Abstract
Autoimmune encephalitides constitute a diverse group of immune-mediated central nervous system disorders mainly characterized by the presence of antibodies targeting neuronal or glial antigens. Despite the notable contribution of antibody discovery to the understanding of their physiopathology, the specific immune cells and inflammatory mediators involved in autoimmune encephalitis are still poorly defined. However, cytokines have recently emerged as crucial signalling molecules in the pathogenesis of autoimmune encephalitis. Cytokines are biologically active, soluble, low-molecular-weight proteins or glycoproteins involved in a wide variety of physiological functions, including central nervous system development and homeostasis, immune surveillance, as well as proliferation and maturation of immune cells. Since unbalanced cytokine expression is considered a hallmark of many autoimmune central nervous system disorders, their identification and quantification has become an essential element in personalized medicine applied to the field of neuroimmunology. Several studies have explored the cytokine profile of autoimmune encephalitis, but their interpretation and comparison is challenging due to their small sample sizes and extremely high heterogeneity, especially regarding the cytokines analysed, type of sample used, and associated neural antibody. Only the cytokine profile of anti-N-methyl-D-aspartate receptor encephalitis has extensively been investigated, with findings suggesting that, although humoral immunity is the main effector, T cells may also be relevant for the development of this disorder. A better understanding of cytokine dynamics governing neuroinflammation might offer the opportunity of developing new therapeutic strategies against specific immune cells, cytokines, antibodies, or intracellular signalling cascades, therefore leading to better outcomes and preventing undesired side effects of the presently used strategies. In this review, we first summarize the current knowledge about the role of cytokines in the pathogenesis of autoimmune encephalitis, combining theoretical analysis with experimental validations, to assess their suitability as clinical biomarkers. Second, we discuss the potential applicability of the novel targeted immunotherapies in autoimmune encephalitis depending on the immunobiology of the associated antibody, their limitations, as well as the main limitations that should be addressed in future studies.
Keywords: autoimmune encephalitis, NMDAR, LGI1, cytokines, targeted immunotherapies
Ciano-Petersen et al. extensively review the value of cytokines as clinical biomarkers in autoimmune encephalitis, summarize the current knowledge about their physiopathological role, and additionally discuss in detail the most relevant targeted immunotherapies used in autoimmune encephalitis according to their underlying pathogenesis and associated antibody.
Graphical Abstract
Graphical abstract.
Introduction
Autoimmune encephalitides (AEs) comprise immune-mediated CNS disorders mainly characterized by the presence of antibodies against neuronal or glial antigens. Some of the most recent advances in the knowledge of AE include a more accurate diagnosis thanks to improved antibody detection, the deciphering of the pathogenic roles of these antibodies, and the different responses to current treatments according to the targeted antigen.1 However, the specific immune cells and inflammatory mediators contributing to the development of neuroinflammation have so far been poorly investigated, although cytokines are signalling molecules promising for the understanding of the pathogenesis of AE.
Cytokines constitute a group of small proteins that have a major role in the development of inflammation and immune regulation.2,3 Moreover, since an unbalanced cytokine expression is characteristic of many autoimmune CNS disorders, their quantification has become an essential element in neuroimmunology, acquiring a major role as diagnostic, prognostic, and therapeutic biomarkers.2,4 Their application in the era of ‘biomarker-guided therapy’ has led to a paradigm shift in the field, providing a wide therapeutic arsenal to modulate the specific inflammatory pathways involved in autoimmune CNS disorders.5
In the present article, we provide a theoretical analysis and review experimental validations to evaluate cytokines as clinical biomarkers in AE and summarize the current knowledge about their role in the pathogenesis of these diseases. In addition, we discuss the most relevant targeted immunotherapies in AE according to their underlying pathogenesis and associated antibody, as well as the main limitations that should be addressed in future studies.
Cytokines and chemokines
Cytokines are biologically active, soluble, low-molecular-weight proteins or glycoproteins involved in a wide range of physiological functions, such as CNS development and homeostasis, immune surveillance, as well as proliferation and maturation of immune cells.6,7 Furthermore, they are highly important signalling molecules, not only for immune cells to communicate and coordinate their biological activities but also for neurons and microglia.8 Cytokines can be classified according to their ability to attract immune cells into specific organs. Non-chemoattractant cytokines mainly regulate the proliferation and maturation of immune cells and comprise tumour necrosis factors (TNFs), interleukins (ILs), interferons (IFNs), and growth factors such as transforming growth factor (TGF). In contrast, chemotactic cytokines (chemokines) have the ability to attract and guide leukocyte migration into affected organs.8 These molecules establish a wide biochemical network that coordinates the interaction between innate and adaptive immune cells and can be further classified according to their role in the recruitment, proliferation, and activation of different subsets of immune cells.8, 9
The cytokine microenvironment regulates the proliferation and polarization of precursor immune cells into functional subsets, such as the shift of naïve CD4+ T-cells into Th1-, Th2-, Th9-, Th22-, T follicular helper, and Th17-cells. Among these, Th1-, Th2-, and Th17-cells have been reported to have a role in CNS autoimmunity (Table 1). This transformation is driven by the binding of cytokines to their receptors (CKR), leading to the activation of a cascade of downstream signalling molecules, such as Janus kinases (JAKs), and signal transducers and activators of transcription (STAT), which ultimately regulate gene transcription necessary for specialized tasks.10 Although some cytokines may bind several CKR, most of them exhibit a higher affinity for specific CKR expressed by certain immune cells, and therefore play a role in particular immune responses.8
Table 1.
Interactions between cytokines and different immune cells involved in immune responses
| Immune cells | Function | Attracted by | Induced by | Produce |
|---|---|---|---|---|
| Th1-cells | Regulate cellular immunity, orchestrating expansion of cytotoxic T cells and ASC | CXCL-9–11, CCL3 | IFN-γ, IL-12 | IFN-y, IL-2, TNF-α, CXCL9-11 |
| Th2-cells | Regulate humoral immunity, relevant in allergy | CCL1,8,17, 22 | IL-4 | IL4-6, 13, 25, 31, 33; CCL21 |
| Th17-cells | Major role in neuroinflammatory disorders, can further shift towards Th1 or Th2 phenotype | CXCL-13, CCL22 | TGF-β, IL-6, IL-23 | IL-1β, 6, 8, 17A, 21-23, |
| Treg-cells | Regulate immune responses and immune tolerance | CCL1, CCL22 | TGF-β, IL-2 | IL-10, IL-35, TGF-β |
| B-cells | Regulate humoral immunity effectors | CXCL-10, CXCL-13 | IL-1β, IL-6 | IL-5, 10, 12, 14; TNF-β |
ASC, antibody-secreting cells CCL, CC chemokine ligand; CXCL, C-X-C motif chemokine; IFN, interferon; IL, interleukin; TGF, transforming growth factor; Th, T-helper cell; TNF, tumour necrosis factor; Treg, regulatory T cells.
The characterization of immune cells based on their cytokine signature and CKR has recently gained interest, as particular T-cell subsets may predominate in certain neuroimmune disorders.11 Hence, a better definition of the dynamics of these cells and their cytokine expression could unravel the immunological pathways and mechanisms responsible for the development of AE, which, in turn, is crucial for the identification of candidates for new-targeted immunological therapies.
Methods
For the evaluation of cytokines as clinical biomarkers in AE, we conducted an exhaustive search of articles published from 1st January 1995 to 15th May 2021 in MEDLINE/Pubmed. The following MeSH terms, free text, and related search terms were included: ‘Autoimmune encephalitis’ AND (‘cytokine’ OR ‘chemokine’ OR ‘interleukin’ OR ‘inflammatory mediator’). The first screening of results was performed by titles and abstracts, and only studies with full text available in English language were included. Additional articles were retrieved by searching through the reference lists of all studies. Only studies analysing cytokines in the CSF and including at least 20 patients with the same type of AE were selected, regardless of the methodology used to quantify cytokine levels. Despite myelin oligodendrocyte glycoprotein antibody-associated disease and neuromyelitis optica spectrum disorders (NMOSD) being also associated with acute disseminated encephalomyelitis and other encephalitic syndromes, they were not included in this study.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
The role of cytokine networks in autoimmune encephalitides
Most studies reviewed examined cytokine profiles in AE with immunoassay-based methodologies. Depending on the cytokine analysed and the commercial kit used, cytokines were assessed by either a single plex assay using conventional sandwich ELISA, or by multiplexing strategies with fluorescent bead-based immunoassays that allow the detection of several cytokines in a small amount of sample. However, the limited size of the study populations, as well as the heterogeneity of cytokines assessed, types of samples, and patient characteristics make it difficult to interpret and compare the reported results. For these reasons, in an attempt to homogeneously present these data and ease its understanding, we focused on cytokines analysed in CSF and in a significant number of patients with the same AE, and summarized findings according to the associated neural antibody and immune response involved.
Anti-N-methyl-D-aspartate receptor encephalitis
Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis is an autoimmune disorder characterized by the presence of IgG1 antibodies against NMDAR.12,13 Meaningful investigations have been conducted on cultured neurons12,14 and in vivo models,15 demonstrating the pathogenic role of the antibodies that provoke the internalization of NMDAR and disrupt its interaction with Ephrin B2 receptor.16 The active role of these antibodies and the expansion of CD19+ B-cells and CD19 + CD138 + plasma cells in CSF suggest that humoral immunity is key to anti-NMDAR encephalitis.17–20 However, although histopathological studies found intense infiltration of CD138 + plasma cells in the brain parenchyma and mild infiltrations of B-cells in perivascular spaces, CD3+ T-cells were also identified, reflecting that cellular immunity may play also a role. Nevertheless, there is no evidence of antibody and complement deposition or neuronal loss, which may explain the generally good outcome with immunotherapy.21, 22
Several cytokines implicated in B- and T-cell activation have been found in the CSF of patients with anti-NMDAR encephalitis23 (Table 2). Among cytokines regulating the B-cell axis, C-X-C motif chemokine (CXCL) 13 is considered the main B-cell recruiter (via CXCR5) into the CNS, although it is also involved in Th17/Treg ratio regulation and Th17-B-cell interactions necessary for antibody production.9 High intrathecal CXCL-13 levels have been reported in a total of 271 patients with anti-NMDAR encephalitis.24–29 Intriguingly, a paired serum-CSF study found higher concentrations in serum than in CSF,25 whereas another found increased levels only in the CSF.24 The CXCL-13 CSF/serum ratio is considered a measure of chemotactic gradient that may change during the disease course, likely explaining the differences in serum CXCL-13 levels.8 Interestingly, high CSF CXCL-13 levels have been reported to correlate with high intrathecal titers of anti-NMDAR antibodies, clinical severity at admission, relapses, worse treatment response, and greater long-term disability, highlighting its potential value as a prognostic and therapeutic biomarker.24,25,27 In addition, B-cell-activating factor (BAFF) and proliferation-inducing ligand (APRIL) are two other molecules from the TNF cytokine superfamily that play a role in B-cell survival, differentiation, and maturation, as well as in antibody synthesis.30 High serum and CSF levels of both molecules have been found in patients with NMOSD, Behçet disease, paraneoplastic opsoclonus-myoclonus (OM), myasthenia gravis (MG), systemic lupus erythematosus (SLE), Sjögren syndrome (SS), and rheumatoid arthritis (RA).31–34 Moreover, BAFF and APRIL levels were elevated in the acute phase of 40 patients with anti-NMDAR encephalitis and presented a positive correlation, thus reflecting a synergic role in neuroinflammation. Furthermore, high levels were associated with worse long-term outcomes.35 Conversely, other studies found no significant difference in BAFF and APRIL levels between anti-NMDAR patients and controls.25,27,32
Table 2.
