Abstract
Objective
Papillary thyroid cancer (PTC) is the most common endocrine malignancy with a steadily increasing incidence. Researches have reported that tumor multifocality occurs in an extensive number of cases. Nevertheless, the clinical characteristics and prognostic value remained controversial. This study was performed to investigate the relationship between multifocal PTC and adverse clinicopathologic features and the prognosis.
Methods
A systematic review and meta‐analysis were conducted based on three electronic databases up to December 31, 2021. Parameters of interest included five clinical features (extrathyroidal extension, lymphovascular invasion, central lymph node metastasis, lateral lymph node metastasis, distant metastasis) and were pooled into risk ratios (RRs). Time‐to‐event data (recurrence‐free survival and all‐cause mortality) were evaluated using hazard ratios (HRs). Publication bias was examined using funnel plots and Egger's test.
Results
A total of 23 articles were included according to the inclusion criteria; all of the studies were retrospective cohorts. In comparison with unifocality, multifocality showed an increased risk of extrathyroidal extension (RR 1.38, 95% CI 1.25–1.53), lymphovascular invasion (RR 1.27, 95% CI 1.04–1.55), central lymph node metastasis (RR 1.21, 95% CI 1.12–1.30), lateral lymph node metastasis (RR 1.86, 95% CI 1.62–2.14), and distant metastasis (RR 1.35, 95% CI 1.03–1.76). Multifocal patients were predisposed to postoperative recurrence (HR 1.76, 95% CI 1.50–2.07). The rate of all‐cause mortality did not reach a statistical difference.
Level of Evidence
2.
Conclusion
Multifocal PTC is more aggressive in contrast to unifocal PTC and is accompanied by an increased risk of recurrence. They were usually diagnosed in higher grades and stages. To achieve the maximal benefit, we recommend personalized therapy and close follow‐up for multifocal PTC patients. Further prospective studies will clarify the best‐fitted treatment plans.
Keywords: clinical performance, multifocality, papillary thyroid cancer, risk factor
We evaluated the robust statistical data from several retrospective cohort trials, regarding patients with multifocal papillary thyroid cancer. The results were with a relatively high level of evidence, indicating that multifocal PTC is more aggressive in contrast to unifocal PTC and is accompanied by an increased risk of recurrence.

1. INTRODUCTION
Papillary thyroid cancer (PTC) accounts for 80%–90% of thyroid neoplasms and the incidence continues to increase. 1 Although patients with PTC have a much better outcome than individuals with other pathological subtypes of thyroid cancer, 2 personalized regimens for low‐to‐medium risk patients remain to be determined. In clinical practice, controversies regarding the treatment of low‐to‐medium risk PTC include but are not limited to: dynamic surveillance, surgery extent, and postoperative radioactive iodine (RAI). 3 , 4
The American Thyroid Association (ATA) declared several clinicopathological characteristics as the stratification criterion for recurrence in differentiated thyroid cancer to help with clinical strategies. To date, the 8th American Joint Committee on Cancer/Union for International Cancer Control (AJCC) TNM staging system still considers sex, age, tumor size and lymph node metastasis as independent factors for determining tumor prognosis. 5 Risk for recurrence increases with the presence of tissue invasion, local/distal metastasis as well as microscopic/gross infiltration. Response to initial surgery and RAI therapy are also closely related with clinicopathological features. 6 In present clinical practice, advanced age (over 55 years), minor extrathyroidal extension (mETE) and central/mediastinal (VI&VII compartment: pretracheal/paratracheal/prelaryngeal and mediastinal region) lymph node metastasis are well‐established indicators for PTC persistent/recurrence. 7
Tumor multifocality, whether unilateral or bilateral, is not rare in thyroid cancer. However, the clinical evolution and the prognostic significance of multifocal PTCs are debated. 8 The presence of multifocality is regarded as an unfavorable event that implies tumor deterioration. Related studies have reported the existence of tumor multifocality in small cell lung cancer (SCLC), medulloblastoma and prostate cancer, the curative effect and prognosis of them were inferior to those with unifocal disease. 9 , 10 , 11 , 12 Moreover, it is estimated that approximately 18%–87% of PTC patients were present with tumor multifocality. 13 , 14 McCarthy 15 stated that the separate tumor foci usually originated from the same clone. However, an alternative view has suggested that multifocal PTCs developed from discrete clones with irrelevant genetic backgrounds. 16 , 17 In addition, scattered tumor lesions usually exist as microcarcinomas of less than 1 cm. The mean tumor size of multifocal PTCs is smaller than that of a solitary tumor, 18 and thus the risk of multifocal PTCs might be misinterpreted.
Several studies have shown that central lymph node metastasis (CLNM) was correlated with tumor multifocality in comparison with unifocality. 18 , 19 , 20 A retrospective analysis 21 of 150 pediatric thyroid cancer patients revealed a higher recurrence rate than adult patients with multifocal tumors. However, inconsistencies 14 , 22 in the reported clinical outcomes of multifocal PTCs led to confusion and dilemmas, which tended to depend on one's empirical understanding. In addition, the lack of existing consensus about the prognostic value for multifocal PTCs has impeded decision‐making. Currently, only two institutions 23 emphasize multifocality as a risk of disease‐specific mortality. It is crucial to understand other indicators of disease screening.
In this study, we conducted a comprehensive systematic review and meta‐analysis to identify the association between multifocality and adverse clinicopathologic outcomes in PTCs.
2. METHODS
This report was implemented in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA Statement). 24 , 25 MEDLINE, Embase and Web of Science databases were retrieved up to December 31, 2021. The search was restricted to original studies concerning multifocality for PTC patients. Search terms are shown in Table 1. The study was performed with the following PICOS strategy
Population: patients presenting with PTC for the first time.
Intervention: pathologically proved multifocal lesions.
Comparison: a single unilateral tumor.
Outcome: adverse clinicopathological performance, postoperative recurrence, and all‐cause mortality.
Study design: retrospective cohorts.
TABLE 1.
