Abstract
Essential oils and aromatic extracts (oleoresins, absolutes, concretes, resinoids) are often used as food flavorings and constituents of fragrance compositions. The flavor and fragrance industry observed significant growth in the sales of some natural materials during the COVID-19 outbreak. Some companies worldwide are making false claims regarding the effectiveness of their essential oils or blends (or indirectly point toward this conclusion) against coronaviruses, even though the available data on the activity of plant materials against highly pathogenic human coronaviruses are very scarce. Our exploratory study aimed to develop pioneering knowledge and provide the first experimental results on the inhibitory properties of hundreds of flavor and fragrance materials against SARS-CoV-2 main and papain-like proteases and the antiviral potential of the most active protease inhibitors. As essential oils are volatile products, they could provide an interesting therapeutic strategy for subsidiary inhalation in the long term.
Subject terms: Proteases, SARS-CoV-2
Introduction
Essential oils (EOs) are plant-derived products and often consist of dozens to hundreds of volatile compounds. They are produced only by physical methods such as mechanical pressing or distillation (hydrodistillation, steam distillation)1. Aromatic extracts (AEs), conversely, are obtained by extraction with organic solvents by classical solid–liquid extraction, solid–solid extraction (enfleurage), or modern equipment (supercritical fluid and microwave extractors). Essential oils and aromatic extracts are often used as food flavorings, perfuming agents, pharmaceuticals, and the former for aromatherapy. The complexity of EO and AE matrices is an undesirable trait in drug discovery, but they are useful in some areas of medical therapy and can be a promising source of new drugs2. Essential oils and aromatic extracts are not considered highly effective antiviral agents, and in the flavor and fragrance scientific community, interest has shifted more toward the assessment of the antimicrobial properties of these plant-derived products. Studies regarding the antiviral properties of volatile natural materials mostly concern in vitro evaluations3; in vivo testing of these materials is very rare4, and in vivo human data are virtually nonexistent.
The flavor and fragrance industry observed significant growth in the sales of some natural materials during the coronavirus disease-2019 (COVID-19) outbreak. This growth was attributed in part to the aromatherapeutic properties of the selected products but also in part to fraudulent marketing. Some companies worldwide are making false claims regarding the effectiveness of their essential oils (or indirectly point toward this conclusion) against coronaviruses and (in the same marketing message) their ability to boost the immune system during respiratory illnesses.
Coronaviruses (fam. Coronaviridae) are crown-like enveloped viruses comprising single-stranded, positive-sense RNA. Host targets for coronaviruses are animals and humans. Before the current outbreak, HCoV-229E, HCoV-NL63, HCoV-OC43, HCoV-HKU1, SARS-CoV, and MERS-CoV were known to be able to infect humans5. The first four are the cause of (in most cases) mild illnesses (common colds), whereas infections with SARS-CoV and MERS-CoV can develop into a severe respiratory disease that could be fatal. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is responsible for the current outbreak of the disease (COVID-19), is the seventh coronavirus known to infect humans6. Due to international travel and human–human interactions, the virus spread rapidly, which has resulted in more than 500 million infections and over 6 000 000 deaths (according to Worldometers Info, accessed June 30, 2022). The world scientific community and pharmaceutical industry are devoted to finding an effective therapy for COVID-19. In addition to the existing protective vaccinations against COVID-19, targeted drug therapy plays a very important role; however, despite many efforts, it has not been sufficiently developed thus far. A preliminary study showed that drugs from many groups, such as antibacterials, antiprotozoals, immunomodulators, angiotensin II receptor blockers, bradykinin B2 receptor antagonists, corticosteroids, anthelmintics, H2 blockers, and anticoagulants, are considered potential therapeutics in COVID-19 treatment7.
Two of the very well characterized and promising drug targets are the main protease (Mpro, 3CLpro) and the papain-like protease (PLpro), which play key roles in viral replication and transcription8,9. SARS-CoV-2 PLpro also exhibits deubiquitinating and deISGylating activities, which are implicated in suppressing host innate immune responses9,10. Therefore, research efforts are focused on the rapid development of SARS-CoV-2 Mpro and PLpro inhibitors as drug candidates. Several approaches have been utilized in coronaviral protease inhibitor-drug discovery, including structure-based drug design8,11–15, high-throughput (HT) screening of structurally diverse compound libraries16–18, in silico studies19–21, and drug repurposing22–25. Despite substantial efforts, only one compound (PF-07321332, brand name Paxlovid) has been approved by the US Food and Drug Administration (FDA) for the treatment of mild-to-moderate coronavirus disease26, and one (S-217622) is in late clinical trials (NCT05305547)21. Both compounds act as SARS-CoV-2 Mpro inhibitors and are administered orally. Unlike Mpro, few SARS-CoV-2 PLpro inhibitors with experimentally proven efficacy have been reported17,27–29. Due to the restricted binding pockets at the P1 and P2 substrate-binding sites and the preference for relatively large ubiquitin-like proteins over short peptide substrates, inhibitor-drug design toward SARS-CoV-2 PLpro is much more challenging10,30.
In the present study, we wanted to address the question of whether common flavor and fragrance materials are inhibitors or have the potential to be a source for effective inhibitors of the main and papain-like proteases of SARS-CoV-2 and whether these inhibitors exhibit in vitro antiviral properties. As essential oils are volatile products, they could provide an interesting therapeutic strategy for subsidiary inhalation in the long term. To the best of our knowledge, this is the first experimental study31 regarding the evaluation of the potential of flavors and fragrances as a part of the search for recent anti-coronaviral strategies.
Materials and methods
The molecular modeling studies were prepared with the protocol of our earlier studies32. Crystal structures of SARS-CoV-2 Mpro (PDB ID: 6XBH33) and SARS-CoV-2 PLpro (PDB ID: 6WX410) were obtained from the Research Collaboratory for Structural Bioinformatics Protein Data Bank (RCSB-PDB). Waters, ligands, and ions were deleted, and the proteins were protonated at the experimental pH and optimized34. Before docking, the structures of the inhibitors were protonated in the experimental pH typical for each enzyme and optimized by LigPrep35. The Induced Fit Docking protocol was used for molecular docking studies. The site of the enclosing box was set at 20 Å on the centroid of the catalytic cysteines36. The VSGB (variable-dielectric generalized Born) model, which incorporates residue-dependent effects, was used. The solvent was water. The active center amino acids were optimized within 5.0 Å of ligand poses, and Glide redocking was carried out with the XP (extra precision) algorithm. The top three poses for the ligand were saved. The last steps, rePrime refinement, and MM-GBSA (molecular mechanics-generalized Born surface area) calculations were performed to calculate the Gibbs free energies with protein flexibility, and the distance from the ligand was also set to 5.0 Å. The one with the lowest free binding energy was considered for publication. The protocol and all the parameters were the same for the second docking, but the ligands were forced to interact with the catalytic cysteine (Cys145 or Cys111) during the IFD.
Reagents
Essential oils and related products
All samples of investigated plant volatile products were generously donated by members of the industry – manufacturers of the products at the best possible quality. Nomenclature of the products according to specifications.
They were:
A. Fakhry & Co.
Essential oils of bitter orange (fruit) distilled, petitgrain bigarade sur fleurs, cabbage rose, carrot seed, celery seed, celery leaf, chamomile blue, cinnamon basil, tropical basil, sweet basil, lemon basil, clary sage, coriander seed, coriander leaf, dill leaf, jasmine, anise seed, cumin, lantana, leek, fennel bitter, fennel sweet, neroli, neroli BdN, parsley seed, parsley leaf, petitgrain bigarade, petitgrain key lime, petitgrain mandarin, winter savory, summer savory, tagetes, geranium, petitgrain mandarin, bitter orange distilled, rosemary, caraway, aniseed, spearmint, sweet marjoram, mature bitter orange (“red”) expressed, immature bitter orange (“green”) expressed, onion, and garlic, absolutes of artichoke, cabbage rose, cabbage rose leaves, calendula, carnation, carrot leaf, cassie, Rosa damascena, bitter orange blossom hydrolate, honeysuckle, jasmine, mango leaf, nasturtium, nettle, olive leaf, rocket, spinach, strawberry leaf, tomato leaf, tropical basil, sweet basil, lemon basil, tropical basil hydrolate, petitgrain bigarade, tagetes, geranium, carrot seed, coriander leaves, fenugreek, geranium hydrolate, rosemary, caraway, sweet marjoram, blue chamomile, cumin, orange flower water, violet leaves, and clary sage, concretes of cabbage rose, jasmine, lemon basil, sweet basil, tropical basil, geranium, thyme, blue chamomile, cumin, violet leaves, nettle, calendula, artichoke, nasturtium, mango leaves, cassie, strawberry leaves, olive leaves, clary sage, carnation, rocket, and carrot leaves, and hydroalcoholic extract of licorice.
Albert Vieille
Essential oils of traditional and complete cistus and absolutes of cistus, cistus SEV, and tonka bean.