CSF cytokine profile of patients with anti-NMDAR encephalitis
| Immune cell | Cytokine | Sample, n | Findings | References |
|---|---|---|---|---|
| B-cell | CXCL-13 | 271 | Elevated in CSF during the acute phase | 24,25,27–29 |
| Elevated in CSF during relapses | 24,27 | |||
| Correlated with CSF titres of NMDAR antibodies | 24 | |||
| Correlated with CSF pleocytosis | 25 | |||
| Correlated with clinical severity | 27 | |||
| Correlated with outcomes | 24,27 | |||
| BAFF and APRIL | 40 | Elevated in CSF during the acute phase | 35 | |
| Associated with outcomes | 35 | |||
| Th1 | IFN-γ | 46 | Elevated in CSF during the acute phase | 25,29 |
| Persistently increased over months | 25 | |||
| TNF-α | 125 | Elevated in CSF during the acute phase | 25,27,29,36–38 | |
| Persistently increased over months | 25 | |||
| Only increased in CSF of paraneoplastic cases | 39 | |||
| CXCL-10 | 30 | Elevated in CSF during the acute phase | 25,27,29 | |
| Persistently increased over months | 25 | |||
| Elevated in CSF during relapses | 27 | |||
| Correlated with CSF pleocytosis | 25 | |||
| Correlated with clinical severity | 27,29 | |||
| Correlated with outcomes | 27 | |||
| IL-2 | 24 | Elevated in serum, which inversely correlated with CSF IL-6 and IL-17A | 26 | |
| Decreased significantly after treatment | 29 | |||
| Th2 | CCL22 | 70 | Elevated in CSF during the acute phase | 28,29 |
| Correlated with clinical severity | 28,29 | |||
| Th17 | IL-1β | 109 | Elevated in CSF during the acute phase | 28,40,41 |
| Correlated NLRP3 levels | 40 | |||
| IL-6 | 223 | Elevated in CSF during the acute phase | 26–28,37,38,40,42,43 | |
| Correlated with clinical severity | 28,29,40,44 | |||
| Correlated with and NLRP3 levels and HMGB1 | 40,43 | |||
| IL-7 | 52 | Elevated in CSF during the acute phase | 25,29 | |
| Persistently increased over months | 25 | |||
| IL-17A | 189 | Elevated in CSF during the acute phase | 25,26,28,40–43 | |
| Persistently increased over months | 25 | |||
| Correlated with NLRP3 and HMGB1 levels | 40,43 | |||
| Correlated with clinical severity | 40,42 | |||
| Correlated with outcomes | 28 | |||
| Elevated in CSF during relapses | 28 | |||
| Treg | IL-10 | 80 | Elevated in CSF during the acute phase | 27,29,37,38 |
| Only elevated in CSF of paraneoplastic cases | 39 | |||
| Correlated with clinical severity | 45 |
APRIL, proliferation-inducing ligand; BAFF, B cell-activating factor; CCL, C-C motif chemokine ligand; CSF, cerebrospinal fluid; CXCL, C-X-C motif chemokine; HMGB1, high-mobility group box 1; IFN, interferon; IL, interleukin; NLRP3, NOD-like receptor family, pyrin domain-containing 3; NMDAR, N-methyl-D-aspartate receptor; TNF, tumour necrosis factor; Th, T-helper cells; Treg, regulatory T cells.
Despite being mostly driven by humoral immunity, T cells may also have a role in anti-NMDAR encephalitis by orchestrating the ensuing inflammatory cascade and assisting B-cells in their maturation towards memory B-cells and long-lived plasma cells.8,9,26,29 Cellular immune responses recruit different T-cell subsets according to the nature of the antigen and the phase of the response.11 For instance, Th1-cells play a preferential role in the regulation of cytotoxic T cells and antibody-secreting cells (ASCs) through the production of different cytokines.11 Among them, TNF-α, which is broadly involved in the initiation and regulation of immune responses, was found at higher CSF concentrations in 125 patients with anti-NMDAR encephalitis compared to controls.25,27,29,36–38 Similarly, interferon-γ (IFN-γ), another pleiotropic cytokine produced by Th1-cells that stimulate ASC within the CNS by increasing their susceptibility to CXCL-109, was elevated in the CSF of 46 anti-NMDAR patients.25,29 Moreover, IFN-γ was also persistently elevated in patients that developed anti-NMDAR encephalitis after herpetic encephalitis, suggesting that a long-lasting stimulation of B-cells could lead to the synthesis of autoantibodies.46 In addition, IFN-γ and TNF-α induce the expression of the receptor CXCR3 in immune cells as well as the secretion of its ligand CXCL-10, the main recruiter of Th1-cells into the CNS.29 Interestingly, CXCL-10 was elevated in the CSF of 30 patients with anti-NMDAR encephalitis,25,27,29 and remained elevated longer than CXCL-13, implying that after an initial B-cell response Th1-cell activation is needed to maintain the autoimmune reaction.25 Furthermore, the levels of CXCL-10 were associated with CSF pleocytosis, clinical severity, and relapses, reflecting its potential as prognostic biomarker.25,27,29 Strikingly, a single case report found that pleocytosis, CXCL-10, and CXCL-13 progressively increased in parallel with the patient’s clinical worsening until coma; furthermore, the increase in CXCL-10 levels preceded that of CXCL-13 and BAFF, suggesting a role of astrocytes in the initial phases.9,25 In addition, Th1-cells also produce IL-2, a pleiotropic cytokine that has a role in the proliferation of cytotoxic T cells, although it also induces anti-inflammatory feedback by stimulating the proliferation of Treg cells.9 IL-2 may limit uncontrolled inflammatory responses by interfering with IL-6-dependent signalling and inhibiting Th17-cell differentiation.47 Accordingly, high serum IL-2 levels negatively correlated with IL-6 and IL-17A CSF levels in a small cohort of patients with anti-NMDAR encephalitis.26 Surprisingly, although Th2-cells exert a major role in humoral immunity, only few chemokines involved in Th2-cell recruitment have been analysed in anti-NMDAR encephalitis.28,45 Among them, C-C motif chemokine ligand (CCL) 22 was elevated in 70 patients,28,29 and its levels positively correlated with clinical severity.29 Moreover, CCL22 plays a role in the recruitment of Th17-cells, reflecting its pleiotropic properties and supporting a synergic role of both types of T-cell responses in anti-NMDAR encephalitis.28
An increased TH17/Treg ratio is considered a hallmark of autoimmunity and is associated with disease activity in psoriasis, inflammatory bowel disease, RA, and multiple sclerosis.4 Interestingly, high CSF Th17-cells levels have also been found in 60 patients with anti-NMDAR encephalitis.28 The balance between Th17 and Treg-cells is tightly regulated by the surrounding cytokine microenvironment: in the presence of TGF-β and IL-2, naïve CD4+ T cells polarize into Treg-cells, which mainly produce anti-inflammatory cytokines such as IL-10, while TGF-β and IL-6 drive the differentiation into Th17-cells that mainly produce IL-1β, 6, 7, 8, and 17.4,7 The activation of nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome induces the migration of Th1 and Th17 cells into the CNS via IL-1β production, which was found elevated in the CSF of 109 patients with anti-NMDAR encephalitis.28,40,41 As it has been mentioned before, IL-6 is a pleiotropic cytokine that regulates Th17/Treg balance, stimulates B-cell differentiation and antibody production, and regulates the survival of plasmablasts implicated in CNS disorders.48 High intrathecal IL-6 levels have been found in 223 patients with anti-NMDAR encephalitis, which were associated with a worse clinical status.26–29,37,38,40,42,44 Additionally, IL-7, a critical survival factor of regular and autoreactive T cells, was found to be increased in 52 patients with anti-NMDAR encephalitis.25,29 Furthermore, IL-17A, a cytokine that stimulates the expression of IL-6 and facilitates leukocyte trafficking across the blood-brain barrier (BBB),43 was found to be elevated in 189 anti-NMDAR patients and associated with clinical severity, relapses, and outcomes.25,26,28,40–42,44 Therefore, novel immunotherapies aimed to restore the Th17/Treg-cell balance could be promising in future therapeutic strategies for anti-NMDAR encephalitis.
However, some of these cytokine deviations are not exclusive of anti-NMDAR encephalitis, and may partially overlap with other neuroimmune and neuroinfectious disorders, such us TNF-α, IL-6, IL-10, CXCL-13, CXCL-10, and IL-1. These findings suggest shared immunological pathways involving humoral and cellular immunity, and might offer novel treatment strategies for both immune and infectious disorders.27
Anti-leucine-rich glioma-inactivated 1 encephalitis
Anti-leucine-rich glioma-inactivated 1 (LGI1) encephalitis is characterized by the presence of antibodies against the glycoprotein LGI1, predominantly of the IgG4 subclass although IgG1 is also frequent in both serum and CSF.49 LGI1 is a secreted protein that interacts with presynaptic ‘A disintegrin and metalloproteinase domain-containing protein’ (ADAM) 23 as well as postsynaptic ADAM22, regulating the function of presynaptic Kv1.1 potassium channels and postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR).50,51 Interestingly, nearly 20% of patients have LGI1 antibodies detectable only in the serum, and 50% have no inflammatory CSF abnormalities, likely reflecting a different immune mechanism compared to other AE driven by neural cell-surface antibodies such as anti-NMDAR encephalitis.49 These findings are in agreement with the scarce lymphocytic infiltrates and immunoglobulin deposition described in histopathological studies. However, mild B- and T-cell infiltrates and heavy IgG and complement deposits may be found, leading to focal areas of neuronal loss that may explain the frequent residual cognitive deficits21,52–54.
In concordance with the scarce inflammatory reaction distinctive of anti-LGI1 encephalitis, only a few studies found significant differences in intrathecal cytokine levels compared to controls.55,56 High CSF levels of CXCL-13 were observed despite an unremarkable CSF analysis, highlighting that a humoral CNS immune response may be present even in the absence of an evident inflammatory CSF. However, serum levels were generally higher, indicating a peripheral synthesis and a subsequent leakage into the CNS.55,57 In addition, Th17-cell-related cytokines such as IL-17A were increased in patients with anti-LGI1 encephalitis, and associated with disease severity and risk of ICU admission at onset,56 suggesting that cellular immunity may also play a pathogenic role, likely assisting B-cells in antibody production.55 However, other studies failed to find significant differences in CSF cytokine levels compared to controls.26
Other encephalitides
The cytokine profile of AE associated with antibodies against Hu, Yo, glutamic acid decarboxylase (GAD), γ-Aminobutyric acid-B (GABAB) receptor, GABAA receptor, contactin-associated protein-like 2 (CASPR2), immunoglobulin-like cell adhesion molecule 5 (IgLON5), and adenylate kinase 5 (AK5) have also been explored in several studies32,55,56,58,59. However, no firm conclusions can be drawn due to the small size of the samples and the different nature of these diseases. Moreover, the heterogeneity of involved cytokines and pathways hamper the understanding of actionable pathogenic mechanism.32,55,56,58,59 Nevertheless, it is noteworthy that patients with anti-Yo and anti-Hu paraneoplastic cerebellar degeneration had enrichment of CXCR3+ T cells and CXCL-10 in the CSF, supporting a major role of cellular immunity in the pathogenesis of these disorders.58
A general approach to targeted immunotherapies
The current therapeutic approach of AE is based on retrospective studies suggesting a benefit of immunotherapy, but no prospective clinical trials have been conducted yet in AE to support this practice.60 Consequently, most immunotherapies in AE are given empirically and in a non-specific manner, independently of the suspected underlying pathogenic mechanisms. For instance, after the failure of the so-labelled first-line therapies, it is frequent to combine ASC-depleting agents, such as rituximab, with a wide-spectrum drug such as cyclophosphamide, in both antibody and T-cell mediated AE.61 However, this strategy likely increases the risk of side effects and may even affect the anti-tumour response in paraneoplastic cases. Hence, novel therapeutic regimens tailored to modulate the specific underlying immune response are required to improve outcomes and prevent undesired events in patients with AE.