Search strategy.
| MEDLINE | thyroid cancer, papillary [Mesh] AND (“multifocal”[Title/Abstract] OR “multifocality”[Title/Abstract]) |
| Web of Science | TI = (papillary thyroid cancer) OR TI = (papillary thyroid carcinoma) OR TI = (papillary thyroid neoplasm)) AND (TS = multifocal OR TS = multifocality |
| Embase | multifocality: ab, ti OR multifocal: ab, ti) AND (“papillary thyroid cancer”: ti OR “papillary thyroid carcinoma”: ti OR “papillary thyroid neoplasm”: ti |
2.1. Literature selection and quality assessment
Inclusion criteria were as follows: (a) patients undergone thyroid surgery for the first time, (b) the pathologic findings were confirmed as multifocal/unifocal PTC, and (c) studies reported both multifocality and unifocality. Level of confidence was determined according to the Oxford Centre for Evidence‐Based Medicine, Levels of Evidence (OCEBM Levels of Evidence 2009). 26
Exclusion criteria were as follows: (a) non‐English articles; (b) insufficient data; (c) overlapping reports in multiple publications; (d) case report, editorials, letters or meeting abstracts; (e) patients with non‐neoplastic thyroid disease were included; and (f) restricted pathological subtypes or genetic background.
The Newcastle–Ottawa Scale (NOS) was used by two investigators (LK Cui and CF Zhu) according to the Cochrane collaboration. 27 Stars were awarded based on patient selection (four items), comparability (one item) and the evaluation of outcomes (three items). In cases of disagreement, another investigator (QY Li) provided assessment.
2.2. Data extraction and statistical analysis
Two investigators (LK Cui and DD Feng) independently extracted the original data as referred to the predetermined criteria. Extracted data include study design, patient demographics, clinicopathological features and follow‐up data. Disagreement was addressed through a review of the full‐text article and input from a third investigator (CF Zhu). Any disagreement was discussed in our group. Meta‐analysis was conducted using Stata 14.0 (Stata Corporation, College Station, TX), RevMan 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen) and Comprehensive Meta‐Analysis 2.0 (Biostat, Englewood, NJ). Relative risk (RR), hazard ratio (HR) and the corresponding 95% confidence interval (CI) were calculated. To avoid confounding factors, only multivariant adjusted time‐to‐effect data were collected.
We quantified the heterogeneity across studies with the I 2 statistic. 28 An I 2 ≥ 50% indicates that there was medium to high heterogeneity among eligible studies. A random‐effects model was used for heterogeneous trials. Sources of the inconsistency among studies were identified by: (a) subgroup analysis based on countries and (b) sensitivity analysis by eliminating each of the included studies. Publication bias was presented with funnel plot and Egger's test in each analysis which included over nine articles. Egger's linear regression method was used to detect asymmetry, and a p value less than .05 was considered the existence of publication bias. The trim and fill method 29 , 30 was further applied to confirm the stability of our estimates.
3. RESULTS
3.1. Study selection and quality assessment
We identified 1194 records after the initial retrieval. After evaluating the remaining articles according to the selection criteria, 23 studies published from 2006 to 2021 were identified for subsequent analysis (Figure 1).
FIGURE 1.
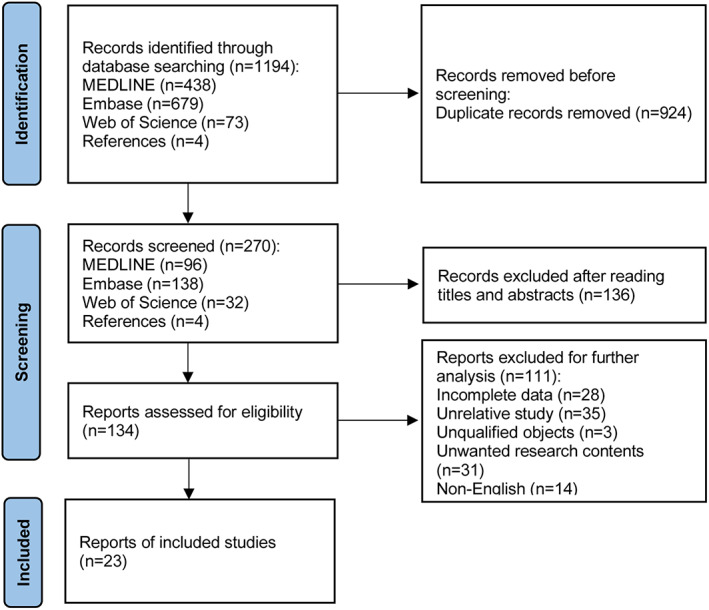
Overview of studies search and selection.
All of the 23 studies were hospital‐based studies. Two articles 18 , 31 only reported papillary thyroid microcarcinomas (in which the maximal diameters were less than 1 cm) and the rest reported PTC patients. 14 , 22 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 The eligible studies were retrospective cohorts. Cao et al. 50 conducted a case–control study, so we did not include this article for further evaluation. Baseline characteristics and NOS evaluation are shown in Tables 2 and 3. According to the included studies, all the subjects were from local medical centers, which may lead to great selection bias, and therefore we did not employ the first two scoring items (“representativeness of the exposed cohort,” “selection of the non‐exposed cohort”). Consequently, studies could be awarded a maximum of seven stars. A study with equal to or greater than five stars was considered as a high‐quality study. Three studies 38 , 47 , 51 were classified as “moderate quality” (four stars), and the rest ranged from five to seven stars.
TABLE 2.
