Abstract
Colorectal cancer (CRC) is a leading cause of cancer-related death for both men and women, highlighting the need for new treatment strategies. Advanced disease is often treated with a combination of radiation and cytotoxic agents, such as DNA damage repair inhibitors and DNA damaging agents. To optimize the therapeutic window of these multimodal therapies, advanced nanomaterials have been investigated to deliver sensitizing agents or enhance local radiation dose deposition. In this study, we demonstrate the feasibility of employing a radiation-induced inflammation targeting nanoscale metal-organic framework (nMOF) platform to enhance CRC treatment. This novel formulation incorporates a fucoidan surface coating to preferentially target P-selectin, which is over-expressed or translocated in irradiated tumors. Using this radiation stimulated delivery strategy, a combination PARP inhibitor talazoparib and chemotherapeutic temozolomide drug-loaded hafnium and 1,4-dicarboxybenzene (Hf-BDC) nMOF was evaluated both in vitro and in vivo. Significantly, these P-selectin targeted nMOFs (TT@Hf-BDC-Fuco) show improved tumoral accumulation over multiple controls and subsequently enhanced therapeutic effects. The integrated radiation and nanoformulation treatment demonstrated improved tumor control (reduced volume, density, and growth rate) and increased survival in a syngeneic CRC mouse model. Overall, the data from this study support the continued investigation of radiation-priming for targeted drug delivery and further consideration of nanomedicine strategies in the clinical management of advanced CRC.
Keywords: Hafnium, MOF, Inflammation, Talazoparib, Temozolomide
1. Introduction
In patients with locally advanced or unresectable colorectal cancer (CRC), chemoradiation enhances loco-regional control and may increase disease-free survival both as adjuvant and neoadjuvant treatments.(1,2) However, radiation therapy (RT) is associated with late-toxicities, such as bowel dysfunction, fecal incontinence, and pelvic fractures. To reduce radiation dose, the use of DNA damage repair inhibitors (DDRi’s), such as poly-ADP ribose polymerase inhibitors (PARPi’s), have been incorporated to increase the therapeutic window. By capitalizing on their DNA damage repair inhibition, PARPi’s concomitant application as a sensitizer for DNA-damaging agents (such as chemotherapy and RT) has emerged as a promising strategy for homologous recombination (HR)-proficient cancers, including CRC.(3,4) This has led to promising results upon combining PARPi’s with the alkylating agent temozolomide (TMZ) in various cancers including CRC, glioblastoma, small cell lung, prostate, sarcoma, and leukemia.(3,5–13)
In addition to characteristic enzymatic inhibition, PARPi’s have been investigated more recently for their ability to form cytotoxic PARP-DNA complexes. (14) This mechanism has been linked to both chemo- and radiosensitization suggesting that a more potent PARP-trapping agent may prove to be a superior radio- and chemo-potentiator.(15) A combination of the most potent PARP trapping agent, talazoparib (Tal), with TMZ has not yet been applied to CRC; however, PARP trapping potency may reduce tolerability of the TMZ/Tal combination.(6,16) To overcome chemotherapeutic toxicity, nanoformulation has been widely employed. Furthermore, nanoformulation has proven to reduce the toxicity of TMZ/Tal combinations specifically.(9) Recently, materials such as nanoscale metal organic frameworks (nMOFs) have been investigated for combination drug delivery and radiation dose enhancement.(17,18) nMOFs which consist of metal ions connected via organic linkers, in this case hafnium and 1,4-dicarboxybenze (Hf-BDC) respectively, are a promising class of radiation dose enhancers.(19–22) However, these systems rely on passive tumoral accumulation resulting in sub-optimal nanoparticle and drug concentrations in the tumor. Active targeting strategies have been employed to overcome these issues, however they have yet to achieve broad success.(23,24)
The increased precision of RT by technologic advancements facilitates the use of radiation as an external stimulus to induce targeted tissue-level changes that can be exploited to enhance nanoparticle accumulation. Radiation induces various molecular changes, some characteristic of inflammation, including the upregulation of several cellular adhesion molecules.(25,26) Of particular interest in this study is the cellular adhesion molecule, P-selectin (P-sel). Various studies have demonstrated the specific radiation-induced upregulation of endothelial P-sel and subsequent targeting of irradiated tissue through the use of a specific P-sel ligand.(27–30) Furthermore, Shamay et al. found evidence of enhanced P-sel expression in human tumors with minimal expression in normal tissues.(31) Fucoidan (Fuco), a natural polysaccharide derived from brown algae, demonstrates a stronger affinity for P-sel than its native ligand P-sel glycoprotein ligand-1 (PSGL-1).(32) Due to the specificity of this ligand-receptor pair, significant interest has arisen for Fuco-based and Fuco-coated nanomaterials to target both endogenous and radiation-induced P-sel expression.(31,33–36)
In this report, we utilize radiation to prime tumors for the targeting of a TMZ/Tal-loaded, Fuco-coated Hf-BDC nMOF (TT@Hf-BDC-Fuco) to increase therapeutic efficacy. The CT26.wt cell line was chosen as the CRC model, as the aggressive nature of these cells more closely mimics human disease and the syngeneic nature of the tumor enables use of immunocompetent mice. This formulation resulted in enhanced cellular uptake and tumor accumulation (with and without IR), increased median survival, reduced tumor volume, density, and growth rate, and inhibited the development of a hypoxic/necrotic core. Furthermore, the aggressive treatment regimen produced no toxicity, indicating the potential clinical utility of this combination. We suggest that the functionalized, drug-loaded nMOF will present an effective therapeutic treatment for CRC.
2. Materials and methods
2.1. Cell culture and reagents
Murine CT26.wt and bEnd.3 cells were purchased from ATCC and maintained in RPMI or DMEM, respectively, at 37°C and 5% CO2. Media was supplemented with 10% (v/v) fetal bovine serum and 1% (v/v) penicillin/streptomycin. Further cell authentication and Mycoplasma testing has not been performed. Cells were used within 20 passages. Talazoparib and temozolomide were purchased from MedChemExpress (Monmouth Junction, NJ, USA). HPLC grade acetonitrile and methanol were purchased from Fisher Scientific (Hampton, NH, USA). Cyanine7.5 carboxylic acid was purchased from Lumiprobe (Hunt Valley, MD, USA). Phosphate buffered saline (PBS) 1X was purchased from Corning Inc. (Corning, NY, USA). DAPI (4’,6-Diamidino-2-Phenylindole, Dilactate), Alexa Fluor™ 488 Phalloidin, RPMI 1640, DMEM (high glucose, pyruvate) and Geltrex (LDEV-Free Reduced Growth Factor Basement Membrane Matrix) were purchased from Thermo Fisher Scientific (Carlsbad, CA, USA). Fucoidan (Fucus vesiculosus >95%) and dextran sulfate sodium salt (MW 7,000–20,000) were purchased from Sigma Aldrich (St. Louis, MO, USA). Glacial acetic acid, terephthalic acid (1,4-benzenedicarboxylic acid -BDC), DMF, hafnium (IV) chloride, and recombinant TNF alpha were purchased from Fischer Scientific.
2.2. Surface modification and drug loading of Hf-BDC nMOF
nMOFs were suspended in either 18 MΩ water (generation 1) or methanol (generation 2) at 5 mg/mL depending on colloidal stability. nMOF redispersed in water at 5 mg/mL was combined with fucoidan or dextran (both at 1 mg/mL in 18 MΩ water) at a 1:1 v/v ratio, sonicated for 1 hour, and filtered. nMOF redispersed in methanol at 5 mg/mL was combined with fucoidan or dextran (both at 1 mg/mL in 18 MΩ water) at a 1:1 v/v ratio, sonicated for 15 mins and stirred open overnight. After evaporation nMOFs were redispersed to 2.5 mg/mL nMOF in water and subsequently filtered. For drug loading, all steps remained the same except that drug dissolved in DMSO (Tal and/or TMZ or Cyanine 7.5) was added to the nMOF in water or methanol, sonicated for one hour, and washed by centrifuge twice (collecting the supernatant for quantification) before continuing with either surface coating procedure.
2.3. P-sel expression
P-sel expression was evaluated in vitro using bEnd.3 and CT26.wt cells. 5 × 104 bEnd.3 cells were plated into 4-well chamber slides, allowed to settle overnight, irradiated with 6 Gy, fixed 20 hrs later, and stained for P-sel (1/50 dilution) and DAPI. The same protocol was repeated in CT26.wt cells (1.5 × 105 cells/well). P-sel expression was also evaluated in CT26.wt tumors harvested from BALB/c mice. Subcutaneous CT26.wt tumor xenografts were established by mixing an equal volume of cell suspension (2 × 106 cells/mL) with Geltrex and injecting 50 µL (5 × 104 cells) into both hind flanks of BALB/c mice (Charles River Laboratories, Wilmington, MA). Twenty days later, mice (n=3) were irradiated with 0, 2, or 6 Gy and tissue harvested at 4-or 24-hrs post-irradiation (IR). Tissues were prepared for histologic examination as described in the histology section.
2.4. In Vitro Cytotoxicity
Free drug cytotoxicity was investigated with Tal and TMZ as individual and combination agents. To determine the optimal TMZ:Tal ratio, CT26.wt cells were plated at 4 × 103 cells per well in a 96-well plate and allowed to settle overnight. Cells were then treated with TMZ (0, 10, 30, 150, 300, 600 or 1000 µM), Tal (0, 1, 5, 10, or 100 µM), or TMZ:Tal at each of the concentrations combined (ratios ranging from 1000:1 to 1:10) and incubated for 72 hrs. Combination indexes (CIs) were determined for all combinations (including only those points on the curve with Fa values between 0.1 and 0.9) using CompuSyn (ComboSyn Inc., Paramus, NJ, USA).
2.5. Clonogenic Assay
One million CT26.wt cells were seeded into six T25 flasks and allowed to settle overnight. 24 hrs later, flasks were treated as follows: no treatment, Hf-BDC-Fuco, Tal@Hf-BDC-Fuco, TMZ@Hf-BDC-Fuco, TT@Hf-BDC-Fuco, or Free Tal/TMZ. The TT@Hf-BDC-Fuco nMOF was diluted 200-fold from the stock (2.5 mg/mL Hf-BDC-Fuco, 7.07 mM TMZ, and 272 µM Tal). Individually loaded nMOFs and free Tal/TMZ were treated to match their counterpart in the TT@Hf-BDC-Fuco treatment arm. After incubating for 20 hrs, cells were washed once with PBS, trypsinized, seeded into 6-well plates, and irradiated (CellRad X-Ray Cabinet Irradiator, Precision X-Ray Irradiation, 130 kV, 5 mA, 0.5 mm aluminum filter, ~1.2 Gy/min). The number of cells seeded varied based on the dose of radiation (0, 1, 2, 3, 4, 5 Gy) and drug treatment conditions ranging from 100 cells/well to 1500 cells/well. Seven days later, colonies were washed with PBS, fixed with 4% v/v formalin, and stained with 0.01 mg/mL crystal violet dye. After staining, plates were washed with DI water, dried, and colonies counted. First, the plating efficiency (PE) of each treatment was calculated by dividing the number of colonies formed by the number of cells seeded. Next, the PE of each treatment was divided by the PE of the unirradiated counterpart to determine the survival fraction (SF).
