Abstract
Background
The prognostic value of the hemoglobin/red blood cell distribution width ratio in primary hepatocellular carcinoma remains unknown. This study aimed to analyze the correlation between hemoglobin/red blood cell distribution width ratio and hepatocellular carcinoma prognosis.
Material/Methods
Medical records of hepatocellular carcinoma patients were analyzed retrospectively. The hemoglobin/red blood cell distribution width ratio cut-off value was determined as 0.987 by receiver operating characteristic curve analysis. Patients were divided into high- and low-level groups, and the clinical data were compared. The correlation among the ratio levels, progression-free survival, and overall survival was measured with univariate and multivariate Cox regression analyses. The prognostic utility of the ratio combined with alpha-fetoprotein was analyzed using Kaplan-Meier survival curves and log-rank detection, and the correlation between the ratio and tumor staging was studied using one-way analysis of variance.
Results
This study included 252 patients. Sex, smoking and alcohol consumption history, body mass index, surgery, staging, platelet/lymphocyte ratio, and hemoglobin/red blood cell distribution width ratio were associated with progression-free and overall survival (P<0.05). The ratio, alpha-fetoprotein, hemoglobin, staging, and surgery were independent risk factors for progression-free survival (P<0.05), and the ratio, alpha-fetoprotein, hemoglobin, body mass index, HBsAg, staging, and surgery were independent risk factors for overall survival (P<0.05). Patients with low ratio levels and high alpha-fetoprotein levels had the worst prognosis.
Conclusions
Low hemoglobin/red blood cell distribution width ratio levels are associated with poor patient prognosis and are potential tumor prognosis markers.
Keywords: Hemoglobin (64-76), Liver Neoplasms, Prognosis
Background
According to the 2020 Global Cancer Report, primary liver cancer is the sixth most common malignancy, with high morbidity and mortality rates. Hepatocellular carcinoma (HCC), its most common pathological type, accounts for about 75% of patient diagnoses [1]. The initial symptoms of HCC are not obvious, and progression is rapid. About 40% of patients are diagnosed at an advanced stage when surgery is no longer a treatment option. The current commonly used treatments are targeted therapy and immunotherapy. However, despite being widely used, their efficacy is affected by low target expression, secondary drug resistance, and other factors limiting the survival and quality of life of HCC patients [2].
Tumor-associated inflammation, one of the 10 characteristics of malignancy [3], is defined by a large number of inflammatory cells present in tumor tissue and peripheral blood and promotes tumor development by disrupting homeostasis of the immune microenvironment [4,5]. Since complete blood count (CBC) is a routine and easy detection method, it is widely used as a predictor of tumor prognosis due to its high reproducibility and good clinical application value. System immune-inflammation index, lymphocyte ratio, and neutrophil count-to-lymphocyte ratio (NLR) are related to the survival rate of patients with multiple malignant tumors [6–9]. Hemoglobin (Hb) is a common indicator that can reflect the immunity of patients. Hb is associated with the clinical stage and prognosis of patients with nasopharyngeal carcinoma, non-small cell lung cancer, gastric cancer, and other tumors [10–12]. Red blood cell distribution width (RDW) is a quantitative measure of the size variability of circulating red blood cells (RBCs). Previous studies found that RDW is closely related to patient prognosis in a variety of malignancies, including esophageal, breast, and kidney cancer [13–16]. The Hb/RDW ratio (HRR) is a novel biomarker that uses the composition of 2 hematologic parameters – Hb and RDW – and has higher specificity and sensitivity compared with Hb or RDW used individually; therefore, it can reflect patients’ inflammation levels more accurately and assess the correlation with cancer progression [17]. The negative correlation among HRR, overall survival (OS), and progression-free survival (PFS) in patients with renal cancer, lung adenocarcinoma, small cell lung cancer, nasopharyngeal carcinoma, and head and neck squamous cell carcinoma has been confirmed [18–24]. Recently, a study confirmed that HRR was negatively correlated with the occurrence of liver metastases in patients with gastric cancer, and its predictive value is higher than the platelet-to-lymphocyte ratio (PLR), suggesting that HRR may have a better prognostic predictive role in some tumors [25]. However, the utility of HRR in HCC patient prognosis is unclear. This study retrospectively analyzed the correlation between HRR and the prognosis of 252 patients with HCC.
Material and Methods
Research Subjects
This retrospective study analyzed HCC patients who visited the First Affiliated Hospital of Anhui Medical University between June 1, 2017 and June 30, 2021. The inclusion criteria were: (a) >18 years old, (b) complete clinical data and histopathologically confirmed primary HCC, (c) no previous anti-tumor treatment, and (d) no severe liver or kidney dysfunction nor bone marrow hematopoietic abnormalities. The exclusion criteria were: (a) a second primary carcinoma, (b) complicated by severe infection, and (c) complicated by severe anemia or other blood diseases. This study obtained informed consent from all patients and their families and was approved by the Ethics Review Committee of the First Affiliated Hospital of Anhui Medical University (Ethics Approval number: Quick-PJ 2022-05-34).
Research Method
This retrospective study collected general clinical features of patients, including sex, age, smoking and drinking history, body mass index (BMI), and whether surgery was performed. Routine measurements of peripheral blood within 3 weeks of the first diagnosis and before receiving antineoplastic therapy were also collected, including white blood cell (WBC) count, neutrophil count, lymphocyte count, platelet count (BPC), Hb, and RDW. Two oncologists were in charge of blood collection and all study participants had peripheral vein blood samples taken from the upper limb, which were collected between 6 am and 8 am. The blood samples were then handed over to the 2 laboratory physicians for sorting and counting, using an Automatic Blood Cell Counter Plus Crp (HORIBA, Japan). Alpha-fetoprotein (AFP) level was tested using an enzyme-linked immunosorbent assay kit (Abcam, USA).