Baseline characteristics of included studies.
| Author | Year | Country | Study design | Cases | Pathology a | Age (average ± SD) | Gender (M/F) | Follow up (year, average ± SD) | Multifocality (M/U b ) | Size (mm) |
|---|---|---|---|---|---|---|---|---|---|---|
| Kim JM 38 | 2006 | Korea | Retrospective cohort | 662 | PTC | 44.8 | 77/585 | 5.7 (0.25–9) | 266/396 | – |
| Grogan 34 | 2013 | America | Retrospective cohort | 269 | PTC | 35.9 ± 15.5 | 89/180 | 7.6 ± 8.1 (11–27) | 121/148 | – |
| Kim KJ 30 | 2015 | Korea | Retrospective cohort | 1661 | PTMC | 45.5 ± 11.5 (13–83) | 392/1917 | 5.6 ± 0.9 (0.1–7.3) | 549/1112 | – |
| Kim HJ 50 | 2015 | Korea | Retrospective cohort | 2095 | PTC | 46 ± 13 | 275/1820 | 7 (0.1–7.3) | 672/1423 | 16 ± 12 |
| Qu 42 | 2016 | China | Retrospective cohort | 496 | PTC | 43.8 ± 17.3 (7–85) | 160/336 | 10.4 ± 5.7 (0.8–28.6) | 209/287 | – |
| Tam 45 | 2016 | Turkey | Retrospective cohort | 912 | PTC | 49.2 ± 12.5 | 193/723 | 3.1 (0.5–8.3) | 308/604 | – |
| Wang W 46 | 2016 | China | Retrospective cohort | 2211 | PTC | 44.3 ± 11.8/44.4 ± 12.3 d | 507/1704 | 6 (0.5–15) | 636/1575 | – |
| Kim SK 39 | 2016 | Korea | Retrospective cohort | 5656 | PTC | 48.0 ± 10.4 | 1002/4654 | 5.1 (0.5–17.8) | 1529/4427 | 6 ± 2 |
| Wang F 21 | 2017 | Multinational c | Retrospective cohort | 2624 | PTC | 46 (35–58) | 385/2239 | 4.8 (2.2–8.9) | 1000/1624 | 15 (10–25) |
| Hwangbo 35 | 2017 | Korea | Retrospective cohort | 3282 | PTC | 47 ± 11 | 2897/385 | 5.8 (1.0–10.2) | 1285/1985 | 1.1 (0.1–2.0) |
| Kim Y 40 | 2017 | Korea | Retrospective cohort | 1928 | PTC | 53 (15–86) | 355/1573 | 7.8 (2–11.1) | 623/1305 | – |
| Khan 37 | 2018 | Pakistan | Retrospective cohort | 209 | PTC | 35.6 ± 13.8 (12–74) | 63/146 | 4.1 (1–16.3) | 87/122 | – |
| Xu 47 | 2018 | China | Retrospective cohort | 3607 | PTC | 47.5 ± 2 | 868/2739 | 5.7 (2.1–11.5) | 675/2932 | 6 ± 30 |
| Gui 17 | 2018 | China | Retrospective cohort | 541 | PTMC | 47.2 ± 12.3 | 128/413 | 3.5 (2–5) | 146/395 | 5.8 ± 2.4 |
| Li 33 | 2018 | China | Retrospective cohort | 570 | PTC | 45.3 ± 10.5/43.3 ± 11.5 d | 160/410 | 1.6 (1–2.2) | 285/285 | – |
| Ryu 44 | 2018 | Korea | Retrospective cohort | 390 | PTC | 46 (17–80) | 118/272 | 6.75 (0.5–13) | 142/248 | 1.61 ± 0.97 |
| Nam 41 | 2018 | Korea | Retrospective cohort | 2384 | PTC | 52 (12–86) | 495/1889 | 7.8 (2–10.9) | 142/248 | – |
| Geron 13 | 2019 | Israel | Retrospective cohort | 1039 | PTC | 48.4 ± 15.3 | 222/817 | 10.1 (4.7–16.3) | 534/505 | 15 (10–25) |
| Feng 33 | 2019 | China | Retrospective cohort | 442 | PTC | 45.4 ± 12.3 | 109/333 | 3.6 (0.9–8.25) | 119/323 | 12.3 ± 9.3 |
| Choi 31 | 2019 | Korea | Retrospective cohort | 2390 | PTC | 52 (12–88) | 516/1874 | 7.7 (2–11.9) | 892/1498 | 1.3 (0.8–1.8) |
| Shin 51 | 2020 | Korea | Retrospective cohort | 2902 | PTC | 51 (43–58) | 619/2283 | 7.4 (5.3–10.3) | 1580/1322 | 11 (7–14) |
| Jiang 36 | 2020 | China | Retrospective cohort | 4107 | PTC | 45.21 (12–82) | 909/3198 | 3.75 (2–11.9) | 1058/3826 | 9.2 (1–80) |
| Woo 48 | 2021 | Korea | Retrospective cohort | 1249 | PTC | 47.4 ± 11.4 | 154/1095 | 5.5 ± 2.7 | 487/762 | 10 ± 7 |
PTC, papillary thyroid carcinoma; PTMC, papillary thyroid microcarcinoma.
M/U, multifocal/unifocal.
America, China, Italy, Poland, Australia, Spain.
The data were presented separately.
TABLE 3.
Quality assessment.