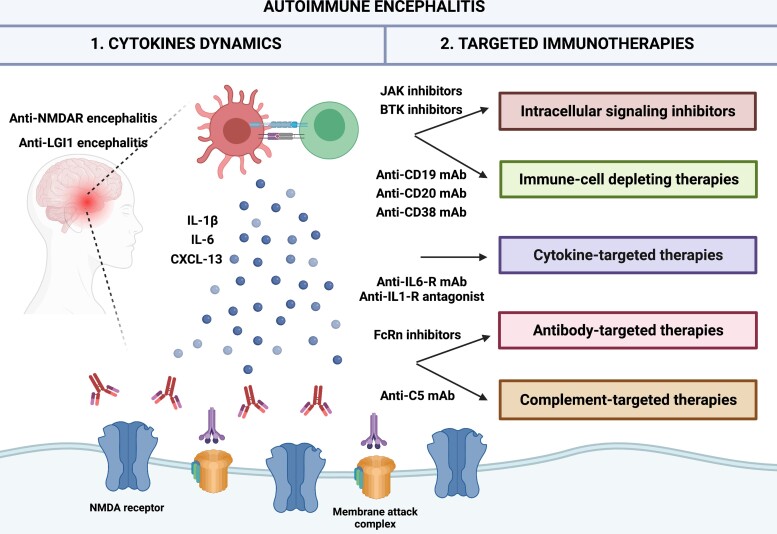
Countless novel immunotherapies have recently been developed to block specific immune pathways at different points of the signalling cascade driving neuroinflammation. A first approach is to deplete specific immune cells subpopulations such as B-cells, plasma cells, or cytotoxic T cells, by targeting their specific cluster of differentiation (CD) with monoclonal antibodies (mAbs), or interfering with their metabolism and inducing apoptosis. Secondly, because excessive recruitment of leukocytes and lymphocytes to affected tissues and organs is a key feature of autoimmune disorders, specific inhibition of this process would be an ideal anti-inflammatory strategy. Therefore, circulating signalling molecules such as cytokines and chemokines produced by immune cells may also be targeted by soluble receptors, neutralizing proteins or mAbs in order to prevent the interaction with their CKR (Fig. 1). Additionally, recombinant fusion proteins and mAbs can be directed against these receptors to prevent their activation and subsequent intracellular signalling cascades or even antagonize their function. These intracellular pathways comprise several cytoplasmic proteins that can also be selectively inhibited to prevent the transcription of selected genes (Fig. 2). Lastly, soluble immune effectors, including antibodies and complement proteins, could also be removed or blocked by mAbs and fusion proteins.
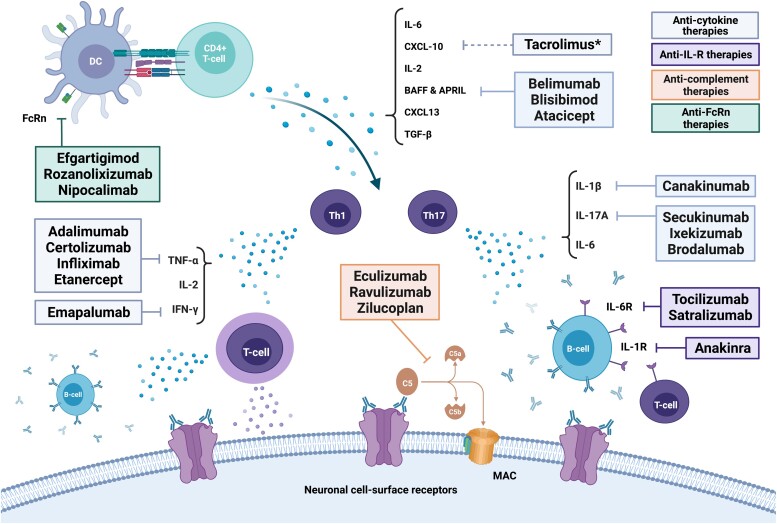
Figure 1.
Targeted immunotherapies against soluble biomarkers. *Tacrolimus is not selectively directed against CXCL-10 but it has been reported to decrease intrathecal CXCL-10 levels. APRIL, proliferation-inducing ligand; BAFF, B-cell-activating factor; CXCL, C-X-C motif chemokine; DC, dendritic cells; FcRn, neonatal Fc receptor; IFN, interferon; IgG, immunoglobulin G; IL, interleukin; IL-R, interleukin receptor; MAC, membrane attack complex; NMDAR, N-methyl-D-aspartate receptor; TGF, transforming growth factor; Th, T-helper cells; TNF, tumour necrosis factor.
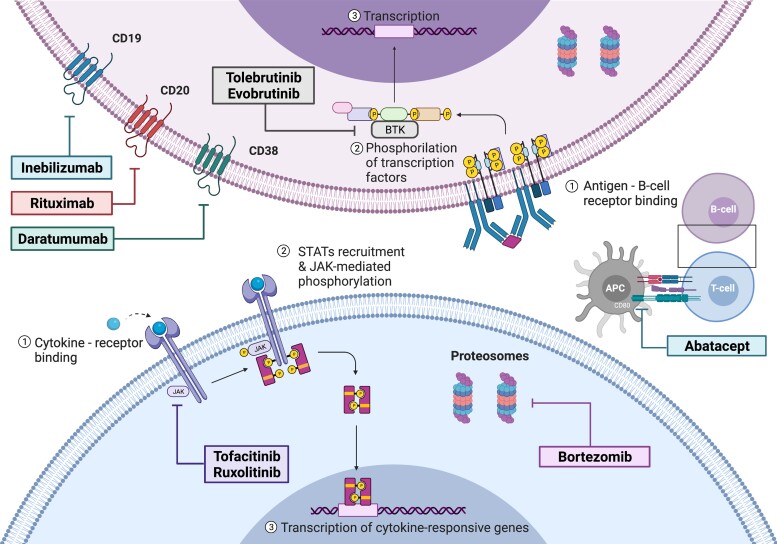
Figure 2.
Targeted immunotherapies against membrane or intracellular proteins. APC, antigen-presenting cell; BTK, Bruton’s tyrosine kinases; JAK, Janus kinases; STAT, signal transducers and activators of transcription.
Potential targeted immunotherapies in autoimmune encephalitides
In this section, we comprehensively review the potential therapeutic options to modulate the immune mechanisms driving AE from a theoretical point of view according to the results presented in the section entitled ‘The role of cytokine networks in AE’. We then summarize the current knowledge regarding their use in other systemic and neurological immune disorders, and, if available, experience in selected patients with AE. All these treatments are further classified according to their predominant effect on the humoral or cellular immunity, the nature of the targeted molecule, and the immune pathways in which they are involved. Supplementary Table 1 presents the main immunotherapies considered potentially relevant in the near future for the management of AE.
Humoral immunomodulators
Several agents have recently been used in antibody-mediated disorders to modulate selectively the humoral immune system. Herein, we describe these therapeutic alternatives according to their mechanisms, which include acting directly on ASC, blocking soluble mediators relevant for B-cell proliferation, removing the final effectors of the humoral response, and modulating intracellular signalling inhibitors such as Bruton’s kinase (BTK) inhibitors.
Antibody-secreting cells depletion therapies
Autoimmune CNS disorders mediated by antibodies have successfully been treated with B-cell depleting therapies such as rituximab in large retrospective.62,63Another anti-CD20 agent, ocrelizumab, has also been suggested to be effective in a prospective clinical trial for AE, although it was prematurely closed due to poor recruitment64, which highlights the need of international collaborative clinical trials to achieve enough sample sizes to assess alternative immunotherapies in AE. However, it has been suggested that refractory cases are driven by CD19 + CD20- long-lived plasma cells (LLPC), explaining why some cases refractory to rituximab respond to plasma cell depletion therapies such as anti-CD19 or anti-CD38 agents.65–67 Accordingly, intrathecal CD20 + memory B-cells, CD38+ ASC, and CD19 + CD138 + plasma cells have been found in patients with anti-NMDAR, anti-GABAbR, and anti-LGI1 encephalitis.20,68,69 Therefore, novel anti-CD19 therapies have been proposed to treat antibody-mediated disorders such as NMOSD, for which inebilizumab, a mAb against CD19, has recently been approved70 and is soon going to be assessed in a trial for anti-NMDAR encephalitis (NCT04372615), while chimeric antigen receptor-T (CAR-T) cells targeting CD19+ B-cells are currently being assessed in a clinical trial for NMOSD (NCT03605238) and MG (NCT04146051). However, anti-CD19 CAR-T cells have been reported to enhance disease progression in a mouse model of multiple sclerosis.71 These therapies could lead to a broad depletion of late B-cell stages including ASC, plasmablasts, and early plasma cells; however, terminally differentiated plasma cells may not be depleted as they can lose the expression of CD19 and CD20.72 In order to remove these terminally differentiated plasma cells, other therapies used for multiple myeloma have been proposed; for instance, daratumumab, which targets CD38, has been reported to be effective in small case series of anti-NMDAR and anti-CASPR2 encephalitis.73,74 This selective depletion of ASC may lead to higher effectiveness and safety compared to conventional anti-CD20 therapies. However, there is limited evidence of the impact of ASC-depleting therapies on intrathecal or parenchymal ASC, especially relevant in AE with B-cell CNS infiltration and intrathecal antibody synthesis.
Cytokine-targeted immunotherapies
Among the different types of drugs directed against cytokines, anti-IL therapies are the most relevant since they have revolutionized the management of several immune and infectious diseases such as RA or psoriasis, and even more recently COVID-19.75 IL-6 is a tempting target for antibody-mediated disorders as the blockage of its receptor restricts B-cell antibody production. Tocilizumab is an anti-IL-6R mAb already used for RA and other juvenile arthritis, giant cell arteritis, and CAR-T cell-induced cytokine release syndrome.76 Regarding AE, tocilizumab has been reported to be effective in nearly 100 patients unresponsive to rituximab, showing higher effectiveness compared to extended administration of rituximab and a surprisingly fast response (within the first weeks).77–79 In addition, the administration within the first month of corticosteroids, intravenous immunoglobulins, rituximab, and tocilizumab, along with teratoma removal if present (T-SIRT protocol) was associated with a better outcome at 1-year follow-up in anti-NMDAR encephalitis.80 However, adverse events such as leukopaenia and pneumonia were commonly observed with this strategy.80 Although there is limited evidence to broadly recommend tocilizumab in AE, recent expert consensus recommendations are considering its use in severe, refractory adult and paediatric cases.61,81 Another mAb against the IL-6R is satralizumab, which has recently been approved for NMOSD due to its enhanced antigen-specificity and a longer plasma half-life than tocilizumab.82
A different approach to modulate humoral immunity is the blockade of BAFF and APRIL pathways with several agents that have mainly been used in SLE. Belimumab is an anti-BAFF mAb that has been reported to be effective and safe in patients with SLE.83 Conversely, it was not effective for MG in a recent clinical trial,84 although patients with antibodies against muscle-specific tyrosine kinase (MuSK) were not included, and they likely represent the subgroup more prompt to respond to anti-B-cell therapies.85 Additionally, blisibimod is a molecule with features of both peptides and antibodies that exerts a selective BAFF antagonism that has been reported to be effective in SLE.86 Although no therapies have been developed to exclusively target the APRIL pathway, atacicept is a human recombinant fusion protein performing a dual blockade of BAFF and APRIL that increased relapses in patients with multiple sclerosis, hence its benefit in other immune CNS disorders remains unclear.87
In addition, novel mAbs against the major chemokine involved in B-cell recruitment and Th17-cell regulation, CXCL-13, have been reported to have good tolerance and efficacy in murine models of relapsing-remitting experimental autoimmune encephalomyelitis, RA, and lupus nephritis, suggesting that they could be useful in the treatment of their homologous human disorders.88,89 In addition, combining BAFF and CXCL-13 inhibitors may constitute another synergic strategy for blocking B-cell pathways, which was found to have higher efficacy in a murine model of SS than targeting BAFF alone.90 Thus, the combination of different immunotherapies that synergically block specific immune pathways in a tailored manner could be a promising strategy in the management of AE.