Clinical staging was done according to the China liver cancer staging (CNLC) guidelines (Supplementary Table 1) [26]. HRR is the ratio of Hb to RDW, with 0.987 as the cut-off value obtained by receiver operating characteristic curve analysis (Supplementary Figure 1). NLR is the ratio of the neutrophil value to the lymphocyte value, with 3.85 as the cut-off value, and PLR is the ratio of BPC to the lymphocyte value, with 171.09 as the cut-off value (Supplementary Figure 1). For HRR, NLR, and PLR, all patients were divided into high- and low-level groups.
Follow-Up
Outpatient, inpatient, or telephone follow-ups were used to obtain survival data until the patient’s death or follow-up deadline (November 30, 2021). Patients who survived at the follow-up endpoint were recorded as survival, and those who died were recorded as death. The median follow-up period was 24.73 (1.0–48.9) months. PFS refers to the time between first diagnosis and the time of tumor progression, death, or follow-up. Progression is defined as an increase of approximately 25% in the vertical diameter of a tumor, the emergence of a new tumor, or a significant progression of the disease. OS refers to the time between first diagnosis and the time of death due to any reason.
Statistical Analysis
SPSS 22.0 statistical software was used to perform data analyses. Data involving 2 groups were compared using the chi-square test or Fisher’s precision test and multi-group data were compared using one-way analysis of variance. Independent risk factors for PFS and OS were assessed using the Cox proportional risk regression model. Survival curves were plotted according to the Kaplan-Meier (K-M) curve, and the log-rank test was used for inter-group comparisons. P<0.05 was set as the level of statistical significance.
Results
Baseline Clinical Features of Patients
A total of 252 patients with HCC were retrospectively included in this study, with a mean age of 64.67±10.38 years, including 62 females and 190 males. The proportion of patients with a history of smoking, and drinking, and those who were overweight (BMI >25) was 24.6%, 23.01%, and 21.43%, respectively. In terms of staging, CNLC stages Ia, Ib, IIa, IIb, IIIa, IIIb, and IV accounted for 7.14%, 7.94%, 12.70%, 35.32%, 17.46%, 7.54%, and 11.9% of patients, respectively, and 80.16% of patients underwent surgical treatment. The percentage of patients that had AFP >25 ng/mL was 70.20%, and 81.75% of patients were HBsAg-positive. The CBC of 252 patients with HCC was analyzed, and the results showed that 84.92% of patients had a WBC count <8.65×109/L, 92.06% had BPC count ≤92.06×109/L, 9.56% had Hb ≤11.4 g/dl, 80.16% had NLR <3.85, and 92.06% had PLR <171.09. According to the HRR cut-off value (0.987), 106 patients were in the high-level HRR group and 146 patients were in low-level HRR group. All clinical features of the 2 groups were compared, and there were significant differences in the drinking history, BMI, CNLC staging, surgery, and Hb levels between the high- and low-level HRR groups (P<0.05). The clinical features and laboratory indicators of the 2 groups are listed in Table 1.
Table 1.
HCC patients (N=252) categorized by HRR and their clinical baseline characteristics.
| Variables | All Patients (N 252) | HRR ≤0.987 (N=146) | HRR >0.987 (N=106) | χ2 | p Value | |||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| Age (mean±SD) | 64.67±10.38 | 64.53±10.17 | 64.87±10.78 | 0.276 | 0.006 | |||
| Gender | ||||||||
| Female | 62 | 24.6 | 44 | 30.14 | 18 | 16.98 | 5.730 | 0.017* |
| Male | 190 | 75.4 | 102 | 69.86 | 88 | 83.02 | ||
| Smoke | ||||||||
| No | 62 | 24.6 | 24 | 16.44 | 38 | 35.85 | 0.380 | 0.538 |
| Yes | 190 | 75.4 | 82 | 83.56 | 108 | 64.15 | ||
| Drink | ||||||||
| No | 39 | 23.01 | 16 | 10.96 | 23 | 21.70 | 7.060 | 0.008* |
| Yes | 152 | 76.89 | 90 | 89.04 | 72 | 78.30 | ||
| BMI | ||||||||
| ≤25 | 196 | 78.57 | 138 | 94.52 | 60 | 56.60 | 52.440 | <0.001* |
| >25 | 54 | 21.43 | 8 | 5.48 | 46 | 43.40 | ||
| CNLC | ||||||||
| Ia | 18 | 7.14 | 18 | 12.33 | 0 | 0 | 91.784 | <0.001* |
