | Choking on secretions, 1–2 bibs/day, dysphagia | 0 | 0 | None | Laryngeal cleft repair | N/A |
| 7 | Choking on secretions, 4–5 bibs/day, recurrent admissions for respiratory symptoms | 10 | 3 | None | Combined laryngeal cleft repair and four duct ligation | None |
| 8 | Increased tracheostomy secretions, 1–2 bibs/day, recurrent respiratory secretions | 2 | 1 | None | Laryngeal cleft repair | N/A |
| 9 | Choking and gagging on secretions, respiratory infections, 1–2 bibs/day | 1 | 1 | None | No subsequent procedures needed | N/A |
All patients presented with coughing and choking on secretions. Subsequent treatment was based on response to combined Sal‐Bot and LCIL as shown in Figure 1. Patient 1 had no change in coughing and choking within the first 2 weeks but had improvement during week 3 as drooling decreased. For this patient, since effect was driven by Sal‐Bot, a repeat salivary gland botulinum toxin injection was recommended, and the patient eventually underwent bilateral submandibular gland excision.
FIGURE 1.
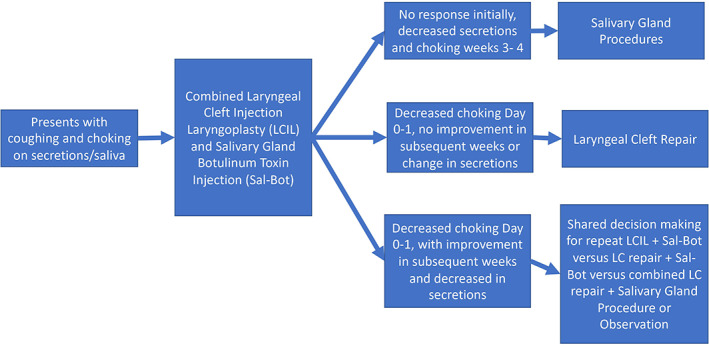
Schema of patients.
Patients 6 and 8 had improved coughing and choking on secretions within days of Sal‐Bot and LCIL but had no effect on drooling or no improvement in the subsequent weeks. These patients went on to have endoscopic laryngeal cleft repairs only.
Five patients had improved coughing and choking within a day of initial combined treatments and then had further improvements over the first 3–4 weeks and/or decreased drooling over that time. With shared decision‐making further treatments were tailored. Patient 2 had a planned repeat Sal‐Bot and LCIL that was deferred due to progressive hypotonia, hypoventilation, and obstructive sleep apnea over the course of 1 year necessitating tracheostomy. Patients 3 and 9 had durable improvement in drooling and choking on secretions and have not required any additional interventions. Patient 4 underwent laryngeal cleft repair and Sal‐Bot. Patient 7 who had 10 episodes of respiratory hospitalizations in the preceding year, underwent combined laryngeal cleft repair and four duct ligation a year after LCIL and Sal‐Bot injections. Patient 5 had no response to treatment had worsening respiratory infections and hypoventilation as a natural progression of his underlying disease. Hospice care was recommended, and the patient subsequently died.
4. DISCUSSION
Patients with underlying neurologic diagnoses in additional to sialorrhea and laryngeal clefts are at risk of aspiration of secretions and associated respiratory complications. Controversy exists in the efficacy of thin liquid intake restriction in prevention of aspiration related lung disease. 18 At the time of interventions all patients in our study had a restriction of thin liquids, but had choking on their secretions. Although there are multiple options for treatment, there are no clear standards for systematically diagnosing and treating patients with both conditions.
In this study, type 1 laryngeal clefts were diagnosed by the common technique of palpating the cleft with a right‐angle probe. In 2020, Newberry et al. noted that there may be poor inter‐rater reliability of otolaryngologists diagnosing type 1 laryngeal clefts when presented with images of type 1 laryngeal clefts palpated with a probe. 19 However, Newberry et al. did state that the use of images and inability for raters to physically feel the laryngeal cleft with the probe may have affected results. Coppess et al. proposed a modification of the technique which they call the Interarytenoid Assessment Protocol (IAAP) which involves palpating the laryngeal cleft with a right‐angle probe and then swinging the probe anterolaterally to compare the depth of the cleft to anatomical laryngeal reference points. 19 The authors of the IAAP showed good inter‐rater reliability, and use of this modified probe technique may help standardize diagnosis of type 1 laryngeal clefts. Additionally, there was most likely some variability in the decision to inject a Type 1 laryngeal cleft. No clear guidelines exist for patients who may be NPO, and intervention for injection laryngoplasty.