2.6. Xenograft tumor inoculation and growth
All animal studies were approved by and conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Oregon Health and Sciences University (OHSU). Subcutaneous CT26.wt tumor xenografts were established by mixing an equal volume of cell suspension (2 × 106 cells/mL) with Geltrex and injecting 50 µL (5 × 104 cells) into the right flank of BALB/c mice (Charles River Laboratories, Wilmington, MA). For all tumor studies, body weight and average tumor volume (1/2 × length × width2) measurements were taken at least three times weekly. When endpoints were reached per IACUC guidelines [(1) tumor ulceration, (2) weight loss reached, (3) 20% of starting weight, or (4) any diameter reached 2 cm] mice were euthanized by cervical dislocation under anesthesia. Relative tumor volume curves were developed to compare starting tumor volume to the volume measured at each day. Kaplan-Meier curves were developed for each treatment group to examine differences in survival. For all imaging studies and when noted, mice were anesthetized using 2–3% isoflurane (Piramal Enterprises Limited, Telangana, India).
2.7. Efficacy and survival study
Eleven days after inoculation, 28 mice were randomized evenly (n=7) into four treatment groups with average tumor sizes of 55–68 mm3. Treatment groups were as follows: (1) Hf-BDC-Fuco, (2) Hf-BDC-Fuco + RT, (3) TT@Hf-BDC-Fuco, or (4) TT@Hf-BDC-Fuco + RT. Particles were administered via tail vein injection b.i.d. five consecutive days starting on day zero. nMOF and fucoidan concentrations were 9.25 and 1.85 mg/kg per administration and the corresponding TMZ and Tal concentrations were 4.4 and 0.36 mg/kg. Mice in groups two and four were irradiated with 2 Gy on days zero and two for a total of 4 Gy. Radiation was delivered (CellRad, Precision X-Ray Irradiation, 130 kV, 5 mA, 0.5 mm aluminum filter, ~1.2 Gy/min) selectively to tumors using half-moon cutout lead shields (Precision X-Ray, North Branford, CT). Terminal blood samples were collected via cardiac puncture for clinical chemistry, performed by IDEXX Laboratories (Westbrook, ME). Organs were collected, weighed, and fixed. Formalin-fixed tumors obtained from days 14 (all treatments) and 21 (all treatments except control) were submitted to the OHSU Histopathology Shared Resource for procedures described in Tissue Histology. Tumor mass was divided by caliper tumor volume to obtain tumor density. For mice was sacrificed due to ulceration, tumors were not included in average tumor density calculations. Tumor volumes were converted to a base-10 logarithmic scale and linear fits were applied to each treatment group. The resulting slope represents the average tumor growth rate for each group. Tumor growth inhibition percent was determined using the tumor growth rate values applied to the formula (1-(growth rate of treatment group)/(growth rate of control group)) × 100%.
2.8. Tissue Histology and Stain Quantification
Tissue was formalin (10% v/v) fixed for 24 hrs and then transferred to 70% ethanol. Samples were then processed and stained by by the OHSU Histopathology Shared Resource Core; stains included P-sel (RB40.34, BD Biosciences, 0.2 mg/mL at 1:100 dilution), CD31 (ab28364, Abcam, supplier recommendations), and Hematoxylin and Eosin (H&E). Imaging was performed on a Zeiss Axio Scan.Z1 Slide Scanner by the OHSU Advanced Light Microscopy Core at 20 × (P-sel & CD31) or 40 × (H&E). Whole-tumor images were analyzed in FIJI using color threshold. For any given marker (H, E, P-sel, CD31) the RGB settings utilized to select the entire tissue and the marker-stained tissue were applied uniformly to all images. After applying the threshold settings, tissue was selected and area measured. For each image the area stained was divided by the whole tissue area to determine a fraction of tissue stained for each marker.
2.9. Statistical analysis
All data are expressed as mean ± SEM. Statistical differences and significance were evaluated using two-way ANOVA with multiple comparisons, one-way ANOVA with multiple comparisons, or logrank (Mantel-Cox) test for survival curves in the GraphPad Prism 8. p < 0.05 was considered statistically significant and represented by *.
3. Results
3.1. Synergistic combination of Talazoparib and Temozolomide
Viability experiments were performed to assess and identify synergistic ratios of TMZ and Tal in CT26.wt cells (Supplementary Fig. S1). Strong synergy (CI values less than 0.35) was observed across high, medium, and low total drug concentrations (31, 155, 310 µM) at a 30:1 TMZ:Tal ratio indicating a robust synergistic effect for this combination.(37)
Once a ratio (30:1, TMZ:Tal) was selected, further cytotoxicity and synergy evaluation was performed. Viability curves and subsequent IC50 values were determined for Tal, TMZ, and TMZ:Tal at a 30:1 ratio (Fig. 1A–B; Supplementary Table 1). Tal was found to be moderately effective as a single agent (IC50 = 5.1 µM) while TMZ was less effective (IC50 = 34 µM). Importantly, combining TMZ and Tal produces an overall IC50 value of 10.7 µM, correlating to concentrations of 0.35 µM Tal and 10.35 µM TMZ. Compared to their individual drug counterparts, these were IC50 decreases of 14.6- and 3.3-fold for Tal and TMZ respectively, demonstrating increased efficacy of the combination. Synergy was found at all tested concentrations using the Chou-Talalay method (Fig. 1C). Despite the oral bioavailability of Tal and TMZ, solubility limitations of Tal make it an ideal candidate for nanoparticle encapsulation (Supplementary Fig. 2).
Figure 1.
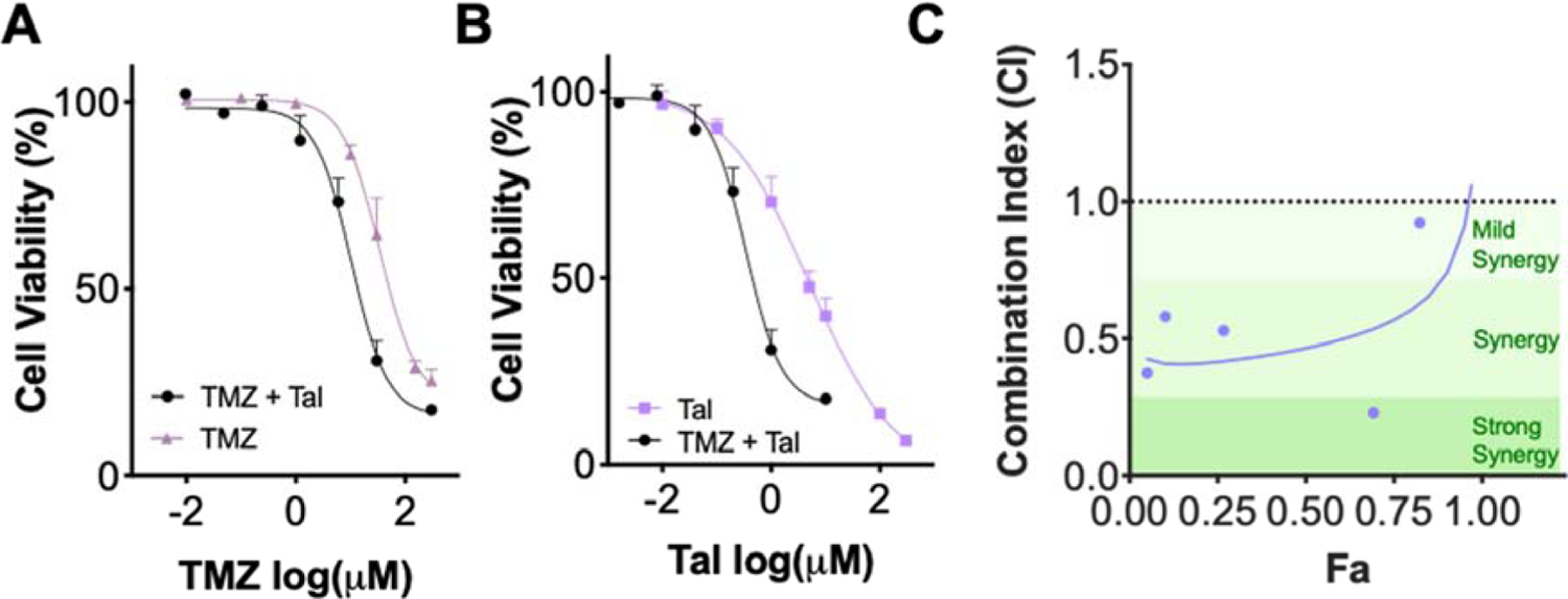
A. Cell viability curves of TMZ and TMZ:Tal at a 30:1 ratio graphed against TMZ concentration. B. Cell viability curves of Tal and TMZ:Tal at a 30:1 ratio graphed against Tal concentration C. Combination Index plot demonstrating synergy for various combinations of TMZ:Tal (30:1) utilizing Fa values between 0.1 and 0.9 from A and B.
3.2. Characterization of TT@Hf-BDC-Fuco
To enhance solubility and circulation, the drugs were encapsulated in a nMOF (Hf-BDC). This nMOF was coated with fucoidan (Hf-BDC-Fuco) as an inflammation-targeting (P-sel) agent or dextran (Hf-BDC-Dex), a non-sulfonated polysaccharide as a non-targeting control.
Evaluation of the various Hf-BDC nMOFs by dynamic light scattering (DLS) showed the size of an uncoated nMOF to be ~84 nm, with each coating and drug loading slightly increasing the nMOF size (Fig. 2A; Supplementary Fig. 3 and Table 2). Both Hf-BDC and Hf-BDC-Fuco revealed size ranges of 30 – 70 nm by transmission electron microscopy (TEM) (Fig. 2B). Bare Hf-BDC nMOFs demonstrated a positive ζ potential and all polysaccharide-coated nMOFs (Hf-BDC-Dex, Hf-BDC-Fuco, TT@Hf-BDC-Fuco) possessed negative ζ potentials (Fig. 2A; Supplementary Fig. 3A–B and Table 2). Notably, this data agrees with previous iterations of this platform.(38) Furthermore, minimal differences were observed between the two coating strategies (Supplementary Table 2). Both drugs loaded effectively in the nMOF with average encapsulation efficiencies (EE) of 90% and 54% for TMZ and Tal (Supplementary Table 3). Furthermore, at neutral pH (7.4, PBS buffer) both drugs release somewhat rapidly over the first 24 hrs reaching approximately 40% release (Supplementary Fig. 3C). Interestingly, Tal reaches 100% release in ~7 days while TMZ only reaches approximately 60% release in the same time period, indicating that more frequent dosing may be important to maintain steady state concentrations for TMZ.
