Abstract
Aim
Metabolic syndrome (MetS) coexists with the occurrence and even death of cardiovascular disease and diabetes mellitus. It is essential to study the factors in the dynamic progression of MetS in the interest of prevention and control.
Purpose
The aim of this study was to analyze the dynamic progression of Mets and explore the potential factors influencing the progression or reversal of MetS.
Patients and Methods
This study involved 5581 individuals from two waves of the China Health and Retirement Longitudinal Study: 2011 and 2015. A multistate Markov model containing 4 states (free of metabolic disorder (FMD), mild metabolic disorder (MMD), severe metabolic disorder (SMD) and MetS) was adopted to study the dynamic progression of MetS and its influencing factors.
Results
After follow-up, a total of 2862 cases (50.28% of the total number) had disease state transition. The intensity of transition from MetS to SMD is the same as that from SMD to MMD, and is greater than that from MMD to Mets (0.06 vs 0.05). For the MetS state, a mean of 1/0.08=12.5 years was spent in the MetS state before recovery. The exercise, smoke, drink, BMI level, hyperuricemia had statistically significant effects on progression of MetS status (P<0.05). The obesity or overweight, little exercise, smoke, drink and hyperuricemia increased the risk of forward progression of MetS disease status. There were significant nonmodifiable (age, gender) and modifiable factors (exercise, drink, BMI level, or high HbA1c) associated with reversion of MetS state.
Conclusion
The likelihood of progression from MMD to MetS is less likely than that of reversion from MetS to SMD and SMD to MMD. Old females were more resistant to recover from worse states than males. Prevention and intervention measures should be adopted early when MMD or SMD onset occurs.
Keywords: metabolic syndrome, multi-state Markov model, forward progression, backward reversal
Introduction
Metabolic syndrome (MetS) is a cluster of syndromes with central obesity, hypertension, hyperglycemia and dyslipidemia as metabolic disorders.1,2 Its essence of occurrence is the abnormal aggregation of a variety of disease states and potential risk factors related to cardiovascular disease. These potential risk factors cooperate with disease states and seriously threaten human health.3 Studies have demonstrated this disorder is in connection with the occurrence of cardiovascular disease, type 2 diabetes, and cancer, as well as the occurrence of death from heart disease and all-cause mortality.4,5 As one of the increasingly serious global public health challenge the prevention and control of MetS has become an urgent task today.6
Based on the definition of the Joint Interim Statement (JIS),7 three of the five components (central obesity, hypertension, hyperglycemia, hypertriglyceride, low high density lipoprotein cholesterol) are fulfilled and will be diagnosed as MetS. Its occurrence and development experienced several states: health state→one abnormal component→two abnormal components→Mets. Health and different components can form 31 different disease states. Depending on their gene, environment and personal lifestyle, one can have a different combination of the syndrome components at any given time and one can transit from one state to another.8 The occurrence and development of MetS is an extremely complex and difficult to clearly define dynamic process.9 Among the existing studies have illustrated the natural histories of Mets with Markov model.8–12 The model can accurately capture the transfer of various states of the disease under discrete stages, including positive progression and reverse reversal,13 but these studies have provided contradictory findings and mainly focused on the natural history of MetS of different gender and age. In view of the fact that potential factors such as lifestyle and health status may play a key role in the development and reversal of MetS, further research on MetS disease progression trajectory and its potential factors adapted to the actual clinical situation remains to be carried out.
Thus, based on the high quality nationally representative sample of Chinese residents of China Health retirement longitudinal study (CHARLS), this study constructs a Markov model of four states to fit the dynamic development process of MetS, screen and analyze the impact of potential factors on its dynamic progression, so as to provide evidence information for delaying and reversing MetS and related chronic diseases. In this way, exploring the feasibility and application value of Markov models in disease dynamic progression and risk assessment.
Materials and Methods
Data Source
Data for this study were obtained from the two waves of the CHARLS survey conducted by the National School of Development at Peking University in 2011 and 2015. The study is an ongoing nationally representative longitudinal survey in China.14 A design of four-stage, stratified, cluster sampling was applied. The baseline of CHARLS was conducted between 2011 and 2012. The blood samples were collected in 2011 and 2015 through a standardized process which was described in detail elsewhere (http://charls.pku.edu.cn/).
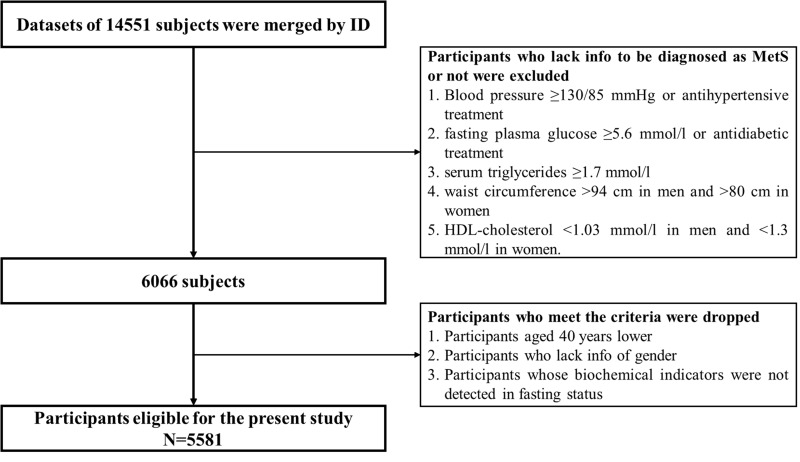
This article selected the national survey data in 2011–2015 to scan the dynamic development process and associated factors of MetS by downloading modules comprising Biomarker, Blood, Demographic background and Health status. After merging these modules by ID, further data cleansing was performed. After eliminating the untraceable samples and any missing key information, we finally get 5581 valid cases (Figure 1). The CHARLS study was approved by the Biomedical Ethics Review Committee of Peking University. All participants gave written informed consent before participation.
Figure 1.
Flow chart for participants selection.
Variables
This study selected demographic, lifestyle and health status as independent variables to explore the impact of factors on the dynamic disease progression of MetS.
Demographic: ① Gender; ② Age: 40 ~ 54 years old, 55 ~ 64 years old and ≥65 years old; ③ Marital status: married or other situations (including divorce, widowhood, separation and unmarried); ④ Education: illiterate, primary school, junior middle school and ≥senior high school.
Lifestyle: ① Smoke: define “smoked but quit smoking”, “never smoked” as non-smoking, and “smoked and smoked all the time” as smoking; Drinking: If alcohol was not consumed in the past 1 year (including beer and wine), it was designated as “no alcohol” and the rest was drinking. ③ Exercise: divided into often, occasionally and little; ④ Sleep time: according to the sleep time at night, it is divided into <6 h, 6–8 h and >8 h.