Antibody-targeted therapies
Humoral immunity can also be modulated directly by interfering with its main effectors. Interestingly, the neonatal Fc receptor (FcRn) system allows the recycling of serum IgGs, avoiding lysosomal degradation, by transporting them back to circulation and prolonging their survival.91 Thus, reversing this process with FcRn antagonists decreases IgG levels up to 85% from baseline without affecting other immune cells, therefore preventing severe and complete immunosuppression.92 Efgartigimod is an engineered human IgG1 antibody Fc-fragment designed to block the FcRn that has been reported to cause a rapid and long-lasting improvement in 75% of patients with MG.93 Novel mAbs have been designed to recognize FcRn, such as rozanolixizumab and nipocalimab, leading to a rapid depletion of 75–90% of IgG levels.94 These therapies are showing promising results in MG (NCT0397142, NCT04951622),95 which led to the design of a new clinical trial with rozanolixizumab for anti-LGI1 encephalitis (NCT04875975). However, the application of these therapies in AE could be controversial as FcRn plays a different role in the CNS, where it removes intrathecal IgG by transporting them to the circulation through reverse transcytosis across the BBB.96 Further research to selectively block peripheral FcRn or stimulate central FcRn could be promising in the future management of AE and other CNS antibody-mediated disorders.
Complement-targeted therapies
The complement system is an essential element of both innate and adaptive immune responses that amplifies the function of phagocytes and antibodies. Anti-complement therapies are effective in systemic and neurological autoimmune disorders, suggesting its potential benefit in AE with evidence of complement activation.85,97 Eculizumab, an anti-C5 mAb that inhibits the C5 cleavage into C5a and C5b preventing complement activation, has recently been approved for refractory anti-acetylcholine receptor MG and anti-aquaporine 4 NMOSD.98,99 In addition, ravulizumab, a second-generation anti-C5 mAb with a longer plasma half-life, is also currently undergoing a clinical trial for MG (NCT03920293). Another therapy to inhibit complement-mediated tissue damage currently being evaluated in MG is zilucoplan, a synthetic macrocyclic peptide that binds C5 with sub-nanomolar affinity inhibiting the cleavage into C5a and C5b (NCT04115293).94 Although anti-complement immunotherapies are promising for AE with complement activation such as anti-AK5 AE,100 their efficacy in IgG4-mediated disorders is unclear, as this IgG subclass cannot theoretically activate complement due to an inefficient binding to C1q.97 However, this assumption should be carefully considered since complement deposition has been described in histopathological studies of AE traditionally thought to be driven by IgG4, such as anti-LGI1 and anti-CASPR2 encephalitis,21,101 which moreover commonly present other IgG subclasses in serum and CSF such as IgG1.49,102
Therapies targeting B-cell intracellular signalling molecules
Alternative agents proposed for the management of autoimmune CNS disorders are BTK inhibitors, mainly due to their ability to cross the BBB.103 BTK are cytoplasmic tyrosine kinases that play a central role in B-cell development and production of cytokines and antibodies by regulating the interaction between specific cell-surface receptors and their downstream signalling pathways.104 BTK is present in most haematopoietic cells except for T-, natural killer-, and plasma cells. Therefore, they have primarily been used in B-cell malignancies, although evobrutinib and tolebrutinib have been reported to have good efficacy and tolerability in multiple sclerosis.105,106 Since BTK is not present in plasma cells, BTK inhibitors could be useful in the early phases of AE that are predominantly driven by humoral responses, but not in late stages when plasma cells are responsible for antibody production.
Cellular immunomodulators
As reviewed in the section entitled ‘The role of cytokine networks in AE’, the cellular immunity seems to play a key role not only in AE associated with antibodies against intracellular antigens, but also in AE driven by neural cell-surface antibodies by assisting humoral immune cells in antibody production. A broad variety of agents that modulate different points of the cellular immune cascade are summarized in the present section, including T-cell depleting therapies, cytokine and CKR blockers, and intracellular signalling inhibitors as JAK inhibitors.
T-cell depleting therapies
A decrease in T-cell populations can be achieved through a wide variety of therapies. However, most of these therapies are not selective for T cells and also affect B-cell populations. Cyclophosphamide is an alkylating antineoplastic agent used in several autoimmune disorders due to its ability to slow T-cell proliferation,107 and its use in AE is supported by solid evidence from retrospective studies.62 Bortezomib is a reversible ubiquitin-proteasome inhibitor used in lymphomas and multiple myeloma, and which induces apoptosis of activated T cells and antibody-secreting plasma cells.108,109 Small series have shown its efficacy in anti-NMDAR encephalitis after the failure of more widely used treatments,110–112 yet some physicians select it instead of cyclophosphamide in young patients.113 However, there is currently no solid evidence supporting its wide application but a clinical trial in AE will soon provide additional data (NCT03993262). Another option reported to be effective in small series of anti-NMDAR encephalitis is intrathecal methotrexate, a folate antagonist widely used in haematological malignancies that inhibits the enzyme dihydrofolate-reductase. Its intrathecal administration could be an asset in AE with intrathecal synthesis of antibodies, but further studies should assess its safety and efficacy.25,114–116 Another T- and B-cell depleting therapy proposed for AE owing to the previous experience in multiple sclerosis is alemtuzumab, a mAb against CD52.117 However, its use should be carefully considered as its main safety concern is the development of secondary autoimmune disorders including anti-GABAA receptor and anti-glutamate receptor 3 (GluR3) AE.118,119
Cytokine-targeted immunotherapies
Anti-TNF-α mAbs such as adalimumab, certolizumab, and infliximab, as well as etanercept, a recombinant fusion protein that blocks circulating TNF-α, are used in several autoimmune and inflammatory disorders including RA, Crohn’s disease, ankylosing spondylitis, and neurosarcoidosis.120 Despite being effective and overall safe for these indications, they have been associated with central and peripheral demyelinating diseases, lupus-like syndromes, and vasculitis.121 Hence, although they could be effective in AE, further studies should clarify the nature of these adverse events before their application in immune CNS disorders. Likewise, IFN-γ can also be targeted by emapalumab, a mAb successfully used in patients with primary hemophagocytic lymphohistiocytosis.122
Since a Th17/Treg-cell imbalance in favour of the former has been related to the development of autoimmunity, the blockade of cytokines that drive Th17-cell proliferation could be a future therapeutic approach in AE.123 IL-1β can be targeted by canakinumab, a mAb used to treat cryopyrin-associated periodic syndromes and gout.124 Moreover, IL-1R can be antagonized with anakinra, a recombinant protein that has been reported to attenuate seizures in an animal model of anti-NMDAR encephalitis,125 and in patients with new-onset refractory status epilepticus (NORSE) as well as those with febrile infection-related epilepsy syndrome (FIRES),126,127 two poorly understood entities that might have some pathogenic similarities with AE. Interestingly, IL-1 blockage is considered to promote anti-tumour immunity and synergize with immunotherapy, which could be particularly useful for paraneoplastic cases.128,129 Additionally, anti-IL-6 therapies are also feasible candidates not only to modulate cellular immunity, but also to treat paraneoplastic cases as they seem to enhance anti-tumour immunity.128,130
Similarly, IL-17 can be targeted with mAbs such as secukinumab, a therapy broadly used in psoriasis and ankylosing spondylitis that seems to be also effective in multiple sclerosis,131 suggesting its potential benefit in AE. In addition, novel anti-IL17A therapies such as ixekizumab and brodalumab could also be proposed to antagonize this pathway, although the use of anti-IL17A therapies in paraneoplastic cases should be carefully considered until further studies clarifies its impact on anti-tumour immune response.132
Moreover, the administration of cytokines with anti-inflammatory properties such as IL-2 or IL-10 could also be used to ensure the balance between Th17- and Treg-cells.133 Indeed, the administration of low doses of IL-2 to selectively stimulate Treg cells has been reported to be safe and effective for refractory AE,134 as Treg cells present a lower activation threshold than T cells.135 Similarly, daclizumab, which is a mAb acting as an agonist of the IL-2R, was reported to normalize the proportions of T and B cells in the CSF of patients with multiple sclerosis.136
In addition, several immunotherapies directed against other cytokines such as IL-12/IL-23 could also be applied in AE. However, we consider these treatments out of the scope of this review since there is currently little evidence regarding the role of these cytokines in AE or in other neuroinflammatory contexts.
Therapies targeting intracellular signalling molecules
JAKs are multidomain non-receptor tyrosine kinases that govern intracellular cytokine signals relevant for B- and T-cell proliferation and maturation by regulating transcription and gene expression.137 Since all CKR transmit their signals through JAK1, JAK2, JAK3, or TYK2, their selective blockade with JAK inhibitors allows the simultaneous modulation of multiple cytokine signalling pathways. Moreover, due to their ability to cross the BBB, they offer an interesting therapeutic option from a theoretical point of view, although the evidence regarding their efficacy in neuroimmune disorders is scarce. Tofacitinib is a JAK 1–3 inhibitor used in RA, psoriasis, and ulcerative colitis that seemed to be effective in selected cases of refractory AE,138 whereas ruxolitinib, a dual JAK1 and JAK2 inhibitor approved for myelofibrosis, was effective in single case reports of anti-MuSK MG, NMOSD and immune checkpoint inhibitor-induced myelitis.139 Therefore, this multi-cytokine blockade seems promising for AE, although its administration in patients with active cancer is currently not recommended due to concerns about its impact on anti-tumour immune response and effectiveness of immunotherapy. JAK inhibitors have already been approved for multiple rheumatological conditions and they are currently being evaluated in several clinical trials for solid non-haematological malignancies.140–145.