| Ib | 20 | 7.94 | 20 | 13.70 | 0 | 0 | ||
| IIa | 32 | 12.70 | 26 | 17.81 | 6 | 5.66 | ||
| IIb | 89 | 35.32 | 59 | 40.41 | 30 | 28.30 | ||
| IIIa | 44 | 17.46 | 14 | 9.59 | 30 | 28.30 | ||
| IIIb | 19 | 7.54 | 9 | 6.16 | 10 | 9.43 | ||
| IV | 30 | 11.90 | 0 | 0 | 30 | 28.31 | ||
| Surgery | ||||||||
| Yes | 202 | 80.16 | 66 | 45.21 | 136 | 93.15 | 36.836 | <0.001* |
| No | 50 | 19.84 | 40 | 54.79 | 10 | 6.29 | ||
| WBC | ||||||||
| ≤8.65×105 | 214 | 84.92 | 88 | 60.28 | 126 | 86.30 | 0.517 | 0.472 |
| >8.65×105 | 38 | 15.08 | 18 | 39.73 | 20 | 13.70 | ||
| BPC | ||||||||
| ≤3.09×105 | 232 | 92.06 | 96 | 90.57 | 136 | 93.15 | 0.561 | 0.454 |
| >3.09×105 | 20 | 7.94 | 10 | 9.43 | 10 | 3.85 | ||
| Hb (g/dL) | ||||||||
| ≤11.4 g/dl | 24 | 9.52 | 10 | 9.43 | 14 | 9.59 | 9.670 | 0.002* |
| >11.4 g/dl | 228 | 90.48 | 96 | 90.57 | 132 | 90.41 | ||
| AFP (ng/mL) | 26.797 | <0.001* | ||||||
| ≤25 | 75 | 29.80 | 13 | 12.26 | 62 | 42.47 | ||
| >25 | 177 | 70.20 | 92 | 87.84 | 83 | 57.54 | ||
| HBsAg | 0.064 | 0.801 | ||||||
| Negative | 46 | 18.25 | 19 | 17.92 | 27 | 18.50 | ||
| Positive | 206 | 81.75 | 87 | 82.08 | 119 | 81.51 | ||
| NLR | ||||||||
| ≤3.85 | 202 | 80.16 | 96 | 90.57 | 106 | 72.60 | 12.460 | <0.001* |
| >3.85 | 50 | 19.84 | 10 | 9.43 | 40 | 27.40 | ||
| PLR | ||||||||
| ≤171.09 | 232 | 92.06 | 96 | 90.57 | 136 | 93.15 | 0.561 | 0.454 |
| >171.09 | 20 | 7.94 | 10 | 9.43 | 10 | 6.85 | ||
Correlation Analysis of HRR with Survival Time
Univariate Cox survival analysis showed that drinking history, BMI, CNLC staging, surgical treatment, BPC, Hb, AFP, NLR, and HRR (HR=3.979, 95% CI: 2.955–5.358, P<0.001) were associated with PFS (Table 2). In addition, BMI, CNLC staging, surgical treatment, Hb, AFP, HBsAg-positive, and HRR (HR=4.613, 95% CI: 3.236–6.575) were correlated with OS (P<0.001, Table 2).
Table 2.
Comparative univariate survival analyses of 252 patients with HCC.
| Univariate analysis | Progression-free survival | Overall survival | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age, Years (mean±SD) | 1.123 (0.872, 1.447) | 0.369 | 1.026 (0.746, 1.412) | 0.874 |
| Gender | 0.205 | 0.100 | ||
| Female | 1 | 1 | ||
| Male | 1.209 (0.901, 1.621) | 1.355 (0.944, 1.945) | ||
| Smoke | 0.643 | 0.613 | ||
| Yes | 1 | 1 | ||
| No | 1.065 (0.795, 1.427) | 1.094 (0.772, 1.552) | ||
| Drink | 0.002* | 0.268 | ||
| No | 1 | 1 | ||
| Yes | 1.615 (1.192, 2.186) | 1.299 (0.854, 1.769) | ||
| BMI | <0.001* | <0.001* | ||
| ≤23.9 | 1 | 1 | ||
| >23.9 | 2.05 (1.497, 2.807) | 3.056 (1.73, 5.397) | ||
| CNLC | <0.001* | <0.001* | ||
| I, II | 1 | 1 | ||
| III, IV | 14.036 (9.719, 20.269) | 4.492 (3.130, 6.448) | ||
| Surgery | <0.001* | <0.001* | ||
| No | 1 | 1 | ||
| Yes | 66.67 (0.06, 0.037) | 2.624 (1.768, 3.893) | ||
| WBC | 0.288 | 0.985 | ||
| ≤8.65×109 | 1 | 1 | ||
| >8.65×109 | 1.259 (0.823, 1.924) | 1.015 (0.598, 1.689) | ||
| PBC | 0.021* | 0.078 | ||
| ≤3.09×109 | 1 | 1 | ||
| >3.09×109 | 1.722 (1.085, 2.733) | 1.704 (0.942, 3.080) | ||
| Hb (g/dL) | 0.012* | 0.031* | ||
| ≤11.4 | 1 | 1 | ||
| >11.4 | 1.571 (1.103, 2.238) | 1.552 (1.040, 2.316) | ||
| AFP (ng/mL) | <0.001* | <0.001* | ||
| ≤25 | 1 | 1 | ||
| >25 | 6.280 (4.358, 9.050) | 3.270 (2.210, 4.837) | ||
| HBsAg | 0.010 | 0.035* | ||
| Negative | 1 | 1 | ||
| Positive | 1.520 (1.104, 2.093) | 1.601 (1.035, 2.477) | ||
| NLR | 0.002* | 0.095 | ||
| ≤3.85 | 1 | 1 | ||
| >3.85 | 1.508 (0.435, 0.823) | 1.385 (0.945, 2.030) | ||
| PLR | 0.155 | 0.630 | ||
| ≤171.08 | 1 | 1 | ||
| >171.08 | 1.24 (0.592, 1.087) | 1.095 (0.758, 1.581) | ||
| HRR | <0.001* | <0.001* | ||
| ≤0.987 | 1 | 1 | ||
| >0.987 | 3.979 (2.955, 5.358) | 4.613 (3.236, 6.575) | ||
The multifactor Cox analysis results showed that CNLC staging, surgical treatment, AFP, Hb, and HRR (HR=0.407, 95% CI: 0.280–0.593, P<0.001) were independent factors influencing PFS in patients with HCC (Table 3). BMI, CNLC staging, surgical treatment, Hb, and HRR (HR=0.433, 95% CI: 0.281–0.665, P<0.001) were independent factors influencing OS in patients with HCC (Table 3).
Table 3.