Health status: ① functional loss: measured by the self-care ability of daily living (ADL) scale.15 The scale consists of six independent options and is divided into four levels: “no difficulty = 1”, “difficulty but still can be completed = 2”, “difficulty but need help = 3” and “unable to complete = 4”. The comprehensive score range is 6 ~ 24 points. If the comprehensive score of ADL is greater than 6 points, it means that the overall daily activity function is lost; ② Depression: measured by the Center for Epidemiological Studies Depression Scale-10 (CES-D10) in the CHARLS questionnaire.16 The CES-D10 comprised 10 questions about depression, and the total score was 0 ~ 30. The higher score indicated higher depression. More than 10 points can be considered to be accompanied by depression; ③ Body mass index (BMI) level: weight (kg)/height (m)2. It is divided into: thin (BMI <18.5 kg/m2), normal body weight (18.5kg/m2≤ BMI < 24.0 kg/m2), overweight (24.0kg/m2≤ BMI < 28.0 kg/m2), obesity (BMI ≥28.0 kg/m2); ④ High total cholesterol (TC): TC ≥5.20 mmol/l; ⑤High low density lipoprotein cholesterol (LDL-C): LDL-C ≥3.40 mmol/l; ⑥ Hyperuricemia: male blood uric acid concentration ≥420 μmol/L, female blood uric acid concentration ≥350 μmol/L; ⑦ High glycated hemoglobin (HbA1c): HbA1c ≥6%; ⑧High C-reactive protein (CRP): CRP ≥5 mg/L.
Definition of Metabolic Syndrome
The outcome MetS was based on the definition of the Joint Interim Statement (JIS) was adopted to defined MetS according to the following categories: (1) Hypertension (Blood pressure ≥130/85 mmHg or antihypertensive treatment); (2) Hyperglycemia (fasting plasma glucose ≥5.6 mmol/l or antidiabetic treatment); (3) Hypertriglyceride (serum triglycerides ≥1.7 mmol/l); (4) Central obesity (waist circumference >94 cm in men and >80 cm in women); (5) Low high density lipoprotein cholesterol (low HDL-C) (HDL-C <1.03 mmol/l in men and <1.3 mmol/l in women). Three of the mentioned five criteria should be fulfilled for MetS.7
The Markov Model
The Markov model as a recognized method for simulating the natural progression of chronic diseases can simultaneously consider all states and the transition time between states to fit the dynamic development process of the disease. A Markov process is a random model for describing a sequence of probable events in which the probability of each event depends only on the present time, not preceding event. It can be fully described by 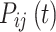 which is the probability that a system is in
which is the probability that a system is in 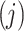 state at time
state at time 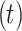 , provided that the process starts from time (t = 0) and state
, provided that the process starts from time (t = 0) and state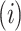 . Hence, when
. Hence, when 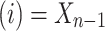 , then one can write a Markov process as follows:8
, then one can write a Markov process as follows:8
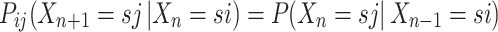 |
Transition intensity refers to the instantaneous transfer risk. 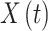 denote an individual’s states at time
denote an individual’s states at time 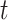 . The transition intensity
. The transition intensity 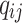 is the instantaneous risk of transitioning from state
is the instantaneous risk of transitioning from state 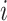 to state
to state 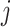 at time
at time 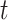 :17
:17
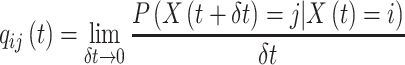 |
All possible intensities form the transition matrix 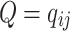 . The diagonal entries are defined by
. The diagonal entries are defined by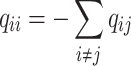 and a single period of occupancy (or sojourn time) in state
and a single period of occupancy (or sojourn time) in state 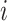 can be calculated by
can be calculated by 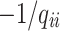 All entries can be estimated by inserting data into the Markov model. The covariates vector
All entries can be estimated by inserting data into the Markov model. The covariates vector 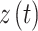 for transition intensity
for transition intensity 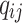 can be investigated by modeling the intensity with the following function:17
can be investigated by modeling the intensity with the following function:17
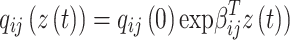 |
Where 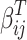 is the coefficient vector representing the effects of covariates on the transition from state
is the coefficient vector representing the effects of covariates on the transition from state 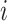 to state
to state 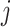 and can be interpreted similarly to those in the ordinary Cox regression model in terms of hazard ratios.
and can be interpreted similarly to those in the ordinary Cox regression model in terms of hazard ratios.
Four-State Markov Model
In this study, the Markov model included four states: free of metabolic disorder (FMD, with no abnormal metabolic factors), mild metabolic disorder (MMD, with 1 abnormal metabolic factor), severe metabolic disorder (SMD, with 2 abnormal metabolic factors), metabolic syndrome (MetS). The MMD state was defined as the state in which any one isolated component occurred simultaneously. The SMD state was defined as the state in which any two isolated components occurred simultaneously. These four states are mutually exclusive, and there was no absorbing state. The Markov model in this study is a reversible multistate model because transitions between any two states were permitted.
Statistical Analysis
Data were cleaned and managed with Excel 2016. Percentages were used to describe discrete variables. Transition intensities between states and mean sojourn time in each state were estimated. The covariates in the Markov model include demographic, lifestyle and health status. The effects of covariates on forward and backward transitions of MetS states were assessed. First, univariate models were used to determine the effect of each factor. Second, significant factors in the univariate models were entered into the multivariate model.
Results
Population Characteristics
A total of 5581 individuals were included in this study, and the average length of follow-up was 4 years. The demographic, lifestyle and health status variables of the individuals at baseline are summarized in Table 1.
Table 1.
Baseline Information of 5581 Subjects
| Variables | Group | Definition | n(%) |
|---|---|---|---|
| Gender | Female | 1 | 3481(62.4) |
| Male | 2 | 2100(37.6) | |
| Age(year) | 40~ | 1 | 2015(36.1) |
| 55~ | 2 | 2192(39.3) | |
| ≥65 | 3 | 1374(24.6) | |
| Marital status | Married | 1 | 4931(88.4) |
| Other | 2 | 650(11.6) | |
| Education | Illiteracy | 1 | 2790(50.0) |
| Primary school | 2 | 1232(22.1) | |
| Junior high school | 3 | 1057(18.9) | |
| ≥Senior high school | 4 | 502(9.0) | |
| Exercise | Often | 1 | 1718(30.8) |
| Occasionally | 2 | 2139(38.3) | |
| Little | 3 | 1724(30.9) | |
| Sleep time (h) | <6 | 1 | 1674(30.0) |
| 6~8 | 2 | 2285(40.9) | |
| ≥8 | 3 | 1622(29.1) | |
| BMI level | Normal | 1 | 2782(49.8) |
| Thin | 2 | 357(6.4) | |
| Overweight/Obesity | 3 | 2442(43.8) | |
| Smoke | No | 0 | 4157(74.5) |
| Yes | 1 | 1424(25.5) | |
| Drink | No | 0 | 3753(67.2) |
| Yes | 1 | 1828(32.8) | |
| Depression | No | 0 | 3385(60.7) |
| Yes | 1 | 2196(39.3) | |
| Functional loss | No | 0 | 4200(75.3) |
| Yes | 1 | 1381(24.7) | |
| Hyperuricemia | No | 0 | 5175(92.7) |
| Yes | 1 | 406(7.3) | |
| High TC | No | 0 | 4404(78.9) |
| Yes | 1 | 1177(21.1) | |
| High LDL-C | No | 0 | 3386(60.7) |
| Yes | 1 | 2195(39.3) | |
| High HbA1c | No | 0 | 5196(93.1) |
| Yes | 1 | 385(6.9) | |
| High CRP | No | 0 | 4589(91.8) |
| Yes | 1 | 408(8.2) |
Abbreviations: BMI, body Mass Index; TC, total cholesterol; LDL-C, low density lipoprotein cholesterol; HbA1c, glycated hemoglobin; CRP, C-reactive protein.