Amigo & Arditi
Essential oils of cabreuva red, guaiac wood, citronella organic, and petitgrain bitter orange.
Ashna Samai
Essential oils of Irish lace, spiked pepper, copal, ishpingo, and palo santo.
Berje Inc.
Essential oils of angelica root, angelica seed, anise (Pimpinella anisum), Artemisia afra, rosewood, buchu leaf betulina, buchu leaf crenulata, calamus, cascarilla bark, catnip, coffee, cognac white, cubeb, dill seed, Eucalyptus smithii, fir needle China, lavandin abrialis, lovage leaf, lovage root, mandarin red, milfoil, Ocotea cymbarum, parsley leaf, Rosa damascena Bulgaria, Rosa damascena Turkey, peppermint Yakima, Pinus pumilio, perilla, sassafras, savory, siam wood, tagetes, valerian root, and wormwood European, and fir balsam, absolutes of spruce, Rosa damascena Morocco and Bulgaria, and orris root concrete, and gums of labdanum and storax.
Bordas S.A.
Essential oils of labdacistus, rue, bitter fennel, clementine, cypriol, laurel, onion, tarragon, tangerine, petitgrain lemon, petitgrain mandarin, vetiver Java, ylang-ylang 2nd, citronella Java type, orange CP Valencia, and thyme red, absolutes of labdanum, jasmine, spike lavender, thyme red, and thyme gray, and oleoresins of cumin and turmeric.
BoreA Canada
Essential oils of black spruce twigs and leaves, bark, and wood, jack pine twigs and leaves, and wood, balsam poplar, balsam fir bark, hops, larch tamarack, white spruce, and Labrador tea.
Brüder Unterweger
Essential oils of black pine, maritime pine, silver fir cones, hops, hyssop, yarrow blue 3% and 15% chamazulene, and Venice turpentine I.
Christodoulou Bros S.A.
Essential oils of bitter orange, lemon, mandarin, and white grapefruit.
Citrus and Allied Essences Ltd.
Essential oils of fir needle Siberian, ginger China, lemon California type CP Extra, lemon Argentina Tucuman CP, orange Valencia CP, orange Brazil CP, orange Brazil fivefold, corn mint India (partially dementholized, Mentha arvensis), tangerine Dancy, and geranium China.
D.V. Deo Industries
Essential oils of palmarosa, cardamom, gingergrass vetiver, cypriol, angelica root, spikenard, ginger, betel leaf, lemongrass, galangal root, chaulmoogra, and cedarwood.
Dutjahn Sandalwood Oils.
Santalum spicatum essential oil.
Essential Oils and Herbs
Melissa and yarrow essential oils.
Essential Oils of Tasmania
Essential oils of bitter fennel, coastal tea tree, fennel (70% fenchone), kunzea, lavender, parsley herb, parsley seed, peppermint, rosemary, big badja gum, smoky tea tree, and southern Rosalina, absolutes of Boronia, Boronia leaves, and Tasmanian pepperberry, and Tasmanian pepperberry extract.
Eucaforest
Essential oils of rose geranium, tea tree, lemon-scented tea tree, Eucalyptus smithii, Eucalyptus radiata, and tagete.
Hierbas Patagónicas S.R. L
Essential oils of fabiana, Douglas fir, paramela, and Pinus ponderosa.
Jacarandas International
Essential oils of psiadia, ylang-ylang I, II, III, and complete, clove bud and leaf, ginger fresh, black pepper, Helichrysum gymnocephalum, Helichrysum bracteiferum, Eucalyptus citriodora, geranium, niaouli, and ravintsara.
Kallin Ltd.
Essential oils of citronella China, garlic China, geranium China, geranium Egypt, ginger China, neroli Morocco, peppermint arvensis China, petitgrain bigarade, sandalwood India, shiu (Ho wood) China, vetiver Bourbon, and ylang-ylang, absolutes of coffee, fenugreek, jasmine India, orris, and orange flower, and ginger China and pink pepper CO2 extracts.
Lebermuth
Essential oils of allspice, blood orange, catnip, cedarwood Himalayan, Eucalyptus polybractea, goldenrod, grapefruit red, Lavandula stoechas, mandarin red, Peru balsam, Rosa damascena, black spruce, tea tree, ylang-ylang I, and ylang-ylang II, and neem oil.
Les Arômes du Maroc
Mastic and neroli essential oils, orange flower water absolute, orange flower and bran concretes, and orris germanica butter.
Lluch Essence
Essential oils of cedarleaf, cedarwood Texas, citronella Java type, geranium Egypt, ginger China, ginger Nigeria, gurjun balsam, lavender fresh Bulgarian, lemon Spain, sweet orange CP Valencia, sweet orange CP Brazil, pennyroyal, peppermint Arvensis, thuja, thyme red, star aniseed, cassie, citrate, cognac green, coriander seed, cumin “ex-distilled”, dill leaf, Eucalyptus radiata, lemongrass, key lime distilled type Mexico, key lime expressed type Mexico, mandarin green, myrtle, nutmeg, parsley seed, patchouli molecular distillation, patchouli super dark, pimento berry, pimento leaf, pine needle, scotch pine, ravintsara, sweet basil, tropical basil, verbena, ylang-ylang I, and ylang-ylang III and oleoresins of basil, capsicum 1,000,000 SHU, paprika 40,000 SHU, paprika 60,000 SHU, paprika 80,000 SHU, pepper black 40/20, and thyme red.
Mane Kancor Ingredients Private Limited
Essential oils of carrot seed, ajowan, black pepper CO2, capsicum CO2, ginger CO2, cumin seed, black cumin, celery seed, fennel, and mace and tuberose absolute and concrete.
Metainy
Cotula and neroli essential oils.
Nelixia
Cardamom essential oil, storax gum and raw storax gums, and peru and raw peru balsams.
New Zealand Mānuka Group
Essential oils of mānuka (MBTK 5 +), mānuka (MBTK 20 +), mānuka (MBTK 25 +), and kanuka.
Payan Bertrand S.A.
Essential oils of galbanum, myrrh, olibanum, opoponax, and storax, oakmoss absolute, and resinoids of galbanum, myrrh, olibanum, opoponax, storax, benzoin Siam, and benzoin Sumatra.
Plant’s Power
Essential oils of magnolia flower, goldenrod, catnip, rhododendron, fir balsam needle, Labrador tea, sweet fern, black spruce, and yuzu.
Robertet S.A.
Bran, blackcurrant buds, and broom absolutes.
Ultra International
Essential oils of angelica root, artemisia, Artemisia taurica, blood orange, blue cypress, buchu leaf, buddha wood, coriander herb, Eucalyptus kochii, hinoki, kumquat, kunzea, lemon myrtle, and Rosalina and CO2 extracts of juniper berry, star anise, turmeric, vetiver, black seed, cardamom green, coffee arabica, coffee robusta, and ginger.
Van Aroma
Essential oils of clove stem 85% MD, clove stem 85% dark, and cajeput and CO2 extracts of cocoa butter, black pepper, clove bud, turmeric, cubeb, cassia bark, ginger, and benzoin crystals.
Vessel Essential Oils
Essential oils of lavender organic, oregano liquid fire, oregano organic, lavandin organic, peppermint organic, sweet orange organic, lemon organic, mandarin organic, pine organic, sea fennel organic, melissa organic, helichrysum organic, thyme organic, laurel organic, sage organic, rosemary organic, white fir organic, blue chamomile organic, mastic organic, rose organic, wild carrot seed organic, sweet marjoram organic, cypress organic, geranium organic, clary sage organic, yarrow organic, sweet fennel organic, juniper berry organic, anise organic, dill organic, cistus organic, Roman chamomile, spearmint, grapefruit organic, coriander seed oil, petitgrain organic, basil organic ct linalool, inula, frankinscense Somalia, Emerald frankincense, frankincense Ethiopia, frankincense India, and frankincense Oman.
The remaining samples of essential oils and other aromatic materials were purchased from PerfumersWorld Ltd. Essential oils were named according to the ISO 4720:2018 norm—Essential oils—Nomenclature where possible.
Enzymes
The expression of recombinant enzymes SARS-CoV-2 Mpro and PLpro is described in our previous works 8,10.
ACC-labeled substrates
The synthesis of ACC-labeled substrate for Mpro (Ac-Abu-Tle-Leu-Gln-ACC) and PLpro (Ac-Leu-Arg-Gly-Gly-ACC) is described in our previous works 10,37.