Furthermore, the blockade of signalling molecules responsible for T-cell recruitment into the CNS could be applied in the initial phases of AE to prevent the full development of the immune response. Tacrolimus, a calcineurin inhibitor used in several autoimmune disorders, has been reported to selectively deplete CSF CXCL-10 in rapidly progressive cerebellar syndrome associated with Yo and Hu antibodies.58
Other targeted immunotherapies
The recruitment and migration of immune cells across the BBB is a crucial process orchestrated by multiple chemokines and other molecules involved in neuroinflammation. Thus, agents preventing the entry of T cells into the CNS could be useful in AE. Natalizumab, a mAb against the subunit α4 of adhesion integrins, has been reported to reduce intrathecal and peripheral levels of several cytokines in patients with multiple sclerosis, and could therefore potentially be effective in AE, particularly in paraneoplastic cases in which the peripheral anti-tumour response should not be compromised.146 Nevertheless, hampering the CNS immune surveillance could increase the risk of CNS metastasis. Recently, a Phase II trial in Hu-associated paraneoplastic syndromes found partial improvement with natalizumab, although most patients had very severe subacute sensory neuropathies.147
Another promising strategy is the positive modulation of immune checkpoints to increase peripheral self-tolerance and decrease self-reactive immune responses. Abatacept is a fusion protein composed by cytotoxic T-lymphocyte antigen 4 (CTLA4) and the Fc region of IgG1 that binds to CD80 or CD86, thus suppressing the second T-cell activation signal and downregulating cellular immunity. In addition, abatacept may also modulate B-cell activity by internalizing CD80/CD86 and decreasing plasmablasts and IgG levels.148 Hence, it seems likely that its applicability in AE and neurological complications of immune checkpoint inhibitors could be effective, although previous reports in immune checkpoint inhibitor-induced myocarditis found opposing results.149
Conclusions and future perspectives
Recent improvements in cytokine identification and quantification provide new insights into the immune mechanism underlying neuroinflammation. In this review, we summarized several studies that explored their dynamics and suitability as prognostic and therapeutic biomarkers in AE, chiefly anti-NMDAR and anti-LGI1 encephalitis, reflecting the synergic pathogenic role of humoral and cellular immunity. However, the interpretation and comparison of the aforementioned studies is challenging due to several methodological limitations that should be considered in the design of future cytokine studies.
First, since AE are rare conditions, most studies included a small sample of patients and therefore solid conclusions cannot be reached. However, the incidence of AE has significantly increased in the last decade probably due to improvements in their diagnosis.150,151 Second, the CSF/serum cytokine gradient and BBB dysfunction were rarely reported despite being crucial to determine intrathecal production. Hence, future studies should study cytokines in paired CSF/serum samples at similar time-points, as the concentration and location of cytokines may change during the course of the disease and after the administration of immunomodulators. Otherwise, the results obtained may lead to misinterpretations that might explain the high heterogeneity observed so far. Furthermore, the use of cytokines as biomarkers is challenging due to their complex nature, and their interpretation requires the understanding of their pleiotropic activities, interindividual variability, dynamics in relation to the course and severity of the disease, and their interactions with cytokine-binding proteins and soluble receptors that may underestimate their quantification.152 Thus, all these confounding factors should be considered when designing new cytokine studies and tailoring immunotherapeutic strategies for AE.
Currently, AE are treated empirically and independently of the underlying pathogenic mechanisms, mainly due to the absence of clinical trials on these rare disorders. However, the increasing knowledge on the cytokine dynamics summarized in this review offers a promising opportunity to treat patients in a personalized manner that could change the present paradigm of AE management. In the era of ‘biomarker-guided therapy’, cytokines might be useful in the diagnosis of neuroimmune disorders and to identify subsets of patients that could potentially benefit from novel therapeutic strategies, thus improving outcomes and minimizing side effects.153,154 However, these new therapies should be administered as early as possible in the disease course to avoid misinterpretation, since their targets are dynamic and activate downstream signalling cascades that may underestimate their efficacy if administered in an advanced stage of the disease. Additionally, the blockade of a sole pathway may be insufficient to control the entire immune reaction, especially if not applied before the full response has been developed. Indeed, the simultaneous blockade of cytokines and their CKR, or the concomitant blockade of inflammatory pathways and activation of anti-inflammatory processes have been proposed to be more effective.5 Similarly, the interruption of intracellular signalling pathways relevant for multiple cytokines with JAK or BTK inhibitors, instead of a single pathway inhibition, could be more effective. Hence, future therapeutic strategies for AE should comprise combinations of immunotherapies to regulate various key points of the immune system considering the clinical characteristics and comorbidities of the patient, such as the type of neural antibodies associated, cancer, and cytokine profile, in order to tailor personalized synergic therapies directed against B- and/or T cells, cytokines or CKRs, antibodies, complement, or specific signalling molecules.
Future studies on the cytokine profile of AE should comprise international, collaborative, prospective designs to avoid current limitations related to small samples and cytokine dynamics during the course of the disease. Concurrently, prospective clinical trials assessing the safety and efficacy of novel targeted immunotherapies are required to reach stronger evidence and improve outcomes in patients with AE.
Supplementary Material
Acknowledgements
We gratefully acknowledge Philip Robinson for help in manuscript preparation (Direction de la Recherche Clinique, Hospices Civils de Lyon).
Abbreviations
- ADAM =
A disintegrin and metalloproteinase domain-containing protein
- AE =
autoimmune encephalitides
- AK5 =
adenylate kinase 5
- AMPAR =
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- APRIL =
proliferation-inducing ligand
- ASC =
antibody-secreting cell
- BAFF =
B cell-activating factor
- BBB =
blood-brain barrier
- BTK =
Bruton’s tyrosine kinase
- CAR-T =
Chimeric Antigenic Receptor-T
- CASPR2 =
contactin-associated protein-like 2
- CCL =
C-C motif chemokine ligand
- CKR =
cytokine receptor
- CXCL =
C-X-C motif chemokine
- FcRn =
neonatal Fc receptor
- GAD =
glutamic acid decarboxylase
- HMGB1 =
high–mobility group box 1
- IFN =
interferon
- IgLON5 =
immunoglobulin-like cell adhesion molecule 5
- IL =
interleukin
- JAK =
Janus kinase
- LGI1 =
leucine-rich glioma-inactivated 1
- mAb =
monoclonal antibody
- MG =
myasthenia gravis
- MUSK =
muscle-specific tyrosine kinase
- NMDAR =
N-methyl-D-aspartate receptor
- NMOSD =
neuromyelitis optica spectrum disorder
- NLRP3 =
NOD-like receptor family, pyrin domain-containing 3
- OM =
opsoclonus-myoclonus
- RA =
rheumatoid arthritis
- SLE =
systemic lupus erythematosus
- SS =
Sjögren syndrome
- STAT =
signal transducers and activators of transcription
- TGF =
transforming growth factor
- TNF =
tumour necrosis factor
- Th =
T-helper cells
- Treg =
regulatory T cells
Contributor Information
Nicolás Lundahl Ciano-Petersen, French Reference Center on Paraneoplastic Neurological Syndromes and Autoimmune Encephalitis, Hospices Civils de Lyon, Hôpital Neurologique, Bron, France; SynatAc Team, Institute MeLiS, INSERM U1314/CNRS UMR 5284, Université de Lyon, Université Claude Bernard Lyon 1, Lyon, France; Neuroimmunology and Neuroinflammation group. Biomedical Research Institute of Málaga (IBIMA), Málaga, Spain; Red Andaluza de Investigación Clínica y Traslacional en Neurología (Neuro-RECA). Hospital Regional Universitario de Málaga, Málaga, Spain.
Sergio Muñiz-Castrillo, French Reference Center on Paraneoplastic Neurological Syndromes and Autoimmune Encephalitis, Hospices Civils de Lyon, Hôpital Neurologique, Bron, France; SynatAc Team, Institute MeLiS, INSERM U1314/CNRS UMR 5284, Université de Lyon, Université Claude Bernard Lyon 1, Lyon, France.
Cristina Birzu, Service de Neurologie 2-Mazarin, Centre de Recherche de l’Institut du Cerveau et de la Moelle Epinière, Groupe Hospitalier Pitie-Salpetrière et Université Pierre et Marie Curie-Paris 6, AP-HP, Paris, France.
Alberto Vogrig, French Reference Center on Paraneoplastic Neurological Syndromes and Autoimmune Encephalitis, Hospices Civils de Lyon, Hôpital Neurologique, Bron, France; SynatAc Team, Institute MeLiS, INSERM U1314/CNRS UMR 5284, Université de Lyon, Université Claude Bernard Lyon 1, Lyon, France.
Antonio Farina, French Reference Center on Paraneoplastic Neurological Syndromes and Autoimmune Encephalitis, Hospices Civils de Lyon, Hôpital Neurologique, Bron, France; SynatAc Team, Institute MeLiS, INSERM U1314/CNRS UMR 5284, Université de Lyon, Université Claude Bernard Lyon 1, Lyon, France.
Macarena Villagrán-García, French Reference Center on Paraneoplastic Neurological Syndromes and Autoimmune Encephalitis, Hospices Civils de Lyon, Hôpital Neurologique, Bron, France; SynatAc Team, Institute MeLiS, INSERM U1314/CNRS UMR 5284, Université de Lyon, Université Claude Bernard Lyon 1, Lyon, France.
Bastien Joubert, French Reference Center on Paraneoplastic Neurological Syndromes and Autoimmune Encephalitis, Hospices Civils de Lyon, Hôpital Neurologique, Bron, France; SynatAc Team, Institute MeLiS, INSERM U1314/CNRS UMR 5284, Université de Lyon, Université Claude Bernard Lyon 1, Lyon, France.
Dimitri Psimaras, Service de Neurologie 2-Mazarin, Centre de Recherche de l’Institut du Cerveau et de la Moelle Epinière, Groupe Hospitalier Pitie-Salpetrière et Université Pierre et Marie Curie-Paris 6, AP-HP, Paris, France.
Jérôme Honnorat, French Reference Center on Paraneoplastic Neurological Syndromes and Autoimmune Encephalitis, Hospices Civils de Lyon, Hôpital Neurologique, Bron, France; SynatAc Team, Institute MeLiS, INSERM U1314/CNRS UMR 5284, Université de Lyon, Université Claude Bernard Lyon 1, Lyon, France.
Funding
This study is supported by Fondation pour la recherche médicale (FRM-DQ20170336751). This work has been developed within the Biomarkers in autoimmune Encephalitis and paraneoplastic neurological syndromes (BETPSY) project, which is supported by a public grant overseen by the Agence Nationale de la Recherche (ANR) as part of the second Investissements d ´Avenir programme (ANR-18-RHUS-0012).
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain Communications online.