Longitudinal Change of MetS States
Longitudinal change of MetS states between baseline and follow-up is displayed in Table 2 and Figure 2. After follow-up investigation, a total of 2862 cases (50.28% of the total number) had disease state transfer. One thousand three hundred and nineteen cases (46.09% of the changed number) had reverse improvement of MetS, of which 142 cases (10.77% of the improved number) had changed from MMD to FMD, the improvement rate of MMD was 11.98%, that of SMD was 28.24%, and that of MetS was 31.07%; Within four years, 1543 states of MetS forward developed (53.91% of the changed number), the development rate of FMD was 74.58%, that of MMD was 52.58%, and that of SMD was 39.21%.
Table 2.
Transition Frequency Between MetS Disease States During Follow-Up
| Original States | Follow-Up States | ||||
|---|---|---|---|---|---|
| FMD | MMD | SMD | MetS | Total | |
| FMD | 121(25.42) | 188(39.50) | 114(23.95) | 53(11.13) | 476 |
| MMD | 142(11.98) | 420(35.44) | 378(31.90) | 245(20.68) | 1185 |
| SMD | 67(4.65) | 340(23.59) | 469(32.55) | 565(39.21) | 1441 |
| MetS | 22(0.89) | 197(7.95) | 551(22.23) | 1709(68.93) | 2479 |
| Total | 352 | 1145 | 1512 | 2572 | 5581 |
Abbreviations: FMD, free of metabolic disorder; MMD, mild metabolic disorder; SMD, severe metabolic disorder; MetS, metabolic syndrome.
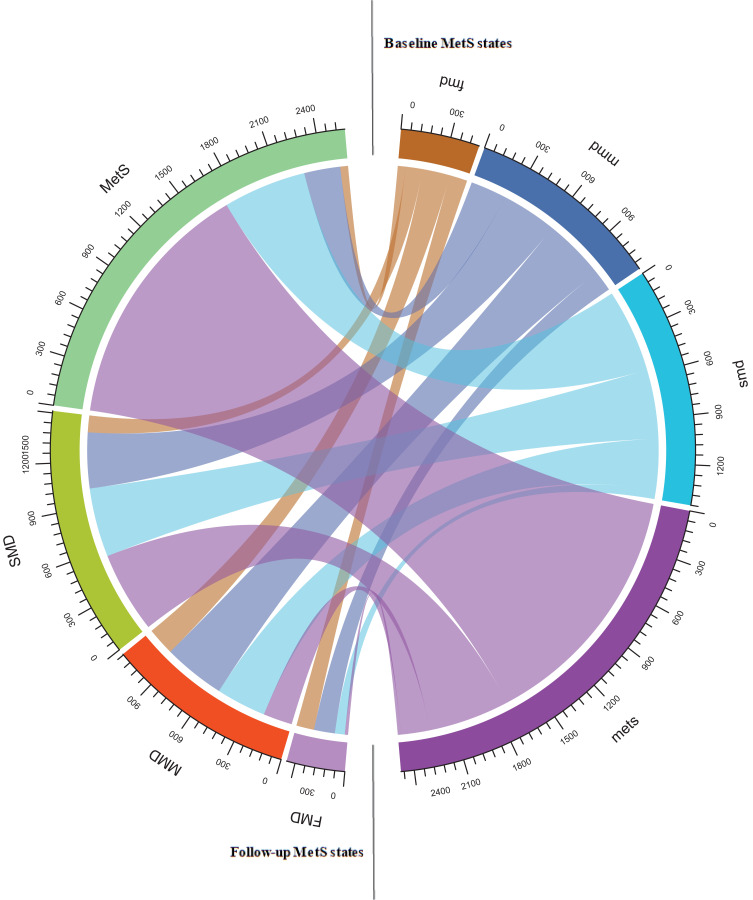
Figure 2.
Transition frequency of MetS states from Baseline to follow-up.
Abbreviations: FMD, free of metabolic disorder; MMD, mild metabolic disorder; SMD, severe metabolic disorder; MetS, metabolic syndrome.
Transition Intensities of States of MetS
The transition intensities estimated by the multistate model are presented in Table 3. The intensity of transition from FMD to MMD and SMD to MetS is the highest, followed by MMD to SMD, which are the forward development of MetS disease state. It is noteworthy that the intensity of transition from MetS to SMD is the same as that from SMD to MMD, and is greater than that from MMD to Mets (0.06 vs 0.05), with a mean of 1/0.16=6.25 years is spent in the MMD state before recovery or progression. For the Mets state, a mean of 1/0.08=12.5 years was spent in the MetS state before recovery.
Table 3.
The Transition Intensities Estimated by the Multistate Model
| Original States | Follow-Up States | Mean Sojourn Time (Years) (95% CI) | |||
|---|---|---|---|---|---|
| FMD | MMD | SMD | MetS | ||
| FMD | −0.19 | 0.10 | 0.06 | 0.03 | 5.26(4.80–5.89) |
| MMD | 0.03 | −0.16 | 0.08 | 0.05 | 6.25(5.74–6.62) |
| SMD | 0.01 | 0.06 | −0.17 | 0.10 | 5.88(5.54–6.28) |
| MetS | <0.01 | 0.02 | 0.06 | −0.08 | 12.50(11.94–13.74) |
Abbreviations: FMD, free of metabolic disorder; MMD, mild metabolic disorder; SMD, severe metabolic disorder; MetS, metabolic syndrome.
Effect of Each Factor on the Dynamic Progression of MetS
Sixteen indicators of demographic (gender, age, education, marital status), lifestyle (smoke, drink, exercise, sleep time) and health status (BMI level, depression, function loss, hyperuricemia, high LDL-C, high HbA1c, high Tc, high CRP) were included in the Markov model to analyze their impact on the forward development and backward reversal of MetS disease states, their definition of relevant factors is shown in Table 1.
The results showed that exercise, smoke, drink, BMI level, function loss, hyperuricemia and high LDL-C had statistically significant effects on the forward transitions of MetS disease status in Table 4. It is worth noting that hyperuricemia, overweight or obesity have an impact on the forward development of FMD→SMD. Among them, the risk of SMD in patients with hyperuricemia is 1.89 times higher than that in patients without disease. Little exercise, smoking, drinking, overweight/obesity and high LDL-C all are risk factors for promoting the development from metabolic disorder to MetS.
Table 4.