| Selection | Comparability | Outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Studies | Representativeness of the exposed cohort a | Selection of the non‐exposed cohort b | Ascertainment of exposure | Outcome of interest was not present at start of study c | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Follow‐up duration | Adequacy of follow up of cohorts | Total |
| Kim JM 39 | – | – | ★ | ★ | ★ | – | ★ | ★ | 5 |
| Grogan 35 | – | – | ★ | ★ | – | ★ | ★ | ★ | 5 |
| Kim KJ 31 | – | – | ★ | ★ | ★★ | ★ | ★ | – | 6 |
| Kim HJ 51 | – | – | ★ | ★ | ★ | – | – | ★ | 4 |
| Qu 43 | – | – | ★ | ★ | ★ | ★ | ★ | ★ | 6 |
| Tam 46 | – | – | ★ | ★ | ★ | ★ | – | ★ | 5 |
| Wang W 47 | – | – | ★ | ★ | ★ | – | – | ★ | 4 |
| Kim SK 40 | – | – | ★ | ★ | ★ | ★ | ★ | – | 5 |
| Wang F 22 | – | – | ★ | ★ | ★★ | ★ | ★ | – | 6 |
| Hwangbo 36 | – | – | ★ | ★ | – | ★ | ★ | ★ | 5 |
| Kim Y 41 | – | – | ★ | ★ | – | ★ | ★ | ★ | 5 |
| Khan 38 | – | – | ★ | ★ | ★ | – | ★ | – | 4 |
| Xu 48 | – | – | ★ | ★ | ★ | ★ | – | ★ | 5 |
| Gui 18 | – | – | ★ | ★ | – | ★ | ★ | ★ | 5 |
| Li 34 | – | – | ★ | ★ | ★★ | ★ | – | ★ | 6 |
| Ryu 45 | – | – | ★ | ★ | ★ | ★ | ★ | ★ | 6 |
| Nam 42 | – | – | ★ | ★ | ★ | – | ★ | ★ | 5 |
| Geron 14 | – | – | ★ | ★ | ★★ | ★ | ★ | ★ | 7 |
| Feng 33 | – | – | ★ | ★ | ★★ | ★ | – | ★ | 6 |
| Choi 32 | – | – | ★ | ★ | ★★ | ★ | ★ | ★ | 7 |
| Shin 32 | – | – | ★ | ★ | ★ | ★ | ★ | ★ | 6 |
| Jiang 37 | – | – | ★ | ★ | ★ | ★ | ★ | ★ | 6 |
| Woo 49 | – | – | ★ | ★ | ★★ | – | ★ | ★ | 6 |
Patients were collected from certain medical institutions (subjects from Kim KJ and Gui were only consisted of PTMC).
Multifocal and unifocal cases were extracted from a single medical center (except for Wang F, Hwangbo and Geron et al.).
Outcome events include recurrence and death.
3.2. The association between multifocality and clinicopathological features
3.2.1. ETE
Thirteen articles 14 , 22 , 31 , 32 , 33 , 35 , 43 , 46 , 47 , 49 , 51 , 52 , 53 reported the results for ETE. The meta‐analysis suggested that multifocality is a risk factor of ETE (RR 1.38, 95% CI 1.25–1.53, p < .001) (Figures 2 and 3A). The statistical heterogeneity was significant (I 2 = 88.2%, p < .001). Similarly, we further deduced a high heterogeneity in studies in China and Korea by subgroup analysis (I 2 = 59.6%, p = .12). Still, sensitivity analysis did not reverse the aforementioned result (the pooled RRs ranged from 1.01 to 2.27, p < .001) (Figure S1).
FIGURE 2.
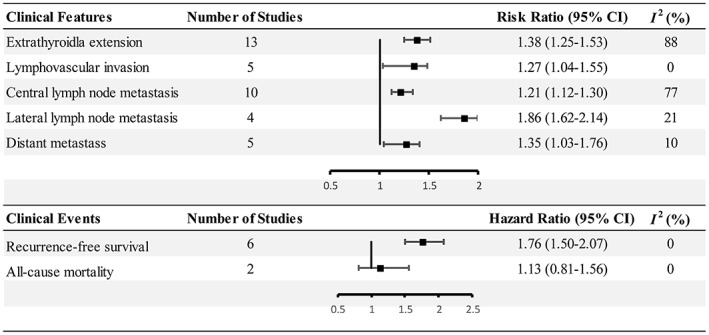
Summary of the results.
FIGURE 3.
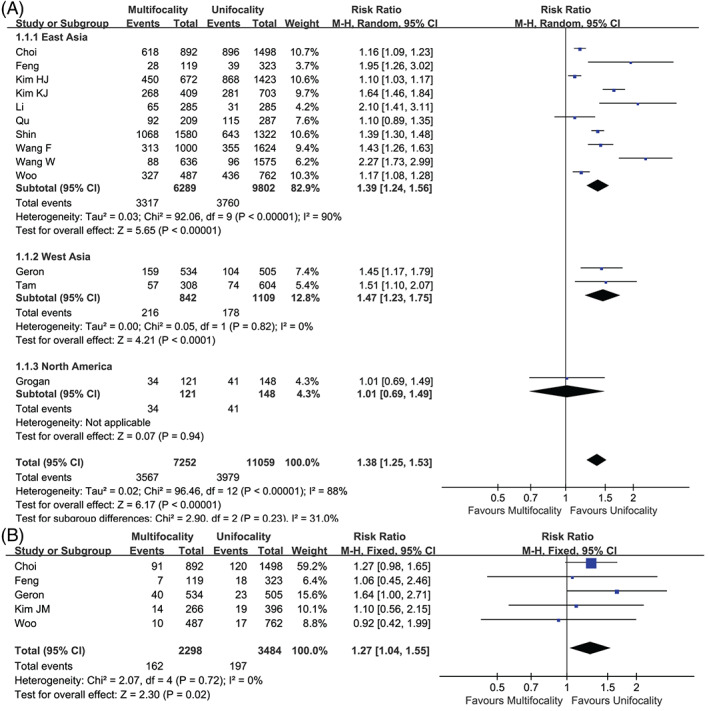
Forest plot of the studies. (A) Extrathyroidal extension and (B) lymphovascular invasion.
3.2.2. Lymphovascular invasion
Six studies 14 , 32 , 33 , 39 , 46 , 49 covered lymphovascular events, and the I 2 was 65.5%. The analysis indicated that the data from Tam et al. 46 was the source of the inconsistency; after excluding this study, the heterogeneity dropped to 0%. Finally, the pooled analysis implied that multifocality was a risk factor for lymphovascular invasion (RR 1.27, 95% CI 1.04–1.55, p = .02) (Figures 2 and 3B).
3.2.3. CLNM
Ten articles 31 , 32 , 33 , 37 , 40 , 43 , 48 , 49 , 51 , 52 reported CLNM, and the pooled result suggested a strong relationship between multifocality and CLNM (RR 1.21, 95% CI 1.12–1.30, p < .001) (Figures 2 and 4A). The I 2 was 75% with a significant difference, especially among East Asia countries. The sensitivity analysis also verified its robustness (the RR ranged from 1.06 to 1.49, p < .001) (Figure S2).