References
- 1. Dalmau J, Graus F. Antibody-mediated encephalitis. N Engl J Med. 2018;378(9):840–851. [DOI] [PubMed] [Google Scholar]
- 2. Dinarello CA. Historical insights into cytokines. Eur J Immunol. 2007;37(Suppl 1):S34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Laing KJ, Secombes CJ. Chemokines. Dev Comp Immunol. 2004;28(5):443–460. [DOI] [PubMed] [Google Scholar]
- 4. Lee GR. The balance of th17 versus treg cells in autoimmunity. Int J Mol Sci. 2018;19(3):730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arron JR, Townsend MJ, Keir ME, Yaspan BL, Chan AC. Stratified medicine in inflammatory disorders: From theory to practice. Clin Immunol. 2015;161(1):11–22. [DOI] [PubMed] [Google Scholar]
- 6. Williams JL, Holman DW, Klein RS. Chemokines in the balance: Maintenance of homeostasis and protection at CNS barriers. Front Cell Neurosci. 2014;8:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Becher B, Spath S, Goverman J. Cytokine networks in neuroinflammation. Nat Rev Immunol. 2017;17(1):49–59. [DOI] [PubMed] [Google Scholar]
- 8. Pranzatelli MR. Advances in biomarker-guided therapy for pediatric- and adult-onset neuroinflammatory disorders: Targeting chemokines/cytokines. Front Immunol. 2018;9:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murphy K, Paul T, Mark W, Charles J. Janeway’s Immunobiology. New York: Garland Science, 2008. [Google Scholar]
- 10. Mclornan DP, Pope JE, Gotlib J, Harrison CN. Current and future status of JAK inhibitors. Lancet. 2021;398(10302):803–816. [DOI] [PubMed] [Google Scholar]
- 11. Raphael I, Nalawade S, Eagar TN, Forsthuber TG. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 2015;74(1):5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dalmau J, Gleichman AJ, Hughes EG, et al. . Anti-NMDA-receptor encephalitis: Case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hughes EG, Peng X, Gleichman AJ, et al. . Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30(17):5866–5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Planagumà J, Leypoldt F, Mannara F, et al. . Human N-methyl D-aspartate receptor antibodies alter memory and behaviour in mice. Brain. 2015;138(1):94–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mikasova L, de Rossi P, Bouchet D, et al. . Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain. 2012;135(5):1606–1621. [DOI] [PubMed] [Google Scholar]
- 17. Camdessanché JP, Streichenberger N, Cavillon G, et al. . Brain immunohistopathological study in a patient with anti-NMDAR encephalitis. Eur J Neurol. 2011;18(6):929–931. [DOI] [PubMed] [Google Scholar]
- 18. Dale RC, Pillai S, Brilot F. Cerebrospinal fluid CD19(+) B-cell expansion in N-methyl-D-aspartate receptor encephalitis. Dev Med Child Neurol. 2013;55(2):191–193. [DOI] [PubMed] [Google Scholar]
- 19. Hachiya Y, Uruha A, Kasai-Yoshida E, et al. . Rituximab ameliorates anti-N-methyl-d-aspartate receptor encephalitis by removal of short-lived plasmablasts. J Neuroimmunol. 2013;265(1-2):128–130. [DOI] [PubMed] [Google Scholar]
- 20. Malviya M, Barman S, Golombeck KS, et al. . NMDAR encephalitis: Passive transfer from man to mouse by a recombinant antibody. Ann Clin Transl Neurol. 2017;4(11):768–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bien CG, Vincent A, Barnett MH, et al. . Immunopathology of autoantibody-associated encephalitides: Clues for pathogenesis. Brain. 2012;135(5):1622–1638. [DOI] [PubMed] [Google Scholar]
- 22. Martinez-Hernandez E, Horvath J, Shiloh-Malawsky Y, Sangha N, Martinez-Lage M, Dalmau J. Analysis of complement and plasma cells in the brain of patients with anti-NMDAR encephalitis. Neurology. 2011;77(6):589–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ciano-Petersen NL, Cabezudo-García P, Muñiz-Castrillo S, Honnorat J, Serrano-Castro PJ, Oliver-Martos B. Current Status of Biomarkers in Anti-N-Methyl-D-Aspartate Receptor Encephalitis. Int J Mol Sci. 2021;22:13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leypoldt F, Höftberger R, Titulaer MJ, et al. . Investigations on CXCL13 in anti-N-methyl-D-aspartate receptor encephalitis: A potential biomarker of treatment response. JAMA Neurology. 2015;72(2):180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liba Z, Kayserova J, Elisak M, et al. . Anti-N-methyl-D-aspartate receptor encephalitis: The clinical course in light of the chemokine and cytokine levels in cerebrospinal fluid. J Neuroinflammation. 2016;13(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Byun JI, Lee ST, Moon J, et al. . Distinct intrathecal interleukin-17/interleukin-6 activation in anti-N-methyl-D-aspartate receptor encephalitis. J Neuroimmunol. 2016;297:141–147. [DOI] [PubMed] [Google Scholar]
- 27. Kothur K, Wienholt L, Mohammad SS, et al. . Utility of CSF cytokine/chemokines as markers of active intrathecal inflammation: Comparison of demyelinating, anti-NMDAR and enteroviral encephalitis. PLoS One. 2016;11(8):e0161656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zeng C, Chen L, Chen B, et al. . Th17 cells were recruited and accumulated in the cerebrospinal fluid and correlated with the poor prognosis of anti-NMDAR encephalitis. Acta Biochim Biophys Sin. 2018;50(12):1266–1273. [DOI] [PubMed] [Google Scholar]
- 29. Liu J, Liu L, Kang W, et al. . Cytokines/Chemokines: Potential biomarkers for Non-paraneoplastic Anti-N-Methyl-D-Aspartate receptor encephalitis. Front Neurol. 2020;11:582296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mackay F, Browning JL. BAFF: A fundamental survival factor for B cells. Nat Rev Immuno. 2002;2(7):465–475. [DOI] [PubMed] [Google Scholar]
- 31. Samy E, Wax S, Huard B, Hess H, Schneider P. Targeting BAFF and APRIL in systemic lupus erythematosus and other antibody-associated diseases. Int Rev Immunol. 2017;36(1):3–19. [DOI] [PubMed] [Google Scholar]
- 32. Kimura A, Yoshikura N, Koumura A, Hayashi Y, Inuzuka T. B-cell-activating factor belonging to the tumor necrosis factor family (BAFF) and a proliferation-inducing ligand (APRIL) levels in cerebrospinal fluid of patients with meningoencephalitis. J Neurol Sci. 2015;352(1-2):79–83. [DOI] [PubMed] [Google Scholar]
- 33. Zhang J, Roschke V, Baker KP, et al. . Cutting edge: A role for B Lymphocyte stimulator in systemic Lupus Erythematosus. J Immunol. 2001;166(1):6–10. [DOI] [PubMed] [Google Scholar]
- 34. Wang H, Wang K, Zhong X, et al. . Cerebrospinal fluid BAFF and APRIL levels in neuromyelitis optica and multiple sclerosis patients during relapse. J Clin Immunol. 2012;32(5):1007–1011. [DOI] [PubMed] [Google Scholar]
- 35. Deng B, Liu XN, Li X, Zhang X, Quan C, Chen XJ. Raised cerebrospinal fluid BAFF and APRIL levels in anti- N -methyl- d -aspartate receptor encephalitis: Correlation with clinical outcome. J Neuroimmunol. 2017;305:84–91. [DOI] [PubMed] [Google Scholar]
- 36. Zhu J, Li Y, Zheng D, et al. . Elevated serum and cerebrospinal fluid CD138 in patients with anti-N-methyl-d-aspartate receptor encephalitis. Front Mol Neurosci. 2019;12:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen J, Ding Y, Zheng D, et al. . Elevation of YKL-40 in the CSF of anti-NMDAR encephalitis patients is associated with poor prognosis. Front Neurol. 2018;9:727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li Q, Chen J, Yin M, et al. . High level of soluble CD146 In cerebrospinal fluid might be a biomarker of severity of Anti-N-Methyl-D-Aspartate receptor encephalitis. Front Immunol. 2021;12:680424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jiang XY, Lei S, Zhang L, et al. . Co-expression of NMDA-receptor subunits NR1, NR2A, and NR2B in dysplastic neurons of teratomas in patients with paraneoplastic NMDA-receptor-encephalitis: A retrospective clinico-pathology study of 159 patients. Acta Neuropathol Commun. 2020;8(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peng Y, Liu B, Pei S, et al. . Higher CSF Levels of NLRP3 inflammasome is associated with poor prognosis of Anti-N-methyl-D-Aspartate receptor encephalitis. Front Immunol. 2019;10:905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li J, Gu Y, An H, et al. . Cerebrospinal fluid light and heavy neurofilament level increased in anti-N-methyl-d-aspartate receptor encephalitis. Brain and Behav. 2019;9(8):e01354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ai P, Zhang X, Xie Z, et al. . The HMGB1 is increased in CSF of patients with an Anti-NMDAR encephalitis. Acta Neurol Scand. 2018;137(2):277–282. [DOI] [PubMed] [Google Scholar]
- 43. Huppert J, Closhen D, Croxford A, et al. . Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J. 2010;24(4):1023–1034. [DOI] [PubMed] [Google Scholar]
- 44. Liu B, Ai P, Zheng D, et al. . Cerebrospinal fluid pentraxin 3 and CD40 ligand in anti- N -menthyl- d -aspartate receptor encephalitis. J Neuroimmunol. 2018;315:40–44. [DOI] [PubMed] [Google Scholar]
- 45. Liu J, Liu L, Kang W, et al. . Cytokines/Chemokines: Potential biomarkers for non-paraneoplastic Anti-N-Methyl-D-Aspartate receptor encephalitis. Front Neurol. 2020;11:582296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ygberg S, Fowler Å, Wickström R. Cytokine and chemokine expression in CSF may differentiate viral and autoimmune NMDAR encephalitis in children. J Child Neurol. 2016;31(13):1450–1456. [DOI] [PubMed] [Google Scholar]
- 47. Laurence A, Tato CM, Davidson TS, et al. . Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26(3):371–381. [DOI] [PubMed] [Google Scholar]
- 48. Kimura A, Kishimoto T. IL-6: Regulator of Treg/Th17 balance. Eur J Immunol. 2010;40(7):1830–1835. [DOI] [PubMed] [Google Scholar]
- 49. Muñiz-Castrillo S, Haesebaert J, Thomas L, et al. . Clinical and prognostic value of immunogenetic characteristics in Anti-LGI1 encephalitis. Neurol Neuroimmunol Neuroinflam. 2021;8(3):e974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Debanne D, El Far O. Pre- and postsynaptic effects of LGI1 autoantibodies in a murine model of limbic encephalitis. Brain. 2018;141(11):3092–3095. [DOI] [PubMed] [Google Scholar]
- 51. Dalmau J, Geis C, Graus F. Autoantibodies to synaptic receptors and neuronal cell surface proteins in autoimmune diseases of the central nervous system. Physiol Rev. 2017;97(2):839–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Khan NL, Jeffree MA, Good C, Macleod W, Al-Sarraj S. Histopathology of VGKC antibody-associated limbic encephalitis. Neurology. 2009;72(19):1703–1705. [DOI] [PubMed] [Google Scholar]
- 53. Park DC, Murman DL, Perry KD, Bruch LA. An autopsy case of limbic encephalitis with voltage-gated potassium channel antibodies. Eur J Neurol. 2007;14(10):e5–6. [DOI] [PubMed] [Google Scholar]
- 54. Dunstan EJ, Winer JB. Autoimmune limbic encephalitis causing fits, rapidly progressive confusion and hyponatraemia. Age and Ageing. 2006;35(5):536–537. [DOI] [PubMed] [Google Scholar]
- 55. Körtvelyessy P, Goihl A, Guttek K, Schraven B, Prüss H, Reinhold D. Serum and CSF cytokine levels mirror different neuroimmunological mechanisms in patients with LGI1 and Caspr2 encephalitis. Cytokine. 2020;135:155226. [DOI] [PubMed] [Google Scholar]
- 56. Levraut M, Bourg V, Capet N, et al. . Cerebrospinal fluid IL-17A could predict acute disease severity in Non-NMDA-receptor autoimmune encephalitis. Front Immunol. 2021;12:673021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lin YT, Yang X, Lv JW, Liu XW, Wang SJ. CXCL13 is a biomarker of anti-leucine-rich Glioma-Inactivated Protein 1 encephalitis patients. Neuropsychiatr Dis Treat. 2019;15:2909–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Roberts WK, Blachère NE, Frank MO, Dousmanis A, Ransohoff RM, Darnell RB. A destructive feedback loop mediated by CXCL10 in central nervous system inflammatory disease. Ann Neurol. 2015;78(4):619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jiang JX, Fewings N, Dervish S, et al. . Novel surrogate markers of CNS inflammation in CSF in the diagnosis of autoimmune encephalitis. Front Neurol. 2020;10:1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ciano-Petersen NL, Muñiz-Castrillo S, Vogrig A, Joubert B, Honnorat J. Immunomodulation in the acute phase of autoimmune encephalitis. Revue Neurologique. 2022;178(1-2):34–47. [DOI] [PubMed] [Google Scholar]