Comparative multivariate survival analyses of 252 patients with HCC.
| Univariate Analysis | Progression-free survival | Overall survival | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Drink | 0.094 | – | ||
| Yes | 1 | – | ||
| No | 1.333 (0.537, 1050) | – | ||
| BMI | 0.708 | 0.008* | ||
| ≤25 | 1 | 1 | ||
| >25 | 1.076 (0.63, 1.368) | 1.806 (1.166, 2.798) | ||
| CNLC stage | <0.001* | 0.010* | ||
| I, II | 1 | 1 | ||
| III, IV | 4.692 (2.899, 7.594) | 1.920 (1.168, 3.157) | ||
| Surgery | <0.001* | 0.025* | ||
| No | 1 | 1 | ||
| Yes | 34.483 (0.014, 0.061) | 1.876 (0.307, 0.925) | ||
| Platelet (/L) | 0.328 | – | ||
| ≤3.09×109 | 1 | – | ||
| >3.09×109 | 1.292 (0.773, 2.159) | – | ||
| Hb (g/dL) | 0.014* | <0.001* | ||
| ≤11.4 | 1 | 1 | ||
| >11.4 | 1.645 (0.408, 0.904) | 1.686 (0.385, 0.913) | ||
| AFP (ng/mL) | <0.001* | 0.023* | ||
| ≤20 | 1 | 1 | ||
| >20 | 3.873 (2.560, 5.858) | 1.765 (1.080, 2.886) | ||
| HBsAg | – | 0.946 | ||
| Negative | – | 1 | ||
| Positive | – | 10.173 (0.605, 1.598) | ||
| NLR | 0.646 | |||
| ≤3.85 | 1 | – | – | |
| >3.85 | 1.083 (0.656, 1.299) | – | ||
| HRR | <0.001* | <0.001* | ||
| ≤0.987 | 1 | 1 | ||
| >0.987 | 2.457 (0.280, 0.593) | 2.31 (0.281, 0.665) | ||
Survival Analysis
The median follow-up period was 24.73 (1.0–48.9) months for the entire group, and 156 patients died during follow-up. For all patients, the median OS was 18.2 (3–48.3) months and the median PFS was 12.7 (0.8–42.5) months.
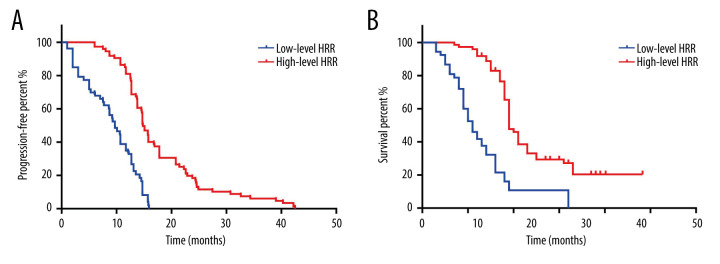
The median PFS in the high-level HRR group was 7.1 months longer than in the low-level group (10.7 [9.17–12.22] months vs 17.80 [16.18–19.41] months) (Figure 1A, P<0.001). In addition, AFP levels, CNLC staging, and surgical treatment also had a significant effect on PFS in HCC patients (Supplementary Figure 2).
Figure 1.
K-M curve for PFS and OS in high- and low-level HRR patients with HCC. (A) K-M curve for PFS in high- and low-level HRR patients with HCC; (B) K-M curve for OS in high- and low-level HRR patients with HCC.
HRR, AFP, Hb, patient CNLC staging, whether surgery was performed, and BMI were all associated with OS in HCC patients (Figure 1B and Supplementary Figure 3). Among them, the median OS of patients with high HRR levels was 10 months longer than that of the low-level group (13 [10.31–15.68] months vs 23 [17.37–26.19] months, P<0.001).
Prognosis of HRR Combined with AFP in Patients with HCC
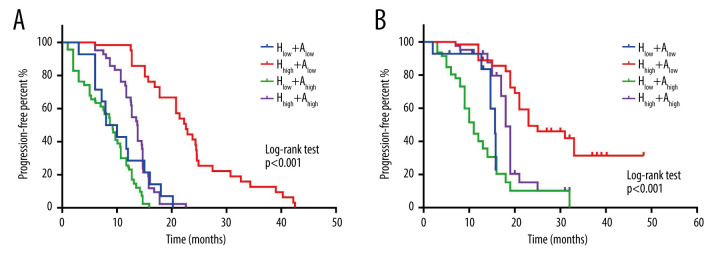
HRR and AFP were independent risk factors for prognosis in patients with HCC. To further explore the effects of different HRR combined with AFP on the prognosis of patients, according to the cut-off values of HRR and AFP, patients were divided into the high HRR+low AFP level group (Hhigh+Alow), low HRR+low AFP level group (Hlow+Alow), low HRR+high AFP level group (Hlow+Ahigh), and high HRR+high AFP level group (Hhigh+Ahigh). The median PFS and OS were 10 (3–20.2) months and 14.7 (2.1–15.8) months, 22.25 (6.3–42.5) months and 23 (7–48.3) months, 9.7 (1.1–15.9) months and 13.5 (3.2–32.7) months, and 13.7 (6–22.6) months and 18 (7–32) months, in the Hlow+Alow, Hhigh+Alow, Hlow+Ahigh, and Hhigh+Ahigh groups, respectively. PFS and OS in the Hlow+Ahigh group were the worst, while the PFS and OS in the Hhigh+Alow group were the best. The K-M curve and log-rank test found that the patients in the Hlow+Ahigh group had the worst prognosis, while the patients in the Hhigh+Alow group had the best prognosis (Figure 2, both P<0.001). All results suggest that the prognosis is poor when HCC patients have HRR >0.987 and AFP >25 μg/mL.
Figure 2.
Effect of combined hemoglobin/red blood cell distribution width ratio (HRR) and alpha-fetoprotein (AFP) on progression-free survival (PFS) and overall survival (OS) in hepatocellular carcinoma (HCC) patients. (A) K-M curve for the PFS of combined HRR and AFP in HCC patients; (B) K-M curve for the OS of combined HRR and AFP in HCC patients.