FIGURE 4.
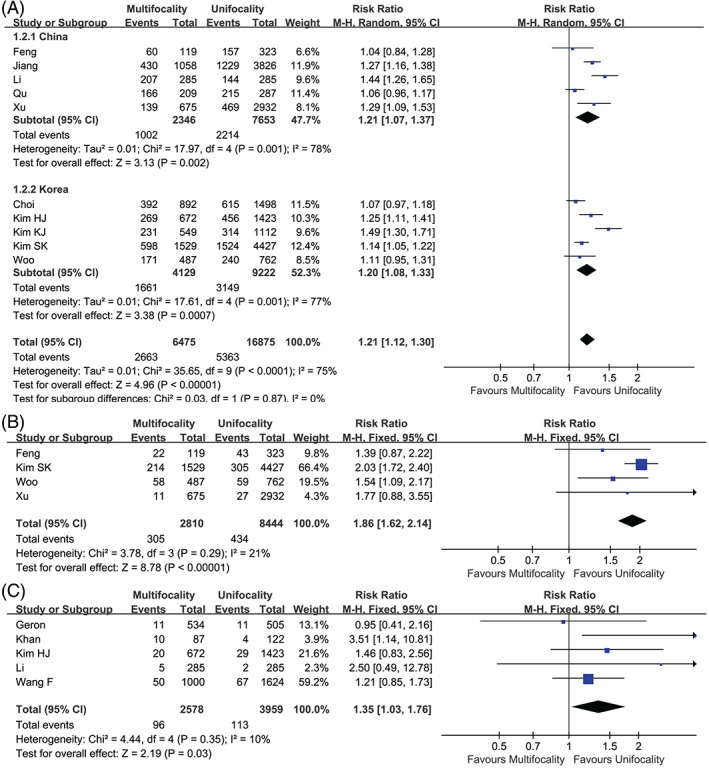
Forest plot of the studies. (A) Central lymph node metastasis, (B) lateral lymph node metastasis, and (C) distant metastasis.
3.2.4. LLNM
We used a fixed effect model to evaluate the risk of multifocality for lateral lymph node metastasis (LLNM) among four studies. 33 , 40 , 48 , 49 The analysis showed that there was a significant association between tumor multifocality and LLNM (RR 1.86, 95% CI 1.62–2.14, p < .001) (Figures 2 and 4B).
3.2.5. Distant metastasis
Five studies 14 , 22 , 34 , 38 , 51 indicated that multifocality was related to distant metastasis, and the propensity of metastasis was higher in multifocal PTCs (RR 1.35, 95% CI 1.03–1.76, p = .03) (Figures 2 and 4C).
3.2.6. Recurrence‐free survival
Six studies 14 , 32 , 33 , 41 , 42 , 53 provided multivariate adjusted data concerning the recurrence rate. Multifocal PTCs were easier to get involved in disease recurrence (HR 1.76, 95% CI 1.50–2.07, p < .001) (Figures 2 and 5A).
FIGURE 5.
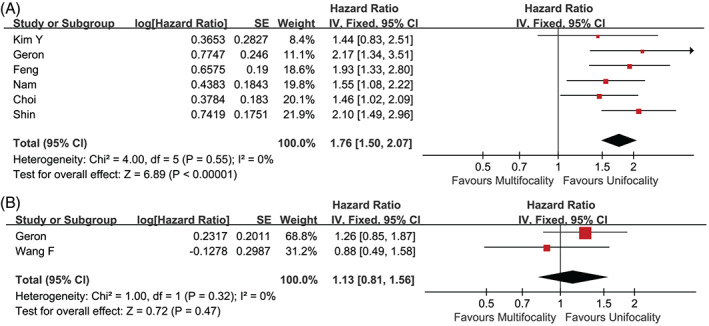
Forest plot of the studies. (A) Recurrence‐free survival and (B) all‐cause mortality.
3.2.7. All‐cause mortality
We failed to detect any predisposition in mortality for multifocal PTCs (p = .47) (Figures 2 and 5B). 14 , 22 More follow‐up data are required to investigate this association.
3.2.8. Publication bias
Funnel plots and Egger's tests did not detect a significant publication bias when confronted with ETE and CLNM (p values were .09 and .45, respectively; Figures S3 and S4). After using the trim and fill method, the results were still robust.
4. DISCUSSION
The natural evolution and specific clinical significance of multifocal PTCs remain inconsistent. This report was to summarize the clinicopathological performance of multifocal PTCs. To our knowledge, the results from 23 studies involving 41,616 patients revealed higher cumulative risks for multifocal PTCs, in developing into disease progression (Figure 2). Accordingly, multifocal PTCs are recommended to accept an intensive surveillance in case further intervention is required.
In a study by Choi 32 et al., multifocality is an independent risk factor for PTC recurrence. These patients usually present with advanced TNM staging. In contrast, Greca et al. 54 argued that disease persistence was rare after total thyroidectomy in patients with multifocal PTCs. According to the ATA, 3 before prophylactic cervical dissection, the risk factors for metastasis and recurrence (such as advanced/young age, lager tumor size, multiple sites, ETE, and LLNM) should be carefully considered. Notably, multifocal PTC has been classified in the moderate‐to‐high risk group. Moreover, except for multifocal PTCs larger than 1 cm, the latest ATA guideline 3 did not recommend aggressive approaches for multifocal papillary thyroid microcarcinomas, for instance, postoperative radioiodine ablation. In our research, multifocal patients accounted for 36% of the PTC population, which is consistent with the findings of Natalia et al. 55 Additionally, surgical approaches are heterogenous in different clinical centers for low‐risk patients. It was not until 2015 that the ATA guidelines 3 negated prophylactic lymph node dissection as a routine choice. And the rate of local recurrence varies across studies because of this discrepancy. We did not include any cross‐sectional studies other than retrospective cohort studies to pursue intact time‐effect data with a higher level of evidence.