- 61. Abboud H, Probasco JC, Irani S, et al. . Autoimmune encephalitis: Proposed best practice recommendations for diagnosis and acute management. J Neurol Neurosurg Psychiatry. 2021;92(7):757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Titulaer MJ, McCracken L, Gabilondo I, et al. . Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: An observational cohort study. Lancet Neurol. 2013;12(2):157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lee WJ, Lee ST, Byun JI, et al. . Rituximab treatment for autoimmune limbic encephalitis in an institutional cohort. Neurology. 2016;86(18):1683–1691. [DOI] [PubMed] [Google Scholar]
- 64. Blackburn KM, Denney DA, Hopkins SC, Vernino SA. Low recruitment in a double-blind, placebo-controlled trial of ocrelizumab for autoimmune encephalitis: A case series and review of lessons learned. Neurol Ther. 2022;11(2):893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen D, Ireland SJ, Davis LS, et al. . Autoreactive CD19 + CD20—Plasma Cells contribute to disease severity of experimental autoimmune encephalomyelitis. J Immunol. 2016;196(4):1541–1549. [DOI] [PubMed] [Google Scholar]
- 66. Winter O, Dame C, Jundt F, Hiepe F. Pathogenic long-lived plasma cells and their survival niches in autoimmunity, malignancy, and allergy. J Immunol. 2012;189(11):5105–5111. [DOI] [PubMed] [Google Scholar]
- 67. Hiepe F, Dörner T, Hauser AE, Hoyer BF, Mei H, Radbruch A. Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat Rev Rheumatol. 2011;7(3):170–178. [DOI] [PubMed] [Google Scholar]
- 68. Golombeck KS, Bönte K, Mönig C, et al. . Evidence of a pathogenic role for CD8+ T cells in anti-GABAB receptor limbic encephalitis. Neurol Neuroimmunol NeuroInflamm. 2016;3(3):e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kornau HC, Kreye J, Stumpf A, et al. . Human cerebrospinal fluid monoclonal LGI1 autoantibodies increase neuronal excitability. Ann Neurol. 2020;87(3):405–418. [DOI] [PubMed] [Google Scholar]
- 70. Cree BAC, Bennett JL, Kim HJ, et al. . Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): A double-blind, randomised placebo-controlled phase 2/3 trial. The Lancet. 2019;394(10206):1352–1363. [DOI] [PubMed] [Google Scholar]
- 71. Mitsdoerffer M, Di Liberto G, Dötsch S, et al. . Formation and immunomodulatory function of meningeal B cell aggregates in progressive CNS autoimmunity. Brain. 2021;144(6):1697–1710. [DOI] [PubMed] [Google Scholar]
- 72. Robillard N, Wuillème S, Moreau P, Béné MC. Immunophenotype of normal and myelomatous plasma-cell subsets. Front Immunol. 2014;5:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ratuszny D, Skripuletz T, Wegner F, et al. . Case report: Daratumumab in a patient with severe refractory Anti-NMDA receptor encephalitis. Front Neurol. 2020;11:602102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Scheibe F, Ostendorf L, Reincke SM, et al. . Daratumumab treatment for therapy-refractory anti-CASPR2 encephalitis. J Neurol. 2020;267(2):317–323. [DOI] [PubMed] [Google Scholar]
- 75. Gupta S, Wang W, Hayek SS, et al. . Association between early treatment with tocilizumab and mortality among critically Ill Patients with COVID-19. JAMA Intern Med. 2021;181(1):41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Brod SA. Review of approved NMO therapies based on mechanism of action, efficacy and long-term effects. Mult Scler Relat Disord. 2020;46:102538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lee WJ, Lee ST, Moon J, et al. . Tocilizumab in autoimmune encephalitis refractory to rituximab: An institutional cohort study. Neurotherapeutics. 2016;13(4):824–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Randell RL, Adams AV., Van Mater H. Tocilizumab in refractory autoimmune encephalitis: A series of pediatric cases. Pediatr Neurol. 2018;86:66–68. [DOI] [PubMed] [Google Scholar]
- 79. Jaafar F, Haddad L, Koleilat N, Sharara-Chami R, Shbarou R. Super refractory status epilepticus secondary to anti-GAD antibody encephalitis successfully treated with aggressive immunotherapy. Epilepsy Behav Rep. 2020;14:100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lee WJ, Lee ST, Shin YW, et al. . Teratoma removal, steroid, IVIG, rituximab and tocilizumab (T-SIRT) in Anti-NMDAR encephalitis. Neurotherapeutics. 2021;18(1):474–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nosadini M, Thomas T, Eyre M, et al. . International consensus recommendations for the treatment of pediatric NMDAR antibody encephalitis. Neurol Neuroimmunol Neuroinflamm. 2021;8(5):e1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Traboulsee A, Greenberg BM, Bennett JL, et al. . Safety and efficacy of satralizumab monotherapy in neuromyelitis optica spectrum disorder: A randomised, double-blind, multicentre, placebo-controlled phase 3 trial. Lancet Neurol. 2020;19(5):402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Blair HA, Duggan ST. Belimumab: A review in systemic lupus erythematosus. Drugs. 2018;78(3):355–366. [DOI] [PubMed] [Google Scholar]
- 84. Hewett K, Sanders DB, Grove RA, et al. . Randomized study of adjunctive belimumab in participants with generalized myasthenia gravis. Neurology. 2018;90(16):E1425–E1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dalakas MC. Immunotherapy in myasthenia gravis in the era of biologics. Nat Rev Neurol. 2019;15(2):113–124. [DOI] [PubMed] [Google Scholar]
- 86. Merrill JT, Shanahan WR, Scheinberg M, Kalunian KC, Wofsy D, Martin RS. Phase III trial results with blisibimod, a selective inhibitor of B-cell activating factor, in subjects with systemic lupus erythematosus (SLE): Results from a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2018;77(6):883–889. [DOI] [PubMed] [Google Scholar]
- 87. Kappos L, Hartung HP, Freedman MS, et al. . Atacicept in multiple sclerosis (ATAMS): A randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Neurol. 2014;13(4):353–363. [DOI] [PubMed] [Google Scholar]
- 88. Wu X, Guo J, Ding R, Lv B, Bi L. CXCL13 blockade attenuates lupus nephritis of MRL/lpr mice. Acta Histochem. 2015;117(8):732–737. [DOI] [PubMed] [Google Scholar]
- 89. Klimatcheva E, Pandina T, Reilly C, et al. . CXCL13 antibody for the treatment of autoimmune disorders. BMC Immunol. 2015;16(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sharma A, Kiripolsky J, Klimatcheva E, et al. . Early BAFF receptor blockade mitigates murine Sjögren’s syndrome: Concomitant targeting of CXCL13 and the BAFF receptor prevents salivary hypofunction. Clinical Immunol. 2016;164:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Patel DD, Bussel JB. Neonatal Fc receptor in human immunity: Function and role in therapeutic intervention. J Allergy Clin Immunol. 2020;146(3):467–478. [DOI] [PubMed] [Google Scholar]
- 92. Dalakas MC, Spaeth PJ. The importance of FcRn in neuro-immunotherapies: From IgG catabolism, FCGRT gene polymorphisms, IVIg dosing and efficiency to specific FcRn inhibitors. Ther Adv Neurol Disord. 2021;14:1756286421997381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Howard JF Jr, Bril V, Vu T, et al. . Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): A multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2021; 20(7):526–536. [DOI] [PubMed] [Google Scholar]
- 94. Dalakas MC. Progress in the therapy of myasthenia gravis: Getting closer to effective targeted immunotherapies. Curr Opin Neurol. 2020;33(5):545–552. [DOI] [PubMed] [Google Scholar]
- 95. Bril V, Benatar M, Andersen H, et al. . Efficacy and safety of rozanolixizumab in moderate to severe generalized myasthenia gravis: A phase 2 randomized control trial. Neurology. 2021;96(6):e853–e865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Cooper PR, Ciambrone GJ, Kliwinski CM, et al. . Efflux of monoclonal antibodies from rat brain by neonatal Fc receptor, FcRn. Brain Res. 2013;1534:13–21. [DOI] [PubMed] [Google Scholar]
- 97. Dalakas MC, Alexopoulos H, Spaeth PJ. Complement in neurological disorders and emerging complement-targeted therapeutics. Nat Rev Neurol. 2020;16(11):601–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Pittock SJ, Berthele A, Fujihara K, et al. . Eculizumab in aquaporin-4–positive neuromyelitis optica spectrum disorder. N Engl J Med. 2019;381(7):614–625. [DOI] [PubMed] [Google Scholar]
- 99. Muppidi S, Utsugisawa K, Benatar M, et al. . Long-term safety and efficacy of eculizumab in generalized myasthenia gravis. Muscle Nerve. 2019;60(1):14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Muñiz-Castrillo S, Hedou JJ, Ambati A, et al. . Distinctive clinical presentation and pathogenic specificities of anti-AK5 encephalitis. Brain. 2021;144(9):2709–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Körtvelyessy P, Bauer J, Stoppel CM, et al. . Complement-associated neuronal loss in a patient with CASPR2 antibody-associated encephalitis. Neurol Neuroimmunol Neuroinflamm. 2015;2(2):e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Muñiz-Castrillo S, Joubert B, Elsensohn MH, et al. . Anti-CASPR2 clinical phenotypes correlate with HLA and immunological features. J Neurol Neurosurg Psychiatry. 2020;91(10):1076–1084. [DOI] [PubMed] [Google Scholar]
- 103. Wen T, Wang J, Shi Y, Qian H, Liu P. Inhibitors targeting Bruton’s tyrosine kinase in cancers: Drug development advances. Leukemia. 2021;35(2):312–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Rip J, Van EK, Ploeg D, Hendriks RW, Corneth OBJ. The role of Bruton’s Tyrosine Kinase in immune cell signaling and systemic autoimmunity. Crit Rev Immunol. 2018;38(1):17–62. [DOI] [PubMed] [Google Scholar]
- 105. Montalban X, Arnold DL, Weber MS, et al. . Placebo-controlled trial of an Oral BTK inhibitor in multiple sclerosis. N Engl J Med. 2019;380(25):2406–2417. [DOI] [PubMed] [Google Scholar]
- 106. Reich DS, Arnold DL, Vermersch P, et al. . Safety and efficacy of tolebrutinib, an oral brain-penetrant BTK inhibitor, in relapsing multiple sclerosis: A Phase 2b, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2021;20(9):729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Shin YW, Lee ST, Park KI, et al. . Treatment strategies for autoimmune encephalitis. Ther Adv Neurol Disord. 2017;11:1756285617722347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sell J, Haselmann H, Hallermann S, Hust M, Geis C. Autoimmune encephalitis: Novel therapeutic targets at the preclinical level. Expert Opin Ther Targets. 2021;25(1):37–47. [DOI] [PubMed] [Google Scholar]
- 109. Lovas S, Varga G, Farkas P, et al. . Real-world data on the efficacy and safety of daratumumab treatment in Hungarian relapsed/refractory multiple myeloma patients. Int J Hematol. 2019;110(5):559–565. [DOI] [PubMed] [Google Scholar]
- 110. Behrendt V, Krogias C, Reinacher-Schick A, Gold R, Kleiter I. Bortezomib treatment for patients with anti-N-methyl-D-aspartate receptor encephalitis. JAMA Neurol. 2016;73(10):1251–1253. [DOI] [PubMed] [Google Scholar]
- 111. Keddie S, Crisp SJ, Blackaby J, et al. . Plasma cell depletion with bortezomib in the treatment of refractory N-methyl-d-aspartate (NMDA) receptor antibody encephalitis. Rational developments in neuroimmunological treatment. Eur J Neurol. 2018;25(11):1384–1388. [DOI] [PubMed] [Google Scholar]
- 112. Cordani R, Micalizzi C, Giacomini T, et al. . Bortezomib-responsive refractory Anti-N-Methyl-D-Aspartate receptor encephalitis. Pediatr Neurol. 2020;103:61–64. [DOI] [PubMed] [Google Scholar]