Figure 2.
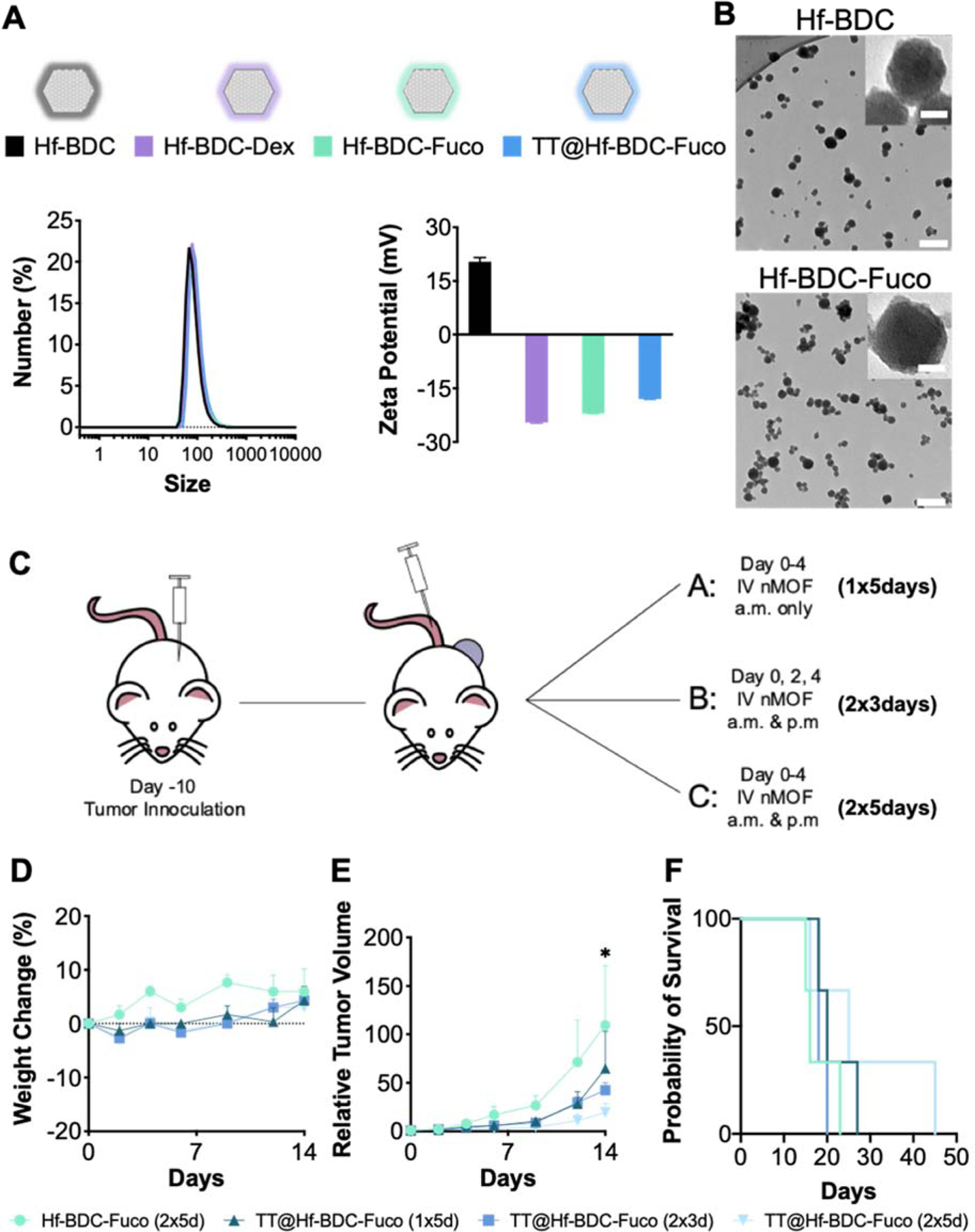
A. Representative nanopartide coatings symbols utilized throughout study (top). ζ potential (bottom left) and size by number (bottom right) measurements for the various nMOF coatings and drug loading B. TEM image of Hf=BDC (top) and Hf-BDC-Fuco (bottom). Scale bar represents 200 nm in the main image and 20 nm in the inset image. In vivo pilot study to determine optimal nMOF dosing regimen before incorporating radiation. C. Schematic representation of the three treatment schedules utilized for proof of concept study. D. Weight measurements over course of injections to evaluate treatment tolerability. E. Tumor growth curves for mice treated with various dosing schemes. F. Kaplan-Meier survival curve to evaluate treatment efficacy. Asterisk (*) represent statistically significant difference (p<0.05) between the control (Hf-BDC-Fuco) and the 2×3 and 2×5 day treatment regimens analyzed by two-way ANOVA.
To confirm the efficacy and determine the optimal in vivo treatment regimen of the TT@Hf-BDC-Fuco nMOF, a proof-of-concept study was performed. Tumor-bearing mice were administered TT@Hf-BDC-Fuco (1 dose ×5 days (d), 2×3 d, or 2×5 d) or Hf-BDC-Fuco (2×5 d) and evaluated for weight changes, tumor growth inhibition, and survival (Fig. 2C). All regimens were non-toxic, with the two most aggressive regimens (2×3 d and 2×5 d) demonstrating a significant reduction in relative tumor volume compared to the control nMOF (Fig. 2D–F). Furthermore, median survival was increased from 16 to 25 days with the 2×5 d treatment regimen. As such, this treatment regimen was selected for further investigation with radiation.
3.3. In vitro & in vivo P-sel expression and targeted uptake
To leverage the P-sel targeting moiety Fuco, baseline and IR-stimulated P-sel expression was investigated in cancer (CT26.wt) and endothelial (bEnd.3) cells. Prior to IR, cancer cells displayed strong puncta in the cytoplasm with minimal diffuse cytoplasmic staining (Fig. 3A). After 6 Gy, puncta are less identifiable and a more diffuse cytoplasmic stain is apparent, indicating translocation. Endothelial cells demonstrate a similar trend of P-sel translocation after 2 Gy (Supplementary Fig. 4).
Figure 3.
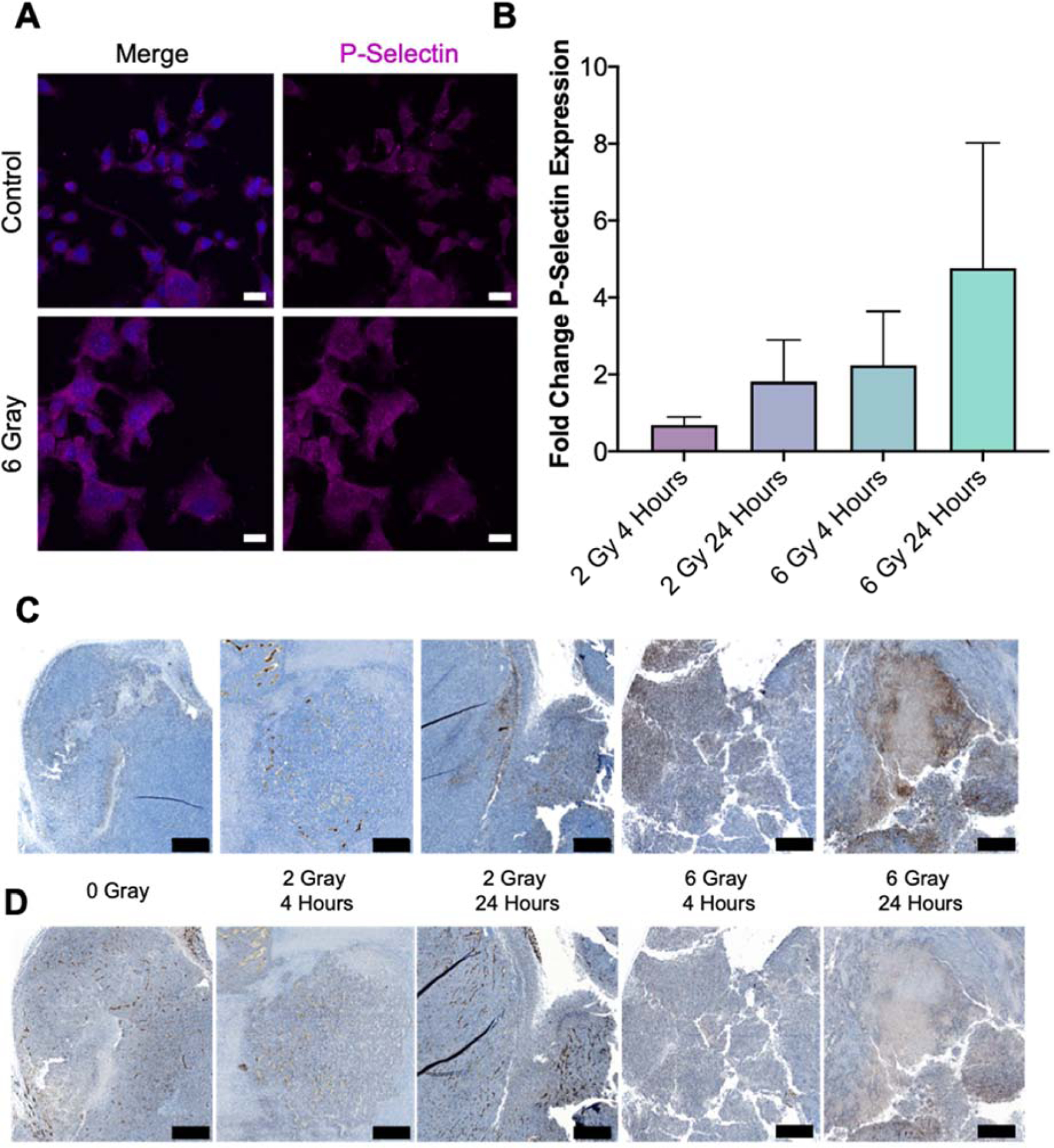
A. P-selectin (pink) expression of DAPI (blue) stained CT26.wt cells before and after radiation demonstrate a decrease in puncta and increase in overal cytoplasmic expression after a radiation dose of 6 gray. Scale bars represent 20 μm. B. Quantification of fold-change in P-selectin expression as a fraction of total tissue for various treatments when compared to no radiation demonstrate in increase in P-selectin expression with respect to IR dose and time after IR. C. Representative sections of tissues analyzed for B zoomed to highlight maximal P-selectm staining (brown). D. Corresponding CD31 stained sections to images in C. Scale bars represent 500 μm.