Forward Transitions of MetS States by Different Factors: Univariate Models [Hazard Ratios (95% CI)]
| Variables | FMD→MMD | FMD→SMD | FMD→MetS | MMD→SMD | MMD→MetS | SMD→MetS |
|---|---|---|---|---|---|---|
| Gender | 1.26(0.94,1.68) | 1.08(0.75,1.56) | 0.67(0.39,1.15) | 1.02(0.83,1.24) | 1.01(0.79,1.30) | 0.95(0.80,1.12) |
| Age (year) | ||||||
| 55~64 | 1.03(0.75,1.41) | 1.02(0.67,1.54) | 1.35(0.76,2.39) | 1.01(0.80,1.26) | 1.23(0.92,1.63) | 0.98(0.81,1.19) |
| ≥65 | 1.05(0.71,1.56) | 1.15(0.70,1.89) | 0.67(0.27,1.65) | 0.98(0.75,1.29) | 1.04(0.74,1.48) | 1.02(0.82,1.26) |
| Marital status | 0.93(0.49,1.76) | 0.76(0.31,1.86) | 1.35(0.49,3.74) | 0.99(0.71,1.39) | 0.79(0.50,1.25) | 0.83(0.64,1.08) |
| Education | ||||||
| Primary school | 0.99(0.70,1.43) | 1.25(0.79,1.98) | 1.01(0.49,2.07) | 1.14(0.89,1.46) | 1.06(0.77,1.46) | 0.99(0.80,1.23) |
| Junior high school | 0.91(0.62,1.32) | 0.95(0.57,1.58) | 1.44(0.75,2.77) | 1.03(0.78,1.36) | 1.01(0.71,1.44) | 1.16(0.94,1.43) |
| ≥Senior high school | 0.72(0.40,1.28) | 1.57(0.88,2.79) | 0.89(0.31,2.57) | 0.93(0.64,1.35) | 1.37(0.92,2.05) | 1.07(0.80,1.42) |
| Exercise | ||||||
| Occasionally | 1.12(0.83,1.51) | 1.27(0.85,1.89) | 0.76(0.42,1.38) | 0.96(0.77,1.21) | 1.33(0.98,1.80) | 1.10(0.89,1.37) |
| Little | 0.76(0.45,1.25) | 1.33(0.76,2.32) | 1.07(0.49,2.35) | 0.99(0.75,1.30) | 1.91(1.37,2.65)* | 1.56(1.27,1.91)* |
| Sleep time(h) | ||||||
| 6–8 | 1.03(0.72,1.46) | 1.05(0.67,1.63) | 1.26(0.65,2.47) | 1.06(0.84,1.36) | 1.09(0.81,1.47) | 1.01(0.83,1.24) |
| ≥8 | 1.10(0.75,1.60) | 0.95(0.58,1.56) | 1.13(0.54,2.38) | 1.03(0.79,1.34) | 0.92(0.66,1.28) | 1.10(0.89,1.36) |
| Smoke | 1.13(0.84,1.51) | 1.16(0.80,1.70) | 0.60(0.32,1.13) | 1.00(0.81,1.24) | 1.40(1.08,1.80)* | 1.10(0.92,1.32) |
| Drink | 1.13(0.85,1.51) | 1.31(0.91,1.90) | 0.68(0.38,1.19) | 1.08(0.88,1.32) | 1.10(0.85,1.41) | 1.21(1.02,1.43)* |
| Depression | 0.91(0.67,1.22) | 0.74(0.50,1.10) | 1.33(0.77,2.28) | 1.05(0.86,1.29) | 0.97(0.75,1.26) | 1.00(0.85,1.19) |
| Functional loss | 0.69(0.48,0.99)* | 1.40(0.95,2.08) | 1.50(0.85,2.65) | 0.95(0.75,1.20) | 0.93(0.69,1.24) | 0.95(0.79,1.16) |
| Hyperuricemia | 1.19(0.67,2.09) | 1.89(1.04,3.44)* | 0.63(0.15,2.59) | 1.13(0.72,1.77) | 1.51 (0.92,2.46) | 1.22(0.90,1.66) |
| BMI level | ||||||
| Thin | 1.15(0.80,1.64) | 0.52(0.28,0.97)* | 1.04(0.52,2.08) | 0.78(0.55,1.10) | 0.68(0.41,1.10) | 0.67 (0.42,1.07) |
| Overweight/Obesity | 0.83(0.42,1.62) | 1.87(1.05,3.35)* | 0.63(0.15,2.61) | 0.96(0.74,1.24) | 1.79(1.36,2.36)* | 1.77(1.49,2.09)* |
| High LDL-C | 0.89(0.65,1.23) | 0.89(0.59,1.35) | 1.34(0.76,2.34) | 0.97(0.78,1.20) | 1.27 (0.98,1.63) | 1.18(1.01,1.40)* |
| High HbA1c | 0.96(0.43,2.16) | 0.52(0.13,2.10) | 1.14(0.28,4.68) | 1.17(0.72,1.90) | 0.95 (0.49,1.84) | 0.96 (0.64,1.44) |
| High TC | 0.73(0.45,1.19) | 1.13(0.67,1.92) | 1.42(0.69,2.90) | 0.85(0.63,1.16) | 0.90 (0.62,1.31) | 1.07 (0.87,1.30) |
| High CRP | 1.06(0.50,2.26) | 0.99(0.37,2.70) | 1.06(0.26,4.38) | 1.08(0.73,1.61) | 0.85 (0.49,1.46) | 1.11 (0.79,1.55) |
Note: *P < 0.05.
Abbreviations: FMD, free of metabolic disorder; MMD, mild metabolic disorder; SMD, severe metabolic disorder; MetS, metabolic syndrome; BMI, body Mass Index; LDL-C, low density lipoprotein cholesterol; HbA1c, glycated hemoglobin; TC, total cholesterol; CRP, C-reactive protein; CI, confidence interval.
On the other hand, analysis of the reversion from MetS to FMD highlighted that beyond the age of 65 there were hardly any cases. Furthermore, those individuals with thin weight were twice as likely to revert their SMD state. For patients with MetS, male is easier to recover than female. However, individuals with overweight/obesity or high HbA1c symptoms are difficult to recover from MetS, individuals with little exercise or high LDL symptoms are difficult to recover from SMD/MMD (Table 5).
Table 5.