We observed an issue with heterogeneity between research in China and Korea. Differences in the diagnostic criteria might be an explanation of this inconsistency. The proportion of thyroid microcarcinoma has gradually increased, which can be another source of the heterogeneity. Shin, 53 Choi, 32 and Li 34 excluded patients with radiation exposure, and Shin 53 and Choi 32 did not pool the results for T4 staging or poorly differentiated PTCs. Kim JM 39 and Jiang 37 each selected their surgical planning. The operations were performed by Kim JM et al. prior to 2009, after that the ATA published guidelines for differentiated thyroid cancer. 39 Jiang 37 et al. performed operations according to Guidelines for the Chinese Thyroid Association. 56 , 57 ATA and NCCN guidelines emphasized preoperative fine needle aspiration (FNA) while Chinese clinicians focus on the distinguishing of undetermined nodules, which rely on intraoperative frozen section examinations. Furthermore, most Chinese surgeons hold a more positive attitude toward prophylactic central lymph node dissection. Additionally, the 2016 Korea Thyroid Association (2016 KTA/KSThR) 58 advocated a tendentious recommendation for FNA for low risk nodules. From 2009 to 2013, inconsistencies have existed among the clinical centers in the Asian‐Pacific region. 59 With the implementation of the 2015 ATA guidelines, surgical strategies for low‐to‐moderate PTCs will become standardized.
Joseph, 60 Kim 61 and Zhang 62 et al. performed meta‐analyses in 2018 and 2021, which reported that multifocality is positively correlated with lymph node metastasis (both in central and lateral compartments), advanced TNM stage and recurrence. Here, we conducted a more comprehensive analysis concerning unfavorable clinicopathologic features and time‐to‐event outcomes result from multifocal PTC, of which should be classified as a higher risk category. Similarly, the presence of multifocality strongly indicates an increased risk of recurrence. As with the results, we intended to address the value of close surveillance to assist personalized therapy, especially for suspicious nodules and regional lymph nodes of multifocal PTCs. Nevertheless, the extent of surgery and the postoperative follow‐up strategy require further investigation.
5. LIMITATION
Nevertheless, 23 studies in our research were performed retrospectively, which may exhibit selection bias and withdraw bias to some extent. Cases with recurrence or deterioration were more easily recorded. Given the restriction of clinical screening methods, postoperative occult lymph nodes may not be detected. On the other hand, the time‐to‐event data were constrained by different length of follow‐up. The representativity of patients was limited, for instance, geographical distribution and radiation exposure. Undoubtedly, many high‐risk features were associated with tumor invasion and local/distant metastasis for PTC, including but not restricted to multifocality alone. Therefore, the results should be treated with caution.
6. CONCLUSION
In summary, this study found that multifocal PTCs are predisposed to disease metastasis and recurrence. ETE and lymphovascular invasion are more likely to be concomitant with these patients. When possible, active surveillance should be considered. We look forward to subsequent prospective studies to guide personalized treatment and postoperative follow‐up.
FUNDING INFORMATION
This work was supported by the National Key Research and Development Program of China (Grant No. 2019YFC0119200).
CONFLICT OF INTEREST
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Supporting information
Supplementary Figure S1 Extrathyroidal extension (sensitivity analysis).
Supplementary Figure S2 Central lymph node metastasis (sensitivity analysis).
Supplementary Figure S3 Extrathyroidal extension (funnel plot).
Supplementary Figure S4 Central lymph node metastasis (funnel plot).
Cui L, Feng D, Zhu C, Li Q, Li W, Liu B. Clinical outcomes of multifocal papillary thyroid cancer: A systematic review and meta‐analysis. Laryngoscope Investigative Otolaryngology. 2022;7(4):1224‐1234. doi: 10.1002/lio2.824
Funding information the National Key Research and Development Program of China, Grant/Award Number: 2019YFC0119200
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Wang TS, Sosa JA. Thyroid surgery for differentiated thyroid cancer – recent advances and future directions. Nat Rev Endocrinol. 2018;14:670‐683. [DOI] [PubMed] [Google Scholar]
- 3. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McLeod DSA, Zhang L, Durante C, Cooper DS. Contemporary debates in adult papillary thyroid cancer management. Endocr Rev. 2019;40:1481‐1499. [DOI] [PubMed] [Google Scholar]
- 5. Gan T, Huang B, Chen Q, et al. Risk of recurrence in differentiated thyroid cancer: a population‐based comparison of the 7th and 8th editions of the American Joint Committee on Cancer Staging Systems. Ann Surg Oncol. 2019;26:2703‐2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pitoia F, Jerkovich F. Dynamic risk assessment in patients with differentiated thyroid cancer. Endocr Relat Cancer. 2019;26:R553‐R566. [DOI] [PubMed] [Google Scholar]
- 7. Vaisman F, Tuttle RM. Clinical assessment and risk stratification in differentiated thyroid cancer. Endocrinol Metab Clin North Am. 2019;48:99‐108. [DOI] [PubMed] [Google Scholar]
- 8. Shaha AR, Poorten VV, Tuttle RM. Multifocality in papillary thyroid carcinoma—an unresolved controversy. Eur J Surg Oncol. 2020;46:1777‐1778. [DOI] [PubMed] [Google Scholar]