The ability of radiation to induce an inflammatory environment and translocate P-sel expression in vitro was confirmed in vivo by evaluating the changes in P-sel and CD31 (endothelial cell marker) in irradiated tumors. Mice were exposed to one of three radiation doses (0, 2, 6 Gy) and tissue harvested either 4 or 24 hrs after radiation. With the exception of 2 Gy - 4 hrs, both increasing radiation dose (2 to 6 Gy) and time post-IR (4 to 24 hrs) resulted in relative increase in fractional P-sel expression (Fig. 3B). Fractional P-sel and CD31 expression was determined by dividing the area of tissue stained by the total area of tissue from whole tumor slices (Supplementary Fig. 5 left, middle). Qualitative differences for both markers could also be observed at higher magnification (Fig. 3C–D). In analysis of a tumor from the 6 Gy - 24 hrs group, increases were noted for both P-sel (7.07-fold) and CD31 (2.05-fold) in sequential slices (Supplementary Fig. 5, right). Contrastingly, 2 Gy - 4 hrs demonstrated a reduction in P-sel (0.66-fold) and slight increase (1.18-fold) in CD31 while 2 Gy - 24 hrs and 6 Gy - 4 hrs demonstrated increases in P-sel (3.82- and 4-fold) and slight decreases in CD31 (0.78- and 0.91-fold).
Utilizing a low dose of IR (2 Gy), the ability of -Fuco to enhance nanoparticle homing and uptake was evaluated. Six hrs after treatment cells (CT26.wt or bEnd.3) were incubated with media, Cy7.5@Hf-BDC-Fuco, Cy7.5@Hf-BDC-Dex, or Cy7.5@Hf-BDC overnight. Employing average pixel intensity as a surrogate for particle uptake, surface modification alone is observed to increase uptake in endothelial cells, but not CRC cells (Fig. 4A–C). However, treatment with 2 Gy significantly increases uptake of Cy7.5@Hf-BDC-Fuco compared to the Cy7.5@Hf-BDC in both cell lines. For irradiated CRC cells, but not irradiated endothelial cells, uptake was reduced for both Cy7.5@Hf-BDC and Cy7.5@Hf-BDC-Dex formulations compared to baseline (Cy7.5@Hf-BDC without IR).
Figure 4.
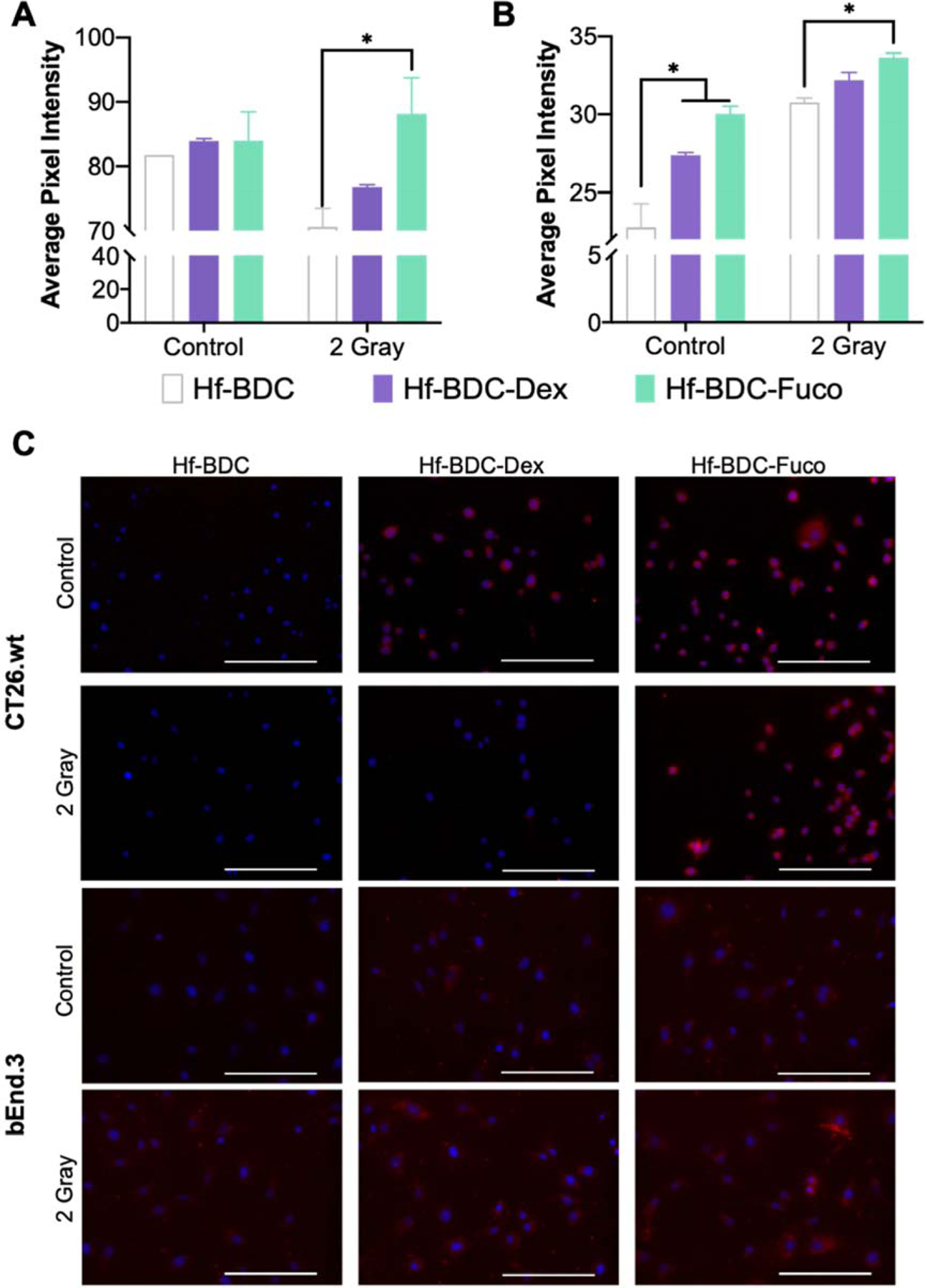
Average pixel intensity of multiple images for A. CT26.wt amd B. bEnd.3 cells treated with uncoated, dextran coated, or fucoidan coated nMOFs with or without 2 gray. C. Representative images of CT26.wt (top two rows) and bEnd.3 (bottom two rows) cellular uptake used for quantification in A and B. Blue DAPI stain demonstrates nuclei and red shows Cy7.5 loaded nMOF. Scale bars represent 200 μm. Asterisk (*) represents statistically significant difference (p<0.05) analyzed by two-way ANOVA with multiple comparisons.
3.4. In vitro toxicity
CT26.wt cells were incubated with free Fuco or Hf-BDC-Fuco and treated with 0 or 2 Gy to determine the influence of Fuco on the cellular response to IR. Free Fuco concentrations were chosen based on the amount of Fuco coating in each nMOF treatment. Following 2 Gy, cell viability decreased approximately 20% across all Fuco concentrations tested (Fig. 5A). Importantly, Fuco coating does not inhibit radioenhancing ability of Hf-BDC at any of the concentrations tested. A nMOF concentration of 15 µg/mL was chosen for subsequent studies, for its lack of baseline toxicity and radioenhancing potential (Supplementary Fig. 6A).
Figure 5.
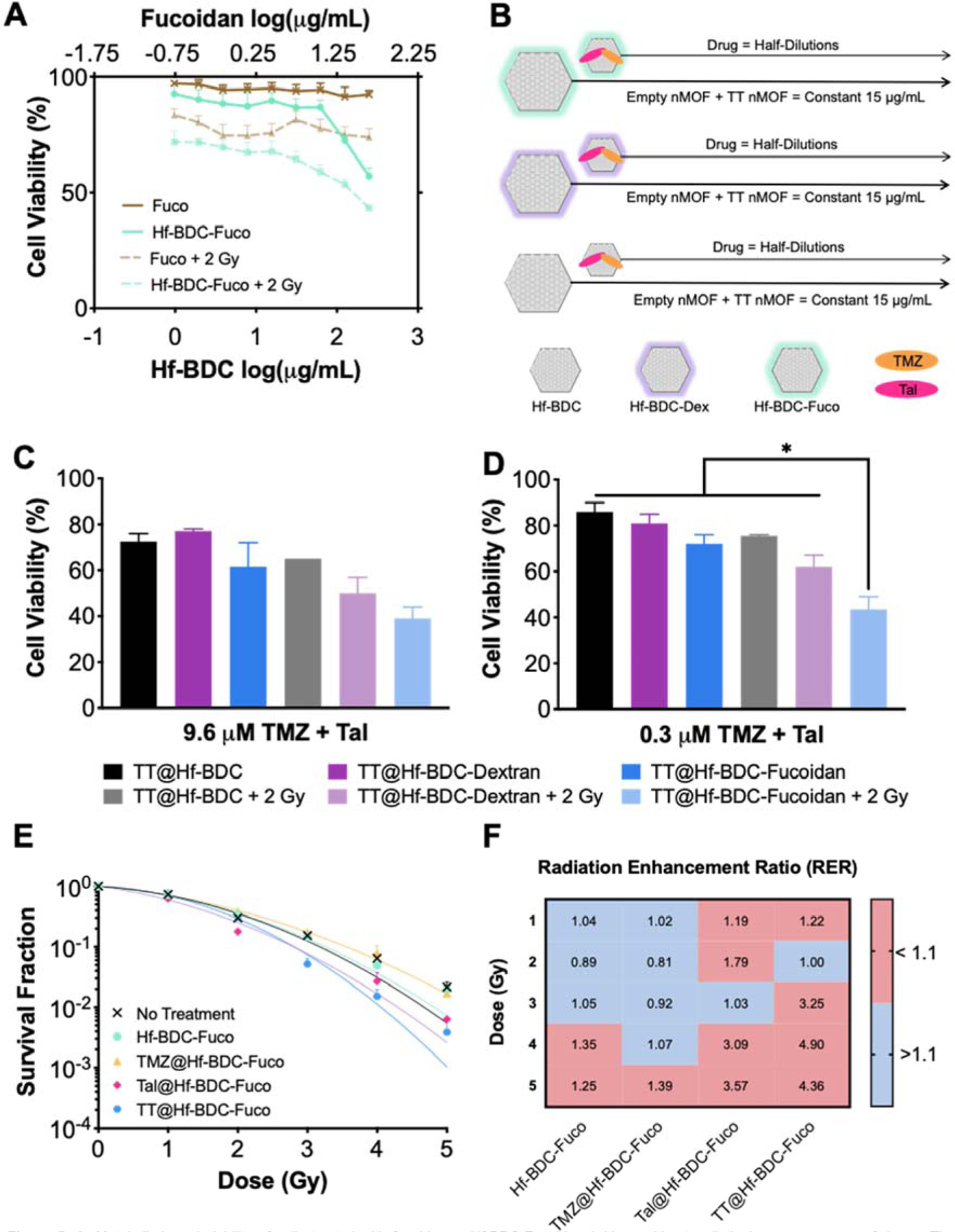
A. Metabolic-based viability of cells treated with fucoidan or Hf-BDC-Fucoidan (with or without radiation) across a range of doses. The concentration of free fucoidan is matched to the concentration of fucoidan on the nanopartide surface. B. Experimental plan for C-D demonstrating that nMOF concentration was kept constant across all wells (15 μg/mL) while dual-drug loaded nMOF was spiked into wells. In vitro viability for CT26.wt cells treated with a constant concentration of Hf-RDC and three coating conditions (uncoated, dextran coated, and fucoidan coated) with or without 2 gray and a TT concentration of C. 0.3 μM or D. 9.6 μM Asterisks (*) represent statistically significant difference (p<0.05) analyzed by one-ANOVA. E. Clonogenic assay investigating the radiosensitizing potential of various Hf-BDC-Fucoidan loaded partides. F. Radiation Enhancement Ratio values for all treatments in E compared to no treatment at a given RT dose.