Backward Transitions of MetS States by Different Factors: Univariate Models [Hazard Ratios (95% CI)]
| Variables | MMD→FMD | SMD→FMD | SMD→MMD | MetS→FMD | MetS→MMD | MetS→SMD |
|---|---|---|---|---|---|---|
| Gender | 0.83(0.60,1.16) | 1.40(0.87,2.26) | 1.21(0.98,1.49) | 2.29(0.99,5.31) | 1.73(1.30,2.30)* | 1.32(1.11,1.58)* |
| Age (year) | ||||||
| 55~64 | 1.15(0.80,1.67) | 0.78(0.45,1.34) | 1.12(0.88,1.43) | 0.87(0.37,2.05) | 0.81(0.59,1.13) | 0.93(0.77,1.14) |
| ≥65 | 0.97(0.61,1.53) | 0.77(0.41,1.45) | 1.14(0.86,1.50) | 0.11(0.02,0.89)* | 0.89(0.63,1.27) | 0.94(0.76,1.17) |
| Marital status | 1.05(0.62,1.80) | 1.35(0.71,2.57) | 1.10(0.81,1.50) | 0.31(0.04,2.39) | 0.81(0.51,1.27) | 0.98(0.77,1.27) |
| Education | ||||||
| Primary school | 0.83(0.55,1.26) | 0.91(0.49,1.66) | 1.07(0.82,1.39) | 0.61(0.17,2.17) | 0.99(0.68,1.44) | 1.07(0.86,1.33) |
| Junior high school | 0.56(0.33,1.04) | 0.61(0.29,1.27) | 0.98(0.74,1.30) | 1.21(0.42,3.42) | 1.42(0.99,2.01) | 1.12(0.89,1.40) |
| ≥Senior high school | 0.64(0.33,1.23) | 1.29(0.62,2.69) | 0.88(0.60,1.31) | 1.04(0.23,4.64) | 1.26(0.77,2.06) | 1.16(0.86,1.56) |
| Exercise | ||||||
| Occasionally | 0.87(0.61,1.24) | 1.12(0.66,1.90) | 0.87(0.69,1.11) | 0.82(0.31,2.20) | 0.87(0.61,1.23) | 0.83(0.67,1.03) |
| Little | 0.58(0.34,0.96)* | 0.50(0.25,0.99)* | 0.54(0.41,0.72)* | 0.52(0.17,1.54) | 0.74(0.52,1.05) | 0.87(0.71,1.08) |
| Sleep time (h) | ||||||
| 6–8 | 0.55 (0.37,0.83) | 0.99(0.56,1.75) | 0.93(0.72,1.20) | 0.47(0.18,1.20) | 1.00(0.72,1.40) | 0.90(0.74,1.10) |
| ≥8 | 1.02(0.69,1.50) | 0.86(0.46,1.61) | 0.92(0.70,1.21) | 0.38(0.12,1.19) | 0.95(0.66,1.37) | 0.97(0.79,1.20) |
| Smoke | 0.69 (0.48,1.00) | 0.71(0.39,1.28) | 0.98(0.77,1.25) | 1.30(0.48,3.53) | 1.13(0.80,1.60) | 1.17(0.95,1.44) |
| Drink | 0.86(0.61,1.21) | 0.86(0.52,1.43) | 0.85(0.68,1.07) | 0.87(0.32,2.36) | 0.93(0.67,1.29) | 1.17(0.97,1.41) |
| Depression | 1.00(0.71,1.39) | 0.99(0.61,1.62) | 1.02(0.82,1.2) | 1.11(0.48,2.60) | 1.03(0.77,1.37) | 1.04(0.88,1.24) |
| Functional loss | 1.04(0.72,1.51) | 1.09(0.64,1.88) | 0.98(0.76,1.25) | 0.94(0.35,2.55) | 1.27(0.93,1.73) | 1.01(0.83,1.23) |
| Hyperuricemia | 1.04 (0.49,2.23) | 0.69 (0.22,2.18) | 0.67 (0.40,1.12) | 0.46 (0.06,3.48) | 0.70 (0.40,1.22) | 0.77 (0.56,1.06) |
| BMI level | ||||||
| Thin | 1.46(0.97,2.22)* | 2.21(1.20,4.08)* | 1.44(1.03,2.02)* | 2.03(0.26,15.62) | 1.29(0.57, 2.94) | 1.56(0.91, 2.69) |
| Overweight/Obesity | 0.17(0.07,0.41) | 0.14(0.06,0.35) | 0.40(0.31,0.53)* | 0.38(0.16,0.91)* | 0.35(0.26,0.47)* | 0.74(0.63,0.88)* |
| High LDL-C | 0.87(0.61,1.23) | 0.55(0.32,0.94)* | 0.87(0.70,1.08) | 0.50(0.20,1.28) | 0.80(0.60,1.07) | 0.89(0.75,1.06) |
| High HbA1c | 0.72(0.27,1.94) | 0.33(0.05,2.35) | 0.86(0.49,1.49) | 0.41(0.06,3.03) | 0.46(0.24,0.86)* | 0.42(0.29,0.63)* |
| High TC | 1.10 (0.70,1.74) | 0.96(0.53,1.76) | 0.93 (0.71,1.22) | 0.44 (0.13,1.47) | 0.91(0.66,1.26) | 0.90 (0.75,1.10) |
| High CRP | 0.49(0.20,1.19) | 1.81(0.82,3.98) | 1.05(0.67,1.63) | 1.96 (0.56,6.81) | 0.56 (0.30,1.06) | 0.77(0.56,1.07) |
Note: *P < 0.05.
Abbreviations: FMD, free of metabolic disorder; MMD, mild metabolic disorder; SMD, severe metabolic disorder; MetS, metabolic syndrome; BMI, body Mass Index; LDL-C, low density lipoprotein cholesterol; HbA1c, glycated hemoglobin; TC, total cholesterol; CRP, C-reactive protein; CI, confidence interval.
Influence of Multiple Factors on the Dynamic Progress of MetS Under the Joint Action of Multiple Factors
A multivariate analysis was carried out to include the variables that had been significant in the univariate models. Table 6 shows that obesity or overweight and hyperuricemia increased the risk of SMD in FMD. Hyperuricemia increased the risk of positive progression of FMD by 2.01 times, and obesity or overweight increased the risk by 1.93 times; Smoking, obesity or overweight and less exercise have an impact on the progress of MMD→MetS, which are the influencing factors to promote the development of MMD into MetS; Alcohol consumption, obesity or overweight, and less exercise increased the risk of MetS in SMD. In addition, male with SMD were less likely to develop Mets than female.
Table 6.