For patients with sialorrhea and Type 1 laryngeal clefts, we focus on combined laryngeal cleft injection laryngoplasty and salivary botulinum injections as diagnostic methods to guide follow‐up treatment. Based on the timing of symptomatic response, it was determined whether the laryngeal cleft effect or the drooling effect was the dominant cause of symptoms. In rare cases, patients may have lasting resolution of symptoms after Sal‐Bot or LCIL and require no more intervention. Most of the time, Sal‐Bot and LCIL are temporary measures and eventually have resumption of symptoms. Therefore, the results of Sal‐Bot and LCIL are used to inform further treatment such as a formal laryngeal cleft repair or salivary gland surgery.
The treatment paradigm is based on following symptoms over time. There are many proposed assessments of posterior drooling and salivary aspiration, but assessments are usually subjective and are mostly unvalidated. Nuclear aspiration studies can detect salivary aspiration but may not be able to differentiate between excessive salivation and an anatomical defect such as a laryngeal cleft. 20 Shoval et al described an unvalidated posterior drooling scale based on the presence or absence of coughing and choking while sitting or lying down. 21 For interpreting videofluoroscopic studies, the penetration‐aspiration scale is an 8‐point scale which can be used to interpret by characterizing depth and response to airway invasion. Borders and Brates performed a systematic review of the PAS literature in 2019 and found that studies using the PAS had poor interrater reliability and discrepancies in statistical treatment. 22 Nevertheless, they concluded the PAS may have value in interpreting videofluoroscopic studies if statistical approaches are consistent across studies. Dye testing with a small amount of dye placed in the oral cavity with endoscopic assessment of the hypopharynx such as the Modified Evan's blue dye test (MEBDT) may be helpful as an aspiration assessment in patients who are NPO or where FEES is contraindicated. 23 Surveys can be subjective methods of monitoring symptoms after laryngeal cleft interventions. The feeding swallowing impact survey (FSIS) is a validated survey in for quality of life in children after treatment which can indicate need for further interventions. 24
For patients whose symptoms are primarily caused by posterior drooling, salivary gland surgery such as submandibular gland excision, sublingual gland excision, or duct ligation are more effective than botulinum injection. 25 Nevertheless, surgery has inherent risk, and there is a possibility of surgery failing to improve symptoms due to drooling coming from a source unamenable to gland excision or ductal ligation. 26
Because definitive surgical interventions carry increased risk over Sal‐Bot, it is necessary to determine whether the patient's respiratory symptoms and dysphagia are due to the laryngeal cleft effect, the drooling effect, both, or due to other causes. If there was no response after combined injections in the first few weeks and a gradual improvement in coughing and choking as drooling decreased, then the botulinum injection was determined to improve the symptoms. The laryngeal cleft injection laryngoplasty, which usually works immediately, had no effect. In this situation, the drooling effect predominated in the patient, and a submandibular excision was performed.
If the response occurred in the first few days and there was no further improvement over subsequent weeks, then the laryngeal cleft injection laryngoplasty was effective, and the botulinum injection was ineffective. Since the laryngeal cleft effect was dominant, a laryngeal cleft repair was done. However, a small number of patients may develop respiratory distress, cough, or stridor as complications of LCIL which can obfuscate the improvement of symptoms. 27 In these cases, the complications should be noted and the patient's ongoing symptoms should be followed more closely to determine which effect was dominant.
In the case where there was initial improvement in the first few days and further improvement over weeks with decreased drooling, both injections were effective, and combined treatments were considered. The combined treatments performed included repeat combined LCIL and Sal‐Bot, LC repair and Sal‐Bot injections, and LC repair and a salivary gland procedure. A summary of our treatment paradigm is shown in Figure 1.
We acknowledge that this study was limited by its sample size of nine patients. As a result, it was not possible to demonstrate efficacy of the proposed management plan with statistical analysis. Furthermore, objective data such as modified barium swallow findings or drooling severity and frequency scale were not reported for all patients in the sample, so symptomatic improvement was determined by qualitative results. Consequently, this study presents our experiences in managing the symptoms of these nine. Additionally, the treatment paradigm outlines possible routes for further intervention for patients but does not dictate whether those interventions should be done. Physicians must still use their clinical judgment to determine whether in a specific patient the benefits of invasive intervention outweigh the risks. The treatment schema proposed can be further developed with future studies that look at the marginal benefit of prescribed interventions in this patient population.