Next, CT26.wt cells were treated with TT@Hf-BDC, TT@Hf-BDC-Dex, or TT@Hf-BDC-Fuco and radiation to further examine the influence of surface coating. For these studies, nMOF concentration was kept constant (15 µg/mL Hf-BDC) while total drug concentrations ranged from 9.6–0.075 µM at a 1:1 ratio (Fig. 5B). Among unirradiated conditions, cells treated with TT@Hf-BDC-Fuco (solid blue line) demonstrate the greatest cytotoxicity compared to the other -IR treatments (black and purple solid lines). Interestingly, TT@Hf-BDC-Fuco -IR was equally efficacious to irradiated TT@Hf-BDC and TT@Hf-BDC-Dex treated cells (Supplementary Fig. 6B). Furthermore, TT@Hf-BDC-Fuco + IR resulted in the strongest cytotoxicity compared to all other treatment groups, suggesting P-sel targeting is enhancing efficacy. Closer evaluation of a single high and low total drug concentration (9.6 and 0.3 µM) revealed the potential for highly cytotoxic doses in non-targeted nMOFs to overcome increased delivery by targeted particles. The lower concentrations led to significant decreases in viability of the TT@Hf-BDC-Fuco + IR treatment group versus all other treatments whereas the higher concentration did not (Fig. 5C and D). Although obtained independently, comparison of Hf-BDC-Fuco and TT@Hf-BDC-Fuco studies demonstrate the cytotoxic influence of drug-loading both with and without IR (Supplementary Fig. 6C).
As metabolic-based viability assays do not evaluate radiobiologic cell death, additional screening was performed using the clonogenic assay. Cells were exposed to various treatments (control, Hf-BDC-Fuco, TMZ@Hf-BDC-Fuco, Tal@Hf-BDC-Fuco, or TT@Hf-BDC-Fuco) and increasing doses of radiation (0, 1, 2, 3, 4, 5 Gy). As seen in Figure 5E and F, as radiation dose increases, TT@Hf-BDC-Fuco displays enhanced radiosensitization compared to other treatments. Further evaluation of individual radiation doses reveals that compared to the control, TT@Hf-BDC-Fuco significantly decreases survival at 3 and 5 Gy (Supplementary Fig. 6D–F).
3.5. Biodistribution
Following in vitro studies, baseline and radiation-enhanced Hf-BDC accumulation was investigated. Four hours after receiving 6 Gy (or no IR), tumor-bearing mice were administered Cy7.5@Hf-BDC-Fuco which were allowed to accumulate for 20 hrs followed by ex vivo fluorescence imaging. Results revealed a slight increase in tumoral Cy7.5@Hf-BDC-Fuco accumulation after IR, indicating potential targeting (Supplementary Fig. 7A–B). Based on the enhanced in vitro accumulation of Hf-BDC-Fuco without radiation, a follow-up study assessed the enhanced accumulation against an untargeted negative control (Hf-BDC-Dex). Here, 24 hrs following 6 Gy, tumor-bearing mice were administered Cy7.5@Hf-BDC-Fuco or Cy7.5@Hf-BDC-Dex which were allowed to accumulation for an additional 24 hrs. In this case, Cy7.5@Hf-BDC-Fuco displays higher tumoral accumulation than Cy7.5@Hf-BDC-Dex both with and without IR (Fig. 6A–B).
Figure 6.
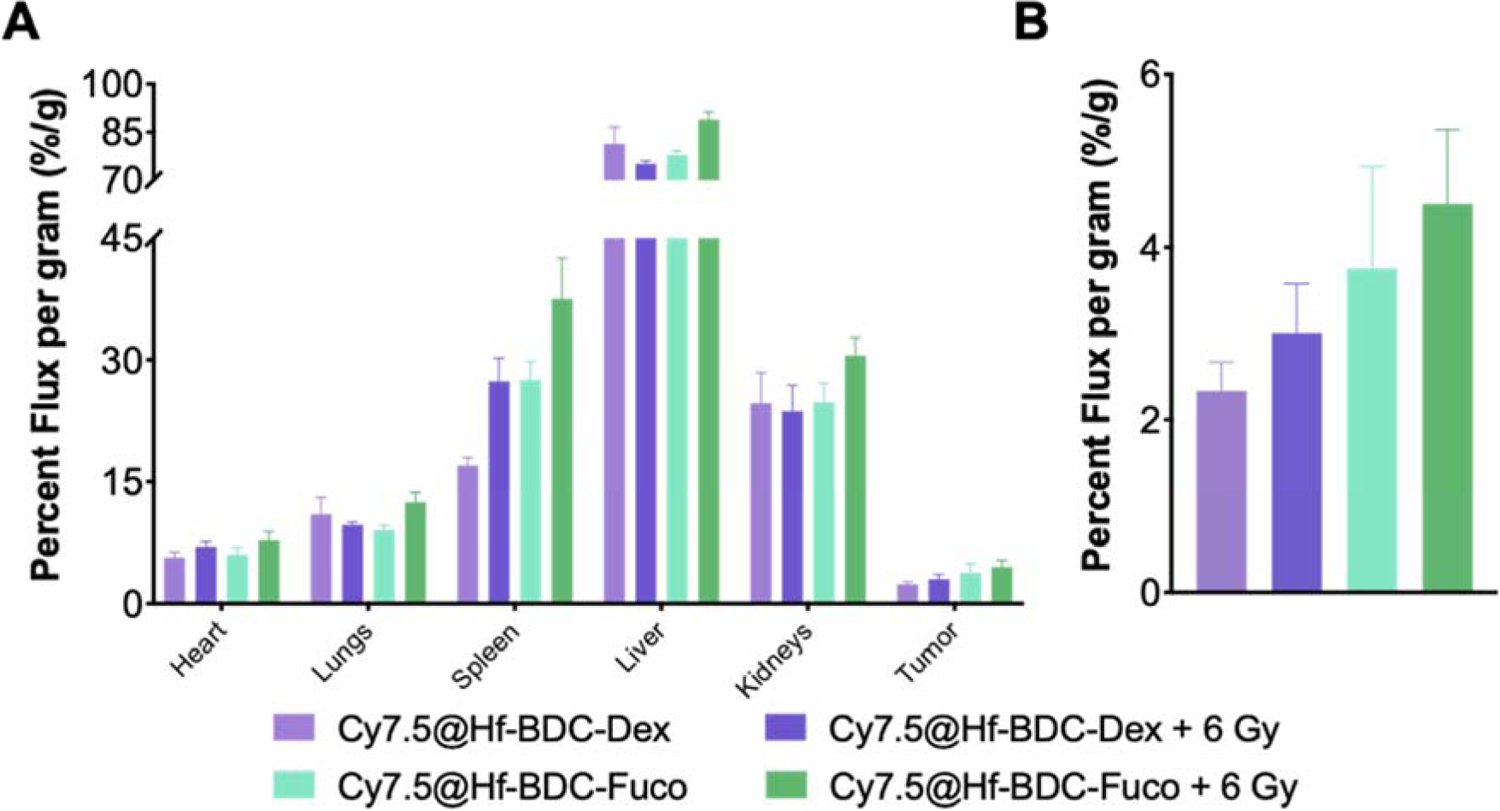
A Whole-organ biodistribution as quantified by IVIS imaging for mice treated with (6 gy) or without radiation and administered either non-targeting Hf-BDC-Dex nMOfs or P-selectin targeting Hf-BDC-Fuco nMOFs. B. Tumor-only biodistribution.
3.6. In vivo efficacy and survival
To evaluate therapeutic efficacy, tumor-bearing mice were administered Hf-BDC-Fuco or TT@Hf-BDC-Fuco twice daily, for 5 consecutive days (Fig. 7A). The first nMOF administration coincided with the first of two RT fractions of 2 Gy. Comparing tumor-growth curves, the combination of TT@Hf-BDC-Fuco + IR leads to a significant decrease in relative tumor volume and increase in survival (23 vs 16 days) compared to Hf-BDC-Fuco (Fig. 7B–C). Furthermore, TT@Hf-BDC-Fuco + IR resulted in decreased tumor density, growth rate, and hypoxia/necrosis compared to the Hf-BDC-Fuco control (Fig. 7D–F, Supplementary Figs. 8–9).(39) The only instance of complete response was observed in the TT@Hf-BDC-Fuco + IR treatment group (Supplementary Fig. 10).
Figure 7.