Forward and Backward Transitions of MetS States by Different Factors: Multivariate Models [Hazard Ratios (95% CI)]
| Variables | Forward Transitions | Backward Transitions | |||||
|---|---|---|---|---|---|---|---|
| FMD→SMD | MMD→MetS | SMD→MetS | SMD→FMD | SMD→MMD | MetS→MMD | MetS→SMD | |
| Gender | 0.74(0.42,1.32) | 0.74(0.53,1.05) | 0.75(0.59,0.95)* | 1.93(1.03,3.64)* | 1.40(1.05,1.86)* | 2.35(1.60,3.44)* | 1.34(1.04,1.72)* |
| Age | |||||||
| 55~64 | 1.22(0.77,1.91) | 1.31(0.97,1.76) | 1.08(0.88,1.32) | 0.64(0.36,1.15) | 1.02(0.79,1.32) | 0.77(0.55,1.09) | 0.93(0.76,1.15) |
| ≥65 | 1.33(0.77,2.28) | 1.18(0.81,1.72) | 1.23(0.98,1.55) | 0.43(0.22,0.86)* | 0.92(0.68,1.25) | 0.81(0.55,1.19) | 0.94(0.74,1.18) |
| Exercise | |||||||
| Occasionally | 1.24(0.82,1.89) | 1.30(0.95,1.76) | 1.06(0.86,1.32) | 1.39(0.80,2.41) | 0.93(0.73,1.18) | 0.93(0.65,1.33) | 0.88(0.71,1.09) |
| Little | 1.36(0.74,2.50) | 1.60(1.14,2.26)* | 1.36(1.10,1.69)* | 0.81(0.39,1.6) | 0.67(0.50,0.89)* | 0.81(0.56,1.16) | 0.88(0.71,1.09) |
| Smoke | 1.32(0.81,2.15) | 1.66(1.22,2.26)* | 1.16(0.93,1.45) | 0.56(0.30,1.07) | 0.90(0.68,1.18) | 0.80(0.53,1.21) | 0.97(0.75,1.24) |
| Drink | 1.32(0.87,2.02) | 1.10(0.83,1.46) | 1.30(1.07,1.57)* | 0.75(0.42,1.34) | 0.76(0.59,0.98)* | 0.70(0.48,1.02) | 1.03(0.83,1.28) |
| Functional loss | 1.37(0.91,2.06) | 0.95(0.71,1.28) | 0.99(0.81,1.20) | 1.03(0.60,1.78) | 0.95(0.74,1.21) | 1.21(0.89,1.66) | 0.97(0.80,1.19) |
| Hyperuricemia | 2.01(1.07,3.76)* | 1.40(0.84,2.33) | 1.08(0.78,1.48) | 0.89(0.27,2.90) | 0.76(0.45,1.29) | 0.71(0.40,1.26) | 0.74(0.54,1.03) |
| BMI level | |||||||
| Thin | 0.53(0.28,1.01) | 0.65(0.40,1.08) | 0.66(0.41,1.06) | 2.44(1.29,4.59)* | 1.43(1.01,2.01)* | 1.28(0.56,2.95) | 1.51(0.88,2.61) |
| Overweight/Obesity | 1.93(1.06.3.53)* | 1.68(1.25,2.25)* | 1.68(1.41,2.00)* | 0.15(0.06,0.37)* | 0.44(0.33,0.58)* | 0.36(0.27,0.48)* | 0.76(0.63,0.90)* |
| High LDL-C | 0.90(0.59,1.37) | 1.19(0.92,1.55) | 1.11(0.94,1.32) | 0.67(0.39,1.18) | 0.97(0.77,1.22) | 1.00(0.74,1.35) | 0.98(0.82,1.17) |
| High HbA1c | 0.47(0.11,1.95) | 1.07(0.54,2.10) | 0.97(0.64,1.46) | 0.28(0.04,2.08) | 0.79(0.45,1.37) | 0.54(0.29,1.03) | 0.45(0.30,0.67)* |
Note: *P < 0.05.
Abbreviations: FMD, free of metabolic disorder; MMD, mild metabolic disorder; SMD, severe metabolic disorder; MetS, metabolic syndrome; BMI, body Mass Index; LDL-C, low density lipoprotein cholesterol; HbA1c, glycated hemoglobin; TC, total cholesterol; CI, confidence interval.
For MetS patients, female and obesity/overweight were the risk factors to delay the improvement of MetS to MMD and SMD. In addition, high HbA1c was the risk factor to delay the improvement of MetS to SMD, with HR of 0.45 (0.30,0.67); A female and beyond the age of 65, both non-modifiable factors, reduced the possibility of SMD recovery. Regarding the progression from SMD to MMD, overweight or obese individuals who had a history of drinking with little exercise had delayed its recovery. It is important that individuals with thin weight were 2.44 times as likely to revert their SMD state.
Discussion
The study of natural history of chronic diseases is doubly complex due to their complex nature and multifactorial causality.8,18 The aim of a study on natural history is to clarify the factors that affect the overall risk of transition from one stage to another as a diseases progresses (or regresses).19 The natural history of MetS is extremely complex and its disease status is interrelated. With the change of time there is two-way conversion between states. Therefore, our study aimed to describe the dynamic evolution of MetS using Markov model based on CHARLS longitudinal dataset and to identify the associations between a set of variables that have been found to be related to spontaneous progression and reversion to MetS states.
Our results indicated that approximately a half states of MetS (50.28%) had disease state transfer. It is noteworthy that 1319 cases (46.09% of the changed number) had reverse improvement of MetS. The diagnosis of MetS is complicated. Each state is the aggregation of five components. It can be improved because MetS does not follow a continuous progression mode.20 Interestingly, our 4-year follow-up results also highlighted that the probability and intensity of transition from MetS to SMD is the same as that from SMD to MMD and is greater than that from MMD to MetS. No matter what the baseline state of the study object is, it may be improved. The metabolic components of the MetS have a close association and shared roots, and they are overlapping in their pathogenic activities.21 The more metabolic disorder components are, it will be easier to develop into other chronic diseases that are not easy to recover. Therefore, mild metabolic disorder is an important disease state, it is advisable to check the other components carefully and periodically for people who usually have one of the components, which will be conducive to the prevention of the occurrence and development of MetS.22
In relation to the forward progression of MetS states, individuals who had a history of drinking and smoking, obesity or overweight and hyperuricemia, less exercise showed a higher risk of progressing MetS, which is consistent with the findings of previous research.23–26 Consider the variables of health status, these results can be explained by the fact that hyperuricemia is closely related to many risk factors of MetS, such as obesity, abnormal lipid metabolism, hypertension, etc., and they may have a common pathophysiological process.25,27 Obesity is closely related to inflammation and fat factors, leading to the occurrence of MetS and related diseases.28 The mechanism may be that genes related to lipid metabolism, obesity and insulin resistance are related to MetS and single nucleotide polymorphism.29 Focusing on lifestyle, various toxic substances from cigarette stimulate the release of adrenaline, which increases blood pressure and heart rate,30 and cause abnormalities in lipoprotein metabolism, endothelial cell dysfunction and insulin resistance, which increase the risk of MetS.26,31 The less physical exercise, the higher the risk of overweight, obesity and MetS.32 Smokers generally drink too much and do not exercise well, and thus these poor lifestyle habits further increase the risk of MetS.20 Therefore, taking patients with obesity and hyperuricemia as high-risk groups, and healthy lifestyle should be followed to prevent, block and delay the progress of metabolic syndrome and its components. The most effective strategy is to change personal behavior, such as weight loss, regular exercise,33 healthy diet, smoking cessation and moderate alcohol consumption.21
Regarding the reversion of MetS, very few female over the age of 65 years experienced this transition. Previous studies have shown that the prevalence of MetS in women is higher than men,34,35 and the prevalence of all components of MetS is higher in women than men.36 Thus, the speed of the MetS development is expected to be higher (as the prevalence of the MetS) in women than men and it is more difficult to return to normal. Studies suggest that metabolic syndrome is more common in postmenopausal elderly women.37 With the increase of age, the metabolic function, hormone level and enzymes of cells, tissues, organs and the whole body have degenerative changes, and are more prone to chronic diseases. In general, hormonal changes in older women contribute to the occurrence of MetS.38 As for such immutable factors, we should strengthen preventive treatment. As for modifiable variables, our results demonstrate that exercise, drinking, BMI and high HbA1c factors are related to reversion of MetS. The important thing is that people with lean weight have a higher probability of improvement, which can restore from severe metabolic syndrome. On the contrary, overweight or obesity, drinking history, high HbA1c and less exercise will hinder the reverse recovery of metabolic syndrome. The evidence has indicated that HbA1c is effective in identifying high-risk group for MetS.39 Therefore, we should also pay attention to the HbA1c in the physical examination population. Studies have shown that lifestyle changes have improved the occurrence of all components of MetS and reduced the incidence rate of diabetes and cardiovascular diseases.40 Indeed, the primary and most effective strategy to control each component of MetS is lifestyle change such as losing body weight, keeping regular exercise, adopting a healthy diet, quitting smoking and alcohol drinking in moderation.21
Of note, unlike models dealing only with the progression from normal to MetS, the Markov model pays particular attention to random changes, its advantage is that the disease development process is divided into several states to dynamically evaluated bidirectional transitions between the different states of MetS, which overcomes the limitations of Cox regression ignoring the reversible characteristics between multiple outcome states and logistic regression ignoring the influence of time factors on the development of events.41 In addition, the model has unique advantages in identify significant factors for progression and regression and helped to develop prevention and intervention targeting strategies.42,43 This analysis approach could explain why variables that are traditionally associated with a risk of MetS in our multivariate study are only associated with the transitions between specific states.