Inhibitor screening
Experimental conditions (concentration of enzyme and substrate, time) were tailored to obtain optimal enzyme activity during measurement like in our previous communications: PLpro10 and Mpro38. Control experiments were carried out in the absence of the inhibitor (100% activity), enzyme or substrate. SARS-CoV-2 Mpro (75 nM) was preincubated in assay buffer containing 20 mM Tris, 150 mM NaCl, 1 mM EDTA, and 1 mM DTT, pH 7.3, for 10 min at 37 °C. Then, the enzyme was added to wells containing inhibitors (50 µg/mL essential oils or aromatic extracts), and the mixture was incubated for 30 min at 37 °C. After the incubation period, fluorogenic substrate (Ac-Abu-Tle-Leu-Gln-ACC) was added to the wells (final concentration 50 μM). ACC liberation was monitored for 30 min at 37 °C (λex = 355 nm, λem = 460 nm) using a Molecular Devices Spectramax Gemini XPS spectrofluorometer. Each experiment was repeated twice (inhibition ≤ 50%) or five times (inhibition > 50%). The same experiments were performed for SARS-CoV-2 PLpro. SARS-CoV-2 PLpro (150 nM) was preincubated in assay buffer containing 50 mM Tris, 5 mM NaCl, 0.075% BSA, and 5 mM DTT, pH 7.5, for 10 min at 37 °C. The assay conditions were the same as described above (Ac-Leu-Arg-Gly-Gly-ACC was used as the substrate to measure SARS-CoV-2 PLpro residual activity).
IC50 determination
Experimental conditions (concentration of enzyme and substrate, time) were tailored to obtain optimal enzyme activity during measurement like in our previous communications: PLpro10 and Mpro38. Control experiments were carried out in the absence of the inhibitor (100% activity), enzyme or substrate. For selected inhibitors, the IC50 value was determined. Serial dilutions of inhibitors in assay buffer were prepared on 96-well plates (20 µL of each dilution in wells). SARS-CoV-2 Mpro (75 nM) or SARS-CoV-2 PLpro (100 nM) was preincubated in assay buffer (20 mM Tris, 150 mM NaCl, 1 mM EDTA, 1 mM DTT, pH 7.3; 50 mM Tris, 5 mM NaCl, 0.075% BSA, 5 mM DTT, pH 7.5) for 10 min at 37 °C. Then, 60 µL of the enzyme was added to the wells containing serial dilutions of inhibitors (ranging from 1 µg/mL to 80 µg/mL), and the mixture was incubated for 30 min at 37 °C. After that time, 20 µL of the substrate (Ac-Abu-Tle-Leu-Gln-ACC for SARS-CoV-2 Mpro or Ac-Leu-Arg-Gly-Gly-ACC for SARS-CoV-2 PLpro) was added to each well. Measurements were carried out at 37 °C for 40 min (λex = 355 nm, λem = 460 nm). The experiments were repeated three times. IC50 values were determined in GraphPad Prism software using nonlinear regression (dose–response – inhibition equation) and presented as relative enzyme activity vs. inhibitor concentration.
Antiviral activity of essential oils and aromatic extracts toward a SARS-CoV-2 strain in VeroE6-GFP cell culture
VeroE6-GFP cells were seeded at a density of 25,000 cells/well in 96-well plates (Greiner Bio One, catalog no. 655090) and pretreated with threefold serial dilutions of the compounds overnight. On the next day (Day 0), cells were infected with the SARS-CoV-2 inoculum at a multiplicity of infection (MOI) of 0.001 tissue culture infectious dose (TCID50) per cell. The number of fluorescent pixels of the GFP signal determined by high-content imaging (HCI) on Day 4 postinfection (p. i.) was used as a read-out. The percent inhibition was calculated by subtracting the background (untreated-infected control wells) and normalizing to the untreated-uninfected control wells (also background subtracted). Experiments were carried out twice. The 50% effective concentration (EC50) was determined using logarithmic interpolation. The potential toxicity of compounds was assessed in a similar setup in treated-uninfected cultures, where metabolic activity was quantified at Day 5 using the MTS assay as described earlier39. The 50% cytotoxic concentration (CC50) was calculated by logarithmic interpolation.
Gas chromatography (FID)
GC analyses were performed using a Shimadzu GC-2010 Plus gas chromatograph equipped with an FID detector and DB-5 (0.25 mm i.d. × 30 m, 0.25 μm film thickness, Agilent, Santa Clara, USA) capillary column. The injection port was maintained at 250 °C. The split ratio was set as 25:1, and 1 μL of the sample was injected. The oven temperature was set at 40 °C and increased to 300 °C at a rate of 2 °C/min, with a constant nitrogen carrier gas flow of 1.5 mL/min. The linear retention indices (RIs) of the compounds were calculated using the retention times of n-alkanes from C8 to C26.
Gas chromatography–mass spectrometry (GC–MS)
GC–MS analyses were performed using an Agilent 7890A gas chromatograph equipped with an HP-5-MS capillary column (0.25 mm i.d. × 30 m, 0.25 μm film thickness, Agilent, Santa Clara, USA) combined with a WATERS GCT high-resolution mass spectrometer (TOF, EI +). The injection port was maintained at 250 °C. One microliter of the sample was injected in splitless mode. The oven temperature was held at 40 °C and raised to 300 °C at a rate of 2 °C/min, with a constant helium carrier gas flow of 1.5 mL/min. Mass spectra in electron impact (EI) mode were recorded at 70 eV ionization energy.
Essential oil fractionation
Distillation of the petitgrain mandarin essential oil was carried out using a Buchi B-585 oven equipped with a Kugelrohr accessory. Eleven grams of the essential oil was placed in a 40 mL round-bottom flask and fractionated under a controlled distillation setup (2 mbar, 40–260 °C). Three fractions (3.22 g, 2.09 g, 3.91 g) and a residue (1.78 g) were analyzed using GC-FID and GC–MS.
Results and discussion
Natural flavorants and fragrances exhibit different inhibitory activities on both SARS-CoV-2 cysteine proteases.
A total of 538 samples of essential oils, aromatic extracts, and F&F raw materials of various origins were screened for their inhibitory properties on SARS-CoV-2 Mpro and PLpro. Screening (spectrofluorimetric enzymatic assay) was carried out with fluorogenic substrates designed by our group in previous works on SARS-CoV-2 proteases10,37. Detailed results for each material are presented in the supplementary data (Table S1). No or the lowest inhibitory activities were observed for all materials with high monoterpene and monoterpenoid contents. The most active natural flavorants and fragrances (inhibitory activity > 70%, Table 1) constitute mostly materials with both volatile and nonvolatile fractions, such as resinoids (storax, benzoin Sumatra and Siam, galbanum, and tolu), gums (elemi, storax, and labdanum), and absolutes (rocket, spinach, and oakmoss). The inhibitory potential of complex mixtures such as natural F&F materials is directly related to their composition, which depends on factors such as source plant species, country of origin, part of the plants used, and method of isolation. Clear differences can be observed, for example, between lovage root and leaf essential oils, where the former exhibits higher inhibitory activity on both SARS-CoV-2 proteases (77% and 39% for Mpro and PLpro, respectively, Table 1) than the latter (16% and 0% for Mpro and PLpro, respectively, Table S1, entry 52).
Table 1.
Inhibitory activities of selected F&F materials against SARS-CoV-2 cysteine proteases.