Correlation of HRR with Tumor Staging in Patients with HCC
Multivariate analysis showed that HRR and tumor staging were independent risk factors affecting the prognosis of patients with HCC. To explore the potential link between the 2, this study analyzed HRR in patients at different stages. The results showed that the average HRR of HCC patients in stages Ia and Ib, IIa and IIb, IIIa, and IIIb, as well as IV, was 1.824±1.899 and 1.689±0.373, 1.286±0.124 and 1.01±0.151, 0.947±0.149 and 0.892±0.128, and 0.838±0.109, respectively, suggesting that HRR is inversely correlated with tumor staging (Figure 3, P<0.001).
Figure 3.
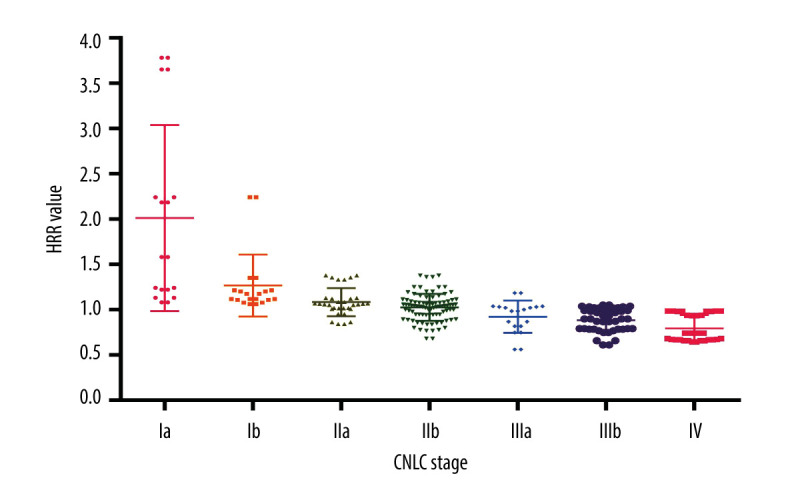
Relationship between hemoglobin/red blood cell distribution width ratio (HRR) and China liver cancer stage.
Discussion
In this study, we investigated the correlation between HRR in peripheral blood and prognosis of 252 patients with HCC. The cut-off value of HRR was used to divide the 252 patients into low- and high-level groups. Patients with low-level HRR often had a history of alcohol consumption and hepatitis B, an overweight BMI (≥25), elevated AFP, anemia, and stage II or III cancer. Univariate and multivariate analysis found that patients with high AFP levels and low HRR levels had a significantly worse prognosis than those in the high-level HRR group. Analysis of patient prognosis by combining HRR and AFP showed that patients with HCC had the worst prognosis when they had both low and high levels of AFP. In addition, the study demonstrated that HRR is inversely correlated with CNLC staging in HCC patients, which may be one of the potential mechanisms by which HRR affects patient prognosis. The results of this study suggest that the prognosis of patients with a variety of malignancies, including HCC, is associated with HRR, a potential marker of tumor prognosis. The combination of HRR and AFP more accurately reflects patient prognosis.
Anemia is one of the common complications in tumor patients. Bi et al [27] compared the Hb levels in peripheral blood before treatment in 152 patients with bladder cancer and showed that 52% had varying degrees of anemia and poor OS. In another regression analysis study, the analysis of Hb levels in 2163 patients with preoperative gastric cancer found that preoperative anemia was an independent risk factor for gastric cancer prognosis [28]. Consistent with previous studies, our study found poor prognosis in patients with Hb <11.4 μg/mL. The effect of anemia on the prognosis of patients with tumors is regulated by numerous factors. First, Hb reflects the nutritional status of patients, and good nutrition helps to activate the immune system and reduce adverse reactions caused by drug treatment. Second, anemia leads to a decrease in blood oxygen saturation, resulting in “hypoxia in the body”. Under hypoxic conditions, the expression of angiogenesis factors, such as HIF1 and ANGPTL4, is elevated to promote neovascularization, and the rich vascular network provides a metastatic pathway for tumor cells [29,30]. Due to the many factors affecting Hb levels, such as chronic inflammation, aging, and menstruation, the sensitivity and specificity of prognosis in patients with Hb-responsive tumors are not high.
RDW is another common indicator of CBC that reflects RBC heterogeneity [31]. In previous studies, RDW levels were found to be increased in the peripheral blood of patients with chronic diseases such as cardiovascular disease, COPD, diabetes, and cancers [32]. In HCC, Smirne et al [33] confirmed that median survival was significantly shortened and positively correlated with mortality when patients had an RDW ≥14.6%. Howell et al found that high RDW was inversely associated with prognosis in 442 HCC patients treated with sorafenib by measuring their pretreatment RDW levels [34]. Although RDW may be associated with prognosis in patients with HCC, the specific mechanism remains unclear. Goyal et al [35] believe it may be related to inflammation and oxidative stress. Inflammatory factors, such as interleukin-6 (IL-6), are highly expressed in the tumor microenvironment, and increase erythrocyte heterogeneity by inhibiting erythropoietin and inducing immature RBC release from the bone marrow into the peripheral blood [36]. However, studies have found that RDW varies greatly between people with long-term exercise vs those who are inactive [37], which may affect the specificity of RDW in predicting prognosis levels in patients with tumors.
HRR – the ratio of Hb to RDW – is a novel inflammatory marker that more stably reflects the body’s oxidative stress and the degree of the systemic inflammatory response [38]. In 2016, Sun et al first found that patients with high levels of HRR had a better prognosis by calculating peripheral blood HRR in patients with esophageal squamous carcinoma. This finding was subsequently confirmed in patients with head and neck cancer, NSCLC, bladder cancer, and other malignancies [18–24]. In the meta-analysis by Chi et al, a total of 2985 patients with malignancies from 11 retrospective analyses were included. This study found that patients with malignancies in the low-HRR group were twice as likely to have malignant events such as recurrence and metastasis when compared to those in the high-HRR group. In addition, Zhai et al confirmed that HRR was a good predictor of prognosis for patients with liver metastases from gastric cancer, and its predictive power was further improved when combined with PLR [25]. In our study, we found that the patients with HCC in the high-HRR group had a better prognosis, and combined detection of HRR and AFP improved the ability to predict prognosis in HCC patients. These results suggest that HRR is a valid prognostic marker, and its use in combination with other valid biomarkers may further enhance the predictive validity.