The study has some limitations. Although the diagnosis of MetS is multifactorial and explained by reversible and non-reversible variables, the absence of information about some biomarkers and dietary habits makes it difficult to determine the etiology of each particular state of MetS. After identifying the factors affecting the state transition of MetS, we will also advocate paying attention to high-risk groups and corresponding lifestyle changes to reduce the risk of MetS. Future research will aim to investigate more variables to explore the etiology of each particular state of MetS. Furthermore, the “natural” transition probability might not be completely natural because some individuals might have received interventions through health services.
Conclusion
Multistate models are crucial in providing the general disease trajectories through intermediates states to alert program response before an adverse event occurs. Our study found the likelihood of progression from mild metabolic disorder to metabolic syndrome is less likely than that of reversion from metabolic syndrome to severe metabolic disorder and severe metabolic disorder to mild metabolic disorder. It is prudent to target early metabolic syndrome screening initiation strengthen the daily monitoring of blood pressure, blood lipid, blood glucose, waist circumference and other indicators of individuals to prevent subsequent progression. Old females were more resistant to recover from worse states than males, so special attention should be paid to such groups. Once this is achieved, it is expected to find mild metabolic disorder, severe metabolic disorder and reduce the risk of developing metabolic syndrome. Targeted screening and health education should be carried out to guide people to reduce their risk of chronic metabolic diseases such as diabetes and dyslipidemia through rational diet and active participation in physical exercise.
Acknowledgments
We appreciate all the individuals who took part in CHARLS and all the researchers, clinicians, administrative staff and coordinators at the data sites who have enabled this survey to be carried out.
Funding Statement
This study was supported by the National Natural Science Foundation of China (project no. 71663053).
Abbreviations
CHARLS, China Health and Retirement Longitudinal Study; MetS, Metabolic syndrome; FMD, free of metabolic disorder; MMD, mild metabolic disorder; SMD, severe metabolic disorder; BMI, body Mass Index; Low HDL-C, low high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; ADL, ability of daily living; CES-D10, Center for Epidemiological Studies Depression Scale-10; TC, total cholesterol; HbA1c, glycated hemoglobin; CRP, C-reactive protein; HbA1c, glycated hemoglobin; CI, confidence interval; HR, Hazard Ratios.
Data Sharing Statement
The datasets generated and analysed during the current study are available in the CHARLS repository [http://charls.pku.edu.cn/].
Ethics Approval
The CHARLS survey was approved by the Institutional Review Board of Peking University, China (IRB00001052-11014 and IRB00001052-11015). This study was approved by the Ethics Committee of the Xinjiang Medical University, China (XJYKDXR20220302045).
Consent for Publication
Consent for publication was obtained from all the authors.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in relation to this work.
References
- 1.Bagheri P, Khalili D, Seif M, et al. Dynamic behavior of metabolic syndrome progression: a comprehensive systematic review on recent discoveries. BMC Endocr Disord. 2021;21(1):54. doi: 10.1186/s12902-021-00716-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin TY, Chien KL, Chiu YH, et al. Dynamics of detailed components of metabolic syndrome associated with the risk of cardiovascular disease and death. Sci Rep. 2021;11(1):3677. doi: 10.1038/s41598-021-83118-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prasun P. Mitochondrial dysfunction in metabolic syndrome. Biochim Biophys Acta Mol Basis Dis. 2020;1866(10):165838. doi: 10.1016/j.bbadis.2020.165838 [DOI] [PubMed] [Google Scholar]
- 4.Esposito K, Chiodini P, Capuano A, et al. Metabolic syndrome and endometrial cancer: a meta-analysis. Endocrine. 2014;45(1):28–36. doi: 10.1007/s12020-013-9973-3 [DOI] [PubMed] [Google Scholar]
- 5.Louters M, Pearlman M, Solsrud E, et al. Functional hypogonadism among patients with obesity, diabetes, and metabolic syndrome. Int J Impot Res. 2021. doi: 10.1038/s41443-021-00496-7 [DOI] [PubMed] [Google Scholar]
- 6.Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):12. doi: 10.1007/s11906-018-0812-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 8.Rezaianzadeh A, Morasae EK, Khalili D, et al. Predicting the natural history of metabolic syndrome with a Markov-system dynamic model: a novel approach. BMC Med Res Methodol. 2021;21(1):260. doi: 10.1186/s12874-021-01456-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia X, Chen Q, Wu P, et al. Dynamic development of metabolic syndrome and its risk prediction in Chinese population: a longitudinal study using Markov model. Diabetol Metab Syndr. 2018;10:24. doi: 10.1186/s13098-018-0328-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Chen Q, Chen L, et al. Description and prediction of the development of metabolic syndrome in Dongying City: a longitudinal analysis using the Markov model. BMC Public Health. 2014;14(1):1033. doi: 10.1186/1471-2458-14-1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang LC, Bai CH, You SL, et al. Description and prediction of the development of metabolic syndrome: a longitudinal analysis using a Markov model approach. PLoS One. 2013;8(6):e67436. doi: 10.1371/journal.pone.0067436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang X, Liu Q. Prediction of the development of metabolic syndrome by the Markov model based on a longitudinal study in Dalian City. BMC Public Health. 2018;18(1):707. doi: 10.1186/s12889-018-5599-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanz-Blasco R, Ruiz-Sanchez de Leon JM, Avila-Villanueva M, et al. Transition from mild cognitive impairment to normal cognition: determining the predictors of reversion with multi-state Markov models. Alzheimers Dement. 2022;18(6):1177–1185. doi: 10.1002/alz.12448 [DOI] [PubMed] [Google Scholar]
- 14.Gao K, Ma W, Huck S, et al. Association between sarcopenia and depressive symptoms in Chinese older adults: evidence from the China Health and retirement longitudinal study. Front Med. 2021;8:755705. doi: 10.3389/fmed.2021.755705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su D, Chen Z, Chang J, et al. Effect of social participation on the physical functioning and depression of empty-nest elderly in China: evidence from the China Health and Retirement Longitudinal Survey (CHARLS). Int J Environ Res Public Health. 2020;17(24):9438. doi: 10.3390/ijerph17249438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Y, Hu Y, Smith J, et al. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol. 2014;43(1):61–68. doi: 10.1093/ije/dys203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang X, Zhang H, Zhao Y, et al. Transition patterns of weight status and their predictive lipid markers among Chinese adults: a longitudinal cohort study using the Multistate Markov Model. Diabetes Metab Syndr Obes. 2021;14:2661–2671. doi: 10.2147/DMSO.S308913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bynum B. A history of chronic diseases. Lancet. 2015;385(9963):105–106. doi: 10.1016/S0140-6736(15)60007-1 [DOI] [Google Scholar]