| English common name | Source plant botanical name | Main compounds of the natural product | Country of origin | Mpro (%)a | PLpro (%)a |
|---|---|---|---|---|---|
| Altingiaceae | |||||
| Storax gum | Liquidambar styraciflua |
Cinnamyl cinnamate 3-Phenylpropyl cinnamate |
Honduras | 90.5 ± 3.6 | 8 |
| Storax resinoid | Liquidambar orientalis | Honduras | 93.1 ± 0.7 | 13 | |
| Amaranthaceae | |||||
| Spinach absolute | Spinacia oleracea | Flavonoids | Egypt | 76.6 ± 5.9 | 15 |
| Apiaceae | |||||
| Galbanum resinoid |
Ferula gummosa Boiss syn. Ferula galbaniflua Boiss. et Buhse |
(+)-Nopinone (+)-Eremorphilene β-Amyrin |
Iran | 78.2 ± 0.8 | 11 |
| Lovage root EO | Levisticum officinale Koch | (Z)-Ligustilide | Hungary | 76.5 ± 1.9 | 39 |
| Brassicaceae | |||||
| Rocket absolute | Eruca vesicaria | Erucic acid | Egypt | 100 | 26 |
| Burseraceae | |||||
| Elemi gum | Canarium luzonicum (Blume) A. Gray |
β-Amyrin, brein, elemadienoic acid β-phellandrene Elemol Elemicin |
Philippines | 97.1 ± 1.9 | 17 |
| Cistaceae | |||||
| Labdanum gum refined | Cistus ladanifer | Labdanoic acid | Spain | 73.7 ± 2.2 | 0 |
| Cupressaceae | |||||
| Blue cypress EO | Callitris intratropica |
(+)-Bulnesol ( −)-Guaiol |
Australia | 73.1 ± 2.7 | 0 |
| Cade crude ex-sabine EO | Juniperus phoenicea |
α-Pinene δ − 3-Carene β-Phellandrene |
Spain | 94.0 ± 0.6 | 31 |
| Siam wood EO | Fokienia hodginsii |
Fokienol (E)-Nerolidol |
Vietnam | 83.8 ± 1.5 | 3 |
| Fabaceae | |||||
| Cabreuva red EO | Myrocarpus fastigiatus Allemao | (E)-Nerolidol | Paraguay | 78.6 ± 0.9 | 19 |
| Tolu resinoid | Myroxylon balsamum (L.) Harms |
Benzyl benzoate Benzyl cinnamate |
Venezuela | 77.3 ± 4.6 | 15 |
| Parmeliaceae | |||||
| Oakmoss absolute | Evernia Prunastri |
Evernin, Atranorin β-Orcinolcarboxylate |
North Macedonia | 71.4 ± 3.1 | 16 |
| Poaceae | |||||
| Vetiver EO |
Chrysopogon zizanioides (L.) Roberty syn. Vetiveria zizanioides (L.) Nash |
Khusimol Vetiselinenol |
Haiti | 74.2 ± 4.3 | 0 |
| Rutaceae | |||||
| Petitgrain mandarin (PM) EO |
Citrus reticulata Blanco syn. Citrus nobilis Andrews |
Dimethyl anthranilate | Egypt, Spain | 85.3 ± 6.2 | 100 |
| Santalaceae | |||||
| Sandalwood EO | Santalum austro-caledonicum | α-Santalol | New Caledonia | 70.9 ± 3.3 | 4 |
| Styracaceae | |||||
| Benzoin Siam resinoid | Styrax tonkinensis |
Benzoic acid Benzoates |
Laos | 99.7 ± 0.4 | 17 |
| Benzoin Sumatra resinoid | Styrax benzoin |
Cinnamic acid Cinnamates |
Indonesia | 72.9 ± 2.5 | 9 |
| Zingiberaceae | |||||
| Turmeric oleoresin | Curcuma longa |
Curcuminoids β-Phellandrene ar-Turmerone |
India | 89.0 ± 0.7 | 64.3 ± 1.6 |
| Zygophyllaceae | |||||
| Guaiacwood EO | Bulnesia sarmientoi |
(+)-Bulnesol ( −)-Guaiol |
Paraguay | 72.0 ± 5.1 | 15 |
aInhibitory activity of each F&F material at a concentration of 50 µg/mL. Numbers represent the mean value from two experiments (inhibition < 50%) or five experiments (inhibition > 50%).
Flavorants and fragrances derived from Cistus ladanifer are very good examples showing the distribution of activity within materials derived from one plant species. Cistus ladanifer is a pyrophoric plant and a source of highly prized labdanum gum. When the plant’s flowers begin to fade, the shrub develops leafy twigs. Its branches are covered with secretory hairs, which release abundant quantities of gum with an amber-like fragrance. The gum was historically harvested by combing goats, whose coats were covered with the exudate as they crisscrossed the hillsides40. Currently, cistus branches are gathered and processed industrially. Hydrodistillation or steam diffusion of cistus branches results in cistus traditional essential oil, which has a lemony, fresh, and amber-like olfactory profile. The addition of an extract from distillation water to the traditional oil gives ciste (full) essential oil with predominant amber-like, warm, and gourmand notes. Extraction of cistus twigs and branches with hydrocarbon-based solvent leads to a cistus concrete, which can be processed by ethanolic extraction that gives traditional cistus absolute with dry wood and sweet notes or by molecular distillation resulting in cistus SEV absolute with balsamic, smoky, and hot notes. Young cistus branches can also be dipped in a hot solution of sodium carbonate. This solution was acidified with sulfuric acid, and raw labdanum gum was obtained. Its hydrodistillation results in labdanum oil with intensive amber-like, woody notes. Extraction of gum with a hydrocarbon solvent leads to labdanum concrete, which after ethanolic extraction gives labdanum absolute, which possesses alcoholic, balsamic, and mild notes. Ethanolic extraction of raw gum leads to labdanum resinoids with balsamic, sweet, and amber-like olfactory profiles. The acid fraction of a mixture of labdanum gum and resinoid processed by molecular distillation leads to a labdasur-aroma material with animalic and cheese-like notes. It should be noted that despite having the same plant origin, cistus isolates present different olfactory properties. This means that some constituents are absent in one isolate but present in another, which may result in different biological activities. In this case, the inhibitory activities of various cistus isolates on SARS-CoV-2 Mpro are different, e.g., labdanum gum—74%, labdanum resinoid—33%, ciste EO—34%, cistus absolute—0%, and cistus SEV absolute—42%.
In some materials, the nonvolatile fraction is mostly responsible for the inhibitory activity. This is evident in the case of elemi (Canarium luzonicum) gum (97% Mpro inhibition vs. 33% in the case of elemi essential oil) and galbanum (Ferula galbaniflua) resinoid (78% vs. 18% (EO) Mpro inhibition). Very illustrative examples of the influence of the method of isolation are turmeric-derived flavorants and fragrances, where the most likely polar volatile part is responsible for the Mpro inhibitory activity (essential oil—64%; isolation method: water/steam distillation vs. CO2 extract—33% inhibition) and the polar nonvolatile part for PLpro inhibitory activity (essential oil—6% and CO2 extract—5% vs. oleoresin 64% inhibition). In the case of turmeric oleoresin, its polar nonvolatile fraction also enhances the Mpro inhibitory activity (89%).
The Cuprassaceae family constitutes a more abundant example of active essential oils. Cade crude ex-sabine EO was the most active (94%) against Mpro, together with blue cypress and Siam wood essential oils. These activities were also retained for Chinese cedarwood (Cupressus funebris) and Virginian cedarwood (Juniperus virginiana) EOs (Table S1, 57% and 60% inhibition, respectively).
Most of the activity of the tested samples was attributed to the inhibition of the SARS-CoV-2 main protease. Mpro is the primary protease of the virus. The papain-like protease has a regulatory role, and both are essential for the processing of the polyprotein. In addition, PLpro has an important role in counteracting the innate immunity of the host cell9. Ultimately, the best inhibitor would be the one that has high activities against one or both enzymes. There were only two examples (out of 400 tested) of such high activities—turmeric oleoresin (89% Mpro and 64% PLpro inhibition) and petitgrain mandarin essential oil (85% Mpro and 100% PLpro inhibition).
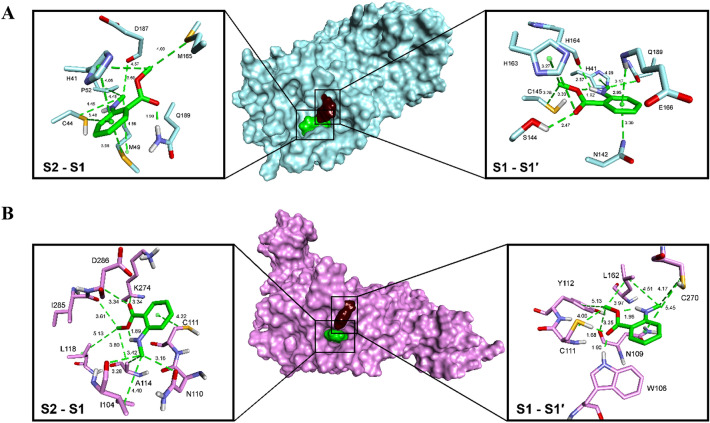
All the main compounds of oils, gums, and resinoids listed in Table 1 have been studied to determine whether they can be considered potent inhibitors of SARS-CoV-2 Mpro and SARS-CoV-2 PLpro. They were docked in the active center of the enzymes in the S1 cavity but with large binding freedom (20 Å) due to their large shape. Mostly they bind at the S1-S1′ cavity but are also shifted toward further cavities on the substrates and the products on both sides (Fig. S1A and B).
The structural similarity of the main compounds outside families is noted, and they can be divided into six main groups: benzoates and cinnamates, aliphatic acids, aromatic oxygenated compounds, low-molecular cyclic oxygenated compounds, terpene hydrocarbons, and terpenoid alcohols. The first group of compounds includes derivatives of organic acids based on the skeleton of benzoic or cinnamic acid. The extension of the ester or skeleton length could have a positive effect, increasing the inhibitory activity. Cinnamic acid esters could be more active than their shorter benzoate counterparts. Complex matrices of the remaining materials do not allow a direct comparison of the structure-inhibition relationship. The additional oxygen, hydroxy, or methoxy moieties or heteroatoms in other main groups of major constituents of the most active natural materials could not directly increase the inhibition of the enzyme. It is difficult to say whether it is certain, especially when a given natural product has an equal amount of individual ingredients.
However, the main constituents can be important for inhibitory activity; for example, (+)-bulnesol and (−)-guaiol prevail in blue cypress and guaiac wood essential oils. The results of inhibition for Mpro are almost the same, but for PLpro, the additional ingredients of guaiac wood oil increase its activity against this enzyme. The variation in the inhibition could be related to the different ratios of the two major compounds in blue cypress and guaiac wood essential oils.
One compound contributes to the overall inhibitory activity of the petitgrain mandarin essential oil.