- 113. Turnbull MT, Siegel JL, Becker TL, Stephens AJ, Lopez-Chiriboga AS, Freeman WD. Early bortezomib therapy for refractory Anti-NMDA receptor encephalitis. Front Neurol. 2020;11:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Yang XZ, Zhu HD, Ren HT, et al. . Utility and safety of intrathecal methotrexate treatment in severe Anti-N-methyl-D-aspartate receptor encephalitis: A pilot study. Chin Med J. 2018;131(2):156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wang D, Wu Y, Ji Z, et al. . A refractory anti-NMDA receptor encephalitis successfully treated by bilateral salpingo-oophorectomy and intrathecal injection of methotrexate and dexamethasone: A case report. J Int Med Res. 2020;48(10):300060520925666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tatencloux S, Chretien P, Rogemond V, Honnorat J, Tardieu M, Deiva K. Intrathecal treatment of anti-N-Methyl-d-aspartate receptor encephalitis in children. Dev Med Child Neurol. 2015;57(1):95–99. [DOI] [PubMed] [Google Scholar]
- 117. Coles AJ, Twyman CL, Arnold DL, et al. . Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: A randomised controlled phase 3 trial. Lancet. 2012;380.(9856): 1829–1839. [DOI] [PubMed] [Google Scholar]
- 118. Buscarinu MC, Fornasiero A, Pellicciari G, et al. . Autoimmune encephalitis and CSF anti-glur3 antibodies in an MS patient after alemtuzumab treatment. Brain Sci. 2019;9(11):299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Maniscalco GT, Mariotto S, Höftberger R, et al. . GABAA receptor autoimmunity after alemtuzumab treatment for multiple sclerosis. Neurology. 2020;95(9):399–401. [DOI] [PubMed] [Google Scholar]
- 120. Gelfand JM, Bradshaw MJ, Stern BJ, et al. . Infliximab for the treatment of CNS Sarcoidosis a multi-institutional series. Neurology. 2017;89(20):2092–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kemanetzoglou E, Andreadou E. CNS demyelination with TNF-α blockers. Curr Neurol Neurosci Rep. 2017;17(4):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Locatelli F, Jordan MB, Allen C, et al. . Emapalumab in children with primary hemophagocytic lymphohistiocytosis. N Engl J Med. 2020;382(19):1811–1822. [DOI] [PubMed] [Google Scholar]
- 123. Fasching P, Stradner M, Graninger W, Dejaco C, Fessler J. Therapeutic potential of targeting the Th17/Treg axis in autoimmune disorders. Molecules. 2017;22(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Jesus AA, Goldbach-Mansky R. IL-1 blockade in autoinflammatory syndromes. Ann Rev Med. 2014;65:223–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Taraschenko O, Fox HS, Zekeridou A, et al. . Seizures and memory impairment induced by patient-derived anti-N-methyl-D-aspartate receptor antibodies in mice are attenuated by anakinra, an interleukin-1 receptor antagonist. Epilepsia. 2021;62(3):671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Specchio N, Pietrafusa N. New-onset refractory status epilepticus and febrile infection-related epilepsy syndrome. Dev Med Child Neurol. 2020;62(8):897–905. [DOI] [PubMed] [Google Scholar]
- 127. Lai YC, Muscal E, Wells E, et al. . Anakinra usage in febrile infection related epilepsy syndrome: An international cohort. Ann Clin Transl Neurol. 2020;7(12):2467–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Martins F, Sykiotis GP, Maillard M, et al. . New therapeutic perspectives to manage refractory immune checkpoint-related toxicities. Lancet Oncol. 2019;20(1):e54–e64. [DOI] [PubMed] [Google Scholar]
- 129. Kaplanov I, Carmi Y, Kornetsky R, et al. . Blocking IL-1β reverses the immunosuppression in mouse breast cancer and synergizes with anti–PD-1 for tumor abrogation. Proc Natl Acad Sci U S A. 2019;116(4):1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kim J, Chuang HC, Wolf NK, Nicolai CJ, Raulet DH, Saijo KBD. Tumor-induced disruption of the blood-brain barrier promotes host death. Dev Cell. 2021;56(19):2712–2721.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Havrdová E, Belova A, Goloborodko A, et al. . Activity of secukinumab, an anti-IL-17A antibody, on brain lesions in RRMS: Results from a randomized, proof-of-concept study. J Neurol. 2016;263(7):1287–1295. [DOI] [PubMed] [Google Scholar]
- 132. Esfahani K, Miller WH. Reversal of autoimmune toxicity and loss of tumor response by Interleukin-17 Blockade. N Engl J Med. 2017;376(20):1989–1991. [DOI] [PubMed] [Google Scholar]
- 133. Florek AG, Nardone B, Thareja S, Tran G, Giles FJ, West DP. Malignancies and ustekinumab: An analysis of the U.S. food and drug administration adverse event reporting system and the European Union drug regulating authorities pharmacovigilance database. Br J Dermatol. 2017;177(5):e220–e221. [DOI] [PubMed] [Google Scholar]
- 134. Wei H, Li B, Sun A, Guo F. Interleukin-10 family cytokines immunobiology and structure. Adv Exp Med Biol. 2019;11(72):79–96. [DOI] [PubMed] [Google Scholar]
- 135. Lim JA, Lee ST, Moon J, et al. . New feasible treatment for refractory autoimmune encephalitis: Low-dose interleukin-2. J Neuroimmunol. 2016;299:107–111. [DOI] [PubMed] [Google Scholar]
- 136. Yu A, Zhu L, Altman NH, Malek TR. A low Interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity. 2009;30(2):204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lin YC, Winokur P, Blake A, Wu T, Romm E, Bielekova B. Daclizumab reverses intrathecal immune cell abnormalities in multiple sclerosis. Ann Clin Transl Neurol. 2015;2(5):445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Seif F, Khoshmirsafa M, Aazami H, Mohsenzadegan M, Sedighi G, Bahar M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Jang Y, Lee WJ, Lee HS, Chu K, Lee SK, Lee ST. Tofacitinib treatment for refractory autoimmune encephalitis. Epilepsia. 2021;62(4):e53–e59. [DOI] [PubMed] [Google Scholar]
- 140. Hodecker SC, Stellmann JP, Rosenkranz SC, et al. . Ruxolitinib treatment in a patient with neuromyelitis optica: A case report. Neurol Neuroimmunol Neuroinflamm. 2017;4(2):e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Alboini PE, Evoli A, Damato V, Iorio R, Bartoccioni E. Remission of myasthenia gravis with MuSK antibodies during ruxolitinib treatment. Muscle Nerve. 2017;55(3):E12–E13. [DOI] [PubMed] [Google Scholar]
- 142. Picca A, Valyraki N, Birzu C, et al. . Anti–Interleukin-6 and Janus Kinase inhibitors for severe neurologic toxicity of checkpoint inhibitors. Neurol Neuroimmunol Neuroinflamm. 2021;8(6):e1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Martins F, Obeid M. Personalized treatment of immune-related adverse events—balance is required. Nat Rev Clin Oncol. 2020;17:517. [DOI] [PubMed] [Google Scholar]
- 144. Shin DS, Zaretsky JM, Escuin-Ordinas H, et al. . Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 2017;7(2):188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Tzeng HT, Chyuan IT, Lai JH. Targeting the JAK-STAT pathway in autoimmune diseases and cancers: A focus on molecular mechanisms and therapeutic potential. Biochem Pharmacol. 2021;193:114760. [DOI] [PubMed] [Google Scholar]
- 146. Mellerård J, Edström M, Vrethem M, Ernerudh J, Dahle C. Natalizumab treatment in multiple sclerosis: Marked decline of chemokines and cytokines in cerebrospinal fluid. Mult Scler. 2010;16(2):208–217. [DOI] [PubMed] [Google Scholar]
- 147. Bastiaansen AEM, De Jongste AHC, De Bruijn MAAM, et al. . Phase II trial of natalizumab for the treatment of anti-Hu associated paraneoplastic neurological syndromes. Neurooncol Adv. 2021;3(1):vdab145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Hottinger AF, De Micheli R, Guido V, Karampera A, Hagmann P, Du Pasquier R. Natalizumab may control immune checkpoint inhibitor-induced limbic encephalitis. Neurol Neuroimmunol Neuroinflamm. 2018;5(2):e439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Lorenzetti R, Janowska I, Smulski CR, et al. . Abatacept modulates CD80 and CD86 expression and memory formation in human B-cells. J Autoimmun. 2019;101:145–152. [DOI] [PubMed] [Google Scholar]
- 150. Salem JE, Allenbach Y, Vozy A, et al. . Abatacept for severe immune checkpoint inhibitor–associated myocarditis. N Engl J Med. 2019;380(24):2377–2379. [DOI] [PubMed] [Google Scholar]
- 151. Liu S, Chan J, Brinc D, et al. . Immune checkpoint inhibitor-associated myocarditis with persistent troponin elevation despite abatacept and prolonged immunosuppression. JACC Cardio Oncol. 2020;2(5):800–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Hébert J, Riche B, Vogrig A, et al. . Epidemiology of paraneoplastic neurologic syndromes and autoimmune encephalitides in France. Neurol Neuroimmunol Neuroinflamm. 2020;7(6):e883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kothur K, Wienholt L, Brilot F, Dale RC. CSF cytokines/chemokines as biomarkers in neuroinflammatory CNS disorders: A systematic review. Cytokine. 2016;77:227–237. [DOI] [PubMed] [Google Scholar]
- 154. Liu C, Chu D, Kalantar-Zadeh K, George J, Young HA, Liu G. Cytokines: From clinical significance to quantification. Adv Sci. 2021;8(15):e2004433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Accessed 11 September 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103705s5367s5388lbl.pdf.
- 156. Accessed 11 September 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761142s000lbl.pdf.
- 157. Accessed 11 September 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021602s040lbl.pdf.
- 158. Accessed 11 September 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761036s013lbl.pdf.
- 159. Accessed 11 September 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/BLA125319_858687lbl.pdf.
- 160. Accessed 11 September 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103950s5136lbl.pdf.
- 161. Accessed 11 September 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761029s007lbl.pdf.
- 162. Accessed 11 September 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125276s114lbl.pdf.
- 163. Accessed 11 September 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761149s000lbl.pdf.
- 164. Accessed 11 September 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125504s013lbl.pdf.
- 165. Accessed 11 September 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125521s004lbl.pdf.
- 166. Accessed 11 September 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf.
- 167. Accessed 11 September 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/125370s016lbl.pdf.
- 168. Accessed 11 September 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125057s410lbl.pdf.
- 169. Accessed 11 September 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125160s270lbl.pdf.
- 170. Accessed 11 September 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/103772s5359lbl.pdf.
- 171. Accessed 11 September 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103795s5503lbl.pdf.
- 172. Accessed 11 September 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125166s172lbl.pdf.
- 173. Accessed 11 September 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761108s000lbl.pdf.
- 174. Howard JF, Nowak RJ, Wolfe GI, et al. . Clinical effects of the self-administered subcutaneous complement inhibitor Zilucoplan in patients with moderate to severe generalized Myasthenia Gravis: Results of a Phase 2 randomized, double-blind, placebo-controlled, multicenter clinical trial. JAMA Neurol. 2020;77(5):582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Accessed 11 September 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203214s018lbl.pdf.
- 176. Accessed 11 September 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202192lbl.pdf.
- 177. Accessed 11 September 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/125104s0576lbl.pdf.
- 178. Accessed 11 September 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125118s171lbl.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.