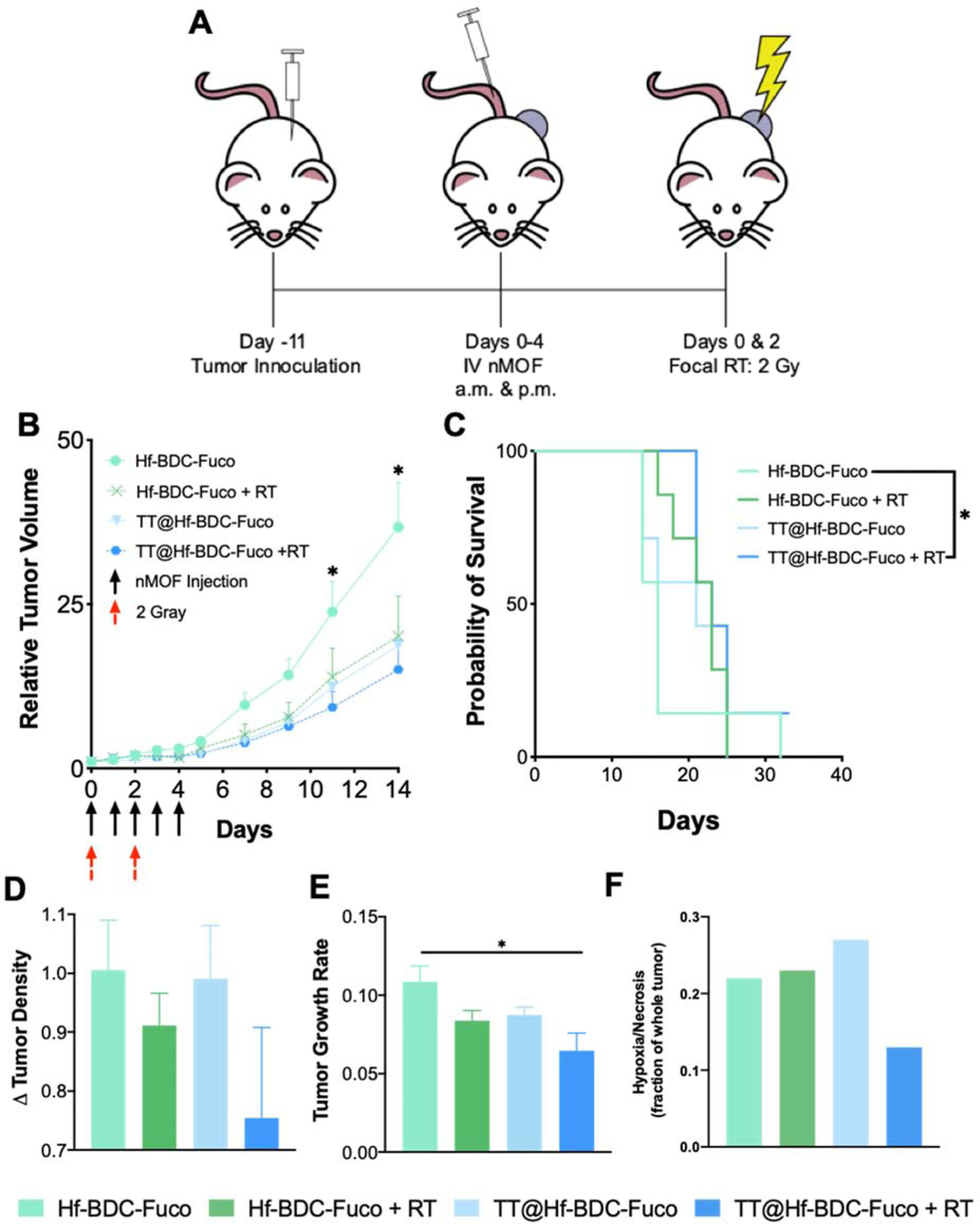
A. Schematic representation of treatment schedule for efficacy study. B. Tumor growth curves for mice treated with various agents. Asterisks (*) represent statistically significant difference (p<0.05) between Hf-BDC-Fuco and all other groups as analyzed by two-way ANOVA with multiple comparisons. C. Kaplan-Meier survival curve to evaluated treatment efficacy. Asterisks (*) represent statistically significant difference (p<0.05) between Hf-BDC-Fuco and TT@Ht-BDC-Fuco + RT as analyzed by Log-rank (Mantel-Cox) test D. Tumor density values for each treatment group determined by dividing final tumor mass by final tumor volume. E. Tumor growth rate values for each treatment group as determined using base-10 logarithmic scale. Asterisks (*) represent statistically significant difference (p<0.05) between Hf-BDC-Fuco and all other groups as analyzed by one-way ANOVA with multiple comparisons. F. Quantification of hypoxic/necrotic regions as a fraction of whole tumor slice in Supplementary Fig. 8.
3.7. In vivo safety
Safety evaluation of the therapeutic efficacy study did not uncover unusual changes in body or organ weight indicating good overall biocompatibility (Fig. 8A, Supplementary Fig. 11A). Furthermore, complete blood count (CBC) and clinical chemistry panels indicated lack of hematologic, hepatic, or renal toxicity (Fig. 8B–C, Supplementary Fig. 11B).
Figure 8.
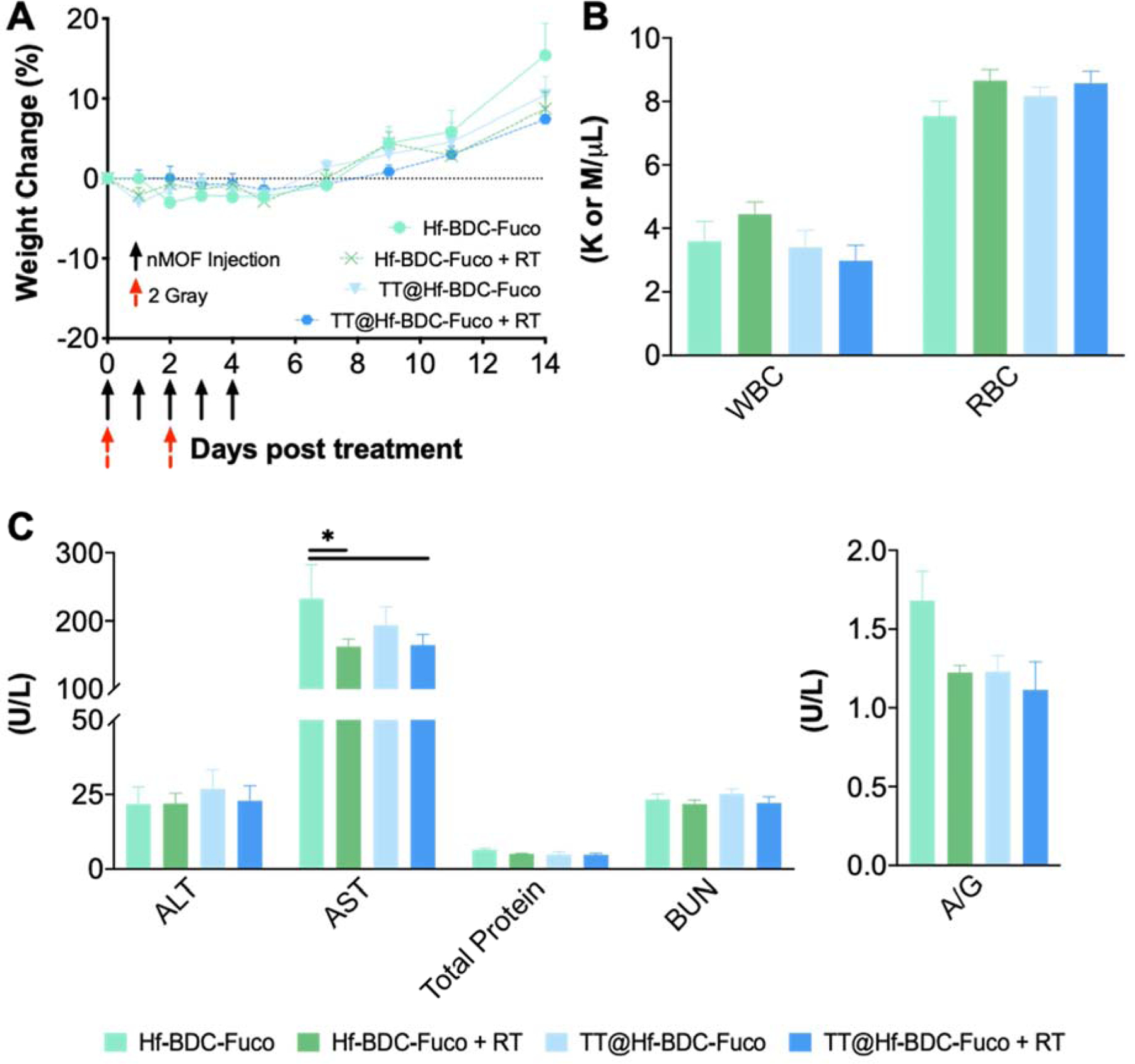
A. Mouse body weight evaluation to evaluate treatment-associated toxicity. B. Complete blood count for mice in each treatment group to evaluate hematologic toxicity. C. Clinical chemistry evaluation of Liver (ALT, AST, Total Protein) and Kidney (BUN, A/G) function. Asterisks (*) represent statistically significant difference (p<0.05).
4. Discussion
In this study, drug synergy was identified across a broad range of ratios and concentrations for TMZ and Tal. Subsequently, several factors were considered in the selection of an optimal delivery vehicle and ratio of TMZ to Tal (30:1). Despite the oral bioavailability of TMZ and Tal, both agents demonstrate hydrophobic characteristics and dose-limiting toxicities which can be overcome through encapsulation.(9) Initial Hf-BDC nMOF loading trials indicated higher loading capacity of TMZ versus Tal (data not shown). This is advantageous as prior studies found synergy at various ratios of TMZ in excess of Tal.(10) Although potent, combining strong PARP-trappers, such as Tal, with TMZ may result in low tolerability.(11) Therefore, a strategy for dual-encapsulation and spatiotemporal drug-release may enhance therapy and reduce unwanted toxicities.(40)
Interest in tissue specific targeting agents has grown as reports have noted the limitation of nanoparticle delivery to tumors.(23,24) An ideal target would have minimal baseline expression and naturally high or externally stimulated expression in target tissue. P-sel expression plays a critical role in the recruitment of inflammatory cells and may be increased in inflammatory cancers such as the CT26.wt model.(41,42) This was first demonstrated by Shamay et al. where high base levels of P-sel were identified in several human cancer types.(31) Furthermore, P-sel expression can be modulated utilizing a therapeutic external modality (RT).(28) In the present study, P-sel expression and subsequent nanoparticle targeting was evaluated at select timepoints and radiation doses. P-sel expression appeared time-dependent both in vitro and in vivo. Additionally, fucoidan coating provided enhanced uptake in P-sel expressing cells or tissue regardless of IR administration over a control. However, comparing CT26.wt and bEnd.3 cells revealed interesting differences in nMOF uptake following a single dose of 2 Gy. IR stimulated increased uptake for all nMOF coatings in bEnd.3 cells, with Hf-BDC-Fuco eliciting the maximal increase. On the other hand, IR decreased uptake of Hf-BDC and Hf-BDC-Dex in CT26.wt cells while Hf-BDC-Fuco still saw an increase, possibly indicating an alteration in endocytic processing. In theory, receptor recycling pathways including P-sel, could be upregulated after IR while other non-specific endocytic pathways may be down-regulated.(43,44) Differential alterations in endosomal activity between cancer and endothelial cells may explain the selective increase in post-IR Hf-BDC-Fuc uptake in CT26 cells compared to other coatings. Studies evaluating the influence of IR on various endocytic pathways and receptor recycling may be warranted when optimizing tumoral accumulation of Fuco-modified nMOFs.