The essential oil isolated from mandarin leaves was the most potent in the preliminary screening studies. It comprises 6 main constituents (Table 2): α-pinene, β-pinene, p-cymene, (+)-limonene, γ-terpinene, and dimethyl anthranilate (DMA), with the last constituent being the most abundant.
Table 2.
The main constituents of the petitgrain mandarin (Citrus reticulata blanco var. Mandarin) oils.
| RIa | Aread [%] | Identification methode | |||||
|---|---|---|---|---|---|---|---|
| DB-5 | Lit. DB-5 | EO | LFr | HFr | |||
| 1 | α-pinene | 931 | 93241 | 2.19 | 3.65 | 2.42 | RI, MS, ref |
| 2 | β-pinene | 973 | 97242 | 2.18 | 3.51 | 1.92 | RI, MS, ref |
| 3 | p-cymene | 1023 | 102543 | 14.7 | 9.09 | 2.19 | RI, MS, ref |
| 4 | (+)-limonene | 1027 | 102444 | 8.26 | 11.3 | 8.34 | RI, MS, ref |
| 5 | γ-terpinene | 1057 | 1056b45 | 17.9 | 30.2 | 20.2 | RI, MS, ref |
| 6 | dimethyl anthranilate (DMA) | 1409 | 1407c46 | 43.5 | 35.1 | 60.6 | RI, MS, ref |
aExperimental and literature RIs relative to n-alkanes on a DB-5 capillary column unless otherwise stated. b RI for the DB-5MS column. c RI for the SLB-5MS column. d Area percentages of the main components; EO essential oil, LFr a light fraction of the oil, HFr a heavy fraction of the oil. eMethods: RI identification based on RI comparison with literature data, MS identification based on mass spectra comparison with those of the Wiley Registry of Mass Spectral Data library (8th edition) and the Adams library of essential oil components (edition 4.1), ref. coinjection with an authentic sample.
Petitgrain mandarin essential oil was fractionated on Kugelrohr, three fractions and distillation residue were analyzed chromatographically, and their activities on both SARS-CoV-2 proteases were assessed (Table 3, the activity of fractions determined at the same concentration as the raw material during the screening phase). Before fractionation, the hypothesis was that the anthranilate part of the oil is responsible for the inhibitory activity (other materials with high monoterpene content exhibited low or no activity). The hypothesis was correct, as the inhibition rate increases with increasing dimethyl anthranilate content. The same can be observed for two fractions of PM essential oil (during the production of the oil, two fractions emerge—a fraction lighter than water, and a fraction heavier than water—the combination of both gives the commercial PM oil). This was further confirmed with the evaluation of pure natural dimethyl anthranilate, which fully inhibits both SARS-CoV-2 proteases under the same experimental conditions.
Table 3.
Discovery of the most active constituents of the petitgrain mandarin (Citrus reticulata blanco var. Mandarin) oil against SARS-CoV-2 Mpro and PLpro.
| Areaa [%] | LFr | HFr | DMA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| EO | Fr. 1 | Fr. 2 | Fr. 3 | Res | |||||
| 1 | α-pinene | 2.19 | 4.07 | 0.7 | 0.03 | 0.0 | 3.65 | 2.42 | – |
| 2 | β-pinene | 2.18 | 4.36 | 1.2 | 0.03 | 0.0 | 3.51 | 1.92 | – |
| 3 | p-cymene | 14.7 | 27.5 | 20.2 | 0.6 | 0.06 | 9.09 | 2.19 | – |
| 4 | limonene | 8.26 | 16.4 | 9.9 | 0.2 | 0.03 | 11.3 | 8.34 | – |
| 5 | γ-terpinene | 17.9 | 33.5 | 27.1 | 0.8 | 0.05 | 30.2 | 20.2 | – |
| 6 | dimethyl anthranilate (DMA) | 43.5 | 7.0 | 33.8 | 93.0 | 92.7 | 35.1 | 60.6 | 100 |
| Mpro inh. [%] | 85 | 3 | 62 | 100 | 100 | 70 | 87 | 100 | |
| PLpro inh. [%] | 100 | 79 | 100 | 100 | 100 | 100 | 100 | 100 | |
aArea percentages of the main components of the petitgrain mandarin oil and its fractions: Fr. distillation fraction number, Res. distillation residue, EO essential oil, LFr light fraction of the oil, HFr heavy fraction of the oil.
The detailed inhibition properties of the petitgrain mandarin essential oil, natural dimethyl anthranilate, and other most active essential oils and aromatic extracts were assessed (Table 4). As suspected from the fractionation studies, PM oil and DMA were significantly stronger inhibitors of SARS-CoV-2 papain-like protease. The best inhibitors of Mpro were benzoin Siam and Sumatra resinoids and rocket extracts (absolute and concrete) with IC50 values in range of 3.41 to 5.24 µg/mL.
Table 4.
Inhibition parameters of essential oils and aromatic extracts and SARS-CoV-2 Mpro and PLpro.
| Natural material | SARS-CoV-2 Mpro IC50 (µg/mL) | SARS-CoV-2 PLpro IC50 (µg/mL) ([µM]) |
|---|---|---|
| Essential oils | ||
| Petitgrain mandarin EO | > 100 | 22.9 ± 4.6 |
| DMA | > 100 | 5.20 ± 1.22 (31.5 ± 7.4) |
| Guaiacwood EO | 48.01 ± 4.51 | – |
| Blue cypress EO | 48.22 ± 3.02 | – |
| Lovage root EO | 70.86 ± 4.65 | – |
| Siam wood EO | 76.10 ± 1.23 | – |
| Extracts | ||
| Benzoin Siam resinoid | 5.24 ± 0.22 | – |
| Benzoin Sumatra resinoid | 4.20 ± 0.15 | – |
| Galbanum resinoid | 34.52 ± 2.27 | – |
| Storax resinoid | 46.31 ± 3.52 | – |
| Turmeric oleoresin | 12.01 ± 1.31 | 96.15 ± 2.09 |
| Labdanum gum refined | 47.44 ± 1.60 | – |
| Nasturtium absolute | 72.79 ± 1.12 | – |
| Rocket absolute | 3.41 ± 0.32 | – |
| Rocket concrete | 4.79 ± 0.04 | – |
| Tasmania lanceolata extract | 49.98 ± 1.99 | – |
Our exploratory study aimed to develop pioneering knowledge and provide the first experimental results on the inhibitory properties of hundreds of flavor and fragrance materials against SARS-CoV-2 main and papain-like proteases. The majority of available information is related to SARS-CoV. Only several plant extracts have been evaluated47–49 for their activity against the key proteases, and they exhibited moderate to low inhibitory potential (Table S2, entries 3–5) compared to two natural plant constituents, 3-isotheaflavin-3-gallate and tannic acid50, which had relatively good inhibitory activities against the SARS-CoV main protease (Table S2, entries 1 and 2).
Molecular docking studies reveal the detailed binding method of DMA in the active sites of both enzymes.
Dimethyl anthranilate was docked in the active centers of the enzymes to investigate ligand-enzyme interactions. Two types of binding were considered. The ligand was allowed to bind freely 20 Å from the catalytic cysteine (Cys145 for Mpro and Cys111 for PLpro) or was forced to interact with the mentioned amino acid. In the first step of docking, DMA interacted with the catalytic groups of SARS-CoV-2 Mpro in both docking pathways. The S1-S1′ pocket is not filled tightly by the ligand (Fig. 1A), which is directed with the methyl group of the ester to Cys145. The placement is stabilized by the hydrophobic interactions of Cys145–-Me (3.28 Å) and His163–-Me (3.27 Å). The carbonyl oxygen atom also interacts with histidine (3.33 Å). Its position is determined by a strong hydrogen bond with Ser144 (2.47 Å). Another strong hydrogen bond is noticeable for the amino group of the inhibitor with His164 (2.57 Å). The position of the phenyl ring and the N-methyl group seems to be rather constrained, and the interactions of the amino acids with them are weaker compared to the ester of the carboxyl group. In addition, their positions might be influenced by a strong intermolecular interaction of neighboring COOMe and NHMe groups (1.92 Å).
Figure 1.
The interactions of dimethyl anthranilate in the active centers of SARS-CoV-2 Mpro (A) and SARS-CoV-2 PLpro (B). The binding for each enzyme is shown for the S1-S1′ and S2-S1 pockets. The surface of the enzymes is colored light blue (Mpro) or light pink (PLpro). The surface of methyl N-methylanthranilate is colored dark brown for the S1-S1′ pockets and green for the S2-S1 pockets. The ligands and the side chains of the amino acids are shown as sticks, and the bond order is not shown.