5. CONCLUSION
We present the first study of patients for concurrent laryngeal cleft injection laryngoplasty and salivary gland botulinum toxin injections for patients who cough and choke on their oropharyngeal secretions. We highlight strategies for choosing subsequent procedures, when needed.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Nguyen J, Ongkasuwan J, Anand G, Lambert EM. Combined laryngeal cleft injection laryngoplasty and salivary botulinum toxin for saliva aspiration. Laryngoscope Investigative Otolaryngology. 2022;7(4):1194‐1199. doi: 10.1002/lio2.823
Presentations/Meetings: SENTAC (December 5th 2020, virtual).
REFERENCES
- 1. Johnston DR, Watters K, Ferrari LR, Rahbar R. Laryngeal cleft: evaluation and management. Int J Pediatr Otorhinolaryngol. 2014;78(6):905‐911. doi: 10.1016/j.ijporl.2014.03.015 [DOI] [PubMed] [Google Scholar]
- 2. Leboulanger N, Garabédian EN. Laryngo‐tracheo‐oesophageal clefts. Orphanet J Rare Dis. 2011;6(1):81. doi: 10.1186/1750-1172-6-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benjamin B, Inglis A. Minor congenital laryngeal clefts: diagnosis and classification. Ann Otol Rhinol Laryngol. 1989;98(6):417‐420. doi: 10.1177/000348948909800603 [DOI] [PubMed] [Google Scholar]
- 4. Ojha S, Ashland JE, Hersh C, Ramakrishna J, Maurer R, Hartnick CJ. Type 1 laryngeal cleft a multidimensional management algorithm. JAMA Otolaryngol: Head Neck Surg. 2014;140(1):34‐40. doi: 10.1001/jamaoto.2013.5739 [DOI] [PubMed] [Google Scholar]
- 5. Miglani A, Schraff S, Clarke PY, et al. An aerodigestive approach to laryngeal clefts and dysphagia using injection laryngoplasty in young children. Curr Gastroenterol Rep. 2017;19(12):1‐10. doi: 10.1007/s11894-017-0599-0 [DOI] [PubMed] [Google Scholar]
- 6. Cohen MS, Zhuang L, Simons JP, Chi DH, Maguire RC, Mehta DK. Injection laryngoplasty for type 1 laryngeal cleft in children. Otolaryngol: Head Neck Surg. 2011;144(5):789‐793. doi: 10.1177/0194599810395082 [DOI] [PubMed] [Google Scholar]
- 7. Mangat HS, El‐Hakim H. Injection augmentation of type 1 laryngeal clefts. Otolaryngol: Head Neck Surg (USA). 2012;146(5):764‐768. doi: 10.1177/0194599811434004 [DOI] [PubMed] [Google Scholar]
- 8. Horn DL, DeMarre K, Parikh SR. Interarytenoid sodium carboxymethylcellulose gel injection for management of pediatric aspiration. Ann Otol Rhinol Laryngol. 2014;123(12):852‐858. doi: 10.1177/0003489414539129 [DOI] [PubMed] [Google Scholar]
- 9. Khan WU, Campisi P, Nadarajah S, et al. Botulinum toxin A for treatment of sialorrhea in children: an effective, minimally invasive approach. Arch Otolaryngol: Head Neck Surg. 2011;137(4):339‐344. doi: 10.1001/archoto.2010.240 [DOI] [PubMed] [Google Scholar]
- 10. Gubbay A, Marie BA. Effects of salivary gland botulinum toxin‐A on drooling and respiratory morbidity in children with neurological dysfunction. Int J Pediatr Otorhinolaryngol. 2019;124:124‐128. doi: 10.1016/j.ijporl.2019.05.044 [DOI] [PubMed] [Google Scholar]
- 11. Himmelmann K, Sundh V. Survival with cerebral palsy over five decades in western Sweden. Dev Med Child Neurol. 2015;57(8):762‐767. doi: 10.1111/dmcn.12718 [DOI] [PubMed] [Google Scholar]
- 12. Lakraj AA, Moghimi N, Jabbari B. Sialorrhea: anatomy, pathophysiology and treatment with emphasis on the role of botulinum toxins. Toxins (Basel). 2013;5(5):1010‐1031. doi: 10.3390/toxins5051010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rodwell K, Edwards P, Ware RS, Boyd R. Salivary gland botulinum toxin injections for drooling in children with cerebral palsy and neurodevelopmental disability: a systematic review. Dev Med Child Neurol. 2012;54(11):977‐987. doi: 10.1111/j.1469-8749.2012.04370.x [DOI] [PubMed] [Google Scholar]