Our study found that HRR is inversely correlated with CNLC staging, suggesting that HRR may affect patient outcomes by promoting tumor progression, but its specific mechanism is not well understood. Anemia is a common symptom in patients with malignant tumors, which can be caused by a variety of factors such as tumor rupture, bleeding, and treatment adverse effects [39]. Anemia occurs with increased neovascularization and elevated levels of tumor invasion metastasis, which may be one of the potential mechanisms by which HRR promotes tumor progression in HCC. Some studies proposed that this phenomenon may be used to improve the effectiveness of chemotherapy by entering the systemic circulation through Hb-based oxygen carriers to alleviate solid tumor hypoxia [40], and this may be a potential way to use HRR to improve patient treatment efficiency. Perhaps in future studies, we can use this finding to further investigate whether modulating HRR in peripheral blood can promote the treatment outcome and survival of cancer patients.
The effect of HRR on tumor prognosis may be related to RBC levels and variability. Yin et al [41] found that RBCs and Hb can act as endogenous signals to activate the ROS-NFγB pathway in tumor cells, thereby regulating ABCB1 gene expression, inducing cytokine release, promoting macrophage recruitment and polarization, and then promoting tumor cell proliferation and tumor resistance.
Tham et al [42] showed that BMI is positively correlated with prognosis of cancer patients, while this study found that in overweight patients with a BMI of >25, HRR levels were low and patients had a poor prognosis. This may be because the liver, as the central organ of metabolism, secretes large amounts of lipid metabolism regulator proteins, such as bile and ANGPTLs to promote lipid metabolism [43]. In overweight people, the incidence of nonalcoholic fatty liver disease increases, and liver function is impaired, which is positively associated with the incidence of liver cancer [44]. In these patients, those without fatty liver function impairment are more likely to a have poor prognosis. Second, 19.44% of the patients in this study were already in stage IIIb or IV at the time of initial diagnosis, and ascites caused by distant metastasis of the peritoneum and other parts may also cause patients to have a BMI >25.
Conclusions
This study is the first to confirm the relevance of HRR in the prognosis of patients with liver cancer. HRR will be an important biomarker in predicting the prognosis of patients with HCC.
However, our study has some limitations. First, it was a single-center study with a small sample size, so there may be some bias. Second, it was also a retrospective study, which did not distinguish between patients treated with different drugs and did not explore the effect of different treatment regimens on patient prognosis in HCC patients with high and low levels of HRR.
Supplementary Data
Supplementary Table 1.
CNLC grading method for patients with HCC.
| Physical activity status score | Child-Pugh grade | Tumor number | Maximal diameter | Vascular invasion or extrahepatic metastases | |
|---|---|---|---|---|---|
| Ia | 0–2 | A/B | 1 | ≤5 cm | No |
| Ib | 0–2 | A/B | 1 | >5 cm, or 2–3 tumors ≤3 cm | No |
| IIa | 0–2 | A/B | 2–3 | >3 cm | No |
| IIb | 0–2 | A/B | ≤4 | Regardless of tumor diameter | No |
| IIIa | 0–2 | A/B | Regardless o tumor number | Regardless of tumor diameter | With vascular invasion |
| IIIb | 0–2 | A/B | Regardless of tumor number | Regardless of tumor diameter | Regardless of vascular invasion but extrahepatic metastases exist |
| IV | 3–4 | C | Regardless of tumor number | Regardless of tumor diameter | Regardless of vascular invasion and extrahepatic metastases |
ROC curve of NLR, PLR and HRR.
Different factors affecting PFS in patients with HCC. (A) K-M curve for PFS in high-and low-level Hb patients with HCC; (B) K-M curve for PFS in high-and low-level AFP patients with HCC; (C) K-M curve for OS in patients with HCC who have undergone surgery or not; (D) K-M curve for PFS in different CNLC stage patients with HCC.