- 9. Yang D, Denny SK, Greenside PG, et al. Intertumoral heterogeneity in SCLC is influenced by the cell type of origin. Cancer Discov. 2018;8:1316‐1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cavalli FMG, Remke M, Rampasek L, et al. Intertumoral heterogeneity within medulloblastoma subgroups. Cancer Cell. 2017;31:737‐754 e736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Simonsen TG, Gaustad JV, Rofstad EK. Intertumor heterogeneity in vascularity and invasiveness of artificial melanoma brain metastases. J Exp Clin Cancer Res. 2015;34:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Le JD, Tan N, Shkolyar E, et al. Multifocality and prostate cancer detection by multiparametric magnetic resonance imaging: correlation with whole‐mount histopathology. Eur Urol. 2015;67:569‐576. [DOI] [PubMed] [Google Scholar]
- 13. Iacobone M, Jansson S, Barczynski M, Goretzki P. Multifocal papillary thyroid carcinoma—a consensus report of the European Society of Endocrine Surgeons (ESES). Langenbecks Arch Surg. 2014;399:141‐154. [DOI] [PubMed] [Google Scholar]
- 14. Geron Y, Benbassat C, Shteinshneider M, et al. Multifocality is not an independent prognostic factor in papillary thyroid cancer: a propensity score‐matching analysis. Thyroid. 2019;29:513‐522. [DOI] [PubMed] [Google Scholar]
- 15. McCarthy RP, Wang M, Jones TD, Strate RW, Cheng L. Molecular evidence for the same clonal origin of multifocal papillary thyroid carcinomas. Clin Cancer Res. 2006;12:2414‐2418. [DOI] [PubMed] [Google Scholar]
- 16. Chow SM, Law SC, Chan JK, Au SK, Yau S, Lau WH. Papillary microcarcinoma of the thyroid‐prognostic significance of lymph node metastasis and multifocality. Cancer. 2003;98:31‐40. [DOI] [PubMed] [Google Scholar]
- 17. Lin JD, Chao TC, Hsueh C, Kuo SF. High recurrent rate of multicentric papillary thyroid carcinoma. Ann Surg Oncol. 2009;16:2609‐2616. [DOI] [PubMed] [Google Scholar]
- 18. Gui CY, Qiu SL, Peng ZH, Wang M. Clinical and pathologic predictors of central lymph node metastasis in papillary thyroid microcarcinoma: a retrospective cohort study. J Endocrinol Invest. 2018;41:403‐409. [DOI] [PubMed] [Google Scholar]
- 19. Siddiqui S, White MG, Antic T, et al. Clinical and pathologic predictors of lymph node metastasis and recurrence in papillary thyroid microcarcinoma. Thyroid. 2016;26:807‐815. [DOI] [PubMed] [Google Scholar]
- 20. Thompson AM, Turner RM, Hayen A, et al. A preoperative nomogram for the prediction of ipsilateral central compartment lymph node metastases in papillary thyroid cancer. Thyroid. 2014;24:675‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee YA, Jung HW, Kim HY, et al. Pediatric patients with multifocal papillary thyroid cancer have higher recurrence rates than adult patients: a retrospective analysis of a large pediatric thyroid cancer cohort over 33 years. J Clin Endocrinol Metab. 2015;100:1619‐1629. [DOI] [PubMed] [Google Scholar]
- 22. Wang F, Yu X, Shen X, et al. The prognostic value of tumor multifocality in clinical outcomes of papillary thyroid cancer. J Clin Endocrinol Metab. 2017;102:3241‐3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Momesso DP, Tuttle RM. Update on differentiated thyroid cancer staging. Endocrinol Metab Clin North Am. 2014;43:401‐421. [DOI] [PubMed] [Google Scholar]
- 24. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1‐34. [DOI] [PubMed] [Google Scholar]
- 26. Oxford Centre for evidence‐based medicine – levels of evidence (March 2009). March 2009. https://www.cebm.net/2009/06/oxford-centre-evidencebased-medicine-levels-evidence-march-2009/
- 27. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25:603‐605. [DOI] [PubMed] [Google Scholar]
- 28. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539‐1558. [DOI] [PubMed] [Google Scholar]
- 29. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56:455‐463. [DOI] [PubMed] [Google Scholar]
- 30. Shi L, Lin L. The trim‐and‐fill method for publication bias: practical guidelines and recommendations based on a large database of meta‐analyses. Medicine (Baltimore). 2019;98:e15987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim KJ, Kim SM, Lee YS, Chung WY, Chang HS, Park CS. Prognostic significance of tumor multifocality in papillary thyroid carcinoma and its relationship with primary tumor size: a retrospective study of 2,309 consecutive patients. Ann Surg Oncol. 2015;22:125‐131. [DOI] [PubMed] [Google Scholar]
- 32. Choi WR, Roh JL, Gong G, et al. Multifocality of papillary thyroid carcinoma as a risk factor for disease recurrence. Oral Oncol. 2019;94:106‐110. [DOI] [PubMed] [Google Scholar]
- 33. Feng JW, Pan H, Wang L, Ye J, Jiang Y, Qu Z. Total tumor diameter: the neglected value in papillary thyroid microcarcinoma. J Endocrinol Invest. 2020;43:601‐613. [DOI] [PubMed] [Google Scholar]
- 34. Genpeng L, Jianyong L, Jiaying Y, et al. Independent predictors and lymph node metastasis characteristics of multifocal papillary thyroid cancer. Medicine (Baltimore). 2018;97:e9619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grogan RH, Kaplan SP, Cao H, et al. A study of recurrence and death from papillary thyroid cancer with 27 years of median follow‐up. Surgery. 2013;154:1436‐1446. discussion 1446‐1437. [DOI] [PubMed] [Google Scholar]
- 36. Hwangbo Y, Kim JM, Park YJ, et al. Long‐term recurrence of small papillary thyroid cancer and its risk factors in a Korean multicenter study. J Clin Endocrinol Metab. 2017;102:625‐633. [DOI] [PubMed] [Google Scholar]
- 37. Jiang LH, Yin KX, Wen QL, Chen C, Ge MH, Tan Z. Predictive risk‐scoring model for central lymph node metastasis and predictors of recurrence in papillary thyroid carcinoma. Sci Rep. 2020;10:710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Khan M, Syed AA, Khan AI, Hussain SR, Urooj N. Association of tumor size and focality with recurrence/persistence in papillary thyroid cancer patients treated with total thyroidectomy along with radioactive‐iodine ablation and TSH suppression. Updates Surg. 2018;70:121‐127. [DOI] [PubMed] [Google Scholar]