- 14. Pena AH, Cahill AM, Gonzalez L, Baskin KM, Kim H, Towbin RB. Botulinum toxin a injection of salivary glands in children with drooling and chronic aspiration. J Vasc Interv Radiol. 2009;20(3):368‐373. doi: 10.1016/j.jvir.2008.11.011 [DOI] [PubMed] [Google Scholar]
- 15. Bekkers S, Delsing CP, Kok SE, et al. Randomized controlled trial comparing botulinum vs surgery for drooling in neurodisabilities. Neurology. 2019;92(11):e1195‐e1204. doi: 10.1212/WNL.0000000000007081 [DOI] [PubMed] [Google Scholar]
- 16. Miller AL, Hersh CJ, Johnson KE, Hartnick CJ. Short‐term swallowing outcomes following type 1 laryngeal cleft injection. Int J Pediatr Otorhinolaryngol. 2019;116:159‐163. doi: 10.1016/j.ijporl.2018.10.040 [DOI] [PubMed] [Google Scholar]
- 17. Thottam PJ, Georg M, Chi D, Mehta DK. Outcomes and predictors of surgical management in type 1 laryngeal cleft swallowing dysfunction. Laryngoscope. 2016;126(12):2838‐2843. doi: 10.1002/lary.26069 [DOI] [PubMed] [Google Scholar]
- 18. Weir K, McMahon S, Chang AB. Restriction of oral intake of water for aspiration lung disease in children. Cochrane Database Syst Rev. 2012;2012(9):CD005303. doi: 10.1002/14651858.cd005303.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Newberry CI, Carpenter P, McCrary H, Casazza G, Skirko J, Meier J. Inter‐rater reliability in diagnosis and treatment of type one laryngeal cleft: a blinded observational study. Int J Pediatr Otorhinolaryngol. 2020;139:110475. doi: 10.1016/J.IJPORL.2020.110475 [DOI] [PubMed] [Google Scholar]
- 20. Drubach LA, Zurakowski D, Palmer EL, Tracy DA, Lee EY. Utility of salivagram in pulmonary aspiration in pediatric patients: comparison of salivagram and chest radiography. Am J Roentgenol. 2013;200(2):437‐441. doi: 10.2214/AJR.12.8792 [DOI] [PubMed] [Google Scholar]
- 21. Shoval HA, Antelis E, Hillman A, et al. Onabotulinum toxin a injections into the salivary glands for spinal muscle atrophy type I: a prospective case series of 4 patients. Am J Phys Med Rehabil. 2018;97(12):873‐878. doi: 10.1097/PHM.0000000000000989 [DOI] [PubMed] [Google Scholar]
- 22. Borders JC, Brates D. Use of the penetration‐aspiration scale in dysphagia research: a systematic review. Dysphagia. 2020;35(4):583‐597. doi: 10.1007/S00455-019-10064-3/TABLES/3 [DOI] [PubMed] [Google Scholar]
- 23. Béchet S, Hill F, Gilheaney Ó, Walshe M. Diagnostic accuracy of the modified Evan's blue dye test in detecting aspiration in patients with tracheostomy: a systematic review of the evidence. Dysphagia. 2016;31(6):721‐729. doi: 10.1007/S00455-016-9737-3 [DOI] [PubMed] [Google Scholar]
- 24. Fracchia MS, Diercks G, Yamasaki A, et al. Assessment of the feeding swallowing impact survey as a quality of life measure in children with laryngeal cleft before and after repair. Int J Pediatr Otorhinolaryngol. 2017;99:73‐77. doi: 10.1016/J.IJPORL.2017.05.016 [DOI] [PubMed] [Google Scholar]
- 25. Weitzman RE, Kawai K, Nuss R, Hughes A. A 10‐year retrospective review of botulinum toxin injections and surgical management of Sialorrhea. Cureus. 2020;12(5):e7916. doi: 10.7759/cureus.7916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Delsing CPA, Bekkers S, van Hulst K, Erasmus CE, van den Hoogen FJA. Unsuccessful submandibular duct surgery for anterior drooling: surgical failure or parotid gland salivation? Int J Pediatr Otorhinolaryngol. 2019;123:132‐137. doi: 10.1016/j.ijporl.2019.04.036 [DOI] [PubMed] [Google Scholar]
- 27. Ramazani F, Isaac A, Johannsen W, El‐Hakim H. Side effects and complications of injection laryngoplasty for treatment of congenital type 1 laryngeal clefts. Int J Pediatr Otorhinolaryngol. 2020;131:109886. doi: 10.1016/J.IJPORL.2020.109886 [DOI] [PubMed] [Google Scholar]