The optimization of the ligand-enzyme complex moved the dimethyl anthranilate toward the S2-S1 pocket (Fig. 1A). The phenyl ring finds itself in the middle of a hydrophobic area of a pocket built by His41 (4.05 Å), Cys44 (5.48 Å), and Met49 (3.98 Å). Intermolecular interactions are not formed because the side chain of Gln189 creates a strong hydrogen bond (1.90 Å) with the carbonyl oxygen atom, while the methyl ester group interacts with His41 (3.60 Å) and Met165 (4.03 Å). Such positioning of the ligand seems more natural and comparable to the enzyme–substrate complex. This is also confirmed by the large difference in the binding energy ΔGS1-S1′ = -16.70 kcal/mol vs. ΔGS2-S1 = -37.08 kcal/mol.
The amino acids building the S1 and S1′ active pockets of SARS-CoV-2 PLpro are close, and the catalytic Cys111 is involved in the construction of both. Dimethyl anthranilate is placed very well in the S1 area (Fig. 1B). It creates strong hydrogen bonds between its carbonyl oxygen atom and the catalytic amino acids Trp106 (1.90 Å) and Cys111 (3.25 Å). The methyl ester group plays the role of a dovetail interlocking the compound in its position with hydrophobic interactions with Asn109 (3.25 Å), Tyr112 (5.13 Å), and Leu162 (3.97 Å). The leucine interacts hydrophobically with the N-methyl group (4.17 Å) likewise. A small role in the binding of the inhibitor is played by Cys270, which arranges the aromatic part of DMA (5.45 Å). Intramolecular interaction of COOMe and NHMe (1.96 Å) also occurs.
In the S2-S1 pocket, placement is different than in the Mpro (Fig. 1B). The phenyl ring interacts with Cys111 (4.22 Å), while Asn110 (3.16 Å) interacts with the hydrophobic amino acids Ile104 (4.40 Å, 3.28 Å for C=O–-Me) and Ala114 (3.42 Å). The alanine (3.80 Å), Leu118 (5.13 Å) and Ile285 (3.61 Å) position the methyl carboxylate. The carbonyl oxygen atom interacts with the carbonyl oxygen atom of Asp286 (3.34 Å) and through the carbon–hydrogen bond with Lys274 (3.34 Å), but those bonds come from recurring intermolecular interactions (1.89 Å). The binding energy determines that the S2-S1 pocket is also preferred: ΔGS1-S1′ = − 17.02 kcal/mol vs. ΔGS2-S1 = − 23.59 kcal/mol. The compound binds well in both selected areas of both tested enzymes; however, the binding energy confirms that they prefer S2-S1.
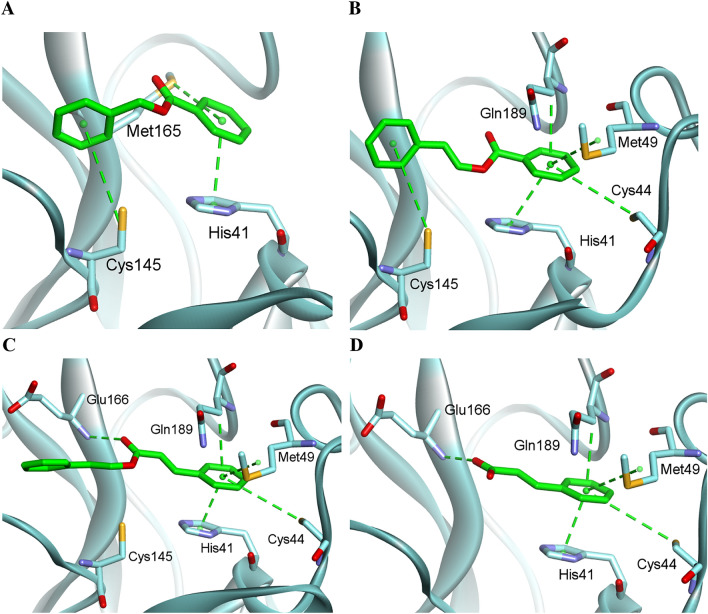
Benzoin resinoids (Siam and Sumatra) demonstrated very promising inhibitory activity against Mpro. They are comprised of derivatives of benzoic and cinnamic acids. Four main constituents were evaluated in silico on SARS-CoV-2 Mpro (Fig. 2): benzyl benzoate (Fig. 2A), benzyl cinnamate (Fig. 2B), cinnamyl cinnamate (Fig. 2C), and cinnamic acid (Fig. 2D). For the general nature of the inhibition, we assumed that the unsaturated parts of the compounds are trans-isomers.
Figure 2.
The interactions of benzyl benzoate (A), benzyl cinnamate (B), cinnamyl cinnamate (C), and cinnamic acid (D) in the active center of SARS-CoV-2 Mpro. The ligands and the side chains of the amino acids are shown as sticks, and the bond order is not shown.
Benzyl derivatives (Fig. 2A and B) are directed by the phenyl ring of the acid part toward the deep part of the S2 pocket of the active center. Arenes are stabilized by multiple hydrophobic interactions with lipophilic amino acids, such as His41 and Met49. However, none of the amino acids create hydrogen bonds between the ligand and protein. Hydrogen interaction occurs when the backbone is elongated. Carbonyl oxygen interacts with the amino group’s hydrogen atom from Glu166. The attracted ligands are too distant to form a weak phenyl-Cys145 interaction. Nevertheless, it may expose the double bond from the ester and create the possibility of forming a covalent bond between the aforementioned cysteine and the backbone of the compound.
Benzoin Sumatra resinoid has the most prominent antiviral activity.
The most active protease inhibitors were also tested for their antiviral activity using a fully replication-competent SARS-CoV-2 strain in VeroE6-GFP cell culture (Table S3). The benzoin Sumatra resinoid showed selective inhibition (half-maximal effective concentration (EC50) = 31.5 ± 2.4 µg/mL) with limited toxicity (50% cytotoxic concentration (CC50) = 85.5 ± 1.9 µg/mL). The best PLpro inhibitor, petitgrain mandarin essential oil, showed lower antiviral activity (> 100 µg/mL).
Available data on the activity of plant materials against highly pathogenic human coronaviruses are scarce. A few studies have been conducted on the anti-SARS-CoV activities of plant extracts and essential oils (Table S4). Evaluation of cinnamon bark and clove bud aqueous extracts (Table S4, entries 1–5) showed that the hydroalcoholic extract of cinnamon bark and its butanol fraction possess moderate inhibitory activity against SARS-CoV infection51. Screening of 121 Chinese herbs resulted in the identification of two compounds (Table S4, entries 6 and 7), tetra-O-galloyl-β-D-glucose from Galla chinensis and luteolin from Veronica linariifolia, which could inhibit SARS-CoV infection in a dose-dependent manner52. Only one reference addresses the evaluation of anti-SARS-CoV activities of essential oils53 (Table S4, entries 10–16), with laurel EO being the most active (modestly).
In addition to the in vitro evaluations, several randomized controlled trials showed some promise of Chinese herbal medicine combined with conventional medicine for the treatment of SARS, but the evidence was insufficient due to the low methodological quality of the trials54.
Relevant data on the activity of plant materials against MERS-CoV and SARS-CoV-2 are even more scarce, as most of the references are focused on in silico evaluations of natural products (including essential oil constituents)55 or perspectives in this field56. Two of the few meaningful references describe the antiviral value of the hydroalcoholic extract of Echinacea purpurea herb and roots (Echinaforce®) against MERS-CoV and SARS-CoV-257. Fifty micrograms/ml Echinaforce® (the final concentration of ethanol was 0.2%, and it was proven that the tested coronaviruses were not sensitive to this residual concentration) fully blocked the infectivity of MERS-CoV and SARS-CoV-2. Most recently, one study described the anti-SARS-CoV-2 activity of some Lamiaceae EOs and their monoterpene constituents58. The most active essential oil (Mentha vilosa) exhibited moderate inhibitory activity (127.00 ± 4.63 ppm), as did carvacrol (80.23 ± 6.07 µM). The main omission of the abovementioned study is the lack of stereochemical descriptors of the evaluated optically active monoterpenes. In addition, toxicological aspects of pennyroyal (Mentha pulegium) essential oil, menthofuran, and pulegone are not discussed in enough detail. The results clearly show no or limited toxicity of the abovementioned materials to Vero 76 cells, but they are known to be highly toxic to humans.
Despite the lack of experimental in vitro data on the potential of flavor and fragrance materials against SARS-CoV-2, two clinical trials evaluated the potential of a commercial product containing essential oil constituents (Listerine®—originally EO-based, now comprising a mixture of eucalyptol, (−)-menthol, thymol, and methyl salicylate) to eliminate SARS-CoV-2 in the throat and nasopharynx of COVID-19 patients. The first trial (59, NCT04410159) was completed and showed high viral clearance [two negative RT–PCR (of swabs from the oropharynx and nasopharynx) results at least 24 h apart] rates for 1% iodine-povidone (Betadine®) and “essential oils” (Listerine®) gargles (100% and 80%, respectively) 4 days after intervention (median time without intervention—14 days). These results should be treated with caution, as the testing groups were very small (5 patients in each gargle group). The second “gargle” clinical trial (NCT04584684), “Antiviral Efficacy and Acceptability of Therapeutic Antiseptic Mouth Rinses for Inactivation of COVID SARS-2 Virus”, is ongoing. Its goal is to test the efficacy of antiseptic mouth rinses (e.g., Listerine®) to inactivate SARS-CoV-2 in the saliva of COVID-19-positive patients (480 patients). Listerine® is a hydroalcoholic mixture of essential oil constituents. The ethanol concentration is at the level of 21%, which is significant, but it has been proven that the concentration of ethyl alcohol for the complete inactivation of SARS-CoV-2 must be ≥ 30% (60, 20% EtOH concentration resulted in a reduction factor of only 1.1).