- 19.Jewell NP. Natural history of diseases: statistical designs and issues. Clin Pharmacol Ther. 2016;100(4):353–361. doi: 10.1002/cpt.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guzmán A, Navarro E, Obando L, et al. Effectiveness of interventions for the reversal of a metabolic syndrome diagnosis: an update of a meta-analysis of mixed treatment comparison studies. Biomedica. 2019;39(4):647–662. doi: 10.7705/biomedica.4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HL, Chung J, Kim KJ, et al. Lifestyle modification in the management of metabolic syndrome: statement from Korean Society of CardioMetabolic Syndrome (KSCMS). Korean Circ J. 2022;52(2):93–109. doi: 10.4070/kcj.2021.0328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aman A, Rasyid H, Bakri S, et al. The association between parents history of type 2 diabetes with metabolic syndrome component and insulin resistance in non-diabetic young adult male. Acta Med Indones. 2018;50(4):309–313. [PubMed] [Google Scholar]
- 23.Renninger M, Hansen BH, Steene-Johannessen J, et al. Associations between accelerometry measured physical activity and sedentary time and the metabolic syndrome: a meta-analysis of more than 6000 children and adolescents. Pediatr Obes. 2020;15(1):e12578. doi: 10.1111/ijpo.12578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chin YR, So ES. The effects of weight fluctuation on the components of metabolic syndrome: a 16-year prospective cohort study in South Korea. Arch Public Health. 2021;79(1):21. doi: 10.1186/s13690-021-00539-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen S, Wu NW, Yu C, et al. Association between baseline and changes in serum uric acid and incident metabolic syndrome: a nation-wide cohort study and updated meta-analysis. Nutr Metab. 2021;18(1):59. doi: 10.1186/s12986-021-00584-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang JY, Bai Y, Zeng ZH, et al. Association between life-course cigarette smoking and metabolic syndrome: a discovery-replication strategy. Diabetol Metab Syndr. 2022;14(1):11. doi: 10.1186/s13098-022-00784-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu D, Ding Y, Zhao Y, Miao S, Qu Q. Positively increased visceral adiposity index in hyperuricemia free of metabolic syndrome. Lipids Health Dis. 2018;17(1):101. doi: 10.1186/s12944-018-0761-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Litwin M, Kulaga Z. Obesity, metabolic syndrome, and primary hypertension. Pediatr Nephrol. 2021;36(4):825–837. doi: 10.1007/s00467-020-04579-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Velazquez-Roman J, Angulo-Zamudio UA, Leon-Sicairos N, et al. Association of FTO, ABCA1, ADRB3, and PPARG variants with obesity, type 2 diabetes, and metabolic syndrome in a Northwest Mexican adult population. J Diabetes Complications. 2021;35(11):108025. doi: 10.1016/j.jdiacomp.2021.108025 [DOI] [PubMed] [Google Scholar]
- 30.Accardo A, Silveri G, Ajcevic M, et al. Influence of smoking and other cardiovascular risk factors on heart rate circadian rhythm in normotensive and hypertensive subjects. PLoS One. 2021;16(9):e0257660. doi: 10.1371/journal.pone.0257660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris KK, Zopey M, Friedman TC. Metabolic effects of smoking cessation. Nat Rev Endocrinol. 2016;12(5):299–308. doi: 10.1038/nrendo.2016.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alizaei Yousefabadi H, Niyazi A, Alaee S, et al. Anti-inflammatory effects of exercise on metabolic syndrome patients: a systematic review and meta-analysis. Biol Res Nurs. 2021;23(2):280–292. doi: 10.1177/1099800420958068 [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Tan Q, Guo Y, et al. The influence of exercise training on endothelial function, serum irisin and inflammatory markers in the elderly with metabolic. Clin Lab. 2021;67(3). doi: 10.7754/Clin.Lab.2020.200446 [DOI] [PubMed] [Google Scholar]
- 34.Hirode G, Wong RJ. Trends in the prevalence of metabolic syndrome in the United States, 2011–2016. JAMA. 2020;323(24):2526–2528. doi: 10.1001/jama.2020.4501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ying X, Yang S, Li S, et al. Prevalences of metabolic syndrome and its sex-specific association with socioeconomic status in rural China: a cross-sectional study. Bmc Public Health. 2021;21(1):2033. doi: 10.1186/s12889-021-12074-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khosravi A, Akhavan Tabib A, Golshadi I, et al. The relationship between weight and CVD risk factors in a sample population from central Iran (Based on IHHP). ARYA Atheroscler. 2012;8(2):82–89. [PMC free article] [PubMed] [Google Scholar]
- 37.Harraqui K, Oudghiri DE, Hannoun Z, et al. Frequency of metabolic syndrome and study of anthropometric, clinical and biological characteristics in peri- and postmenopausal Women in the City of Ksar El Kebir (Northern Morocco). Int J Environ Res Public Health. 2022;19:10. doi: 10.3390/ijerph19106109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chedraui P, Escobar GS, Perez-Lopez FR, et al. Angiogenesis, inflammation and endothelial function in postmenopausal women screened for the metabolic syndrome. Maturitas. 2014;77(4):370–374. doi: 10.1016/j.maturitas.2014.01.014 [DOI] [PubMed] [Google Scholar]
- 39.Jung JY, Ryoo JH, Chung PW, et al. Association of fasting glucose and glycated hemoglobin with the long-term risk of incident metabolic syndrome: Korean Genome and Epidemiology Study (KoGES). Acta Diabetol. 2019;56(5):551–559. doi: 10.1007/s00592-019-01290-0 [DOI] [PubMed] [Google Scholar]
- 40.Jones J, Reneau P, Dos Santos J. Metabolically healthy obese vs. Metabolic syndrome - The crosslink between nutritional exposure to bisphenols and physical exercise. Med Hypotheses. 2021;149:110542. doi: 10.1016/j.mehy.2021.110542 [DOI] [PubMed] [Google Scholar]
- 41.Taguchi A, Hara K, Tomio J, et al. Multistate Markov model to predict the prognosis of high-risk human papillomavirus-related cervical lesions. Cancers. 2020;12(2):270. doi: 10.3390/cancers12020270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ornstein KA, Liu SH, Husain M, et al. Prospective assessment of dementia on transitions in homeboundness using multistate Markov models. J Am Geriatr Soc. 2021;70(4):1117–1126. doi: 10.1111/jgs.17631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu T, Song G, Liu Q, et al. Transition patterns of weight status and their associated factors among elementary school children: a longitudinal cohort study using Multistate Markov Model. Child Obes. 2019;15(5):306–312. doi: 10.1089/chi.2018.0345 [DOI] [PubMed] [Google Scholar]