- 39. Kim JM, Kim TY, Kim WB, et al. Lymphovascular invasion is associated with lateral cervical lymph node metastasis in papillary thyroid carcinoma. Laryngoscope. 2006;116:2081‐2085. [DOI] [PubMed] [Google Scholar]
- 40. Kim SK, Park I, Woo JW, et al. Predictive factors for lymph node metastasis in papillary thyroid microcarcinoma. Ann Surg Oncol. 2016;23:2866‐2873. [DOI] [PubMed] [Google Scholar]
- 41. Kim Y, Roh JL, Gong G, et al. Risk factors for lateral neck recurrence of N0/N1a papillary thyroid cancer. Ann Surg Oncol. 2017;24:3609‐3616. [DOI] [PubMed] [Google Scholar]
- 42. Nam SH, Roh JL, Gong G, et al. Nodal factors predictive of recurrence after thyroidectomy and neck dissection for papillary thyroid carcinoma. Thyroid. 2018;28:88‐95. [DOI] [PubMed] [Google Scholar]
- 43. Qu N, Zhang L, Ji QH, et al. Number of tumor foci predicts prognosis in papillary thyroid cancer. BMC Cancer. 2014;14:914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Qu N, Zhang L, Wu WL, et al. Bilaterality weighs more than unilateral multifocality in predicting prognosis in papillary thyroid cancer. Tumour Biol. 2016;37:8783‐8789. [DOI] [PubMed] [Google Scholar]
- 45. Ryu YJ, Cho JS, Yoon JH, Park MH. Identifying risk factors for recurrence of papillary thyroid cancer in patients who underwent modified radical neck dissection. World J Surg Oncol. 2018;16:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tam AA, Ozdemir D, Cuhaci N, et al. Association of multifocality, tumor number, and total tumor diameter with clinicopathological features in papillary thyroid cancer. Endocrine. 2016;53:774‐783. [DOI] [PubMed] [Google Scholar]
- 47. Wang W, Su X, He K, et al. Comparison of the clinicopathologic features and prognosis of bilateral versus unilateral multifocal papillary thyroid cancer: an updated study with more than 2000 consecutive patients. Cancer. 2016;122:198‐206. [DOI] [PubMed] [Google Scholar]
- 48. Xu Y, Xu L, Wang J. Clinical predictors of lymph node metastasis and survival rate in papillary thyroid microcarcinoma: analysis of 3607 patients at a single institution. J Surg Res. 2018;221:128‐134. [DOI] [PubMed] [Google Scholar]
- 49. Woo J, Kim H, Kwon H. Impact of multifocality on the recurrence of papillary thyroid carcinoma. J Clin Med. 2021;10:5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cao J, Hu JL, Chen C, et al. Vascular invasion is an independent prognostic factor for distant recurrence‐free survival in papillary thyroid carcinoma: a matched‐case comparative study. J Clin Pathol. 2016;69:872‐877. [DOI] [PubMed] [Google Scholar]
- 51. Kim HJ, Park HK, Byun DW, et al. Number of tumor foci as predictor of lateral lymph node metastasis in papillary thyroid carcinoma. Head Neck. 2015;37:650‐654. [DOI] [PubMed] [Google Scholar]
- 52. Li G, Lei J, You J, et al. Independent predictors and lymph node metastasis characteristics of multifocal papillary thyroid cancer. Medicine. 2018;97:e9619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shin CH, Roh JL, Song DE, et al. Prognostic value of tumor size and minimal extrathyroidal extension in papillary thyroid carcinoma. Am J Surg. 2020;220:925‐931. [DOI] [PubMed] [Google Scholar]
- 54. La Greca A, Xu B, Ghossein R, Tuttle RM, Sabra MM. Patients with multifocal macroscopic papillary thyroid carcinoma have a low risk of recurrence at early follow‐up after total thyroidectomy and radioactive iodine treatment. Eur Thyroid J. 2017;6:31‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pstrag N, Ziemnicka K, Bluyssen H, Wesoly J. Thyroid cancers of follicular origin in a genomic light: in‐depth overview of common and unique molecular marker candidates. Mol Cancer. 2018;17:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu ST, Zhang X. Epidemiology and management guidelines of thyroid cancer. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016;51:146‐149. [DOI] [PubMed] [Google Scholar]
- 57. Ya M. Interpretation of the management guidelines for patients with thyroid nodules and differentiated thyroid cancer (2012 Chinese edition). Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013;27:917‐920. [PubMed] [Google Scholar]
- 58. Ha EJ, Na DG, Moon W‐J, Lee YH, Choi N. Correction to: Diagnostic performance of ultrasound‐based risk‐stratification systems for thyroid nodules: comparison of the 2015 American Thyroid Association Guidelines with the 2016 Korean Thyroid Association/Korean Society of Thyroid Radiology and 2017 American College of Radiology Guidelines. Thyroid. 2018;28:1532‐1537. doi: 10.1089/thy.2018.0094 Thyroid 2019; 29:159. [DOI] [PubMed] [Google Scholar]
- 59. Yang SP, Ying LS, Saw S, Tuttle RM, Venkataraman K, Su‐Ynn C. Practical barriers to implementation of thyroid cancer guidelines in the Asia‐Pacific region. Endocr Pract. 2015;21:1255‐1268. [DOI] [PubMed] [Google Scholar]
- 60. Joseph KR, Edirimanne S, Eslick GD. Multifocality as a prognostic factor in thyroid cancer: a meta‐analysis. Int J Surg. 2018;50:121‐125. [DOI] [PubMed] [Google Scholar]
- 61. Kim H, Kwon H, Moon BI. Association of multifocality with Prognosis of papillary thyroid carcinoma: a systematic review and meta‐analysis. JAMA Otolaryngol Head Neck Surg. 2021;147:847‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang T, He L, Wang Z, et al. The differences between multifocal and unifocal papillary thyroid carcinoma in unilateral lobe: a meta‐analysis. Front Oncol. 2021;11:657237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1 Extrathyroidal extension (sensitivity analysis).
Supplementary Figure S2 Central lymph node metastasis (sensitivity analysis).
Supplementary Figure S3 Extrathyroidal extension (funnel plot).
Supplementary Figure S4 Central lymph node metastasis (funnel plot).