Different factors affecting OS in patients with HCC. (A) K-M curve for OS in high- and low-level AFP patients with HCC; (B) K-M curve for OS in high-and low-level Hb patients with HCC; (C) K-M curve for OS in different CNLC stage patients with HCC; (D) K-M curve for OS in patients with HCC who have undergone surgery or not; (E) K-M curve for OS in patient who have different BMI. Normal weight: BMI <25, Overweight: BMI ≥25
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Footnotes
Conflict of interest: None declared
Department and Institution Where Work Was Done
All work was done in the Department of Oncology, Graduate Student Affairs Office, the First Affiliated Hospital of Anhui Medical University.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: This study was supported by First Affiliated Hospital of Anhui Medical University Youth Cultivation Foundation (2020kj21)
References
- 1.Xu F, Jin T, Zhu Y, Dai C. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res. 2018;37:110. doi: 10.1186/s13046-018-0777-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 4.Hibino S, Kawazoe T, Kasahara H, et al. Inflammation-Induced tumorigenesis and metastasis. Int J Mol Sci. 2021;22:5421. doi: 10.3390/ijms22115421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arvanitakis K, Mitroulis I, Germanidis G. Tumor-Associated neutrophils in hepatocellular carcinoma pathogenesis, prognosis, and therapy. Cancers. 2021;13:2899. doi: 10.3390/cancers13122899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin Y, Wang J, Wang X, et al. Prognostic value of the neutrophil to lymphocyte ratio in lung cancer: A meta-analysis. Clinics (Sao Paulo) 2015;70:524–30. doi: 10.6061/clinics/2015(07)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toda M, Tsukioka T, Izumi N, et al. Platelet-to-lymphocyte ratio predicts the prognosis of patients with non-small cell lung cancer treated with surgery and postoperative adjuvant chemotherapy. Thorac Cancer. 2018;9:112–19. doi: 10.1111/1759-7714.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe K, Yasumoto A, Amano Y, et al. Mean platelet volume and lymphocyte-to-monocyte ratio are associated with shorter progression-free survival in EGFR-mutant lung adenocarcinoma treated by EGFR tyrosine kinase inhibitor. PLoS One. 2018;13:e0203625. doi: 10.1371/journal.pone.0203625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yılmaz A, Mirili C, Tekin SB, et al. The ratio of hemoglobin to red cell distribution width predicts survival in patients with gastric cancer treated by neoadjuvant FLOT: A retrospective study. Ir J Med Sci. 2020;189:91–102. doi: 10.1007/s11845-019-02153-x. [DOI] [PubMed] [Google Scholar]
- 10.Huang XZ, Yang YC, Chen Y, et al. Preoperative anemia or low hemoglobin predicts poor prognosis in gastric cancer patients: A meta-analysis. Dis Markers. 2019;2019:7606128. doi: 10.1155/2019/7606128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Y, Su C, Jiang H, et al. The association between pretreatment anemia and overall survival in advanced non-small cell lung cancer: A retrospective cohort study using propensity score matching. J Cancer. 2022;13:51–61. doi: 10.7150/jca.55159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo S, Tang L, Chen Q, et al. Is hemoglobin level in patients with nasopharyngeal carcinoma still a significant prognostic factor in the era of Intensity-Modulated radiotherapy technology? PLoS One. 2015;10:e136033. doi: 10.1371/journal.pone.0136033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu WY, Yang XB, Wang WQ, et al. Prognostic impact of the red cell distribution width in esophageal cancer patients: A systematic review and meta-analysis. World J Gastroenterol. 2018;24:2120–29. doi: 10.3748/wjg.v24.i19.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Divsalar B, Heydari P, Habibollah G, et al. Hematological parameters changes in patients with breast cancer. Clin Lab. 2021:201103. doi: 10.7754/Clin.Lab.2020.201103. [DOI] [PubMed] [Google Scholar]
- 15.Hu L, Li M, Ding Y, et al. Prognostic value of RDW in cancers: A systematic review and meta-analysis. Oncotarget. 2017;8:16027–35. doi: 10.18632/oncotarget.13784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Życzkowski M, Rajwa P, Gabrys E, et al. The relationship between red cell distribution width and cancer-specific survival in patients with renal cell carcinoma treated with partial and radical nephrectomy. Clin Genitourin Cancer. 2018;16:e677–83. doi: 10.1016/j.clgc.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Sun P, Zhang F, Chen C, et al. The ratio of hemoglobin to red cell distribution width as a novel prognostic parameter in esophageal squamous cell carcinoma: A retrospective study from southern China. Oncotarget. 2016;7:42650–60. doi: 10.18632/oncotarget.9516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yılmaz H, Yılmaz A, Demirağ G. Prognostic significance of hemoglobin-to-red cell distribution width ratio in patients with metastatic renal cancer. Future Oncol. 2021;17:3853–64. doi: 10.2217/fon-2021-0040. [DOI] [PubMed] [Google Scholar]
- 19.Wu F, Yang S, Tang X, et al. Prognostic value of baseline hemoglobin-to-red blood cell distribution width ratio in small cell lung cancer: A retrospective analysis. Thorac Cancer. 2020;11:888–97. doi: 10.1111/1759-7714.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrella F, Casiraghi M, Radice D, et al. Prognostic value of the hemoglobin/red cell distribution width ratio in resected lung adenocarcinoma. Cancers (Basel) 2021;13:710. doi: 10.3390/cancers13040710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Z, Zhang X, Luo Y, et al. The value of hemoglobin-to-red blood cell distribution width ratio (Hb/RDW), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) for the diagnosis of nasopharyngeal cancer. Medicine (Baltimore) 2021;100(28):e26537. doi: 10.1097/MD.0000000000026537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su YC, Wen SC, Li CC, et al. Low hemoglobin-to-red cell distribution width ratio is associated with disease progression and poor prognosis in upper tract urothelial carcinoma. Biomedicines. 2021;9(6):672. doi: 10.3390/biomedicines9060672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bi H, Huang Y, Wang G, et al. Impact of body mass index and pretreatment hemoglobin level on prognosis following radical cystectomy for bladder cancer in males and females. Urol Int. 2020;104:28–35. doi: 10.1159/000500561. [DOI] [PubMed] [Google Scholar]