Conclusion
F&F materials are important for society because they are commonly used in various areas of human interest, have established toxicological profiles and are relatively safe. Until recently, the tools to assess the inhibitory potential of chemicals against the key SARS-CoV-2 proteases were limited. Our recent developments10,37 provided a framework for the discovery of new materials/molecules with potential therapeutic value. The results of this study give the scientific community the selection criteria for further in vitro and in vivo testing of the most promising EOs, AEs, and isolated natural compounds. They can also be used for the rational design of SARS-CoV-2 protease inhibitors. Even though important countermeasures for the spread of SARS-CoV-2 are in motion (i.e., the introduction of vaccines), these results will be important to the field because though coronaviruses can change, the coronaviral proteases are conserved (broad application of results in potential future outbreaks).
Supplementary Information
Acknowledgements
D.J.S. was supported by the project “Synthesis of new fragrances from raw materials of a natural origin with application in perfumery, cosmetics, and household chemistry” (SYNFRA; LIDER Programme, LIDER/4/0099/L-7/15/NCBR/2016) financed by the National Centre for Research and Development—Poland; M.D. was supported by the Medical Research Agency in Poland through its Own Project (grant 2020/ABM/SARS/1), by the National Science Center grant UMO-2020/01/0/NZ1/00063, and the “TEAM/2017-4/32” project, which is carried out within the TEAM program of the Foundation for Polish Science, co-financed by the European Union under the European Regional Development Fund. The authors acknowledge Schrödinger, Inc., an American multinational biopharmaceutical and technology company, for providing a trial license. Discovery Studio Visualizer was used for the preparation of the figures. We would like to express our gratitude to the International Federation of Essential Oils and Aroma Trades (IFEAT), IFEAT President Mr. Alastair Hitchen, IFEAT Chairman of Executive Committee Mr. Hussein Fakhry, IFEAT Chair of the Scientific Committee & New Industry Projects Task Leader Mr. Alain Frix, and IFEAT Chief Scientific Officer Dr. Jonatan Bonello for networking support and fruitful discussions regarding various aspects of the essential oils industry. We would like to thank representatives of the essential oils industry and IFEAT Members: A. Fakhry & Co., Albert Vieille, Amigo & Arditi, Ashna Samai, Berje Inc., Bordas S.A., BoreA Canada, Brüder Unterweger, Christodoulou Bros S.A., Citrus and Allied Essences Ltd, D.V. Deo Industries, Dutjahn Sandalwood Oils, Essential Oils and Herbs, Essential Oils of Tasmania, Eucaforest, Hierbas Patagónicas S.R. L, Kallin ltd, Jacarandas International, Mane Kancor Ingredients Private Limited, Lebermuth, Les Arômes du Maroc, Lluch Essence, Metainy, Nelixia, New Zealand Mānuka Group, Payan Bertrand S.A., Plant’s Power, Van Aroma, Robertet S.A., Ultra International, and Vessel Essential Oils for the donation of most of the samples of natural flavor and fragrance materials for this study.
Abbreviations
- Abu
2-Aminobutanoic acid
- ACC
7-Amino-4-carbamoylmethylcoumarin
- RFU
Relative fluorescence unit
- Tle
Tert-Leucine
- EO
Essential oil
- AE
Aromatic extract
- MBTK
Manuka oil beta triketones
- DMA
Dimethyl anthranilate
Author contributions
Conceptualization: D.J.S.; project administration: D.J.S., M.S.; investigation: D.J.S., W.R., M.Z., M.S., D.J., L.V.; methodology: D.J.S., W.R., M.Z., D.J.; molecular modeling studies: M.T.; obtainment of natural samples: D.J.S., W.B.; enzyme expression: L.Z., X.S., Z.L., D.N., R.H., S.O.; statistical analysis: D.J.S., M.Z.; writing - original draft preparation: D.J.S, M.T., W.R., M.S.,; writing - review and editing: D.J.S, M.T., W.R., M.Z., M.S., W.B., R.H., S.O., D.J., M.D.; funding acquisition: D.J.S., M.D. All authors have read and agreed to the published version of the manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Daniel Jan Strub, Email: daniel.strub@pwr.edu.pl.
Marcin Drąg, Email: marcin.drag@pwr.edu.pl.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-18676-w.
References:
- 1.Baser, K. H. C. & Buchbauer, G. Handbook of Essential Oils: Science, Technology, and Applications, Second Edition. (CRC Press, 2015).
- 2.Feyaerts AF, Luyten W, Van Dijck P. Striking essential oil: tapping into a largely unexplored source for drug discovery. Sci. Rep. 2020;10:2867. doi: 10.1038/s41598-020-59332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Logu A, Loy G, Pellerano ML, Bonsignore L, Schivo ML. Inactivation of HSV-1 and HSV-2 and prevention of cell-to-cell virus spread by Santolina insularis essential oil. Antiviral Res. 2000;48:177–185. doi: 10.1016/S0166-3542(00)00127-3. [DOI] [PubMed] [Google Scholar]
- 4.Yin J, Li G, Li J, Yang Q, Ren X. In vitro and in vivo effects of Houttuynia cordata on infectious bronchitis virus. Avian Pathol. 2011;40:491–498. doi: 10.1080/03079457.2011.605107. [DOI] [PubMed] [Google Scholar]
- 5.Chen B, et al. Overview of lethal human coronaviruses. Signal Transduct. Target. Ther. 2020;5:89. doi: 10.1038/s41392-020-0190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarighi P, et al. A review of potential suggested drugs for coronavirus disease (COVID-19) treatment. Eur. J. Pharmacol. 2021;895:173890. doi: 10.1016/j.ejphar.2021.173890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368:409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin D, et al. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature. 2020;587:657–662. doi: 10.1038/s41586-020-2601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rut W, et al. Activity profiling and crystal structures of inhibitor-bound SARS-CoV-2 papain-like protease: A framework for anti–COVID-19 drug design. Sci. Adv. 2020;6:eabd4596. doi: 10.1126/sciadv.abd4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin Z, et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 12.Kitamura N, et al. Expedited Approach toward the Rational Design of Noncovalent SARS-CoV-2 Main Protease Inhibitors. J. Med. Chem. 2022;65:2848–2865. doi: 10.1021/acs.jmedchem.1c00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma C, et al. Discovery of Di- and trihaloacetamides as covalent SARS-CoV-2 main protease inhibitors with high target specificity. J. Am. Chem. Soc. 2021;143:20697–20709. doi: 10.1021/jacs.1c08060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai W, et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368:1331–1335. doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quan B-X, et al. An orally available Mpro inhibitor is effective against wild-type SARS-CoV-2 and variants including Omicron. Nat. Microbiol. 2022;7:716–725. doi: 10.1038/s41564-022-01119-7. [DOI] [PubMed] [Google Scholar]
- 16.Zhu W, et al. Identification of SARS-CoV-2 3CL Protease Inhibitors by a Quantitative High-Throughput Screening. ACS Pharmacol. Trans. Sci. 2020;3:1008–1016. doi: 10.1021/acsptsci.0c00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma C, et al. Discovery of SARS-CoV-2 papain-like protease inhibitors through a combination of high-throughput screening and a FlipGFP-based reporter assay. ACS Cent. Sci. 2021;7:1245–1260. doi: 10.1021/acscentsci.1c00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma C, et al. Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. Cell Res. 2020;30:678–692. doi: 10.1038/s41422-020-0356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaidman D, et al. An automatic pipeline for the design of irreversible derivatives identifies a potent SARS-CoV-2 M<sup>pro</sup> inhibitor. Cell Chem. Biol. 2021;28:1795–1806.e1795. doi: 10.1016/j.chembiol.2021.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva JRA, Kruger HG, Molfetta FA. Drug repurposing and computational modeling for discovery of inhibitors of the main protease (Mpro) of SARS-CoV-2. RSC Adv. 2021;11:23450–23458. doi: 10.1039/D1RA03956C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unoh Y, et al. Discovery of S-217622, a noncovalent oral SARS-CoV-2 3CL protease inhibitor clinical candidate for treating COVID-19. J. Med. Chem. 2022;65:6499–6512. doi: 10.1021/acs.jmedchem.2c00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drayman N, et al. Masitinib is a broad coronavirus 3CL inhibitor that blocks replication of SARS-CoV-2. Science. 2021;373:931–936. doi: 10.1126/science.abg5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jang WD, Jeon S, Kim S, Lee SY. Drugs repurposed for COVID-19 by virtual screening of 6,218 drugs and cell-based assay. Proc. Natl. Acad. Sci. 2021;118:e2024302118. doi: 10.1073/pnas.2024302118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinzi L, Tinivella A, Caporuscio F, Rastelli G. Drug Repurposing and Polypharmacology to Fight SARS-CoV-2 Through Inhibition of the Main Protease. Front. Pharmacol. 2021 doi: 10.3389/fphar.2021.636989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narayanan A, et al. Identification of SARS-CoV-2 inhibitors targeting Mpro and PLpro using in-cell-protease assay. Commun. Biol. 2022;5:169. doi: 10.1038/s42003-022-03090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owen DR, et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374:1586–1593. doi: 10.1126/science.abl4784. [DOI] [PubMed] [Google Scholar]