To gain further insight into the radiobiologic influence of treatment, radiation enhancement ratio (RER) values were calculated and graphed.(45) Here, RER>1 indicates increased efficacy and RER<1 indicatives decreased efficacy. Hf-BDC-Fuco’s radioenhancing ability is demonstrated at 4 and 5 Gy (Fig. 5F), stronger radioenhancement at lower IR doses may be elicited through nMOF concentration optimization. Tal@Hf-BDC-Fuco demonstrates increasing radioenhancement with increasing IR dose. The influence of Tal is clearly evident as TMZ@Hf-BDC-Fuco only demonstrates slight enhancement at the highest IR dose. This may be a result of Tal’s ability to inhibit DNA damage repair and/or its ability to induce G2/M phase cell cycle arrest, as others have found that combining Tal or Tal/TMZ with radiation increases the fraction of cells stalled in G2/M phase.(12) In addition, the increased efficacy of TT@Hf-BDC-Fuco may arise due to the chemopotentiating role of PARP-trapping when Tal and TMZ are combined.(15) The focus of PARPi’s has shifted from application in BRCA deficient models to wider utility and these results further confirm the ability of Tal to act as a radiosensitizer in a BRCA proficient model.
One of the most promising results of TT@Hf-BDC-Fuco + IR was the complete response of a mouse treated with this therapeutic regimen. In a study by Baldwin et al. 40% of mice treated with a nano-formulation of Tal (NanoTLZ) and oral TMZ saw a complete response. (9) In comparison, the cure rate for the present study was 14% for TT@Hf-BDC-Fuco+ IR. Comparing cumulative doses of Tal and TMZ between these studies demonstrates similar Tal dose (3 vs. 3.6 mg/kg) and a 5.5-fold reduction in TMZ dose (250 vs 44 mg/kg). Total dose could be increased to enhance therapeutic efficacy with this system, however, the dose and regimen were selected based on reports of high toxicity for combined TMZ/Tal regimens. (9,13) In this study, a dosing schedule similar to that employed by Kizilbash et al. for free TMZ and Tal in a glioblastoma model was utilized due to its promising results and minimized toxicity.(13) In the aforementioned study, animals were dosed at 5 mg/kg TMZ 1×5d and 0.15 mg/kg Tal 2×5d, repeated every 14 d, while in this study a dosing regimen of 4.4 mg/kg TMZ 2×5d and 0.36 mg/kg Tal 2×5d, not repeated, was utilized. As drug toxicity is typically lower with nanoparticle formulations, we chose to increase both the concentration of Tal and the frequency of TMZ dosing, without adverse responses. Comparably, the model study did not result in any complete responses, while this was possible in the regimen of this study.(13) Importantly, as compared to the pilot study, dosing was not re-initiated 7 days after the final treatment day in the efficacy study due to unforeseen circumstances (COVID-19 restrictions), which may account for the lack of an increase in survival and complete responses.
Further evidence of enhanced efficacy was provided by histological evaluation of H&E stained tumor tissues. Here, histology revealed that 14- and 21-days post-treatment initiation, the development a hypoxic/necrotic core was reduced for TT@Hf-BDC-Fuco + IR (Figure 7F). It is well-understood that hypoxia decreases the efficacy of therapeutic interventions including RT and chemotherapy.(46) As such, the ability of TT@Hf-BDC-Fuco + IR to decrease hypoxia warrants further investigation. Decreased hypoxia in the TT@Hf-BDC-Fuco + IR treatment may be a result of vascular normalization, which is supported by the observed increase in CD31 expression 24 hrs after a dose of 6 Gy. This correlates well with data that radiation can cause vascular normalization and increased therapeutic delivery to tumors.(47) While RT-induced vascular normalization could increase therapeutic efficacy (increased delivery) and minimize chemoresistance (decreased hypoxia), a simultaneous increase in P-sel expression would lead to an even greater amount of therapeutic delivery and efficacy.
In addition to a 14% cure rate, a significantly reduced tumor volume and tumor growth rate, increased survival, and evidence of decreased tumor density were observed. Future studies may benefit from alterations such as reduced administration (once daily while maintaining daily dose from b.i.d. schedule), increased radiation dose (either per fraction or number of fractions), repeated dosing, and/or combinations with immunotherapeutic agents. Furthermore, as this study was performed with a heterotropic colorectal allograft, future studies exploring the therapeutic efficacy in an orthotopic colorectal model are encouraged. With proper optimization of these parameters, this therapeutic regimen may achieve a higher rate of complete responses.
5. Conclusions
While RT has long been a mainstay for the treatment of cancer, the need to improve outcomes through multimodality treatment is ever increasing. Here, we demonstrate the application of the HF-BDC nMOF for use as a radioenhancing and drug delivery vehicle. Fucoidan surface functionalization provided enhanced nMOF accumulation over multiple controls in vitro and in vivo, demonstrating the vast potential for enhanced tumor accumulation and subsequent therapeutic delivery. Furthermore, the combination of TMZ and Tal was synergistic and radio-potentiating as both free drugs and encapsulated agents in vitro and as co-encapsulated agents in vivo. When administered to tumor-bearing mice and activated with IR, these TT@Hf-BDC-Fuco nMOFs demonstrated tumor control (reduced volume, density, and growth rate) and increased survival. Finally, drug-loading and nMOF coating was facile, indicating the potential ease of scale-up for clinical application. Ultimately, this report demonstrates the value of combining Tal and TMZ with P-sel targeted, and radiation dose enhancing nMOFs for enhanced chemoradiotherapy in a heterotopic, syngeneic CRC model.
Supplementary Material
HIGHLIGHTS.
A novel fucoidan-coated nanoscale metal organic framework (nMOF) carrier was developed for targeted drug delivery during chemoradiation.
Co-delivery of a PARP inhibitor talazoparib and chemotherapeutic temozolomide by the nMOF demonstrated treatment efficacy in vitro and in vivo.
This novel formulation preferentially targets P-selectin, which is over-expressed or translocated in irradiated tumors.
The integrated multimodal treatment demonstrated enhanced tumor control and increased survival in a syngeneic colorectal cancer mouse model.
Acknowledgements
The authors thank the Histology, Multiscale Microscopy, and Advanced Light Microscopy Cores at Oregon Health & Sciences University for their assistance with various procedures presented in this study.
Funding Sources
This work was supported by the National Institutes of Health NIGMS Maximizing Investigators’ Research Award 1R35GM119839–01 (C.S.) and Oregon State University College of Pharmacy Start-up Funds.
Abbreviations
- nMOFs
nanoscale metal organic frameworks
- P-sel
P-selectin
- PSGL-1
P-sel glycoprotein ligand-1
- Fuco
fucoidan
- Hf-BDC-Fuco
fucoidan-coated hafnium and 1,4-dicarboxybenze nMOF
- Hf-BDC-Dex
dextran-coated hafnium and 1,4-dicarboxybenze nMOF
- TT@Hf-BDC-Fuco
temozolomide/talazoparib-loaded Hf-BDC-Fuco
- IR
irradiation
- CI
combination index
- DLS
dynamic light scattering
- TEM
transmission electron microscopy
- RER
radiation enhancement ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
SUPPORTING INFORMATION
Supplementary data for this article are available online.