- 24.Chi G, Lee JJ, Montazerin SM, et al. Prognostic value of hemoglobin-to-red cell distribution width ratio in cancer: A systematic review and meta-analysis. Biomark Med. 2022;16:473–82. doi: 10.2217/bmm-2021-0577. [DOI] [PubMed] [Google Scholar]
- 25.Zhai Z, Gao J, Zhu Z, et al. The ratio of the hemoglobin to red cell distribution width combined with the ratio of platelets to lymphocytes can predict the survival of patients with gastric cancer liver metastasis. Biomed Res Int. 2021;2021:8729869. doi: 10.1155/2021/8729869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Health Commission of the People’s Republic of China. [Diagnosis and treatment of primary liver cancer (2019)]. Electronic Journal of Comprehensive Oncology Therapy. 2020;6:55–85. [in Chinese] [Google Scholar]
- 27.Liu X, Qiu H, Huang Y, et al. Impact of preoperative anemia on outcomes in patients undergoing curative resection for gastric cancer: A single-institution retrospective analysis of 2163 Chinese patients. Cancer Med. 2018;7:360–69. doi: 10.1002/cam4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niu Y, Bao L, Chen Y, et al. HIF2-induced long noncoding RNA RAB11B-AS1 promotes hypoxia-mediated angiogenesis and breast cancer metastasis. Cancer Res. 2020;80:964–75. doi: 10.1158/0008-5472.CAN-19-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tse SW, Tan CF, Park JE, et al. Microenvironmental hypoxia induces dynamic changes in lung cancer synthesis and secretion of extracellular vesicles. Cancers (Basel) 2020;12:2917. doi: 10.3390/cancers12102917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salvagno GL, Sanchis-Gomar F, Picanza A, et al. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52:86–105. doi: 10.3109/10408363.2014.992064. [DOI] [PubMed] [Google Scholar]
- 31.Hu L, Li M, Ding Y, et al. Prognostic value of RDW in cancers: A systematic review and meta-analysis. Oncotarget. 2017;8(9):16027–35. doi: 10.18632/oncotarget.13784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smirne C, Grossi G, Pinato DJ, et al. Evaluation of the red cell distribution width as a biomarker of early mortality in hepatocellular carcinoma. Dig Liver Dis. 2015;47:488–94. doi: 10.1016/j.dld.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Howell J, Pinato DJ, Ramaswami R, et al. Integration of the cancer-related inflammatory response as a stratifying biomarker of survival in hepatocellular carcinoma treated with sorafenib. Oncotarget. 2017;8:36161–70. doi: 10.18632/oncotarget.15322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goyal H, Hu ZD. Prognostic value of red blood cell distribution width in hepatocellular carcinoma. Ann Transl Med. 2017;5:271. doi: 10.21037/atm.2017.06.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun P, Zhang F, Chen C, et al. The ratio of hemoglobin to red cell distribution width as a novel prognostic parameter in esophageal squamous cell carcinoma: A retrospective study from southern China. Oncotarget. 2016;7:42650–60. doi: 10.18632/oncotarget.9516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lippi G, Schena F, Salvagno GL, et al. Foot-strike haemolysis after a 60-km ultramarathon. Blood Transfus. 2012;10:377–83. doi: 10.2450/2012.0167-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood. 2019;133:40–50. doi: 10.1182/blood-2018-06-856500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greten FR, Grivennikov SI. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity. 2019;51:27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lucas A, Belcher DA, Munoz C, et al. Polymerized human hemoglobin increases the effectiveness of cisplatin-based chemotherapy in non-small cell lung cancer. Oncotarget. 2020;11:3770–81. doi: 10.18632/oncotarget.27776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin T, He S, Liu X, et al. Extravascular red blood cells and hemoglobin promote tumor growth and therapeutic resistance as endogenous danger signals. J Immunol. 2015;194:429–37. doi: 10.4049/jimmunol.1400643. [DOI] [PubMed] [Google Scholar]
- 41.Tham T, Olson C, Wotman M, et al. Evaluation of the prognostic utility of the hemoglobin-to-red cell distribution width ratio in head and neck cancer. Eur Arch Otorhinolaryngol. 2018;275:2869–78. doi: 10.1007/s00405-018-5144-8. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen P, Leray V, Diez M, et al. Liver lipid metabolism. J Anim Physiol Anim Nutr (Berl) 2008;92:272–83. doi: 10.1111/j.1439-0396.2007.00752.x. [DOI] [PubMed] [Google Scholar]
- 43.Fan KC, Wu HT, Wei JN, et al. Serum angiopoietin-like protein 6, risk of type 2 diabetes, and response to hyperglycemia: A prospective cohort study. J Clin Endocrinol Metab. 2020;105:dgaa103. doi: 10.1210/clinem/dgaa103. [DOI] [PubMed] [Google Scholar]
- 44.Marengo A, Rosso C, Bugianesi E. Liver cancer: Connections with obesity, fatty liver, and cirrhosis. Annu Rev Med. 2016;67:103–17. doi: 10.1146/annurev-med-090514-013832. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
CNLC grading method for patients with HCC.
| Physical activity status score | Child-Pugh grade | Tumor number | Maximal diameter | Vascular invasion or extrahepatic metastases | |
|---|---|---|---|---|---|
| Ia | 0–2 | A/B | 1 | ≤5 cm | No |
| Ib | 0–2 | A/B | 1 | >5 cm, or 2–3 tumors ≤3 cm | No |
| IIa | 0–2 | A/B | 2–3 | >3 cm | No |
| IIb | 0–2 | A/B | ≤4 | Regardless of tumor diameter | No |
| IIIa | 0–2 | A/B | Regardless o tumor number | Regardless of tumor diameter | With vascular invasion |
| IIIb | 0–2 | A/B | Regardless of tumor number | Regardless of tumor diameter | Regardless of vascular invasion but extrahepatic metastases exist |
| IV | 3–4 | C | Regardless of tumor number | Regardless of tumor diameter | Regardless of vascular invasion and extrahepatic metastases |
ROC curve of NLR, PLR and HRR.
Different factors affecting PFS in patients with HCC. (A) K-M curve for PFS in high-and low-level Hb patients with HCC; (B) K-M curve for PFS in high-and low-level AFP patients with HCC; (C) K-M curve for OS in patients with HCC who have undergone surgery or not; (D) K-M curve for PFS in different CNLC stage patients with HCC.
Different factors affecting OS in patients with HCC. (A) K-M curve for OS in high- and low-level AFP patients with HCC; (B) K-M curve for OS in high-and low-level Hb patients with HCC; (C) K-M curve for OS in different CNLC stage patients with HCC; (D) K-M curve for OS in patients with HCC who have undergone surgery or not; (E) K-M curve for OS in patient who have different BMI. Normal weight: BMI <25, Overweight: BMI ≥25