- 27.Freitas BT, et al. Characterization and Noncovalent Inhibition of the Deubiquitinase and deISGylase activity of SARS-CoV-2 papain-like protease. ACS Infect. Dis. 2020;6:2099–2109. doi: 10.1021/acsinfecdis.0c00168. [DOI] [PubMed] [Google Scholar]
- 28.Klemm T, et al. Mechanism and inhibition of the papain-like protease, PLpro, of SARS-CoV-2. EMBO J. 2020;39:e106275. doi: 10.15252/embj.2020106275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen Z, et al. Design of SARS-CoV-2 PLpro inhibitors for COVID-19 antiviral therapy leveraging binding cooperativity. J. Med. Chem. 2022;65:2940–2955. doi: 10.1021/acs.jmedchem.1c01307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steuten K, et al. Challenges for Targeting SARS-CoV-2 Proteases as a Therapeutic Strategy for COVID-19. ACS Infect. Dis. 2021;7:1457–1468. doi: 10.1021/acsinfecdis.0c00815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strub D, et al. Evaluation of the anti-SARS-CoV-2 properties of essential oils and aromatic extracts. PREPRINT (Version 2) Res. Square. 2021 doi: 10.21203/rs.3.rs-997891/v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Talma M, Mucha A. P1′ Residue-oriented virtual screening for potent and selective phosphinic (Dehydro) dipeptide inhibitors of metallo-aminopeptidases. Biomolecules. 2020;10:659. doi: 10.3390/biom10040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sacco MD, et al. Structure and inhibition of the SARS-CoV-2 main protease reveal strategy for developing dual inhibitors against Mpro and cathepsin L. Sci. Adv. 2020;6:eabe0751. doi: 10.1126/sciadv.abe0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schrödinger, L.L.C. Schrödinger Release 2020-1: Schrödinger Suite 2020-1 Protein Preparation Wizard; Epik: New York, NY, USA, (2020).
- 35.Schrödinger LLC. Schrödinger Release 2020-1: LigPrep; Schrödinger LLC: New York, NY, USA, (2020).
- 36.Schrödinger Release 2020-1: Induced Fit; Docking Protocol; Induced Fit Docking protocol; Glide, Schrödinger,LLC: New York, NY, USA, (2020).
- 37.Rut W, et al. SARS-CoV-2 Mpro inhibitors and activity-based probes for patient-sample imaging. Nat. Chem. Biol. 2020 doi: 10.1038/s41589-020-00689-z. [DOI] [PubMed] [Google Scholar]
- 38.Nawrot-Hadzik I, et al. Reynoutria Rhizomes as a Natural Source of SARS-CoV-2 Mpro Inhibitors-Molecular Docking and In Vitro Study. Pharmaceuticals. 2021;14:742. doi: 10.3390/ph14080742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jochmans D, Leyssen P, Neyts J. A novel method for high-throughput screening to quantify antiviral activity against viruses that induce limited CPE. J. Virol. Methods. 2012;183:176–179. doi: 10.1016/j.jviromet.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albert Vieille SAS catalogue, Cistus ladaniferus, Vallauris. (2017).
- 41.Merle H, Morón M, Blázquez MA, Boira H. Taxonomical contribution of essential oils in mandarins cultivars. Biochem. Syst. Ecol. 2004;32:491–497. doi: 10.1016/j.bse.2003.09.010. [DOI] [Google Scholar]
- 42.Apel MA, Sobral M, Zuanazzi JÂS, Henriques AT. Essential oil composition of Calycorectes australis and Calycorectes psidiißorus (Myrtaceae) Flavour Fragr. J. 2006;21:656–658. doi: 10.1002/ffj.1640. [DOI] [Google Scholar]
- 43.Rotman A, et al. Aromatic plants from Yungas. Part III. Composition and antimicrobial activity of Myrrhinium atropurpureum Schott var. octandrum Bentham essential oil. Flavour Fragr. J. 2003;18:211–214. doi: 10.1002/ffj.1189. [DOI] [Google Scholar]
- 44.Limberger RP, et al. Essential oils from some Myrceugenia species (Myrtaceae) Flavour Fragr. J. 2002;17:341–344. doi: 10.1002/ffj.1113. [DOI] [Google Scholar]
- 45.Zoghbi MDGB, Andrade EHA, da Silva MHL, Maia JGS. Volatile constituents of Lippia lupulina Cham. Flavour Fragr. J. 2002;17:29–31. doi: 10.1002/ffj.1032. [DOI] [Google Scholar]
- 46.Lo Presti M, et al. Evaluation of the volatile and chiral composition in Pistacia lentiscus L essential oil. Flavour Fragr. J. 2008;23:249–257. doi: 10.1002/ffj.1878. [DOI] [Google Scholar]
- 47.Lau K-M, et al. Immunomodulatory and anti-SARS activities of Houttuynia cordata. J. Ethnopharmacol. 2008;118:79–85. doi: 10.1016/j.jep.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim DW, et al. Phenolic phytochemical displaying SARS-CoV papain-like protease inhibition from the seeds of Psoralea corylifolia. J. Enzyme Inhib. Med. Chem. 2014;29:59–63. doi: 10.3109/14756366.2012.753591. [DOI] [PubMed] [Google Scholar]
- 49.Luo W, et al. Anti-SARS coronavirus 3C-like protease effects of Rheum palmatum L. extracts. Biosci. Trends. 2009;3:124–126. [PubMed] [Google Scholar]
- 50.Chen C-N, et al. Inhibition of SARS-CoV 3C-like Protease Activity by Theaflavin-3,3'-digallate (TF3) Evid. Based Complement. Altern. Med. 2005;2:209–215. doi: 10.1093/ecam/neh081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhuang M, et al. Procyanidins and butanol extract of Cinnamomi Cortex inhibit SARS-CoV infection. Antiviral Res. 2009;82:73–81. doi: 10.1016/j.antiviral.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yi L, et al. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J. Virol. 2004;78:11334. doi: 10.1128/JVI.78.20.11334-11339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loizzo MR, et al. Phytochemical analysis and in vitro antiviral activities of the essential oils of seven lebanon species. Chem. Biodivers. 2008;5:461–470. doi: 10.1002/cbdv.200890045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu J, Manheimer E, Shi Y, Gluud C. Chinese herbal medicine for severe acute respiratory syndrome: A systematic review and meta-analysis. J. Altern. Complement. Med. 2004;10:1041–1051. doi: 10.1089/acm.2004.10.1041. [DOI] [PubMed] [Google Scholar]
- 55.Thuy BTP, et al. Investigation into SARS-CoV-2 resistance of compounds in garlic essential Oil. ACS Omega. 2020;5:8312–8320. doi: 10.1021/acsomega.0c00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boukhatem MN, Setzer WN. Aromatic herbs, medicinal plant-derived essential oils, and phytochemical extracts as potential therapies for coronaviruses: Future perspectives. Plants. 2020 doi: 10.3390/plants9060800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Signer J, et al. In vitro virucidal activity of Echinaforce®, an Echinacea purpurea preparation, against coronaviruses, including common cold coronavirus 229E and SARS-CoV-2. Virol. J. 2020;17:136. doi: 10.1186/s12985-020-01401-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ćavar Zeljković S, Schadich E, Džubák P, Hajdúch M, Tarkowski P. Antiviral Activity of Selected Lamiaceae Essential Oils and Their Monoterpenes Against SARS-Cov-2. Front. Pharmacol. 2022 doi: 10.3389/fphar.2022.893634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mohamed NA, et al. Early viral clearance among covid-19 patients when gargling with povidone-iodine and essential oils: a pilot clinical trial. medRxiv. 2020 doi: 10.1101/2020.09.07.20180448. [DOI] [Google Scholar]
- 60.Kratzel A, et al. Inactivation of severe acute respiratory syndrome coronavirus 2 by WHO-recommended hand rub formulations and alcohols. Emerg. Infect. Dis. J. 2020;26:1592. doi: 10.3201/eid2607.200915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.