References
- 1.Willett C, Chino F, Palta M, Czito B. Radiation Therapy in Colon Carcinoma. In: Wenz F, editor. Radiation Oncology [Internet]. Cham: Springer International Publishing; 2018. [cited 2019 Nov 18]. page 1–15. Available from: 10.1007/978-3-319-52619-5_46-1 [DOI] [Google Scholar]
- 2.Kamran SC, Mamon HJ, Wo JY. Rectal and Colon Cancer: Radiation Therapy Planning. In: Hong T, Das P, editors. Radiation Therapy for Gastrointestinal Cancers [Internet]. Cham: Springer International Publishing; 2017. [cited 2019 Nov 18]. page 171–80. Available from: 10.1007/978-3-319-43115-4_14 [DOI] [Google Scholar]
- 3.Pishvaian MJ, Slack RS, Jiang W, He AR, Hwang JJ, Hankin A, et al. A phase 2 study of the PARP inhibitor veliparib plus temozolomide in patients with heavily pretreated metastatic colorectal cancer. Cancer. 2018;124:2337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reilly NM, Novara L, Nicolantonio FD, Bardelli A. Exploiting DNA repair defects in colorectal cancer. Molecular Oncology. 2019;13:681–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horton TM, Jenkins G, Pati D, Zhang L, Dolan ME, Ribes-Zamora A, et al. Poly(ADP-ribose) polymerase inhibitor ABT-888 potentiates the cytotoxic activity of temozolomide in leukemia cells: influence of mismatch repair status and O6-methylguanine-DNA methyltransferase activity. Mol Cancer Ther. 2009;8:2232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grignani G, Merlini A, Sangiolo D, D’Ambrosio L, Pignochino Y. Delving into PARP inhibition from bench to bedside and back. Pharmacology & Therapeutics. 2020;206:107446. [DOI] [PubMed] [Google Scholar]
- 7.Donawho CK, Luo Y, Luo Y, Penning TD, Bauch JL, Bouska JJ, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007;13:2728–37. [DOI] [PubMed] [Google Scholar]
- 8.Rationale for Poly(ADP-ribose) Polymerase (PARP) Inhibitors in Combination Therapy with Camptothecins or Temozolomide Based on PARP Trapping versus Catalytic Inhibition | Journal of Pharmacology and Experimental Therapeutics [Internet]. [cited 2020 Jul 17]. Available from: http://jpet.aspetjournals.org.ezproxy.proxy.library.oregonstate.edu/content/349/3/408.short [DOI] [PMC free article] [PubMed]
- 9.Baldwin P, Likhotvorik R, Baig N, Cropper J, Carlson R, Kurmasheva R, et al. Nanoformulation of Talazoparib Increases Maximum Tolerated Doses in Combination With Temozolomide for Treatment of Ewing Sarcoma. Front Oncol [Internet]. Frontiers; 2019. [cited 2020 May 20];9. Available from: 10.3389/fonc.2019.01416/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lok BH, Gardner EE, Schneeberger VE, Ni A, Desmeules P, Rekhtman N, et al. PARP Inhibitor Activity Correlates with SLFN11 Expression and Demonstrates Synergy with Temozolomide in Small Cell Lung Cancer. Clin Cancer Res. 2017;23:523–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta SK, Smith EJ, Mladek AC, Tian S, Decker PA, Kizilbash SH, et al. PARP Inhibitors for Sensitization of Alkylation Chemotherapy in Glioblastoma: Impact of Blood-Brain Barrier and Molecular Heterogeneity. Front Oncol [Internet]. 2019. [cited 2020 Apr 29];8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6349736/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lesueur P, Chevalier F, El-Habr EA, Junier M-P, Chneiweiss H, Castera L, et al. Radiosensitization Effect of Talazoparib, a Parp Inhibitor, on Glioblastoma Stem Cells Exposed to Low and High Linear Energy Transfer Radiation. Scientific Reports. Nature Publishing Group; 2018;8:3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kizilbash SH, Gupta SK, Chang K, Kawashima R, Parrish KE, Carlson BL, et al. Restricted delivery of talazoparib across the blood-brain barrier limits the sensitizing effects of poly (ADP-ribose) polymerase inhibition on temozolomide therapy in glioblastoma. Mol Cancer Ther. 2017;16:2735–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Zhang S, Song L, Qu M, Zou Z. Synergistic lethality between PARP-trapping and alantolactone-induced oxidative DNA damage in homologous recombination-proficient cancer cells. Oncogene. Nature Publishing Group; 2020;39:2905–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.PARP1 Trapping by PARP Inhibitors Drives Cytotoxicity in Both Cancer Cells and Healthy Bone Marrow | Molecular Cancer Research [Internet]. [cited 2020 Jul 17]. Available from: https://mcr-aacrjournals-org.liboff.ohsu.edu/content/17/2/409 [DOI] [PubMed]
- 16.Laird JH, Lok BH, Ma J, Bell A, Stanchina ED, Poirier J, et al. PARP trapping by Talazoparib is a Potent Mechanism of Radiosensitization in Small Cell Lung Cancer Cell Lines and Patient-Derived Xenografts. International Journal of Radiation Oncology • Biology • Physics. Elsevier; 2018;102:e184–5. [Google Scholar]
- 17.Wang L, Zheng M, Xie Z. Nanoscale metal–organic frameworks for drug delivery: a conventional platform with new promise. J Mater Chem B. 2018;6:707–17. [DOI] [PubMed] [Google Scholar]
- 18.Carné A, Carbonell C, Imaz I, Maspoch D. Nanoscale metal–organic materials. Chem Soc Rev. 2010;40:291–305. [DOI] [PubMed] [Google Scholar]
- 19.Lan G, Ni K, Veroneau SS, Song Y, Lin W. Nanoscale Metal–Organic Layers for Radiotherapy–Radiodynamic Therapy. J Am Chem Soc. 2018;140:16971–5. [DOI] [PubMed] [Google Scholar]
- 20.Lu K, He C, Guo N, Chan C, Ni K, Lan G, et al. Low-dose X-ray radiotherapy–radiodynamic therapy via nanoscale metal–organic frameworks enhances checkpoint blockade immunotherapy. Nat Biomed Eng. 2018;2:600–10. [DOI] [PubMed] [Google Scholar]
- 21.Ni K, Lan G, Veroneau SS, Duan X, Song Y, Lin W. Nanoscale metal-organic frameworks for mitochondria-targeted radiotherapy-radiodynamic therapy. Nat Commun. 2018;9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Yang Y, Zhu W, Yi X, Dong Z, Xu X, et al. Nanoscale metal−organic frameworks for combined photodynamic & radiation therapy in cancer treatment. Biomaterials. 2016;97:1–9. [DOI] [PubMed] [Google Scholar]
- 23.Price LSL, Stern ST, Deal AM, Kabanov AV, Zamboni WC. A reanalysis of nanoparticle tumor delivery using classical pharmacokinetic metrics. Science Advances. American Association for the Advancement of Science; 2020;6:eaay9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, et al. Analysis of nanoparticle delivery to tumours. Nature Reviews Materials. Nature Publishing Group; 2016;1:1–12. [Google Scholar]
- 25.Hallahan DE, Virudachalam S. Ionizing radiation mediates expression of cell adhesion molecules in distinct histological patterns within the lung. Cancer Res. 1997;57:2096–9. [PubMed] [Google Scholar]
- 26.Corso CD, Ali AN, Diaz R. Radiation-induced tumor neoantigens: imaging and therapeutic implications. Am J Cancer Res. 2011;1:390–412. [PMC free article] [PubMed] [Google Scholar]
- 27.Hariri G, Yan H, Wang H, Han Z, Hallahan DE. Radiation-guided drug delivery to mouse models of lung cancer. Clin Cancer Res. 2010;16:4968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hallahan DE, Staba-Hogan MJ, Virudachalam S, Kolchinsky A. X-ray-induced P-selectin localization to the lumen of tumor blood vessels. Cancer Res. 1998;58:5216–20. [PubMed] [Google Scholar]
- 29.Mollà M, Gironella M, Salas A, Miquel R, Pérez‐del‐Pulgar S, Conill C, et al. Role of P-selectin in radiation-induced intestinal inflammatory damage. International Journal of Cancer. 2001;96:99–109. [DOI] [PubMed] [Google Scholar]
- 30.Ferber S, Tiram G, Sousa-Herves A, Eldar-Boock A, Krivitsky A, Scomparin A, et al. Co-targeting the tumor endothelium and P-selectin-expressing glioblastoma cells leads to a remarkable therapeutic outcome. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shamay Y, Elkabets M, Li H, Shah J, Brook S, Wang F, et al. P-selectin is a nanotherapeutic delivery target in the tumor microenvironment. Science Translational Medicine. 2016;8:345ra87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bachelet L, Bertholon I, Lavigne D, Vassy R, Jandrot-Perrus M, Chaubet F, et al. Affinity of low molecular weight fucoidan for P-selectin triggers its binding to activated human platelets. Biochimica et Biophysica Acta (BBA) - General Subjects. 2009;1790:141–6. [DOI] [PubMed] [Google Scholar]
- 33.Lu K-Y, Li R, Hsu C-H, Lin C-W, Chou S-C, Tsai M-L, et al. Development of a new type of multifunctional fucoidan-based nanoparticles for anticancer drug delivery. Carbohydrate Polymers. 2017;165:410–20. [DOI] [PubMed] [Google Scholar]
- 34.Xiang Li, Wolfgang Bauer, Ina Israel, Kreissl Michael C, Johannes Weirather, Dominik Richter, et al. Targeting P-Selectin by Gallium-68–Labeled Fucoidan Positron Emission Tomography for Noninvasive Characterization of Vulnerable Plaques. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34:1661–7. [DOI] [PubMed] [Google Scholar]
- 35.Shin S-W, Jung W, Choi C, Kim S-Y, Son A, Kim H, et al. Fucoidan-Manganese Dioxide Nanoparticles Potentiate Radiation Therapy by Co-Targeting Tumor Hypoxia and Angiogenesis. Mar Drugs [Internet]. 2018. [cited 2019 Nov 14];16. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6316049/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mizrachi A, Shamay Y, Shah J, Brook S, Soong J, Rajasekhar VK, et al. Tumour-specific PI3K inhibition via nanoparticle-targeted delivery in head and neck squamous cell carcinoma. Nature Communications. 2017;8:14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shahbazi J, Liu PY, Atmadibrata B, Bradner JE, Marshall GM, Lock RB, et al. The Bromodomain Inhibitor JQ1 and the Histone Deacetylase Inhibitor Panobinostat Synergistically Reduce N-Myc Expression and Induce Anticancer Effects. Clin Cancer Res. American Association for Cancer Research; 2016;22:2534–44. [DOI] [PubMed] [Google Scholar]
- 38.Neufeld MJ, DuRoss AN, Landry MR, Winter H, Goforth AM, Sun C. Co-delivery of PARP and PI3K inhibitors by nanoscale metal–organic frameworks for enhanced tumor chemoradiation. Nano Res [Internet]. 2019. [cited 2019 Nov 13]; Available from: 10.1007/s12274-019-2544-z [DOI]
- 39.Growth rate analysis and efficient experimental design for tumor xenograft studies. - Abstract - Europe PMC [Internet]. [cited 2020 Jul 10]. Available from: https://europepmc.org/article/med/25574127 [DOI] [PMC free article] [PubMed]
- 40.Godsey ME, Suryaprakash S, Leong KW. Materials innovation for co-delivery of diverse therapeutic cargos. RSC Adv. 2013;3:24794–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tuo Y, Zhang Z, Tian C, Hu Q, Xie R, Yang J, et al. Anti-inflammatory and metabolic reprogramming effects of MENK produce antitumor response in CT26 tumor-bearing mice. Journal of Leukocyte Biology [Internet]. John Wiley & Sons, Ltd; 2020. [cited 2020 May 23];n/a. Available from: 10.1002/JLB.3MA0120-578R [DOI] [PubMed] [Google Scholar]
- 42.Miller S, Senior PV, Prakash M, Apostolopoulos V, Sakkal S, Nurgali K. Leukocyte populations and IL-6 in the tumor microenvironment of an orthotopic colorectal cancer model. Acta Biochim Biophys Sin (Shanghai). 2016;48:334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. Journal of Controlled Release. 2010;145:182–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel S, Kim J, Herrera M, Mukherjee A, Kabanov AV, Sahay G. Brief update on endocytosis of nanomedicines. Advanced Drug Delivery Reviews. 2019;144:90–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roeske JC, Nuñez L, Hoggarth M, Labay E, Weichselbaum RR. Characterization of the Theorectical Radiation Dose Enhancement from Nanoparticles: Technology in Cancer Research & Treatment [Internet]. SAGE PublicationsSage CA: Los Angeles, CA; 2016. [cited 2020 May 29]; Available from: 10.1177/153303460700600504 [DOI] [PubMed] [Google Scholar]
- 46.Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment [Internet]. [cited 2020 Jun 21]. Available from: https://www-ncbi-nlm-nih-gov.liboff.ohsu.edu/pmc/articles/PMC6177375/ [DOI] [PMC free article] [PubMed]
- 47.Potiron V, Clément-Colmou K, Jouglar E, Pietri M, Chiavassa S, Delpon G, et al. Tumor vasculature remodeling by radiation therapy increases doxorubicin distribution and efficacy. Cancer Letters. 2019;457:1–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


